Facile Synthesis of Co Nanoparticles Embedded in N-Doped Carbon Nanotubes/Graphitic Nanosheets as Bifunctional Electrocatalysts for Electrocatalytic Water Splitting
Abstract
:1. Introduction
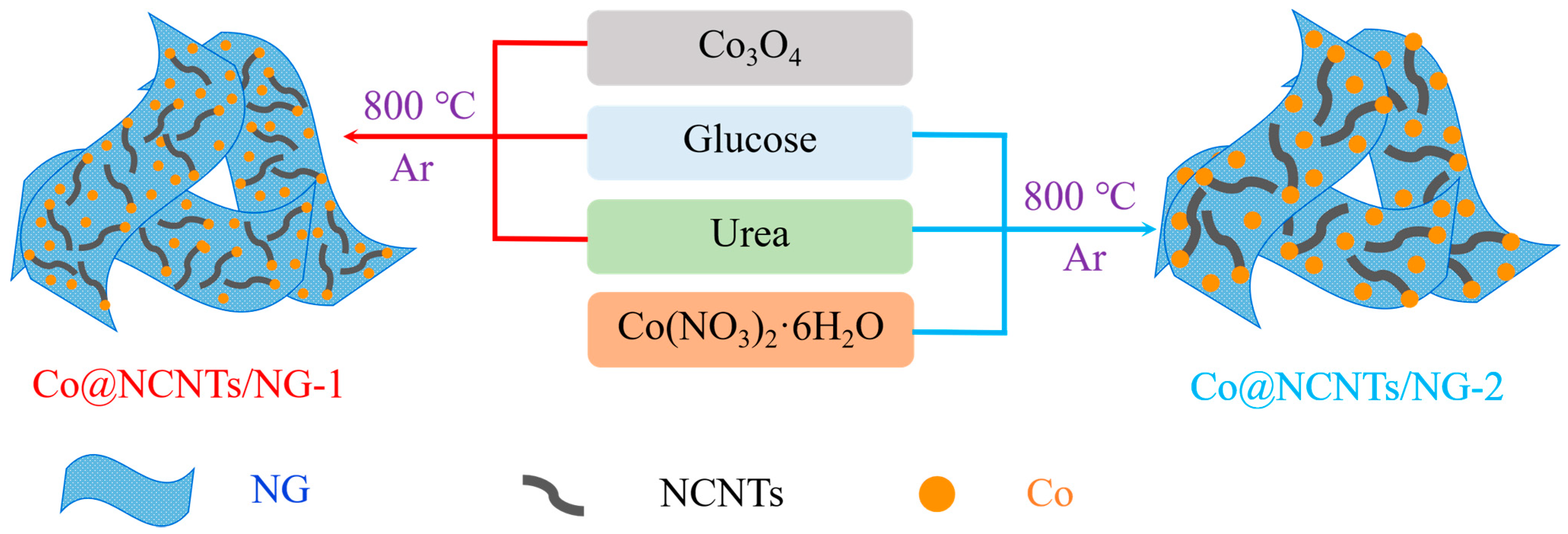
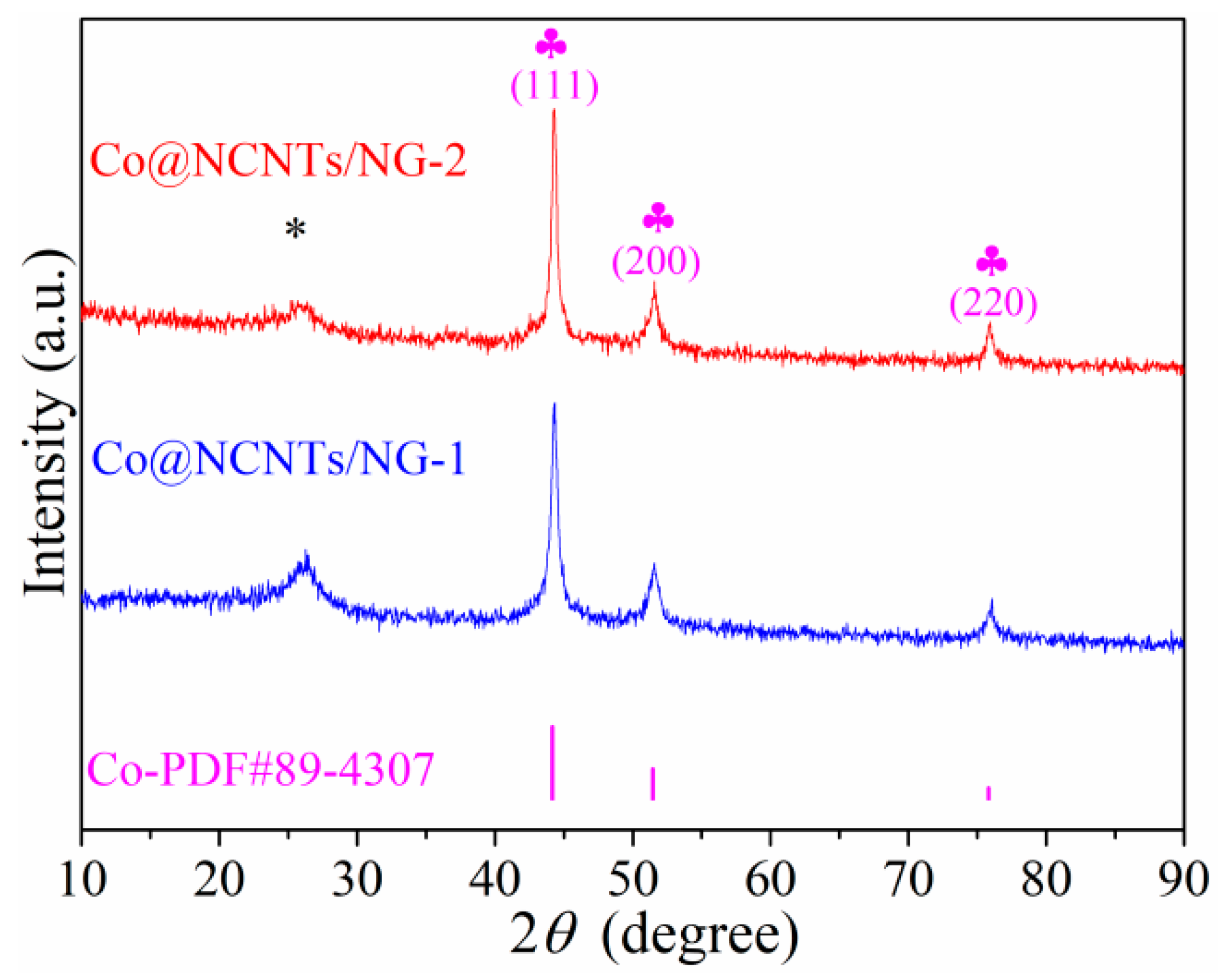
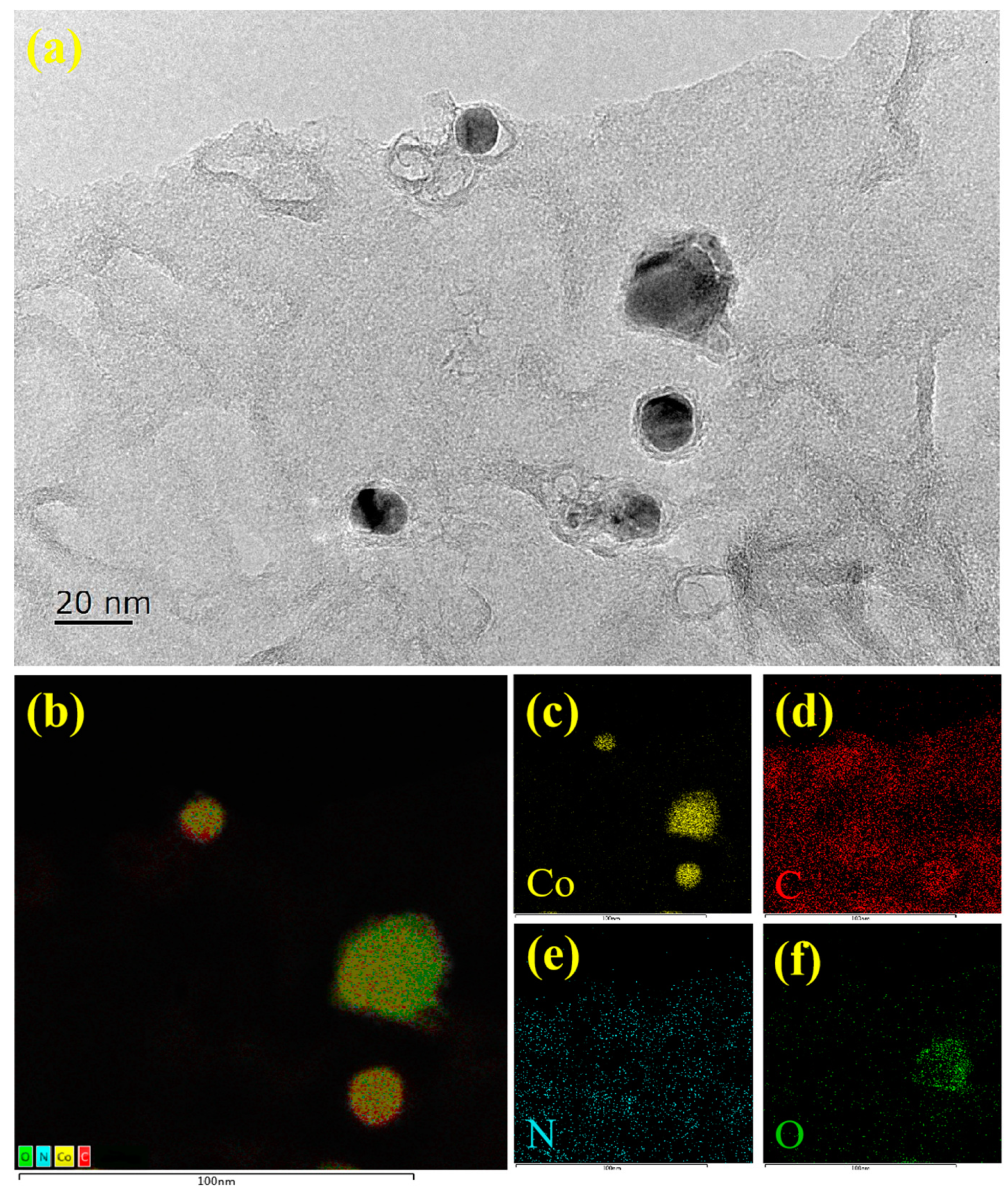
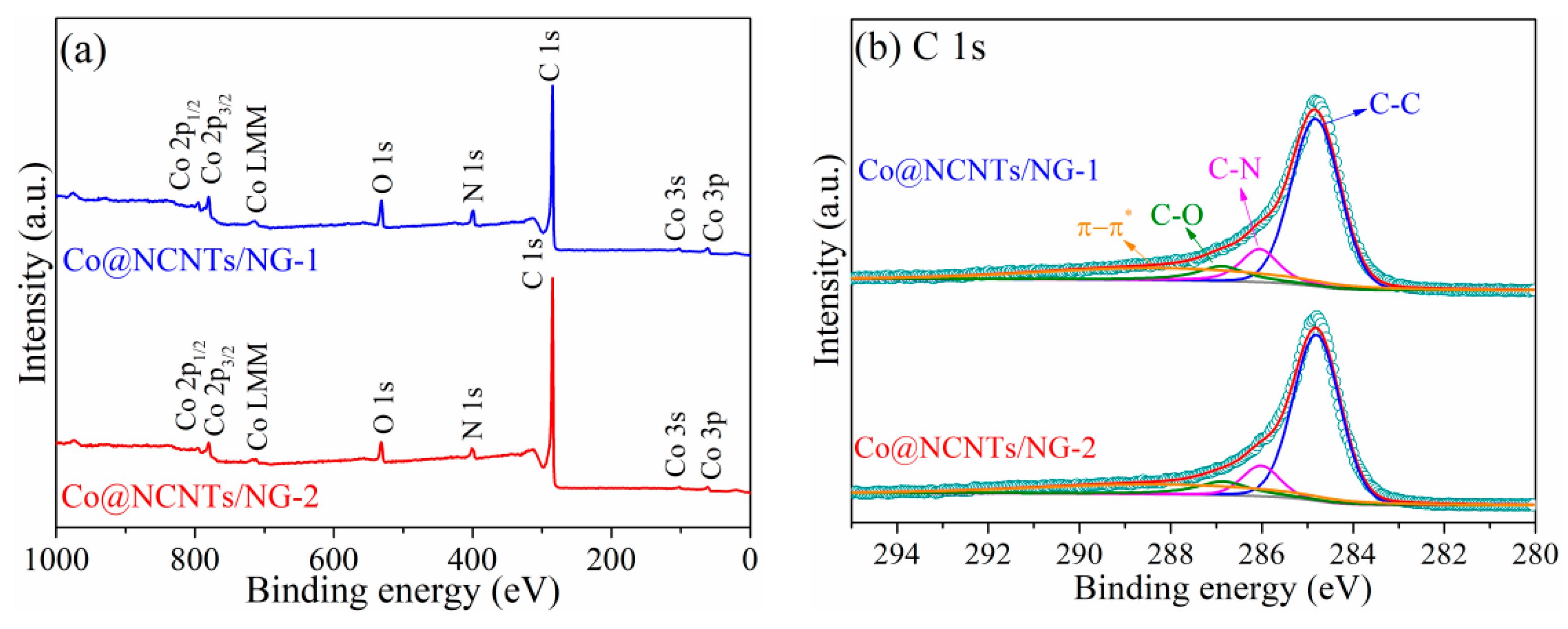
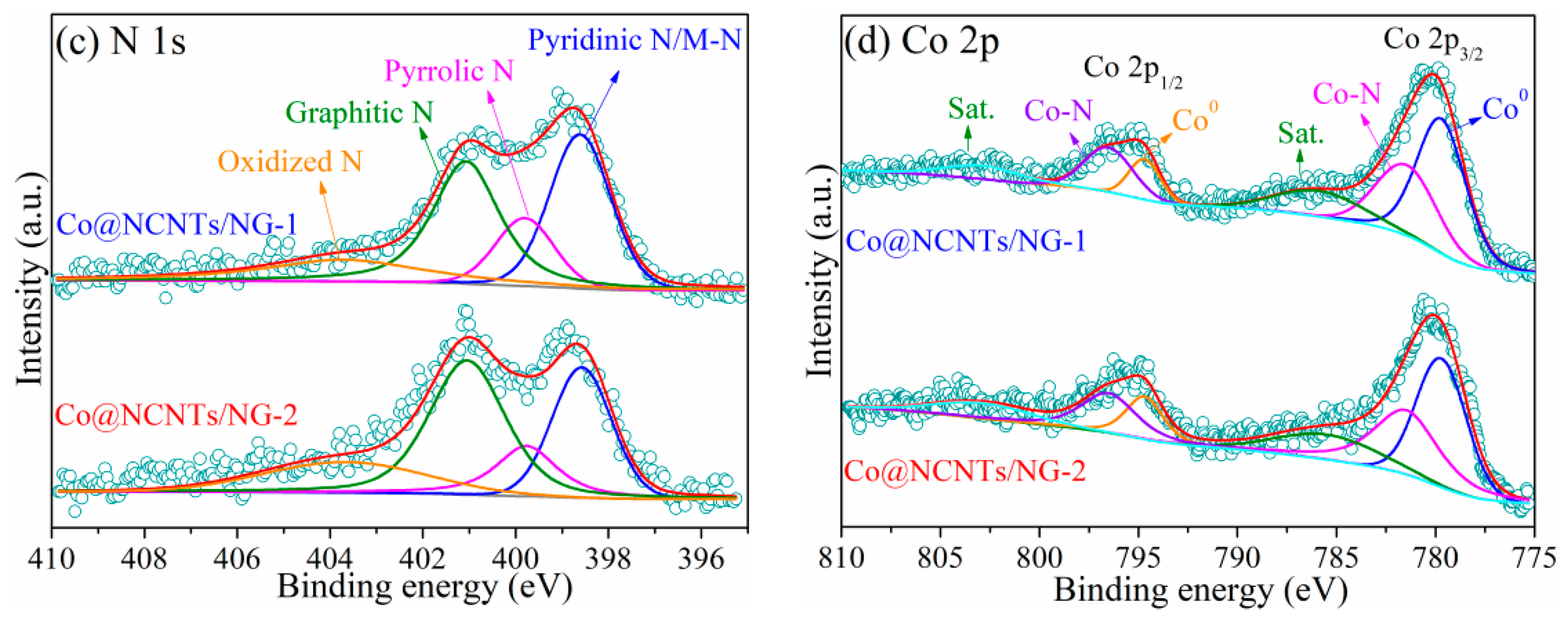
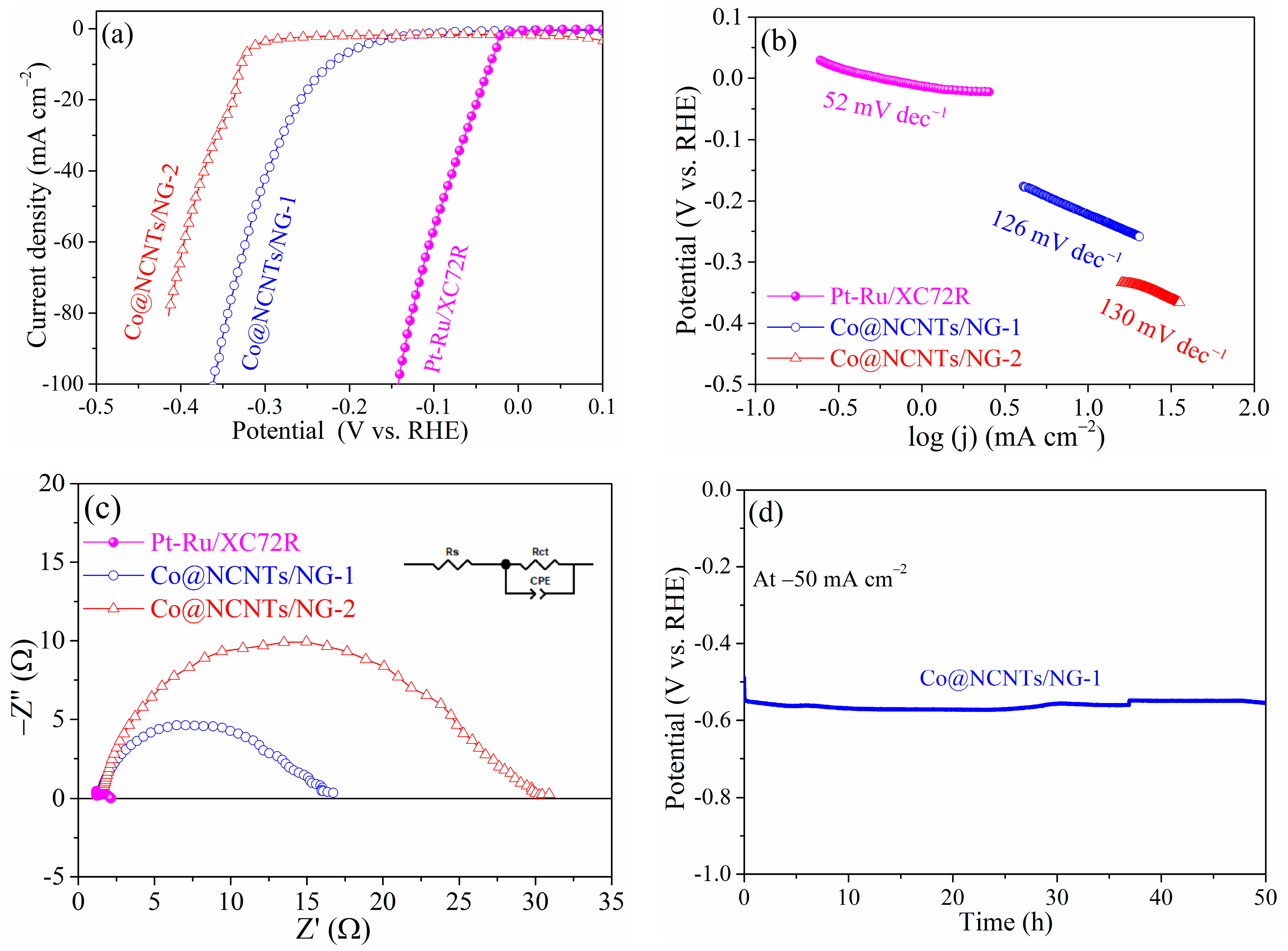
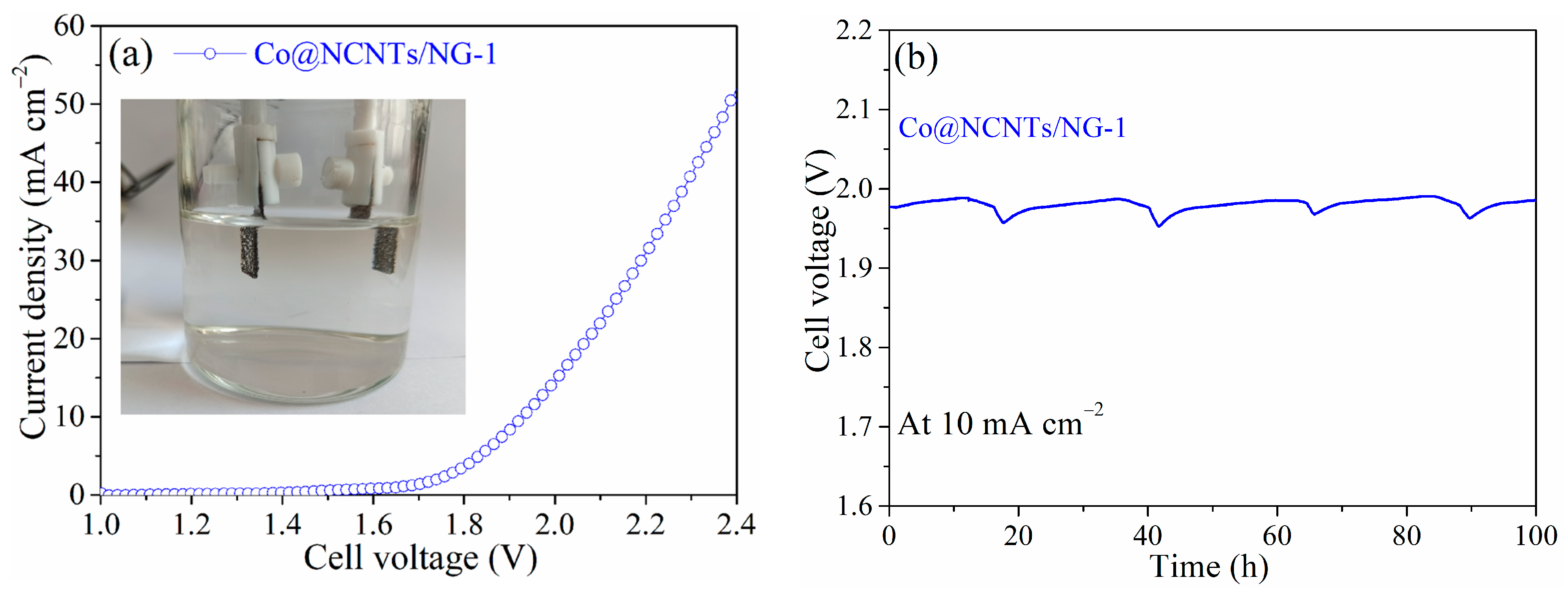
2. Results and Discussion
3. Experimental Details
3.1. Catalyst Synthesis
3.2. Material Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Z.; Huang, J.; Chen, Z.; Li, X.; Feng, Z.; Yang, H.; Huang, S.; Luo, R. Experimental and DFT studies of flower-like Ni-doped Mo2C on carbon fiber paper: A highly efficient and robust HER electrocatalyst modulated by Ni(NO3)2 concentration. J. Adv. Ceram. 2022, 11, 1294–1306. [Google Scholar] [CrossRef]
- Li, B.-Q.; Tang, C.; Wang, H.-F.; Zhu, X.-L.; Zhang, Q. An aqueous preoxidation method for monolithic perovskite electrocatalysts with enhanced water oxidation performance. Sci. Adv. 2016, 2, 1600495. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Wang, M.; Chen, Y.; Luo, L.J.; Yu, W.F.; Zhang, Y.P. Cadmium Doped NiFe LDH Catalyst for Hydrogen Evolution Reaction. J. Ceram. 2023, 44, 736–743. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, M.; Gu, X.; Shi, Y.; Deng, Z.; Cai, N. Water Electrolysis toward Elevated Temperature: Advances, Challenges and Frontiers. Chem. Rev. 2023, 123, 7119–7192. [Google Scholar] [CrossRef]
- Hu, Q.; Li, G.; Han, Z.; Wang, Z.; Huang, X.; Yang, H.; Zhang, Q.; Liu, J.; He, C. Recent progress in the hybrids of transition metals/carbon for electrochemical water splitting. J. Mater. Chem. A 2019, 7, 14380–14390. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER, and Zn-Air Batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhu, Y.; Yang, X.; Li, C. Transitional Metal (Fe, Co, Ni) Encapsulated in Nitrogen-Doped Carbon Nanotubes as Bi-functional Catalysts for Oxygen Electrode Reactions. J. Mater. Chem. A 2016, 4, 1694–1701. [Google Scholar] [CrossRef]
- Daems, N.; Sheng, X.; Vankelecom, I.F.J.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, H.; Wang, J.; Zhang, S. Synthesis of Carbon Nanotubes Via Co-Catalyzed Pyrolysis of Pitch. J. Ceram. 2017, 38, 631–634. [Google Scholar] [CrossRef]
- Sinnott, S.B.; Andrews, R.; Qian, D.; Rao, A.M.; Mao, Z.; Dickey, E.C.; Derbyshire, F. Model of carbon nanotube growth through chemical vapor deposition. Chem. Phys. Lett. 1999, 315, 25–30. [Google Scholar] [CrossRef]
- Zou, X.; Huang, X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmekova, E.; Asefa, T. Cobalt-embedded nitrogen-rich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew. Chem. 2014, 53, 4372–4376. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhou, S.; Yu, F.; Yu, M.; Chiang, C.-Y.; Zhou, W.; Zhao, J.; Qiu, J. Metal-Organic-Framework-Derived Hybrid Carbon Nanocages as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution. Adv. Mater. 2017, 29, 1700874. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Xiang, C.; Ruan, G.; Yan, Z.; Natelson, D.; Tour, J.M. Graphene Nanoribbon and Nanostructured SnO2 Composite Anodes for Lithium Ion Batteries. ACS Nano 2013, 7, 6001–6006. [Google Scholar] [CrossRef]
- Joo, H.; Jung, D.; Sunwoo, S.H.; Koo, J.H.; Kim, D.H. Material Design and Fabrication Strategies for Stretchable Metallic Nanocomposites. Small 2020, 16, 1906270. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Y.; Wang, Z.; Huang, H.; Zhao, Z.; Qiu, J. Towards efficient electrocatalysts for oxygen reduction by doping cobalt into graphene-supported graphitic carbon nitride. J. Mater. Chem. A 2015, 3, 19657–19661. [Google Scholar] [CrossRef]
- Zhou, K.-Y.; Chen, G.-Y.; Liu, J.-A.; Zhang, Z.-P.; Sun, P.; Zhang, W.-Z.; Niu, F.; Zhang, W.-X.; Liang, J.-C. Cobalt nanoparticles encapsulated in N-doped graphene nanoshells as an efficient cathode electrocatalyst for a mechanical rechargeable zinc–air battery. RSC Adv. 2016, 6, 90069–90075. [Google Scholar] [CrossRef]
- Zhu, P.; Gao, J.; Liu, S. Facile in situ coupling CoFe/Co nanoparticles and N-doped carbon nanotubes/graphitic nanosheets as bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. J. Power Sources 2020, 449, 227512. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhou, M.; Bai, J.; Bo, X. Fast and Facile Room-Temperature Synthesis of MOF-Derived Co Nanoparticle/Nitrogen-Doped Porous Graphene in Air Atmosphere for Overall Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 11947–11955. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mate. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, H.; Gao, B.; Wang, Y.; Li, K.; Sun, Y.; Yin, J.; Kan, J. In-situ synthesis of Fe2O3/rGO using different hydrothermal methods as anode materials for lithium-ion batteries. Rev. Adv. Mater. Sci. 2020, 59, 477–486. [Google Scholar] [CrossRef]
- Xu, L.; Wu, S.; Deng, D.; Wang, C.; Qian, J.; Lu, G.; Li, H. Fabricating highly active and stable tungsten carbide electrocatalyst for rechargeable zinc–air batteries: An approach of dual metal Co-adjusted the electronic structure. J. Alloys Compd. 2021, 868, 159236. [Google Scholar] [CrossRef]
- Tian, X.; Hao, X.; Jiang, Z.; Shen, Q.; Zhang, B.; Fang, J.; Fang, Y.; Jiang, Z.-J. Carbon nitride decorated nitrogen doped graphene hollow spheres loaded Ni/Co and corresponding oxides nanoparticles as reversible air electrode catalysts for rechargeable zinc-air batteries. J. Alloys Compd. 2021, 865, 158940. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Cheng, G.; Shuai, Y.; Liu, S.; Liu, Y. CoNi nanoalloys embedded in N-doped carbon nanofibers derived from layered bimetal-organic framework and as efficient oxygen electrocatalyst. J. Alloys Compd. 2021, 888, 161588. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, C.; Qu, Y.; Zhou, H.; Zhou, F.; Wang, J.; Wu, Y.; Li, Y. Trifunctional Self-Supporting Cobalt-Embedded Carbon Nanotube Films for ORR, OER, and HER Triggered by Solid Diffusion from Bulk Metal. Adv. Mater. 2019, 31, e1808043. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Zhang, J.; Zhang, C.; Yoo, S.J.; Kim, J.-G.; Chu, X.; Song, C.; Wang, P.; Zhao, Z.; et al. Highly-dispersed cobalt clusters decorated onto nitrogen-doped carbon nanotubes as multifunctional electrocatalysts for OER, HER and ORR. Carbon 2020, 166, 284–290. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, P.; Wu, W.; Kimura, H.; Shen, Y.; Wu, D.; Xie, X.; Hou, C.; Sun, X.; Yang, X.; et al. Facile synthesis of reduced graphene oxide@Co3O4 composites derived from assisted liquid-phase plasma electrolysis for high-performance hybrid supercapacitors. Appl. Surf. Sci. 2023, 609, 155188. [Google Scholar] [CrossRef]
- Lyu, D.; Du, Y.; Huang, S.; Mollamahale, B.Y.; Zhang, X.; Hasan, S.W.; Yu, F.; Wang, S.; Tian, Z.Q.; Shen, P.K. Highly Efficient Multifunctional Co-N-C Electrocatalysts with Synergistic Effects of Co-N Moieties and Co Metallic Nanoparticles Encapsulated in a N-Doped Carbon Matrix for Water-Splitting and Oxygen Redox Reactions. ACS Appl. Mater. Interfaces 2019, 11, 39809–39819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Tian, J.; Sun, M.; Yuan, D.; Zhang, L. Nitrogen doped CuCo2O4 nanoparticles anchored on beaded-like carbon nanofibers as an efficient bifunctional oxygen catalyst toward zinc-air battery. J. Colloid Interface Sci. 2022, 608 Pt 2, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Feng, J.J.; Yao, Y.Q.; Wang, Z.G.; Zhang, L.; Wang, A.J. Mn, N, P-tridoped bamboo-like carbon nanotubes decorated with ultrafine Co2P/FeCo nanoparticles as bifunctional oxygen electrocatalyst for long-term rechargeable Zn-air battery. J. Colloid Interface Sci. 2021, 590, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, D.; Hou, H.; Ma, Y.; Fang, Z.; Lu, X.; Xu, S.; Hou, P.; Shao, G.; Yang, W.; et al. Embedded FeCo alloy nanoparticles in N-doped mesoporous carbon nanofibers as efficient Bi-functional electrocatalysts for Long-Term rechargeable Zn-Air batteries. Appl. Surf. Sci. 2022, 571, 151292. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, H.; Wu, M. N-Doped-carbon/cobalt-nanoparticle/N-doped-carbon multi-layer sandwich nanohybrids derived from cobalt MOFs having 3D molecular structures as bifunctional electrocatalysts for on-chip solid-state Zn-air batteries. Nanoscale 2020, 12, 3750–3762. [Google Scholar] [CrossRef]
- Niu, H.-J.; Chen, S.-S.; Feng, J.-J.; Zhang, L.; Wang, A.-J. Assembled hollow spheres with CoFe alloyed nanocrystals encapsulated in N, P-doped carbon nanovesicles: An ultra-stable bifunctional oxygen catalyst for rechargeable Zn-air battery. J. Power Sources 2020, 475, 228594. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-Doped Graphene-Based Materials for Energy-Relevant Electrocatalytic Processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Ji, D.; Fan, L.; Li, L.; Mao, N.; Qin, X.; Peng, S.; Ramakrishna, S. Hierarchical catalytic electrodes of cobalt-embedded carbon nanotube/carbon flakes arrays for flexible solid-state zinc-air batteries. Carbon 2019, 142, 379–387. [Google Scholar] [CrossRef]
- Li, Z.; He, H.; Cao, H.; Sun, S.; Diao, W.; Gao, D.; Lu, P.; Zhang, S.; Guo, Z.; Li, M.; et al. Atomic Co/Ni dual sites and Co/Ni alloy nanoparticles in N-doped porous Janus-like carbon frameworks for bifunctional oxygen electrocatalysis. Appl. Catal. B Environ. 2019, 240, 112–121. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Li, K.; Ao, Z.; Wang, C.; Liu, H.; Sun, K.; Wang, G. Electrospun cobalt embedded porous nitrogen doped carbon nanofibers as an efficient catalyst for water splitting. J. Mater. Chem. A 2016, 4, 12818–12824. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Ponraj, J.; Mansour, S.A. Development of Co/Co9S8 metallic nanowire anchored on N-doped CNTs through the pyrolysis of melamine for overall water splitting. Electrochim. Acta 2021, 368, 137642. [Google Scholar] [CrossRef]
- Zeng, M.; Liu, Y.; Zhao, F.; Nie, K.; Han, N.; Wang, X.; Huang, W.; Song, X.; Zhong, J.; Li, Y. Metallic Cobalt Nanoparticles Encapsulated in Nitrogen-Enriched Graphene Shells: Its Bifunctional Electrocatalysis and Application in Zinc-Air Batteries. Adv. Funct. Mater. 2016, 26, 4397–4404. [Google Scholar] [CrossRef]
- Wang, J.; Gao, D.; Wang, G.; Miao, S.; Wu, H.; Li, J.; Bao, X. Cobalt nanoparticles encapsulated in nitrogen-doped carbon as a bifunctional catalyst for water electrolysis. J. Mater. Chem. A 2014, 2, 20067–20074. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, S.; Chen, J.; Zhou, Y.; Zhao, P.; Dai, R.; Zhou, W.; Yang, P.; Zhang, H.; Chen, A. Interface engineering of Fe2P@CoMnP4 heterostructured nanoarrays for efficient and stable overall water splitting. J. Colloid Interface Sci. 2023, 633, 897–906. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Zhang, H.; Zhang, Z.; Zhou, J.; Hou, Y.; Liu, P.; Xu, B.; Zhang, H.; Guo, J. Interface engineering of porous Co(OH)2/La(OH)3@Cu nanowire heterostructures for high efficiency hydrogen evolution and overall water splitting. J. Mater. Chem. A 2023, 11, 4355–4364. [Google Scholar] [CrossRef]
- Hao, M.; Xu, Z.; Liu, X.; Ma, J.; Wang, L.; Li, C.; Wang, W. The in situ growth of FeCo nanoalloys in NiFeCoP nanosheets arrays with superhydrophilicity and surperaerophobicity for efficient overall water splitting. Int. J. Hydrogen Energy 2023, 48, 147–159. [Google Scholar] [CrossRef]
- Zhai, Y.; Kuang, C.; Liu, H.; Li, L. Free-Standing Dendritic Nanostructured Co/CNT/Carbon Nanofiber Composites for Efficient Water Splitting in Alkaline Media. ACS Appl. Nano Mater. 2023, 6, 8593–8602. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, X.; Liu, C.; Lu, Y.; Liu, Y.; Li, D.; Jiang, D. Zinc and fluorine ions dual-modulated NiCoP nanoprism array electrocatalysts for efficient water splitting. J. Colloid Interface Sci. 2023, 630, 559–569. [Google Scholar] [CrossRef]
- Wan, X.; Wang, X.; Lu, D.; Xu, Y.; Liu, G.; Fu, Y.; Shui, T.; Wang, H.; Cheng, Z. Composition and Morphology Modulation of Bimetallic Nitride Nanostructures on Nickel Foams for Efficient Oxygen Evolution Electrocatalysis. Catalysts 2023, 13, 230. [Google Scholar] [CrossRef]
- Liu, H.; Xie, R.; Wang, Q.; Han, J.; Han, Y.; Wang, J.; Fang, H.; Qi, J.; Ding, M.; Ji, W.; et al. Enhanced OER Performance and Dynamic Transition of Surface Reconstruction in LaNiO3 Thin Films with Nanoparticles Decoration. Adv. Sci. 2023, 10, 2207128. [Google Scholar] [CrossRef]
- Ye, C.; Liu, J.; Zhang, Q.; Jin, X.; Zhao, Y.; Pan, Z.; Chen, G.; Qiu, Y.; Ye, D.; Gu, L.; et al. Activating Metal Oxides Nanocatalysts for Electrocatalytic Water Oxidation by Quenching-Induced Near-Surface Metal Atom Functionality. J. Am. Chem. Soc. 2021, 143, 14169–14177. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-D.; Xiang, J.; Zhao, R.-D.; Loy, S.; Li, M.-T.; Ma, D.-M.; Li, J.; Wu, F.-F. Nanoengineering of ZnCo2O4@CoMoO4 heterogeneous structures for supercapacitor and water splitting applications. Ceram. Int. 2023, 49, 4422–4434. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, M.; Lee, L.Y.S. Best Practices in Using Foam-Type Electrodes for Electrocatalytic Performance Benchmark. ACS Energy Lett. 2020, 5, 3260–3264. [Google Scholar] [CrossRef]
- Zhuang, Y.; Cheng, H.; Meng, C.; Chen, B.; Zhou, H. Self-catalyzed Co, N-doped carbon nanotubes-grafted hollow carbon polyhedrons as efficient trifunctional electrocatalysts for zinc-air batteries and self-powered overall water splitting. J. Colloid Interface Sci. 2023, 643, 162–173. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, R.; Huang, X.; Cong, H.; Tang, J.; Xu, W.; Li, M.; Chen, Y.; Wang, Y.; Han, S.; et al. CoS2 quantum dots modified by ZIF-67 and anchored on reduced graphene oxide as an efficient catalyst for hydrogen evolution reaction. Chem. Eng. J. 2022, 430, 132634. [Google Scholar] [CrossRef]
- Kim, S.; Min, K.; Kim, H.; Yoo, R.; Shim, S.E.; Lim, D.; Baeck, S.-H. Bimetallic-metal organic framework-derived Ni3S2/MoS2 hollow spheres as bifunctional electrocatalyst for highly efficient and stable overall water splitting. Int. J. Hydrogen Energy 2022, 47, 8165–8176. [Google Scholar] [CrossRef]
- Han, H.; Paik, J.W.; Ham, M.; Kim, K.M.; Park, J.K.; Jeong, Y.K. Atomic Layer Deposition-Assisted Fabrication of Co-Nanoparticle/N-Doped Carbon Nanotube Hybrids as Efficient Electrocatalysts for the Oxygen Evolution Reaction. Small 2020, 16, e2002427. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Xu, B.Z.; Song, J.; Shi, Q.; Engelhard, M.H.; Li, X.; Beckman, S.P.; Sun, J.; Du, D.; et al. Sugar Blowing-Induced Porous Cobalt Phosphide/Nitrogen-Doped Carbon Nanostructures with Enhanced Electrochemical Oxidation Performance toward Water and Other Small Molecules. Small 2017, 13, 1700796. [Google Scholar] [CrossRef]
- Wang, S.; Jang, H.; Wang, J.; Wu, Z.; Liu, X.; Cho, J. Cobalt-Tannin-Framework-Derived Amorphous Co-P/Co-N-C on N, P Co- Doped Porous Carbon with Abundant Active Moieties for Efficient Oxygen Reactions and Water Splitting. ChemSusChem 2019, 12, 830–838. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Li, H.; Li, P.; Xie, L.; Liu, Y.; Cao, Z.; Tian, C.; Wang, C.-A.; Xie, Z. Facile Synthesis of Co Nanoparticles Embedded in N-Doped Carbon Nanotubes/Graphitic Nanosheets as Bifunctional Electrocatalysts for Electrocatalytic Water Splitting. Molecules 2023, 28, 6709. https://doi.org/10.3390/molecules28186709
Yang W, Li H, Li P, Xie L, Liu Y, Cao Z, Tian C, Wang C-A, Xie Z. Facile Synthesis of Co Nanoparticles Embedded in N-Doped Carbon Nanotubes/Graphitic Nanosheets as Bifunctional Electrocatalysts for Electrocatalytic Water Splitting. Molecules. 2023; 28(18):6709. https://doi.org/10.3390/molecules28186709
Chicago/Turabian StyleYang, Wei, Han Li, Pengzhang Li, Linhua Xie, Yumin Liu, Zhenbao Cao, Chuanjin Tian, Chang-An Wang, and Zhipeng Xie. 2023. "Facile Synthesis of Co Nanoparticles Embedded in N-Doped Carbon Nanotubes/Graphitic Nanosheets as Bifunctional Electrocatalysts for Electrocatalytic Water Splitting" Molecules 28, no. 18: 6709. https://doi.org/10.3390/molecules28186709





