A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films
Abstract
:1. Introduction
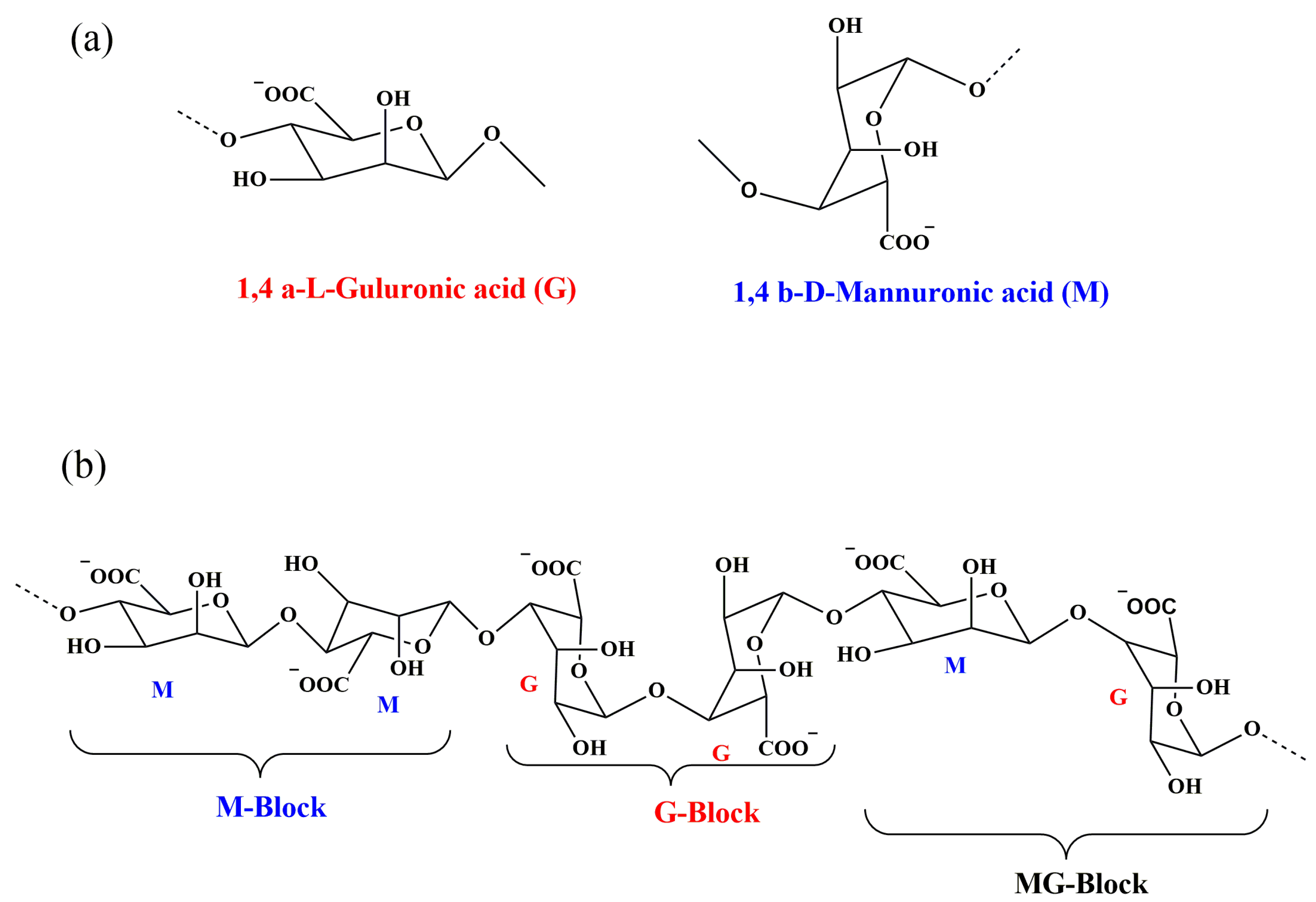
2. Alginate: Structure
3. Alginate: Properties and Application
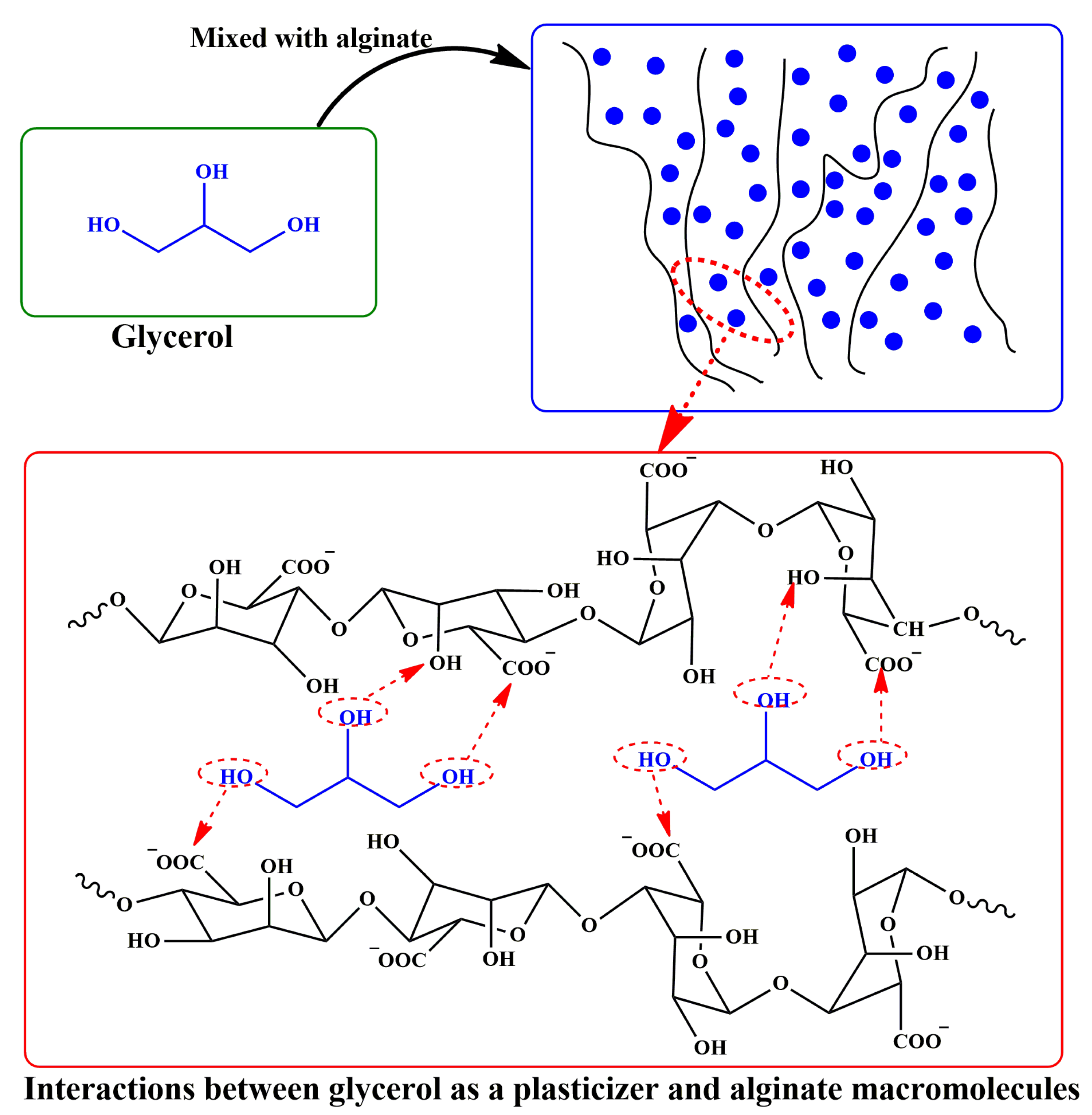
4. Plasticizers
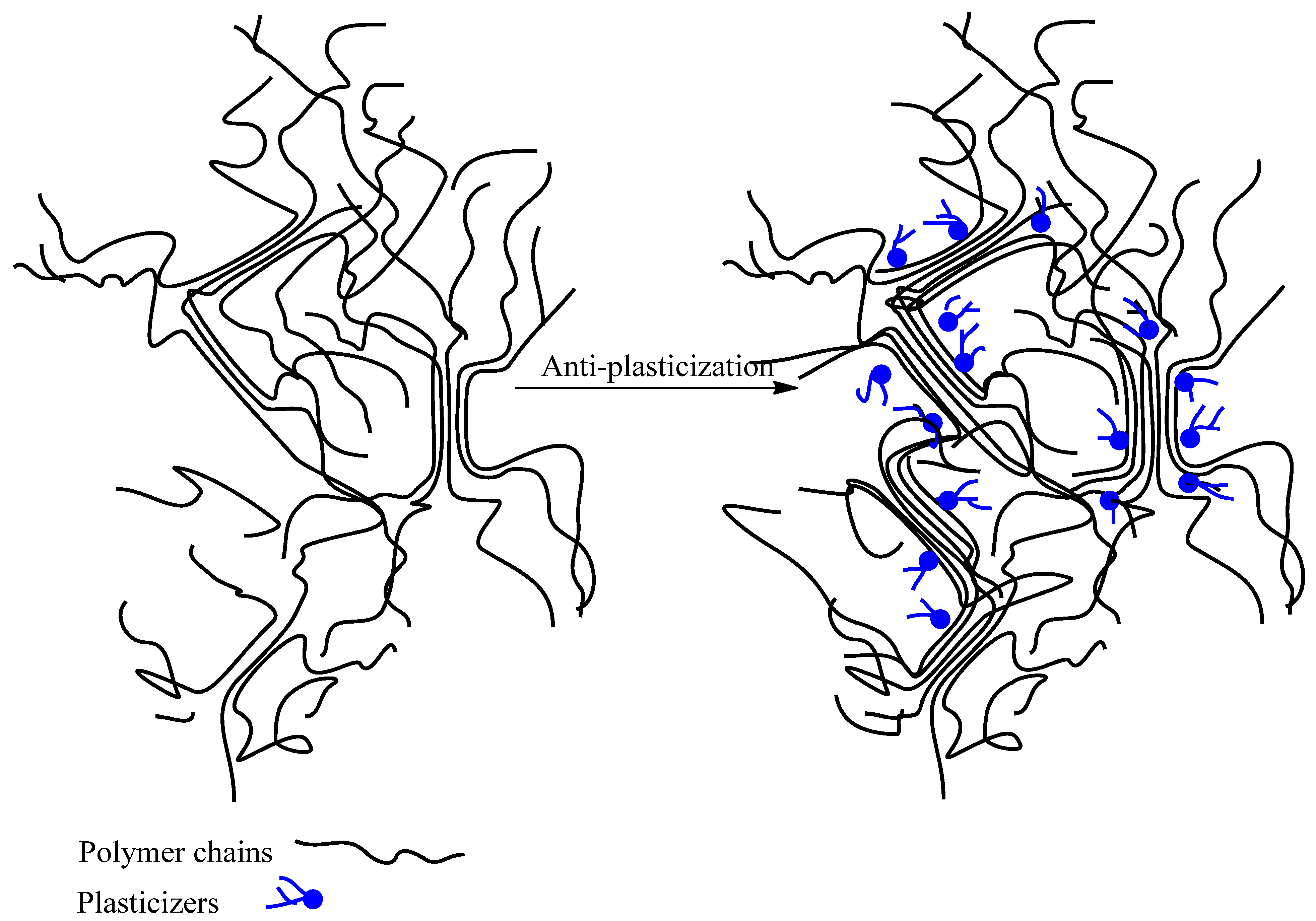
4.1. Plasticization Mechanisms
Free Volume Theory
4.2. Classification of the Plasticizers
4.2.1. Water-Soluble Plasticizers
- Water
- 2.
- Polyols
- Glycerol
- Ethylene glycol
- Sugar alcohols
- 3.
- Sugars
4.2.2. Water-Insoluble Plasticizers
- Oils
- Other water-insoluble plasticizers
5. Drawback Effects of Plasticizers on Alginate-Based Films
6. Other Plasticization Approaches
6.1. Internal Plasticization
6.2. Using Other Polymers as Plasticizers
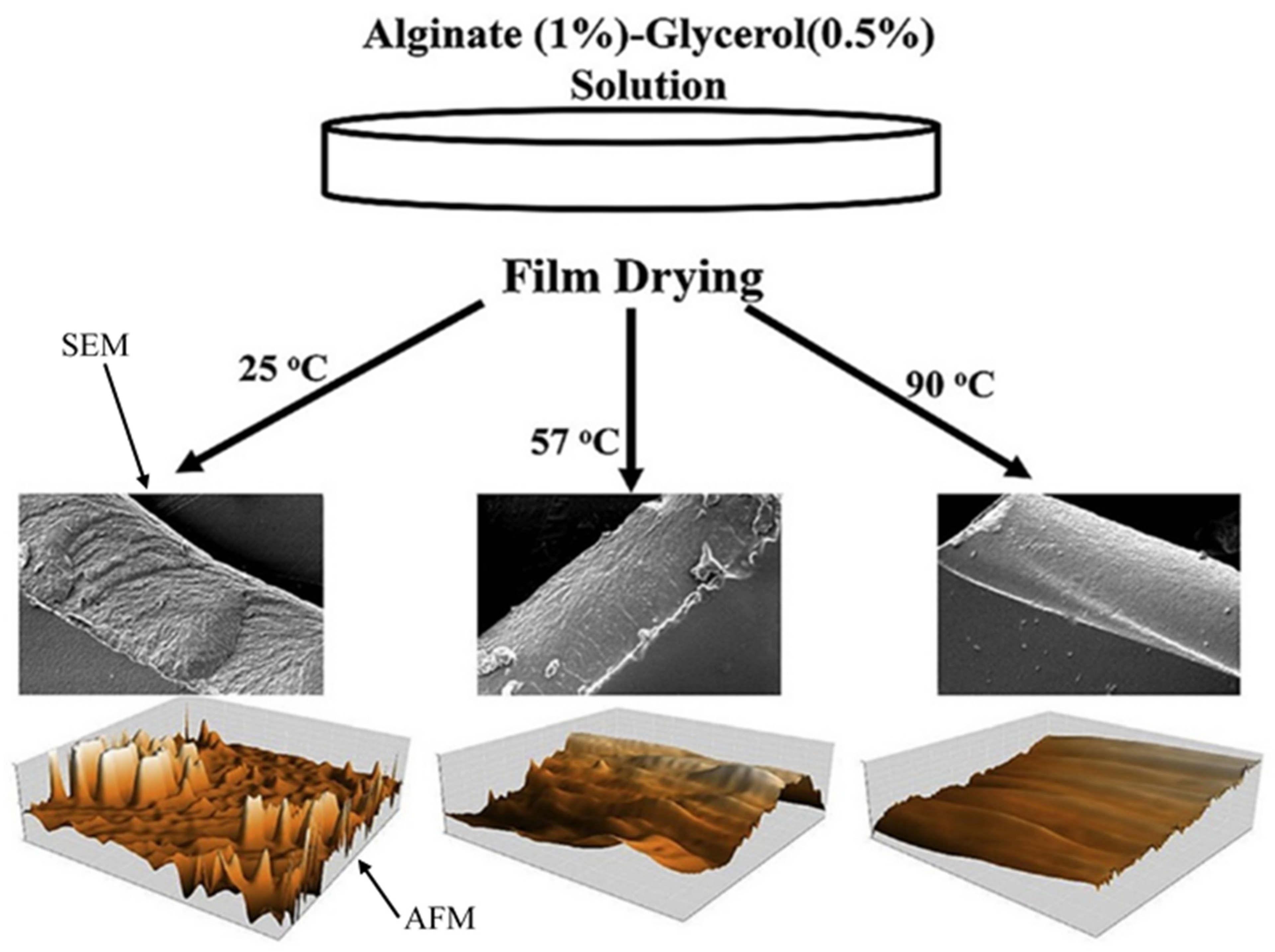
6.3. The Effect of Drying Conditions on the Plasticity of Alginate
7. Discussion
7.1. Scaling Up Challenges
7.2. Lack of Knowledge
8. Conclusions
- Pure alginate films are brittle with poor mechanical properties, and compared with synthetic plastics, possess inferior properties: hence, extra research effort is needed to improve their properties and flexibility to levels comparable with their synthetic counterparts.
- Among the plasticizers used for plasticizing alginate films, glycerol has proved to be the most studied because of its low cost and efficiency. However, glycerol is a very small molecule, highly hygroscopic, and soluble in water. These characteristics limit the use of glycerol-plasticized films in humid environments because of leaching, migration, and increasing the WVP.
- Hydrophobic plasticizers, such as oils, fatty acids, and citric acids, have the potential to decrease the hygroscopic characteristics of alginate films and reduce the WVP to make films comparable with synthetic plastics. Unfortunately, there have only been a few studies on this plasticizer.
- Segregation phenomenon (phase separation), anti-plasticization effects, leaching, migration, and evaporation from the surface are the unexpected problems associated with plasticizers. To avoid the segregation phenomenon and anti-plasticization effects, an optimal amount of plasticizer should be added to the polymer. Leaching, migration, and evaporation from the surface can be avoided by applying an effective plasticization process.
- Most of the alginate films are produced by a solvent casting method at a laboratory scale. This method is unsuitable for scaling up to an industrial scale because of the difficulties in thickness control, blending, long drying time, and high cost. Hence, special attention should be paid to this field in future works.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdullah, N.A.; Mohamad, Z.; Khan, Z.I.; Jusoh, M.; Zakaria, Z.Y.; Ngadi, N. Alginate Based Sustainable Films and Composites for Packaging: A Review. Chem. Eng. Trans. 2021, 83, 271–276. [Google Scholar] [CrossRef]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Castro-Yobal, M.A.; Contreras-Oliva, A.; Saucedo-Rivalcoba, V.; Rivera-Armenta, J.L.; Hernández-Ramírez, G.; Salinas-Ruiz, J.; Herrera-Corredor, A. Evaluation of physicochemical properties of film-based alginate for food packing applications. e-Polymers 2021, 21, 82–95. [Google Scholar] [CrossRef]
- Khairunnisa, S.; Junianto, J.; Zahidah, Z.; Rostini, I. The effect of glycerol concentration as a plasticizer on edible films made from alginate towards its physical characteristic. World Sci. News 2018, 112, 130–141. [Google Scholar]
- Galgano, F.; Condelli, N.; Favati, F.; Bianco, D.; Perretti, G.; Caruso, M. Biodegradable packaging and EDIBLE COATING for fresh-cut fruits and vegetables. Ital. J. Food Sci. 2015, 27, 1–20. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Latha, S.; Rose, M. Industrial Applications of Alginate. In Industrial Applications of Marine Biopolymers; Sudha, P., Ed.; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2017; pp. 545–575. [Google Scholar] [CrossRef]
- Moore, A. (Ed.) Alginic acid: Chemical structure, uses and health benefits. In Chemistry Research and Applications; Nova Publishers: New York, NY, USA, 2015. [Google Scholar]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Omar, A.A.; Hanafi, M.H.M.; Razak, N.H.; Ibrahim, A.; Razak, N.A.A. A Best-Evidence Review of Bio-based Plasticizer and the Effects on the Mechanical Properties of PLA. Chem. Eng. Trans. 2021, 89, 241–246. [Google Scholar] [CrossRef]
- Montilla-Buitrago, C.E.; Gómez-López, R.A.; Solanilla-Duque, J.F.; Serna-Cock, L.; Villada-Castillo, H.S. Effect of Plasticizers on Properties, Retrogradation, and Processing of Extrusion-Obtained Thermoplastic Starch: A Review. Starch Stärke 2021, 73, 2100060. [Google Scholar] [CrossRef]
- Poutanen, K.; Forssell, P. Modification of Starch Properties with Plasticizers. Trends Polym. Sci. 1996, 4, 128–132. [Google Scholar]
- MacHugh, D.J.; McHugh, D.J. (Eds.) Production and utilization of products from commercial seaweeds. In FAO Fisheries Technical Paper; No. 288; Food and Agriculture Organization of the United Nations: Rome, Italy, 1987. [Google Scholar]
- Giz, A.S.; Berberoglu, M.; Bener, S.; Aydelik-Ayazoglu, S.; Bayraktar, H.; Alaca, B.E.; Catalgil-Giz, H. A detailed investigation of the effect of calcium crosslinking and glycerol plasticizing on the physical properties of alginate films. Int. J. Biol. Macromol. 2020, 148, 49–55. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Avella, M.; Di Pace, E.; Immirzi, B.; Impallomeni, G.; Malinconico, M.; Santagata, G. Addition of glycerol plasticizer to seaweeds derived alginates: Influence of microstructure on chemical–physical properties. Carbohydr. Polym. 2007, 69, 503–511. [Google Scholar] [CrossRef]
- Feng, L.; Cao, Y.; Xu, D.; Wang, S.; Zhang, J. Molecular weight distribution, rheological property and structural changes of sodium alginate induced by ultrasound. Ultrason. Sonochemistry 2017, 34, 609–615. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll. 2017, 63, 414–420. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, J.; Zhao, L.; Zhang, J.; Wang, F. Applications of Alginate as a Functional Food Ingredient. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–429. [Google Scholar] [CrossRef]
- Wong, T.W.; Chan, L.W.; Bin Kho, S.; Heng, P.W.S. Aging and microwave effects on alginate/chitosan matrices. J. Control. Release 2005, 104, 461–475. [Google Scholar] [CrossRef]
- Ashikin, W.H.N.S.; Wong, T.W.; Law, C.L. Plasticity of hot air-dried mannuronate- and guluronate-rich alginate films. Carbohydr. Polym. 2010, 81, 104–113. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. IJMS 2022, 23, 9035. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Abu Elgoud, E.M.; Aly, H.F. Alginate modified graphene oxide for rapid and effective sorption of some heavy metal ions from an aqueous solution. Cellulose 2022, 29, 6231–6245. [Google Scholar] [CrossRef]
- Lagoa, R.; Rodrigues, J.R. Evaluation of Dry Protonated Calcium Alginate Beads for Biosorption Applications and Studies of Lead Uptake. Appl. Biochem. Biotechnol. 2007, 143, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Wrobel, K.; Wrobel, K.; Kazunori, S.; Tzu, T.W. Lead Ion Uptake By Sodium Alginate And Calcium Alginate Film: A Comparison Study. In Proceedings of the2012 International Congress on Informatics, Environment, Energy and Applications-IEEA, Singapore, 17–18 March 2012. [Google Scholar]
- Jeevahan, J.J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Joseph, G.B.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Kordjazi, Z.; Ajji, A. Development of TiO2 photocatalyzed EC/HTPB based oxygen scavenging mats by electrospinning method for packaging applications. Food Packag. Shelf Life 2022, 31, 100801. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Puttipipatkhachorn, S. Alginate-magnesium aluminum silicate films: Effect of plasticizers on film properties, drug permeation and drug release from coated tablets. Int. J. Pharm. 2007, 333, 34–44. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT Food Sci. Technol. 2008, 41, 359–366. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Paixão, L.C.; Lopes, I.A.; Filho, A.K.D.B.; Santana, A.A. Alginate biofilms plasticized with hydrophilic and hydrophobic plasticizers for application in food packaging. J. Appl. Polym. Sci. 2019, 136, 48263. [Google Scholar] [CrossRef]
- Sharmin, N.; Sone, I.; Walsh, J.L.; Sivertsvik, M.; Fernández, E.N. Effect of citric acid and plasma activated water on the functional properties of sodium alginate for potential food packaging applications. Food Packag. Shelf Life 2021, 29, 100733. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Tong, Q.; Xiao, Q.; Lim, L.-T. Effects of glycerol, sorbitol, xylitol and fructose plasticisers on mechanical and moisture barrier properties of pullulan-alginate-carboxymethylcellulose blend films. Int. J. Food Sci. Technol. 2013, 48, 870–878. [Google Scholar] [CrossRef]
- Muobom, S.S.; Umar, A.-M.S.; Brolin, A.-P.; Soongseok, Y. Title: A Review on Plasticizers and Eco-Friendly Bioplasticizers: Biomass Sources and Market. IJERT 2020, 9, IJERTV9IS050788. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M. Mechanisms of Plasticizers Action. In Handbook of Plasticizers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 119–134. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.-J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-Order Transition Temperatures and Related Properties of Polystyrene. I. Influence of Molecular Weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- El Miri, N.; Aziz, F.; Aboulkas, A.; El Bouchti, M.; Ben Youcef, H.; El Achaby, M. Effect of plasticizers on physicochemical properties of cellulose nanocrystals filled alginate bionanocomposite films. Adv. Polym. Technol. 2018, 37, 3171–3185. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-forming Liquids. J. Am. Chem. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Unexpected Plasticization Effects on the Structure and Properties of Polyelectrolyte Complexed Chitosan/Alginate Materials. ACS Appl. Polym. Mater. 2020, 2, 2957–2966. [Google Scholar] [CrossRef]
- Russo, R.; Abbate, M.; Malinconico, M.; Santagata, G. Effect of polyglycerol and the crosslinking on the physical properties of a blend alginate-hydroxyethylcellulose. Carbohydr. Polym. 2010, 82, 1061–1067. [Google Scholar] [CrossRef]
- Aadil, K.R.; Jha, H. Physico-chemical properties of lignin–alginate based films in the presence of different plasticizers. Iran Polym. J. 2016, 25, 661–670. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Innovative plasticized alginate obtained by thermo-mechanical mixing: Effect of different biobased polyols systems. Carbohydr. Polym. 2017, 157, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Titow, W.V. Plasticisers. In PVC Plastics; Springer: Dordrecht, The Netherlands, 1990; pp. 177–257. [Google Scholar] [CrossRef]
- Klähn, M.; Krishnan, R.; Phang, J.M.; Lim, F.C.; van Herk, A.M.; Jana, S. Effect of external and internal plasticization on the glass transition temperature of (Meth)acrylate polymers studied with molecular dynamics simulations and calorimetry. Polymer 2019, 179, 121635. [Google Scholar] [CrossRef]
- Chen, H.; Wu, C.; Feng, X.; He, M.; Zhu, X.; Li, Y.; Teng, F. Effects of two fatty acids on soy protein isolate/sodium alginate edible films: Structures and properties. LWT 2022, 159, 113221. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes-Parra, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Seetabhawang, S.; Sanchavanakit, N.; Phisalaphong, M. Fabrication and characterization of novel bacterial cellulose/alginate/gelatin biocomposite film. J. Biomater. Sci. Polym. Ed. 2019, 30, 961–982. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P.; Yamaguchi, M. The Effect of Glycerol/Water and Sorbitol/Water on the Plasticization of Hydroxyethylacryl Chitosan/Sodium Alginate Films. MATEC Web Conf. 2015, 30, 02006. [Google Scholar] [CrossRef]
- Barbut, S.; Harper, B.A. Dried Ca-alginate films: Effects of glycerol, relative humidity, soy fibers, and carrageenan. LWT 2019, 103, 260–265. [Google Scholar] [CrossRef]
- Harper, B.A.; Barbut, S.; Smith, A.; Marcone, M.F. Mechanical and Microstructural Properties of ‘Wet’ Alginate and Composite Films Containing Various Carbohydrates. J. Food Sci. 2015, 80, E84–E92. [Google Scholar] [CrossRef]
- Souza, R.C.R.; Andrade, C.T. Processing and properties of thermoplastic starch and its blends with sodium alginate. J. Appl. Polym. 2001, 81, 412–420. [Google Scholar] [CrossRef]
- Gonzalez-Cuello, R.E.; Mogollon, O.F.C.; Berrio-Guzman, O.J.; Cuevas-Martinez, C.M. Optimization of the mechanical properties of biofilm based on alginate—Gellan plasticized with glycerol. Contemp. Eng. Sci. 2018, 11, 891–905. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Influence of Drying Conditions on Physical Properties of Alginate Films. Dry. Technol. 2012, 30, 72–79. [Google Scholar] [CrossRef]
- Santana, A.A.; Kieckbusch, T.G. Physical evaluation of biodegradable films of calcium alginate plasticized with polyols. Braz. J. Chem. 2013, 30, 835–845. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Schott, M.; Schmid, M.; Müller, K. Effect of Presence and Concentration of Plasticizers, Vegetable Oils, and Surfactants on the Properties of Sodium-Alginate-Based Edible Coatings. IJMS 2018, 19, 742. [Google Scholar] [CrossRef] [PubMed]
- Seixas, F.; Turbiani, F.; Salomão, P.; Souza, R.; Gimenes, M. Biofilms composed of alginate and pectin: Effect of concentration of crosslinker and plasticizer agents. Chem. Eng. Trans. 2013, 32, 1693–1698. [Google Scholar] [CrossRef]
- Kadzińska, J.; Bryś, J.; Ostrowska-Ligęza, E.; Estéve, M.; Janowicz, M. Influence of vegetable oils addition on the selected physical properties of apple–sodium alginate edible films. Polym. Bull. 2020, 77, 883–900. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Salama, H.E.; Sabaa, M.W. Biobased alginate/castor oil edible films for active food packaging. LWT 2018, 96, 455–460. [Google Scholar] [CrossRef]
- Gutiérrez-Jara, C.; Bilbao-Sainz, C.; McHugh, T.; Chiou, B.-S.; Williams, T.; Villalobos-Carvajal, R. Physical, mechanical and transport properties of emulsified films based on alginate with soybean oil: Effects of soybean oil concentration, number of passes and degree of surface crosslinking. Food Hydrocoll. 2020, 109, 106133. [Google Scholar] [CrossRef]
- Marismandani, A.D.P.; Husni, A. Development and Characterization of Biobased Alginate/Glycerol/Virgin Coconut Oil as Biodegradable Packaging. E3S Web Conf. 2020, 147, 03016. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Hasmiyani; Dirpan, A.; Mahendradatta, M. Physical, mechanical, and barrier properties of sodium alginate/gelatin emulsion based-films incorporated with canola oil. IOP Conf. Ser. Earth Environ. 2017, 101, 012019. [Google Scholar] [CrossRef]
- Frank, K.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Alginate Biocomposite Films Incorporated with Cinnamon Essential Oil Nanoemulsions: Physical, Mechanical, and Antibacterial Properties. Int. J. Polym. Sci. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Baek, S.-K.; Kim, S.; Bin Song, K. Characterization of Ecklonia cava Alginate Films Containing Cinnamon Essential Oils. IJMS 2018, 19, 3545. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Avena-Bustillos, R.J.; Olsen, C.; Friedman, M.; Henika, P.R.; Martín-Belloso, O.; Pan, Z.; McHugh, T.H. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate–apple puree edible films. J. Food Eng. 2007, 81, 634–641. [Google Scholar] [CrossRef]
- Işıklan, N.; Kurşun, F.; Inal, M. Graft copolymerization of itaconic acid onto sodium alginate using ceric ammonium nitrate as initiator. J. Appl. Polym. 2009, 114, 40–48. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Pourjavadi, A. New polysaccharide-g-polyacrylonitrile copolymers: Synthesis and thermal characterization. Polym. Technol. 2003, 14, 508–516. [Google Scholar] [CrossRef]
- Di Donato, P.; Taurisano, V.; Poli, A.; D’ayala, G.G.; Nicolaus, B.; Malinconinco, M.; Santagata, G. Vegetable wastes derived polysaccharides as natural eco-friendly plasticizers of sodium alginate. Carbohydr. Polym. 2020, 229, 115427. [Google Scholar] [CrossRef]
- Aadil, K.R.; Prajapati, D.; Jha, H. Improvement of physcio-chemical and functional properties of alginate film by Acacia lignin. Food Packag. Shelf Life 2016, 10, 25–33. [Google Scholar] [CrossRef]
- Bagheri, F.; Radi, M.; Amiri, S. Drying conditions highly influence the characteristics of glycerol-plasticized alginate films. Food Hydrocoll. 2019, 90, 162–171. [Google Scholar] [CrossRef]
- Wong, T.W.; Ashikin, W.H.N.S.; Law, C.L. Evaporation and Diffusion Transport Properties and Mechanical Properties of Alginate Dried Film. Dry. Technol. 2014, 32, 117–125. [Google Scholar] [CrossRef]
- Hambleton, A.; Perpiñan-Saiz, N.; Fabra, M.J.; Voilley, A.; Debeaufort, F. The Schroeder paradox or how the state of water affects the moisture transfer through edible films. Food Chem. 2012, 132, 1671–1678. [Google Scholar] [CrossRef]
- Gontard, N.; Thibault, R.; Cuq, B.; Guilbert, S. Influence of Relative Humidity and Film Composition on Oxygen and Carbon Dioxide Permeabilities of Edible Films. J. Agric. Food Chem. 1996, 44, 1064–1069. [Google Scholar] [CrossRef]
- McHugh, T.H.; Krochta, J.M. Sorbitol- vs. Glycerol-Plasticized Whey Protein Edible Films: Integrated Oxygen Permeability and Tensile Property Evaluation. J. Agric. Food Chem. 1994, 42, 841–845. [Google Scholar] [CrossRef]
- Liu, L.; Kerry, J. Application and assessment of extruded edible casings manufactured from pectin and gelatin/sodium alginate blends for use with breakfast pork sausage. Meat Sci. 2007, 75, 196–202. [Google Scholar] [CrossRef]
- Harper, B.; Barbut, S.; Lim, L.-T.; Marcone, M. Characterization of ‘wet’ alginate and composite films containing gelatin, whey or soy protein. Food Res. Int. 2013, 52, 452–459. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. Starch nano-biocomposites based on needle-like sepiolite clays. Carbohydr. Polym. 2010, 80, 145–153. [Google Scholar] [CrossRef]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Averous, L. Properties of thermoplastic blends: Starch–polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Carbohydr. Polym. 2007, 67, 288–295. [Google Scholar] [CrossRef]
- Tan, H.; Aziz, A.A.; Aroua, M. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Matet, M.; Heuzey, M.-C.; Pollet, E.; Ajji, A.; Avérous, L. Innovative thermoplastic chitosan obtained by thermo-mechanical mixing with polyol plasticizers. Carbohydr. Polym. 2013, 95, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Hulleman, S.H.; Janssen, F.H.; Feil, H. The role of water during plasticization of native starches. Polymer 1998, 39, 2043–2048. [Google Scholar] [CrossRef]
- Myllärinen, P.; Partanen, R.; Seppälä, J.; Forssell, P. Effect of glycerol on behaviour of amylose and amylopectin films. Carbohydr. Polym. 2002, 50, 355–361. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218. [Google Scholar] [CrossRef] [PubMed]
- Remuñán-López, C.; Bodmeier, R. Mechanical and Water Vapor Transmission Properties of Polysaccharide Films. Drug Dev. Ind. Pharm. 1996, 22, 1201–1209. [Google Scholar] [CrossRef]
- Donhowe, I.G.; Fennema, O. The Effects of Plasticizers on Crystallinity, Permeability, and Mechanical Properties of Methylcellulose Films. J. Food Process. Preserv. 1993, 17, 247–257. [Google Scholar] [CrossRef]
- Jangchud, A.; Chinnan, M. Properties of Peanut Protein Film: Sorption Isotherm and Plasticizer Effect. LWT Food Sci. Technol. 1999, 32, 89–94. [Google Scholar] [CrossRef]
- Embuscado, M. Embuscado. Polyols. In Optimising Sweet Taste in Foods; Elsevier: Amsterdam, The Netherlands, 2006; pp. 153–174. [Google Scholar] [CrossRef]
- Chick, J.; Ustunol, Z. Mechanical and Barrier Properties of Lactic Acid and Rennet Precipitated Casein-Based Edible Films. J. Food Sci. 2006, 63, 1024–1027. [Google Scholar] [CrossRef]
- Fairley, P.; Monahan, F.J.; German, J.B.; Krochta, J.M. Mechanical Properties and Water Vapor Permeability of Edible Films from Whey Protein Isolate and Sodium Dodecyl Sulfate. J. Agric. Food Chem. 1996, 44, 438–443. [Google Scholar] [CrossRef]
- Parris, N.; Coffin, D.R. Composition Factors Affecting the Water Vapor Permeability and Tensile Properties of Hydrophilic Zein Films. J. Agric. Food Chem. 1997, 45, 1596–1599. [Google Scholar] [CrossRef]
- Shellhammer, T.; Krochta, J. Whey Protein Emulsion Film Performance as Affected by Lipid Type and Amount. J. Food Sci. 1997, 62, 390–394. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.H. Plasticization of Pea Starch Films with Monosaccharides and Polyols. J. Food Sci. 2006, 71, E253–E261. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Patsaoura, A.; Barkoula, N.-M.; Ladavos, A. A novel solution blending method for using olive oil and corn oil as plasticizers in chitosan based organoclay nanocomposites. Carbohydr. Polym. 2017, 157, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Alhanish, A.; Abu Ghalia, M. Developments of biobased plasticizers for compostable polymers in the green packaging applications: A review. Biotechnol. Prog. 2021, 37, e3210. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Avalos, M.C.; Femenia, A.; Minjares-Fuentes, R.; Contreras-Esquivel, J.C.; Aguilar-González, C.N.; Esparza-Rivera, J.R.; Meza-Velázquez, J.A. Improvement of the Quality and the Shelf Life of Figs (Ficus carica) Using an Alginate–Chitosan Edible Film. Food Bioprocess Technol. 2016, 9, 2114–2124. [Google Scholar] [CrossRef]
- Siddaramaiah; Swamy, T.M.M.; Ramaraj, B.; Lee, J.H. Sodium alginate and its blends with starch: Thermal and morphological properties. J. Appl. Polym. 2008, 109, 4075–4081. [Google Scholar] [CrossRef]
- Russo, R.; Giuliani, A.; Immirzi, B.; Malinconico, M.; Romano, G. Alginate/Polyvinylalcohol Blends for Agricultural Applications: Structure-Properties Correlation, Mechanical Properties and Greenhouse Effect Evaluation. Macromol. Symp. 2001, 169, 241–250. [Google Scholar] [CrossRef]
- Çaykara, T.; Demirci, S. Preparation and Characterization of Blend Films of Poly(Vinyl Alcohol) and Sodium Alginate. J. Macromol. Sci. Part A 2006, 43, 1113–1121. [Google Scholar] [CrossRef]
- Platzer, N. The technology of plasticizers, J. Kern Sears and Joseph R. Darby, SPE Monograph Series, Wiley, New York, 1982, 1166 Price: $130.00. J. Polym. B Polym. Lett. Ed. 1982, 20, 459. [Google Scholar] [CrossRef]
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.; Montero, M.; Gómez-Guillén, M. Physical and functional characterization of active fish gelatin films incorporated with lignin. Food Hydrocoll. 2013, 30, 163–172. [Google Scholar] [CrossRef]
- Wu, R.-L.; Wang, X.-L.; Li, F.; Li, H.-Z.; Wang, Y.-Z. Green composite films prepared from cellulose, starch and lignin in room-temperature ionic liquid. Bioresour. Technol. 2009, 100, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Physical and mechanical properties of chitosan films as affected by drying methods and addition of antimicrobial agent. J. Food Eng. 2013, 119, 140–149. [Google Scholar] [CrossRef]
- Tomka, I. Thermoplastic Starch. In Water Relationships in Foods; Advances in Experimental Medicine and Biology 302; Levine, H., Slade, L., Eds.; Springer: Boston, MA, USA, 1991; pp. 627–637. [Google Scholar] [CrossRef]
- Munhoz, D.R.; Moreira, F.K.; Bresolin, J.D.; Bernardo, M.P.; de Sousa, C.P.; Mattoso, L.H.C. Sustainable Production and In vitro Biodegradability of Edible Films from Yellow Passion Fruit Coproducts via Continuous Casting. ACS Sustain. Chem. 2018, 6, 9883–9892. [Google Scholar] [CrossRef]
- Mojumdar, S.C.; Moresoli, C.; Simon, L.C.; Legge, R.L. Edible wheat gluten (WG) protein films: Preparation, thermal, mechanical and spectral properties. J. Therm. Anal. Calorim. 2011, 104, 929–936. [Google Scholar] [CrossRef]
| Bio-Polymer | Plasticizer | Findings and Results | Refs. |
|---|---|---|---|
| Alginate and gelatin | Glycerol and water | Increasing RH led to increased TS and decreased EB, and glycerol increased the flexibility of the films without altering TS. | [52] |
| Alginate and pectin | 50% glycerol and water (53% RH) | The absorbed water during conditioning had a plasticizing effect on the films. | [53] |
| Chitosan and alginate | 25, 40, and 50% glycerol and sorbitol–water | A dramatic decrease in Tg with the incorporation of glycerol and water. | [54] |
| Alginate (M/G = 0.45 and M/G = 1.5) | Fructose, glycerol, sorbitol, and polyethylene glycol (PEG-8000) | WVP was higher for the films conditioned at higher RH; PEG-plasticized films were opaque because of phase separation. | [31] |
| Alginate | Glycerol and water | Films conditioned at 100% RH had higher EB and lower TS and YM than films conditioned at 57% RH. | [55] |
| Alginate and other carbohydrates | water | Wet alginate films had higher EB than dried films because of the plasticizing effect of water. | [56] |
| Alginate | Glycerol and water | There was a remarkable decrease in the degradation temperature of the film plasticized with 50% glycerol. Increasing glycerol content beyond 30% led to the segregation phenomenon. | [19] |
| Alginate | Glycerol, sorbitol, and water | The glycerol-plasticized film had lower Tg than the sorbitol-plasticized one, The films obtained from the solvent casting method had higher TS and YM, but lower EB compared with the thermo-mechanical mixing method. | [47] |
| Corn starch (CS) and sodium alginate (SA) | 15% glycerol and water | A twin-screw extruder was used for blending the materials, and glycerol and water decreased the processing temperature. | [57] |
| Alginate | Glycerol (20–40%) and sorbitol (30–50%) | Although glycerol is a more effective plasticizer based on mass content, the plasticizing efficiency of sorbitol was higher at the molecular basis. | [15] |
| Alginate | Glycerol | Glycerol and calcium chloride (crosslinker) had a synergistic effect on the mechanical properties of the films, and beyond a certain limit they had a deteriorating effect. | [13] |
| Alginate (high guluronic acid (Ap) and low guluronic acid (Ar)) | Glycerol | Ap polymer was effectively plasticized because of its buckled structure. | [17] |
| Alginate and low acyl gellan | Glycerol | The optimal concentration of glycerol was 8% v/v. | [58] |
| Alginate | Glycerol | At temperatures above 40 °C, a significant amount of glycerol was lost. | [59] |
| Alginate/pectin | Glycerol | When the plasticizer concentration was above a critical limit, phase separation could be observed on the surface of the film. | [29] |
| Alginate | Polyglycerol | Polyglycerol had an anti-plasticization effect on alginate because of the presence of high amounts of hydroxyl groups in polyglycerol. | [45] |
| Alginate | glycerin and polyethylene glycol 400 (PEG400) | Glycerin was a better plasticizer than PEG400 and gave more flexibility to the films because of the lower molecular weight of glycerin. | [30] |
| Alginate | glycerol (GLY), diethylene glycol (DEG), and polyethylene glycol (PEG) | WVP of films plasticized with PEG and DEG was lower than that of GLY-plasticized film. | [42] |
| Alginate | Glycerol, Xylitol, and mannitol | Glycerol and xylitol-plasticized films were more transparent and uniform than the mannitol-plasticized film, but they had higher WVP. | [60] |
| Alginate and vegetable oils | Glycerol and sorbitol (0–20%) | The surface tension did not alter by the addition of the plasticizers, but vegetable oils diminished the surface tension. | [61] |
| Pullulan and alginate | glycerol, sorbitol, xylitol, and fructose | Sorbitol- and fructose-plasticized blend films exhibited the lowest and similar EB at any given plasticizer concentrations compared with glycerol and xylitol-plasticized films, with the fructose-plasticized film being even more brittle with higher TS and lower EB. | [36] |
| Alginate and pectin | Glycerol | Increasing glycerol content promoted the WVP of the films. | [62] |
| Alginate and pectin | 33% Polyglycerol | Higher swelling degree cross-linked film with the addition of polyglycerol. | [8] |
| Alginate and apple puree | Glycerol, rapeseed oil, coconut oil, hazelnut oil, and sugars in the apple puree | The Tg decreased with the addition of vegetable oils and apple puree, so they had a plasticizing effect. | [63] |
| Alginate | Glycerol and oregano essential oil (OEO) | Higher EB and lower WVP and TS observed with the incorporation of OEO. | [51] |
| Alginate | Glycerol, castor oil (CO) | The incorporation of CO led to increased EB and decreased TS and WVP. | [64] |
| Alginate | Glycerol and soybean oil | At high calcium chloride concentrations, the EB of alginate decreased with increasing oil concentrations. WVP decreased with the addition of oil. | [65] |
| Alginate (2–6%) and virgin coconut oil | Glycerol (10%) | To decrease the surface tension of oil and alginate, ethanol was used. | [66] |
| Alginate/gelatin | Glycerol and canola oil | Higher EB and lower WVP and TS observed with the incorporation of canola oil. | [67] |
| Alginate | Glycerol and cinnamon essential oil (CEO) | The incorporation of higher amounts of CEOs led to a decreased EB. | [68] |
| Alginate | Glycerol and cinnamon essential oil (CEO) | The incorporation of the CEO led to an increased EB and WVP and decreased TS. | [69] |
| Alginate/garlic oil | - | Garlic oil increased EB and decreased TS of the film, and WVP increased remarkably with increasing oil content. | [70] |
| Alginate | Glycerol, essential oils (Eos) | Oil droplets had a plasticizing effect by decreasing interactions between chains. | [71] |
| Alginate/apple puree | Glycerol and plant essential oils | EB increased with the addition of the oil, but TS decreased. | [72] |
| Soy protein isolate/alginate | Stearic acid and lauric acid | TS and EB of the films decreased with the incorporation of the fatty acids; however, EB increased at higher concentrations of auric acid. WVP value decreased at lower amounts of fatty acids, but it increased at higher amounts. | [50] |
| Alginate | Glycerol and oleic acid | Oleic acid behaved like a second plasticizer. | [3] |
| Alginate | Glycerol, tri-butyl citrate (TC) | TC-plasticized films were opaque; Tg and TS increased with the addition of TC; EB decreased with the addition of TC. | [33] |
| Alginate | Citric acid (CA) | CA at higher concentrations had a plasticizing effect. | [34] |
| Chitosan/alginate | Triacetin, glycerol, and Ionic liquid | Triacetin-plasticized films were brittle and thermally stable. | [44] |
| Alginate | Graft copolymerization of itaconic acid (internal plasticization) | The Tg value of the grafted alginate film was lower, indicating the plasticizing effect of itaconic acid. | [73] |
| Natural polysaccharides such as alginate | Graft copolymerization of polyacrylonitrile (internal plasticization) | The grafted chains might act as internal plasticizers because of the reduced Tg. | [74] |
| Alginate | Lemon and fennel wastes (contain pectin-like polymers) | Tg and degradation temperature decreased, but the EB and TS of the films increased with the incorporation of the plasticizers. | [75] |
| Alginate/lignin | Glycerol and lignin | Lignin exerts an apparent plasticizing effect on alginate by reducing the intermolecular interaction between chains and decreasing the tensile strength of the films. | [76] |
| Gluronate-rich (MG) and mannuronate-rich (MC) alginate | Water and hot air | Plasticity was decreased by increasing the drying temperature to 60 °C, Hot air at 80 °C induced plasticity because of the formation of bubbles and degradation of alginate molecules. | [22] |
| Alginate | Glycerol | The amount of glycerol in the dried films was decreased by increasing the drying temperature; hence, the properties of the film were affected. | [77] |
| Alginate | - | According to thermo-mechanical analysis, the films prepared at 80 °C were more plasticized than the films produced at lower temperatures. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. https://doi.org/10.3390/molecules28186637
Eslami Z, Elkoun S, Robert M, Adjallé K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules. 2023; 28(18):6637. https://doi.org/10.3390/molecules28186637
Chicago/Turabian StyleEslami, Zahra, Saïd Elkoun, Mathieu Robert, and Kokou Adjallé. 2023. "A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films" Molecules 28, no. 18: 6637. https://doi.org/10.3390/molecules28186637






