Palladium Nanoparticles Grafted onto Phytochemical Functionalized Biochar: A Sustainable Nanozyme for Colorimetric Sensing of Glucose and Glutathione
Abstract
:1. Introduction
2. Results and Discussion
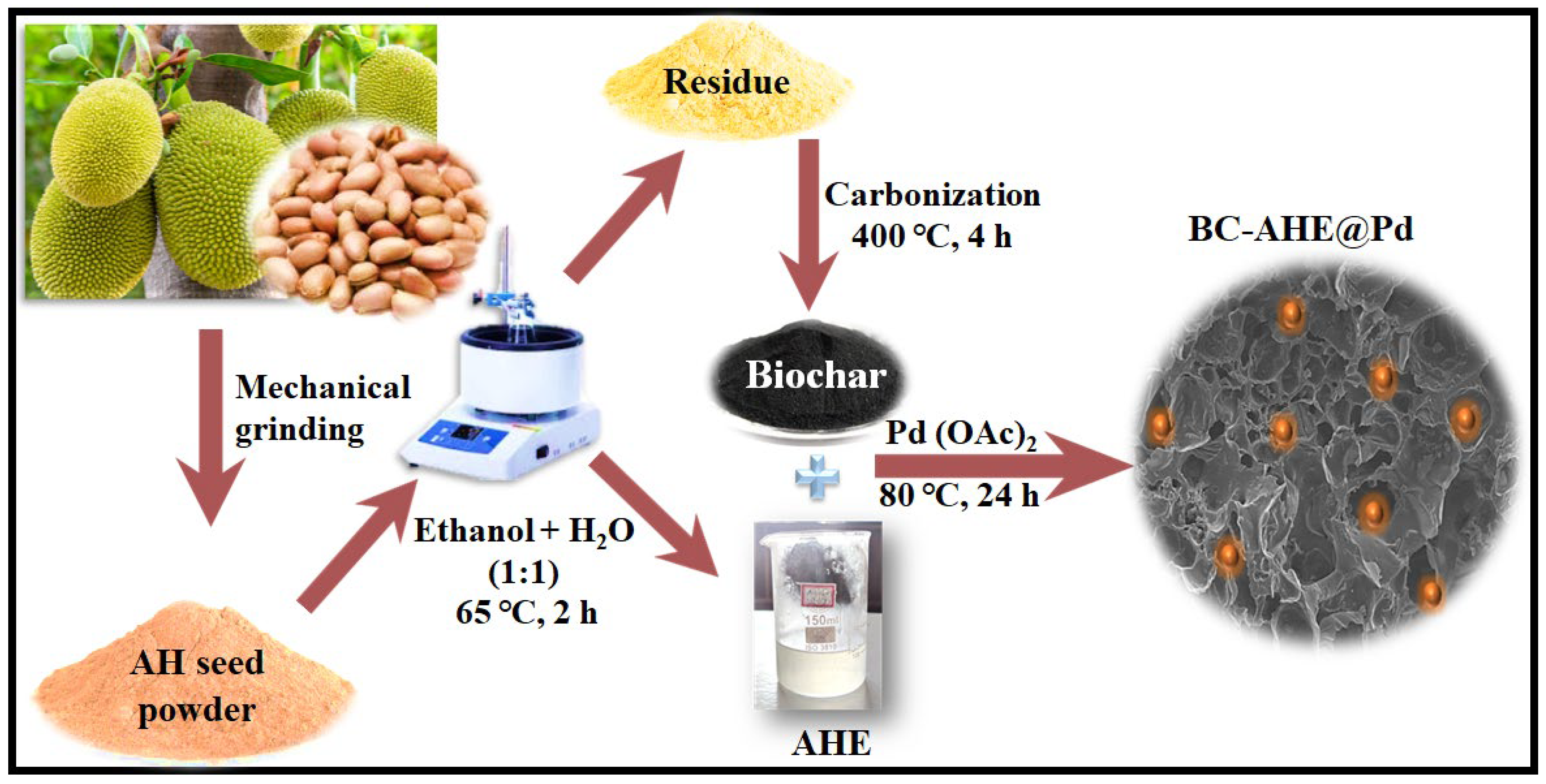
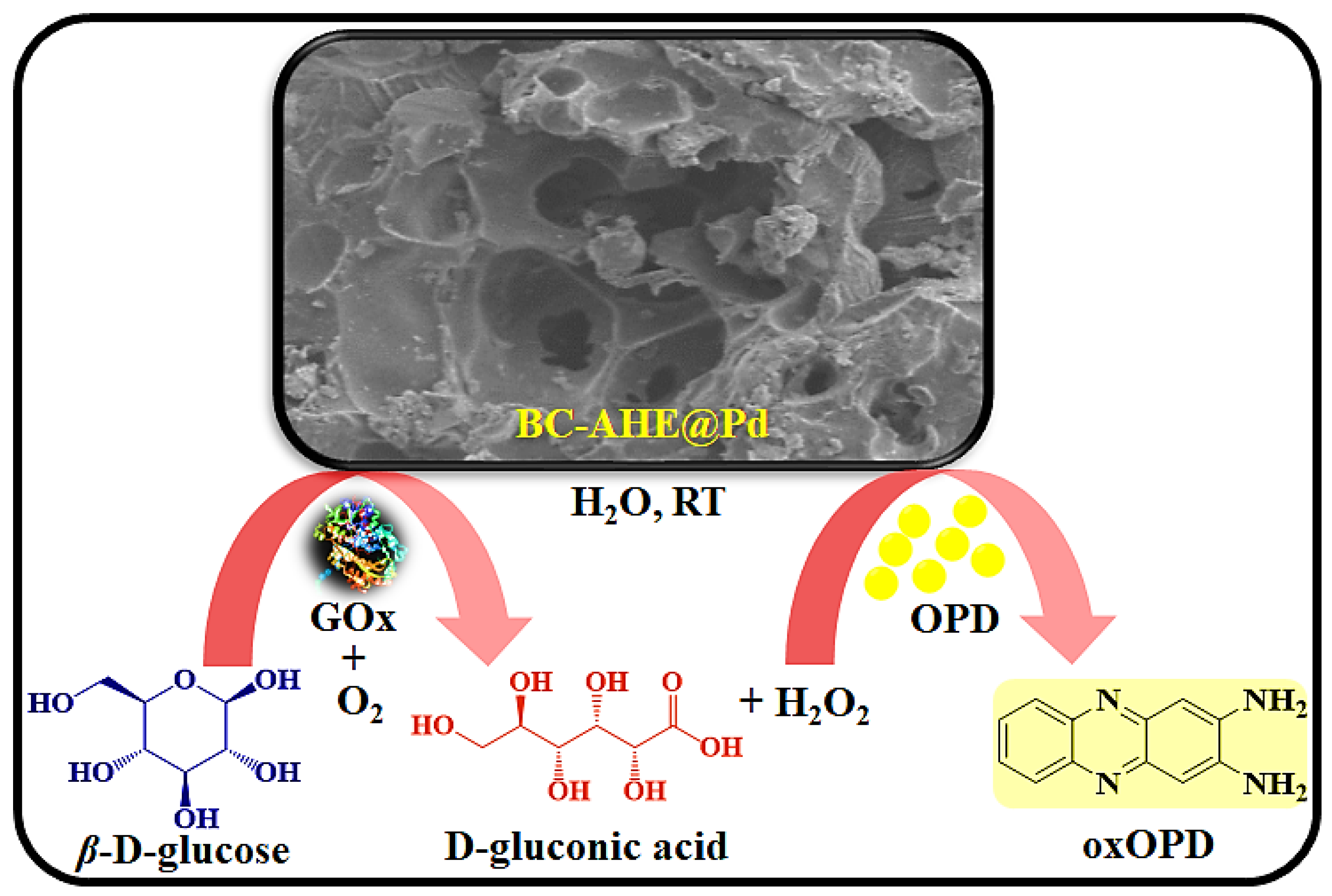
2.1. Synthesis of BC-AHE@Pd Nanocatalyst
2.2. Spectroscopic and Microscopic Analysis of BC-AHE@Pd
2.2.1. GC-MS and Qualitative Analysis of AHE
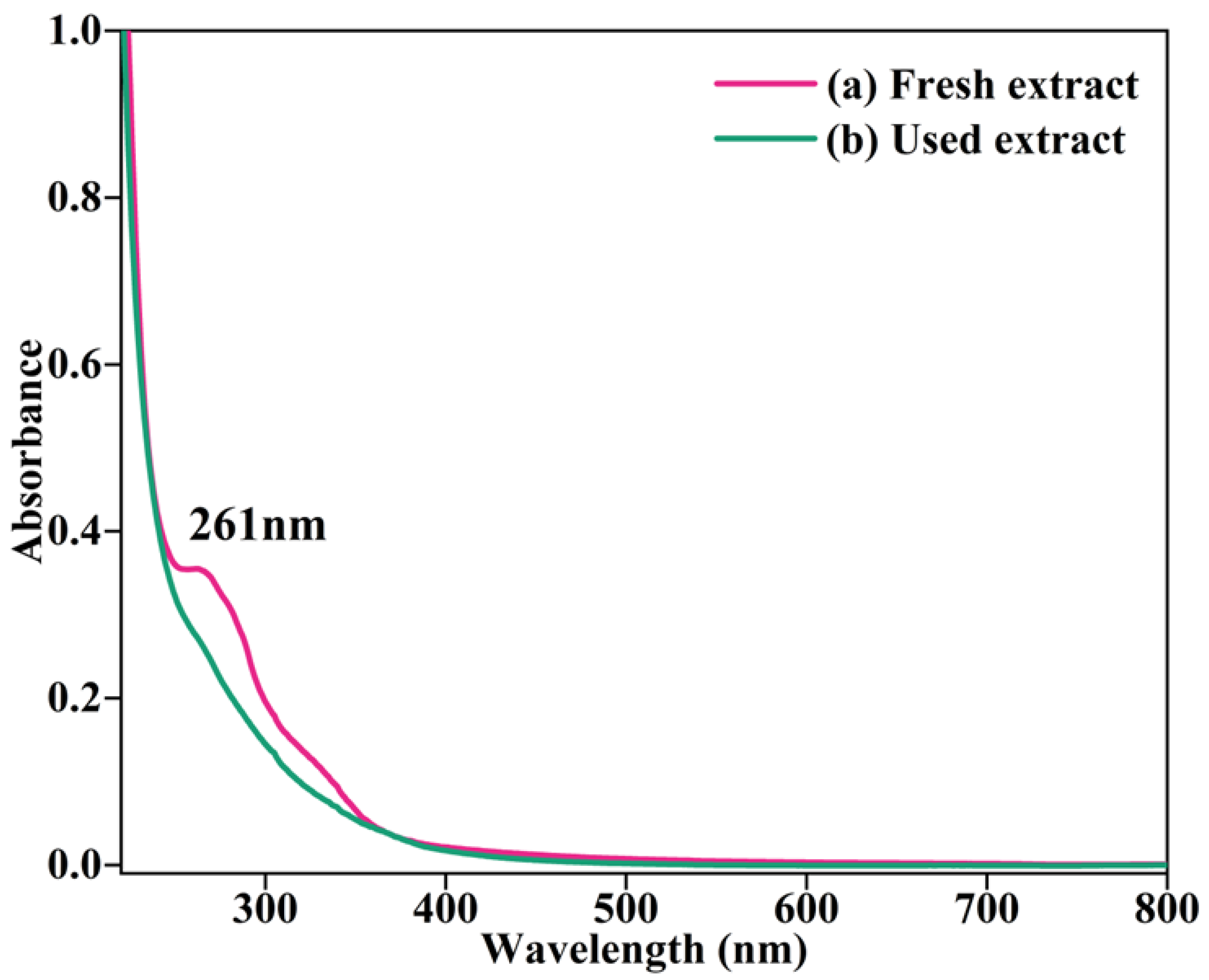
2.2.2. UV–Visible Analysis
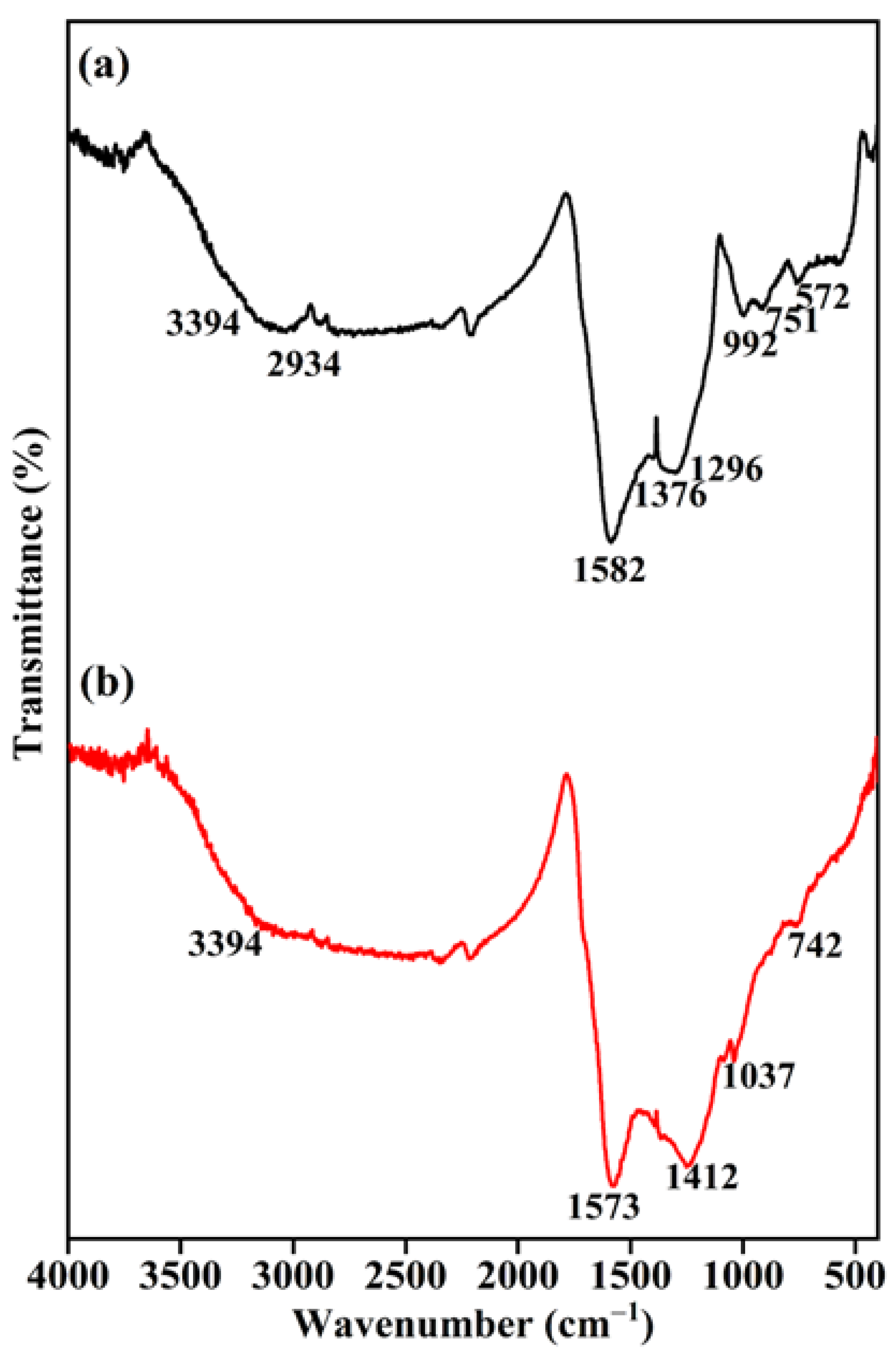
2.2.3. FT-IR Spectroscopy
2.2.4. p-XRD Analysis
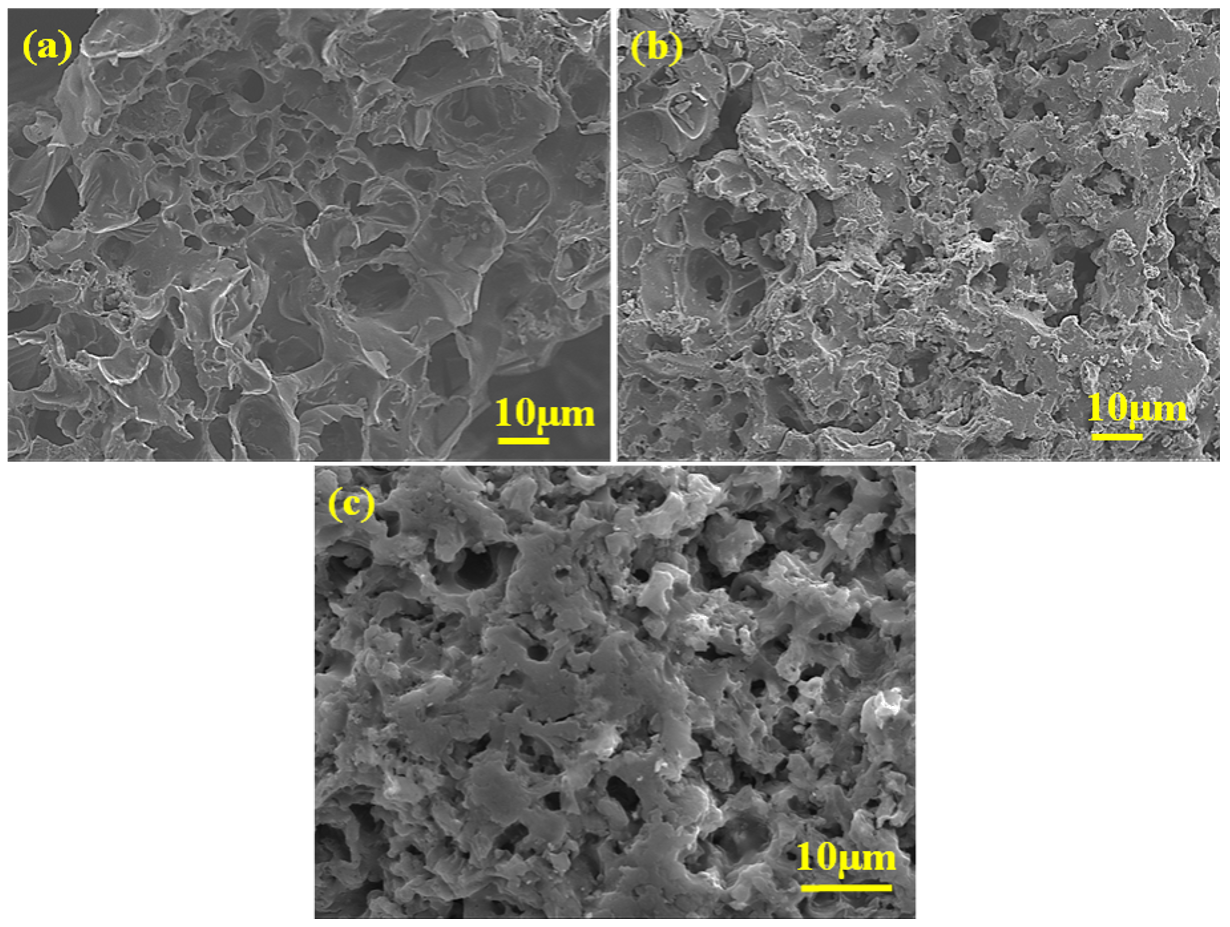
2.2.5. FE-SEM Analysis
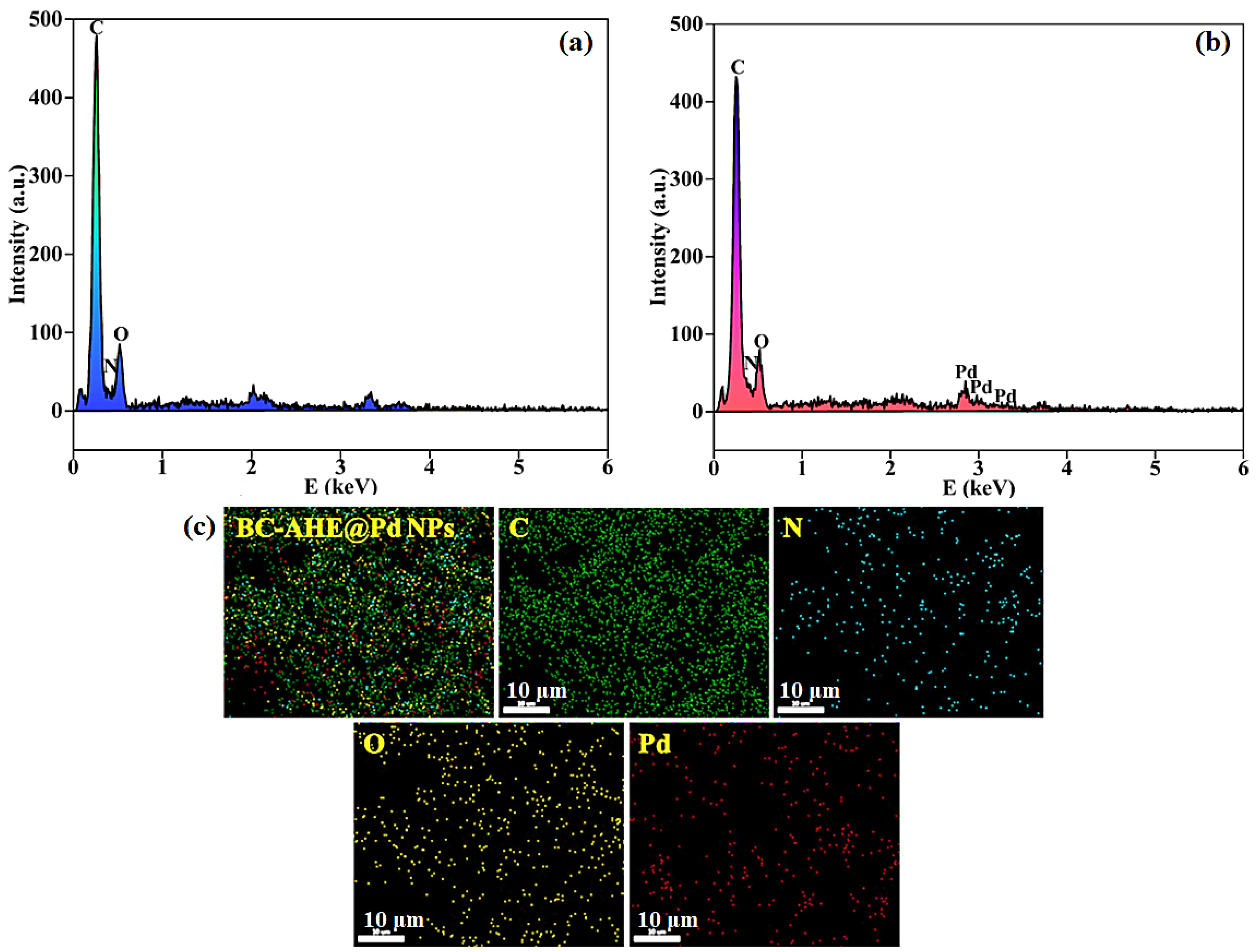
2.2.6. EDS Analysis
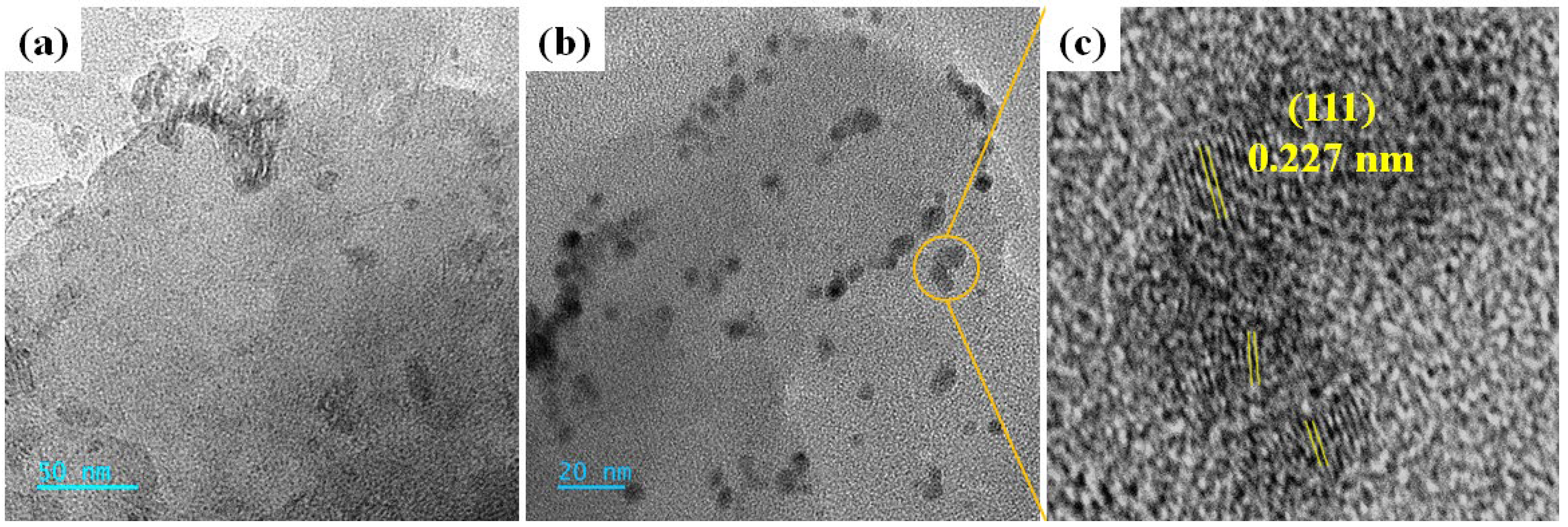
2.2.7. HR-TEM Analysis
2.2.8. ICP-OES Analysis
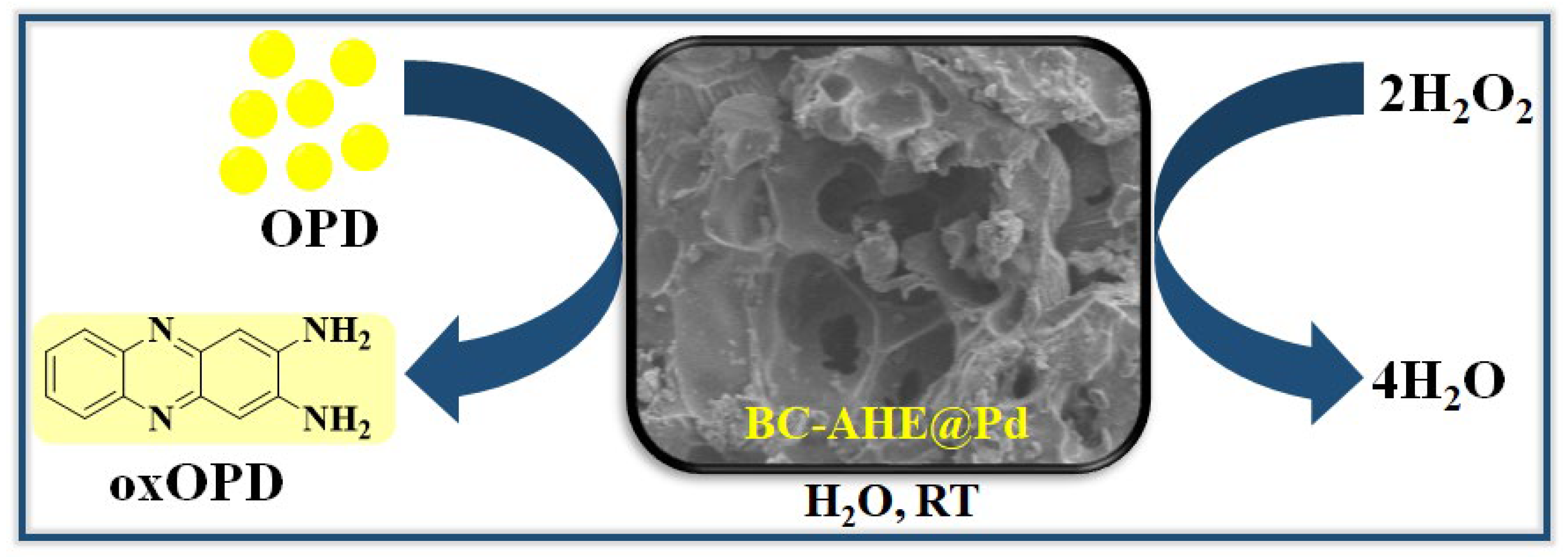
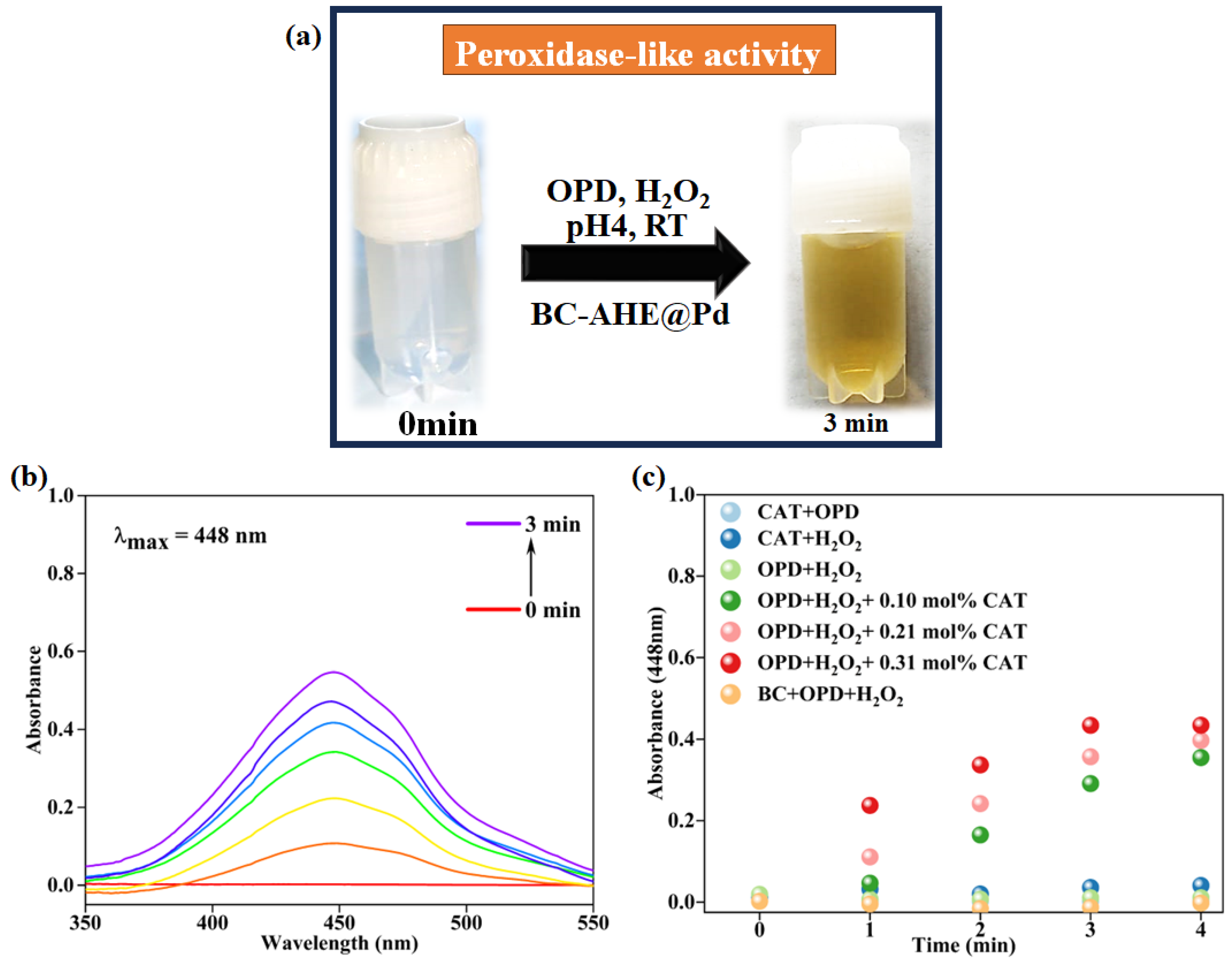
2.3. Peroxidase-like Activity of BC-AHE@Pd in H2O2 Sensing
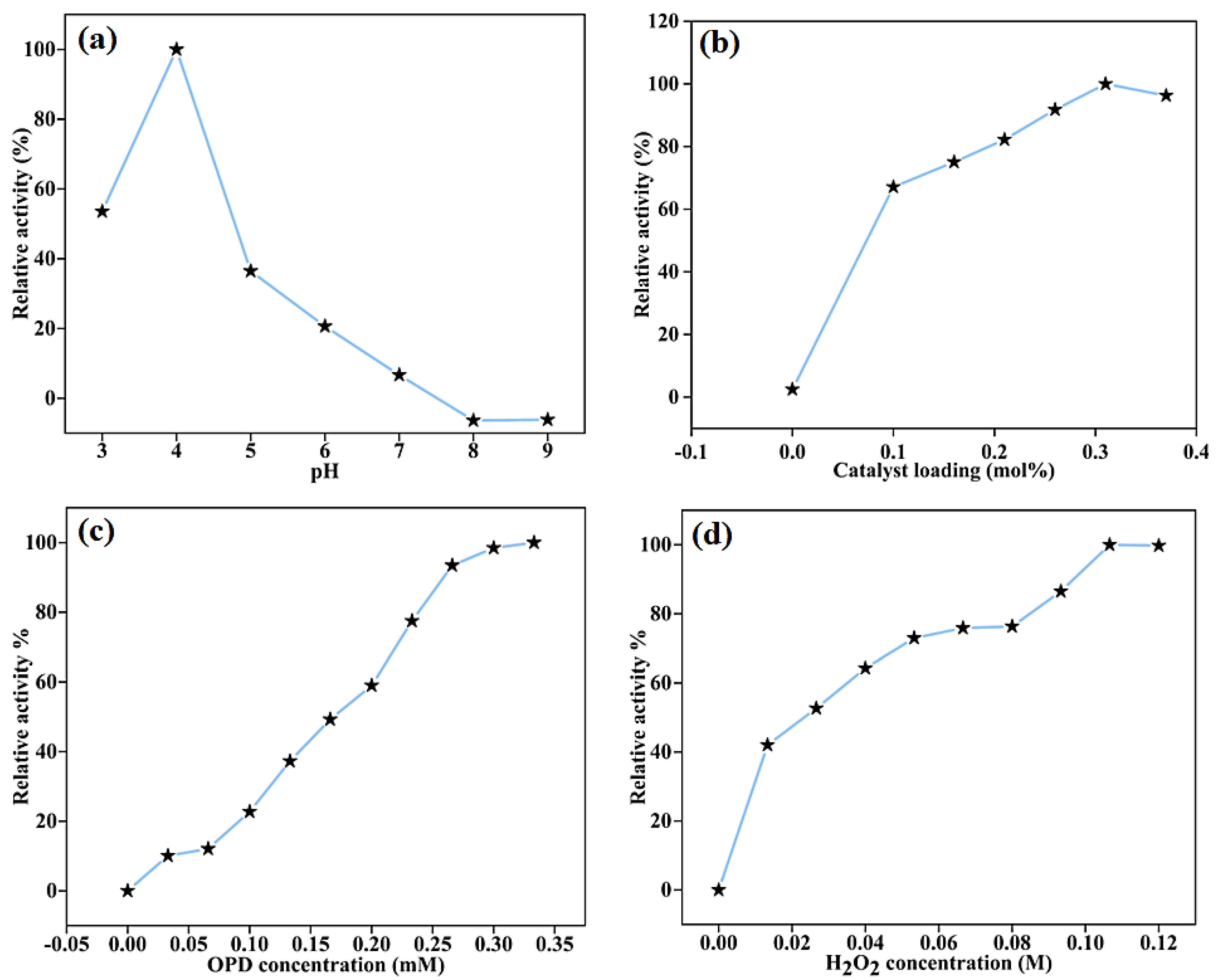
2.3.1. Effect of Reaction Parameters on the Peroxidase-like Activity of BC-AHE@Pd
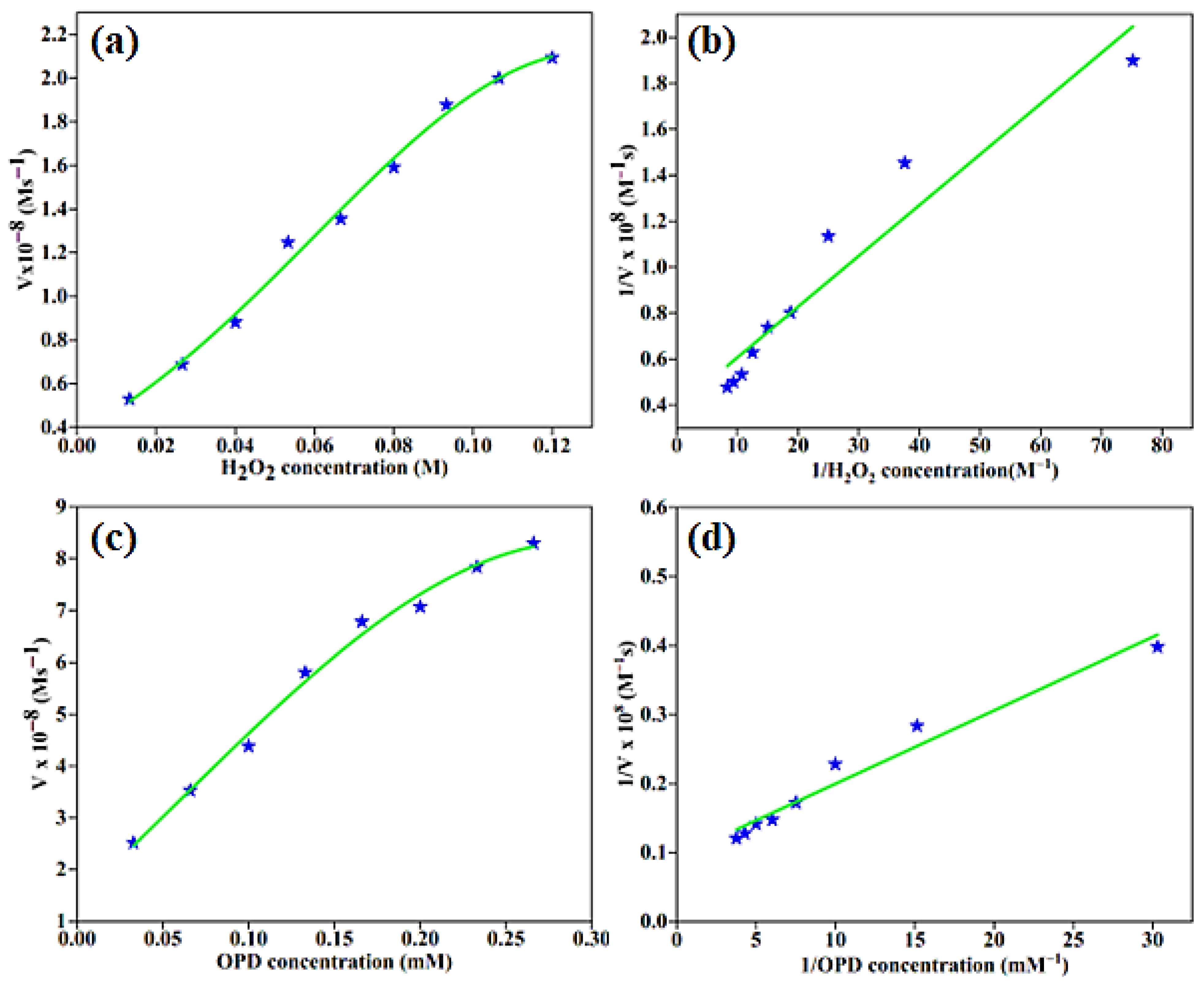
2.3.2. Kinetic Analysis of the Peroxidase-like Activity of BC-AHE@Pd
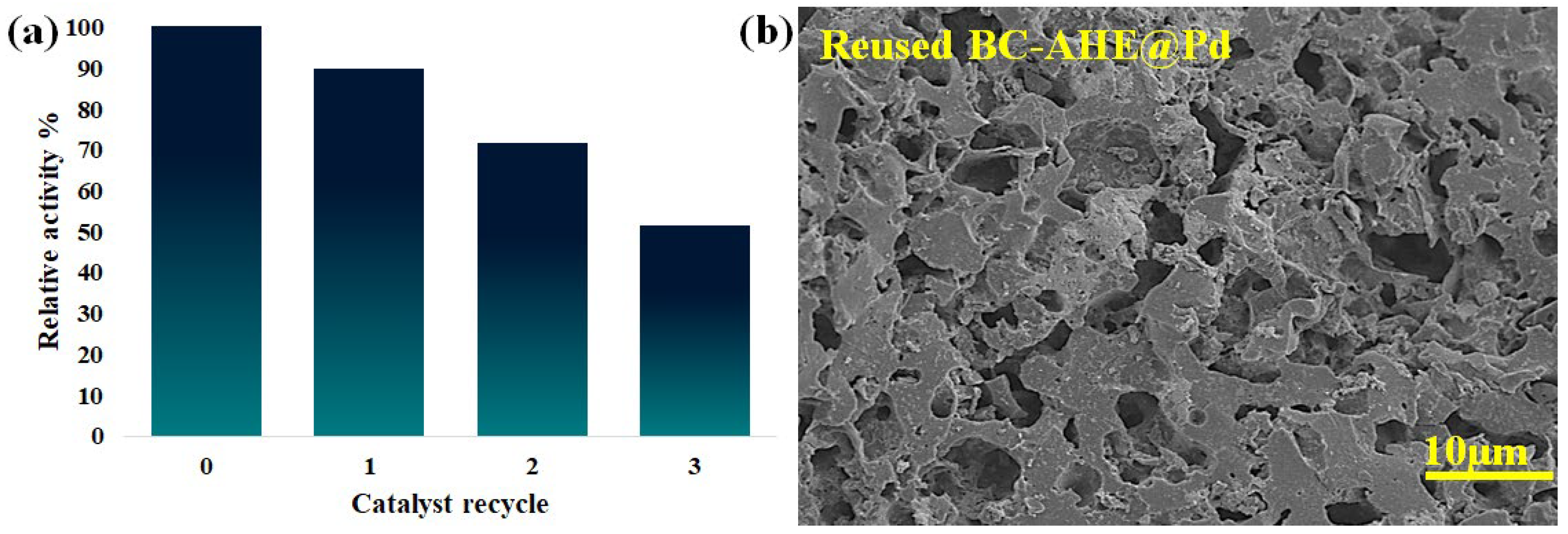
2.3.3. Catalyst Reusability
2.3.4. Comparison of Performance of BC-AHE@Pd
2.4. Sensing of Glucose Using BC-AHE@Pd
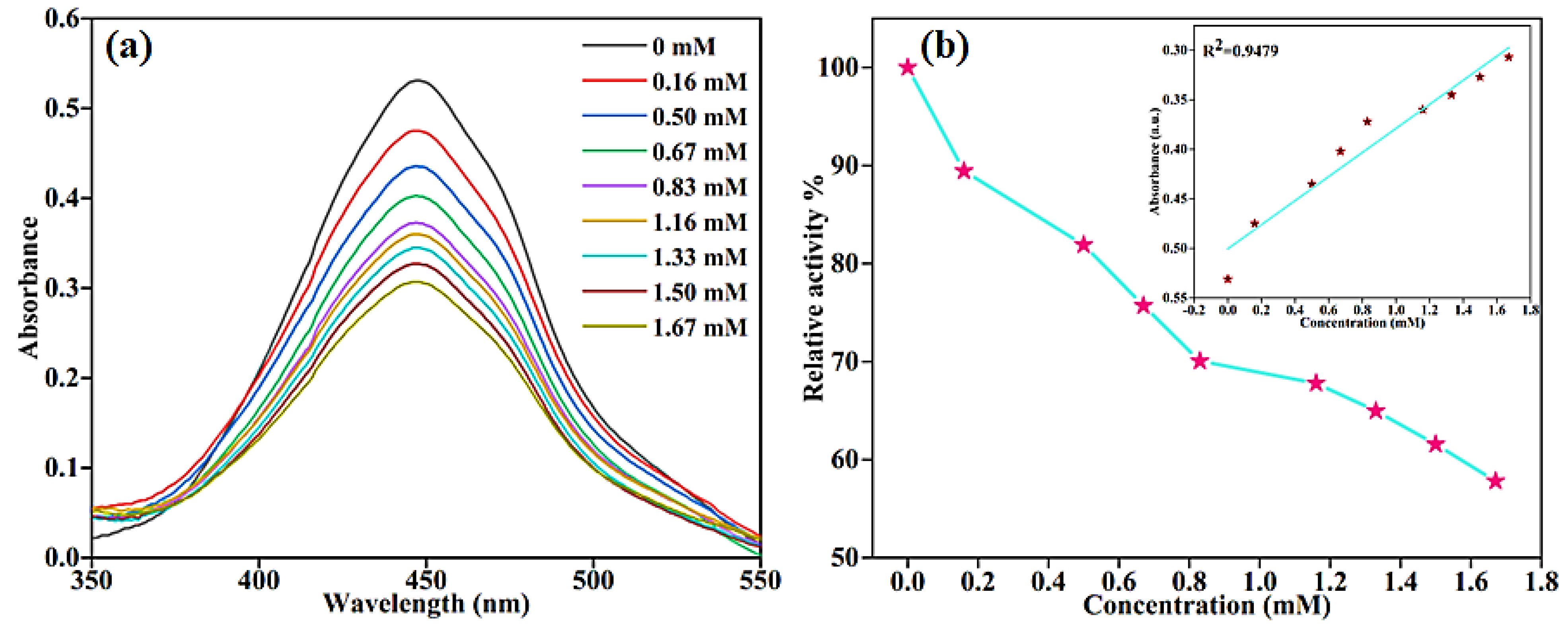
2.5. Sensing of Glutathione Using BC-AHE@Pd
3. Experimental
3.1. Materials
3.2. Instrumentation and Analyses
3.3. Extraction of Artocarpus heterophyllus Seeds (AHE)
3.4. Preparation of Biochar (BC)
3.5. Synthesis of Pd NPs Grafted onto Phytochemical Functionalized BC (BC-AHE@Pd)
3.6. Peroxidase-like Activity of BC-AHE@Pd
3.7. Recovery and Recycling of BC-AHE@Pd
3.8. Glucose Sensing Using BC-AHE@Pd
3.9. Glutathione Sensing Using BC-AHE@Pd
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Linsinger, T.P.; Roebben, G.; Solans, C.; Ramsch, R. Reference materials for measuring the size of nanoparticles. TrAC Trends Anal. Chem. 2011, 30, 18–27. [Google Scholar] [CrossRef]
- Navya, P.; Daima, H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar]
- Biswas, P.; Wu, C.-Y. Nanoparticles and the environment. J. Air Waste Manag. Assoc. 2005, 55, 708–746. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a unique position in medicine. J. Nanomater. 2023, 13, 574. [Google Scholar]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mat. Res. 2008, 20, 4225–4241. [Google Scholar]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar]
- Sápi, A.; Rajkumar, T.; Kiss, J.; Kukovecz, Á.; Kónya, Z.; Somorjai, G.A. Metallic nanoparticles in heterogeneous catalysis. Catal. Lett. 2021, 151, 2153–2175. [Google Scholar]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Materials, Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Limaye, A.S.; Alsaiari, M.; Shinde, P.V.; Ghosh, A.; Jalalah, M.; Rout, C.S.; Patil, S.A.; Harraz, F.A.; Dateer, R.B. Greener Approach for Pd-NPs Synthesis Using Mangifera indica Leaf Extract: Heterogeneous Nano Catalyst for Direct C–H Arylation of (Poly) Fluorobenzene, Hiyama Coupling Reaction and Hydrogen Evolution Reaction Study. Catal. Lett. 2023, 153, 1988–2004. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Wang, L.; Dong, X.; Zhang, J.; Wang, G.; Han, S.; Meng, X.; Zheng, A.; Xiao, F.-S. Importance of zeolite wettability for selective hydrogenation of furfural over Pd@Zeolite catalysts. ACS Catal. 2018, 8, 474–481. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Biochar-supported nanomaterials for environmental applications. J. Ind. Eng. Chem. 2019, 78, 21–33. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Punekar, N.S. Enzymes: Catalysis, Kinetics and Mechanisms; Springer: Singapore, 2018. [Google Scholar]
- Munoz Robles, V.; Ortega-Carrasco, E.; Alonso-Cotchico, L.; Rodriguez-Guerra, J.; Lledos, A.; Marechal, J.-D. Toward the computational design of artificial metalloenzymes: From protein-ligand docking to multiscale approaches. ACS Catal. 2015, 5, 2469–2480. [Google Scholar] [CrossRef]
- Ren, X.; Chen, D.; Wang, Y.; Li, H.; Zhang, Y.; Chen, H.; Li, X.; Huo, M. Nanozymes-Recent development and biomedical applications. J. Nanobiotechnol. 2022, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Nanomaterials Exhibiting Enzyme-Like Properties (Nanozymes): Current Advances and Future Perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, Q.; Cai, S.; Wang, T.; Qi, C.; Yang, R.; Wang, C. Excellent peroxidase mimicking property of CuO/Pt nanocomposites and their application as an ascorbic acid sensor. Analyst 2017, 142, 2500–2506. [Google Scholar] [CrossRef]
- Mishra, S.; Abdal-hay, A.; Rather, S.U.; Tripathi, R.M.; Shekh, F.A. Recent Advances in Silver nanozymes: Concept, Mechanism, and Applications in Detection. Adv. Mater. Interfaces 2022, 9, 2200928. [Google Scholar] [CrossRef]
- Cai, X.; Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Nanozyme-involved biomimetic cascade catalysis for biomedical applications. Mater. Today 2021, 44, 211–228. [Google Scholar] [CrossRef]
- Karim, M.N.; Anderson, S.R.; Singh, S.; Ramanathan, R.; Bansal, V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens. Bioelectron. 2018, 110, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. J. Sens. 2017, 17, 1866. [Google Scholar] [CrossRef]
- Rubino, F.M. Toxicity of Glutathione-Binding Metals: A Review of Targets and Mechanisms. Toxics 2015, 3, 20–62. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Doong, R.-A. The biomimic oxidase activity of layered V2O5 nanozyme for rapid and sensitive nanomolar detection of glutathione. Sens. Actuators B Chem. 2018, 273, 1179–1186. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Technology, Dynamic molecular structure of plant biomass-derived black carbon (biochar). J. Environ. Sci. 2010, 44, 1247–1253. [Google Scholar]
- Haas, T.J.; Nimlos, M.R.; Donohoe, B.S. Real-time and post-reaction microscopic structural analysis of biomass undergoing pyrolysis. Energy Fuels 2009, 23, 3810–3817. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Havener, K.; Zhang, M.; Zhang, L.; Wu, W.; Huang, K. Temperature-Controlled Selectivity of Hydrogenation and Hydrodeoxygenation of Biomass by Superhydrophilic Nitrogen/Oxygen Co-Doped Porous Carbon Nanosphere Supported Pd Nanoparticles. Small 2022, 18, 2106893. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Gao, S.; Wang, H.; He, Z.; Xu, Y.; Huang, K. In situ encapsulated ultrafine Pd nanoparticles in nitrogen-doped porous carbon derived from hyper-crosslinked polymers effectively catalyse hydrogenation. J. Catal. 2021, 396, 342–350. [Google Scholar] [CrossRef]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Jiang, S.-F.; Ling, L.-L.; Xu, Z.; Liu, W.-J.; Jiang, H. Enhancing the catalytic activity and stability of noble metal nanoparticles by the strong interaction of magnetic biochar support. Ind. Eng. Chem. Res. 2018, 57, 13055–13064. [Google Scholar] [CrossRef]
- Santos, J.L.; Megías-Sayago, C.; Ivanova, S.; Centeno, M.Á.; Odriozola, J.A. Structure-sensitivity of formic acid dehydrogenation reaction over additive-free Pd NPs supported on activated carbon. J. Chem. Eng. 2021, 420, 127641. [Google Scholar] [CrossRef]
- Kandathil, V.; Dateer, R.B.; Sasidhar, B.; Patil, S.A.; Patil, S.A. Green synthesis of palladium nanoparticles: Applications in aryl halide cyanation and hiyama cross-coupling reaction under ligand free conditions. J. Catal. 2018, 148, 1562–1578. [Google Scholar] [CrossRef]
- Antony, A.M.; Yelamaggad, C.; Patil, S.A. Palladium nanoparticles decorated on functionalized graphitic carbon nitride as an efficient and retrievable nanocatalyst for organic dye degradation and hydrogen peroxide sensing. Mater. Chem. Phys. 2023, 297, 127370. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, G.J.; Choi, A.-J.; Kim, T.-H.; Kim, T.-I.; Oh, J.-M. Layered double hydroxide nanomaterials encapsulating Angelica gigas Nakai extract for potential anticancer nanomedicine. Front. Pharmacol. 2018, 9, 723. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, W.-J.; Zhang, N.; Li, Y.-S.; Jiang, H.; Sheng, G.-P. Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour. Technol. 2014, 169, 403–408. [Google Scholar] [CrossRef]
- Widjonarko, D.; Rafidah, S.; Elmatiana, E. Slow Pyrolysis of Coconut wood (Cocos nucifera L.) and Bio-Char Compositions. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 4th International Conference on Advanced Material for Better Future 2019 (ICAMBF 2019), Surakarta, Indonesia, 7–8 October 2019; IOP Publishing: Bristol, UK, 2020; p. 012023. [Google Scholar]
- Huang, L.; Zhao, H.; Yi, T.; Qi, M.; Xu, H.; Mo, Q.; Huang, C.; Wang, S.; Liu, Y. Preparation and properties of cassava residue cellulose nanofibril/cassava starch composite films. Nanomaterials 2020, 10, 755. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]
- Balouch, A.; Ali Umar, A.; Mawarnis, E.R.; Md Saad, S.K.; Mat Salleh, M.; Abd Rahman, M.Y.; Kityk, I.; Oyama, M. Synthesis of amorphous platinum nanofibers directly on an ITO substrate and its heterogeneous catalytic hydrogenation characterization. ACS Appl. Mater. Interfaces 2015, 7, 7776–7785. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Pd nanoparticles synthesized in situ with the use of Euphorbia granulate leaf extract: Catalytic properties of the resulting particles. J. Colloid Interface Sci. 2016, 462, 243–251. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Ahn, D.; Kim, Y.M.; Chung, S.J. Enzyme mimetic activity of ZnO-Pd nanosheets synthesized via a green route. Molecules 2020, 25, 2585. [Google Scholar] [CrossRef]
- Liang, Y.; Li, H.; Fan, L.; Li, R.; Cui, Y.; Ji, X.; Xiao, H.; Hu, J.; Wang, L. Zwitterionic daptomycin stabilized palladium nanoparticles with enhanced peroxidase-like properties for glucose detection. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127797. [Google Scholar] [CrossRef]
- Chamanmalik, M.I.; Antony, A.M.; Yelamaggad, C.; Patil, S.A.; Patil, S.A. Biogenic Silver Nanoparticles/Mg-Al Layered Double Hydroxides with Peroxidase-like Activity for Mercury Detection and Antibacterial Activity. Molecules 2023, 28, 5754. [Google Scholar] [CrossRef]
- Han, L.; Li, C.; Zhang, T.; Lang, Q.; Liu, A. Au@Ag heterogeneous nanorods as nanozyme interfaces with peroxidase-like activity and their application for one-pot analysis of glucose at nearly neutral pH. ACS Appl. Mater. Interfaces 2015, 7, 14463–14470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lopes, R.P.; Lüdtke, T.; Di Silvio, D.; Moya, S.; Hamon, J.-R.; Astruc, D. “Click” dendrimer-Pd nanoparticle assemblies as enzyme mimics: Catalytic o-phenylenediamine oxidation and application in colorimetric H2O2 detection. Inorg. Chem. Front. 2021, 8, 3301–3307. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Shu, J.; Li, Y. One-step solvothermal synthesis of Fe3O4@Cu@Cu2O nanocomposite as magnetically recyclable mimetic peroxidase. J. Alloys Compd. 2016, 682, 432–440. [Google Scholar] [CrossRef]
- Yu, H.; Wu, H.; Tian, X.; Zhou, Y.; Ren, C.; Wang, Z. A nano-sized Cu-MOF with high peroxidase-like activity and its potential application in colorimetric detection of H2O2 and glucose. RSC Adv. 2021, 11, 26963–26973. [Google Scholar] [CrossRef]
- Ge, J.; Xing, K.; Geng, X.; Hu, Y.-L.; Shen, X.-P.; Zhang, L.; Li, Z.-H. Human serum albumin templated MnO2 nanosheets are oxidase mimics for colorimetric determination of hydrogen peroxide and for enzymatic determination of glucose. Mikrochim. Acta 2018, 185, 559. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, J.; Zhang, L.; Geng, X.; Ge, J.; Hu, Y.; Li, Z. A novel one-step colorimetric assay for highly sensitive detection of glucose in serum based on MnO2 nanosheets. Anal. Methods 2017, 9, 4275–4281. [Google Scholar] [CrossRef]
- Mandpe, P.; Prabhakar, B.; Gupta, H.; Shende, P. Glucose oxidase-based biosensor for glucose detection from biological fluids. Sens. Rev. 2020, 40, 497–511. [Google Scholar] [CrossRef]
- Lee, G.; Kim, C.; Kim, D.; Hong, C.; Kim, T.; Lee, M.; Lee, K. Multibranched Au–Ag–Pt nanoparticle as a nanozyme for the colorimetric assay of hydrogen peroxide and glucose. ACS Omega 2022, 7, 40973–40982. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, L.; Zhang, R.; Ren, H.; Liu, A. CoO-supported ordered mesoporous carbon nanocomposite based nanozyme with peroxidase-like activity for colorimetric detection of glucose. Biochem. J. 2019, 81, 92–98. [Google Scholar] [CrossRef]
- Jabariyan, S.; Zanjanchi, M.A.; Arvand, M.; Sohrabnezhad, S. Colorimetric detection of glucose using lanthanum-incorporated MCM-41. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 294–300. [Google Scholar] [CrossRef]
- Tsogas, G.Z.; Vlessidis, A.G.; Giokas, D.L. Analyte-mediated formation and growth of nanoparticles for the development of chemical sensors and biosensors. Microchim. Acta 2022, 189, 434. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, C.; Song, J.; Ji, X.; Wang, W. Cytidine-gold nanoclusters as peroxidase mimetic for colorimetric detection of glutathione (GSH), glutathione disulfide (GSSG) and glutathione reductase (GR). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119316. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Ji, F.; Zheng, R.; Ji, X.; Liu, Z.; Wang, L. Biocompatible pericarpium citri reticulatae polysaccharide templated Pd nanoparticles for effectively colorimetric detection of glutathione. Colloids Surf. A Physicochem. Eng. 2022, 650, 129617. [Google Scholar] [CrossRef]
- Cao, X.; Yang, H.; Wei, Q.; Yang, Y.; Liu, M.; Liu, Q.; Zhang, X. Fast colorimetric sensing of H2O2 and glutathione based on Pt deposited on NiCo layered double hydroxide with double peroxidase-/oxidase-like activity. Inorg. Chem. Commun. 2021, 123, 108331. [Google Scholar] [CrossRef]
- Luo, N.; Yang, Z.; Tang, F.; Wang, D.; Feng, M.; Liao, X.; Yang, X. Fe3O4/carbon nanodot hybrid nanoparticles for the indirect colorimetric detection of glutathione. ACS Appl. Nano Mater. 2019, 2, 3951–3959. [Google Scholar] [CrossRef]
- Ojwang, R.; Muge, E.; Mbatia, B.; Mwanza, B.; Ogoyi, D. Comparative Analysis of Phytochemical Composition and Antioxidant Activities of Methanolic Extracts of Leaves, Roots and Bark of Jackfruit (Artocapus heterophyllus) from Selected Regions in Kenya and Uganda. J. Adv. Biol. Biotechnol. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Sreeja Devi, P.S.; Kumar, N.S.; Sabu, K.K. Phytochemical profiling and antioxidant activities of different parts of Artocarpus heterophyllus Lam. (Moraceae): A review on current status of knowledge. FJPS 2021, 7, 30. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, H.S.; Chung, E.; Lee, D.Y. Nanozyme-based colorimetric biosensor with a systemic quantification algorithm for noninvasive glucose monitoring. Theranostics 2022, 12, 6308–6338. [Google Scholar] [CrossRef]
- Xian, Z.; Zhang, L.; Yu, Y.; Lin, B.; Wang, Y.; Guo, M.; Cao, Y. Nanozyme based on CoFe2O4 modified with MoS2 for colorimetric determination of cysteine and glutathione. Mikrochim. Acta 2021, 188, 65. [Google Scholar] [CrossRef] [PubMed]




| Catalyst | Km (mM) | Vmax × 10−8 (Ms−1) | Reference |
|---|---|---|---|
| dendrimer-1-PdNPs | 3.02 | 0.149 | [50] |
| Fe3O4@Cu@Cu2O | 0.85 | 13.1 | [51] |
| Cu-MOF | 0.54 | 7.87 | [52] |
| BC-AHE@Pd | 0.113 | 10.72 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banu, A.; Antony, A.M.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Palladium Nanoparticles Grafted onto Phytochemical Functionalized Biochar: A Sustainable Nanozyme for Colorimetric Sensing of Glucose and Glutathione. Molecules 2023, 28, 6676. https://doi.org/10.3390/molecules28186676
Banu A, Antony AM, Sasidhar BS, Patil SA, Patil SA. Palladium Nanoparticles Grafted onto Phytochemical Functionalized Biochar: A Sustainable Nanozyme for Colorimetric Sensing of Glucose and Glutathione. Molecules. 2023; 28(18):6676. https://doi.org/10.3390/molecules28186676
Chicago/Turabian StyleBanu, Aakhila, Arnet Maria Antony, Balappa Somappa Sasidhar, Shivaputra A. Patil, and Siddappa A. Patil. 2023. "Palladium Nanoparticles Grafted onto Phytochemical Functionalized Biochar: A Sustainable Nanozyme for Colorimetric Sensing of Glucose and Glutathione" Molecules 28, no. 18: 6676. https://doi.org/10.3390/molecules28186676
APA StyleBanu, A., Antony, A. M., Sasidhar, B. S., Patil, S. A., & Patil, S. A. (2023). Palladium Nanoparticles Grafted onto Phytochemical Functionalized Biochar: A Sustainable Nanozyme for Colorimetric Sensing of Glucose and Glutathione. Molecules, 28(18), 6676. https://doi.org/10.3390/molecules28186676







