Application of Iodine as a Catalyst in Aerobic Oxidations: A Sustainable Approach for Thiol Oxidations
Abstract
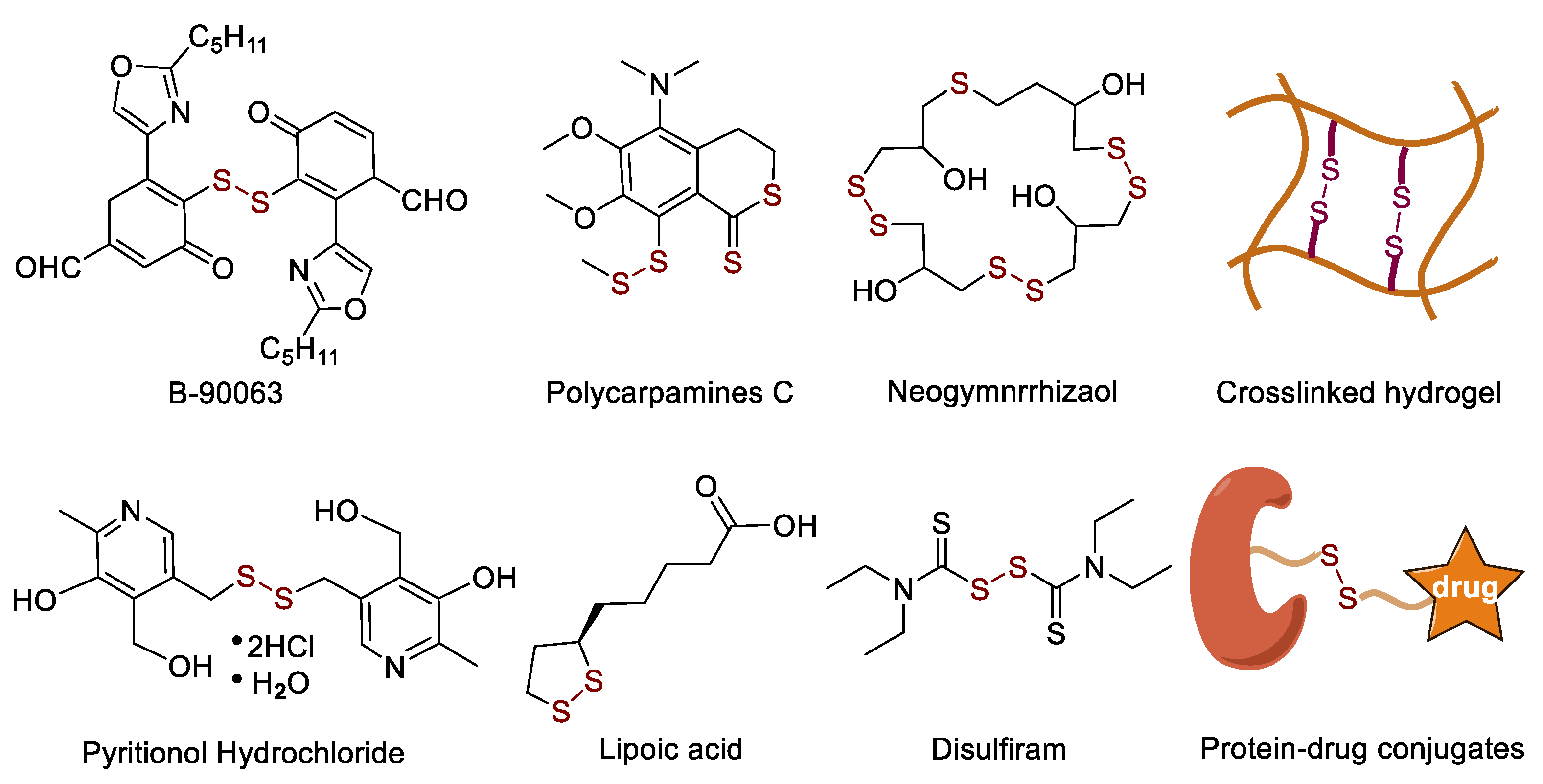
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Calculation of the Yield by Internal Standard Using 1H NMR
3.3. Optimization Studies for the Oxidative Coupling of Thiols (Table 1)
3.4. General Procedure for the Oxidative Coupling of Thiols
- 1,2-Didodecyldisulfane (2a) [38]. According to the general procedure, the oxidation of dodecane-1-thiol (60.7 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane), afforded 59.2 mg of 2a in 98% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 2.68 (t, J = 7.4 Hz, 4H), 1.67 (m, 4H), 1.38 (m, 4H), 1.31–1.22 (m, 32H), 0.88 (t, J = 6.9 Hz, 6H); 13C{1H} NMR (126 MHz, CDCl3) δ 39.3, 32.0, 29.7 (×3), 29.6, 29.4, 29.3 (×2), 28.6, 22.8, 14.2.
- 1,2-Diphenethyldisulfane (2b) [39]. According to the general procedure, the oxidation of 2-phenylethane-1-thiol (41.5 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–12.5% EtOAc/Hexane) afforded 40.3 mg of 2b in 98% yield as a yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.29 (m, 4H), 7.23–7.16 (m, 6H), 3.01–2.95 (m, 4H), 2.95–2.90 (m, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 140.1, 128.7, 128.6, 126.5, 40.3, 35.8.
- 1,2-Dibenzyldisulfane (2c) [40]. According to the general procedure, the oxidation of phenylmethanethiol (37.3 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa), after chromatography (0–10% EtOAc/Hexane), afforded 36.2 mg of 2c in a 98% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.33–7.28 (m, 4H), 7.28–7.25 (m, 2H), 7.25–7.21 (m, 4H), 3.59 (s, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 137.4, 129.5, 128.6, 127.5, 43.4.
- 1,2-Dicyclohexyldisulfane (2d) [41]. According to the general procedure, the oxidation of cyclohexanethiol (34.9 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 33.9 mg of 2d in 98% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 2.68 (m, 2H), 2.12–1.94 (m, 4H), 1.86–1.71 (m, 4H), 1.67–1.52 (m, 2H), 1.39–1.16 (m, 10H); 13C{1H} NMR (126 MHz, CDCl3) δ 50.1, 32.9, 26.2, 25.8.
- 1,2-Bis(4-fluorobenzyl)disulfane (2e) [42]. According to the general procedure, the oxidation of (4-fluorophenyl)methanethiol (42.6 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 41.5 mg of 2e in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.19 (m, 4H), 7.01 (m, 4H), 3.58 (s, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 162.3 (d, JC-F = 246.4 Hz), 133.2 (d, JC-F = 3.1 Hz), 131.0 (d, J C-F= 8.1 Hz), 115.5 (d, JC-F= 21.5 Hz), 42.5.
- 1,2-Bis(4-chlorobenzyl)disulfane (2f) [43]. According to the general procedure, the oxidation of (4-chlorophenyl)methanethiol (47.6 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 46.3 mg of 2f in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.29 (m, 4H), 7.15 (m, 4H), 3.57 (s, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 135.9, 133.5, 130.7, 128.7, 42.6.
- 1,2-Bis(2-chlorobenzyl)disulfane (2g) [38]. According to the general procedure, the oxidation of (2-chlorophenyl)methanethiol (47.6 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–12.5% EtOAc/Hexane) afforded 46.3 mg of 2g in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.37 (m, 2H), 7.26 (m, 2H), 7.24–7.20 (m, 4H), 3.78 (s, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 135.1, 134.2, 131.7, 129.8, 129.0, 126.8, 41.2.
- 1,2-Bis(4-methoxybenzyl)disulfane (2h) [38]. According to the general procedure, the oxidation of (4-methoxyphenyl)methanethiol (46.3 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–12.5% EtOAc/Hexane) afforded 45.0 mg of 2h in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.16 (m, 4H), 6.85 (m, 4H), 3.79 (s, 6H), 3.59 (s, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 159.1, 130.6, 129.5, 114.0, 55.3, 42.8.
- 1,2-Bis(4-(tert-butyl)benzyl)disulfane (2i) [42]. According to the general procedure, the oxidation of (4-(tert-butyl)phenyl)methanethiol (54.1 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–12.5% EtOAc/Hexane) afforded 52.7 mg of 2i in 98% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.33 (m, 4H), 7.17 (m, 4H), 3.59 (s, 4H), 1.31 (s, 18H); 13C{1H} NMR (126 MHz, CDCl3) δ 150.5, 134.3, 129.2, 125.5, 43.1, 34.6, 31.4.
- 1,2-Bis(4-fluorophenyl)disulfane (2j) [40]. According to the general procedure, the oxidation of 4-fluorobenzenethiol (38.4 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 37.4 mg of 2j in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.43 (m, 4H), 7.00 (m, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 162.7 (d, JC-F = 247.8 Hz), 132.3 (d, JC-F = 3.2 Hz), 131.4 (d, JC-F = 8.1 Hz), 116.4 (d, JC-F = 22.5 Hz).
- 1,2-Bis(4-chlorophenyl)disulfane (2k) [40]. According to the general procedure, the oxidation of 4-chlorobenzenethiol (43.4 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 42.2 mg of 2k in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.39 (m, 4H), 7.27 (m, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 135.2, 133.7, 129.4 (×2).
- 1,2-Bis(4-bromophenyl)disulfane (2l) [40]. According to the general procedure, the oxidation of 4-bromobenzenethiol (56.7 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 47.4 mg of 2l in 84% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.41 (m, 4H), 7.32 (m, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 135.8, 132.3, 129.4, 121.6.
- 1,2-Bis(3-bromophenyl)disulfane (2m) [39]. According to the general procedure, the oxidation of 3-bromobenzenethiol (56.7 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 55.3 mg of 2m in a 98% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.62 (m, 2H), 7.41–7.33 (m, 4H), 7.17 (m, 2H); 13C{1H} NMR (126 MHz, CDCl3) δ 138.7, 130.6, 130.5, 130.0, 126.0, 123.2.
- 1,2-Bis(2-bromophenyl)disulfane (2n) [41]. According to the general procedure, the oxidation of 2-bromobenzenethiol (56.7 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 53.6 mg of 2n in a 95% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.55–7.50 (m, 4H), 7.26 (m, 2H), 7.07 (m, 2H); 13C{1H} NMR (126 MHz, CDCl3) δ 136.2, 133.0, 128.3, 128.0, 127.0, 121.1.
- 1,2-Bis(4-methoxyphenyl)disulfane (2o) [40]. According to the general procedure, the oxidation of 4-methoxybenzenethiol (42.1 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–12.5% EtOAc/Hexane) afforded 40.9 mg of 2o in 98% yield as a yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.39 (m, 4H), 6.83 (m, 4H), 3.79 (s, 6H); 13C{1H} NMR (126 MHz, CDCl3) δ 160.0, 132.7, 128.5, 114.7, 55.4.
- 1,2-Bis(2-methoxyphenyl)disulfane (2p) [40]. According to the general procedure, the oxidation of 2-methoxybenzenethiol (42.1 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–12.5% EtOAc/Hexane), afforded 40.9 mg of 2p in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.53 (m, 2H), 7.17 (m, 2H), 6.90 (m, 2H), 6.84 (m, 2H), 3.88 (s, 6H); 13C{1H} NMR (126 MHz, CDCl3) δ 156.7, 127.8, 127.7, 124.6, 121.4, 110.6, 55.9.
- 1,2-Bis(3,4-dimethoxyphenyl)disulfane (2q) [40]. According to the general procedure, the oxidation of 3,4-dimethoxybenzenethiol (51.1 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–12.5% EtOAc/Hexane) afforded 49.8 mg of 2q in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.06 (d, J = 2.1 Hz, 1H), 7.04 (d, J = 2.1 Hz, 1H), 7.01 (m, 2H), 6.79 (s, 1H), 6.78 (s, 1H), 3.87 (s, 6H), 3.83 (s, 6H); 13C{1H} NMR (126 MHz, CDCl3) δ 149.6, 149.2, 128.7, 123.9, 114.1, 111.3, 56.0, 55.9.
- 1,2-Bis(4-isopropylphenyl)disulfane (2r) [44]. According to the general procedure, the oxidation of 4-isopropylbenzenethiol (45.6 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 39.0 mg of 2r in 86% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.42 (m, 4H), 7.16 (m, 4H), 2.87 (m, 2H), 1.22 (d, J = 6.9 Hz, 12H); 13C{1H} NMR (126 MHz, CDCl3) δ 148.4, 134.4, 128.3, 127.3, 33.8, 24.0.
- 1,2-Di-p-tolyldisulfane (2s) [40]. According to the general procedure, the oxidation of 4-methylbenzenethiol (37.3 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 32.2 mg of 2s in 87% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.38 (m, 4H), 7.10 (m, 4H), 2.32 (s, 6H); 13C{1H} NMR (126 MHz, CDCl3) δ 137.5, 134.0, 129.9, 128.6, 21.1.
- N,N′-(Disulfanediylbis(4,1-phenylene))diacetamide (2t) [41]. According to the general procedure, the oxidation of N-(4-mercaptophenyl)acetamide (50.2 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–10% MeOH/EtOAc) afforded 40.4 mg of 2t in 81% yield as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 10.07 (s, 2H), 7.59 (m, 4H), 7.42 (m, 4H), 2.04 (s, 6H); 13C{1H} NMR (126 MHz, DMSO-d6) δ 168.5, 139.5, 130.1, 129.4, 119.7, 24.0.
- 1,2-Di(naphthalen-2-yl)disulfane (2u) [40]. According to the general procedure, the oxidation of naphthalene-2-thiol (48.1 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 46.8 mg of 2u in 98% yield as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.97 (m, 2H), 7.79–7.74 (m, 4H), 7.71 (m, 2H), 7.61 (m, 2H), 7.48–7.40 (m, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 134.3, 133.6, 132.6, 129.1, 127.8, 127.5, 126.8, 126.6, 126.3, 125.7.
- 1,2-Di(thiophen-2-yl)disulfane (2v) [41]. According to the general procedure, the oxidation of thiophene-2-thiol (34.9 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (100% Hexane) afforded 28.3 mg of 2v in 82% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.48 (d, J = 5.2 Hz, 2H), 7.14 (d, J = 3.7 Hz, 2H), 7.00 (dd, J = 5.2, 3.7 Hz, 2H); 13C{1H} NMR (126 MHz, CDCl3) δ 135.8, 135.7, 132.3, 127.8.
- 1,2-Bis(furan-2-ylmethyl)disulfane (2w) [39]. According to the general procedure, the oxidation of furan-2-ylmethanethiol (34.2 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–12.5% EtOAc/Hexane) afforded 33.3 mg of 2w in 98% yield as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.38 (m, 2H), 6.33 (dd, J = 3.2, 2.0 Hz, 2H), 6.22 (d, J = 3.2 Hz, 2H), 3.68 (s, 4H); 13C{1H} NMR (126 MHz, CDCl3) δ 150.3, 142.5, 110.8, 109.0, 35.7.
- Dimethyl 3,3′-disulfanediyl(2R,2′R)-bis(2-((tert-butoxycarbonyl)amino)propanoate) (2x) [45]. According to the general procedure, the oxidation of N-(tert-butoxycarbonyl)-L-cysteine methyl ester (70.6 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–30% EtOAc/Hexane) afforded 46.4 mg of 2y in 66% yield as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 7.36 (d, J = 8.2 Hz, 2H), 4.26 (m, 2H), 3.64 (s, 6H), 3.07 (m, 2H), 2.90 (m, 2H), 1.37 (s, 18H); 13C{1H} NMR (126 MHz, CDCl3) δ 171.4, 155.3, 78.6, 52.7, 52.1, 39.1, 28.1.
- (2R,2′R)-3,3′-disulfanediylbis(2-acetamidopropanoic acid) (2y) [20]. According to the general procedure, the oxidation of N-acetyl-L-cysteine (49.0 mg, 0.300 mmol) is catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa). The reaction mixture was concentrated, then washed with EtOAc (20 mL), affording 47.7 mg of 2z in 98% yield as a white solid. 1H NMR (500 MHz, D2O) δ 4.68 (dd, J = 8.6, 4.3 Hz, 2H), 3.38 (dd, J = 14.1, 4.3 Hz, 2H), 3.02 (dd, J = 14.1, 8.6 Hz, 2H), 2.04 (s, 6H); 13C{1H} NMR (126 MHz, D2O) δ 177.7, 174.1, 53.2, 39.4, 21.8.
- (4R,5R)-1,2-Dithiane-4,5-diol (2z) [46]. According to the general procedure, the oxidation of (2R,3R)-1,4-dimercaptobutane-2,3-diol (46.3 mg, 0.300 mmol) catalyzed by I2 (3.81 mg, 5.00 mol%) under an oxygen balloon (0.3 MPa) after chromatography (0–100% EtOAc/Hexane) afforded 44.8 mg of 2z in 98% yield as a white solid. 1H NMR (500 MHz, CD3OD) δ 3.48–3.31 (m, 2H), 3.02–2.89 (m, 2H), 2.84–2.73 (m, 2H); 13C{1H} NMR (126 MHz, CD3OD) δ 74.09, 40.4.
3.5. Procedure for Control Experiments (Scheme 1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Eom, T.; Khan, A. Disulfides as Mercapto-Precursors in Nucleophilic Ring Opening Reaction of Polymeric Epoxides: Establishing Equimolar Stoichiometric Conditions in a Thiol–Epoxy ‘Click’ Reaction. Chem. Commun. 2020, 56, 7419–7422. [Google Scholar] [CrossRef]
- Yang, S.; Yu, X.; Szostak, M. Divergent Acyl and Decarbonylative Liebeskind−Srogl Cross-Coupling of Thioesters by Cu-Cofactor and Pd–NHC (NHC = N-Heterocyclic Carbene) Catalysis. ACS Catal. 2023, 13, 1848–1855. [Google Scholar] [CrossRef]
- Zhang, R.; Nie, T.; Fang, Y.; Huang, H.; Wu, J. Poly(Disulfide)s: From Synthesis to Drug Delivery. Biomacromolecules 2022, 23, 1–19. [Google Scholar] [CrossRef]
- Li, H.; Peng, M.; Li, J.; Do, H.; Ni, K.; Wang, M.; Yuan, Z.; Wang, L.; Zhao, T.; Zhang, X.; et al. Redox-Click Chemistry for Disulfide Formation from Thiols 2023. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/6442481be4bbbe4bbf0117ad (accessed on 4 September 2023).
- Hou, X.; Li, Y.; Pan, Y.; Jin, Y.; Xiao, H. Controlled Release of Agrochemicals and Heavy Metal Ion Capture Dual-Functional Redox-Responsive Hydrogel for Soil Remediation. Chem. Commun. 2018, 54, 13714–13717. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, X. Sulfur–Sulfur Bond Construction. Top. Curr. Chem. 2018, 376, 14. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Derikvand, F.; Oskooie, H.A.; Shoar, R.H.; Tajbakhsh, M. Silica-Supported Bis (Trimethylsilyl) Chromate: Oxidation of Thiols to Their Corresponding Disulfides. Synth. Commun. 2007, 37, 513–517. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Silva, M.S.; Mendes, S.R.; de Azambuja, F.; Jacob, R.G.; Santos, P.C.S.d.; Perin, G. Synthesis of Beta-Phenylchalcogeno-Alpha, Beta-Unsaturated Esters, Ketones and Nitriles Using Microwave and Solvent-Free Conditions. J. Braz. Chem. Soc. 2007, 18, 943–950. [Google Scholar] [CrossRef]
- Bourles, E.; de Sousa, R.A.; Galardon, E.; Selkti, M.; Tomas, A.; Artaud, I. Cyclic Mono & Bis-Disulfide & Selective Conversion to Mono- and Bis-Thiosulfinate. Tetrahedron 2007, 63, 2466–2471. [Google Scholar]
- Ali, M.H.; McDermott, M. Oxidation of Thiols to Disulfides with Molecular Bromine on Hydrated Silica Gel Support. Tetrahedron Lett. 2002, 43, 6271–6273. [Google Scholar] [CrossRef]
- Karimi, B.; Zareyee, D. Hexamethyldisilazane (HMDS) Promotes Highly Efficient Oxidative Coupling of Thiols by DMSO Under Nearly Neutral Reaction Conditions. Synlett 2002, 2, 0346–0348. [Google Scholar] [CrossRef]
- Leino, R.; Lönnqvist, J.E. A Very Simple Method for the Preparation of Symmetrical Disulfides. Tetrahedron. Lett. 2004, 45, 8489–8491. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Mallakpour, S.E.; Adibi, H. Selective and Efficient Oxidation of Sulfides and Thiols with Benzyltriphenylphosphonium Peroxymonosulfate in Aprotic Solvent. J. Org. Chem. 2002, 67, 8666–8668. [Google Scholar] [CrossRef] [PubMed]
- Harusawa, S.; Yoshida, K.; Kojima, C.; Araki, L.; Kurihara, T. Design and Synthesis of an Aminobenzo-15-Crown-5-Labeled Estradiol Tethered with Disulfide Linkage. Tetrahedron 2004, 60, 11911–11922. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, X.; Wang, H.; Yang, L.; Li, H.; Yuan, B.; Yang, G. Reusable Cobalt-Phthalocyanine in Water: Efficient Catalytic Aerobic Oxidative Coupling of Thiols to Construct S–N/S–S Bonds. Green Chem. 2017, 19, 2491–2495. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Recent Advances in Transition-Metal Catalyzed Reactions Using Molecular Oxygen as the Oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005, 105, 2329–2364. [Google Scholar] [CrossRef] [PubMed]
- Obora, Y.; Ishii, Y. Palladium-Catalyzed Intermolecular Oxidative Amination of Alkenes with Amines, Using Molecular Oxygen as Terminal Oxidant. Catalysts 2013, 3, 794–810. [Google Scholar] [CrossRef]
- Chauhan, S.M.S.; Kumar, A.; Srinivas, K.A. Oxidation of Thiols with Molecular Oxygen Catalyzed by Cobalt(II) Phthalocyanines in Ionic Liquid. Chem. Commun. 2003, 18, 2348–2349. [Google Scholar] [CrossRef]
- Oba, M.; Tanaka, K.; Nishiyama, K.; Ando, W. Aerobic Oxidation of Thiols to Disulfides Catalyzed by Diaryl Tellurides under Photosensitized Conditions. J. Org. Chem. 2011, 76, 4173–4177. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Pan, L.Y.; Zhang, G.F.; Li, Y.S. A Facile Oxidation of Thiols to Disulfides Catalyzed by CoSalen. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 16–21. [Google Scholar] [CrossRef]
- Huang, H.; Ash, J.; Kang, J.Y. Base-Controlled Fe(Pc)-Catalyzed Aerobic Oxidation of Thiols for the Synthesis of S–S and S–P(O) Bonds. Org. Biomol. Chem. 2018, 16, 4236–4242. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.L.; Li, M.C.; Hu, X.Q.; Mo, W.-M.; Shen, Z.-L. An Efficient and Convenient Method for the Preparation of Disulfides from Thiols Using Oxygen as Oxidant Catalyzed by Tert-Butyl Nitrite. Chin. Chem Lett. 2016, 27, 1505–1508. [Google Scholar] [CrossRef]
- Kuciński, K.; Hreczycho, G. Diisopropylamine as a Single Catalyst in the Synthesis of Aryl Disulfides. Green Process. Synth. 2018, 7, 12–15. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Dou, Y.; Zhen, S.; Li, H.; Zhang, P.; Yuan, B.; Yang, G. TEMPO-Catalyzed Aerobic Oxidative Coupling of Thiols for Metal-Free Formation of S−N/S−S Bonds. Asian J. Org. Chem. 2017, 6, 265–268. [Google Scholar] [CrossRef]
- Sathe, M.; Ghorpade, R.; Kaushik, M.P. Oxidation of Thiols to Disulfides Using Silica Chloride as a Heterogeneous Catalyst. Chem. Lett. 2006, 35, 1048–1049. [Google Scholar] [CrossRef]
- Breugst, M.; von der Heiden, D. Mechanisms in Iodine Catalysis. Chem. Eur. J. 2018, 24, 9187–9199. [Google Scholar] [CrossRef]
- Breugst, M.; Detmar, E.; von der Heiden, D. Origin of the Catalytic Effects of Molecular Iodine: A Computational Analysis. ACS Catal. 2016, 6, 3203–3212. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, H.X.; Ali, S.; Xia, X.F.; Liang, Y.M. Iodine-Mediated Regioselective C2-Amination of Indoles and a Concise Total Synthesis of (±)-Folicanthine. Chem. Commun. 2012, 48, 2343–2345. [Google Scholar] [CrossRef]
- Ishihara, K.; Muñiz, K. Iodine Catalysis in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2022; pp. 11–22. [Google Scholar]
- Witt, D. Recent developments in disulfide bond formation. Synthesis 2008, 16, 2491–2509. [Google Scholar] [CrossRef]
- Bettanin, L.; Saba, S.; Galetto, F.Z.; Mike, G.A.; Rafique, J.; Braga, A.L. Solvent and metal-free selective oxidation of thiols to disulfides using I2/DMSO catalytic system. Tetrahedron. Lett. 2017, 58, 4713–4716. [Google Scholar] [CrossRef]
- Kirihara, M.; Asai, Y.; Ogawa, S.; Noguchi, T.; Hatano, A.; Hirai, Y. A mild and environmentally benign oxidation of thiols to disulfides. Synthesis 2007, 21, 3286–3289. [Google Scholar] [CrossRef]
- Iida, H.; Kozako, R.; Iida, H. Green Aerobic Oxidation of Thiols to Disulfides by Flavin-Iodine Coupled Organocatalysis. Synlett 2021, 32, 1227–1230. [Google Scholar] [CrossRef]
- Spiliopoulou, N.; Kokotos, C.G. Photochemical Metal-Free Aerobic Oxidation of Thiols to Disulfides. Green Chem. 2021, 23, 546–551. [Google Scholar] [CrossRef]
- Primas, N.; Lano, G.; Brun, D.; Curti, C.; Sallee, M.; Sampol-Manos, E.; Lamy, E.; Bornet, C.; Burtey, S.; Vanelle, P. Stability Study of Parenteral N-Acetylcysteine, and Chemical Inhibition of Its Dimerization. Pharmaceuticals 2023, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Galanis, A.S.; Albericio, F.; Grotli, M. Solid-Phase Peptide Synthesis in Water Using Microwave-Assisted Heating. Org. Lett. 2009, 11, 4488–4491. [Google Scholar] [CrossRef] [PubMed]
- Asmellash, S.; Stevens, J.L.; Ichimura, T. Modulating the endoplasmic reticulum stress response with trans-4,5-dihydroxy-1,2-dithiane prevents chemically induced renal injury in vivo. Toxicol. Sci. 2005, 11, 576–584. [Google Scholar] [CrossRef]
- Yue, H.; Wang, J.; Xie, Z.; Tian, J.; Sang, D.; Liu, S. 1,3-Diisopropylcarbodiimide-Mediated Synthesis of Disulfides from Thiols. ChemistrySelect 2020, 5, 4273–4277. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.F.; Lang, X. TEMPO Visible Light Photocatalysis: The Selective Aerobic Oxidation of Thiols to Disulfides. Chin. Chem. Lett. 2020, 31, 1520–1524. [Google Scholar] [CrossRef]
- Song, L.; Li, W.; Duan, W.; An, J.; Tang, S.; Li, L.; Yang, G. Natural Gallic Acid Catalyzed Aerobic Oxidative Coupling with the Assistance of MnCO3 for Synthesis of Disulfanes in Water. Green Chem. 2019, 21, 1432–1438. [Google Scholar] [CrossRef]
- Bhattacherjee, D.; Sufian, A.; Mahato, S.K.; Begum, S.; Banerjee, K.; De, S.; Srivastava, H.K.; Bhabak, K.P. Trisulfides over Disulfides: Highly Selective Synthetic Strategies, Anti-Proliferative Activities and Sustained H2S Release Profiles. Chem. Commun. 2019, 55, 13534–13537. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.L.; Schotten, C.; Alston, S.T.; Browne, D.L. Preparation of Difluoromethylthioethers through Difluoromethylation of Disulfides Using TMS-CF2H. Chem. Commun. 2016, 52, 8448–8451. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Okunaga, K.; Nishida, S.; Kawamura, K.; Eda, K. Oxidative Transformation of Thiols to Disulfides Promoted by Activated Carbon–Air System. Tetrahedron. Lett. 2010, 51, 6734–6736. [Google Scholar] [CrossRef]
- Bottecchia, C.; Erdmann, N.; Tijssen, P.M.A.; Milroy, L.G.; Brunsveld, L.; Hessel, V.; Noel, T. Batch and Flow Synthesis of Disulfides by Visible-Light-Induced TiO2 Photocatalysis. Chemsuschem 2016, 9, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Calandra, N.A.; Cheng, Y.L.; Kocak, K.A.; Miller, J.S. Total Synthesis of Spiruchostatin A via Chemoselective Macrocyclization using an Accessible Enantiomerically Pure Latent Thioester. Org. Lett. 2009, 11, 1971–1974. [Google Scholar] [CrossRef] [PubMed]




 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Catalyst (mol%) | Temperature (°C) | Time (h) | Yield a (%) |
| 1 | EtOAc | 10 | 70 | 4 | >98 |
| 2 | EtOAc | 5.0 | 70 | 4 | >98 |
| 3 | EtOAc | 1.0 | 70 | 4 | 46 |
| 4 | EtOAc | 5.0 | 70 | 1 | 49 |
| 5 | DCM | 5.0 | 70 | 4 | 23 |
| 6 | DMF | 5.0 | 70 | 4 | 22 |
| 7 | EtOAc | 5.0 | r.t. | 4 | 53 |
| 8 | EtOAc | no | 70 | 4 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, L.; Qin, Z.; Ni, K.; Li, X.; Yu, Z.; Kuang, Z.; Qin, X.; Duan, H.; An, J. Application of Iodine as a Catalyst in Aerobic Oxidations: A Sustainable Approach for Thiol Oxidations. Molecules 2023, 28, 6789. https://doi.org/10.3390/molecules28196789
Wang L, Chen L, Qin Z, Ni K, Li X, Yu Z, Kuang Z, Qin X, Duan H, An J. Application of Iodine as a Catalyst in Aerobic Oxidations: A Sustainable Approach for Thiol Oxidations. Molecules. 2023; 28(19):6789. https://doi.org/10.3390/molecules28196789
Chicago/Turabian StyleWang, Lijun, Lingxia Chen, Zixuan Qin, Ke Ni, Xiao Li, Zhiyuan Yu, Zichen Kuang, Xinshu Qin, Hongxia Duan, and Jie An. 2023. "Application of Iodine as a Catalyst in Aerobic Oxidations: A Sustainable Approach for Thiol Oxidations" Molecules 28, no. 19: 6789. https://doi.org/10.3390/molecules28196789
APA StyleWang, L., Chen, L., Qin, Z., Ni, K., Li, X., Yu, Z., Kuang, Z., Qin, X., Duan, H., & An, J. (2023). Application of Iodine as a Catalyst in Aerobic Oxidations: A Sustainable Approach for Thiol Oxidations. Molecules, 28(19), 6789. https://doi.org/10.3390/molecules28196789






