Drug Discovery Based on Fluorine-Containing Glycomimetics
Abstract
:1. Introduction
2. Synthesis of Fluorine-Containing Glycomimetics
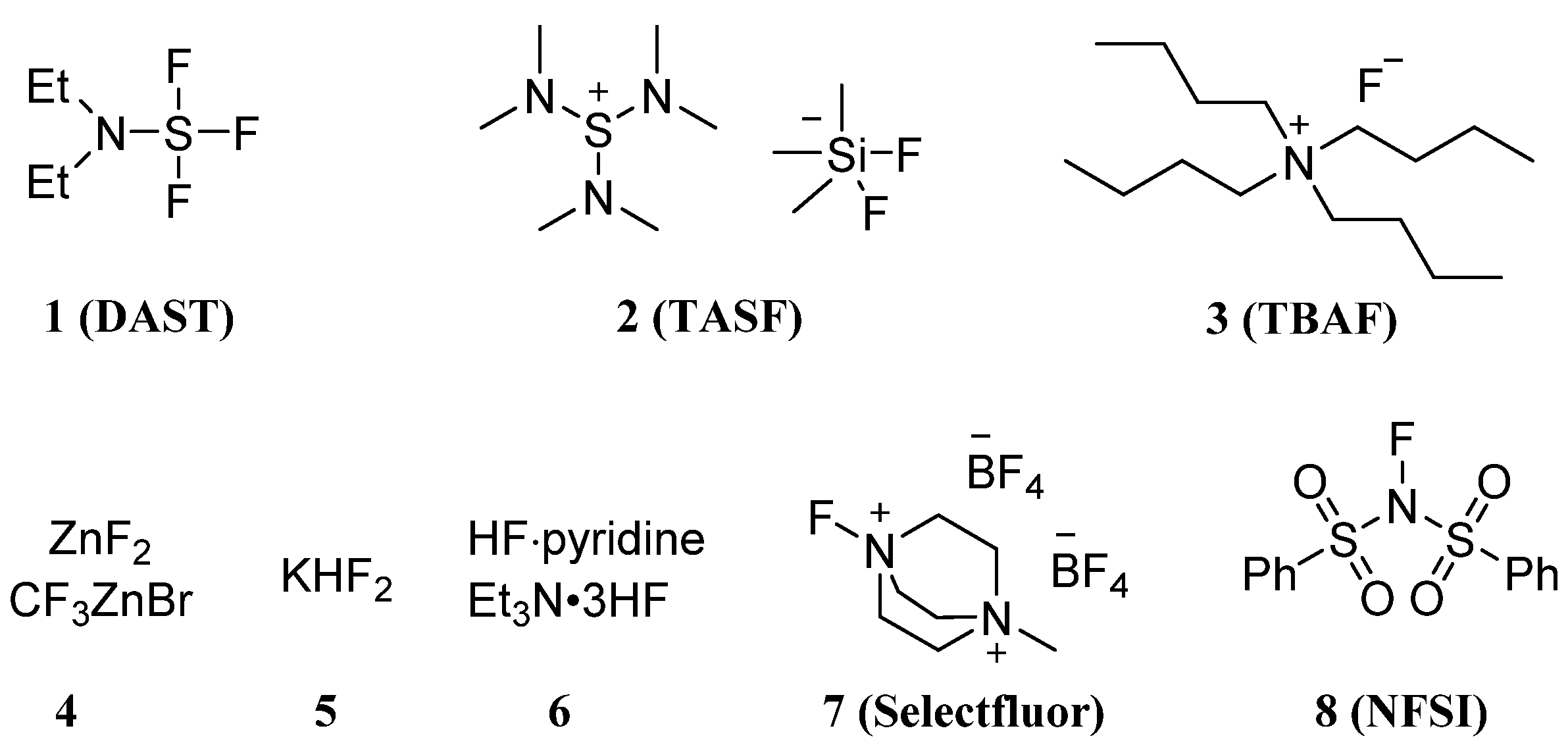
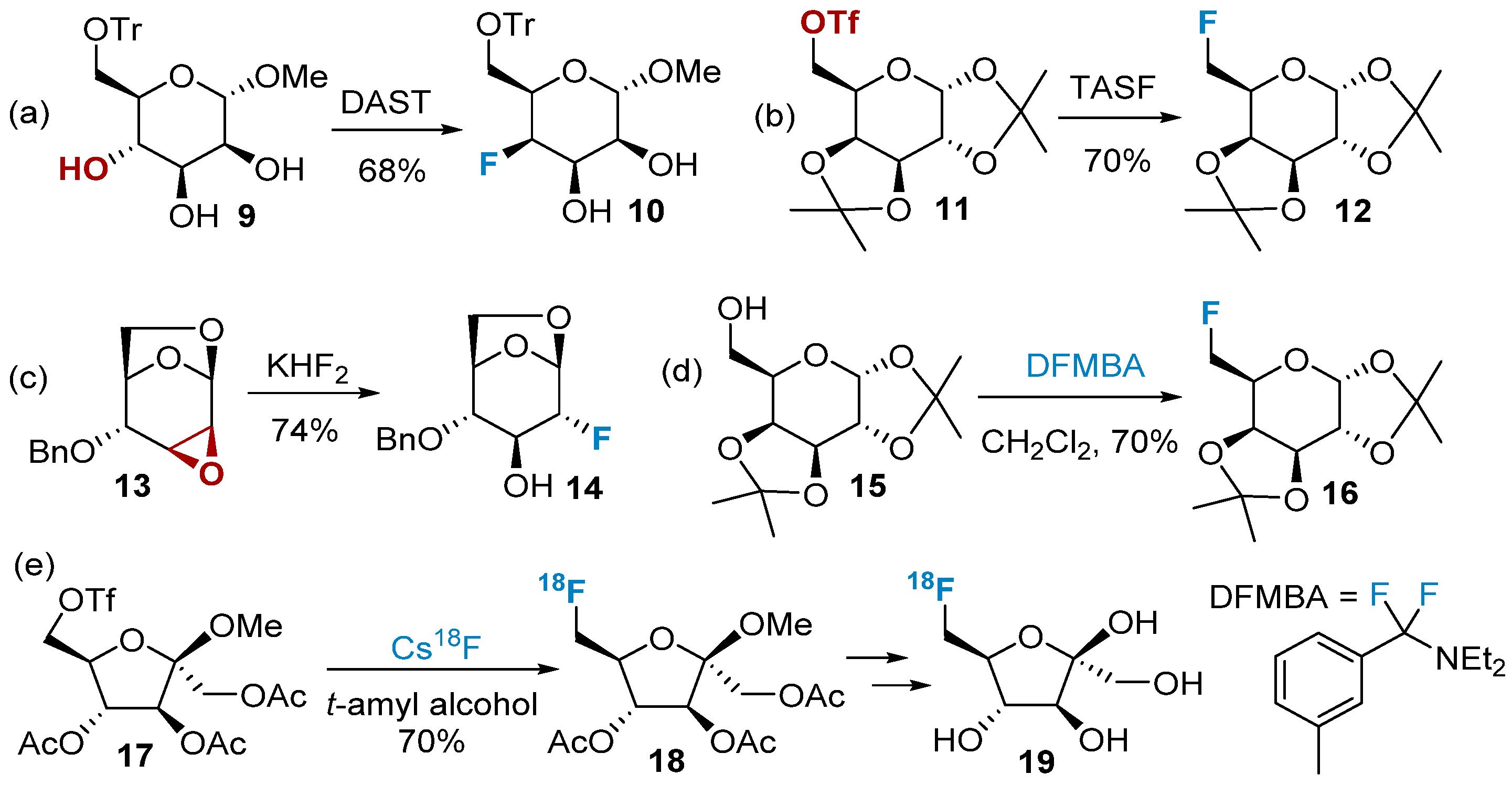
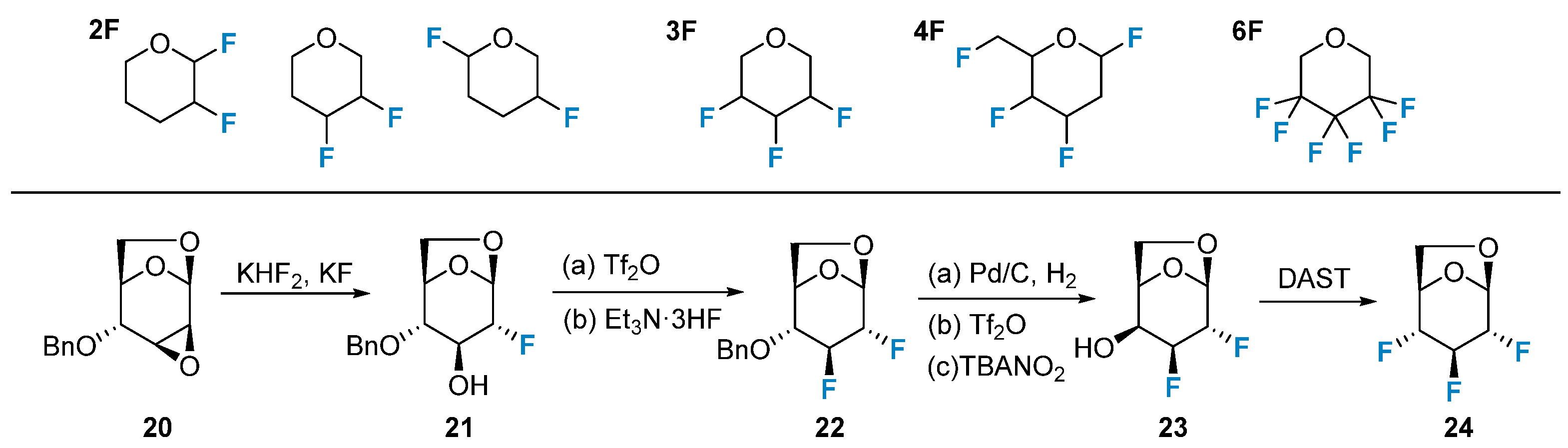
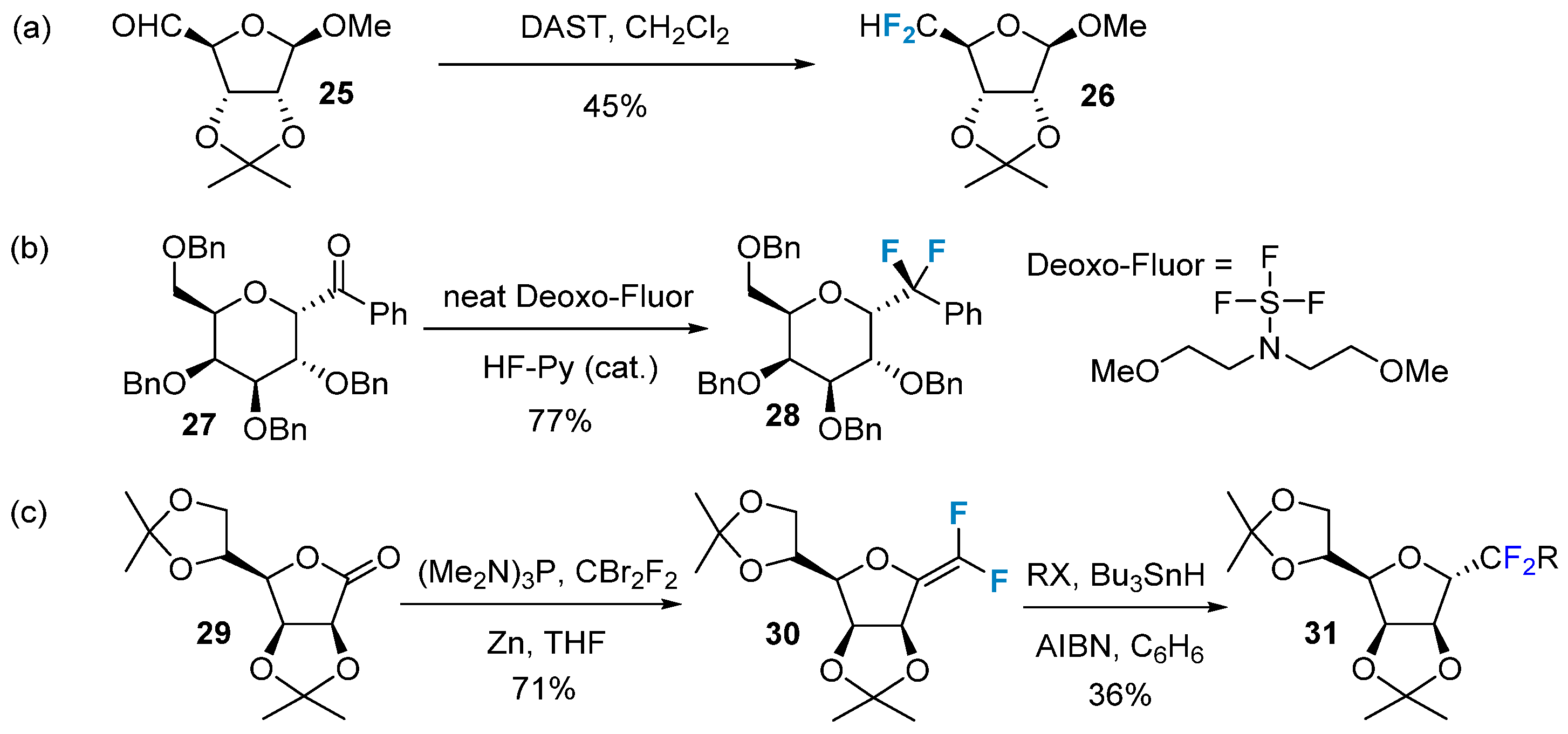
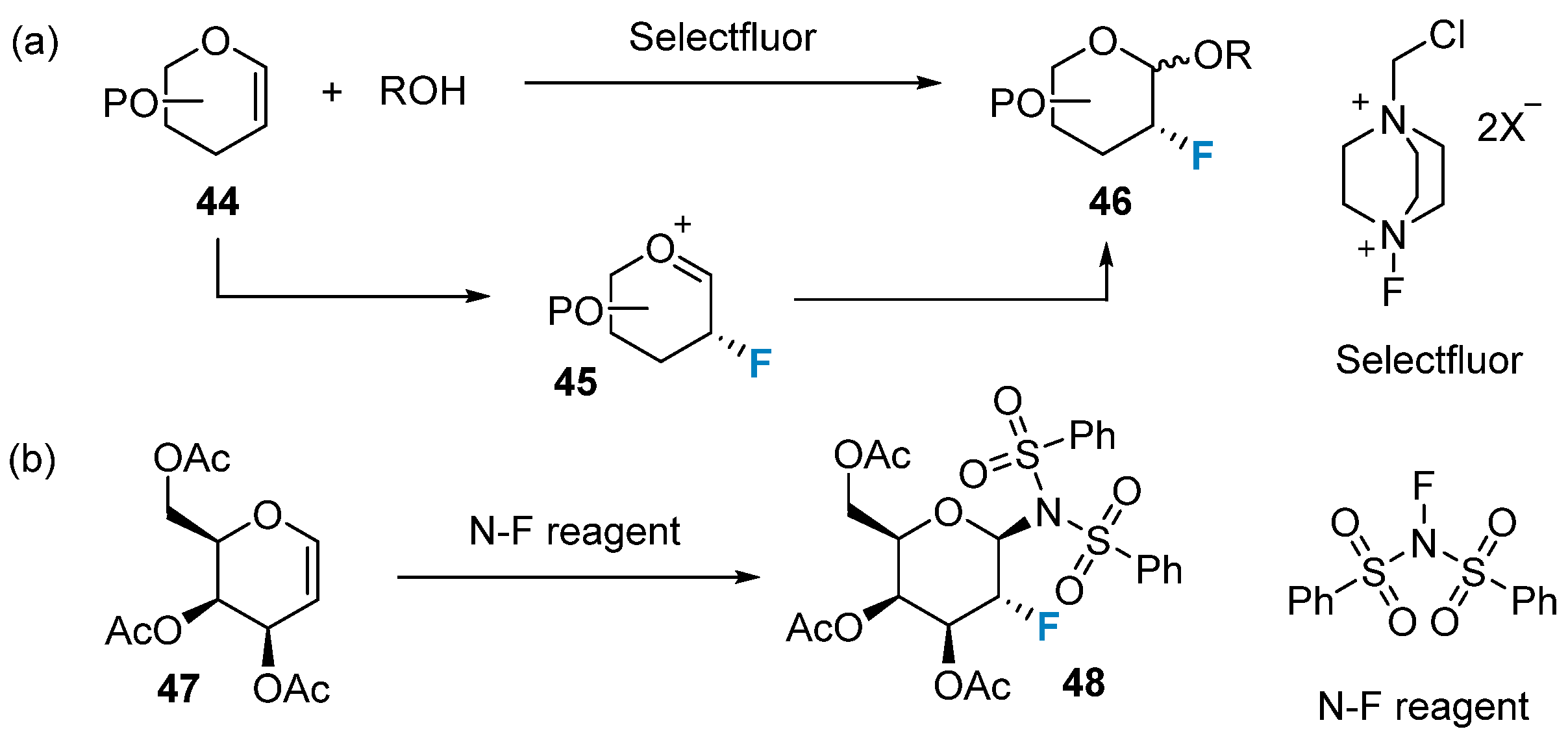
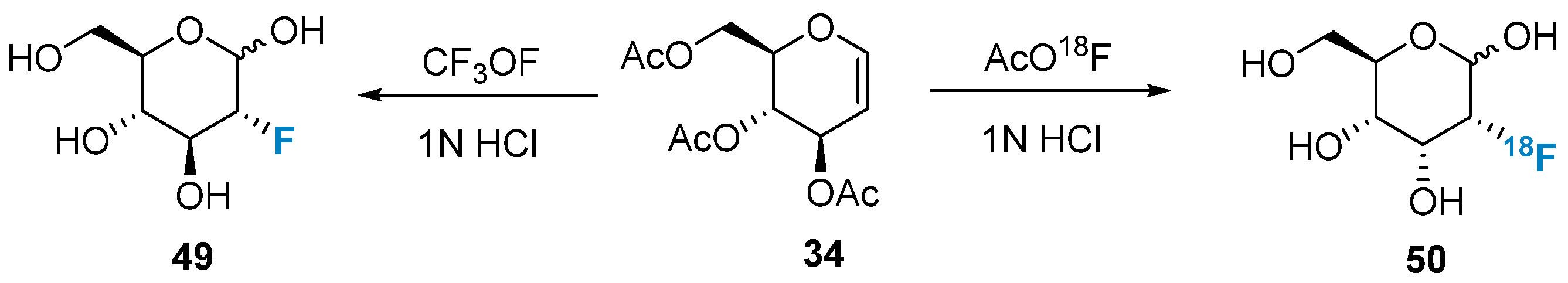
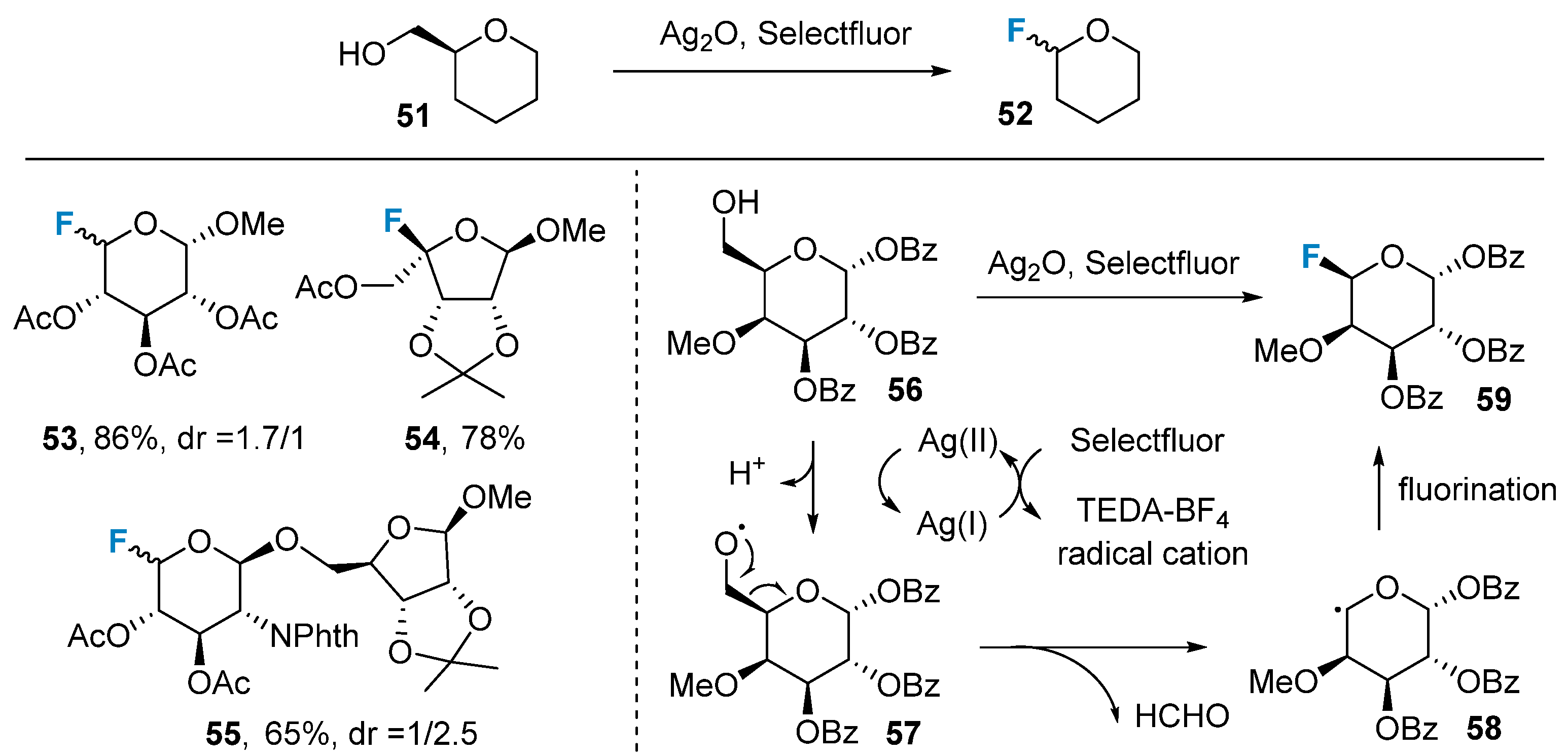
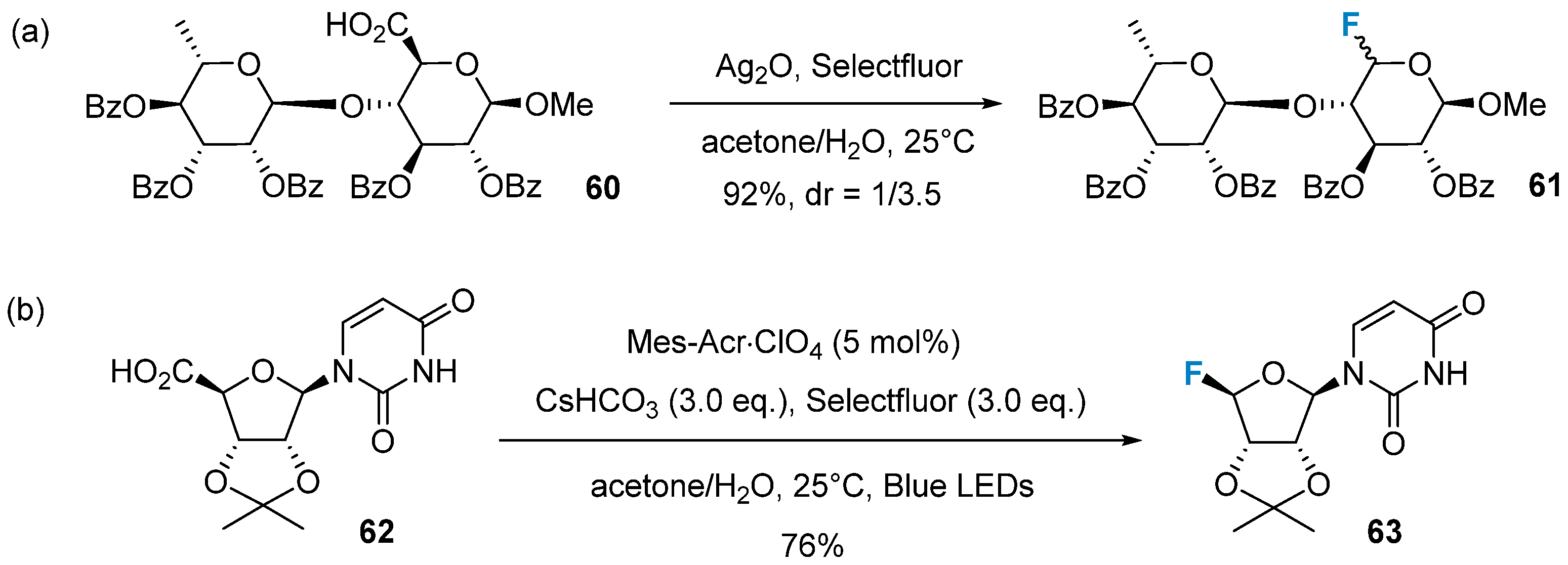
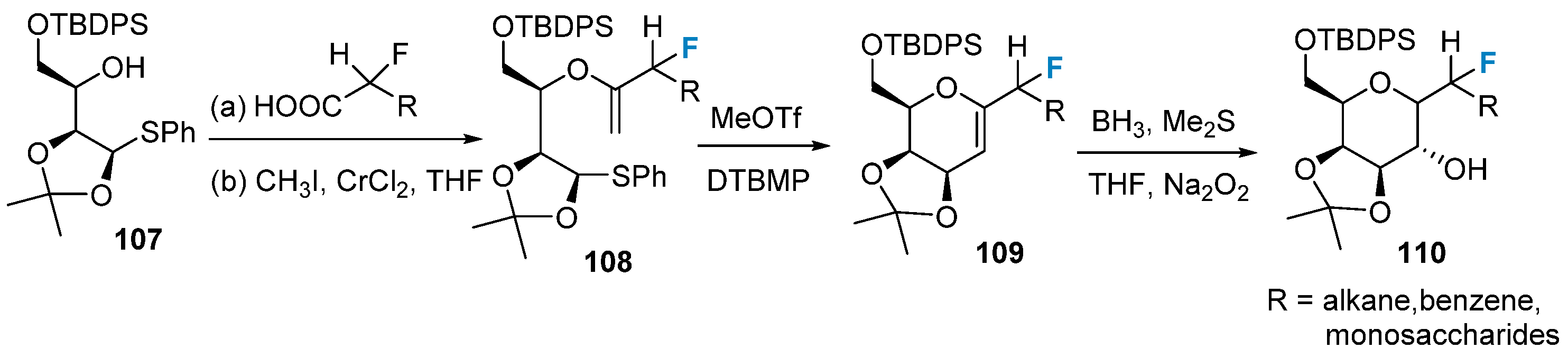
2.1. Fluorination of Carbohydrates via Nucleophilic Fluorination
2.2. Fluorination of Carbohydrates via Electrophilic Addition Reactions
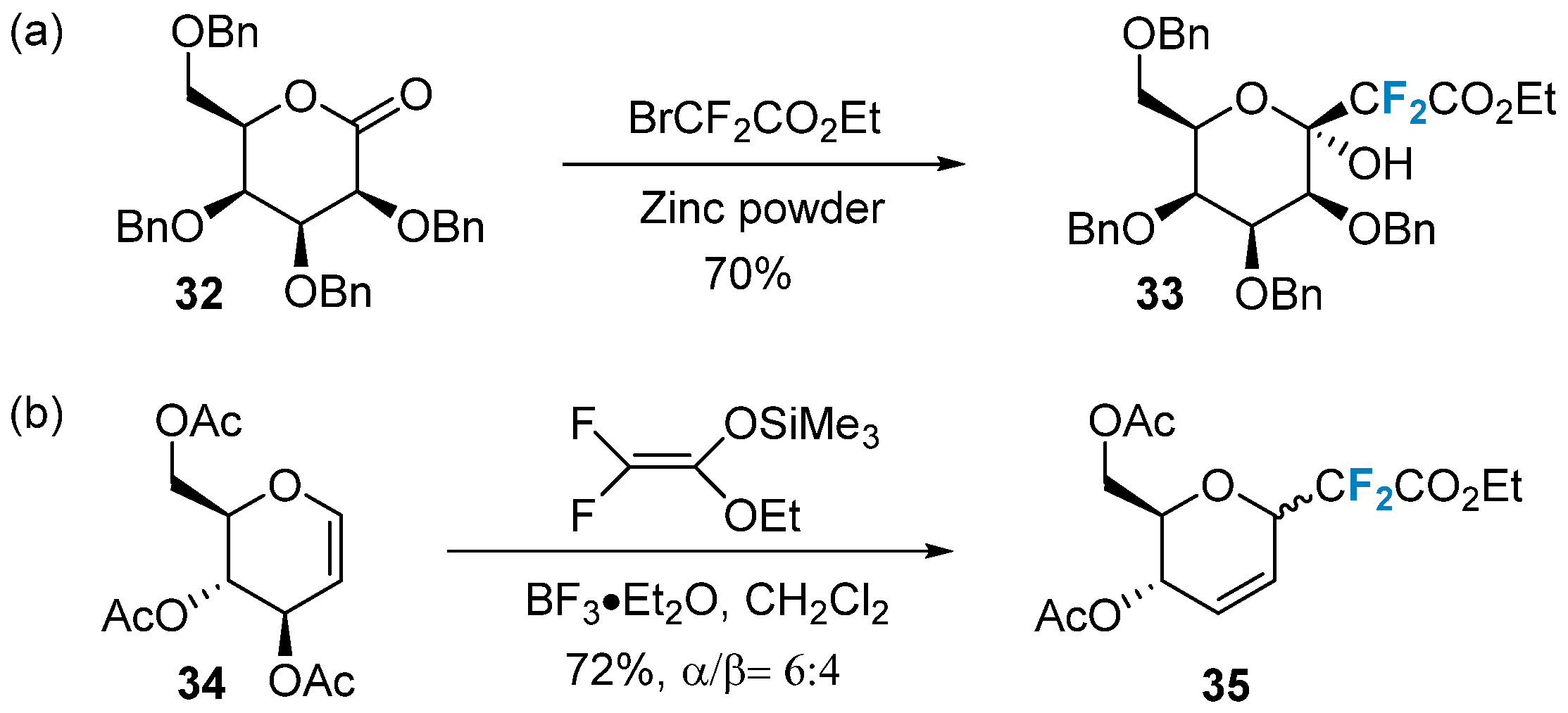
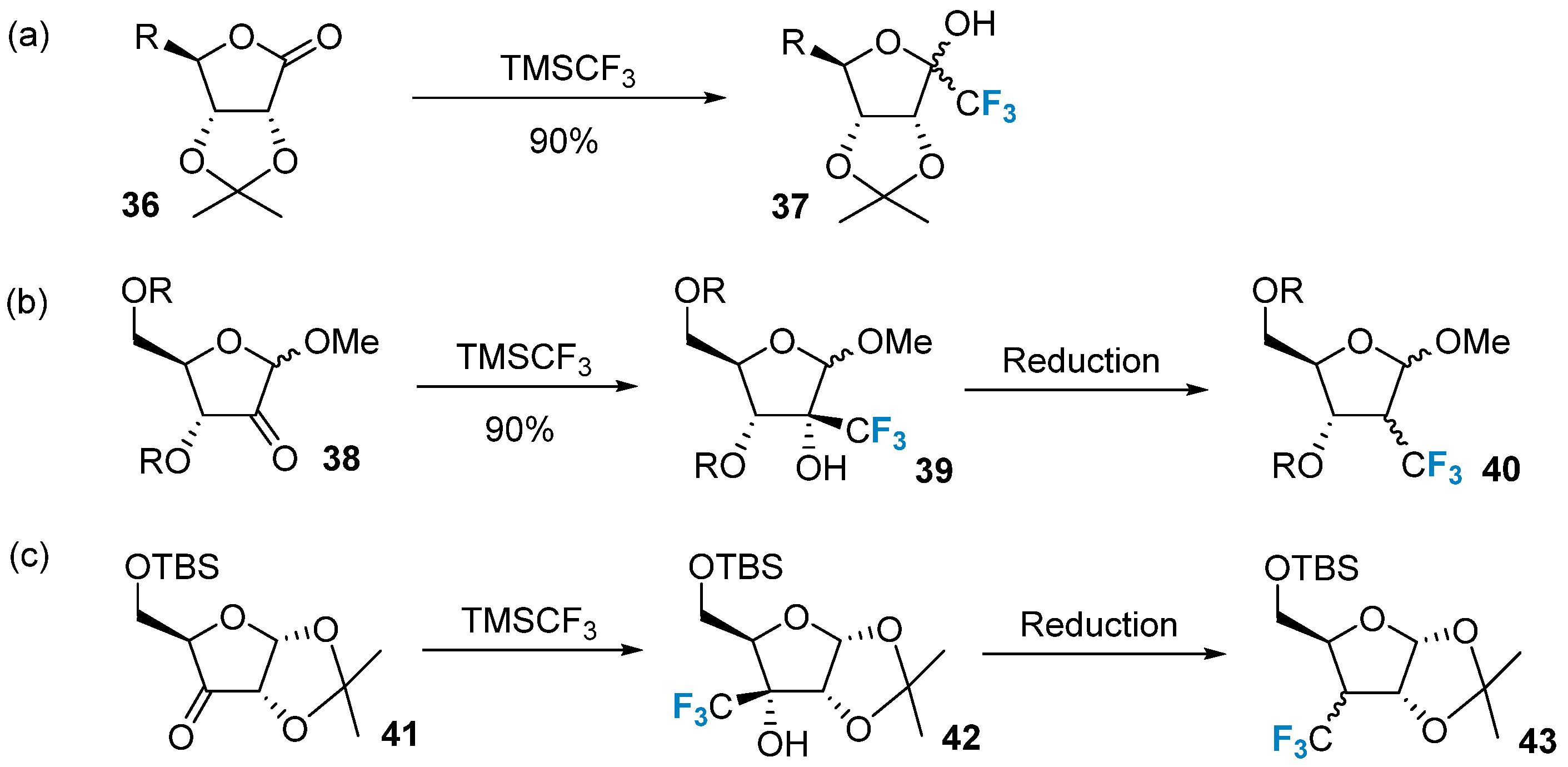
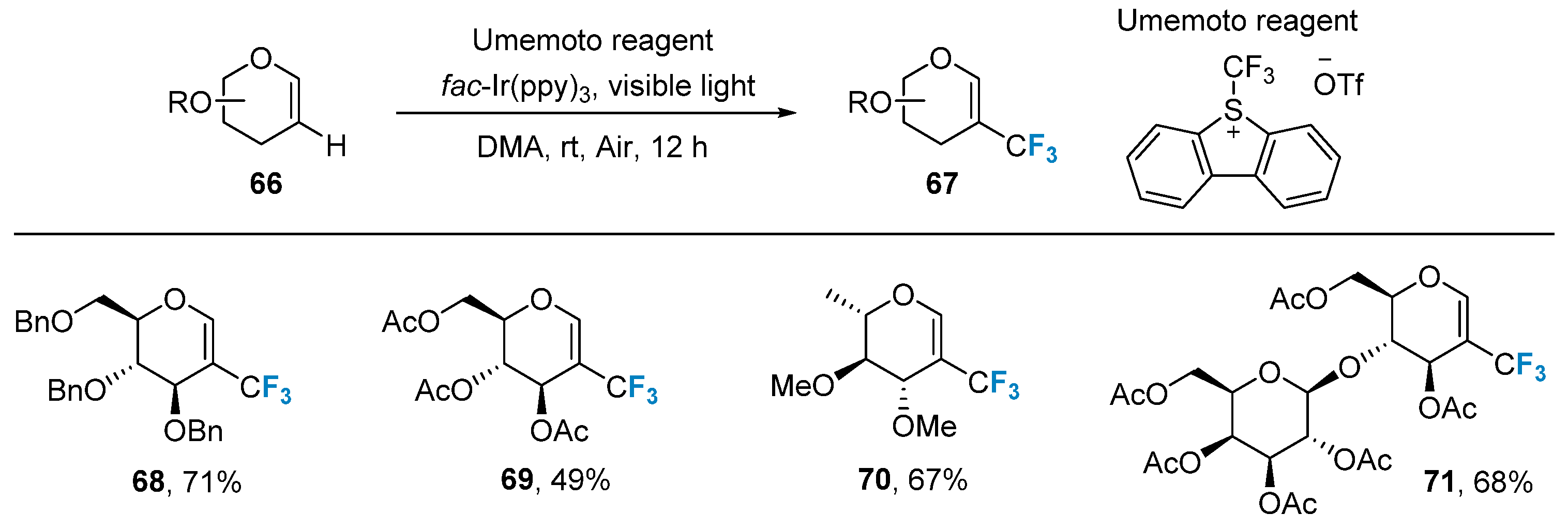
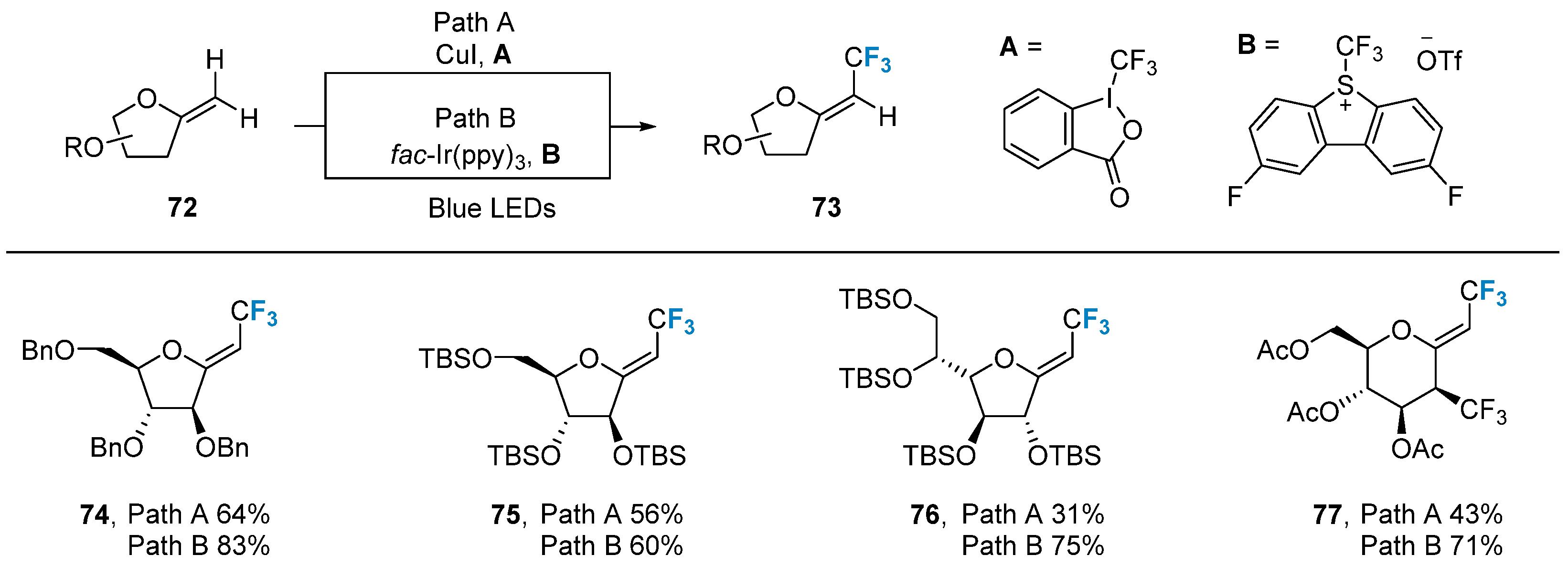
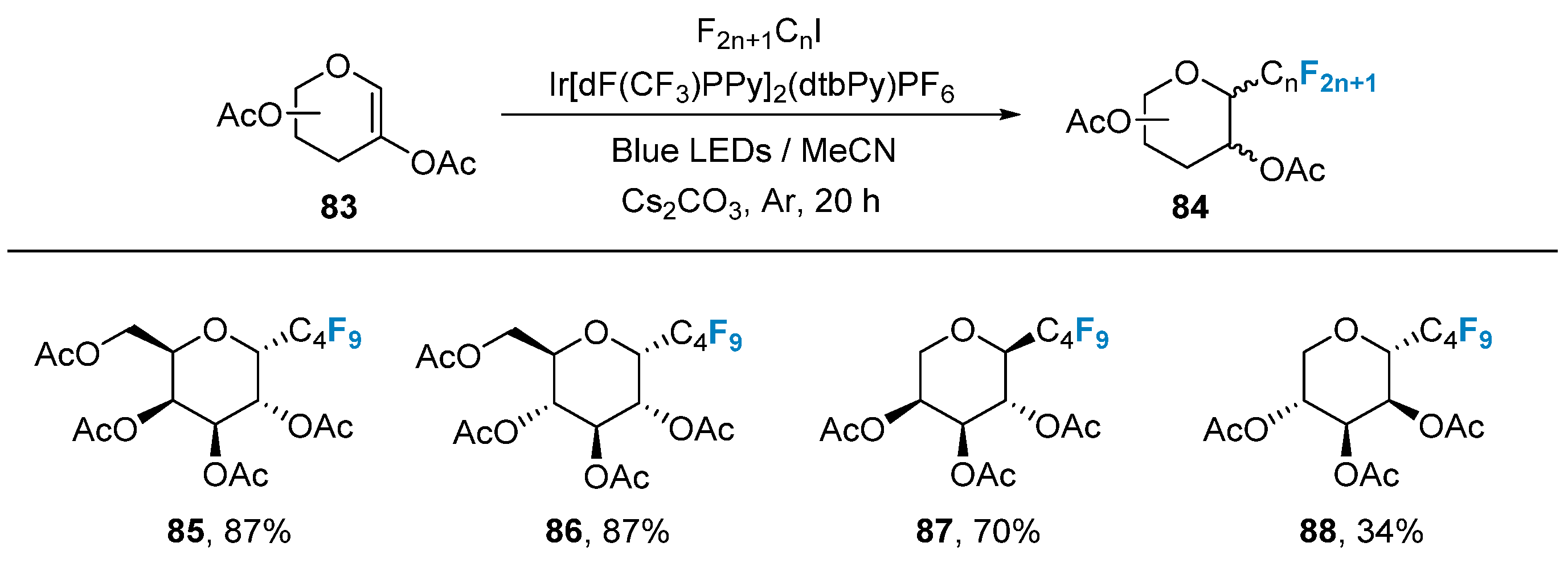
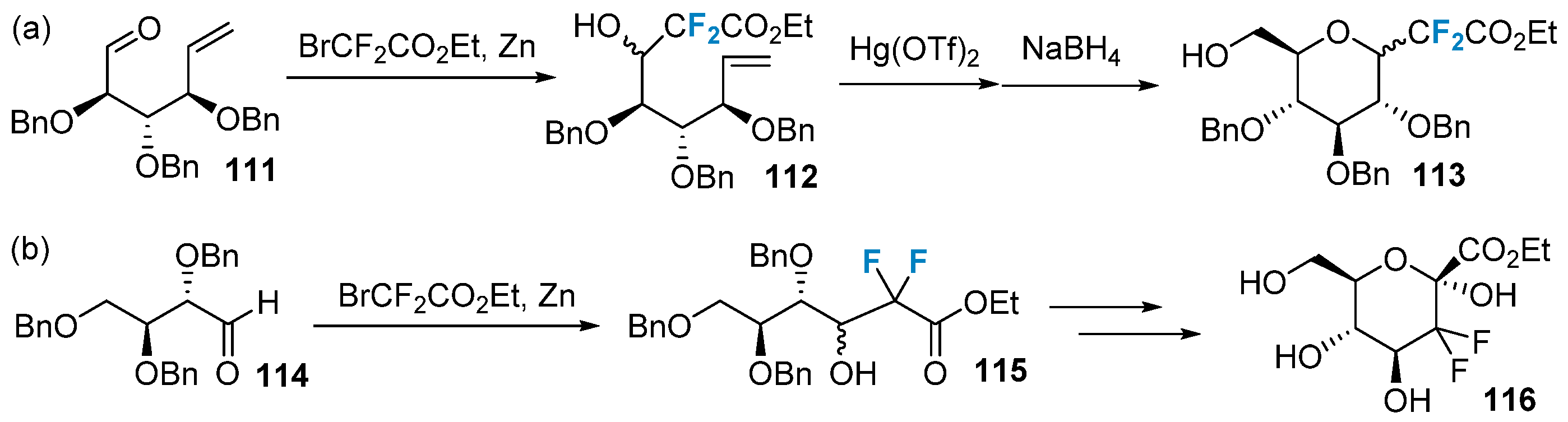
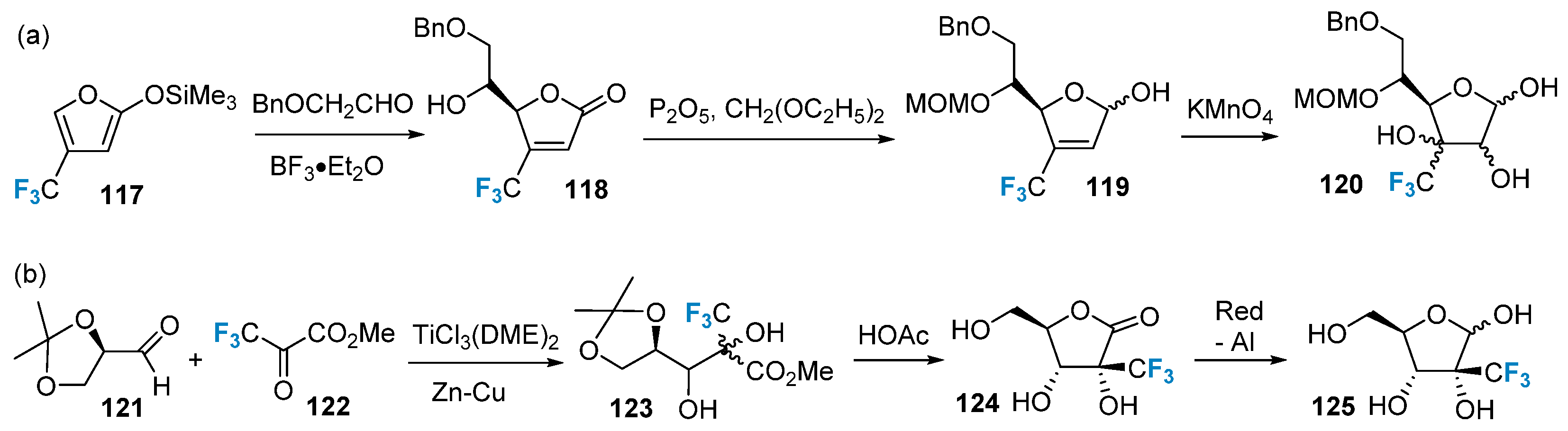
2.3. Fluorination of Carbohydrates via Radical Reactions
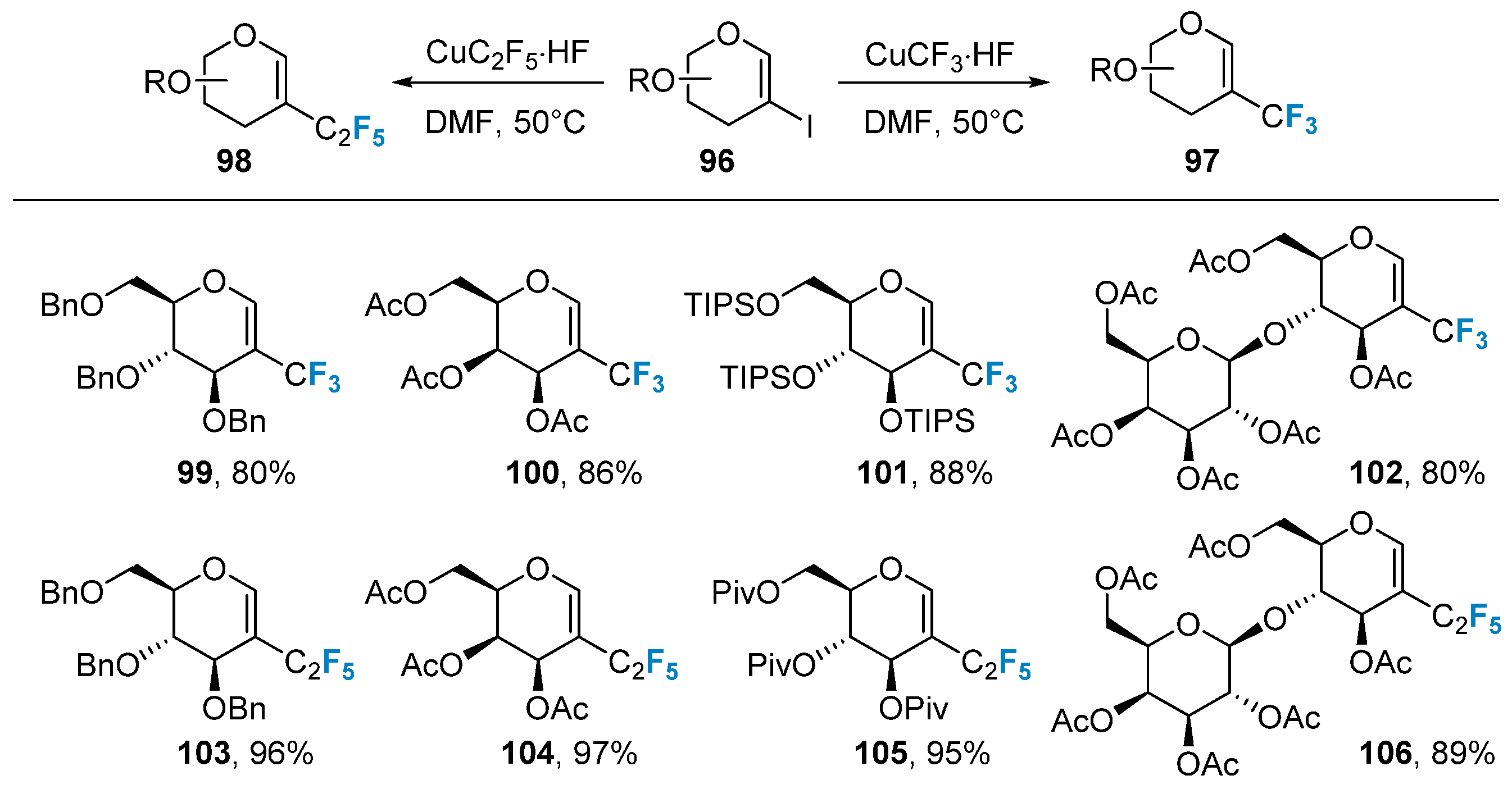
2.4. Fluorination of Carbohydrates via Metal-Catalyzed Coupling Reactions
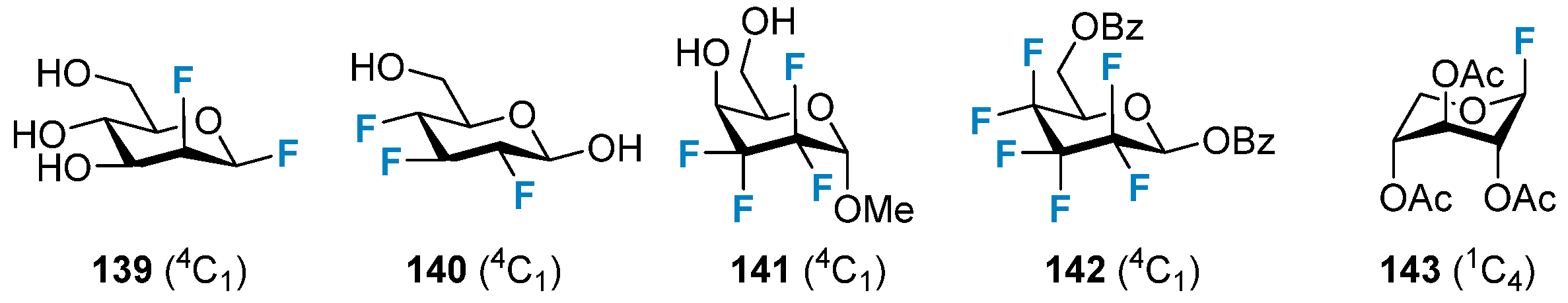
2.5. Fluorination of Carbohydrates via Building Block Strategy
3. Impact of Fluorination on the Physical Properties of Carbohydrates
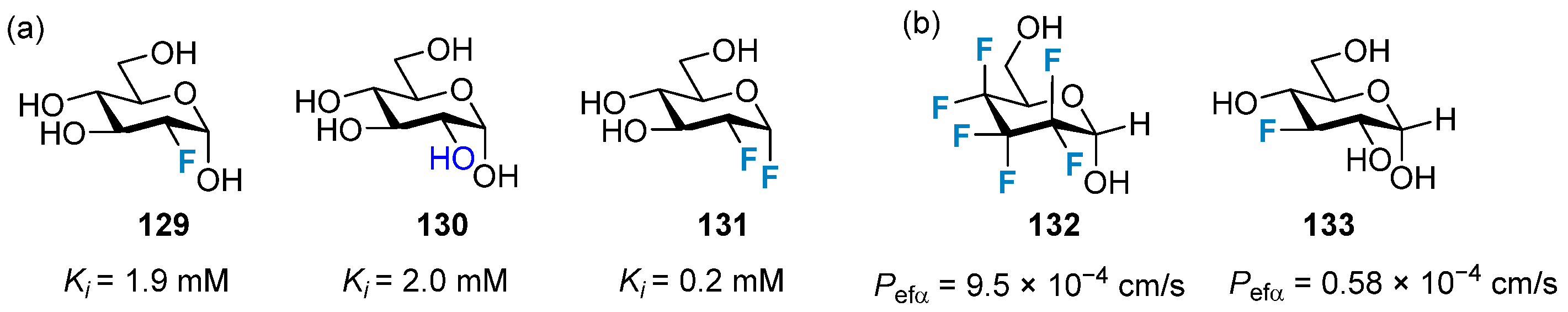
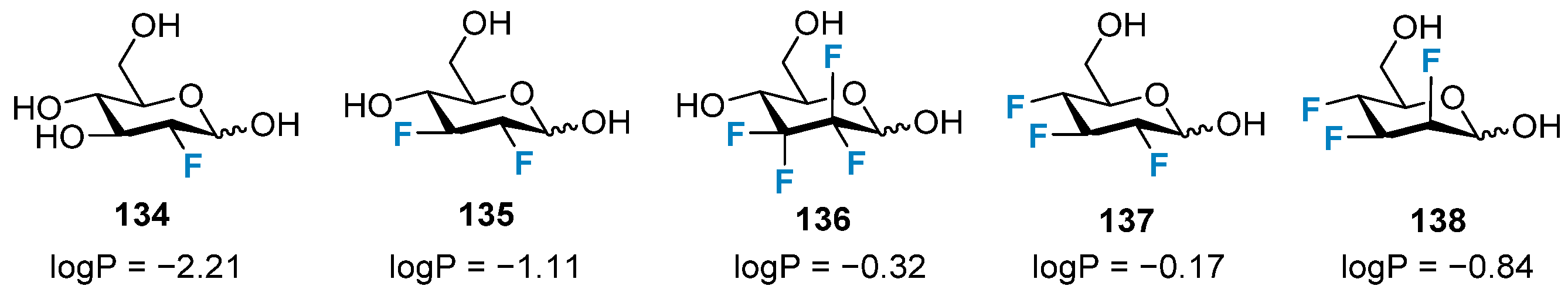
3.1. The Impact on Lipophilicity
3.2. The Impact on Conformation
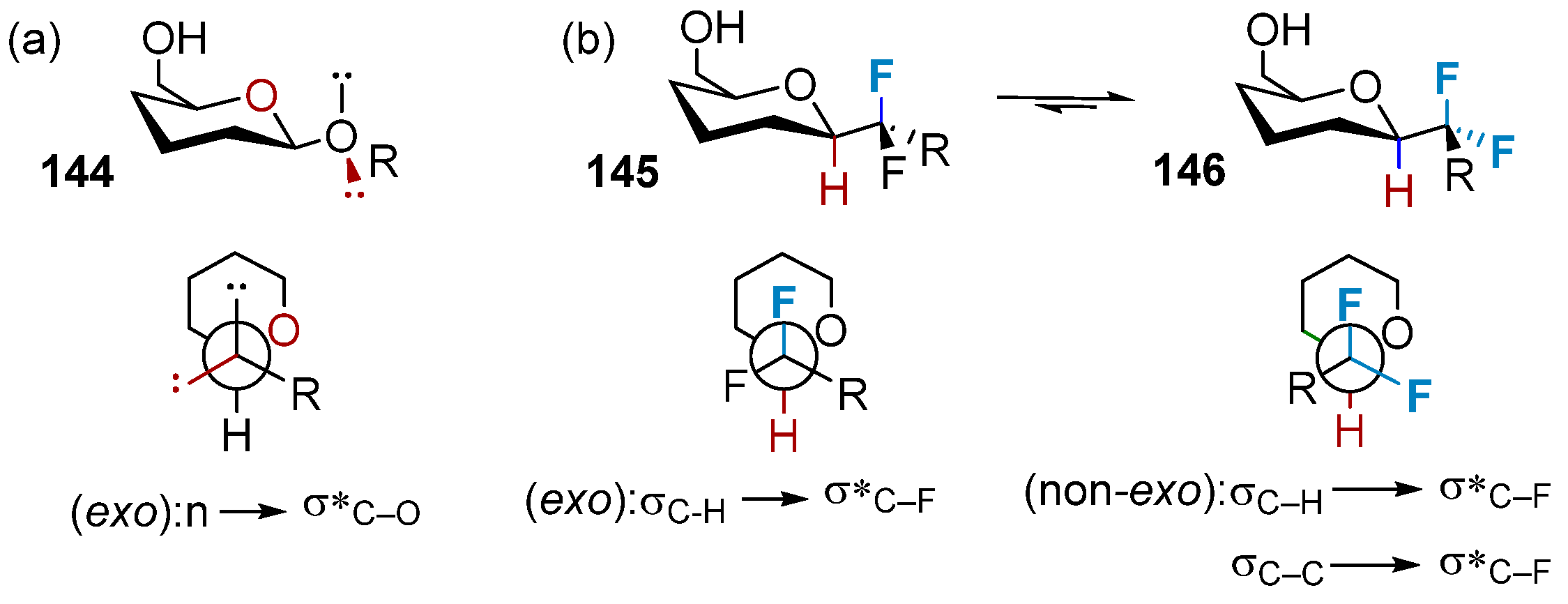
3.2.1. The Impact on the Chair Conformation of Pyranose
3.2.2. The Impact on the Conformation of the Glycosidic Bond
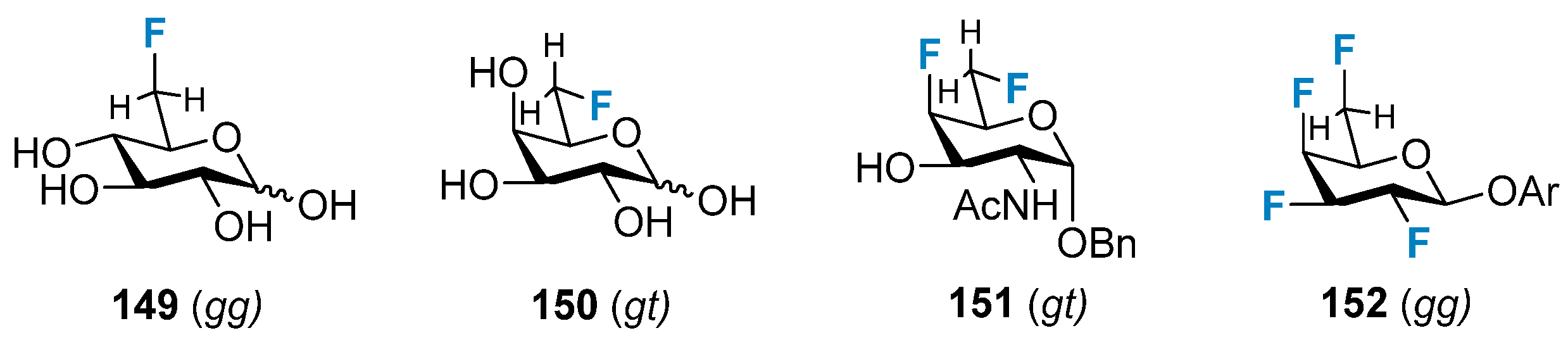
3.2.3. The Impact on the Conformation of the Exocyclic Hydroxymethyl Group
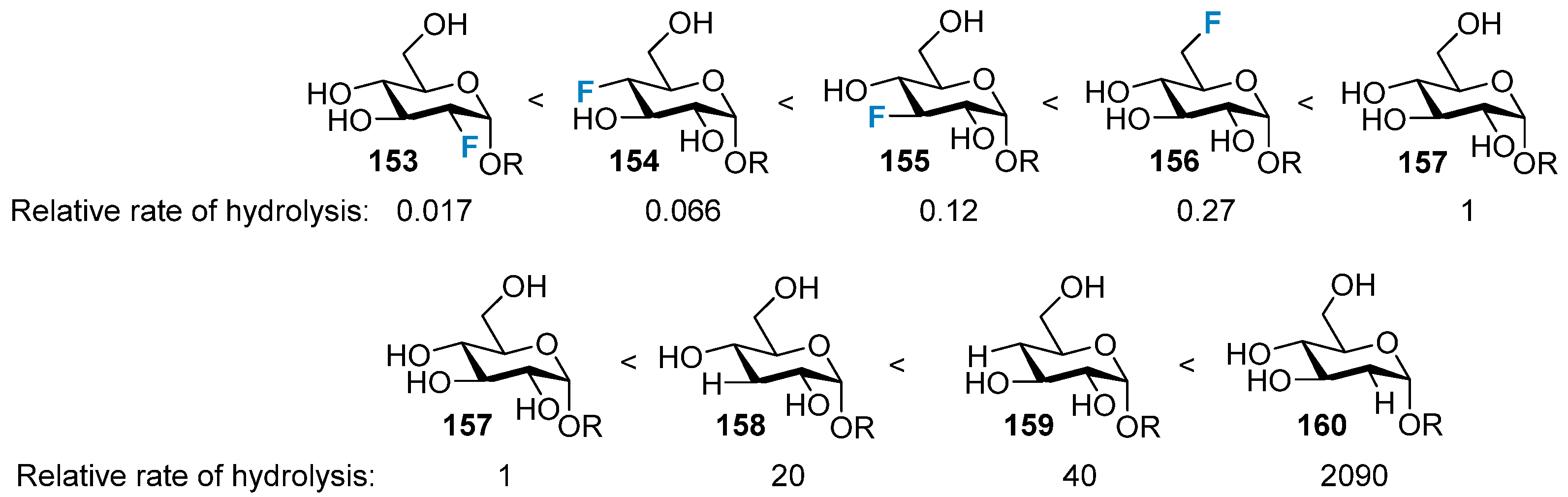
3.3. The Impact on the Stability of the Glycosidic Bond
4. Application
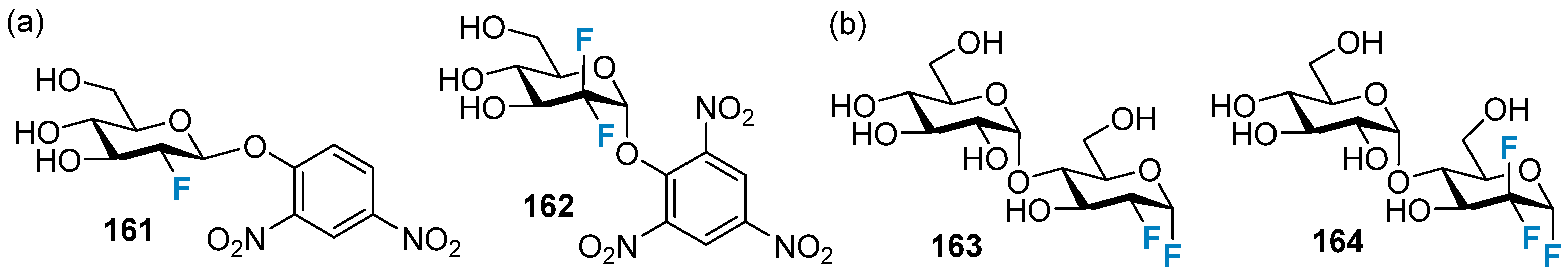
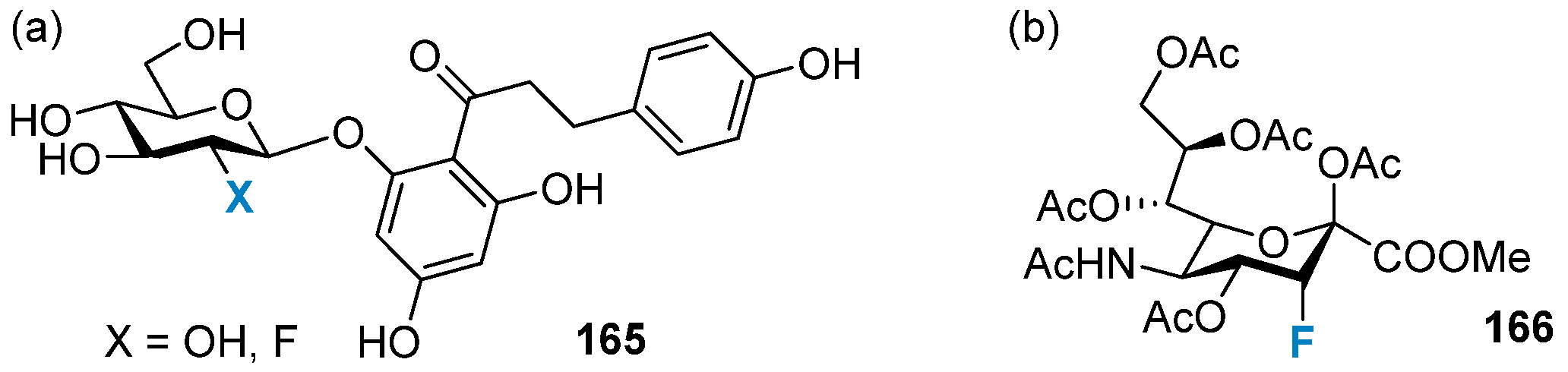
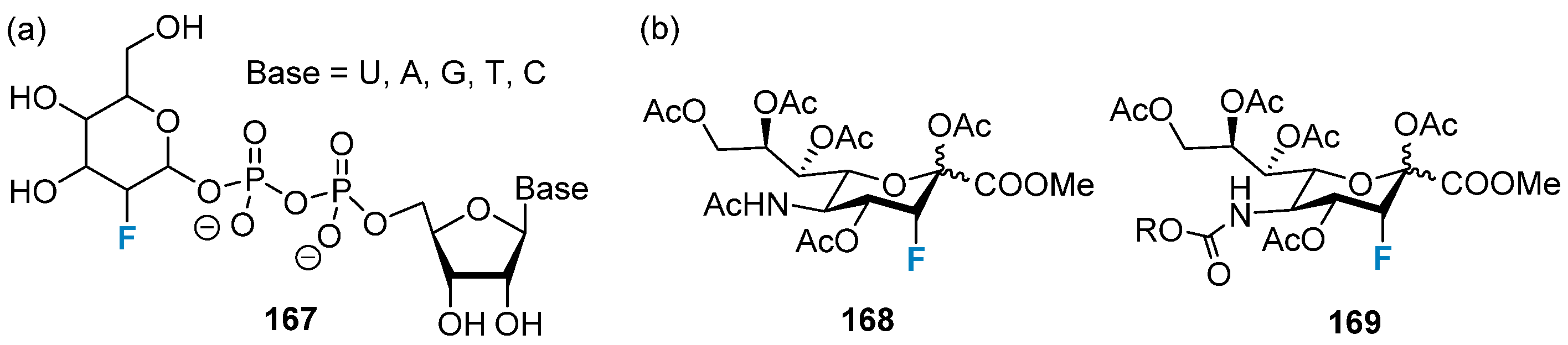
4.1. Fluorine-Containing Sugars as Glycosyltransferase Inhibitors
4.1.1. Fluorosugars as in Cellulose Glycosyltransferase Inhibitors
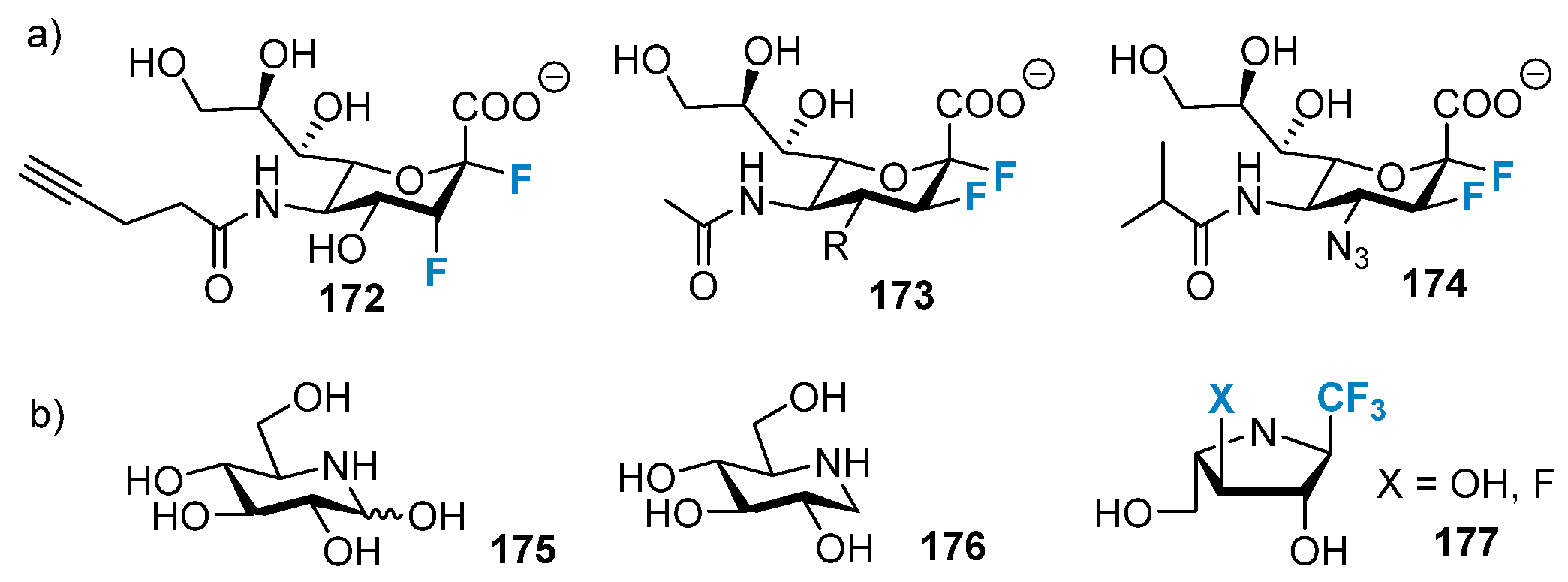
4.1.2. Fluorine-Containing Sugars as Glycosidase Inhibitors
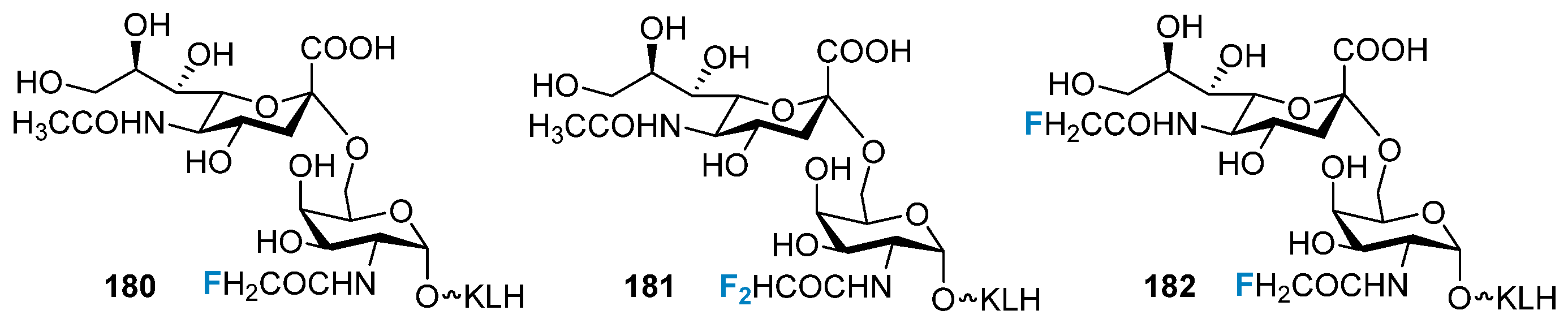
4.2. Synthetic Vaccines and Therapeutic Antibodies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dube, D.H.; Bertozzi, C.R. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; McGuckin, M.A.; Wesselingh, S.; Rogers, G.B. Infection’s sweet tooth: How glycans mediate infection and disease susceptibility. Trends Microbiol. 2018, 26, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.L. Glycomimetic drugs—A new source of therapeutic opportunities. Discov. Med. 2009, 8, 247–252. [Google Scholar] [PubMed]
- Hevey, R. Bioisosteres of Carbohydrate Functional Groups in Glycomimetic Design. Biomimetics 2019, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Hevey, R. Strategies for the Development of Glycomimetic Drug Candidates. Pharmaceuticals 2019, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzyme. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef]
- Biffinger, J.C.; Kim, H.W.; DiMagno, S.G. The polar hydrophobicity of fluorinated compounds. ChemBioChem 2004, 5, 622–627. [Google Scholar] [CrossRef]
- Alabugin. Stereoelectronic effects and general trends in hyperconjugative acceptor ability of σ bonds. J. Am. Chem. Soc. 2002, 124, 3175–3185. [Google Scholar] [CrossRef]
- Glaudemans, C.P.; Kovac, P. Probing the combining site of monoclonal IgA J539 using deoxyfluoro- and other galactosides as ligands. Mol. Immunol. 1985, 22, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Street, I.P.; Armstrong, C.R.; Withers, S.G. Hydrogen bonding and specificity. Fluorodeoxy sugars as probes of hydrogen bonding in the glycogen phosphorylase-glucose complex. Bio Chem. Eur. J. 1986, 25, 6021–6027. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, C.P.J. Mapping of subsites of monoclonal, anti-carbohydrate antibodies using deoxy and deoxyfluoro sugars. Chem. Rev. 2002, 91, 25–33. [Google Scholar] [CrossRef]
- Tysoe, C.; Withers, S.G. Fluorinated mechanism-based inhibitors: Common themes and recent developments. Curr. Top. Med. Chem. 2014, 14, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Rempel, B.P.; Withers, S.G. Covalent inhibitors of glycosidases and their applications in biochemistry and biology. Glycobiology 2008, 18, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Arda, A.; Jimenez-Barbero, J. The recognition of glycans by protein receptors. Insights from NMR spectroscopy. Chem. Commun. 2018, 54, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Tengel, T.; Fex, T.; Emtenas, H.; Almqvist, F.; Sethson, I.; Kihlberg, J. Use of 19F NMR spectroscopy to screen chemical libraries for ligands that bind to proteins. Org. Biomol. Chem. 2004, 2, 725–731. [Google Scholar] [CrossRef]
- Diercks, T.; Ribeiro, J.P.; Canada, F.J.; Andre, S.; Jimenez-Barbero, J.; Gabius, H.J. Fluorinated carbohydrates as lectin ligands: Versatile sensors in 19F-detected saturation transfer difference NMR spectroscopy. Chem. Eur. J. 2009, 15, 5666–5668. [Google Scholar] [CrossRef]
- André, S.; Cañada, F.J.; Shiao, T.C.; Largartera, L.; Diercks, T.; Bergeron-Brlek, M.; Biari, K.; Papadopoulos, A.; Ribeiro, J.P.; Touaibia, M.; et al. Fluorinated carbohydrates as lectin ligands: Biorelevant sensors with capacity to monitor anomer affinity in 19F-NMR-based inhibitor screening. Eur. J. Org. Chem. 2012, 2012, 4354–4364. [Google Scholar] [CrossRef]
- Matei, E.; Andre, S.; Glinschert, A.; Infantino, A.S.; Oscarson, S.; Gabius, H.J.; Gronenborn, A.M. Fluorinated carbohydrates as lectin ligands: Dissecting glycan-cyanovirin interactions by using 19F NMR spectroscopy. Chem. Eur. J. 2013, 19, 5364–5374. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Diercks, T.; Jimenez-Barbero, J.; Andre, S.; Gabius, H.J.; Canada, F.J. Fluorinated carbohydrates as lectin ligands: 19F-based direct STD monitoring for detection of anomeric selectivity. Biomolecules 2015, 5, 3177–3192. [Google Scholar] [CrossRef] [PubMed]
- Diercks, T.; Infantino, A.S.; Unione, L.; Jimenez-Barbero, J.; Oscarson, S.; Gabius, H.J. Fluorinated carbohydrates as lectin ligands: Synthesis of OH/F-Substituted N-Glycan core trimannoside and epitope mapping by 2D STD-TOCSY 19F-NMR spectroscopy. Chem. Eur. J. 2018, 24, 15761–15765. [Google Scholar] [CrossRef] [PubMed]
- Valverde, P.; Quintana, J.I.; Santos, J.I.; Arda, A.; Jimenez-Barbero, J. Novel NMR avenues to explore the conformation and interactions of glycans. ACS Omega 2019, 4, 13618–13630. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.D.; Manzano, A.I.; Calvino, E.; Diego, A.; Rodriguez de Francisco, B.; Romano, C.; Oscarson, S.; Millet, O.; Gabius, H.J.; Jimenez-Barbero, J.; et al. Fluorinated carbohydrates as lectin ligands: Simultaneous screening of a monosaccharide library and chemical mapping by 19F NMR spectroscopy. J. Org. Chem. 2020, 85, 16072–16081. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Roder, A.; Kaiser, A.; Wagner, S.; Gaidzik, N.; Kowalczyk, D.; Westerlind, U.; Gerlitzki, B.; Schmitt, E.; Kunz, H. Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen-Friedenreich antigen and a fluorine-substituted analogue. Angew. Chem. Int. Ed. 2010, 49, 8498–8503. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Roder, A.; Johannes, M. Synthesis of a MUC1-glycopeptide-BSA conjugate vaccine bearing the 3′-deoxy-3′-fluoro-Thomsen-Friedenreich antigen. Chem. Commun. 2011, 47, 9903–9905. [Google Scholar] [CrossRef] [PubMed]
- Oberbillig, T.; Mersch, C.; Wagner, S.; Hoffmann-Roder, A. Antibody recognition of fluorinated MUC1 glycopeptide antigens. Chem. Commun. 2012, 48, 1487–1489. [Google Scholar] [CrossRef]
- Wei, M.M.; Wang, Y.S.; Ye, X.S. Carbohydrate-based vaccines for oncotherapy. Med. Res. Rev. 2018, 38, 1003–1026. [Google Scholar] [CrossRef]
- Anderluh, M.; Berti, F.; Bzducha-Wrobel, A.; Chiodo, F.; Colombo, C.; Compostella, F.; Durlik, K.; Ferhati, X.; Holmdahl, R.; Jovanovic, D.; et al. Recent advances on smart glycoconjugate vaccines in infections and cancer. FEBS J. 2022, 289, 4251–4303. [Google Scholar] [CrossRef]
- Mettu, R.; Chen, C.Y.; Wu, C.Y. Synthetic carbohydrate-based vaccines: Challenges and opportunities. J. Biomed. Sci. 2020, 27, 9. [Google Scholar] [CrossRef]
- Linclau, B.; Arda, A.; Reichardt, N.C.; Sollogoub, M.; Unione, L.; Vincent, S.P.; Jimenez-Barbero, J. Fluorinated carbohydrates as chemical probes for molecular recognition studies. Current status and perspectives. Chem. Soc. Rev. 2020, 49, 3863–3888. [Google Scholar] [CrossRef] [PubMed]
- Hevey, R. The role of fluorine in glycomimetic drug design. Chem. Eur. J. 2021, 27, 2240–2253. [Google Scholar] [CrossRef] [PubMed]
- Markovski, L.N.; Pashinnik, V.E. Applications of dialkylaminosulfur trifluorides for the syntheses of acid fluorides. Synthesis 1975, 12, 801–802. [Google Scholar] [CrossRef]
- Middleton, W.J. New fluorinating reagents. Dialkylaminosulfur fluorides. J. Org. Chem. 1975, 40, 574–578. [Google Scholar] [CrossRef]
- Doboszewski, B.; Hay, G.W.; Szarek, W.A. The rapid synthesis of deoxyfluoro sugars using tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF). Can. J. Chem. 1987, 65, 412–419. [Google Scholar] [CrossRef]
- Card, P.J.; Hitz, W.D. Synthesis of 1′-deoxy-1′-fluorosucrose via sucrose synthetase mediated coupling of 1-deoxy-1-fluorofructose with uridine diphosphate glucose. J. Am. Chem. Soc. 1984, 106, 5348–5350. [Google Scholar] [CrossRef]
- Masataka, Y. Methods of synthesis of glycosyl fluorides. Carbohydr. Res. 2000, 327, 5–14. [Google Scholar]
- Takahashi, Y.; Tsuneda, S.; Tsuchiya, T.; Koyama, Y.; Umezawa, S. Synthesis of 4’-deoxy-4’-fluorokanamycin A and B. Carbohydr. Res. 1992, 232, 89–105. [Google Scholar] [CrossRef]
- Card, P.J. Synthesis of Fluorinated Carbohydrates. J. Carbohydr. Chem. 2007, 4, 451–487. [Google Scholar] [CrossRef]
- Nyffeler, P.T.; Duron, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.H. Selectfluor: Mechanistic insight and applications. Angew. Chem. Int. Ed. 2005, 44, 192–212. [Google Scholar] [CrossRef]
- Cen, Y.; Sauve, A.A. Diastereocontrolled electrophilic fluorinations of 2-deoxyribonolactone: Syntheses of all corresponding 2-deoxy-2-fluorolactones and 2’-deoxy-2’-fluoro-NAD+s. J. Org. Chem. 2009, 74, 5779–5789. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sharon, A.; Chu, C.K. Fluorinated nucleosides: Synthesis and biological implication. J. Fluorine Chem. 2008, 129, 743–766. [Google Scholar] [CrossRef] [PubMed]
- Herdewijn, P.; Aerschot, A.V.; Kerremans, L. Synthesis of nucleosides fluorinated in the sugar moiety. The application of diethylaminosulfur trifluoride to the synthesis of fluorinated nucleosides. Nucleosides Nucleotides 1989, 8, 65–96. [Google Scholar] [CrossRef]
- Uhrig, M.L.; Lantaño, B.; Postigo, A. Synthetic strategies for fluorination of carbohydrates. Org. Biomol. Chem. 2019, 17, 5173–5189. [Google Scholar] [CrossRef] [PubMed]
- Dax, K.; Albert, M.; Ortner, J.; Paul, B.J. Synthesis of deoxyfluoro sugars from carbohydrate precursors. Carbohydr. Res. 2000, 327, 47–86. [Google Scholar] [CrossRef] [PubMed]
- Miethchen, R. Modified natural substances—Fluorinated and fluoroalkylated monosaccharides and inositols. J. Fluorine Chem. 2004, 125, 895–901. [Google Scholar] [CrossRef]
- Lloyd, A.; Coe, P.; Walker, R.; Howarth, O. Some intramolecular rearrangements when pentofuranoses are treated with diethylaminosulphur trifluoride (DAST). J. Fluorine Chem. 1993, 60, 239–250. [Google Scholar] [CrossRef]
- Dmowski, W.; Kamiński, M. Dialkyl-α,α-difluorobenzylamines and dialkyl(trifluoromethyl)-amines-novel fluorinating reagents. J. Fluorine Chem. 1983, 23, 219–228. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yoneda, A.; Fukuhara, T.; Hara, S. Selective synthesis of fluorinated carbohydrates using N,N-diethyl-α,α-difluoro-(m-methylbenzyl) amine. Tetrahedron Lett. 2004, 45, 1287–1289. [Google Scholar] [CrossRef]
- Huonnic, K.; Linclau, B. The synthesis and glycoside formation of polyfluorinated carbohydrates. Chem. Rev. 2022, 122, 15503–15602. [Google Scholar] [CrossRef]
- Sharma, R.; Kavai, I.; Fu, Y.; Bobek, M. Synthesis of gem-difluorosaccharides. Tetrahedron Lett. 1977, 18, 3433–3436. [Google Scholar] [CrossRef]
- Kolympadi, M.; Fontanella, M.; Venturi, C.; André, S.; Gabius, H.J.; Jiménez-Barbero, J.; Vogel, P. Synthesis and conformational analysis of (α-D-galactosyl) phenylmethane and α-, β-difluoromethane analogues: Interactions with the plant lectin viscumin. Chem. Eur. J. 2009, 15, 2861–2873. [Google Scholar] [CrossRef]
- Houlton, J.S.; Motherwell, W.B.; Ross, B.C.; Tozer, M.J.; Williams, D.J.; Slawin, A.M. A convenient strategy for replacement of the anomeric hydroxyl group by difluoromethyl functionality in carbohydrate derivatives. Tetrahedron 1993, 49, 8087–8106. [Google Scholar] [CrossRef]
- Leclerc, E.; Pannecoucke, X. Synthetic efforts towards the synthesis of fluorinated C-glycosidic analogues of α-galactosylceramides. C. R. Chim. 2012, 15, 57–67. [Google Scholar] [CrossRef]
- Cuenca, A.B.; D’Hooge, F.; Gouge, V.; Castelot-Deliencourt, G.; Oulyadi, H.; Leclerc, E.; Jubault, P.; Pannecoucke, X.; Quirion, J.C. Addition of ethyl bromodifluoroacetate to lactones: Reactivity and stereoselectivity. Synlett 2005, 17, 2627–2630. [Google Scholar] [CrossRef]
- Poulain, F.; Leclerc, E.; Quirion, J.C. Approaches to the synthesis of CF2-analogues of 2-deoxy-2-aminoglycosides. Tetrahedron Lett. 2009, 50, 1803–1805. [Google Scholar] [CrossRef]
- Munier, P.; Giudicelli, M.B.; Picq, D.; Anker, D. Synthese des 5-Desoxy-5,5,5-trifluoro-D-et-LPentofuranoses. J. Carbohydr. Chem. 1996, 15, 739–762. [Google Scholar] [CrossRef]
- Lavaire, S.; Plantier-Royon, R.; Portella, C. Synthesis of 3-deoxy-3-C-trifluoromethyl-D-ribose from D-xylose or D-glucose. J. Carbohydr. Chem. 1996, 15, 361–370. [Google Scholar] [CrossRef]
- Lal, G.S.; Pez, G.P.; Syvret, R.G. Electrophilic NF fluorinating agents. Chem. Rev. 1996, 96, 1737–1756. [Google Scholar] [CrossRef]
- Burkart, M.D.; Zhang, Z.Y.; Hung, S.C.; Wong, C.H. A new method for the synthesis of fluorocarbohydrates and glycosides using selectfluor. J. Am. Chem. Soc. 1997, 119, 11743–11746. [Google Scholar] [CrossRef]
- Albert, M.; Dax, K.; Ortner, J. A novel direct route to 2-deoxy-2-fluoro-aldoses and their corresponding derivatives. Tetrahedron 1998, 54, 4839–4848. [Google Scholar] [CrossRef]
- Adamson, J.; Foster, A.B.; Hall, L.D.; Johnson, R.N.; Hesse, R.H. Fluorinated carbohydrates: Part III. 2-deoxy-2-fluoro-D-glucose and 2-deoxy-2-fluoro-D-mannose. Carbohydr. Res. 1970, 15, 351–359. [Google Scholar] [CrossRef]
- Ashique, R.; Chirakal, R.V.; Hughes, D.W.; Schrobilgen, G.J. Two-step regio-and stereoselective syntheses of 19F-and 18F-2-deoxy-2-(R)-fluoro-β-D-allose. Carbohydr. Res. 2006, 341, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ding, H.; Chen, P.; Liu, L.; Sun, Q.; Wang, X.; Wang, P.; Lv, Z.; Li, M. Radical dehydroxymethylative fluorination of carbohydrates and divergent transformations of the resulting reverse glycosyl fluorides. Angew. Chem. Int. Ed. 2020, 59, 4138–4144. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, J.; Zhu, X.; Zhou, X.; Sun, S.; Wang, P.; Cao, H.; Yu, G.; Li, M. Synthesis of Rare 6-Deoxy-D-/L-heptopyranosyl fluorides: Assembly of a hexasaccharide corresponding to campylobacter jejuni strain CG8486 capsular polysaccharide. J. Am. Chem. Soc. 2021, 143, 11171–11179. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, P.; Long, Q.; Ding, H.; Cheng, G.; Li, T.; Li, M. Synthesis of reverse glycosyl fluorides and rare glycosyl fluorides enabled by radical decarboxylative fluorination of uronic acids. Org. Lett. 2020, 22, 9325–9330. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yan, N.; Wang, P.; Song, N.; Sun, Q.; Li, T.; Li, M. Synthesis of reverse glycosyl fluorides via organophotocatalytic decarboxylative fluorination of uronic acids. Org. Chem. Front. 2022, 9, 2808–2814. [Google Scholar] [CrossRef]
- Moreno, B.; Quehen, C.; Rose-Helene, M.; Leclerc, E.; Quirion, J.C. Addition of difluoromethyl radicals to glycals: A new route to α-CF2-D-glycosides. Org. Lett. 2007, 9, 2477–2480. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, D.C.; Ye, X.S. Direct C-H trifluoromethylation of glycals by photoredox catalysis. Org. Lett. 2015, 17, 5698–5701. [Google Scholar] [CrossRef]
- Frédéric, C.J.M.; Cornil, J.; Vandamme, M.; Dumitrescu, L.; Tikad, A.; Robiette, R.; Vincent, S.P. Highly (Z)-diastereoselective synthesis of trifluoromethylated exo-glycals via photoredox and copper catalysis. Org. Lett. 2018, 20, 6769–6773. [Google Scholar] [CrossRef]
- Liu, M.; Luo, Z.X.; Li, T.; Xiong, D.C.; Ye, X.S. Electrochemical trifluoromethylation of glycals. J. Org. Chem. 2021, 86, 16187–16194. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.W.M.; Uhrig, M.L.; Postigo, A. Photocatalyzed reductive fluoroalkylation of 2-acetoxyglycals towards the stereoselective synthesis of α-1-fluoroalkyl-C-glycosyl derivatives. Org. Biomol. Chem. 2020, 18, 8724–8734. [Google Scholar] [CrossRef] [PubMed]
- Belhomme, M.C.; Dru, D.; Xiong, H.Y.; Cahard, D.; Besset, T.; Poisson, T.; Pannecoucke, X. Coppermediated direct functionalization of unsaturated C–C bonds with ethyl bromo (difluoro) acetate: A straightforward access to highly valuable difluoromethylated alkenes. Synthesis 2014, 46, 1859–1870. [Google Scholar]
- Belhomme, M.C.; Poisson, T.; Pannecoucke, X. Copper catalyzed β-difluoroacetylation of dihydropyrans and glycals by means of direct C-H functionalization. Org. Lett. 2013, 15, 3428–3431. [Google Scholar] [CrossRef] [PubMed]
- Mestre, J.; Lishchynskyi, A.; Castillon, S.; Boutureira, O. Trifluoromethylation of electron-rich alkenyl iodides with fluoroform-derived “ligandless” CuCF3. J. Org. Chem. 2018, 83, 8150–8160. [Google Scholar] [CrossRef] [PubMed]
- Mestre, J.; Castillón, S.; Boutureira, O. “Ligandless” pentafluoroethylation of unactivated (hetero) aryl and alkenyl halides enabled by the controlled self-condensation of TMSCF3-derived CuCF3. J. Org. Chem. 2019, 84, 15087–15097. [Google Scholar] [CrossRef] [PubMed]
- Tony, K.A.; Denton, R.W.; Dilhas, A.; Jiménez-Barbero, J.; Mootoo, D.R. Synthesis of β-C-galactopyranosides with fluorine on the pseudoanomeric substituent. Org. Lett. 2007, 9, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Stéphane, M.; François, D.; Sathunuru, R.; Christian, F.; Xavier, P.; Jean-Charles, Q. Synthesis of new α- and β-gem-difluoromethylene C-glycosides in the galactose and glucose series. Tetrahedron Lett. 2001, 42, 5879–5882. [Google Scholar]
- Li, Y.; Drew, M.G.; Welchman, E.V.; Shirvastava, R.K.; Jiang, S.; Valentine, R.; Singh, G. Syntheses of ethyl 3-deoxy-3, 3-difluoro-D-arabino-heptulosonate and analogues. Tetrahedron 2004, 60, 6523–6531. [Google Scholar] [CrossRef]
- Kawada, K.; Kitagawa, O.; Taguchi, T.; Hanzawa, Y.; Kobayashi, Y.; Iitaka, Y. Studies on organic fluorine compounds. XLVII. synthesis of trifluoromethylated sugars through the Aldol Reaction of 2-trimethylsilyloxy-4-trifluoromethylfuran. Chem. Pharm. Bull. 1986, 17, 4216–4222. [Google Scholar] [CrossRef]
- Eilitz, U.; Bottcher, C.; Sieler, J.; Gockel, S.; Haas, A.; Burger, K. Synthesis of 2-C-trifluoromethyl substituted D-ribose. Tetrahedron 2001, 57, 3921–3925. [Google Scholar] [CrossRef]
- N’Go, I.; Golten, S.; Arda, A.; Canada, J.; Jimenez-Barbero, J.; Linclau, B.; Vincent, S.P. Tetrafluorination of sugars as strategy for enhancing protein-carbohydrate affinity: Application to UDP-Galp mutase inhibition. Chem. Eur. J. 2014, 20, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Council, C.E.; Kilpin, K.J.; Gusthart, J.S.; Allman, S.A.; Linclau, B.; Lee, S.S. Enzymatic glycosylation involving fluorinated carbohydrates. Org. Biomol. Chem. 2020, 18, 3423–3451. [Google Scholar] [CrossRef] [PubMed]
- Linclau, B.; Wang, Z.; Compain, G.; Paumelle, V.; Fontenelle, C.Q.; Wells, N.; Weymouth-Wilson, A. Investigating the influence of (deoxy)fluorination on the lipophilicity of non-UV-active fluorinated alkanols and carbohydrates by a new log P determination method. Angew. Chem. Int. Ed. 2016, 55, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Denavit, V.; Lainé, D.; St-Gelais, J.; Johnson, P.A.; Giguère, D. A Chiron approach towards the stereoselective synthesis of polyfluorinated carbohydrates. Nat. Commun. 2018, 9, 4721. [Google Scholar] [CrossRef] [PubMed]
- St-Gelais, J.; Cote, E.; Laine, D.; Johnson, P.A.; Giguere, D. Addressing the structural complexity of fluorinated glucose analogues: Insight into lipophilicities and solvation effects. Chem. Eur. J. 2020, 26, 13499–13506. [Google Scholar] [CrossRef] [PubMed]
- Tarcsay, A.; Keseru, G.M. Contributions of molecular properties to drug promiscuity. J. Med. Chem. 2013, 56, 1789–1795. [Google Scholar] [CrossRef]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef]
- Huchet, Q.A.; Kuhn, B.; Wagner, B.; Kratochwil, N.A.; Fischer, H.; Kansy, M.; Zimmerli, D.; Carreira, E.M.; Muller, K. Fluorination Patterning: A study of structural motifs that impact physicochemical properties of relevance to drug discovery. J. Med. Chem. 2015, 58, 9041–9060. [Google Scholar] [CrossRef]
- Scheidt, F.; Selter, P.; Santschi, N.; Holland, M.C.; Dudenko, D.V.; Daniliuc, C.; Muck-Lichtenfeld, C.; Hansen, M.R.; Gilmour, R. Emulating Natural Product Conformation by Cooperative, Non-Covalent Fluorine Interactions. Chem. Eur. J. 2017, 23, 6142–6149. [Google Scholar] [CrossRef]
- Bresciani, S.; Lebl, T.; Slawin, A.M.; O’Hagan, D. Fluorosugars: Synthesis of the 2,3,4-trideoxy-2,3,4-trifluoro hexose analogues of D-glucose and D-altrose and assessment of their erythrocyte transmembrane transport. Chem. Commun. 2010, 46, 5434–5436. [Google Scholar] [CrossRef] [PubMed]
- Quiquempoix, L.; Wang, Z.; Graton, J.; Latchem, P.G.; Light, M.; Le Questel, J.Y.; Linclau, B. Synthesis of 2,3,4-Trideoxy-2,3,4-trifluoroglucose. J. Org. Chem. 2019, 84, 5899–5906. [Google Scholar] [CrossRef] [PubMed]
- Linclau, B.; Golten, S.; Light, M.; Sebban, M.; Oulyadi, H. The conformation of tetrafluorinated methyl galactoside anomers: Crystallographic and NMR studies. Carbohydr. Res. 2011, 346, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Golten, S.; Fontenelle, C.Q.; Timofte, R.S.; Bailac, L.; Light, M.; Sebban, M.; Oulyadi, H.; Linclau, B. Enantioselective synthesis of dideoxy-tetrafluorinated hexoses. J. Org. Chem. 2016, 81, 4434–4453. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Jagodzinska, M.; Huguenot, F.; Candiani, G.; Zanda, M. Assessing the bioisosterism of the trifluoromethyl group with a protease probe. ChemMedChem. 2009, 4, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L. The C-F bond as a conformational tool in organic and biological chemistry. Beilstein J. Org. Chem. 2010, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Hirai, G.; Watanabe, T.; Yamaguchi, K.; Miyagi, T.; Sodeoka, M. Stereocontrolled and convergent entry to CF2-sialosides: Synthesis of CF2-linked ganglioside GM4. J. Am. Chem. Soc. 2007, 129, 15420–15421. [Google Scholar] [CrossRef]
- Leclerc, E.; Pannecoucke, X.; Etheve-Quelquejeu, M.; Sollogoub, M. Fluoro-C-glycosides and fluoro-carbasugars, hydrolytically stable and synthetically challenging glycomimetics. Chem. Soc. Rev. 2013, 42, 4270–4283. [Google Scholar] [CrossRef]
- Xu, B.; Unione, L.; Sardinha, J.; Wu, S.; Etheve-Quelquejeu, M.; Pilar Rauter, A.; Bleriot, Y.; Zhang, Y.; Martin-Santamaria, S.; Diaz, D.; et al. gem-Difluorocarbadisaccharides: Restoring the exo-anomeric effect. Angew. Chem. Int. Ed. 2014, 53, 9597–9602. [Google Scholar] [CrossRef]
- Turnbull, J.; Powell, A.; Guimond, S. Heparan sulfate: Decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001, 11, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Johnson, D.J.; Esmon, C.T.; Huntington, J.A. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat. Struct. Mol. Biol. 2004, 11, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Canales, A.; Lozano, R.; Lopez-Mendez, B.; Angulo, J.; Ojeda, R.; Nieto, P.M.; Martin-Lomas, M.; Gimenez-Gallego, G.; Jimenez-Barbero, J. Solution NMR structure of a human FGF-1 monomer, activated by a hexasaccharide heparin-analogue. FEBS J. 2006, 273, 4716–4727. [Google Scholar] [CrossRef] [PubMed]
- Sawen, E.; Roslund, M.U.; Cumpstey, I.; Widmalm, G. Synthesis and conformational analysis of carbasugar bioisosteres of alpha-L-iduronic acid and its methyl glycoside. Carbohydr. Res. 2010, 345, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Bernacki, R.J.; Paul, B.; Korytnyk, W. Fluorinated carbohydrates as potential plasma membrane modifiers. Synthesis of 4- and 6-fluoro derivatives of 2-acetamido-2-deoxy-D-hexopyranoses. Carbohydr. Res. 1990, 198, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Lebedel, L.; Arda, A.; Martin, A.; Desire, J.; Mingot, A.; Aufiero, M.; Aiguabella Font, N.; Gilmour, R.; Jimenez-Barbero, J.; Bleriot, Y.; et al. Structural and computational analysis of 2-halogeno-glycosyl cations in the presence of a superacid: An expansive platform. Angew. Chem. Int. Ed. 2019, 58, 13758–13762. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.G.; MacLennan, D.J.; Street, I.P. The synthesis and hydrolysis of a series of deoxyfluoro-D-glucopyranosyl phosphates. Carbohydr. Res. 1986, 154, 127–144. [Google Scholar] [CrossRef]
- Withers, S.G.; Percival, M.D. and Street, I.P. The synthesis and hydrolysis of a series of deoxy- and deoxyfluoro-α-D-“glucopyranosyl” phosphates. Carbohydr. Res. 1989, 187, 43. [Google Scholar] [CrossRef]
- Namchuk, M.N.; McCarter, J.D.; Becalski, A.; Andrews, T.; Withers, S.G. The role of sugar substituents in glycoside hydrolysis. J. Am. Chem. Soc. 2000, 122, 1270–1277. [Google Scholar] [CrossRef]
- Burkart, M.D.; Vincent, S.P.; Duffels, A.; Murray, B.W.; Ley, S.V.; Wong, C.H. Chemo-enzymatic synthesis of fluorinated sugar nucleotide: Useful mechanistic probes for glycosyltransferases. Bioorg. Med. Chem. 2000, 8, 1937–1946. [Google Scholar] [CrossRef]
- Nishimura, S.; Hato, M.; Hyugaji, S.; Feng, F.; Amano, M. Glycomics for drug discovery: Metabolic perturbation in androgen-independent prostate cancer cells induced by unnatural hexosamine mimics. Angew. Chem. Int. Ed. 2012, 51, 3386–3390. [Google Scholar] [CrossRef] [PubMed]
- Marathe, D.D.; Buffone, A.J.; Chandrasekaran, E.V.; Xue, J.; Locke, R.D.; Nasirikenari, M.; Lau, J.T.; Matta, K.L.; Neelamegham, S. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood 2010, 115, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, X.M.; Lawrence, R.; Thijssen, V.L.; van den Broek, S.A.; Troost, R.; van Scherpenzeel, M.; Naidu, N.; Oosterhof, A.; Griffioen, A.W.; Lefeber, D.J.; et al. A common sugar-nucleotide-mediated mechanism of inhibition of (glycosamino)glycan biosynthesis, as evidenced by 6F-GalNAc (Ac3). FASEB J. 2015, 29, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Barthel, S.R.; Antonopoulos, A.; Cedeno-Laurent, F.; Schaffer, L.; Hernandez, G.; Patil, S.A.; North, S.J.; Dell, A.; Matta, K.L.; Neelamegham, S.; et al. Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J. Biol. Chem. 2011, 286, 21717–21731. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.G.; Street, I.P.; Bird, P.; Dolphin, D.H. 2-Deoxy-2-fluoroglucosides: A novel class of mechanism-based glucosidase inhibitors. J. Am. Chem. Soc. 1987, 109, 7530–7531. [Google Scholar] [CrossRef]
- Braun, C.; Brayer, G.D.; Withers, S.G. Mechanism-based inhibition of yeast alpha-glucosidase and human pancreatic alpha-amylase by a new class of inhibitors. 2-Deoxy-2,2-difluoro-alpha-glycosides. J. Biol. Chem. 1995, 270, 26778–26781. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.D.; Howard, S.; Shum, K.; He, S.; Zhu, A.; Withers, S.G. The synthesis, testing and use of 5-fluoro-alpha-D-galactosyl fluoride to trap an intermediate on green coffee bean alpha-galactosidase and identify the catalytic nucleophile. Carbohydr. Res. 2000, 329, 539–547. [Google Scholar] [CrossRef]
- Amaya, M.F.; Watts, A.G.; Damager, I.; Wehenkel, A.; Nguyen, T.; Buschiazzo, A.; Paris, G.; Frasch, A.C.; Withers, S.G.; Alzari, P.M. Structural Insights into the Catalytic Mechanism of Trypanosoma cruzi trans-Sialidase. Structure 2004, 12, 775–784. [Google Scholar] [CrossRef]
- Vocadlo, D.J.; Mayer, C.; He, S.; Withers, S.G. Mechanism of action and identification of Asp242 as the catalytic nucleophile of Vibrio furnisii N-acetyl-beta-D-glucosaminidase using 2-acetamido-2-deoxy-5-fluoro-alpha-L-idopyranosyl fluoride. BioChem. Eur. J. 2000, 39, 117–126. [Google Scholar] [CrossRef]
- Syson, K.; Stevenson, C.E.M.; Rashid, A.M.; Saalbach, G.; Tang, M.; Tuukkanen, A.; Svergun, D.I.; Withers, S.G.; Lawson, D.M.; Bornemann, S. Structural Insight into How Streptomyces coelicolor Maltosyl Transferase GlgE Binds α-Maltose 1-Phosphate and Forms a Maltosyl-enzyme Intermediate. BioChem. Eur. J. 2014, 53, 2494–2504. [Google Scholar] [CrossRef]
- Thanna, S.; Lindenberger, J.J.; Gaitonde, V.V.; Ronning, D.R.; Sucheck, S.J. Synthesis of 2-deoxy-2,2-difluoro-α-maltosyl fluoride and its X-ray structure in complex with Streptomyces coelicolor GlgEI-V279S. Org. Biomol. Chem. 2015, 13, 7542–7550. [Google Scholar] [CrossRef] [PubMed]
- Sadurní, A.; Kehr, G.; Ahlqvist, M.; Wernevik, J.; Sjögren, H.P.; Kankkonen, C.; Knerr, L.; Gilmour, R. Fluorine-Directed Glycosylation Enables the Stereocontrolled Synthesis of Selective SGLT2 Inhibitors for Type II Diabetes. Eur. J. Org. Chem. 2018, 24, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Boltje, T.J.; van Dinther, E.A.; Peters, T.; de Graaf, A.M.; Leusen, J.H.; Kreutz, M.; Figdor, C.G.; den Brok, M.H.; Adema, G.J. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 2015, 9, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Boltje, T.J.; Wassink, M.; de Graaf, A.M.; van Delft, F.L.; den Brok, M.H.; Adema, G.J. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 2013, 12, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Philippe, C.; Olivier, R.M. Carbohydrate mimetics-based glycosyltransferase inhibitors. Bioorg. Med. Chem. 2001, 9, 3077–3092. [Google Scholar]
- Conforti, I.; Marra, A. Iminosugars as glycosyltransferase inhibitors. Org. Biomol. Chem. 2021, 19, 5439–5475. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Lin, Y.N.; Lin, C.H. Development of fucosyltransferase and fucosidase inhibitors. Chem. Soc. Rev. 2013, 42, 4459–4475. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Wu, L.; Sun, X.L. Sialyltransferase inhibition and recent advances. Biochim. Biophys. Acta 2016, 1864, 143–153. [Google Scholar] [CrossRef]
- Breton, C.; Snajdrova, L.; Jeanneau, C.; Koca, J.; Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 2006, 16, 29–37. [Google Scholar] [CrossRef]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef]
- Bull, C.; Boltje, T.J.; Balneger, N.; Weischer, S.M.; Wassink, M.; van Gemst, J.J.; Bloemendal, V.R.; Boon, L.; van der Vlag, J.; Heise, T.; et al. Sialic acid blockade suppresses tumor growth by enhancing T-cell-mediated tumor immunity. Cancer Res. 2018, 78, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Pijnenborg, J.F.A.; Bull, C.; van Hilten, N.; Kers-Rebel, E.D.; Balneger, N.; Elferink, H.; Adema, G.J.; Boltje, T.J. Potent metabolic sialylation inhibitors based on C-5-modified fluorinated sialic acids. J. Med. Chem. 2019, 62, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.W.; Wittmann, V.; Burkart, M.D.; Hung, S.C.; Wong, C.H. Mechanism of human alpha-1,3-fucosyltransferase V: Glycosidic cleavage occurs prior to nucleophilic attack. BioChem. Eur. J. 1997, 36, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Achmatowicz, M.M.; Allen, J.G.; Bio, M.M.; Bartberger, M.D.; Borths, C.J.; Colyer, J.T.; Crockett, R.D.; Hwang, T.L.; Koek, J.N.; Osgood, S.A.; et al. Telescoped process to manufacture 6,6,6-trifluorofucose via diastereoselective transfer hydrogenation: Scalable access to an inhibitor of fucosylation utilized in monoclonal antibody production. J. Org. Chem. 2016, 81, 4736–4743. [Google Scholar] [CrossRef]
- McKenzie, N.C.; Scott, N.E.; John, A.; White, J.M.; Goddard-Borger, E.D. Synthesis and use of 6,6,6-trifluoro-L-fucose to block core-fucosylation in hybridoma cell lines. Carbohydr. Res. 2018, 465, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A.; Pradhan, K.; Paul, A.; Olin, I.R.; Tuck, O.T.; Moulton, K.D.; Kulkarni, S.S.; Dube, D.H. Metabolic inhibitors of bacterial glycan biosynthesis. Chem. Sci. 2020, 11, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, S. Recent advances in synthetic alpha-glucosidase inhibitors. ChemMedChem 2017, 12, 819–829. [Google Scholar] [CrossRef]
- Williams, S.J.; Withers, S.G. Glycosyl fluorides in enzymatic reactions. Carbohydr. Res. 2000, 327, 27–46. [Google Scholar] [CrossRef]
- McCarter, J.D.; Adam, M.J.; Hartman, N.G.; Withers, S.G. In vivo inhibition of beta-glucosidase and beta-mannosidase activity in rats by 2-deoxy-2-fluoro-beta-glycosyl fluorides and recovery of activity in vivo and in vitro. Biochem. J. 1994, 301, 343–348. [Google Scholar] [CrossRef]
- Watts, A.G.; Damager, I.; Amaya, M.L.; Buschiazzo, A.; Alzari, P.; Frasch, A.C.; Withers, S.G. Trypanosoma cruzi trans-sialidase operates through a covalent sialyl-enzyme intermediate: Tyrosine is the catalytic nucleophile. J. Am. Chem. Soc. 2003, 125, 7532–7533. [Google Scholar] [CrossRef]
- Buchini, S.; Buschiazzo, A.; Withers, S.G. A new generation of specific Trypanosoma cruzi trans-sialidase inhibitors. Angew. Chem. Int. Ed. 2008, 47, 2700–2703. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.S.; Yen, H.Y.; Lin, M.I.; Tsai, T.I.; Wang, S.Y.; Huang, W.I.; Hsu, T.L.; Cheng, Y.S.; Fang, J.M.; Wong, C.H. Cell-permeable probe for identification and imaging of sialidases. Proc. Natl. Acad. Sci. USA 2013, 110, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Weck, S.; Robinson, K.; Smith, M.R.; Withers, S.G. Understanding viral neuraminidase inhibition by substituted difluorosialic acids. Chem. Commun. 2015, 51, 2933–2935. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Resende, R.; Wennekes, T.; Chen, H.M.; Bance, N.; Buchini, S.; Watts, A.G.; Pilling, P.; Streltsov, V.A.; Petric, M.; et al. Mechanism-based covalent neuraminidase inhibitors with broad-spectrum influenza antiviral activity. Science 2013, 340, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, H.; Yumoto, H. Nojirimycin, a new antibiotic. II. Isolation, characterization and biological activity. Antibiotics 1967, 20, 66–71. [Google Scholar]
- Inouye, S.; Tsuruoka, T.; Ito, T.; Niida, T. Structure and synthesis of nojirimycin. Tetrahedron 1968, 24, 2125–2144. [Google Scholar] [CrossRef] [PubMed]
- Monika, B.M.; Anna, S.; Bartosz, M. Design, properties and applications of fluorinated and fluoroalkylated N-containing monosaccharides and their analogues. J. Fluorine Chem. 2019, 227, 109364. [Google Scholar]
- Uhrig, M.L.; Mora Flores, E.W.; Postigo, A. Approaches to the synthesis of perfluoroalkyl-modified carbohydrates and derivatives: Thiosugars, iminosugars, and tetrahydro(thio)pyrans. Chem. Eur. J. 2021, 27, 7813–7825. [Google Scholar] [CrossRef]
- Stütz, A.E. (Ed.) Glycosidase Inhibitors: Nojirimycin and Beyond; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Compain, P.; Martin, O.R. (Eds.) Iminosugars, from Synthesis to Therapeutic Applications; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Wagner, S.; Mersch, C.; Hoffmann-Roder, A. Fluorinated glycosyl amino acids for mucin-like glycopeptide antigen analogues. Chem. Eur. J. 2010, 16, 7319–7930. [Google Scholar] [CrossRef]
- Johannes, M.; Oberbillig, T.; Hoffmann-Roder, A. Synthesis of fluorinated Thomsen-Friedenreich antigens: Direct deoxyfluorination of alphaGalNAc-threonine tert-butyl esters. Org. Biomol. Chem. 2011, 9, 5541–5546. [Google Scholar] [CrossRef]
- Johannes, M.; Reindl, M.; Gerlitzki, B.; Schmitt, E.; Hoffmann-Roder, A. Synthesis and biological evaluation of a novel MUC1 glycopeptide conjugate vaccine candidate comprising a 4’-deoxy-4’-fluoro-Thomsen-Friedenreich epitope. Beilstein J. Org. Chem. 2015, 11, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, X.J.; Huo, C.X.; Wang, Y.; Zhang, Y.; Ye, X.S. Enhancement of the immunogenicity of synthetic carbohydrate vaccines by chemical modifications of STn antigen. ACS Chem. Biol. 2011, 6, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zheng, X.J.; Liu, C.C.; Zhou, Y.; Ye, X.S. A cancer vaccine based on fluorine-modified sialyl-Tn induces robust immune responses in a murine model. Oncotarget 2017, 8, 47330–47343. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Yao, W.L.; Yang, H.; Wu, C.Y.; Xiong, D.C.; Yin, Y.X.; Ye, X.S. Total synthesis of tumor-associated KH-1 antigen core nonasaccharide via photo-induced glycosylation. Org. Chem. Front. 2020, 7, 1255–1259. [Google Scholar] [CrossRef]
- Xiao, A.; Zheng, X.J.; Song, C.; Gui, Y.; Huo, C.X.; Ye, X.S. Correction: Synthesis and immunological evaluation of MUC1 glycopeptide conjugates bearing N-acetyl modified STn derivatives as anticancer vaccines. Org. Biomol. Chem. 2017, 15, 2120–2121. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Sun, S.; Huo, C.X.; Li, Q.; Zheng, X.J.; Tai, G.; Zhou, Y.; Ye, X.S. Synthesis and immunological evaluation of N-acyl modified Tn analogues as anticancer vaccine candidates. Bioorg. Med. Chem. 2016, 24, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zheng, X.J.; Huo, C.X.; Song, C.; Li, Q.; Ye, X.S. Synthesis and Evaluation of Glycoconjugates Comprising N-Acyl-Modified Thomsen-Friedenreich Antigens as Anticancer Vaccines. ChemMedChem 2016, 11, 1090–1096. [Google Scholar] [CrossRef]
- Zheng, X.J.; Yang, F.; Zheng, M.; Huo, C.X.; Zhang, Y.; Ye, X.S. Improvement of the immune efficacy of carbohydrate vaccines by chemical modification on the GM3 antigen. Org. Biomol. Chem. 2015, 13, 6399–6406. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Zheng, X.; Xiong, D.; Ye, X. Cancer vaccines based on fluorine-modified KH-1 elicit robust immune response. Molecules 2023, 28, 1934. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chen, C.Y.; Tsai, T.I.; Li, S.T.; Lin, K.H.; Cheng, Y.Y.; Ren, C.T.; Cheng, T.J.; Wu, C.Y.; Wong, C.H. Immunogenicity study of Globo H analogues with modification at the reducing or nonreducing end of the tumor antigen. J. Am. Chem. Soc. 2014, 136, 16844–16853. [Google Scholar] [CrossRef]
- Krauss, I.J.; Joyce, J.G.; Finnefrock, A.C.; Song, H.C.; Dudkin, V.Y.; Geng, X.; Warren, J.D.; Chastain, M.; Shiver, J.W.; Danishefsky, S.J. Fully synthetic carbohydrate HIV antigens designed on the logic of the 2G12 antibody. J. Am. Chem. Soc. 2007, 129, 11042–11044. [Google Scholar] [CrossRef]
- Astronomo, R.D.; Kaltgrad, E.; Udit, A.K.; Wang, S.K.; Doores, K.J.; Huang, C.Y.; Pantophlet, R.; Paulson, J.C.; Wong, C.H.; Finn, M.G.; et al. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem. Biol. 2010, 17, 357–370. [Google Scholar] [CrossRef]
- Joyce, J.G.; Krauss, I.J.; Song, H.C.; Opalka, D.W.; Grimm, K.M.; Nahas, D.D.; Esser, M.T.; Hrin, R.; Feng, M.; Dudkin, V.Y.; et al. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc. Natl. Acad. Sci. USA 2008, 105, 15684–15689. [Google Scholar] [CrossRef]
- Liu, C.C.; Huo, C.X.; Zhai, C.; Zheng, X.J.; Xiong, D.C.; Ye, X.S. Synthesis and immunological evaluation of pentamannose-based HIV-1 vaccine candidates. Bioconjug. Chem. 2022, 33, 807–820. [Google Scholar] [CrossRef]
- Nicholas, D.T. A Proposal That Would Ban Manufacture, Supply, and Use of All Fluoropolymers and Most Fluorinated Reagents within the Entire EU. Org. Process Res. Dev. 2023, 27, 1422–1426. [Google Scholar]
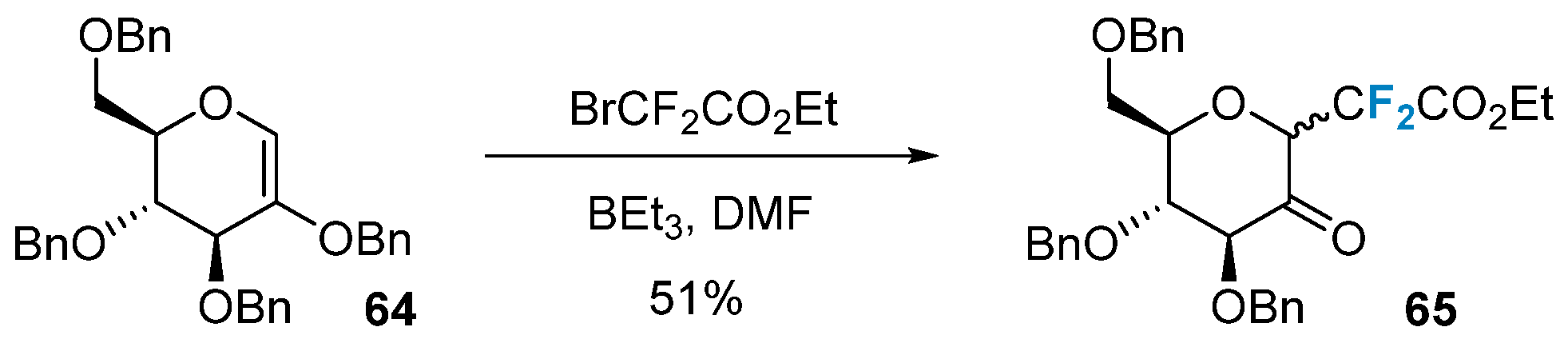





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Wang, P.; Liu, F.; Ye, X.; Xiong, D. Drug Discovery Based on Fluorine-Containing Glycomimetics. Molecules 2023, 28, 6641. https://doi.org/10.3390/molecules28186641
Wei X, Wang P, Liu F, Ye X, Xiong D. Drug Discovery Based on Fluorine-Containing Glycomimetics. Molecules. 2023; 28(18):6641. https://doi.org/10.3390/molecules28186641
Chicago/Turabian StyleWei, Xingxing, Pengyu Wang, Fen Liu, Xinshan Ye, and Decai Xiong. 2023. "Drug Discovery Based on Fluorine-Containing Glycomimetics" Molecules 28, no. 18: 6641. https://doi.org/10.3390/molecules28186641
APA StyleWei, X., Wang, P., Liu, F., Ye, X., & Xiong, D. (2023). Drug Discovery Based on Fluorine-Containing Glycomimetics. Molecules, 28(18), 6641. https://doi.org/10.3390/molecules28186641





