Experimental and Computational Study on Inhibitory Effect and Adsorption Properties of N-Acetylcysteine Amino Acid in Acid Environment
Abstract
:1. Introduction
2. Results and Discussion
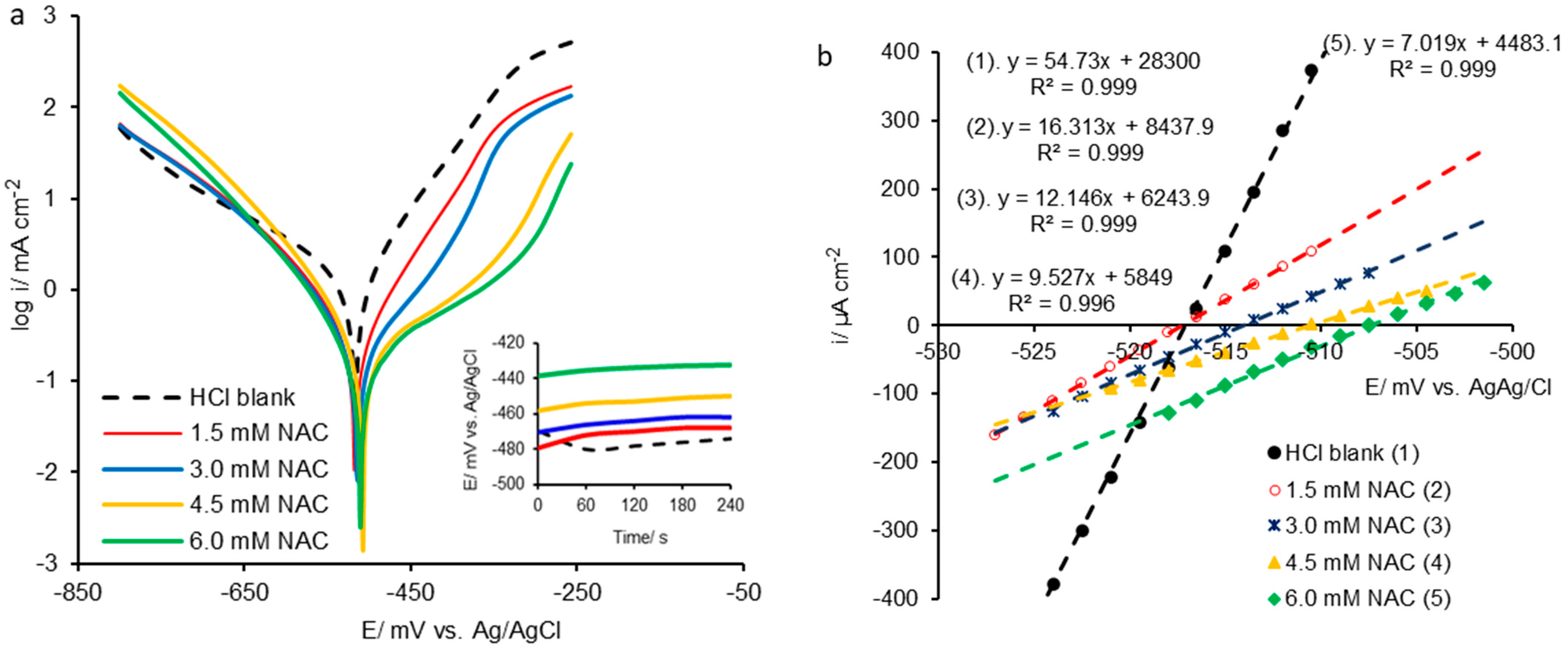
2.1. Potentiodynamic Polarization
2.2. Electrochemical Impedance Spectroscopy (EIS)
2.3. Adsorption Isotherm Approach
2.4. Quantum Chemical Analysis
- ω (electrophilicity index) indicating the molecule’s predisposition to accepting electrons [44].
- ω− (electrodonating power);
- ω+ (electroaccepting power);
2.5. Monte Carlo Simulation
2.6. N-Acetylcysteine Adsorption Mechanism
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Electrochemical Measurements
3.2.2. Potentiodynamic Polarization
3.2.3. Electrochemical Impedance Spectroscopy (EIS)
3.3. Computational Details
3.4. Monte Carlo Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| NAC | N-acetylcysteine |
| PDP | potentiodynamic polarization |
| EIS | electrochemical impedance spectroscopy |
| OCP | open circuit potential |
| icorr | corrosion current density |
| Ecorr | corrosion potential |
| Rp | polarization resistance |
| CR | corrosion rate |
| ba, bc | anodic and cathodic Tafel slopes |
| Rct | charge transfer resistance |
| Cdl | double-layer capacitance |
| Rs | solution resistance |
| IE% | inhibition efficiency |
| K | adsorption–desorption constant |
| standard free adsorption energy | |
| θ | degree of surface coverage |
| χ | electronegativity |
| ε | chemical potential |
| η | hardness |
| σ | softness |
| ω | electrophilicity index |
| ω− | electrodonating power |
| ω+ | electroaccepting power |
| ΔN | fraction of the transferred electron |
References
- Ma, I.A.W.; Ammar, S.; Kumar, S.S.A.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat. Technol. Res. 2022, 19, 241–268. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Zehra, S.; Aslam, J. A comprehensive review of corrosion inhibitors employed to mitigate stainless steel corrosion in different environments. J. Mol. Liq. 2022, 364, 119992. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Nazdracheva, T.; Yavna, V. Comparative Computational Study of L-Amino Acids as Green Corrosion Inhibitors for Mild Steel. Computation 2021, 9, 1. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Q.; Wang, Y.; Tang, J.; Wang, Y.; Wang, H. Molecular dynamic simulation and experimental investigation on the synergistic mechanism and synergistic effect of oleic acid imidazoline and l-cysteine corrosion inhibitors. Corros. Sci. 2021, 185, 109414. [Google Scholar] [CrossRef]
- Bai, P.; Zhao, H.; Zheng, S.; Chen, C. Initiation and developmental stages of steel corrosion in wet H2S environments. Corros. Sci. 2015, 93, 109–119. [Google Scholar] [CrossRef]
- Hamadi, L.; Mansouri, S.; Oulmi, K.; Kareche, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Vaszilcsin, N.; Kellenberger, A.; Laurentiu Dan, M.L.; Duca, D.A.; Ordodi, V.L. Efficiency of Expired Drugs Used as Corrosion Inhibitors: A Review. Materials 2023, 16, 5555. [Google Scholar] [CrossRef]
- Tanwer, S.; Shukla, S.K. Recent advances in the applicability of drugs as corrosion inhibitor on metal surface: A review. Curr. Res. Green Sustain. Chem. (CRGSC) 2022, 5, 100227. [Google Scholar] [CrossRef]
- Alamry, K.A.; Khan, A.; Aslam, J.; Hussein, M.A.; Aslam, R. Corrosion inhibition of mild steel in hydrochloric acid solution by the expired Ampicillin drug. Sci. Rep. 2023, 13, 6724. [Google Scholar] [CrossRef]
- Murungi, P.I.; Sulaimon, A.A. Ideal corrosion inhibitors: A review of plant extracts as corrosion inhibitors for metal surfaces. Corros. Rev. 2022, 40, 127–136. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; El Aboubi, M.; Abbout, S.; Benzekri, Z.; Safi, Z.; Rafik, M.; Bahaj, H.; Kaba, M.; Galai, M.; et al. Synthesis and performance of two ecofriendly epoxy resins as a highly efficient corrosion inhibition for carbon steel in 1 M HCl solution: DFT, RDF, FFV and MD approaches. Chem. Phys. Lett. 2022, 806, 139995. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Polyvinyl alcohol (PVA) as a biodegradable polymeric anticorrosive material: A review on present advancements and future directions. Corros. Eng. Sci. Technol. 2022, 57, 796–812. [Google Scholar] [CrossRef]
- Samide, A.; Stoean, C.; Stoean, R. Surface study of inhibitor films formed by polyvinyl alcohol and silver nanoparticles on stainless steel in hydrochloric acid solution using convolutional neural networks. Appl. Surf. Sci. 2019, 475, 1–5. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Benhiba, F.; Seghiri, R.; Safi, Z.; Kaya, S.; Briche, S.; Serdaroğlu, G.; Erramli, H.; Elbachiri, A.; et al. Insight into the corrosion inhibition of novel macromolecular epoxy resin as highly efficient inhibitor for carbon steel in acidic mediums: Synthesis, characterization, electrochemical techniques, AFM/UV–Visible and computational investigations. J. Mol. Liq. 2021, 337, 116492. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Echihi, S.; Benkhaya, S.; Hilali, M.; Berisha, A.; Briche, S.; Zarrouk, A.; Nouneh, A.; Elharfi, A. New epoxy composite polymers as a potential anticorrosive coatings for carbon steel in 3.5% NaCl solution: Experimental and computational approaches. Chem. Data Collect. (CDC) 2021, 31, 100619. [Google Scholar] [CrossRef]
- Habibullah, M.I.; Veawab, A. Cysteine as an Alternative Eco-Friendly Corrosion Inhibitor for Absorption-Based Carbon Capture Plants. Materials 2023, 16, 3496. [Google Scholar] [CrossRef]
- Farahati, R.; Mousavi-Khoshdel, S.M.; Ghaffarinejad, A.; Behzadi, H. Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Prog. Org. Coat. 2020, 142, 105567. [Google Scholar] [CrossRef]
- Mobin, M.; Zehra, S.; Parveen, M. L-Cysteine as corrosion inhibitor for mild steel in 1M HCl and synergistic effect of anionic, cationic and non-ionic surfactants. J. Mol. Liq. 2016, 216, 598–607. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- Loto, R. Corrosion inhibition effect of non-toxic α-amino acid compound on high carbon steel in low molar concentration of hydrochloric acid. J. Mater. Res. Technol. 2019, 8, 484–493. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Li, Y.Y.; Lei, Y.; Wang, X.; Liu, H.F.; Zhang, G.A. Comparison of the synergistic inhibition mechanism of two eco-friendly amino acids combined corrosion inhibitors for carbon steel pipelines in oil and gas production. Appl. Surf. Sci. 2022, 583, 152559. [Google Scholar] [CrossRef]
- Oubaaqa, M.; Ouakki, M.; Rbaa, M.; Abousalem, A.S.; Maatallah, M.; Benhiba, F.; Jarid, A.; Ebn Touhami, M.; Zarrouk, A. Insight into the corrosion inhibition of new amino-acids as efficient inhibitors for mild steel in HCl solution: Experimental studies and theoretical calculations. J. Mol. Liq. 2021, 334, 116520. [Google Scholar] [CrossRef]
- Mendonça, G.L.F.; Costa, S.N.; Freire, V.N.; Casciano, P.N.S.; Correia, A.N.; de Lima-Neto, P. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods. Corros. Sci. 2017, 115, 41–55. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Zhang, W.; Huang, X.; Ruan, L.; Wu, L. Experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies of methionine and valine as corrosion inhibitors on carbon steel in phase change materials (PCMs) solution. J. Mol. Liq. 2018, 272, 528–538. [Google Scholar] [CrossRef]
- Radovanović, M.; Petrović Mihajlović, M.; Tasić, Ž.; Simonović, A.; Antonijević, M. Inhibitory effect of L-Threonine and L-Lysine and influence of surfactant on stainless steel corrosion in artificial body solution. J. Mol. Liq. 2021, 342, 116939. [Google Scholar] [CrossRef]
- Abdel-Fatah, H.T.M.; Abdel-Samad, H.S.; Hassan, A.A.M.; El-Sehiety, H.E.E. Effect of variation of the structure of amino acids on inhibition of the corrosion of low-alloy steel in ammoniated citric acid solutions. Res. Chem. Intermed. 2014, 40, 1675–1690. [Google Scholar] [CrossRef]
- Dehdab, M.; Shahraki, M.; Habibi-Khorassani, S.M. Theoretical study of inhibition efficiencies of some amino acids on corrosion of carbon steel in acidic media: Green corrosion inhibitors. Amino Acids 2016, 48, 291–306. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Rasul, H.H.; Mamad, D.M.; Azeez, Y.H.; Omer, R.A.; Omer, K.A. Theoretical investigation on corrosion inhibition efficiency of some amino acid compounds. Comput. Theor. Chem. 2023, 1225, 114177. [Google Scholar] [CrossRef]
- Purnima; Goyal, S.; Luxami, V. Exploring the corrosion inhibition mechanism of Serine (Ser) and Cysteine (Cys) in alkaline concrete pore solution simulating carbonated environment. Constr. Build. Mater. 2023, 384, 131433. [Google Scholar] [CrossRef]
- Hussein, A.M.; Abbas, Z.S.; Kadhim, M.M.; Rheima, A.M.; Barzan, M.; Al-Attia, L.H.; Elameer, A.S.; Hachim, S.K.; Hadi, M.A. Inhibitory behavior and adsorption of asparagine dipeptide amino acid on the Fe(111) surface. J. Mol. Model 2023, 29, 162. [Google Scholar] [CrossRef]
- Guerini, M.; Condrò, G.; Friuli, V.; Maggi, L.; Perugini, P. N-acetylcysteine (NAC) and Its Role in Clinical Practice Management of Cystic Fibrosis (CF): A Review. Pharmaceuticals 2022, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Manzano-Pech, L.; Palacios-Chavarría, A.; Valdez-Vázquez, R.R.; Guarner-Lans, V.; Pérez-Torres, I. N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings. Pharmaceuticals 2023, 16, 591. [Google Scholar] [CrossRef] [PubMed]
- Ituen, E.B.; Akaranta, O.; Umoren, S.A. N-acetyl cysteine based corrosion inhibitor formulations for steel protection in 15% HCl solution. J. Mol. Liq. 2017, 246, 112–118. [Google Scholar] [CrossRef]
- Morad, M.S.S.; Hermas, A.; El, -H.A.; Aal, M.S.A. Effect of amino acids containing sulfur on the corrosion of mild steel in phosphoric acid solutions polluted with Cl−, F− and Fe3+ ions–behaviour near and at the corrosion potential. J. Chem. Technol. Biotechnol. (JCTB) 2002, 77, 486–494. [Google Scholar] [CrossRef]
- Morad, M.S. Corrosion inhibition of mild steel in sulfamic acid solution by S-containing amino acids. J. Appl. Electrochem. 2008, 38, 1509–1518. [Google Scholar] [CrossRef]
- El-Hafez, G.M.A.; Badawy, W.A. The use of cysteine, N-acetyl cysteine and methionine as environmentally friendly corrosion inhibitors for Cu–10Al–5Ni alloy in neutral chloride solutions. Electrochem. Acta 2013, 108, 860–866. [Google Scholar] [CrossRef]
- Khaled, K.F.; MEl-Sherik, A. Validation of a Predictive Model for Corrosion inhibition of API 5L X60 Steel in Chloride Solution. Int. J. Electrochem. Sci. 2016, 11, 2377–2391. [Google Scholar] [CrossRef]
- Samide, A.; Bratulescu, G.; Merisanu, C.; Cioatera, N. Anticorrosive coating based on poly(vinyl acetate) formed by electropolymerization on the copper surface. J. Appl. Polym. Sci. 2019, 136, 47320. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Merisanu, C.; Cioatera, N. Thermal analysis: An effective characterization method of polyvinyl acetate films applied in corrosion inhibition field. J. Therm. Anal. Calorim. 2020, 142, 1825–1834. [Google Scholar] [CrossRef]
- Samide, A.; Ilea, P.; Vladu, A.C. Metronidazole Performance as Corrosion Inhibitor for Carbon Steel, 304L Stainless Steel and Aluminum in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2017, 12, 5964–5983. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Dobrițescu, A.; Ilea, P.; Vladu, A.C.; Tigae, C. Electrochemical and theoretical study of metronidazole drug as inhibitor for copper corrosion in hydrochloric acid solution. Int. J. Electrochem. Sci. 2016, 11, 5520–5534. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Varut, R.M.; Oprea, B.; Iordache, S. Interactions of Some Chemotherapeutic Agents as Epirubicin, Gemcitabine and Paclitaxel in Multicomponent Systems Based on Orange Essential Oil. Pharmaceuticals 2021, 14, 619. [Google Scholar] [CrossRef] [PubMed]
- Alar, V.; Stojanović, I.; Mezdić, D. A Comparative Study of Green Inhibitors for Galvanized Steel in Aqueous Solutions. Metals 2020, 10, 448. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef]
- Samide, A.; Iacobescu, G.E.; Tutunaru, B.; Grecu, R.; Tigae, C.; Spînu, C. Inhibitory Properties of Neomycin Thin Film Formed on Carbon Steel in Sulfuric Acid Solution: Electrochemical and AFM Investigation. Coatings 2017, 7, 181. [Google Scholar] [CrossRef]
- Mobin, M.; Basik, M.; Shoeb, M. A Novel Organic-Inorganic Hybrid Complex Based on Cissus Quadrangularis Plant Extract and Zirconium Acetate as a Green Inhibitor for Mild Steel in 1 M HCl Solution. Appl. Surf. Sci. 2019, 469, 387–403. [Google Scholar] [CrossRef]
- Bobina, M.; Kellenberger, A.; Millet, J.-P.; Muntean, M.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of l-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Desimone, M.P.; Grundmeier, G.; Gordillo, G.; Simison, S.N. Amphiphilic amido-amine as an effective corrosion inhibitor for mild steel exposed to CO2 saturated solution: Polarization, EIS and PM-IRRAS studies. Electrochim. Acta 2011, 56, 2990–2998. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.O.; Obot, I.B. Inhibitive properties, thermodynamic and quantum chemical studies of alloxazine on mild steel corrosion in H2SO4. Corros. Sci. 2011, 53, 263–275. [Google Scholar] [CrossRef]
- Kokalj, A. On the use of the Langmuir and other adsorption isotherms in corrosion inhibition. Corros. Sci. 2023, 217, 111112. [Google Scholar] [CrossRef]
- Mobin, M.; Basik, M.; Aslam, J. Boswellia serrata gum as highly efficient and sustainable corrosion inhibitor for low carbon steel in 1 M HCl solution: Experimental and DFT studies. J. Mol. Liq. 2018, 263, 174–186. [Google Scholar] [CrossRef]
- Kokalj, A. Is the analysis of molecular electronic structure of corrosion inhibitors sufficient to predict the trend of their inhibition performance. Electrochim. Acta 2010, 56, 745–755. [Google Scholar] [CrossRef]
- Fazary, A.E.; Awwad, N.S.; Ibrahium, H.A.; Shati, A.A.; Alfaifi, M.Y.; Yi-Hsu, J.; Yi, -H. Protonation Equilibria of N-Acetylcysteine. ACS Omega 2020, 5, 19598–19605. [Google Scholar] [CrossRef]










| C-NAC/ mol L−1 | OCP/ mV | Ecorr/mV | icorr/ mA cm−2 | ba/ mV dec−1 | bc/ mVdec−1 | CR/ mmY−1 | Rp/ Ω cm2 | IE/% | IEm/% | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| from Equation (4) | from Equation (5) cr | from Equation (6) | |||||||||
| 0 | −474 ± 4 | −517.2 ± 2.5 | 1.13 ± 0.19 | 79.3 | −159.3 | 13.3 ± 1.2 | 18.3 ± 1.2 | - | - | - | - |
| 1.5 × 10−3 | −468 ± 3 | −517.3 ± 2.1 | 0.44 ± 0.08 | 85.9 | −110.2 | 5.2 ± 0.6 | 61.3 ± 2.2 | 61.1 | 60.9 | 70.1 | 64.0 ± 0.6 |
| 3.0 × 10−3 | −462 ± 3 | −514.1 ± 2.2 | 0.26 ± 0.08 | 113.3 | −84.5 | 3.1 ± 0.4 | 82.3 ± 4.4 | 76.9 | 77.8 | 77.3 | 77.3 ± 0.4 |
| 4.5 × 10−3 | −450 ± 2 | −510.8 ± 1.9 | 0.19 ± 0.02 | 209.1 | −85.6 | 2.2 ± 0.1 | 114.9 ± 4.2 | 83.2 | 83.5 | 84.1 | 83.6 ± 0.6 |
| 6.0 × 10−3 | −432 ± 2 | −507.5 ± 1.3 | 0.13 ± 0.02 | 213.2 | −87.5 | 1.6 ± 0.1 | 142.5 ± 6.4 | 88.4 | 87.9 | 87.2 | 87.8 ± 0.3 |
| C-NAC/ mol L−1 | Nyquist Parameters | IE/% | ||||
|---|---|---|---|---|---|---|
| Rs/ mΩ cm2 | Cdl/ μF cm−2 | Rct/ Ω cm2 | n | χ2 × 10−4 | ||
| 0 | 403.3 ± 16.1 | 922.6 ± 36.9 | 28.6 ± 1.2 | 0.944 | 1.54 | - |
| 1.5 × 10−3 | 267.8 ± 10.7 | 816.8 ± 32.6 | 140.5 ± 5.6 | 0.987 | 1.86 | 79.64 ± 0.04 |
| 3.0 × 10−3 | 180.2 ± 8.5 | 650.1 ± 19.1 | 194.8 ± 9.3 | 0.978 | 2.48 | 85.3 ± 0.09 |
| 4.5 × 10−3 | 167.5 ± 9.2 | 316.3 ± 17.4 | 251.5 ± 13.8 | 0.969 | 3.36 | 88.6 ± 0.1 |
| 6.0 × 10−3 | 102.8 ± 5.1 | 257.4 ± 12.9 | 309.1 ± 15.5 | 0.97 | 6.22 | 90.7 ± 0.1 |
| C-NAC/ mol L−1 | Bode Parameters | |||||
| log Z | Z/Ωcm2 | Phase/degrees | ||||
| 0 | 1.43 | 26.9 | −61.85 | |||
| 1.5 × 10−3 | 2.15 | 142.8 | −74.77 | |||
| 3.0 × 10−3 | 2.29 | 194.9 | −74.85 | |||
| 4.5 × 10−3 | 2.41 | 257.0 | 73.97 | |||
| 6.0 × 10−3 | 2.50 | 316.2 | −75.65 | |||
| Electrochemical Measurement | Slope | f | α | K/ L mol−1 | R2 | kJ mol−1 |
|---|---|---|---|---|---|---|
| PDP | 0.1712 | 5.84 | −2.92 | 28,566.8 | 0.9956 | −35.3 |
| EIS | 0.1279 | 7.82 | −3.91 | 29,436.8 | 0.9993 | −35.4 |
| Adsorption Isotherm | Linearized form | Slope | Intercept | ||||
|---|---|---|---|---|---|---|---|
| Freundlich | lnθ = f(lnC) | n | lnK | R2 | |||
| PDP | EIS | PDP | EIS | PDP | EIS | ||
| 0.2236 | 0.0947 | 1.0222 | 0.3904 | 0.9917 | 0.9868 | ||
| Langmuir | C/θ = f (C) | 1 | 1/K | R2 | |||
| 0.98 | 1.048 | 0.0009 | 0.0005 | 0.9954 | 0.996 | ||
[θ/(1 − θ)] = f(C) | K | 0 | |||||
| 1180 | 1558 | −0.5 | 0.77 | 0.9946 | 0.9735 | ||
| Flory–Huggins | ln(θ/C) = f(1 − θ) | b | lnK | R2 | |||
| 1.4076 | 1.5916 | 8.7072 | 7.8147 | 0.9506 | 0.9846 | ||
| El-Awady’s model | = f(lnC) | y | lnK | R2 | |||
| 0.9996 | 0.6670 | 7.0521 | 5.6759 | 0.9856 | 0.9915 | ||
| Frumkin | 2α | lnK | R2 | ||||
| NA | −4.3513 | - | 11.322 | - | 0.9751 | ||
| Amino Acid | EHOMO/eV | ELUMO/eV | ΔE/eV | I/eV | A/eV | Reference |
|---|---|---|---|---|---|---|
| N-acetylcysteine | −6.95 | −0.90 | 6.05 | 6.95 | 0.90 | This study |
| Phenylalanine a | −6.94 | −0.431 | 6.48 | - | - | [22] |
| Aspartic acid a | −7.06 | −0.314 | 6.741 | - | - | [22] |
| L-cysteine b | −6.745 | −0.804 | 5.941 | - | - | [4] |
| Serine c | −7.15 | −0.644 | 6.506 | - | - | [27] |
| Tryptophan c | −5.82 | −0.811 | 5.009 | - | - | [27] |
| Tyrosine c | −6.36 | −0.798 | 5.562 | - | - | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samide, A.; Dobriţescu, A.; Tigae, C.; Spînu, C.I.; Oprea, B. Experimental and Computational Study on Inhibitory Effect and Adsorption Properties of N-Acetylcysteine Amino Acid in Acid Environment. Molecules 2023, 28, 6799. https://doi.org/10.3390/molecules28196799
Samide A, Dobriţescu A, Tigae C, Spînu CI, Oprea B. Experimental and Computational Study on Inhibitory Effect and Adsorption Properties of N-Acetylcysteine Amino Acid in Acid Environment. Molecules. 2023; 28(19):6799. https://doi.org/10.3390/molecules28196799
Chicago/Turabian StyleSamide, Adriana, Aurelian Dobriţescu, Cristian Tigae, Cezar Ionuţ Spînu, and Bogdan Oprea. 2023. "Experimental and Computational Study on Inhibitory Effect and Adsorption Properties of N-Acetylcysteine Amino Acid in Acid Environment" Molecules 28, no. 19: 6799. https://doi.org/10.3390/molecules28196799
APA StyleSamide, A., Dobriţescu, A., Tigae, C., Spînu, C. I., & Oprea, B. (2023). Experimental and Computational Study on Inhibitory Effect and Adsorption Properties of N-Acetylcysteine Amino Acid in Acid Environment. Molecules, 28(19), 6799. https://doi.org/10.3390/molecules28196799




