Açai Seeds (Euterpe oleracea Mart) Are Agroindustrial Waste with High Potential to Produce Low-Cost Substrates after Acid Hydrolysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Dried Açai Seeds
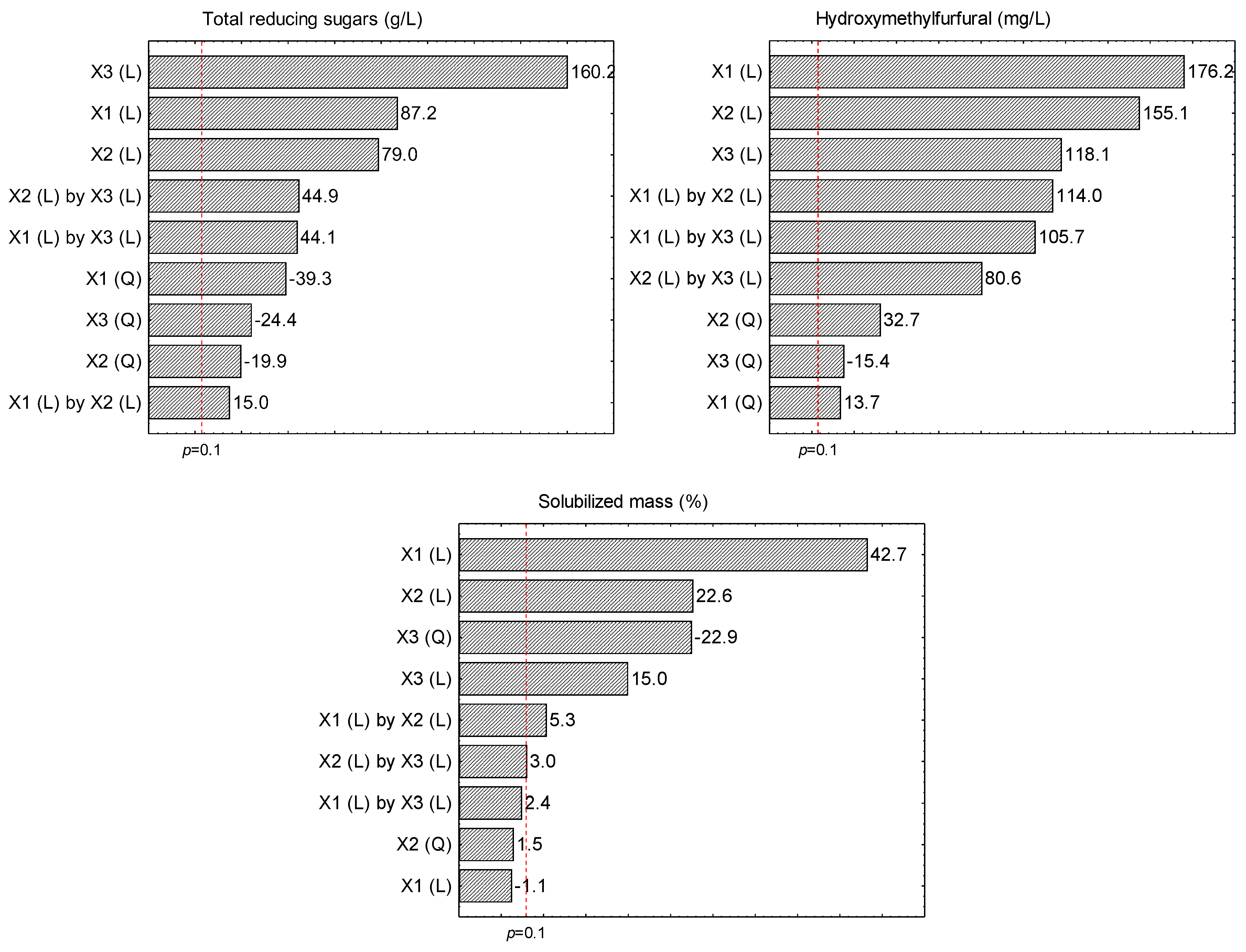
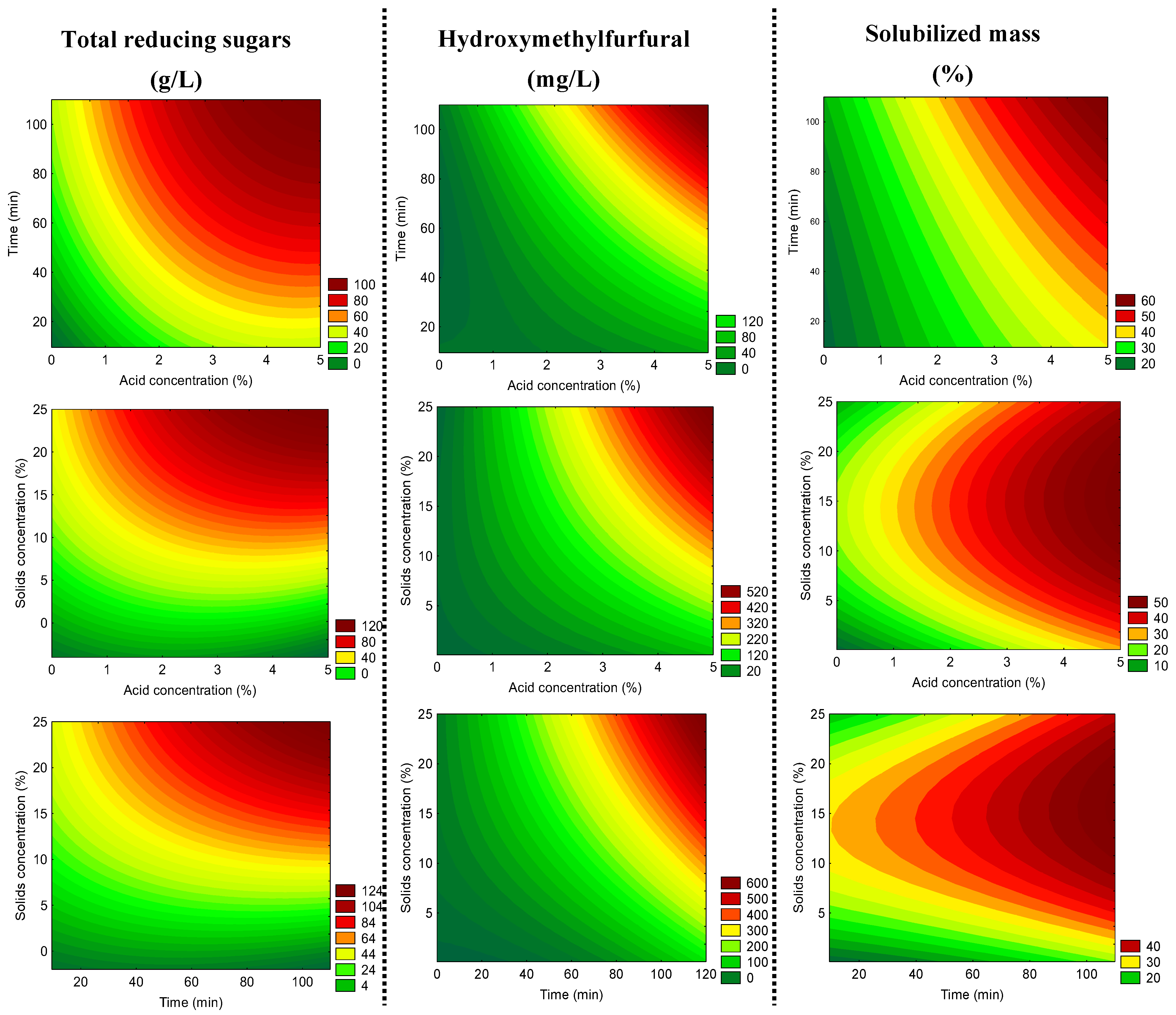
2.2. Effect of the Acid Hydrolysis Treatments on the Dried Açai Seeds
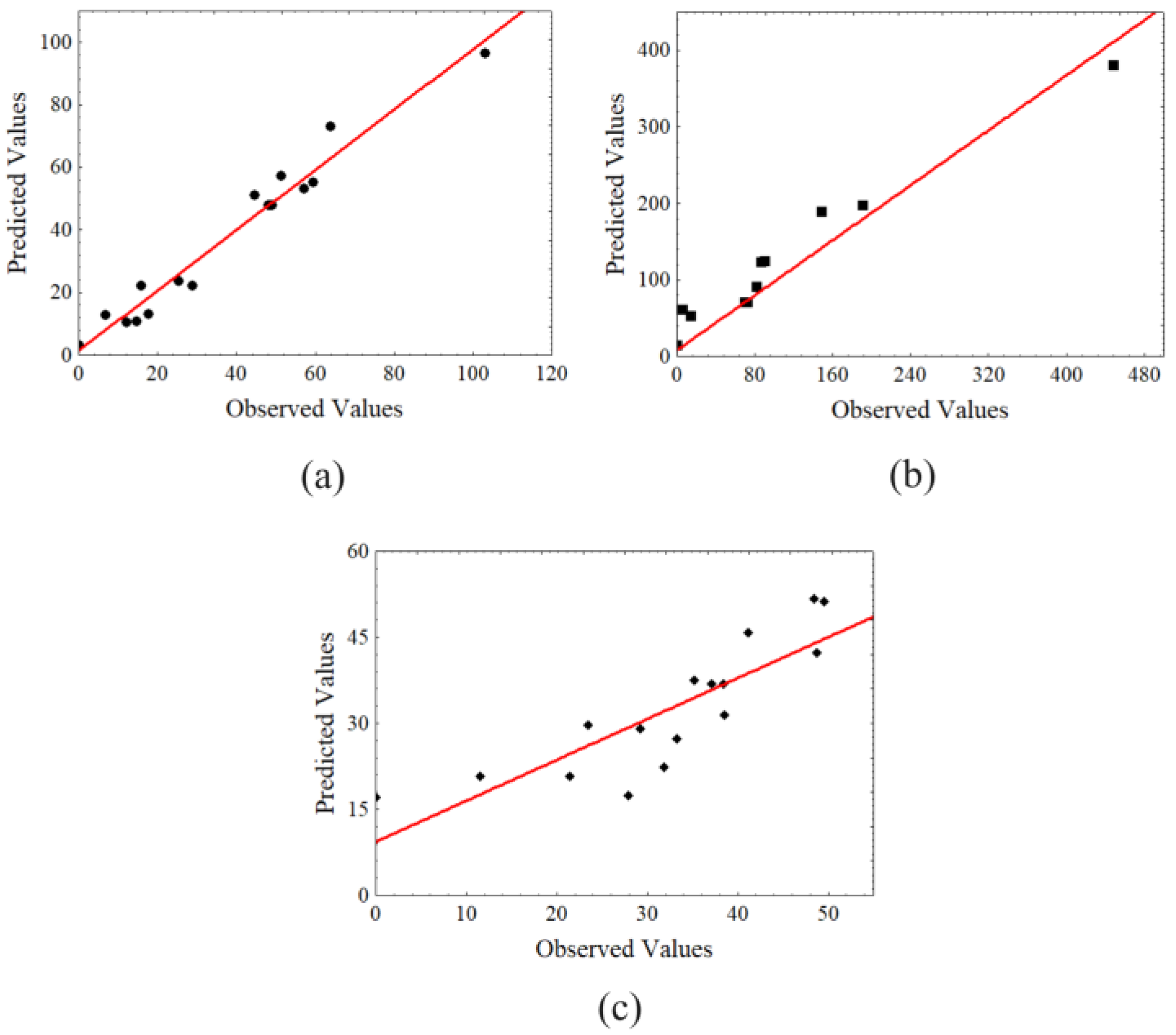
2.3. Optimal Conditions to Produce Liquor from Dried Açai Seeds by Acid Hydrolysis as Pre-Treatment Using H2SO4
2.4. Characterization of Sugars and Potential Microbial Inhibitors in the Newly Hydrolyzed Liquor and the Neutralized Liquor to Be Used as Substrate in Fermentation Processes
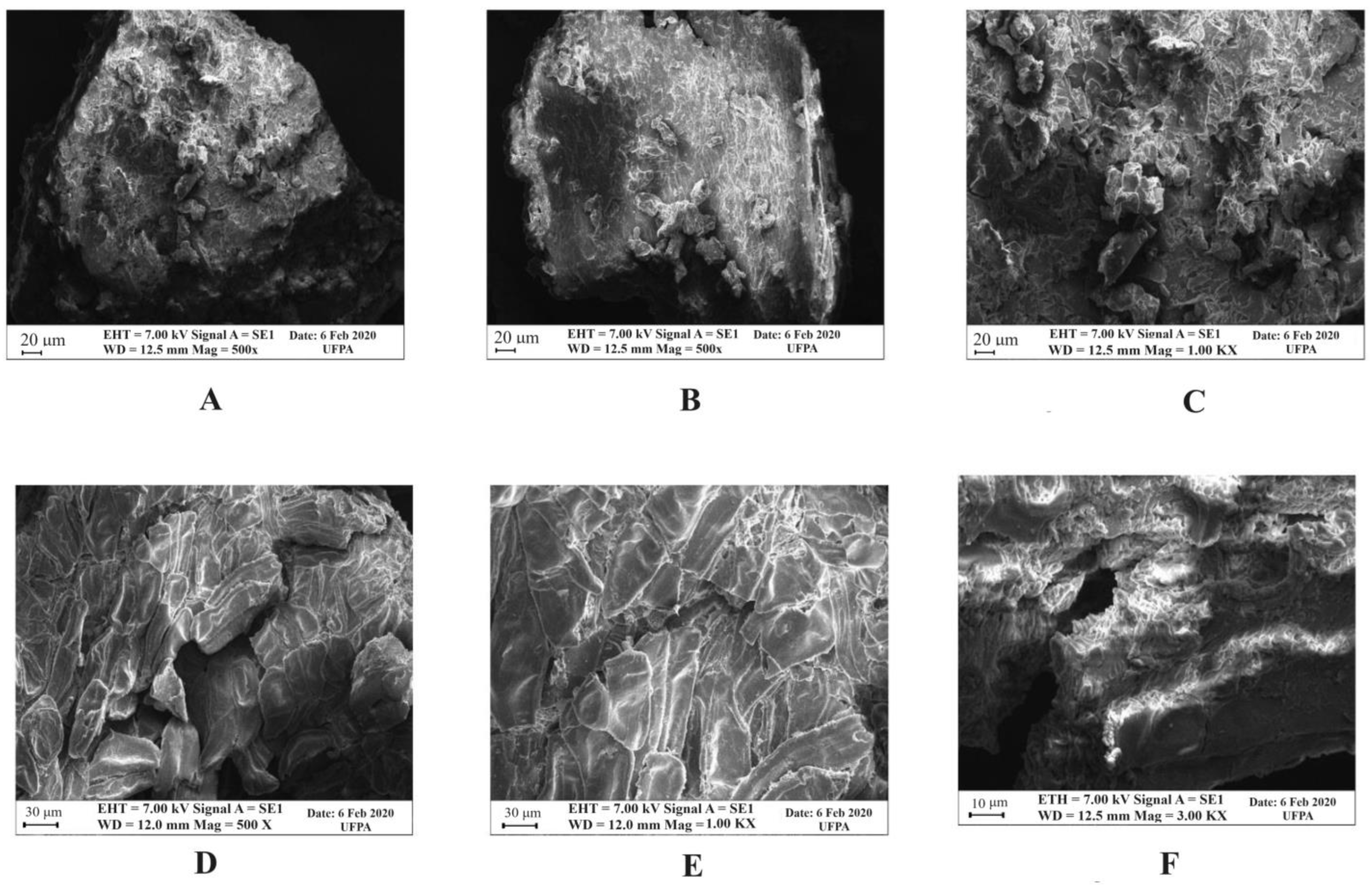
2.5. Scanning Electron Microscopy (SEM) of the Dried Açai Seeds before and after Acid Hydrolysis Treatment with H2SO4
3. Materials and Methods
3.1. Açai Seeds
3.2. Characterization of Dried Açai Seeds
3.3. Determination of Structural Carbohydrates
3.3.1. Determination of Furfural and Hydroxymethylfurfural Content
3.3.2. Determination of Total Lignin
3.3.3. Determination of Monomeric Sugars and Acetic Acid
3.3.4. Calculation of Structural Carbohydrates Contents
3.4. Determination of the Optimal Conditions for the Acid Hydrolysis of Dried Açai Seeds
3.5. Scanning Electron Microscopy (SEM)
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- EMBRAPA. Empresa Brasileira de Pesquisa Agropecuária. Available online: https://www.embrapa.br/busca-de-noticias/-/noticia/52276433/amazonia-em-foco-debate-desenvolvimento-rural-da-regiao?p_auth=XvNXOFir (accessed on 15 March 2023).
- Almeida, A.V.C.; Melo, I.M.; Pinheiro, I.S.; Freitas, J.F.; Melo, A.C.S. Appreciation of acai core of a pulp producer from Ananindeua/PA: Proposal of reverse channel structure oriented by NPSW and reverse logistics. GEPROS Gest. Prod. Oper. Sist. 2017, 3, 59–83. [Google Scholar] [CrossRef]
- Martins, L.H.S.; Rabelo, S.C.; Costa, A.C. Effects of the pretreatment method on high solids enzymatic hydrolysis and ethanol fermentation of the cellulosic fraction of sugarcane bagasse. Bioresour. Technol. 2015, 191, 312–321. [Google Scholar] [CrossRef]
- Rocha, M.V.P.; Rodrigues, T.H.S.; Albuquerque, T.L.; Gonçalves, L.R.B.; Macedo, G.R. Evaluation of dilute acid pretreatment on cashew apple bagasse for ethanol and xylitol production. Chem. Eng. J. 2014, 243, 234–243. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef]
- Oliveira, J.A.R.; Komesu, A.; Filho, R.M. Hydrothermal pretreatment for enhancing enzymatic hydrolysis of seeds of açaí (Euterpe oleracea) and sugar recovery. Chem. Eng. Trans. 2014, 37, 787–792. [Google Scholar] [CrossRef]
- Rambo, M.K.D.; Schmidt, F.L.; Ferreira, M.M.C. Analysis of the lignocellulosic components of biomass residues for biorefinery opportunities. Talanta 2015, 144, 696–703. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Gomes, A.L.; Silva, M.G.; Alves, A.B.; Agnol, W.H.D.; Ferrari, R.A.; Carvalho, P.R.N.; Pacheco, M.T.B. Antioxidant Capacity and Chemical Characterization of Açaí (Euterpe oleracea Mart.) Fruit Fractions. Food Sci. Technol. (HRPUB) 2016, 4, 95–102. [Google Scholar] [CrossRef]
- Arruda, J.C.B.; Fonseca, L.A.B.; Pinto, L.C.P.; Pinheiro, H.C.O.; Monteiro, B.T.O.; Manno, M.C.; Lima, K.R.S.; Lima, A.R. Açaí seed bran in the feed of slow-growth broilers. Acta Amazon. 2018, 48, 298–303. [Google Scholar] [CrossRef]
- Martins, M.A.; Mattoso, L.H.C.; Pessoa, J.D.C. Thermogravimetric evaluation of açaí fruit (Euterpe oleracea Mart.) agroindustry waste. Rev. Bras. Frutic. 2009, 31, 1150–1157. [Google Scholar] [CrossRef]
- Monteiro, A.F.; Miguez, I.S.; Silva, J.P.R.B.; Silva, A.S. High concentration and yield production of mannose from açaí (Euterpe oleracea Mart.) seeds via mannanase-catalyzed hydrolysis. Sci. Rep. 2019, 9, 10939. [Google Scholar] [CrossRef]
- NREL—National Renewable Energy Laboratory. Biodiesel: Handling and Use Guide; TP-540-43672; U.S. Department of Energy: Golden, CO, USA, 2009.
- Ruales-Salcedo, Á.V.; Prado-Rubio, O.A.; Rojas González, A.F. Harnessing lignocellulosic waste for energy storage and generation for off-grid rural areas. Clean Technol. Environ. Policy 2018, 20, 1515–1526. [Google Scholar] [CrossRef]
- Silva, A.S.; Teixeira, R.S.S.; Moutta, R.O.; Ferreira-Leitão, V.S.; de Barros, R.R.O.; Ferrara, M.A.; Bon, E.P.S. Sugarcane and Woody Biomass Pretreatments for Ethanol Production. In Sustainable Degradation of Lignocellulosic Biomass—Techniques, Applications and Commercialization; Chandel, A.K., da Silva, S.S., Eds.; IntechOpen: London, UK, 2013. [Google Scholar]
- López-Linares, J.C.; Cara, C.; Moya, M.; Ruiz, E.; Castro, E.; Romero, I. Fermentable sugar production from rapeseed straw by dilute phosphoric acid pretreatment. Ind. Crops Prod. 2013, 50, 525–531. [Google Scholar] [CrossRef]
- Nair, R.B.; Lundin, M.; Brandberg, T.; Lennartsson, P.R.; Taherzadeh, M.J. Dilute phosphoric acid pretreatment of wheat bran for enzymatic hydrolysis and subsequent ethanol production by edible fungi Neurospora intermedia. Ind. Crops Prod. 2015, 69, 314–323. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Sehnem, N.T.; Machado, A.S.; Leite, F.C.B.; Pita, W.B.; Morais Junior, M.A.; Ayub, M.A.Z. 5-Hydroxymethylfurfural induces ADH7 and ARI1 expression in tolerant industrial Saccharomyces cerevisiae strain P6H9 during bioethanol production. Bioresour. Technol. 2013, 133, 190–196. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Holtzapple, M.T. Lime pretreatment and enzymatic hydrolysis of corn stover. Bioresour. Technol. 2005, 96, 1994–2006. [Google Scholar] [CrossRef]
- Hari, R.P.K.; Patel, T.; Martin, A.A. A new strain of Rhodotorula rubra isolated from yogurt. J. Ind. Microbiol. 1992, 11, 43–51. [Google Scholar] [CrossRef]
- Muncner, D.; Augustin, J. Assimilation of benzoate by Rhodotorula rubra, Rhodotorula glutinis and Rhodosporidium toruloides, as affected by glucose or xylose. World J. Microbiol. Biotechnol. 1995, 11, 240–241. [Google Scholar] [CrossRef]
- Xu, P.; Bura, R.; Doty, S.L. Genetic analysis of D-xylose metabolism by endophytic yeast strains of Rhodotorula graminis and Rhodotorula mucilaginosa. Genet. Mol. Biol. 2011, 34, 471–478. [Google Scholar] [CrossRef]
- Silva, T.L.; Feijão, D.; Roseiro, J.C.; Reis, A. Monitoring Rhodotorula glutinis CCMI 145 physiological response and oil production growing on xylose and glucose using multi-parameter flow cytometry. Bioresour. Technol. 2011, 102, 2998–3006. [Google Scholar] [CrossRef]
- Tiukova, A.I.; Brandenburg, J.; Blomqvist, J.; Sampels, S.; Mikkelsen, N.; Skaugen, M.; Arntzen, M.Ø.; Nielsen, J.; Sandgren, M.; Kerkhoven, E.J. Proteome analysis of xylose metabolism in Rhodotorula toruloides during lipid production. Biotechnol. Biofuels 2019, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Byrtusová, D.; Szotkowski, M.; Kurowska, K.; Shapaval, V.; Márová, I. Rhodotorula kratochvilovae CCY 20-2-26—The Source of Multifunctional Metabolites. Microorganisms 2021, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, Y.; Wu, J.; Liu, H.; Zhang, J. Effect of multiple inhibitions in corncob hydrolysate on the lipid production by Rhodotorula glutinis. Energy Fuels 2017, 31, 12247–12255. [Google Scholar] [CrossRef]
- Vajzovic, A.; Bura, R.; Kohlmeier, K.; Doty, S.L. Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 II: Production of xylitol and ethanol in the presence of inhibitors. J. Ind. Microbiol. Biotechnol. 2012, 39, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.R.; Komesu, A.; Martins, L.H.S.; Maciel Filho, R. Evaluation of microstrutcture of açaí seeds biomass un-treated and treated with H2SO4 and NaOH by SEM, RDX and FTIR. Chem. Eng. Trans. 2016, 50, 379–384. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; AOAC: Washington DC, USA, 1995. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarla, T.A.C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; Technical Report, NREL/TP-510-42619.7; National Renewable Energy Laboratory; U.S. Department of Energy: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report, NREL/TP-510-42618; National Renewable Energy Laboratory; U.S. Department of Energy: Golden, CO, USA, 2011.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; Technical Report, NREL/TP-510-42623; National Renewable Energy Laboratory; U.S. Department of Energy: Golden, CO, USA, 2006.
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 3, 426–428. [Google Scholar] [CrossRef]

| Level of Independent Variables | Dependent Variables | ||||||
|---|---|---|---|---|---|---|---|
| Runs | X1 | X2 | X3 | CSF | TRS (g/L) | HMF (mg/L) | Solubilized Mass (%) |
| 1 | 1 (−1) | 30 (−1) | 5 (−1) | 1.06 | 12.25 | 5.60 | 27.93 |
| 2 | 4 (+1) | 30 (−1) | 5 (−1) | 1.21 | 17.65 | 33.68 | 38.58 |
| 3 | 1 (−1) | 90 (+1) | 5 (−1) | 1.53 | 14.76 | 12.82 | 31.94 |
| 4 | 4 (+1) | 90 (+1) | 5 (−1) | 1.68 | 25.40 | 90.30 | 48.83 |
| 5 | 1 (−1) | 30 (−1) | 20 (+1) | 1.06 | 28.98 | 19.92 | 21.53 |
| 6 | 4 (+1) | 30 (−1) | 20 (+1) | 1.21 | 59.60 | 82.06 | 35.23 |
| 7 | 1 (−1) | 90 (+1) | 20 (+1) | 1.53 | 57.54 | 14.79 | 29.25 |
| 8 | 4 (+1) | 90 (+1) | 20 (+1) | 1.68 | 103.12 | 448.83 | 48.49 |
| 9 | 0 (−1.68) | 60 (0) | 12.5 (0) | NC1 | 6.93 | 1.49 | 11.56 |
| 10 | 5 (+1.68) | 60 (0) | 12.5 (0) | 1.62 | 44.70 | 148.90 | 49.58 |
| 11 | 2.5 (0) | 9.54 (−1.68) | 12.5 (0) | 0.69 | 15.93 | <LOD | 23.52 |
| 12 | 2.5 (0) | 110 (+1.68) | 12.5 (0) | 1.75 | 51.34 | 191.34 | 41.22 |
| 13 | 2.5 (0) | 60 (0) | 0.1 (−1.68) | 1.49 | <LOD | 0.59 | NC2 |
| 14 | 2.5 (0) | 60 (0) | 25 (+1.68) | 1.49 | 63.95 | 87.01 | 33.28 |
| 15 | 2.5 (0) | 60 (0) | 12.5 (0) | 1.49 | 49.06 | 70.05 | 37.13 |
| 16 | 2.5 (0) | 60 (0) | 12.5 (0) | 1.49 | 48.16 | 70.58 | 38.50 |
| 17 | 2.5 (0) | 60 (0) | 12.5 (0) | 1.49 | 48.92 | 72.53 | 37.18 |
| Hydrolyzed Liquor | ||
|---|---|---|
| Newly Hydrolyzed | After Neutralization * | |
| Sugars | ||
| Mannose (g/L) | 51 ± 1 a | 42.1 ± 0.1 b |
| Xylose (g/L) | 1.9 ± 0.2 a | 1.4 ± 0.2 b |
| Glucose (g/L) | 1.3 ± 0.1 a | 1.0 ± 0.1 a |
| Cellobiose (g/L) | 0.9 ± 0.1 a | 0.7 ± 0.1 a |
| Sum of the sugars (g/L) | 55 ± 1 a | 45.2 ± 0.4 b |
| Microbial inhibitors | ||
| Hydroxymethylfurfural (mg/L) | 338 ± 50 a | 238 ± 20 b |
| Furfural (mg/L) | 10 ± 1 a | 9 ± 1 a |
| Acetic acid (g/L) | 1.8 ± 0.1a | 1.76 ± 0.04 a |
| Independent Variable | Level | |||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | +1 | +1.68 | ||
| Acid concentration (%, w/v) | X1 | 0 | 1 | 2.5 | 4 | 5 |
| Hydrolysis time (min) | X2 | 9.5 | 30 | 60 | 90 | 110 |
| Solids concentration (%, w/v) | X3 | 0.1 | 5 | 12.5 | 20 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igreja, W.S.; da Silva Martins, L.H.; de Almeida, R.R.; de Oliveira, J.A.R.; Lopes, A.S.; Chisté, R.C. Açai Seeds (Euterpe oleracea Mart) Are Agroindustrial Waste with High Potential to Produce Low-Cost Substrates after Acid Hydrolysis. Molecules 2023, 28, 6661. https://doi.org/10.3390/molecules28186661
Igreja WS, da Silva Martins LH, de Almeida RR, de Oliveira JAR, Lopes AS, Chisté RC. Açai Seeds (Euterpe oleracea Mart) Are Agroindustrial Waste with High Potential to Produce Low-Cost Substrates after Acid Hydrolysis. Molecules. 2023; 28(18):6661. https://doi.org/10.3390/molecules28186661
Chicago/Turabian StyleIgreja, Willen Silva, Luiza Helena da Silva Martins, Rafaela Rodrigues de Almeida, Johnatt Allan Rocha de Oliveira, Alessandra Santos Lopes, and Renan Campos Chisté. 2023. "Açai Seeds (Euterpe oleracea Mart) Are Agroindustrial Waste with High Potential to Produce Low-Cost Substrates after Acid Hydrolysis" Molecules 28, no. 18: 6661. https://doi.org/10.3390/molecules28186661
APA StyleIgreja, W. S., da Silva Martins, L. H., de Almeida, R. R., de Oliveira, J. A. R., Lopes, A. S., & Chisté, R. C. (2023). Açai Seeds (Euterpe oleracea Mart) Are Agroindustrial Waste with High Potential to Produce Low-Cost Substrates after Acid Hydrolysis. Molecules, 28(18), 6661. https://doi.org/10.3390/molecules28186661









