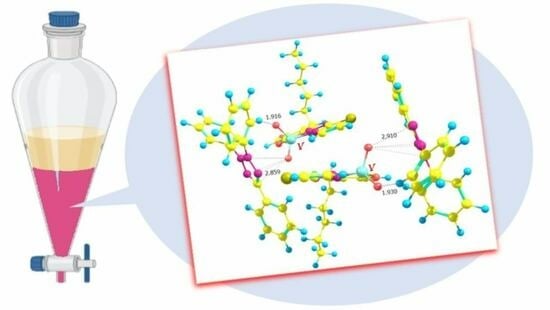
Extractive Spectrophotometric Determination and Theoretical Investigations of Two New Vanadium(V) Complexes
Abstract
1. Introduction
- To study the LLE of the V(V)–HTAR species in the presence of tetrazolium salt;
- To find the ground-state equilibrium geometries of the extracted species using quantum chemical calculations at the B3LYP and HF levels of theory;
- To develop a competitive LLE–spectrophotometric method for determining V(V) in real samples.
2. Results and Discussion
2.1. Absorption Spectra
2.2. Effect of pH and the Amount of Buffer
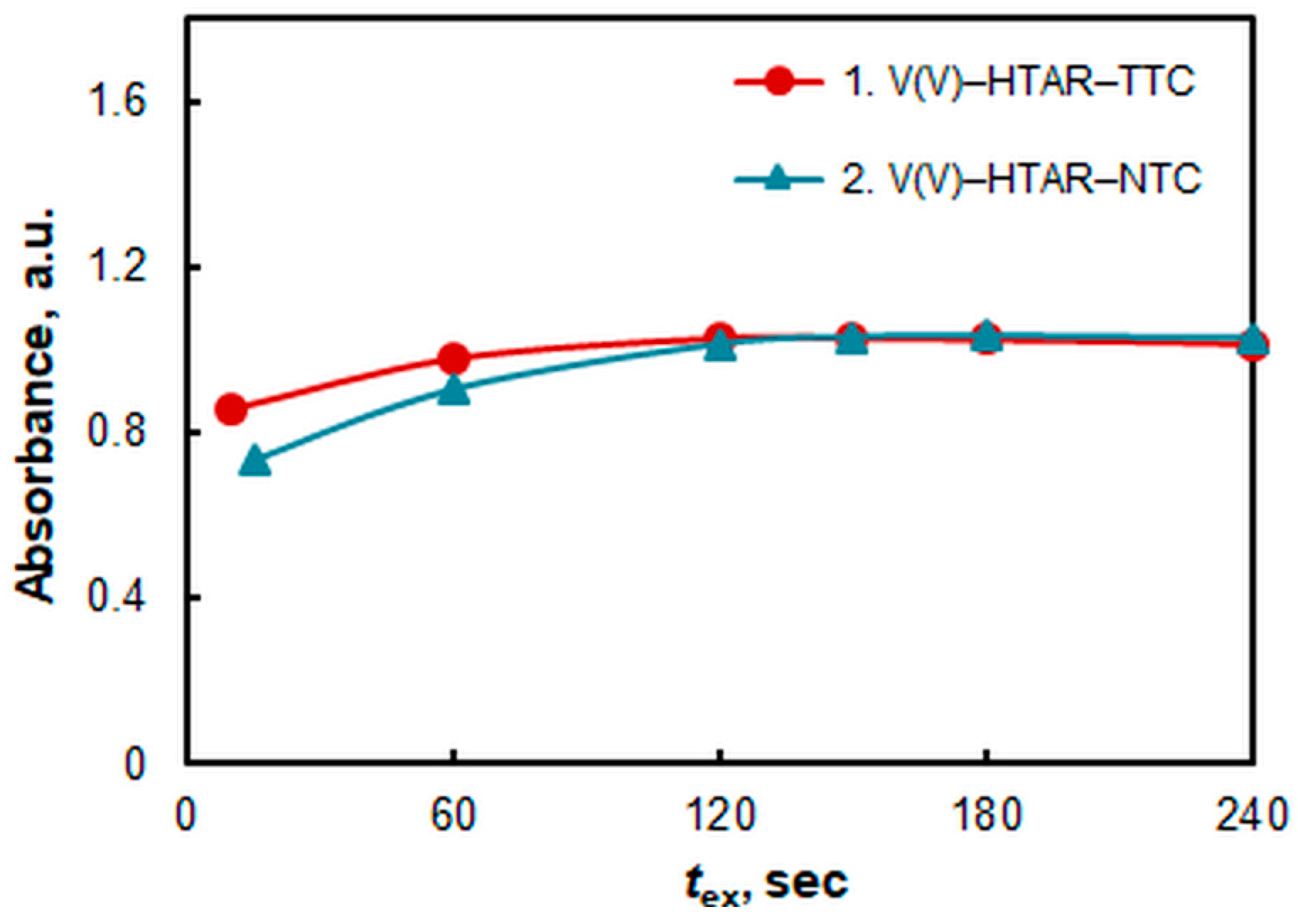
2.3. Effect of Extraction Time
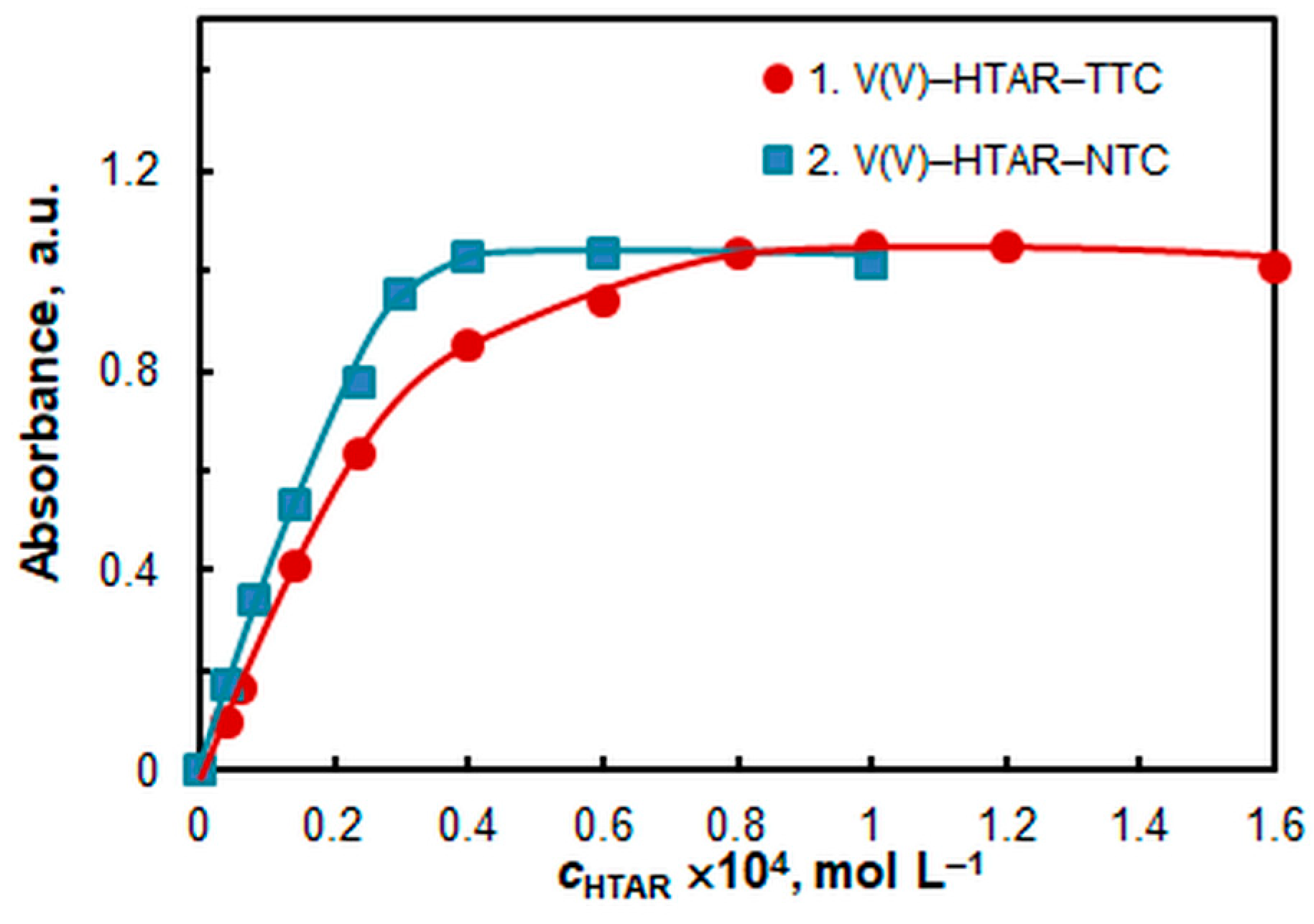
2.4. Effect of HTAR and TS Concentrations
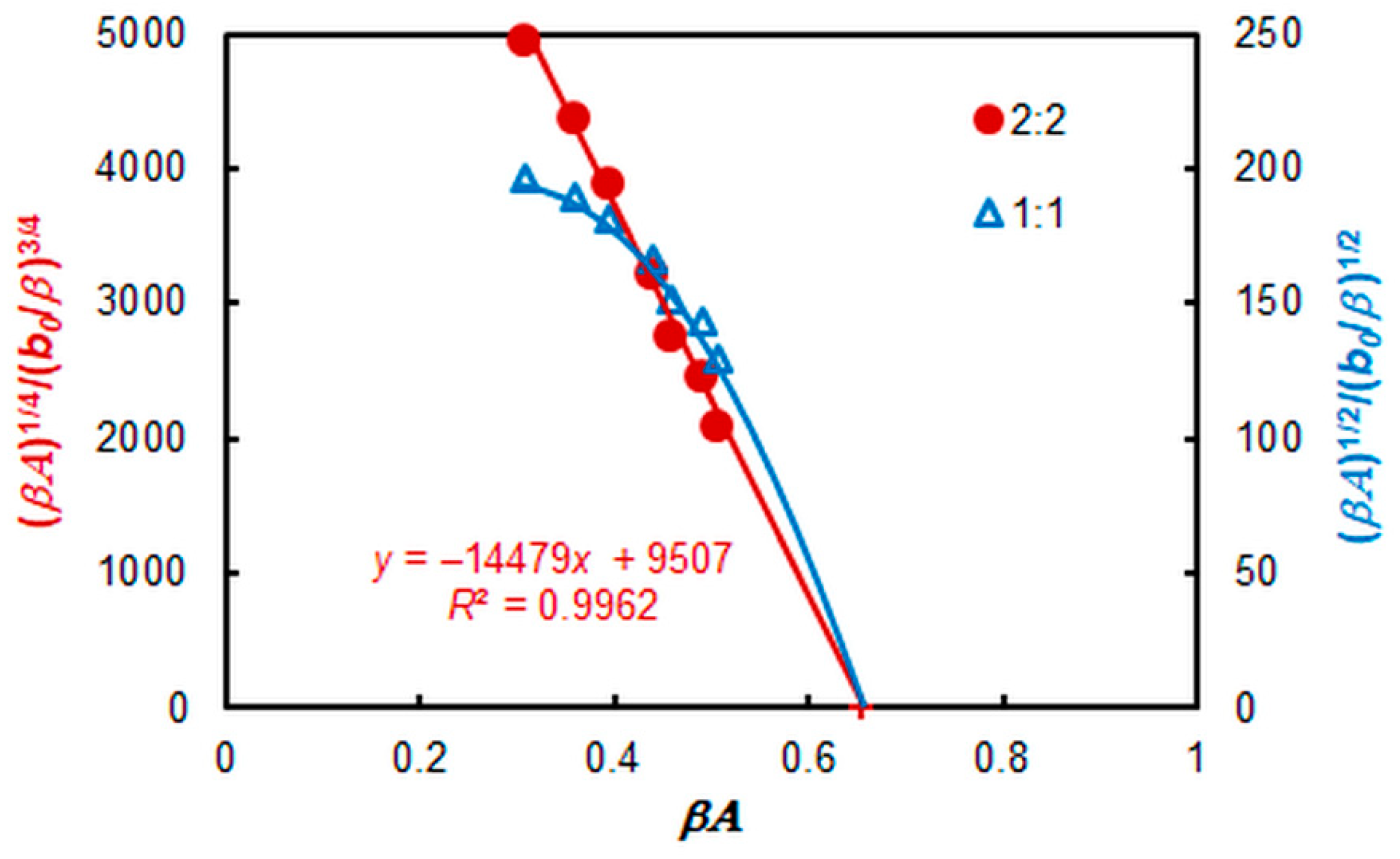
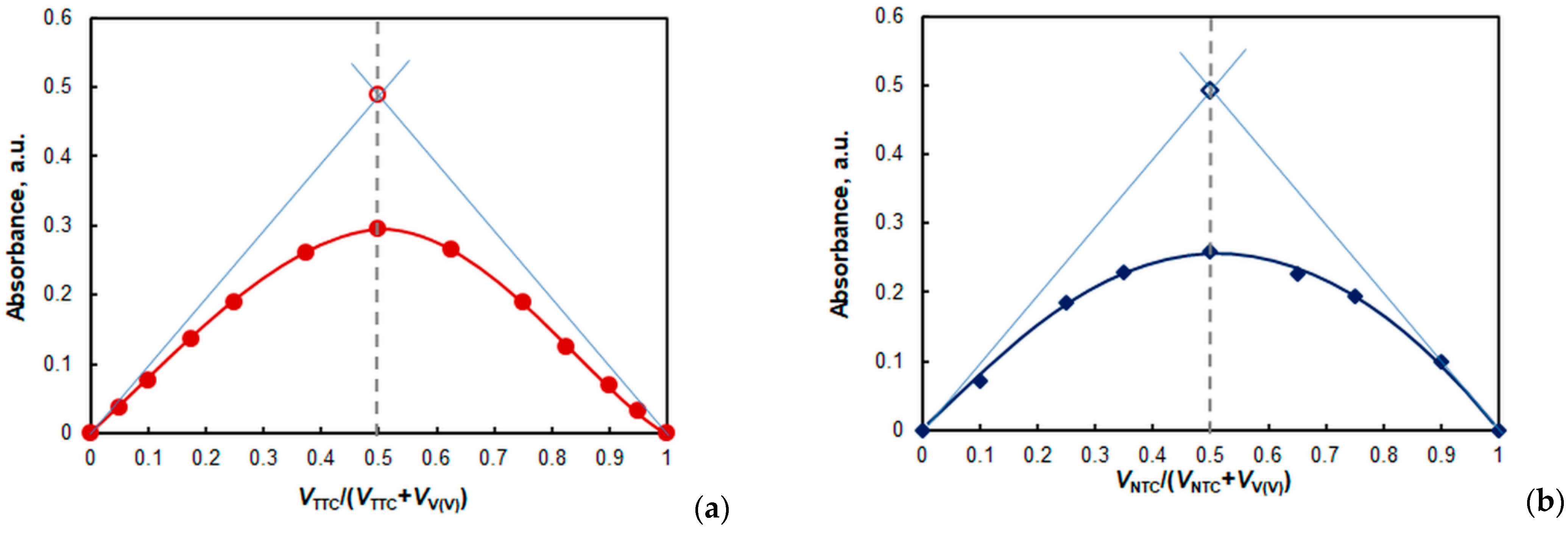
2.5. Stoichiometry, Formulas, and Equations
2.6. Extraction Characteristics
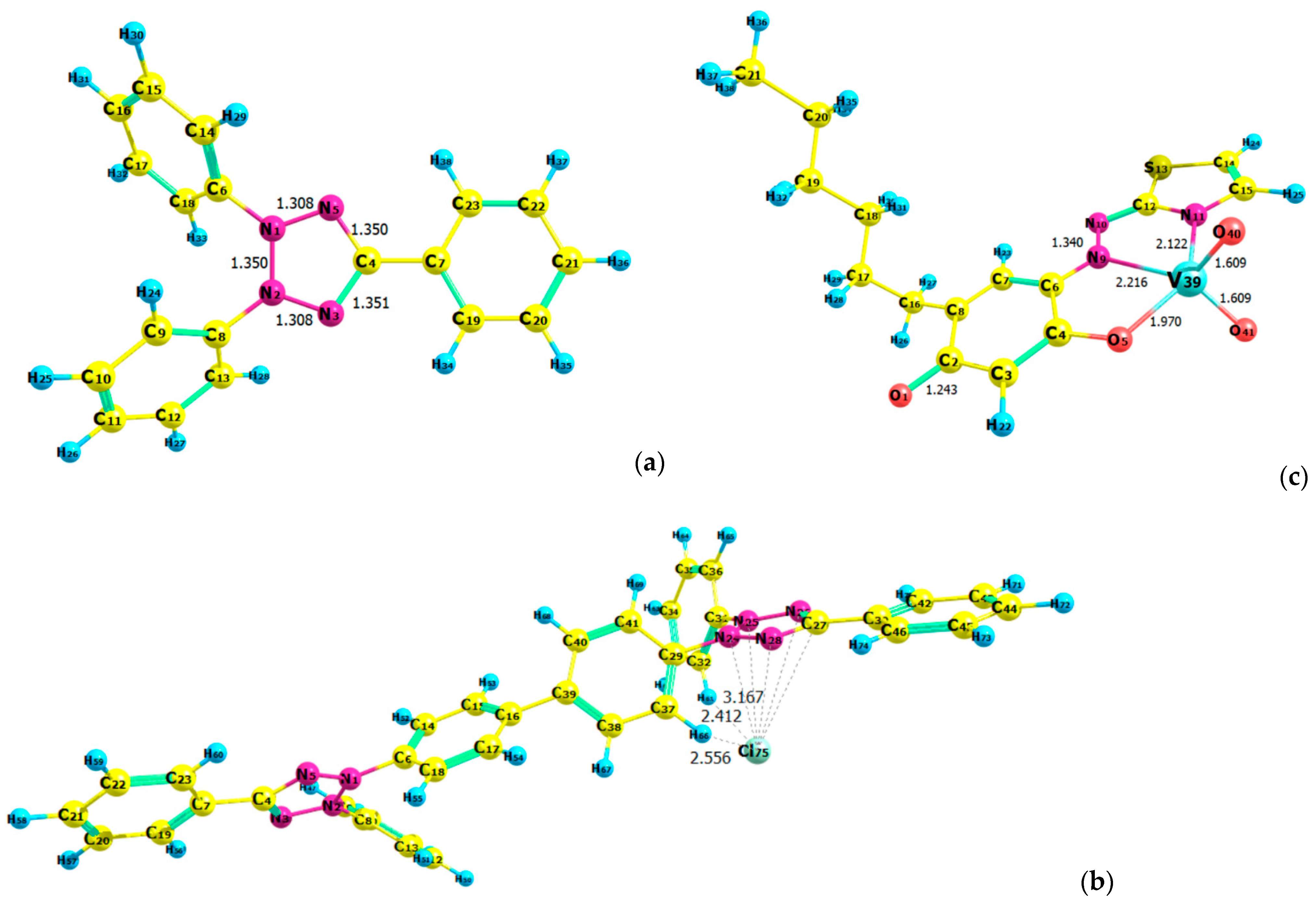
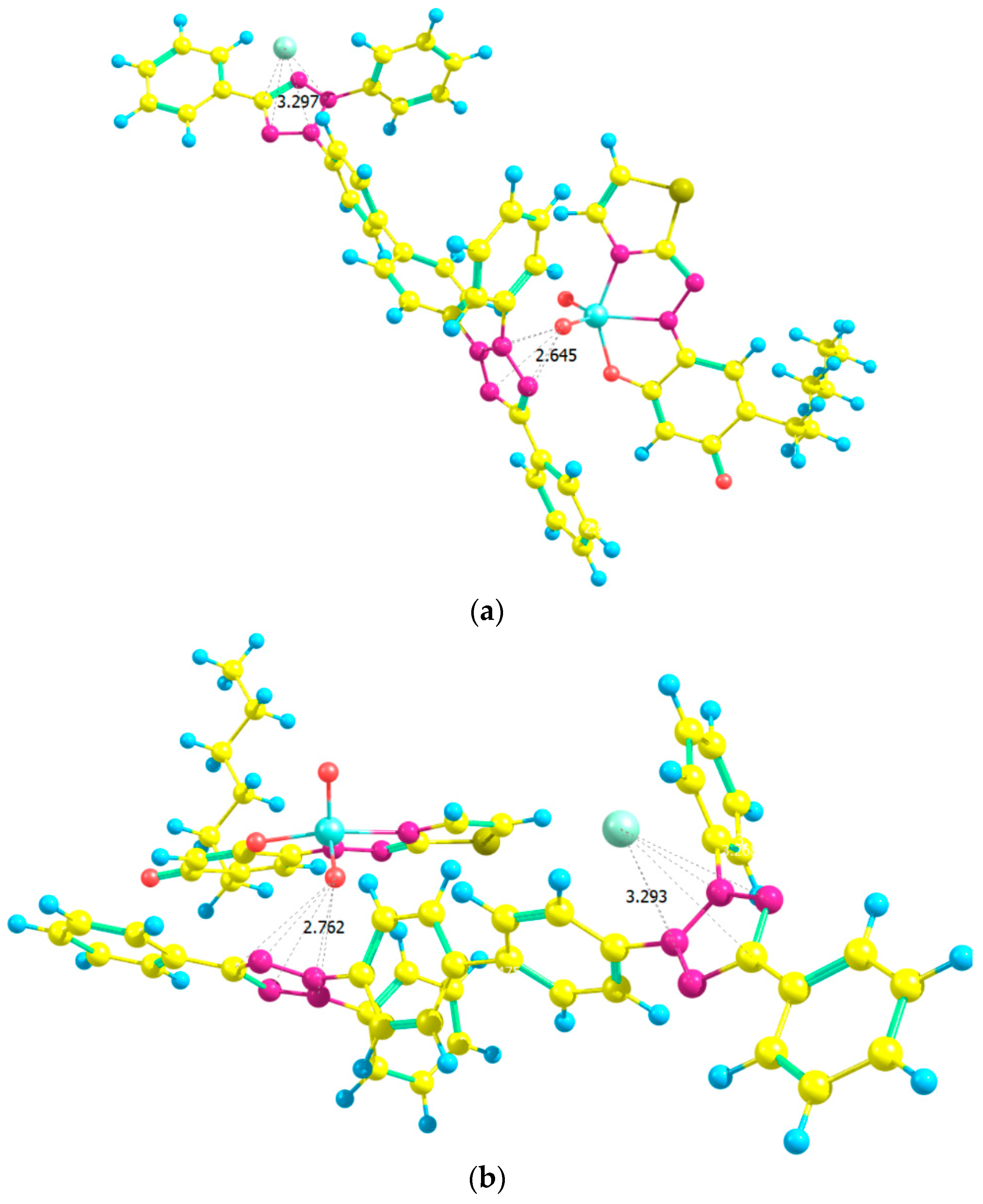
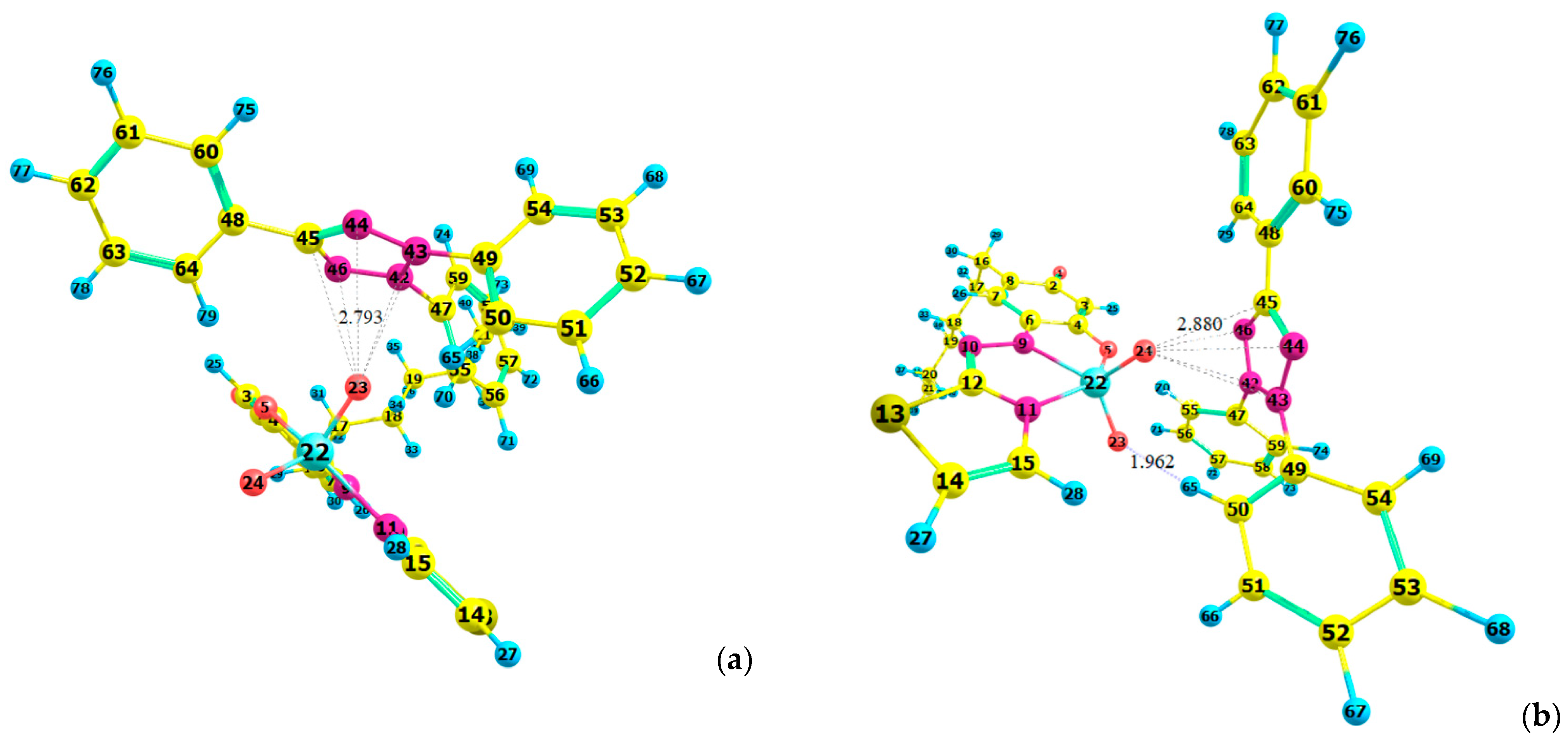
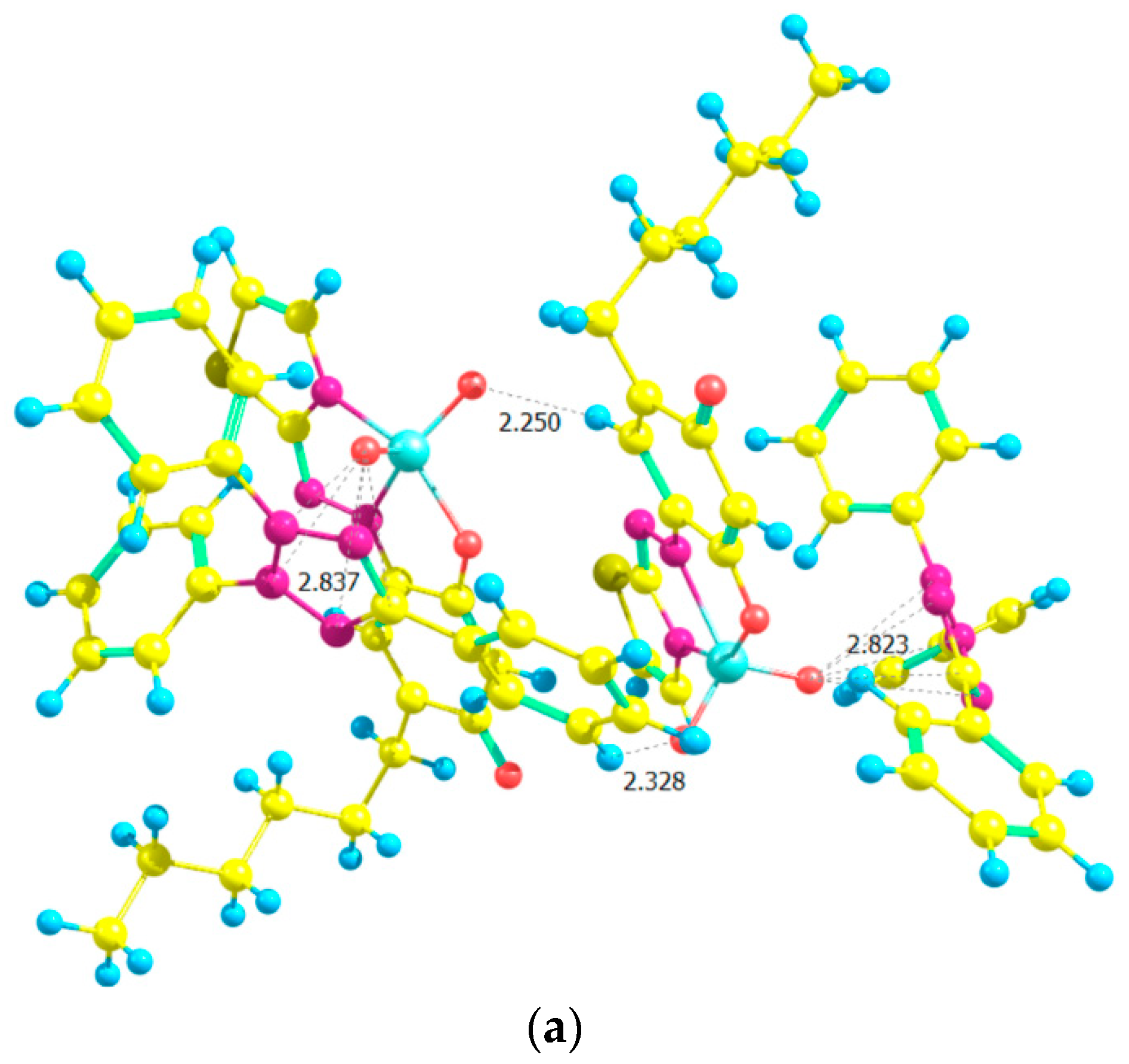
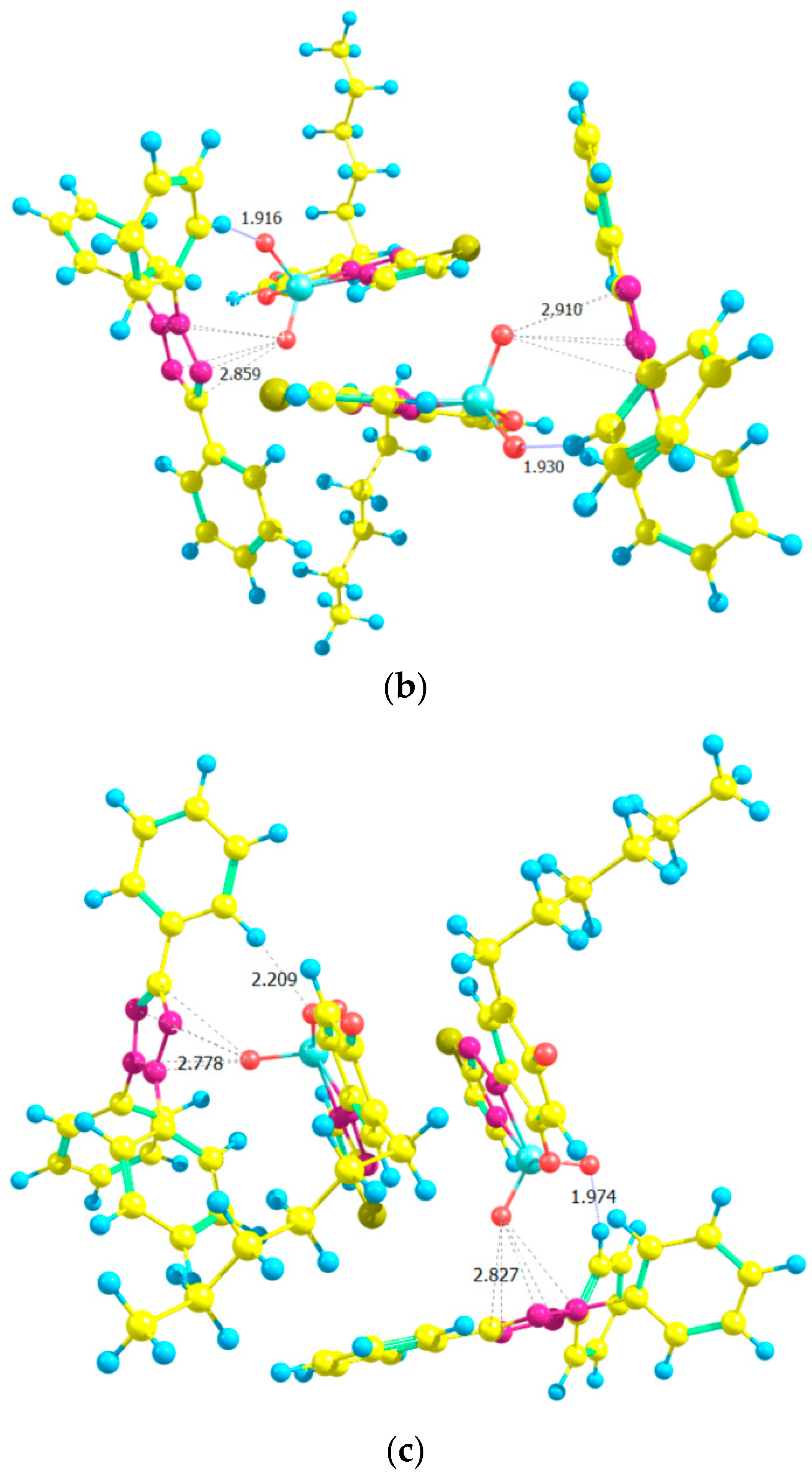
2.7. Ground-State Equilibrium Geometries of the Cations
2.8. Ground-State Equilibrium Geometries of the Complexes
2.9. Analytical Characteristics and Application
2.10. Comparison with Other Spectrophotometric Methods
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Instrumentation
3.3. Samples
3.4. Procedures for Optimization and Determination of Extraction Constants
3.5. Procedure for Determination of Distribution Ratios and Fractions Extracted
3.6. Procedure for Determination of Vanadium(V) with HTAR and TTC
3.7. Procedure for Determination of Vanadium(V) with HTAR and NTC
3.8. Theoretical
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rudnick, R.L.; Gao, S. Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 4, pp. 1–51. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A. Vanadium uptake and toxicity in plants. SF J. Agri. Crop Manag. 2020, 1, 1010. [Google Scholar]
- Hope, B.K. A dynamic model for the global cycling of anthropogenic vanadium. Glob. Biogeochem. Cycles 2008, 22, GB4021. [Google Scholar] [CrossRef]
- Watt, J.A.; Burke, I.T.; Edwards, R.A.; Malcolm, H.M.; Mayes, W.M.; Olszewska, J.P.; Pan, G.; Graham, M.C.; Heal, K.V.; Rose, N.L.; et al. Vanadium: A re-emerging environmental hazard. Environ. Sci. Technol. 2018, 52, 11973–11974. [Google Scholar] [CrossRef] [PubMed]
- Wlazłowska, E.; Grabarczyk, M. Adsorptive Stripping Voltammetry for Determination of Vanadium: A Review. Materials 2023, 16, 3646. [Google Scholar] [CrossRef]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Sci. Total Environ. 2009, 407, 853–868. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Sadeghi Rad, S.; Vilas–Boas, J.A.; Khataee, A. A global systematic review, meta-analysis, and risk assessment of the concentration of vanadium in drinking water resources. Chemosphere 2021, 267, 128904. [Google Scholar] [CrossRef]
- Rayner-Canham, G. Periodic patterns: The Group (n) and Group (n + 10) linkage. Found. Chem. 2013, 15, 229–237. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Rehder, D. Vanadium in health issues. ChemTexts 2018, 4, 20. [Google Scholar] [CrossRef]
- Taylor, M.J.C.; Staden, J.F. Spectrophotometric determination of vanadium(IV) and vanadium(V) in each other’s presence. Analyst 1994, 119, 1263–1276. [Google Scholar] [CrossRef]
- Pyrzyńska, K. Recent developments in spectrophotometric methods for determination of vanadium. Microchim. Acta 2005, 149, 159–164. [Google Scholar] [CrossRef]
- He, W.-Y.; Wang, K.-P.; Yang, J.-Y. Spectrophotometric methods for determination of vanadium: A review. Toxicol. Environ. Chem. 2018, 100, 20–31. [Google Scholar] [CrossRef]
- Kuliyev, K.A.; Verdizade, N.A. Spectroscopic investigation complex formation of vanadium using 2,6-dithiolphenol and hydrofob amins. Am. J. Chem. 2015, 5, 10–18. [Google Scholar]
- Temel, N.K.; Gürkan, R. Preconcentration and determination of trace vanadium(V) in beverages by combination of ultrasound assisted-cloud point extraction with spectrophotometry. Acta Chim. Slov. 2018, 65, 138–149. [Google Scholar] [CrossRef]
- Stefanova, T.S.; Simitchiev, K.K.; Gavazov, K.B. Liquid–liquid extraction and cloud point extraction for spectrophotometric determination of vanadium using 4-(2-pyridylazo) resorcinol. Chem. Pap. 2015, 69, 495–503. [Google Scholar] [CrossRef]
- Bychkova, O.A.; Zavodova, M.N.; Nikitina, T.G.; Nikonorov, V.V. Extraction–photometric determination of vanadium in natural waters. J. Anal. Chem. 2015, 70, 962–966. [Google Scholar] [CrossRef]
- Singh, S.; Nivedita, A.; Parveen, R.; Rajesh, A.; Vikas, K. Molecular dynamics, biological study and extractive spectrophotometric determination of vanadium (V)-2-methyl-8-quinolinol complex. Iran. J. Chem. Chem. Eng. 2021, 40, 207–214. [Google Scholar] [CrossRef]
- Pillai, S.G.; Dwivedi, A.H.; Patni, N. Liquid-liquid extraction and spectrophotometric determination of vanadium(V) with p-carboxy-n-phenyl-calix[4]resorcinarene-hydroxamic acid. Procedia Eng. 2013, 51, 347–354. [Google Scholar] [CrossRef]
- Hristov, D.; Milcheva, N.; Gavazov, K. Extraction-chromogenic systems for vanadium(V) based on azo dyes and xylometazoline hydrochloride. Acta Chim. Slov. 2019, 66, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Kirova, G.K.; Velkova, Z.Y.; Delchev, V.B.; Gavazov, K.B. Vanadium-Containing Anionic Chelate for Spectrophotometric Determination of Hydroxyzine Hydrochloride in Pharmaceuticals. Molecules 2023, 28, 2484. [Google Scholar] [CrossRef] [PubMed]
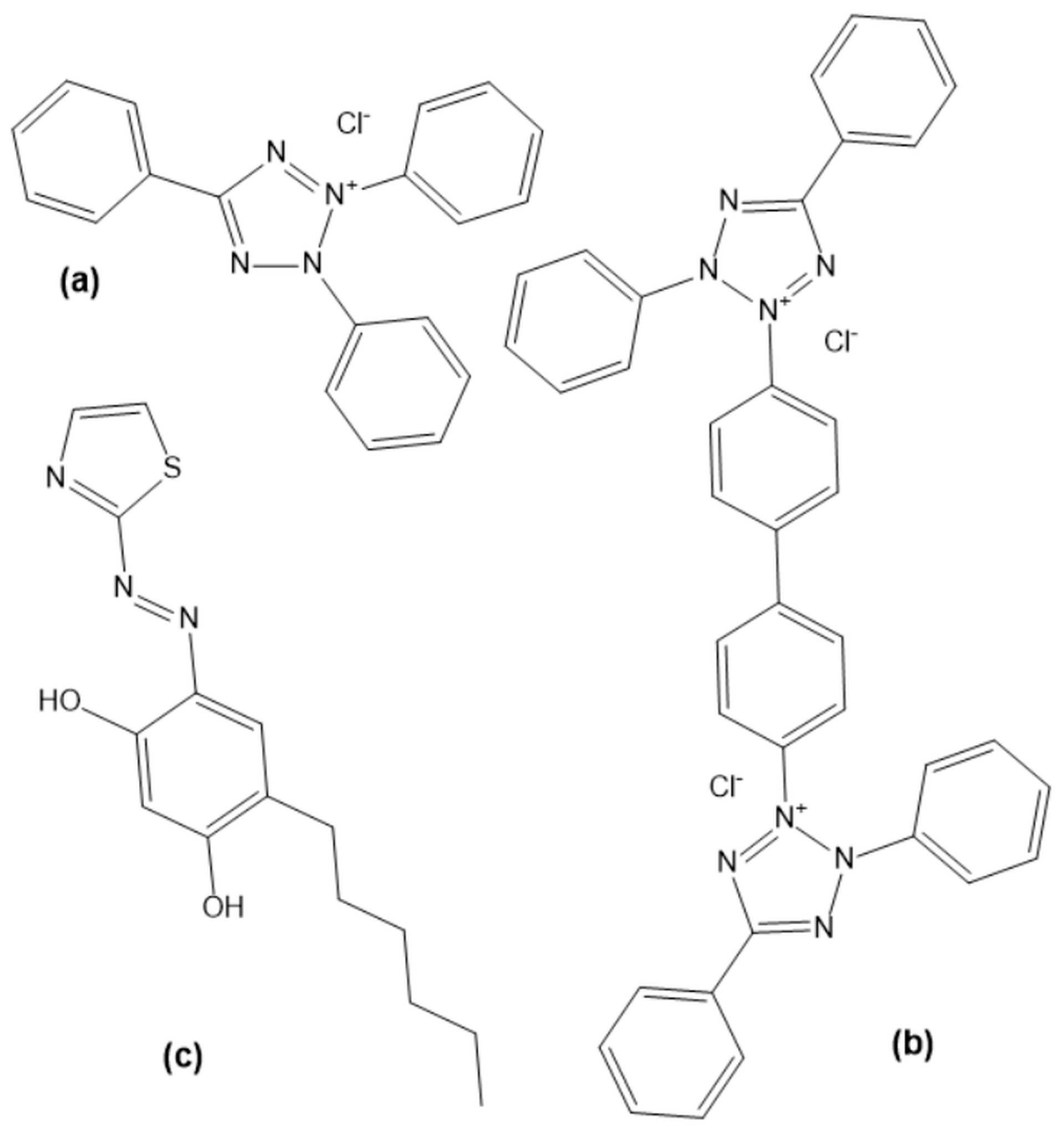
- Daniel, D.S. The chemistry of tetrazolium salts. In Chemistry and Applications of Leuco Dyes; Muthyala, R., Ed.; Kluwer Academic Publishers: New York, NY, USA, 2002; pp. 207–296. [Google Scholar] [CrossRef]
- Gavazov, K.B.; Dimitrov, A.N.; Lekova, V.D. The use of tetrazolium salts in inorganic analysis. Russ. Chem. Rev. 2007, 76, 169–179. [Google Scholar] [CrossRef]
- Creanga, D.; Nadejde, C. Molecular modelling and spectral investigation of some triphenyltetrazolium chloride derivatives. Chem. Pap. 2014, 68, 260–271. [Google Scholar] [CrossRef]
- Voitekhovich, S.V.; Lesnyak, V.; Gaponik, N.; Eychmüller, A. Tetrazoles: Unique Capping Ligands and Precursors for Nanostructured Materials. Small 2015, 11, 5728–5739. [Google Scholar] [CrossRef]
- Penev, K.I.; Wang, M.; Mequanint, K. Tetrazolium salt monomers for gel dosimetry I: Principles. J. Phys. Conf. Ser. 2017, 847, 012048. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Xu, X. Inhibition action of triazolyl blue tetrazolium bromide on cold rolled steel corrosion in three chlorinated acetic acids. J. Mol. Liq. 2019, 274, 77–89. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Ghabbour, H.A.; Hussain, H.; Al-Salahi, R.; Ali, E.A.; Mostafa, G.A.E. Synthesis and computational and X-ray structure of 2,3,5-triphenyl tetrazolium, 5-ethyl-5-phenylbarbituric acid salt. Crystals 2022, 12, 1706. [Google Scholar] [CrossRef]
- Dawi, E.A.; Mustafa, E.; Padervand, M.; Ashames, A.; Hajiahmadi, S.; Saleem, L.; Baghernejad, M.; Nur, O.; Willander, M. Ag/AgCl decorated ionic liquid@tantalum pentoxide nanostructures: Fabrication, photocatalytic activity, and cytotoxicity effects against human brain tumor cells. J. Inorg. Organomet. Polym. 2023. [Google Scholar] [CrossRef]
- Gavazov, K.B.; Toncheva, G.K. Ion-association between some ditetrazolium cations and anions originated from 4-(2-thiazolylazo)resorcinol or 4-(2-pyridylazo)resorcinol. Russ. J. Gen. Chem. 2015, 85, 192–197. [Google Scholar] [CrossRef]
- Alexandrov, A.; Simeonova, Z.; Kamburova, M. A relationship between association constants and the molecular mass of tetrazolium ion association complexes. Bulg. Chem. Commun. 1990, 23, 542–544. [Google Scholar]
- Yuzhakov, V.I. Association of dye molecules and its spectroscopic manifestation. Russ. Chem. Rev. 1979, 48, 1076–1091. [Google Scholar] [CrossRef]
- Konermann, L. Addressing a common misconception: Ammonium acetate as neutral pH “buffer” for native electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Zhiming, Z.; Dongsten, M.; Cunxiao, Y. Mobile equilibrium method for determining composition and stability constant of coordination compounds of the form MmRn. J. Rare Earths 1997, 15, 216–219. [Google Scholar]
- Galan, T.R.; Ramirez, A.A.; Ceba, M.R. A new spectrophotometric method for differentiating mononuclear and polynuclear complexes and for determining their extraction constants. Talanta 1980, 27, 545–547. [Google Scholar] [CrossRef]
- Job, P. Recherches sur la formation de complexes minéraux en solution, et sur leur stabilité. Ann. Chim. 1928, 9, 113–134. [Google Scholar]
- Klausen, K.S.; Langmyhr, F.J. The use of the method of continuous variation for the classification of complexes with mole ratio 1:1. Anal. Chim. Acta 1963, 28, 335–340. [Google Scholar] [CrossRef]
- Asmus, E. Eine neue Methode zur Ermittlung der Zusammensetzung schwacher Komplexe (A new method for the determination of composition of weak complexes). Fresenius’ J. Anal. Chem. 1960, 178, 104–116. [Google Scholar] [CrossRef]
- Bent, H.E.; French, C.L. The structure of ferric thiocyanate and its dissociation in aqueous solution. J. Am. Chem. Soc. 1941, 63, 568–572. [Google Scholar] [CrossRef]
- Sayago, A.; Boccio, M.; Asuero, A.G. Continuous variation data: 1:1 or 2:2 weak complexes? Int. J. Pharm. 2005, 295, 29–34. [Google Scholar] [CrossRef]
- Gavazov, K.B.; Toncheva, G.K.; Delchev, V.B. An extraction-chromogenic system for vanadium(IV,V) based on 2,3-dihydroxynaphtahlene. Open Chem. 2016, 14, 197–205. [Google Scholar] [CrossRef][Green Version]
- Gavazov, K.B.; Delchev, V.B.; Mileva, K.T.; Stefanova, T.S.; Toncheva, G.K. A 2:2:2 complex of vanadium(V) with 4-(2-thiazolylazo)orcinol and 2,3,5-triphenyl-2H-tetrazolium chloride. Acta Chim. Slov. 2016, 63, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Gavazov, K.B.; Stefanova, T.S. Liquid-liquid extraction-spectrophotometric investigations of three ternary complexes of vanadium. Croat. Chem. Acta 2014, 87, 233–240. [Google Scholar] [CrossRef]
- Tôei, K. Ion-association reagents. A review. Anal. Sci. 1987, 3, 479–488. [Google Scholar] [CrossRef]
- Racheva, P.V.; Milcheva, N.P.; Saravanska, A.D.; Gavazov, K.B. Extraction of gallium(III) with a new azo dye in the presence or absence of xylometazoline hydrochloride. Croat. Chem. Acta 2020, 93, 159–165. [Google Scholar] [CrossRef]
- Racheva, P.V.; Milcheva, N.P.; Genc, F.; Gavazov, K.B. A centrifuge-less cloud point extraction-spectrophotometric determination of copper(II) using 6-hexyl-4-(2-thiazolylazo)resorcinol. Spectrochim. Acta Mol. Biomol. Spectrosc. 2021, 262, 120106. [Google Scholar] [CrossRef]
- Likussar, W.; Boltz, D.F. Theory of continuous variations plots and a new method for spectrophotometric determination of extraction and formation constants. Anal. Chem. 1971, 43, 1265–1272. [Google Scholar] [CrossRef]
- Holme, A.; Langmyhr, F.J. A modified and a new straight-line method for determining the composition of weak complexes of the form AmBn. Anal. Chim. Acta 1966, 36, 383–391. [Google Scholar] [CrossRef]
- Harvey, A.E.; Manning, D.L. Spectrophotometric methods of establishing empirical formulas of colored complexes in solution. J. Am. Chem. Soc. 1950, 72, 4488–4493. [Google Scholar] [CrossRef]
- Gjikaj, M.; Xie, T.; Brockner, W. Uncommon compounds in antimony pentachloride—Ionic liquid systems: Synthesis, crystal structure and vibrational spectra of the complexes [TPT][SbCl6] and [Cl-EMIm][SbCl6]. Z. Anorg. Allg. Chem. 2009, 635, 1036–1040. [Google Scholar] [CrossRef]
- Stefanova-Bahchevanska, T.; Milcheva, N.; Zaruba, S.; Andruch, V.; Delchev, V.; Simitchiev, K.; Gavazov, K. A green cloud-point extraction-chromogenic system for vanadium determination. J. Mol. Liq. 2017, 248, 135–142. [Google Scholar] [CrossRef]
- Mortada, W.I.; Zedan, H.E.; Khalifa, M.E. Spectrophotometric determination of trace vanadium in fresh fruit juice samples by ion pair-based surfactant-assisted microextraction procedure with solidification of floating organic drop. Spectrochim. Acta Mol. Biomol. Spectrosc. 2023, 302, 123107. [Google Scholar] [CrossRef] [PubMed]
- Temel, N.K.; Kuş, B.; Gürkan, R. A new ion-pair ultrasound assisted-cloud point extraction approach for determination of trace V(V) and V(IV) in edible vegetal oils and vinegar by spectrophotometry. Microchem. J. 2019, 150, 104139. [Google Scholar] [CrossRef]
- Peroković, V.P.; Ivšić, A.G.; Car, Ž.; Tomić, S. Synthesis of 3-hydroxy-1-(p-methoxyphenyl)-2-methylpyridine-4-one and spectrophotometric extraction studies on its complexation of vanadium(V). Croat. Chem. Acta 2014, 87, 103–109. [Google Scholar] [CrossRef]
- Milcheva, N.P.; Genç, F.; Racheva, P.V.; Delchev, V.B.; Andruch, V.; Gavazov, K.B. An environmentally friendly cloud point extraction–spectrophotometric determination of trace vanadium using a novel reagent. J. Mol. Liq. 2021, 334, 116086. [Google Scholar] [CrossRef]
- Agnihotri, R.; Agnihotri, N.; Kumar, V.; Kamal, R. Synthesis and application of 3-hydroxy-2-[3-(4-methoxyphenyl)-1-phenyl-4-pyrazolyl]-4-oxo-4h-1-benzopyran for extractive spectrophotometric determination of vanadium (V). Der Chem. Sin. 2017, 8, 158–165. [Google Scholar]
- Gavazov, K.B.; Lekova, V.D.; Dimitrov, A.N.; Patronov, G.I. The solvent extraction and spectrophotometric determination of vanadium(V) with 4-(2-thiazolylazo)-resorcinol and tetrazolium salts. Cent. Eur. J. Chem. 2007, 5, 257–270. [Google Scholar] [CrossRef]
- Khazal, H.; Mohammed, H.J.; Khazal, F. Spectrophotometric determination of trace amount of Vanadium in rise and flour using 1-[(4-antipyl azo)] 2-Naphthol as new chromogenic spectrophotometry. J. Phys. Conf. Ser. 2020, 1660, 012024. [Google Scholar] [CrossRef]
- Pasha, C.; Sunil, K.; Stancheva, K. Crystal violet—A new reagent used for the spectrophotometric determination of vanadium. Oxid. Commun. 2022, 45, 503–513. [Google Scholar]
- Gavazov, K.B.; Racheva, P.V.; Milcheva, N.P.; Divarova, V.V.; Kiradzhiyska, D.D.; Genç, F.; Saravanska, A.D. Use of a hydrophobic azo dye for the centrifuge-less cloud point extraction–spectrophotometric determination of cobalt. Molecules 2022, 27, 4725. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- ChemCraft. Available online: http://www.chemcraftprog.com (accessed on 13 September 2023).
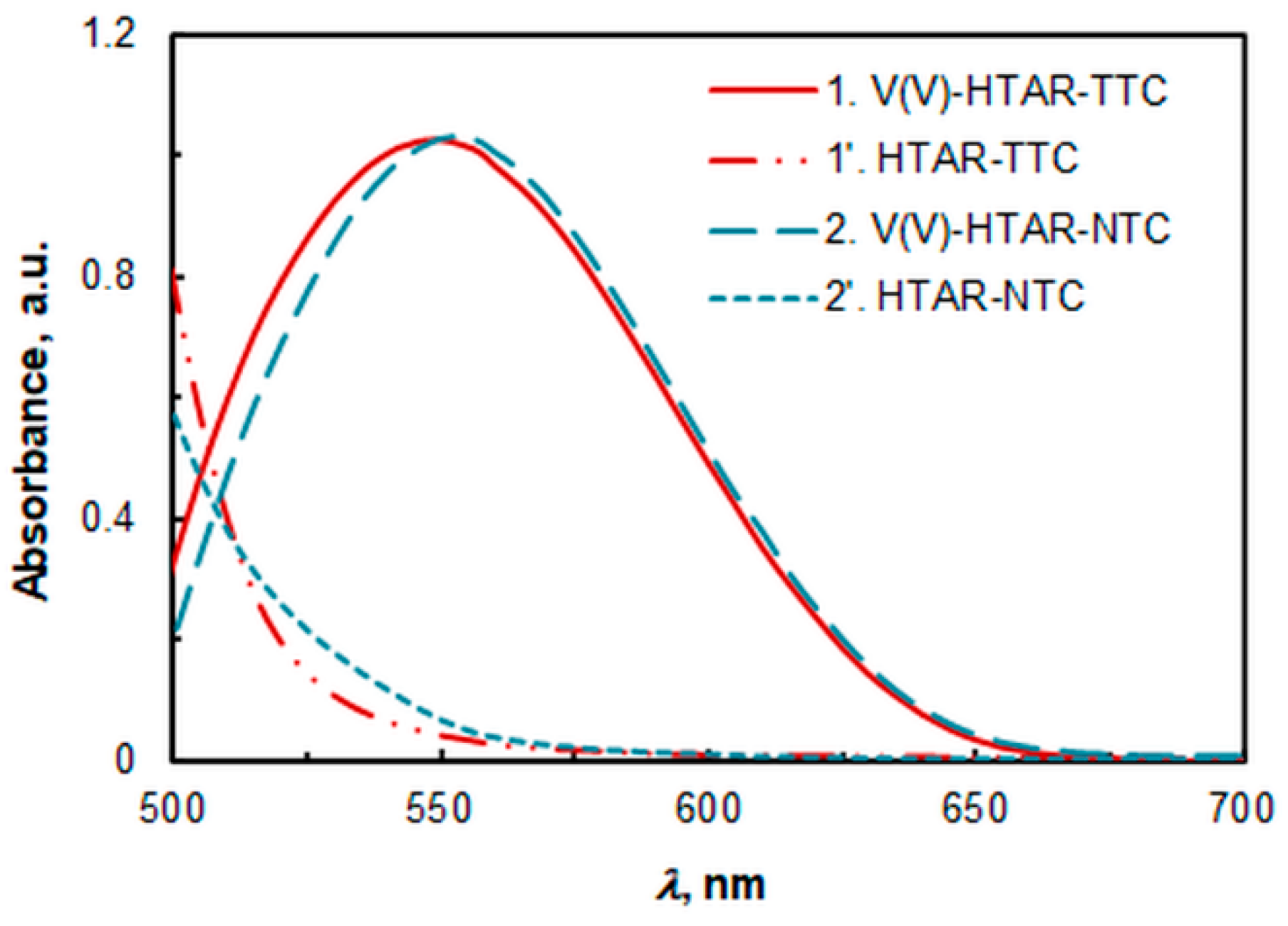
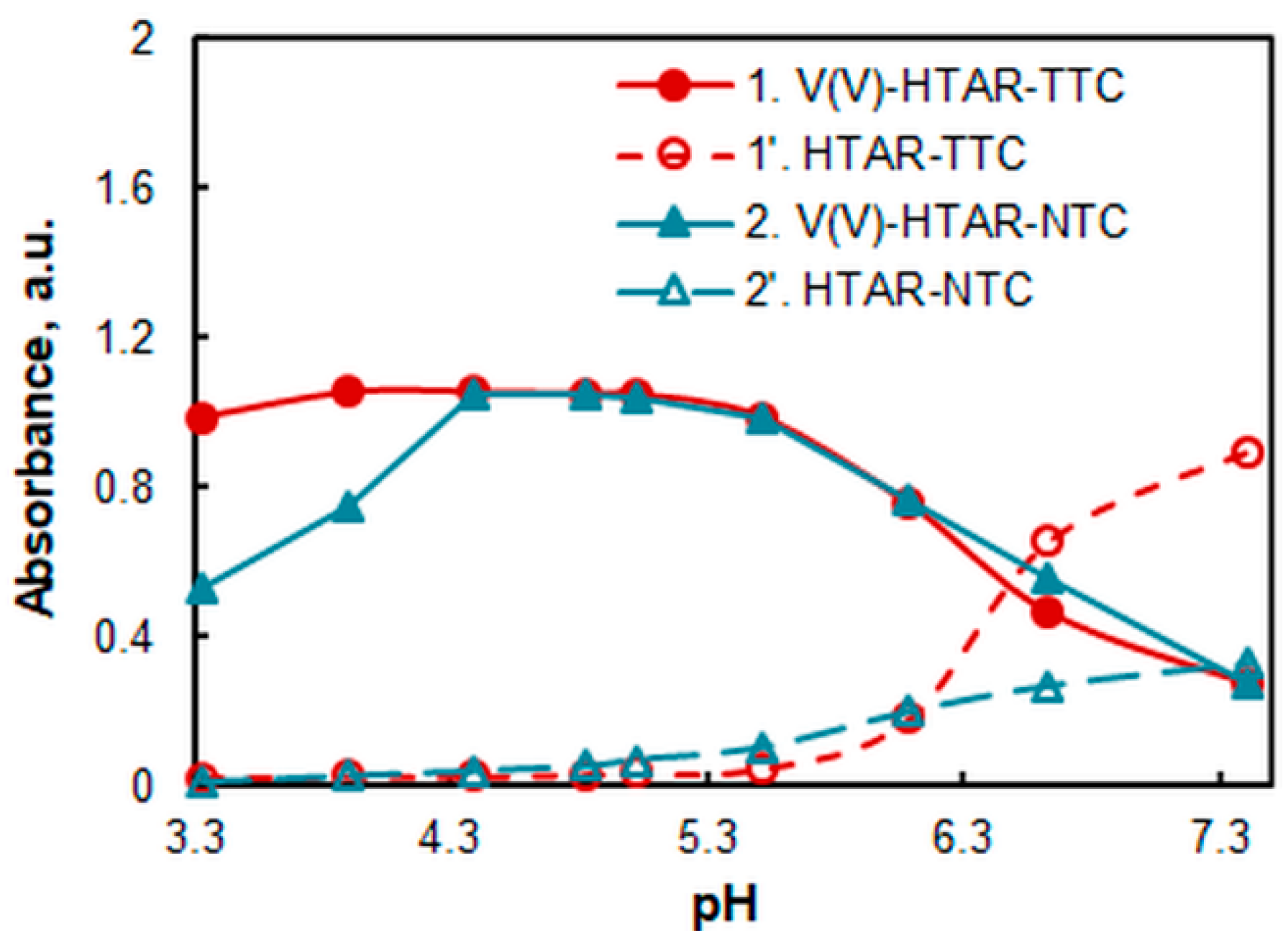



| Extraction System | λmax, nm | pH b | cHTAR, mol L−1 | cTS, mol L−1 | Extraction Time, min |
|---|---|---|---|---|---|
| V(V)–HTAR–TTC | 549 | 4.7 | 8 × 10−5 | 2.4 × 10−4 | 2.5 |
| V(V)–HTAR–NTC | 556 | 4.7 | 4 × 10−5 | 1.4 × 10−4 | 3.0 |
| Molar Ratio | Mobile Equilibrium Method [36] a | Dilution Method [37] a | Job’s Method [38,39] b | Asmus’ Method [40] c | Bent–French Method [41] c |
|---|---|---|---|---|---|
| HTAR:V (TS = TTC) | 2:2 | – | – | 1:1 | 1:1 |
| HTAR:V (TS = NTC) | 1:1 | – | – | 1:1 | 1:1 |
| TTC:V | 2:2 | 2:2 | n:n (n > 1) | 1:1 | 1:1 |
| NTC:V | 1:1 | – | 1:1 | 1:1 |
| Extraction System | Log Kex | Log D | %E | ||||
|---|---|---|---|---|---|---|---|
| Mobile Equilibrium Method [36] | Dilution Method [37] | Likussar–Boltz Method [49] | Holme–Langmyhr Method [50] | Harvey–Manning Method [51] | |||
| V(V)—HTAR—TTC | 15.2 ± 0.4 a | 15.8 ± 0.1 c | 15.2 ± 0.1 d 15.1 ± 0.1 e | – | – | 1.53 ± 0.08 h | 97.1 ± 0.5 h |
| V(V)—HTAR—NTC | 5.0 ± 0.1 b | – | 5.1 ± 0.1 f | 5.0 ± 0.1 g | 5.0 ± 0.1 h | 0.94 ± 0.14 i | 90 ± 3 i |
| Characteristics | HTAR–TTC System | HTAR–NTC System |
|---|---|---|
| Linear regression equation y = ax + b | y = 1.020x + 0.0003 | y = 1.018x − 0.005 |
| Correlation coefficient | 0.9990 (N = 11) | 0.9996 (N = 8) |
| Standard deviations of the slope (a) and y-intercept (b) | 0.015 and 0.017 | 0.012 and 0.008 |
| Linear range, µg mL−1 | 0.015−2.0 | 0.023−1.1 |
| Molar absorptivity coefficient (ε), L mol−1 cm−1 | 5.2 × 104 | 5.2 × 104 |
| Sandell’s sensitivity, ng cm−2 | 0.98 | 0.98 |
| Limit of detection (LOD) a, ng mL−1 | 4.6 | 6.8 |
| Limit of quantitation (LOQ) b, ng mL−1 | 15 | 23 |
| Foreign Ion (FI) Added | Added Salt Formula | HTAR–TTC System | HTAR–NTC System | ||||
|---|---|---|---|---|---|---|---|
| FI:V(V) Mass Ratio | Amount of V(V) Found, μg | R, % | FI:V(V) Mass Ratio | Amount of V(V) Found, μg | R, % | ||
| Al(III) | Al2(SO4)3·7H2O | 1000 | 5.22 | 104 | 500 | 4.84 | 96.7 |
| Ba(II) | Ba(NO3)2 | 2000 a | 4.84 | 96.8 | 100 1000 | 5.01 4.42 | 100 88.5 |
| Br− | NaBr | 2000 a | 4.93 | 98.6 | 250 | 4.93 | 98.5 |
| Ca(II) | Ca(NO3)2·4H2O | 2000 a | 5.01 | 100 | 500 | 4.91 | 98.2 |
| Cd(II) | 3CdSO4·8H2O | 5 500 | 5.13 5.89 | 103 118 | 50 500 | 5.24 4.97 | 105 99.4 |
| Cl− | NaCl | 10,000 a | 5.02 | 100 | 250 2000 | 4.81 4.58 | 96.3 91.6 |
| Co(II) | CoSO4·7H2O | 0.5 | 5.16 | 103 | 0.5 | 5.24 | 105 |
| Cr(III) | Cr2(SO4)3 | 4 | 4.83 | 96.7 | 5 | 5.19 | 104 |
| Cr(VI) | KCrO4 | 500 | 4.87 | 97.4 | 250 | 4.94 | 98.7 |
| Cu(II) | CuSO4·5H2O | 0.5 | 4.84 | 96.7 | 1 | 5.10 | 102 |
| F− | NaF | 500 1000 | 4.84 4.54 | 96.7 90.7 | 2000 5000 | 5.02 4.40 | 100 88.0 |
| Fe(III) | Fe2(SO4)3 | 0.5 | 5.28 | 106 | 0.5 10 b 50 b | 5.42 5.10 5.40 | 108 102 108 |
| HPO42− | Na2HPO4 | 2000 | 5.02 | 100 | 2000 a | 5.17 | 103 |
| Hg(II) | Hg(NO3) | 10 | 5.23 | 105 | 100 | 5.14 | 103 |
| I− | KI | 1000 | 4.84 | 96.9 | 10 | 4.30 | 86.1 |
| Mg(II) | MgCl2 | 1000 | 4.73 | 94.7 | 2000 a | 5.06 | 101 |
| Mn(II) | MnSO4·H2O | 100 500 | 5.04 5.32 | 101 106 | 500 | 5.23 | 105 |
| Mo(VI) | (NH4)6Mo7O24·4H2O | 250 | 4.88 | 97.7 | 10 | 4.74 | 94.9 |
| NO3− | NH4NO3 | 2000 a | 5.10 | 102 | 1000 | 4.71 | 94.4 |
| Ni(II) | NiSO4·6H2O | 0.5 | 5.15 | 103 | 0.5 | 5.10 | 102 |
| Pb(II) | Pb(NO3)2 | 10 | 4.81 | 96.3 | 20 | 4.99 | 99.8 |
| Re(VII) | NH4ReO4 | 250 500 | 4.97 4.71 | 99.4 94.1 | 5 100 | 4.97 4.24 | 99.4 84.7 |
| Tartrate2− | KNaC4H4O6 | 100 | 4.63 | 92.6 | 100 | 3.63 | 72.6 |
| V(IV) | VOSO4·5H2O | 1 | 7.29 | 146 | 1 | 7.10 | 142 |
| W(VI) | Na2WO4·2H2O | 1 | 4.65 | 92.9 | 1 | 4.91 | 98.3 |
| Zn(II) | ZnSO4·7H2O | 500 | 5.06 | 101 | 2000 a | 4.83 | 96.7 |
| Catalyst | Vanadium Found, % | RSD, % | ||
|---|---|---|---|---|
| HTAR—TTC Method | Alternative Method b | HTAR—TTC Method | Alternative Method b | |
| Sample 1 | 2.96 ± 0.07 | 2.89 ± 0.12 | 2.4 | 4.2 |
| Sample 2 | 2.42 ± 0.06 | 2.48 ± 0.11 | 2.5 | 4.4 |
| Sample 3 | 2.99 ± 0.08 | 3.06 ± 0.11 | 2.7 | 3.6 |
| Sample 4 | 1.95 ± 0.04 | 2.03 ± 0.09 | 2.1 | 4.4 |
| Sample | V(V) Spike, ng mL−1 | V(V) Found, ng mL−1 | RSD, % | R, % |
|---|---|---|---|---|
| Sterimar nasal spray | 0 | Not detected | – | – |
| 25 | 24.3 ± 1.1 | 4.5 | 97.2 | |
| 50 | 51.7 ± 1.3 | 2.5 | 103 | |
| 75 | 73.9 ± 1.5 | 2.0 | 98.5 | |
| Marimer inhalation | 0 | Not detected | – | – |
| 25 | 26.3 ± 1.2 | 4.6 | 105 | |
| 50 | 49.4 ± 1.8 | 3.6 | 98.8 | |
| 75 | 75.2 ± 2.4 | 3.2 | 100 | |
| Solution for intravenous infusion | 0 | Not detected | – | – |
| 25 | 25.6 ± 0.8 | 3.1 | 102 | |
| 50 | 50.6 ± 1.3 | 2.6 | 101 | |
| 75 | 74.0 ± 2.3 | 3.1 | 98.6 |
| Reagent(s) | Preconcentration Technique | λmax, nm | ε × 10−4, L mol−1 cm−1 | Linear Range, μg mL−1 | LOD, ng mL−1 | Application | Ref. |
|---|---|---|---|---|---|---|---|
| APANOL | – | 533 | 1.02 | 0.1–4.00 | 54 | Rise and flour | [60] |
| BPHA | LLE | 530 | 0.545 | Up to 1.5 | – | Water samples | [19] |
| CV + KI | – | 588 | 1.27 | 0.1–10.2 | 675 | Soil, biological, and pharmaceutical samples | [61] |
| DTP + Amines | LLE | 615–620 | 2.8–3.0 | 0.05–16 | – | Soils, oil, and oil products | [16] |
| EDTA + ST | UA-CPE | 530 | – | 0.002–0.18 | 0.26 | Vegetal oils and vinegar | [55] |
| HPMEPB | LLE | 415 | 2.7 | Up to 2.2 | 79.2 | Synthetic and technical samples | [58] |
| MQ | LLE | 400 | 0.2449 | Up to 6.2 | 168 | – | [20] |
| PCNPC4RAHA | LLE | 495 | 0.554 | 0.137–9.36 | 8.89 | Steels, environmental and biological samples | [21] |
| PG + ST | UA-CPE | 533 | – | 0.002–0.5 | 0.58 | Beverage samples | [17] |
| TA + CTAB | DLLME-SFOD | 600 | – | 0.006–1 | 1.8 | Fruit juice samples | [54] |
| TAN + H2O2 | MA-CPE | 607 | 8.84 | Up to 0.76 | 1.4 | Mineral water, pharmaceutical, and industrial samples | [53] |
| HTAR + NTC | LLE | 556 | 5.2 | 0.023–1.1 | 6.8 | – | This work |
| HTAR + TTC | LLE | 549 | 5.2 | 0.015–2.0 | 4.6 | Catalysts and pharmaceuticals | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavazov, K.B.; Racheva, P.V.; Saravanska, A.D.; Toncheva, G.K.; Delchev, V.B. Extractive Spectrophotometric Determination and Theoretical Investigations of Two New Vanadium(V) Complexes. Molecules 2023, 28, 6723. https://doi.org/10.3390/molecules28186723
Gavazov KB, Racheva PV, Saravanska AD, Toncheva GK, Delchev VB. Extractive Spectrophotometric Determination and Theoretical Investigations of Two New Vanadium(V) Complexes. Molecules. 2023; 28(18):6723. https://doi.org/10.3390/molecules28186723
Chicago/Turabian StyleGavazov, Kiril B., Petya V. Racheva, Antoaneta D. Saravanska, Galya K. Toncheva, and Vasil B. Delchev. 2023. "Extractive Spectrophotometric Determination and Theoretical Investigations of Two New Vanadium(V) Complexes" Molecules 28, no. 18: 6723. https://doi.org/10.3390/molecules28186723
APA StyleGavazov, K. B., Racheva, P. V., Saravanska, A. D., Toncheva, G. K., & Delchev, V. B. (2023). Extractive Spectrophotometric Determination and Theoretical Investigations of Two New Vanadium(V) Complexes. Molecules, 28(18), 6723. https://doi.org/10.3390/molecules28186723