Preparation and Properties of Poly(vinyl acetate) Adhesive Modified with Vinyl Versatate
Abstract
:1. Introduction
2. Results and Discussion
2.1. ATR-FTIR Spectroscopy of HVPVAc Films
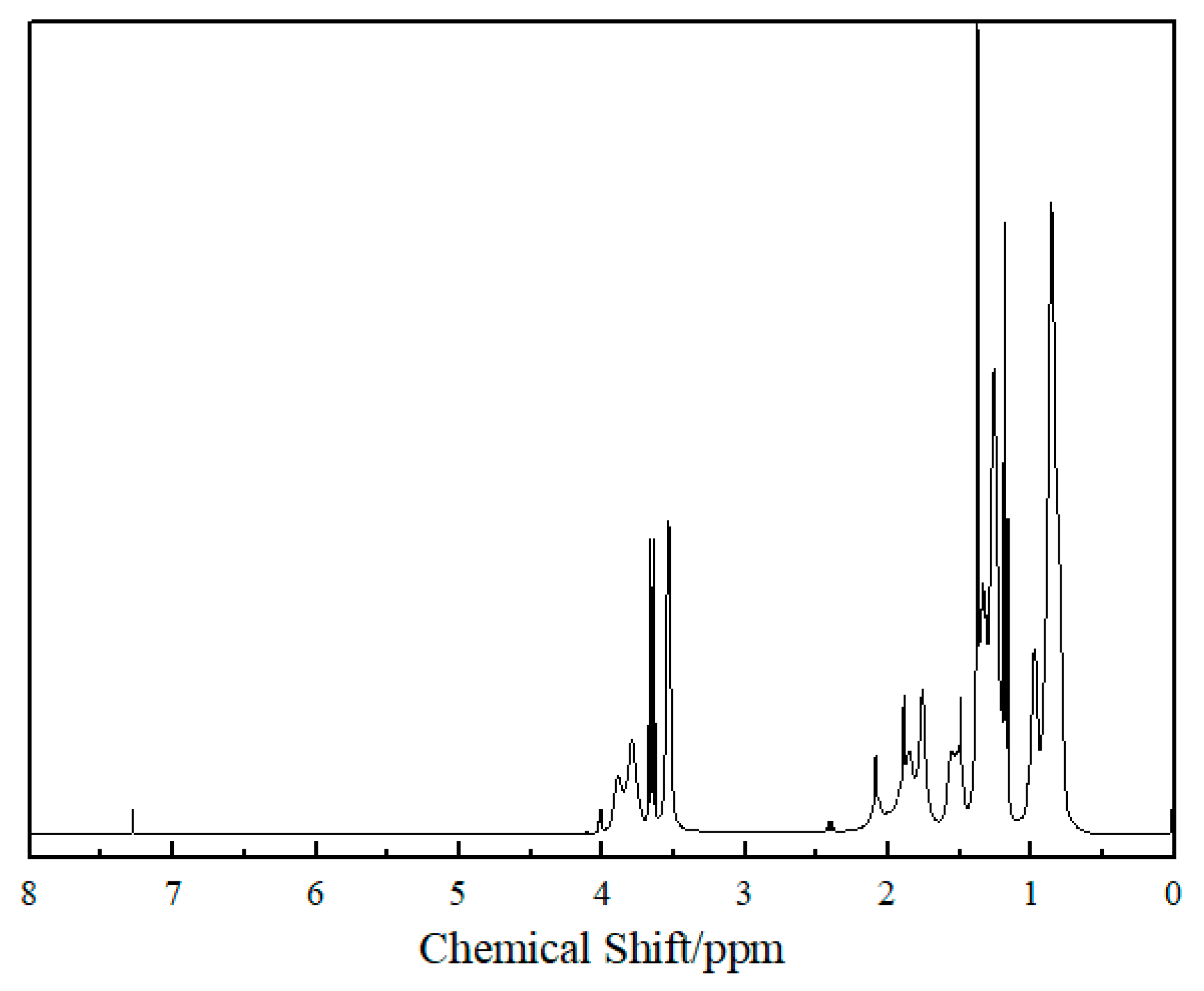
2.2. Nuclear Magnetic Resonance Spectrum of HVPVAc Sample
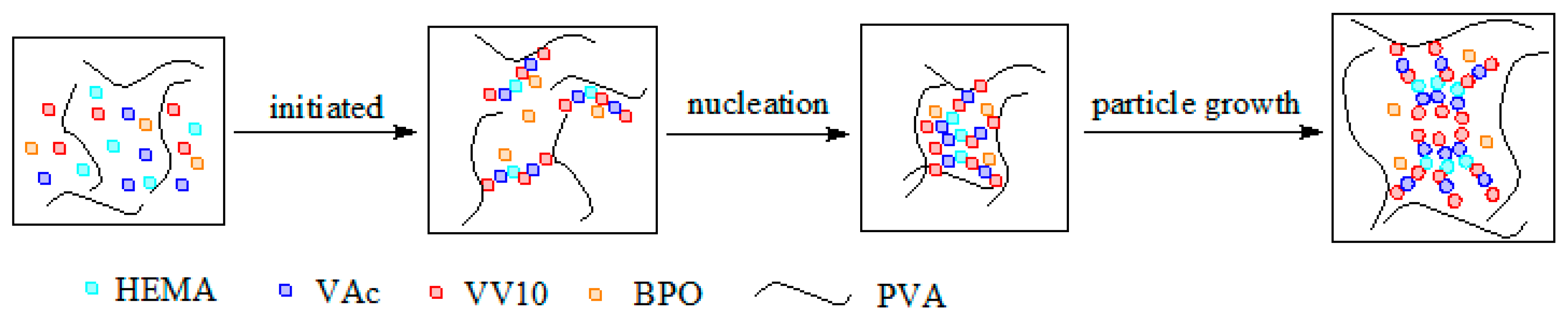
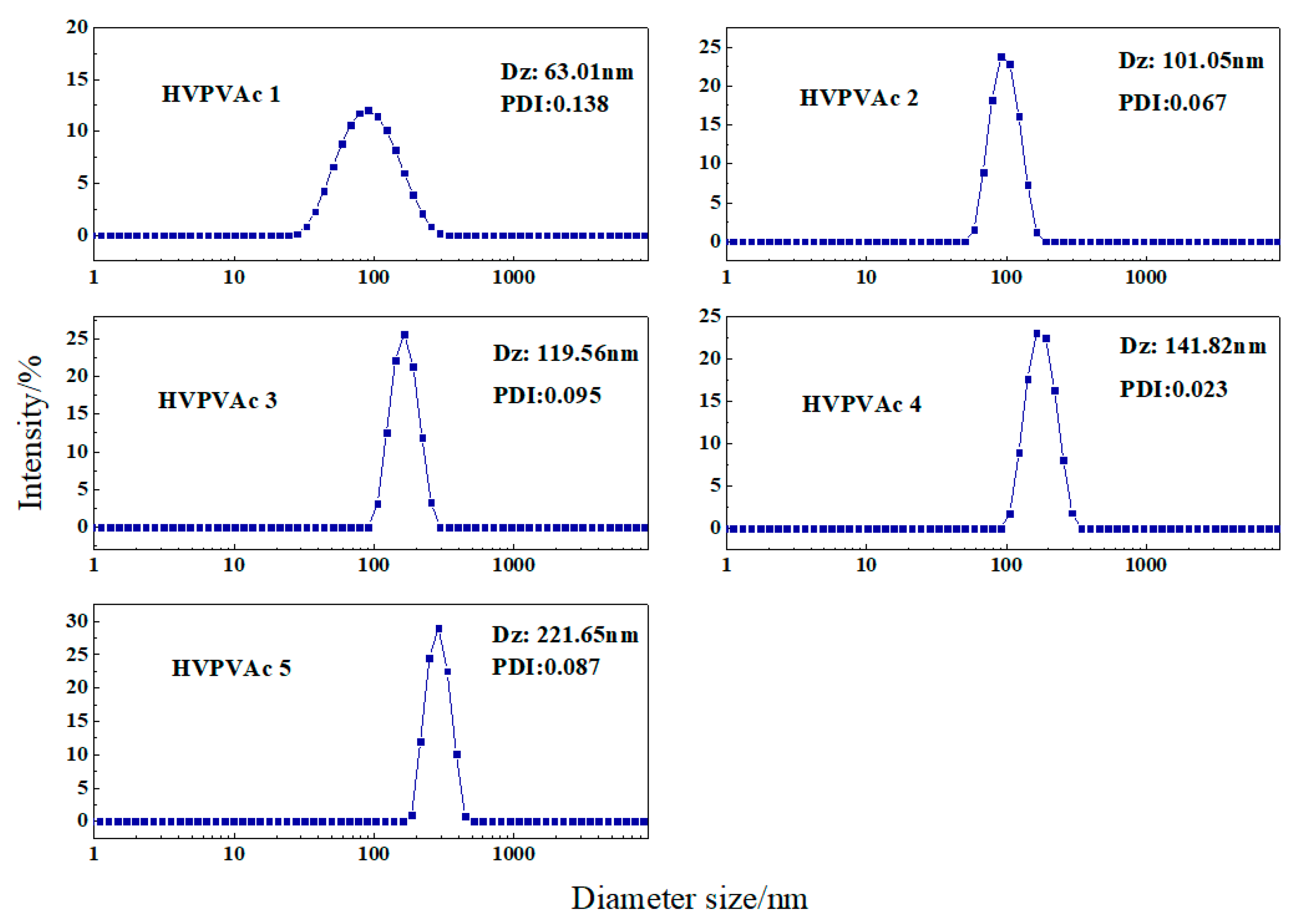
2.3. Effect of the VV10 Content on the Particle Size of the HVPVAc Samples
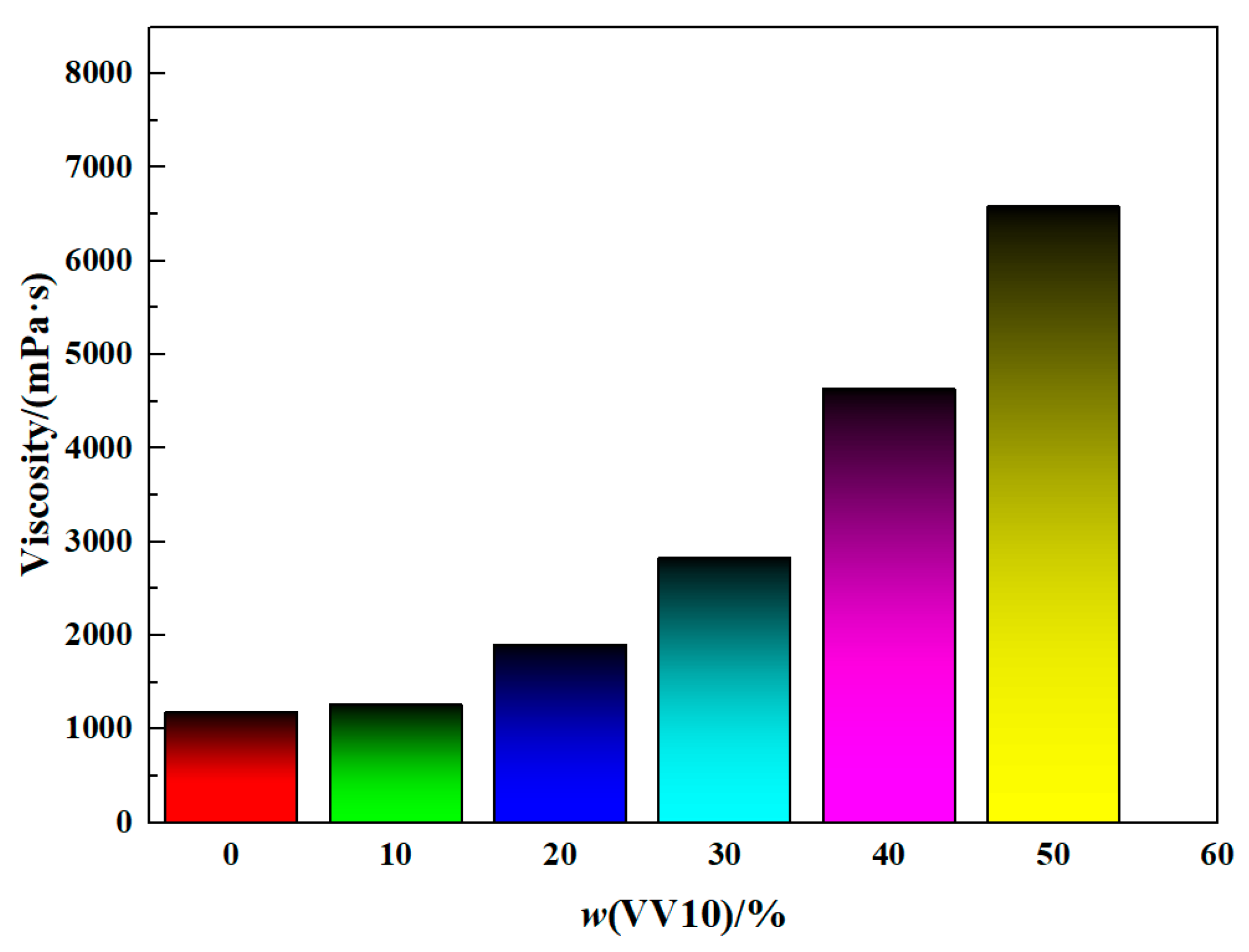
2.4. Effect of the VV10 Content on the Viscosity of HVPVAc Latexes
2.5. Molecular Weight of HVPVAc Using the GPC Method
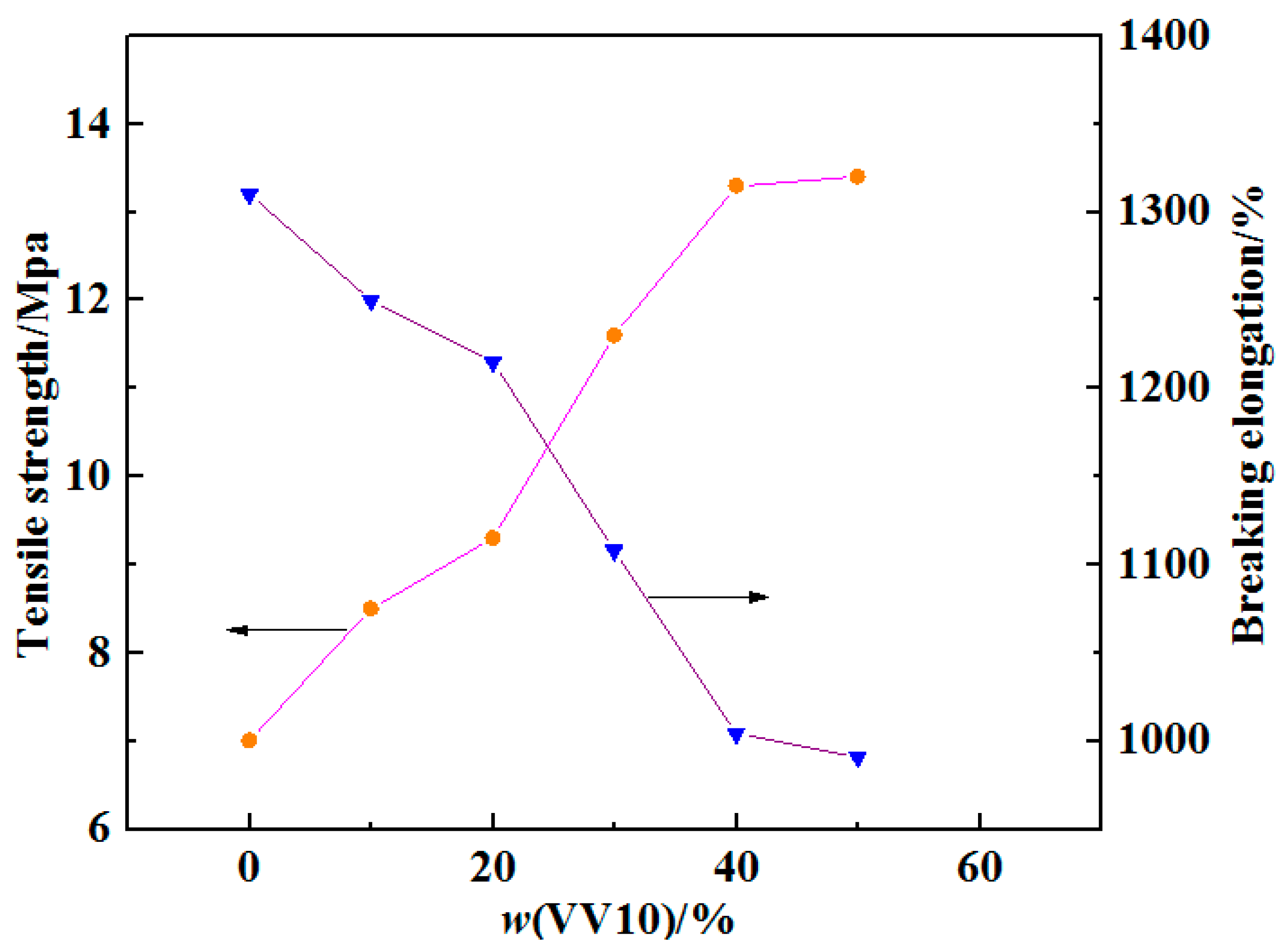
2.6. Effect of the VV10 Content on the Mechanical Properties of the HVPVAc Adhesives
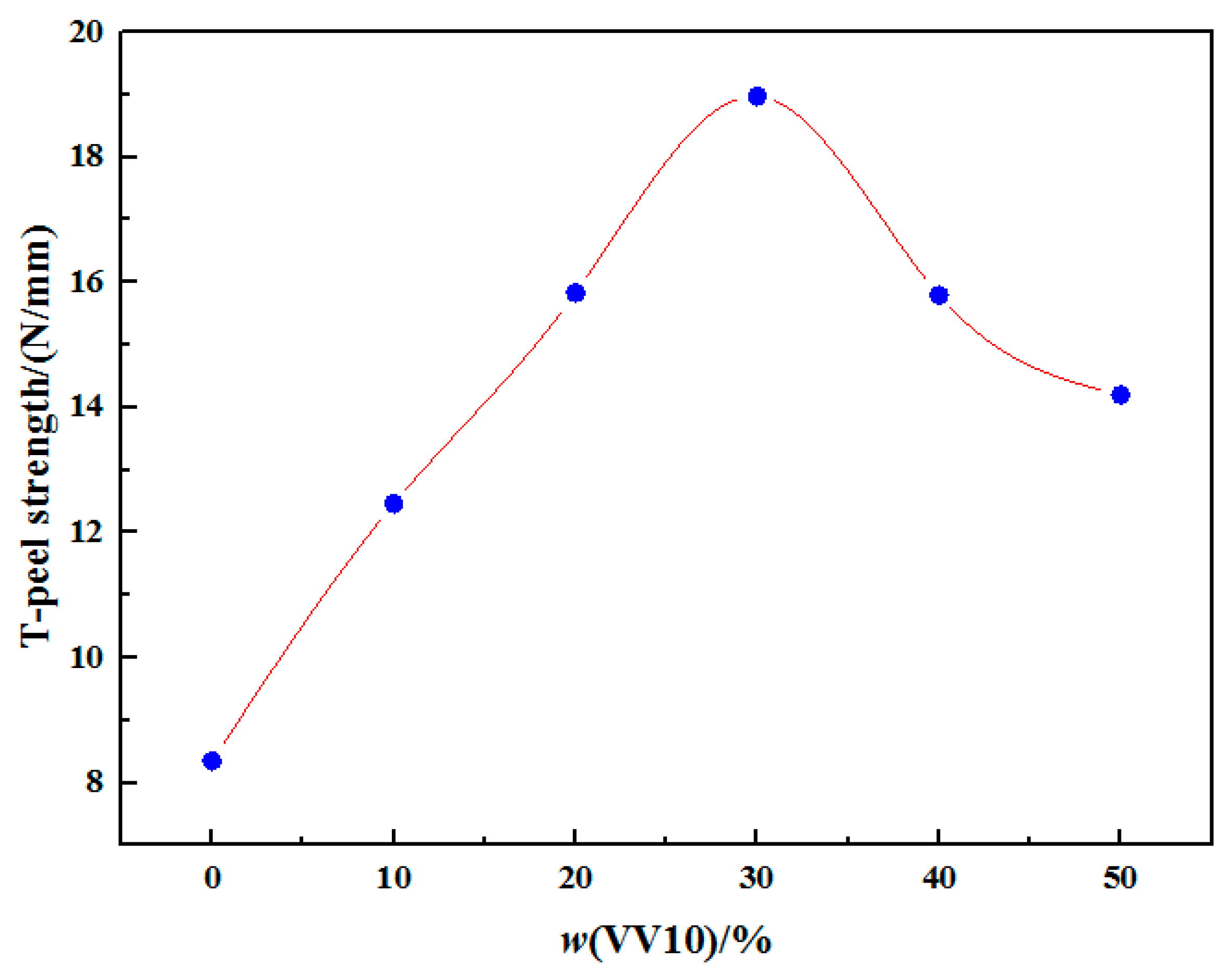
2.7. Effect of the VV10 Content on the T-Peel Strength of the Samples
2.8. Contact Angle of HVPVAc Films with Different VV10 Content
3. Experimental
3.1. Materials
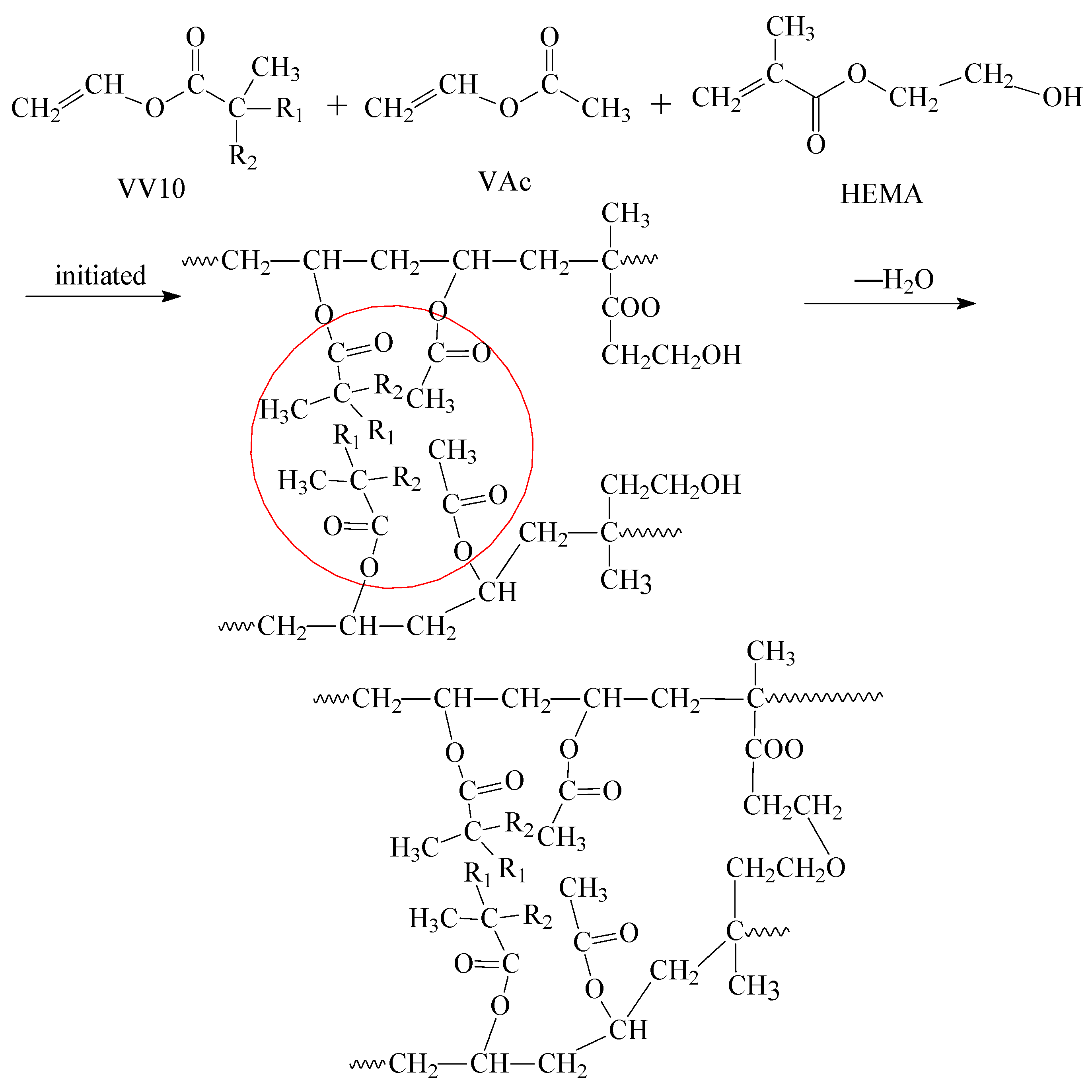
3.2. Preparation of Poly(vinyl acetate) Adhesive Modified with Vinyl Versatate
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cordeiro, C.F.; Petrocelli, F.P. Vinyl Acetate Polymers. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Marmorato, G.C.E.; Pellegrino, F.O.; Roberto, F.M. Influence of vinyl acetate-versatic vinylester copolymer on the microstructural characteristics of cement pastes. Mater. Res. Express 2005, 8, 51–56. [Google Scholar]
- Jewell, G.W. Latex Properties: Effect of Monomer Composition. In Surface Coatings; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Magallanes González, G.S.; Dimonie, V.L.; Sudol, E.D.; Yue, H.J.; Klein, A.; El-Aasser, M.S. Characterization of poly(vinyl alcohol) during the emulsion polymerization of vinyl acetate using poly(vinyl alcohol) as emulsifier. J. Polym. Sci. Pol. Chem. 1996, 34, 849–862. [Google Scholar] [CrossRef]
- Froehling, P.E. Crosslinking of unsaturated polyester resins by combinations of vinyl esters and methacrylates. J. Appl. Polym. Sci. 1982, 27, 3577–3584. [Google Scholar] [CrossRef]
- Guo, J.; Choi, K.Y.; Schork, F.J. Miniemulsion copolymerization of ethylene and vinyl acetate. Macromol. React. Eng. 2009, 3, 412–418. [Google Scholar] [CrossRef]
- Feiteira, J.; Ribeiro, M.S. Polymer action on alkali–silica reaction in cement mortar. Cem. Concr. Res. 2013, 44, 97–105. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, X.; Creighton, A.T.; Peterson, M.M. Effect of styrene–butadiene rubber latex on the chloride permeability and microstructure of Portland cement mortar. Constr. Build. Mater. 2009, 23, 2283–2290. [Google Scholar] [CrossRef]
- Smith, O.W.; Collins, M.J.; Martin, P.S.; Bassett, D.R. New vinyl ester monomers for emulsion polymers. Prog. Org. Coat. 1993, 22, 19–25. [Google Scholar] [CrossRef]
- Geng, W.B.; Shen, Y.H.; Huang, C.; Du, H.Y.; Du, H.X. Effect of poly(vinyl ester of versatic 10-vinyl acetate) emulsion on modified cement mortars. J. Chin. Ceram. Soc. 2015, 43, 1129–1134. [Google Scholar]
- Wu, J.; Li, H.; Winnik, M.A.; Farwaha, R.; Rademacher, J. Poly(vinyl acetate-co-dibutyl maleate) latex films in the presence of grafted and post-added poly(vinyl alcohol). J. Polym. Sci. Pol. Chem. 2004, 42, 5005–5020. [Google Scholar] [CrossRef]
- Rahdar, S.S.; Ahmadi, E.; Abdollahi, M.; Hemmati, M. A comprehensive study on kinetics of free-radical solution copolymerization of vinyl acetate and dibutyl maleate in chloroform. J. Polym. Res. 2014, 21, 582. [Google Scholar] [CrossRef]
- Hennemann, K.K.; Lenz, D.M. Structural methacrylate/epoxy based adhesives for aluminIum joints. Int. J. Adhes. Adhes. 2019, 89, 11–18. [Google Scholar] [CrossRef]
- Liu, B.; Sun, S.; Zhang, M.; Ren, L.; Zhang, H. Facile synthesis of large scale and narrow particle size distribution polymer particles via control particle coagulation during one-step emulsion polymerization. Colloid. Surf. A 2015, 484, 81–88. [Google Scholar] [CrossRef]
- Hitoshi, M.; Tohei, M.; Toshiyuki, A.; Toshiaki, S. New modifications of poly(vinyl alcohol)s and their applications. Br. Poly. J. 1988, 20, 345–351. [Google Scholar]
- Zhang, Z.; Fu, G.; Chen, R.; Wang, C. Study on properties of acrylate post-crosslinking emulsion. Chin. Adhes. 2019, 28, 121–124+129. [Google Scholar]
- Panta, J.; Zhang, Y.X.; Rider, A.N.; Wang, J.; Prusty, G.B. Synergetic effects of carbon nanotubes and triblock copolymer On the lap shear strength of epoxy adhesive joints. Compos. Part B Eng. 2020, 186, 107813. [Google Scholar]
- Wang, X.R.; Ma, G.Y.; Zheng, M.Y. Preparation and characterization of waterborne polyurethane/polyacrylate emulsions containing sulfonate groups. J. Coat. Technol. Res. 2018, 15, 1217–1227. [Google Scholar] [CrossRef]



| Samples | HVPVAc0 | HVPVAc1 | HVPVAc2 | HVPVAc3 | HVPVAc4 | HVPVAc5 |
|---|---|---|---|---|---|---|
| Viscosity/(mPa·s) | 1180 | 1260 | 1900 | 2830 | 4630 | 6580 |
| Samples | HVPVAc0 | HVPVAc1 | HVPVAc2 | HVPVAc3 | HVPVAc4 | HVPVAc5 |
|---|---|---|---|---|---|---|
| Contact angle/° | 50.3 | 60.3 | 62.5 | 66.9 | 67.1 | 67.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Wang, L.; Wang, X.; Wang, C.; Li, X.; Li, L.; Ma, H. Preparation and Properties of Poly(vinyl acetate) Adhesive Modified with Vinyl Versatate. Molecules 2023, 28, 6634. https://doi.org/10.3390/molecules28186634
Ma G, Wang L, Wang X, Wang C, Li X, Li L, Ma H. Preparation and Properties of Poly(vinyl acetate) Adhesive Modified with Vinyl Versatate. Molecules. 2023; 28(18):6634. https://doi.org/10.3390/molecules28186634
Chicago/Turabian StyleMa, Guoyan, Le Wang, Xiaorong Wang, Chengjun Wang, Xi Li, Lu Li, and Hongfei Ma. 2023. "Preparation and Properties of Poly(vinyl acetate) Adhesive Modified with Vinyl Versatate" Molecules 28, no. 18: 6634. https://doi.org/10.3390/molecules28186634






