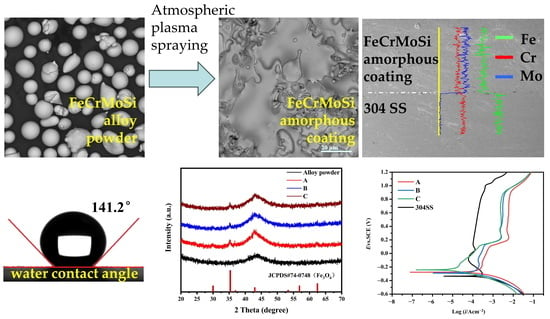
2.1. Structure and Morphology
The phase structure of FeCrMoSi alloy raw materials powder and as-fabricated FeCrMoSi amorphous coatings were examined using X-ray diffraction (XRD); the XRD patterns are presented in
Figure 1a. The XRD pattern of the raw materials exhibited a single broadened and diffused diffraction peak at approximately 43°. This indicates that the FeCrMoSi alloy raw materials are in a completely amorphous state. Similarly, each XRD pattern of the coatings also exhibited a broadened and diffused diffraction peak around 43°, consistent with that of the raw materials, further confirming that the coatings are predominantly in an amorphous state. However, in each XRD pattern of the FeCrMoSi amorphous coatings, a small sharp peak at about 35°, which could be attributed to Fe
3O
4, was observed [
25,
26]. This finding suggests the occurrence of a certain oxidation reaction during the fabrication of the coatings by APS. In addition, the bright field transmission electron microscopy (TEM) images of FeCrMoSi amorphous coatings show a typical amorphous morphology (
Figure 1b) and the corresponding selected area electron diffraction (SAED) pattern only shows a typical diffuse ring. Consequently, both XRD patterns and TEM observation demonstrated that FeCrMoSi amorphous coatings with high amorphous phase content may contain a very small amount of crystalline structure.
The surface morphology of the coatings and the interface between the coatings and the base material were observed using scanning electron microscopy (SEM); the images are depicted in
Figure 2. Each coating (
Figure 2a–c) exhibited partially molten particles and micropores. The number of partially molten particles on the surface gradually decreased with the increase in applied current during APS. Among the coatings, coating C displayed the highest degree of melting under a current of 600 A. The high-temperature molten FeCrMoSi alloy particles experienced rapid impact on the surface of the 304 SS base material during APS. Some particles were fully deformed, forming a typical layered structure and resulting in a certain smooth surface, meanwhile others remained spherical after APS, leading to incomplete packing between molten flat particles and the appearance of a small number of pores. Additionally, the cold shrinkage of molten flat particles during solidification may have contributed to the formation of some pores [
24].
Figure 2d–f show the cross-sectional morphologies of the coatings. With increasing current, the presence of pores in the coatings reduced. However, even at the maximum current, some pores persisted between the coatings and the 304 SS base material. These pores can be found to be oxygen-rich by X-ray energy dispersive spectroscope (EDS) mapping (
Figure 2g,h). These phenomena may be attributed to slight oxidation in air [
27], considering that the raw materials needed to be heated to their melting temperature during APS. Subsequently, the chemical composition of the FeCrMoSi amorphous coatings was examined using EDS and the results are summarized in
Table 1. The chemical composition of the as-fabricated coatings generally reflects that of the FeCrMoSi alloy powder raw materials, with slight oxidation observed. The elemental distribution of coating C and the base material was measured by EDS line scanning and the results are shown in
Figure 2i. It is seen that the coating mainly contains the elements Fe, Cr, and Mo and the distribution of these three elements is uniform. Comparing to 304 SS base materials, the Cr content in the coating is slightly lower than that of 304 SS. The Fe content in the coating is largely lower than that of 304 SS. Meanwhile, 304 SS base material does not contain Mo element. Previous studies have shown that adding Mo to an alloy can form a protective film on the surface, which can effectively prevent further corrosion inside the alloy [
28]. Furthermore, the migration or diffusion of the alloying elements from the base material to the coating and vice versa may occur during the coating process. However, the current EDS line scanning does not detect this phenomenon, which can be further studied in the future.
2.2. Bonding Strength
The bonding strengths between the coatings and the 304 SS base material were measured using the tensile test and the results are summarized in
Table 2. Coatings A, B, and C exhibited bonding strengths of 64.3, 61.6, and 53.1 N/mm
2, respectively. For comparison, Zhang et al. prepared Fe-based amorphous coatings on WE43 magnesium alloy using HVOF spraying, with bonding strength reaching 56 MPa [
29]. In another study, Zhang et al. prepared laminar coatings comprising multi-amorphous Fe48Mo14C15Y2C15B6 layers and a crystalline NiCrAl layer using the HVOF technique and the bonding strength of those coatings was only 40 MPa [
30]. In our work, the bonding strength of the coatings reached up to 64.3 N/mm
2, indicating a strong bonding state between the coating and the base material. SEM images of the fracture surface of the coating and the base material after the bonding strength test revealed some flat FeCrMoSi alloy particles still attached to the base material, demonstrating a robust binding force between them (
Figure 3). Prior to spraying, the base materials were sandblasted to increase the surface roughness, thus enhancing the bonding strength between the coatings and the base material. Notably, FeCrMoSi amorphous alloy particles experienced rapid heating in atmospheric plasma, resulting in inevitable oxidation [
31]. Subsequently, these molten particles were sequentially sprayed onto the base material’s surface, forming laminar patches with oxygenated interfaces on the 304 SS base material (
Figure 2). Based on the above observations and tests, the bonding and failure mechanism of the as-prepared FeCrMoSi amorphous coatings can be analyzed as follows: During the initial APS, the sprayed FeCrMoSi alloy particles combine with the base material and leave cavities between the particles, resulting in a porous structure on the coating surfaces. As atomic diffusion is sufficiently active, the particles undergo plastic deformation, leading to an improved engagement between the coatings and the base material. When the coating is subjected to tensile force, cracks continue to extend along the defective areas due to the presence of some oxides and pores. Consequently, when the extended cracks contact each other, the fracture occurs in the weakest region inside the coating.
2.4. Hydrophobicity
Hydrophobicity was characterized by measuring the water contact angle (WCA) of the FeCrMoSi amorphous coatings and the 304 SS base material, as depicted in
Figure 4. Remarkably, the FeCrMoSi amorphous coatings exhibited excellent hydrophobicity, with WCAs ranging from 136.6° to 141.2°. In contrast, the WCA of the 304 SS was only 77.5°. The hydrophobicity of the FeCrMoSi amorphous coatings surpassed that of the NiCrBSi coating, which recorded a WCA of 128° [
33], and even exceeded the chromium-doped titanium nitride film, with a WCA of only 115.86° [
34].
The hydrophobicity of a coating is closely related to its surface morphology [
35] and the direct attraction of water droplets to the surface materials, which is minimized due to the lower surface energy. Generally, greater surface roughness results in improved hydrophobicity [
36]. Models used to describe hydrophobic states can be divided into two types. The first is called the Wenzel state, wherein water droplets completely penetrate the roughness [
37]. The second is the Cassie–Baxter state, which assumes that only the upper region of the rough surface is in contact with water and an air pocket exists between them [
38]. For the FeCrMoSi amorphous coatings in this study, the hydrophobicity of the FeCrMoSi amorphous coatings suggests that the water droplets cannot fully penetrate any part of the coating surface and can only form an “air wall” at the groove, which traps the air in the groove, creating a slight “air cushion” effect [
39]. This effect supports the water droplets, thereby increasing the hydrophobicity of the coating (
Figure 5). According to SEM observations, numerous small humps are present on the surface of the coatings (
Figure 3), which likely result from the partial melting and simultaneous splashing of the molten or semi-molten alloy particles during APS [
40].
2.5. Corrosion Resistance
The potentiodynamic polarization curves of the FeCrMoSi amorphous coatings and the 304 SS base material in a hydrogen-passing sulfuric acid and hydrofluoric mixed solution (0.5 M H
2SO
4 + 2 ppm HF, 80 °C) are presented in
Figure 6, with the corresponding electrochemical parameters summarized in
Table 4. The results demonstrate an active–passive transition for the 304 SS base material. As the potential increases, the anodic polarization curve of 304 SS forms a stable passivation region at 0.09–1 V. However, as the anode polarization potential further increases, the anode current density rises rapidly, indicating that the passivation film begins to break down, losing its passivation ability in the 1–1.2 V range. In contrast, the FeCrMoSi amorphous coatings exhibit superior corrosion resistance compared to 304 SS, evidenced by their lower corrosion current density (I
corr) and higher corrosion potential value (E
corr). The E
corr of the FeCrMoSi amorphous coatings is approximately 90 mV greater than that of the 304 SS. Particularly noteworthy is the fact that the I
corr of the FeCrMoSi amorphous coatings is lower than that of the 304 SS base material by one to two orders of magnitude. According to the potentiodynamic polarization curves (
Figure 6), the FeCrMoSi amorphous coatings form a passivation film at lower current densities, remaining stable at higher potentials. Coatings A and B exhibit a slow activation process before passivation. Once the E
corr reaches approximately 0.15 V, the I
corr increases rapidly until reaching the equilibrium state of active dissolution and passivation. However, the I
corr of coating C increases rapidly before passivation, and passivation begins near 0.25 V. This active reaction is primarily attributed to the dispersed distribution of particles, oxides, and pores in the coating. Such structural defects often become sites of initial corrosion, leading to a swift increase in the early I
corr of anodic polarization until the appearance of a passivation state.
The surface morphologies of a typical FeCrMoSi amorphous coating C and the 304 SS base material before and after the corrosion resistance test were observed using SEM, as depicted in
Figure 7. Clearly, the 304 SS suffered severe corrosion damage, with the surface covered in corrosion products and corrosion pits (
Figure 7a). In contrast, the overall morphology of the FeCrMoSi amorphous coating did not change significantly, with only a small number of corrosion pits observed on the surface (
Figure 7b). These SEM observations further confirm that the FeCrMoSi amorphous coating exhibits superior corrosion resistance compared with 304 SS.
The chemical state and composition of the corrosion products of the typical FeCrMoSi amorphous coating C before and after the corrosion resistance test were investigated using X-ray photoelectron spectroscopy (XPS). The original XPS data were initially calibrated with carbon 1 s (284.6 eV). Subsequently, XPS data analysis was carried out using the XPS Peak 4.1 program and a Shirley function was employed to subtract the background. The Fe 2p, Cr 2p, and Mo 3d core-level spectra were fitted with Lorentzian–Gaussian curves [
41]. For the Fe 2p
1/2, Fe 2p
3/2, Cr 2p
1/2, and Cr 2p
3/2 core-level spectra, a 1:2 ratio was applied in the curve fitting of all the Fe 2p and Cr 2p XPS spectra. Similarly, the Mo 3d
3/2 and Mo 3d
5/2 signal areas had a 2:3 ratio which was applied in the curve fitting of all the Mo 3d XPS spectra.
The XPS survey spectrum (
Figure 8) clearly indicates that in addition to adventitious carbon, some main elements include Fe, Mo, Cr, Si, and O. The XPS core-level spectra of Fe before the corrosion resistance test can be identified to be composed of Fe 2p
1/2 and Fe 2p
3/2 peaks of Fe
0, Fe
2+, and Fe
3+ states [
42], as depicted in
Figure 9a. The presence of Fe
2+ and Fe
3+ indicates that a small amount of Fe has been oxidized during APS. This reaction corresponds to the appearance of Fe
3O
4 in the XRD patterns (
Figure 1), which is composed of FeO and Fe
2O
3 [
43]. The XPS core-level spectra of Mo before the corrosion resistance test can be identified to be composed of Mo 3d
3/2 and Mo 3d
5/2 peaks of Mo
0 and Mo
6+ states [
44,
45], as displayed in
Figure 9b. The presence of Mo6+ indicates that a small amount of Mo has been oxidized during APS. The XPS core-level spectra of Cr before the corrosion resistance test can be identified to be composed of Cr 2p
1/2 and Cr 2p
3/2 peaks of Cr
0, Cr
3+, and Cr
6+ states [
46], as presented in
Figure 9c. The presence of Cr
3+ indicates that a small amount of Cr has been oxidized during APS.
The XPS core-level spectra of Fe after the corrosion resistance test reveal the presence of Fe 2p
1/2 and Fe 2p
3/2 peaks corresponding to Fe
0 and Fe
3+ states, as marked in
Figure 9d. In comparison to the XPS core-level spectra of Fe before the corrosion resistance test (
Figure 9a), Fe
2+ ions were not found, indicating that Fe
2+ is more soluble in the acidic solution (0.5 M H
2SO
4 + 2 ppm HF, 80 °C). Similarly, the XPS core-level spectra of Mo after the corrosion resistance test exhibit Mo 3d
3/2 and Mo 3d
5/2 peaks corresponding to Mo
0 and Mo
6+ states, as marked in
Figure 9e. Likewise, the XPS core-level spectra of Cr after the corrosion resistance test show Cr 2p
1/2 and Cr 2p
3/2 peaks corresponding to Cr
3+ states only, as marked in
Figure 9f. These spectra are consistent with those before the corrosion resistance test, indicating that the Mo and Cr elements can withstand the corrosion of this specific acidic solution (0.5 M H
2SO
4 + 2 ppm HF, 80 °C).
Based on the above analysis, the passivation film on the surface of the coating is believed to be composed of oxides of Cr, Fe, and Mo, with the main compounds being Fe
3O
4, Cr
2O
3, and MoO
3. These oxides significantly improve the stability of the passivation film and enhance the protective effect of the chromium oxide film [
47]. Xia et al. have proposed that hexavalent chromium dissolves in water and increases the corrosion current density, but it possesses a self-healing ability and can promote the formation of dense chromium oxide [
48]. The presence of Cr ions is crucial for the corrosion resistance of FeCrMoSi amorphous alloy coatings as they can form chromium hydroxide passivation films. Additionally, Cr reacts with H
2O to form Cr
2O
3, which further enhances its corrosion resistance [
49]. This also explains the significant reduction in the intensity of Cr
0 after corrosion. Moreover, Tian et al. have suggested that MoO
3 can accumulate on the surface and hinder the diffusion of corrosion ions into the interior (
Figure 10), effectively preventing pitting corrosion [
43]. Thus, FeCrMoSi amorphous coatings demonstrate better corrosion resistance than 304 SS due to their higher Cr and Mo contents. Furthermore, the chemical composition of the corroded surface of coating C (
Table 5) shows that the O content has doubled compared to that of the as-fabricated coating, indicating a substantial production of oxides on the surface of the coating during the corrosion resistance test.