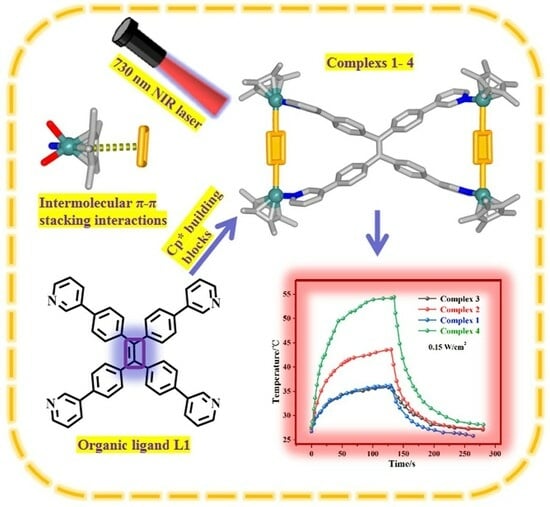
A Coordination-Driven Self-Assembly and NIR Photothermal Conversion Study of Organometallic Handcuffs
Abstract
:1. Introduction
2. Results and Discussion
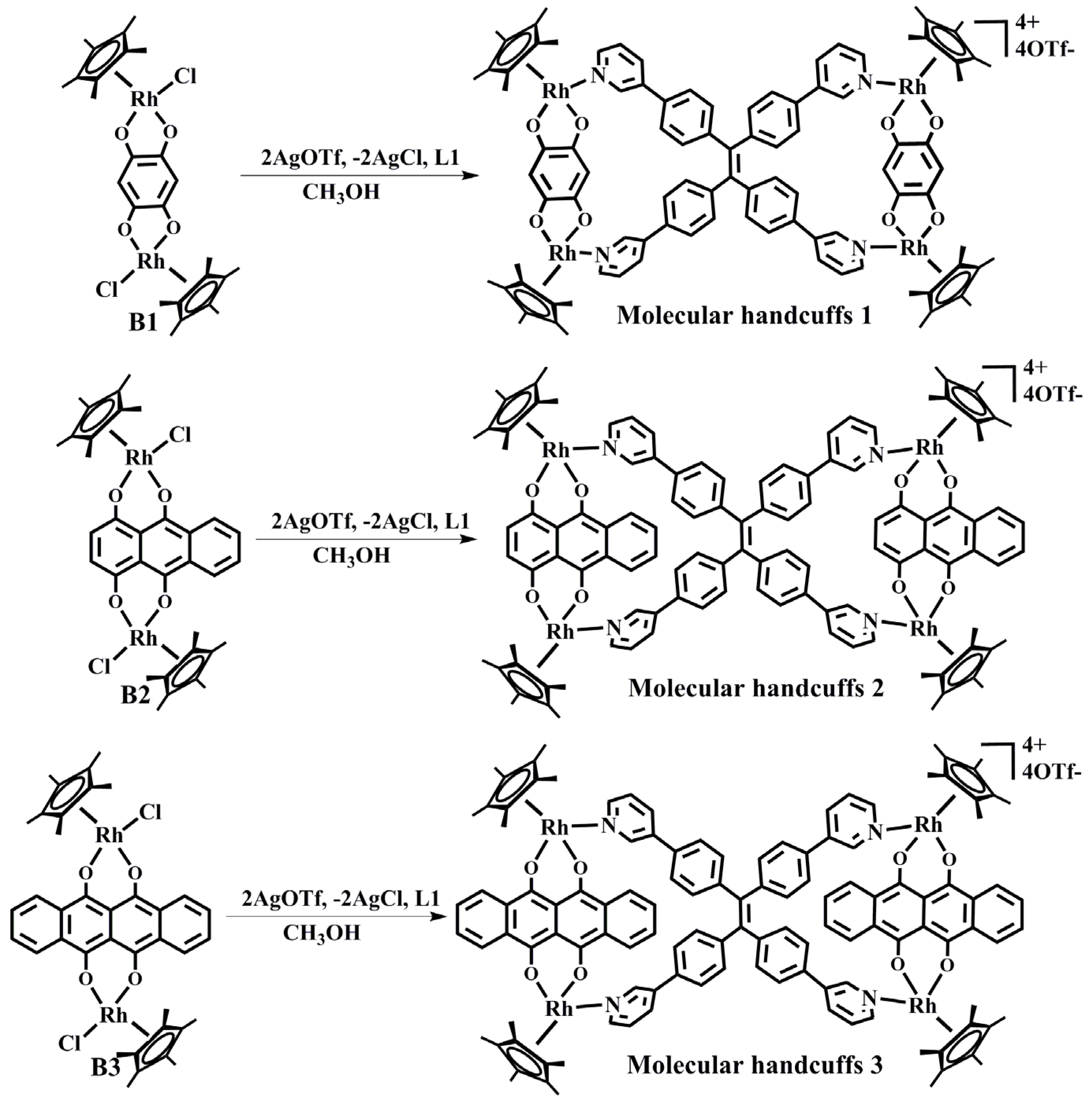
2.1. The Synthesis of Organometallic Handcuffs 1, 2, 3
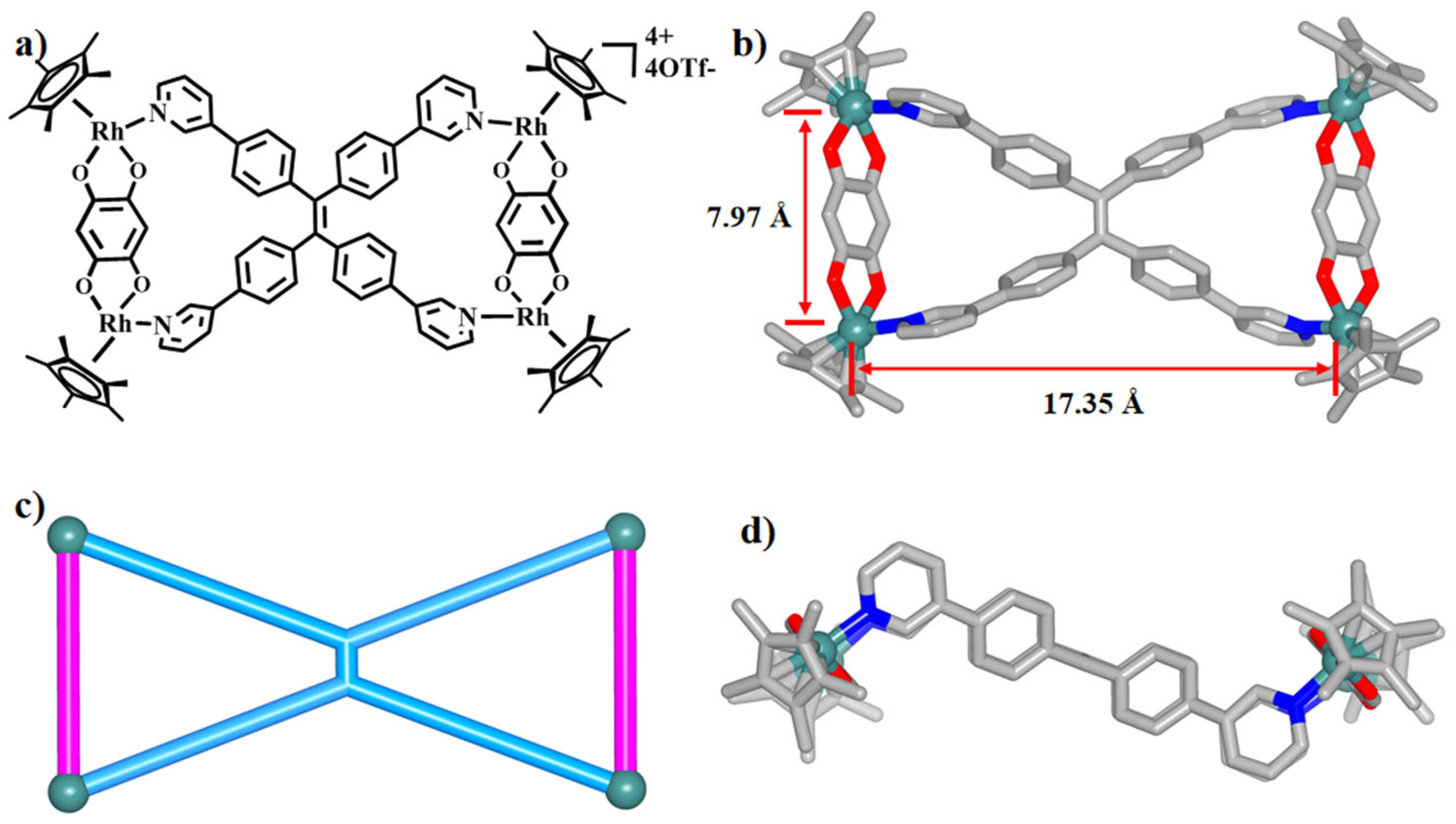
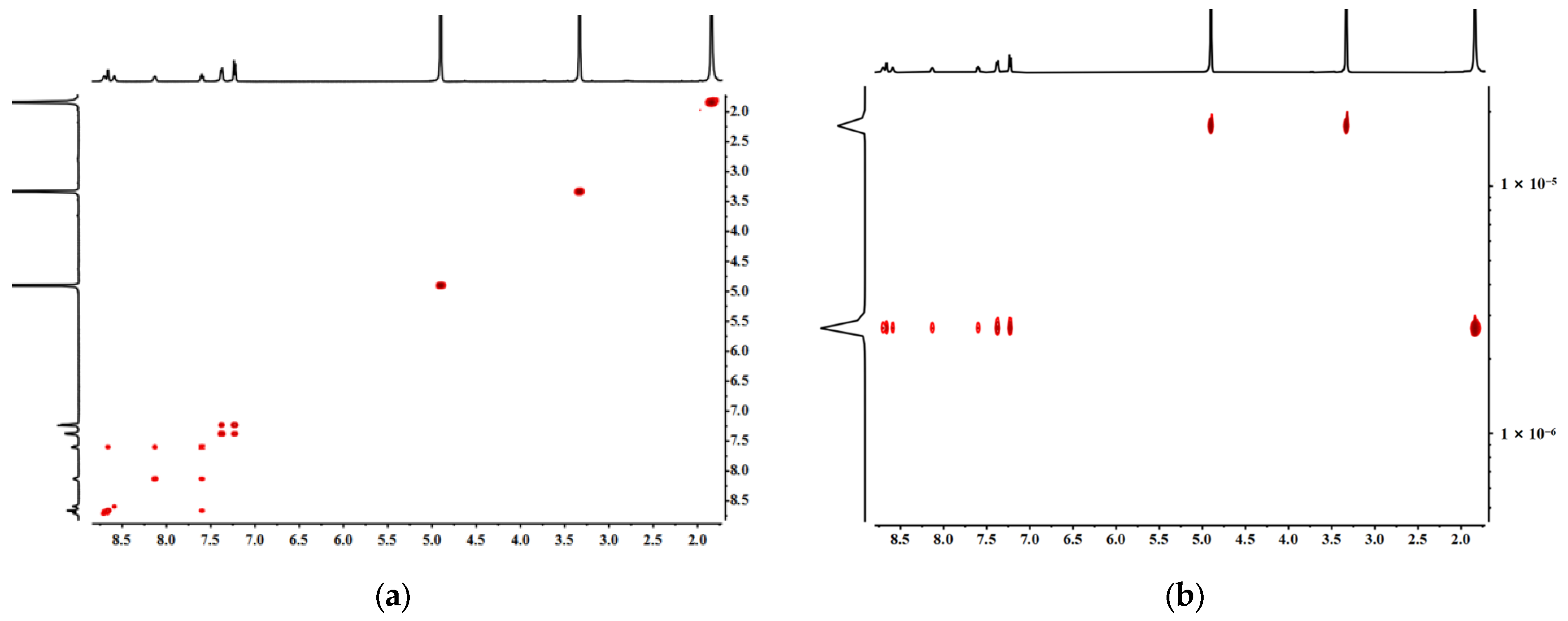
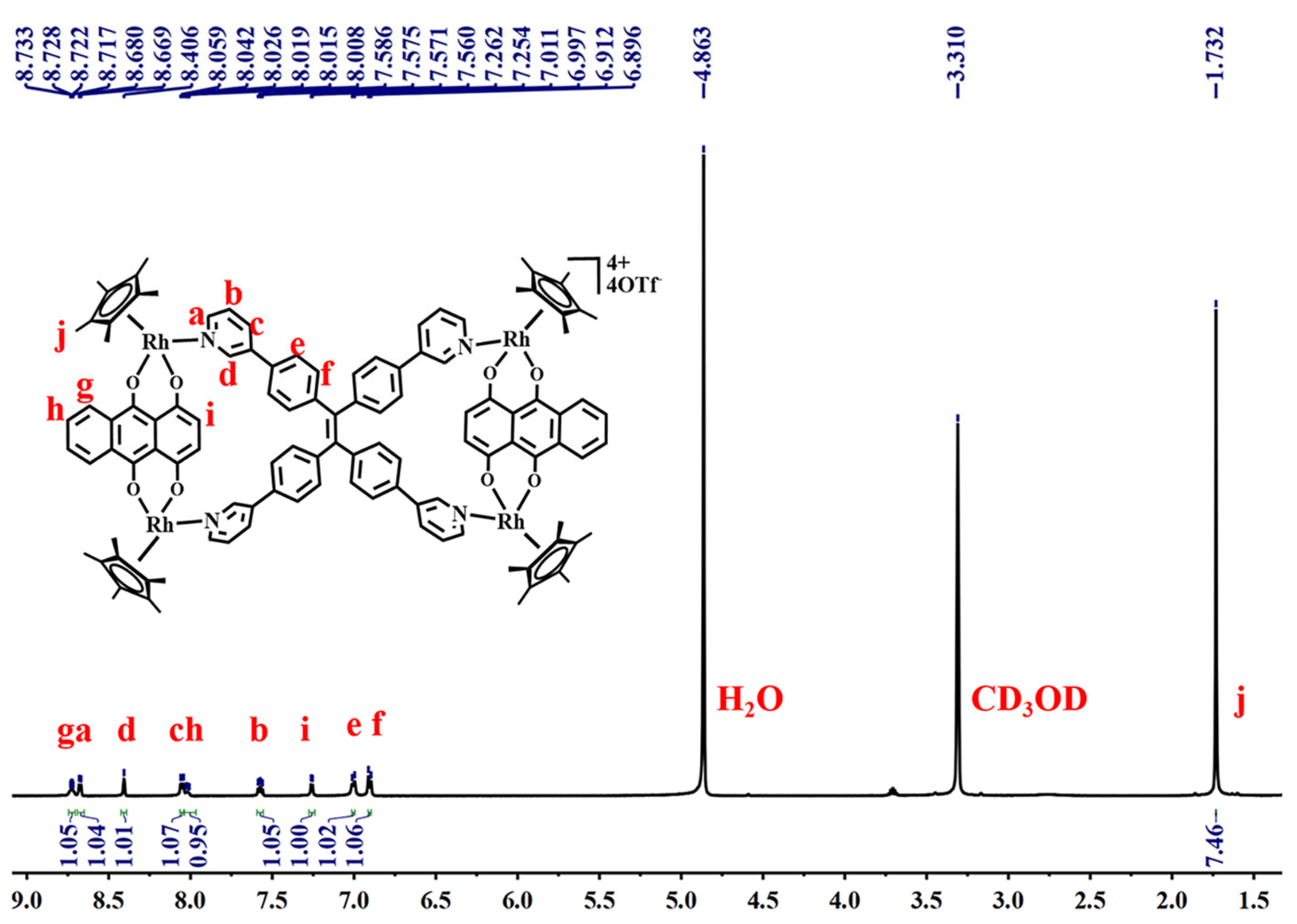
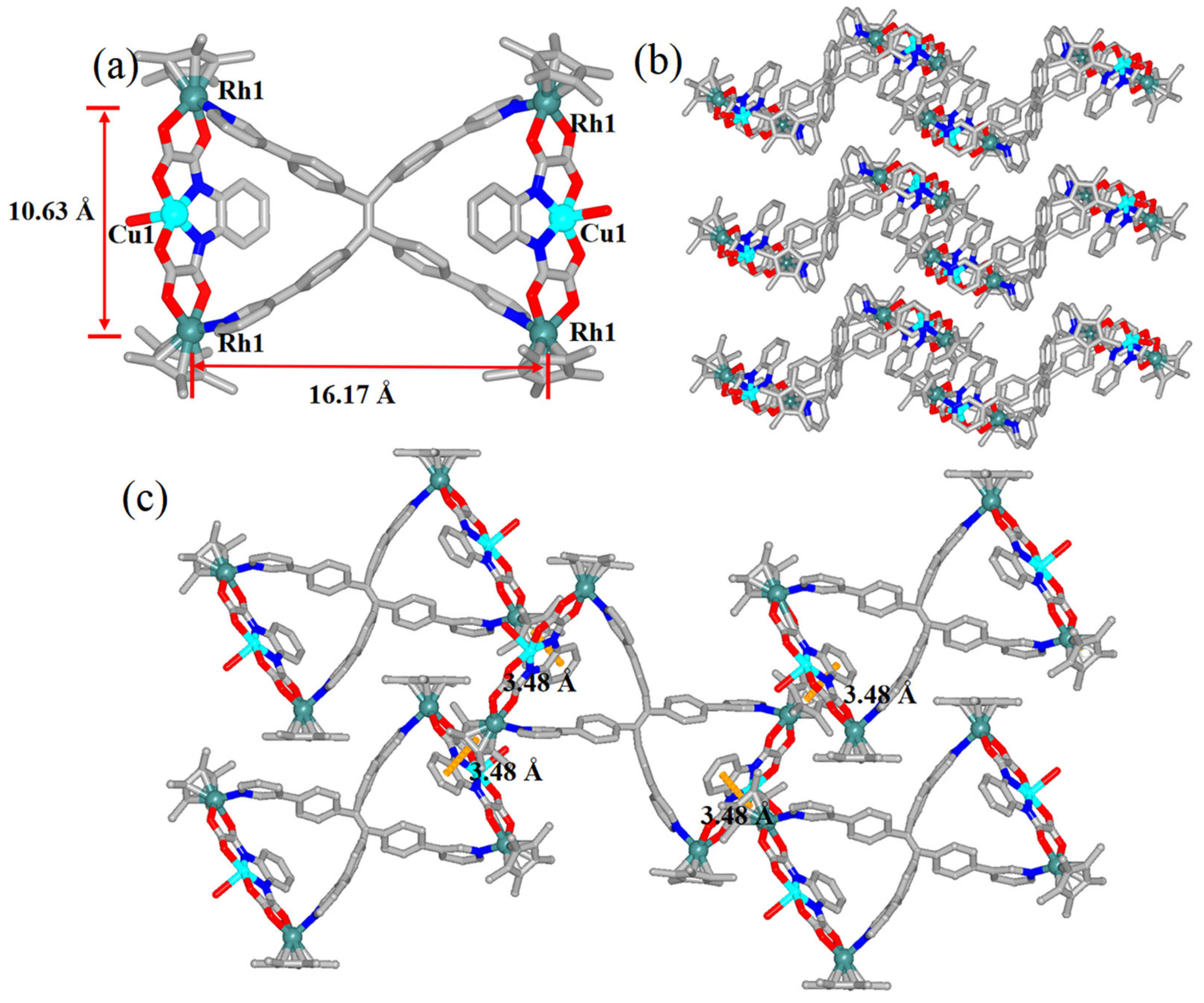
2.2. The Self-Assembly and Structural Analysis of Molecular Handcuffs 1
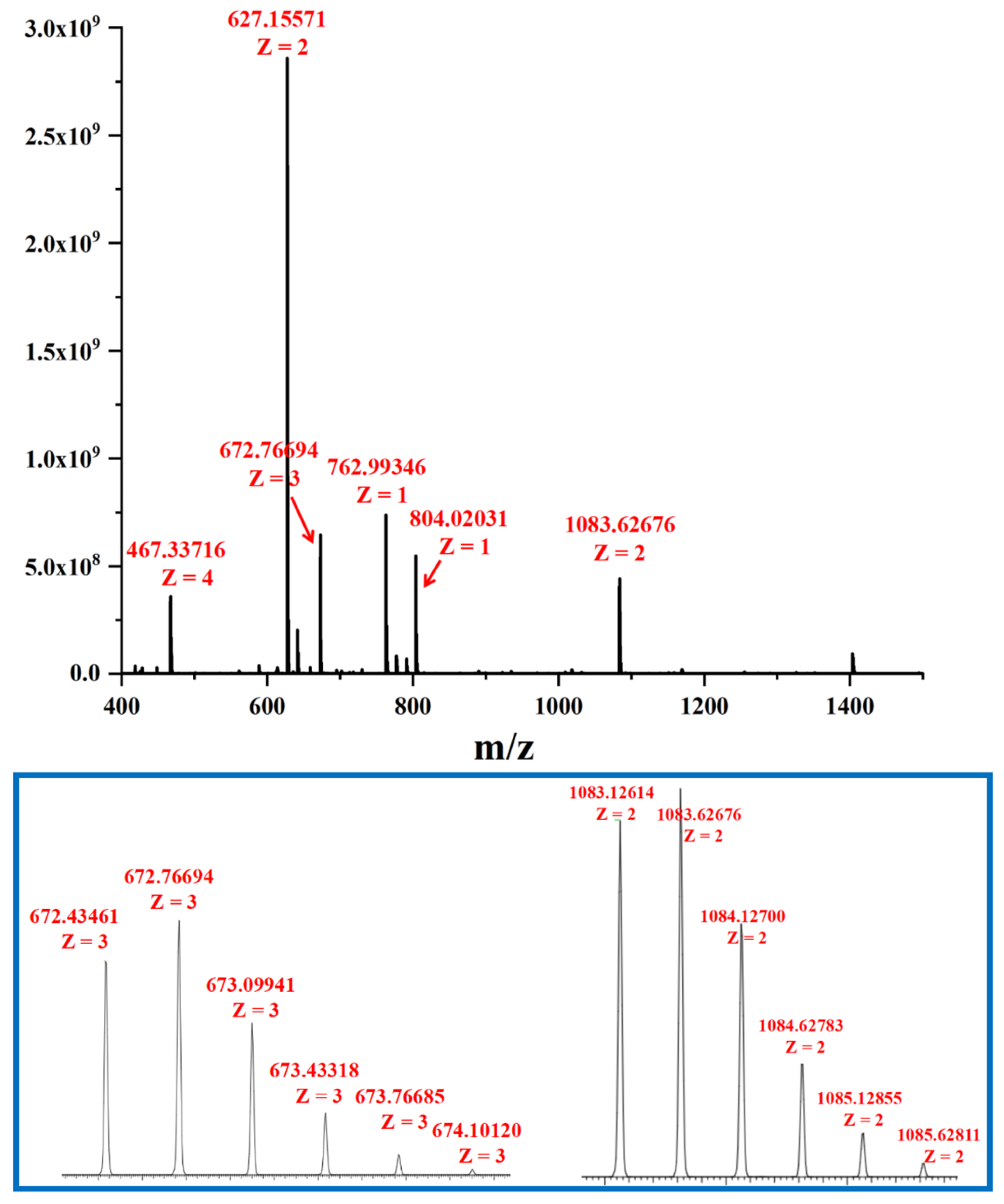
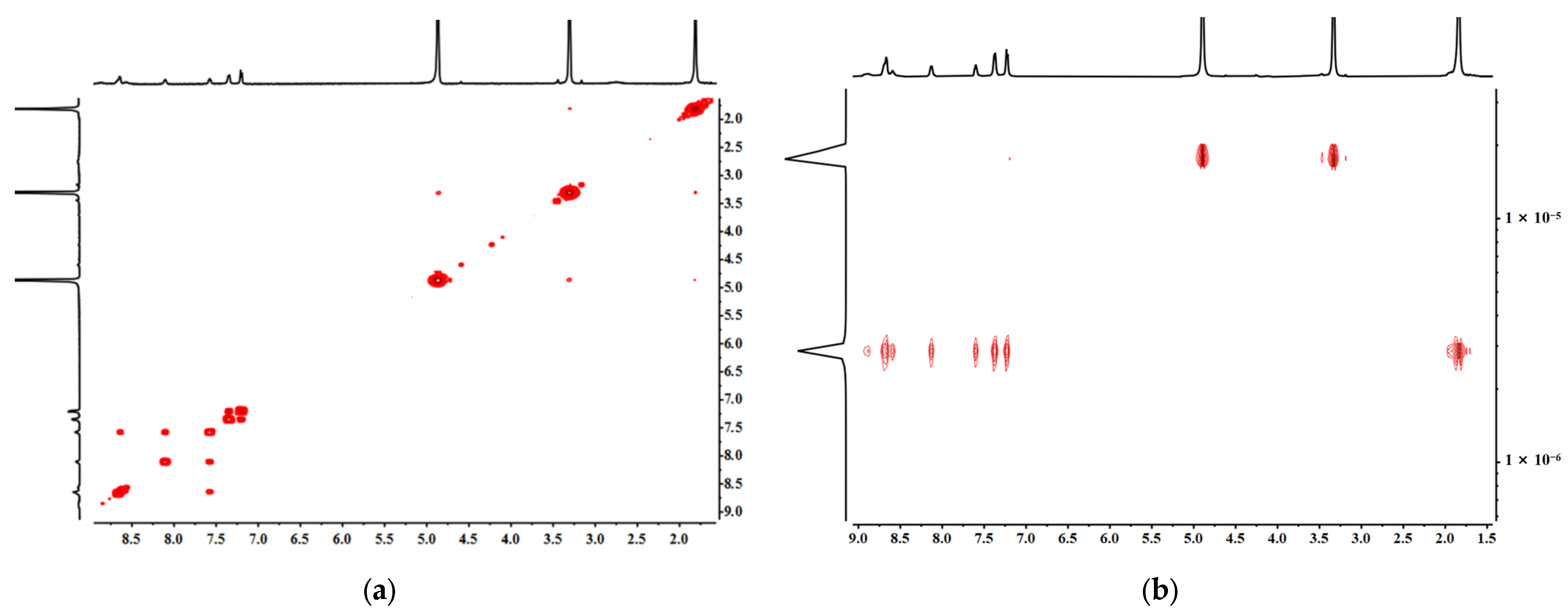
2.3. The Self-Assembly and Structural Analysis of Molecular Handcuffs 2 and 3
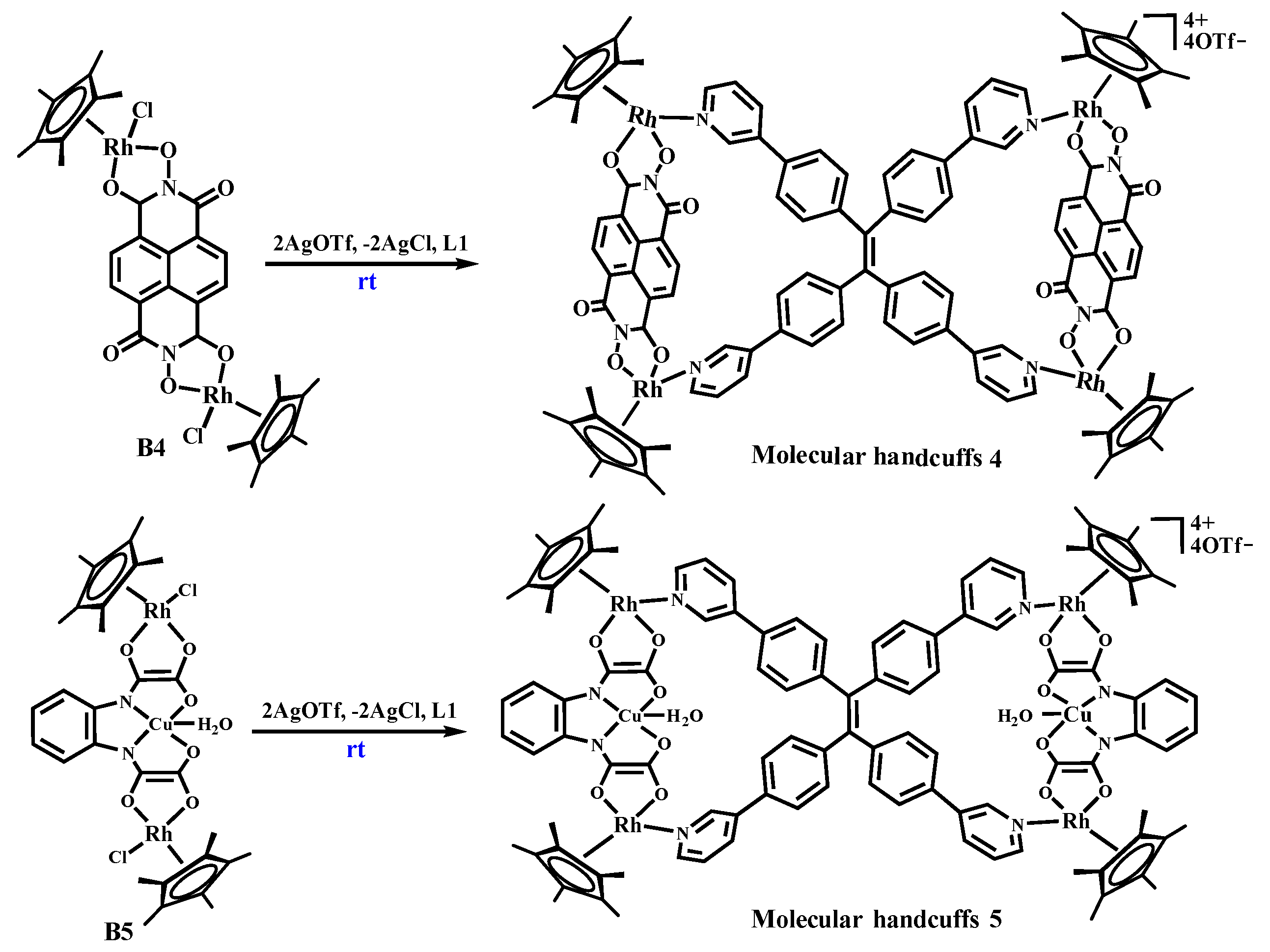
2.4. The Self-Assembly of Molecular Handcuffs 4 and Heterometallic Handcuffs 5
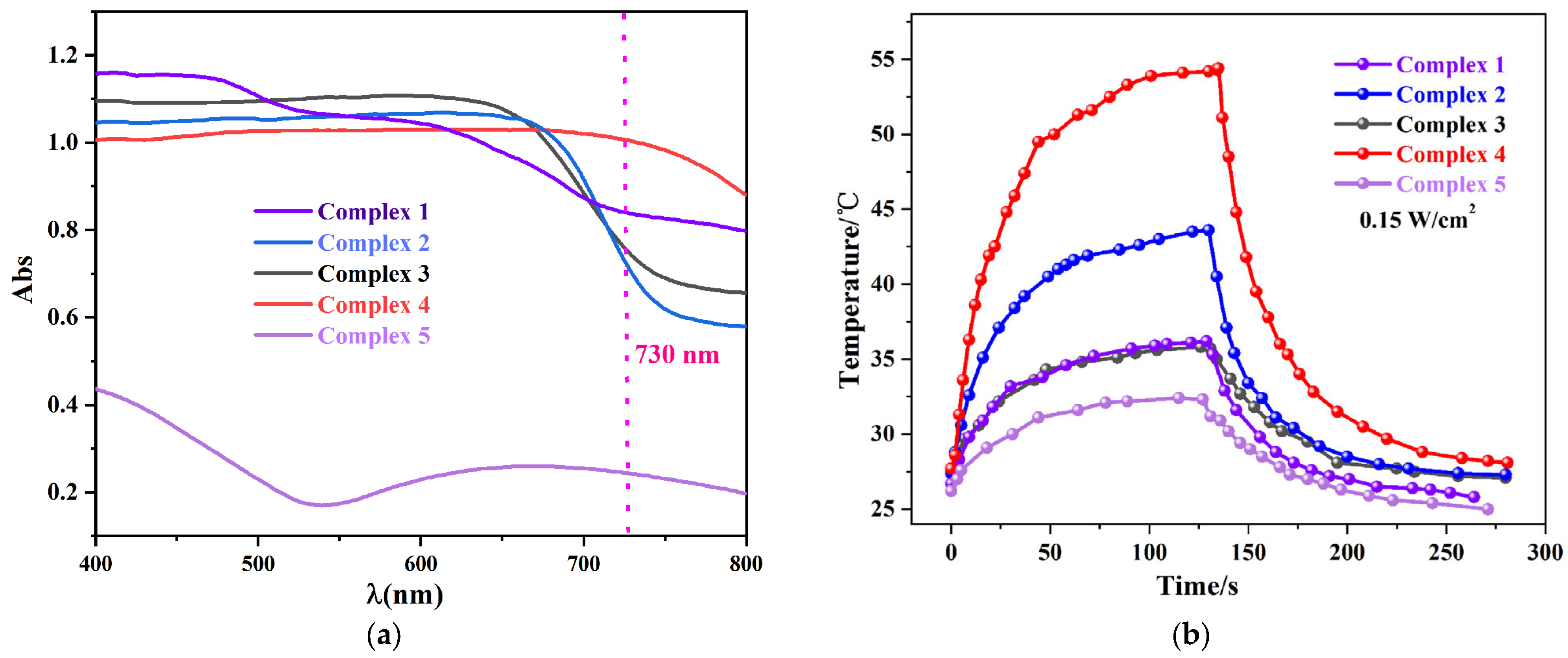
2.5. Photothermal Conversion Studies
3. Experiment
Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Percástegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and applications of Water-Soluble coordination cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef]
- Northrop, B.H.; Zheng, Y.R.; Chi, K.W.; Stang, P.J. Self-Organization in Coordination-Driven Self-Assembly. Acc. Chem. Res. 2009, 42, 1554–1563. [Google Scholar] [CrossRef]
- Clever, G.H.; Punt, P. Cation–Anion arrangement patterns in Self-Assembled Pd2L4 and Pd4L8 coordination cages. Acc. Chem. Res. 2017, 50, 2233–2243. [Google Scholar] [CrossRef]
- Ibáñez, S.; Poyatos, M.; Peris, E. N-Heterocyclic carbenes: A door open to supramolecular organometallic chemistry. Acc. Chem. Res. 2020, 53, 1401–1413. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Peng, F.; Meng, F.; Zha, J.J.; Ma, L.; Du, Y.H.; Peng, N.; Ma, L.F.; Zhang, Q.H.; et al. Intercalation-activated layered MoO3 nanobelts as biodegradable nanozymes for tumor-specific photo-enhanced catalytic therapy. Angew. Chem. Int. Ed. 2022, 61, e202115939. [Google Scholar] [CrossRef]
- Pöthig, A.; Casini, A. Recent developments of supramolecular Metal-based structures for applications in cancer therapy and imaging. Theranostics 2019, 9, 3150–3169. [Google Scholar] [CrossRef]
- Shan, W.L.; Lin, Y.J.; Hahn, F.E.; Jin, G.X. Highly selective synthesis of Iridium(III) metalla [2]catenanes through component Pre-Orientation by π⋅⋅⋅π stacking. Angew. Chem. Int. Ed. 2019, 58, 5882–5886. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-Assembly of finite Two- and Three-Dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Garci, A.; David, A.H.G.; Le Bras, L.; Ovalle, M.; Abid, S.; Young, R.M.; Liu, W.; Azad, C.S.; Brown, P.J.; Wasielewski, M.R.; et al. Thermally Controlled Exciplex Fluorescence in a Dynamic Homo[2]catenane. J. Am. Chem. Soc. 2022, 144, 23551–23559. [Google Scholar] [CrossRef]
- Denis, M.; Goldup, S.M. A [3]Rotaxane host selects between stereoisomers. Angew. Chem. Int. Ed. 2018, 57, 4462–4464. [Google Scholar] [CrossRef]
- Hu, S.J.; Guo, X.Q.; Zhou, L.P.; Yan, D.N.; Cheng, P.M.; Cai, L.X.; Li, X.Z.; Sun, Q.F. Guest-driven self-assembly and chiral induction of photofunctional lanthanide tetrahedral cages. J. Am. Chem. Soc. 2022, 144, 4244–4253. [Google Scholar] [CrossRef]
- Dang, L.L.; Sun, Z.B.; Shan, W.L.; Lin, Y.J.; Li, Z.H.; Jin, G.X. Coordination-driven self-assembly of a molecular figure-eight knot and other topologically complex architectures. Nat. Commun. 2019, 10, 2057. [Google Scholar] [CrossRef]
- Wang, H.; Qin, J.; Huang, C.; Han, Y.; Xu, W.; Hou, H. Mono/bimetallic water-stable lanthanide coordination polymers as luminescent probes for detecting cations, anions and organic solvent molecules. Dalton Trans. 2016, 45, 12710–12716. [Google Scholar] [CrossRef]
- Forgan, R.S.; Sauvage, J.-P.; Stoddart, J.F. Chemical Topology: Complex Molecular Knots, Links, and Entanglements. Chem. Rev. 2011, 111, 5434–5464. [Google Scholar] [CrossRef]
- Sauvage, J.-P. From Chemical Topology to Molecular Machines (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11080–11093. [Google Scholar] [CrossRef]
- Dang, L.L.; Li, T.T.; Zhang, T.T.; Zhao, Y.; Chen, T.; Gao, X.; Ma, L.F.; Jin, G.X. Highly selective synthesis and near-infrared photothermal conversion of metalla-Borromean ring and [2]catenane assemblies. Chem. Sci. 2022, 13, 5130–5140. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, H.-N.; Lin, Y.-J.; Jin, G.-X. Selective Construction of Molecular Borromean Rings And [2]catenane Utilizing Ether Bipyridyl Ligands. Chem. Eur. J. 2023, 29, e202204038. [Google Scholar] [CrossRef]
- Zhou, M.-Y.; Yu, Z.-S.; Deng, W.; Lu, H.-L.; Niu, X.-F.; Tong, J.; Yu, S.-Y.; Fujita, M. [M8L4]8+-Type Squares Self-Assembled by Dipalladium Corners and Bridging Aromatic Dipyrazole Ligands for Iodine Capture. Inorg. Chem. 2023, 62, 10193–10202. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, X.; Lin, Y.-J.; Jin, G.-X. Stereoselective self-assembly of complex chiral radial [5]catenanes using half-sandwich rhodium/iridium building blocks. J. Am. Chem. Soc. 2022, 144, 2379–2386. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, H.-N.; Mu, Q.-S.; Dang, L.-L.; Jin, G.-X. Selective Construction of Borromean Rings and Tweezer-Like Molecular Assembly Featuring Cp*Rh/Ir Clips for Near-Infrared Photothermal Conversion. Chin. J. Chem. 2023, 41, 3229–3237. [Google Scholar] [CrossRef]
- Gao, W.X.; Feng, H.J.; Lin, Y.J.; Jin, G.X. Covalent Post-assembly modification triggers structural transformations of borromean rings. J. Am. Chem. Soc. 2019, 141, 9160–9164. [Google Scholar] [CrossRef]
- Gao, W.X.; Feng, H.J.; Guo, B.B.; Lu, Y.; Jin, G.X. Coordination-Directed Construction of Molecular Links. Chem. Rev. 2020, 120, 6288–6325. [Google Scholar] [CrossRef]
- Cui, Z.; Mu, Q.-S.; Gao, X.; Jin, G.-X. Stereoselective Construction of Chiral Linear [3]Catenanes and [2]Catenanes. J. Am. Chem. Soc. 2023, 145, 725–731. [Google Scholar] [CrossRef]
- Zhang, L.; Stephens, A.J.; Lemonnier, J.F.; Pirvu, L.; Vitorica-Yrezabal, I.J.; Robinson, C.J.; Leigh, D.A. Coordination chemistry of a Molecular pentafoil knot. J. Am. Chem. Soc. 2019, 141, 3952–3958. [Google Scholar] [CrossRef]
- Zhang, L.; Stephens, A.J.; Nussbaumer, A.L.; Lemonnier, J.F.; Jurcek, P.; Vitorica-Yrezabal, I.J.; Leigh, D.A. Stereoselective synthesis of a composite knot with nine crossings. Nat. Chem. 2018, 10, 1083–1088. [Google Scholar] [CrossRef]
- Leigh, D.A.; Schaufelberger, F.; Piruv, L.; Stenlid, J.H.; August, D.P.; Segard, J. Tying different knots in a molecular strand. Nature 2020, 584, 562–568. [Google Scholar] [CrossRef]
- Inomata, Y.; Sawada, T.; Fujita, M. Metal-Peptide Torus Knots from Flexible Short Peptides. Chem 2020, 6, 294–303. [Google Scholar] [CrossRef]
- Leigh, D.A.; Danon, J.J.; Fielden, S.D.P.; Lemonnier, J.-F.; Whitehead, G.F.S.; Woltering, S.L. A molecular endless (74) knot. Nat. Chem. 2021, 13, 117–122. [Google Scholar] [CrossRef]
- Dang, L.L.; Zhang, T.T.; Chen, T.; Zhao, Y.; Gao, X.; Francisco, A.; Ma, L.F.; Jin, G.X. Selective Synthesis and Structural Transformation of a 4-Ravel Containing Four Crossings and Featuring Cp*Rh/Ir Fragments. Angew. Chem. Int. Ed. 2023, 62, e202301516. [Google Scholar] [CrossRef]
- Liu, X.-R.; Cui, P.-F.; Guo, S.-T.; Lin, Y.-J.; Jin, G.-X. “Cage Walking” Synthetic Strategy for Unusual Unsymmetrical Supramolecular Cages. J. Am. Chem. Soc. 2023, 145, 8569–8575. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.; Cui, Z.; Lin, Y.J.; Jin, G.X. Adaptive Self-Assembly and Induced-Fit interconversions between molecular borromean rings, russian dolls and Ring-in-Ring complexes. Chin. J. Chem. 2021, 39, 360–366. [Google Scholar] [CrossRef]
- Liu, G.; Liu, X.-Q.; Sun, L.-B. Recent Advances in Metal-Organic Cages-Based Composite Membranes. Chin. J. Struct. Chem. 2022, 41, 2211100–2211109. [Google Scholar]
- Zhang, H.N.; Yu, W.B.; Lin, Y.J.; Jin, G.X. Stimuli-Responsive topological transformation of a molecular borromean ring via controlled oxidation of thioether moieties. Angew. Chem. Int. Ed. 2021, 60, 15466–15471. [Google Scholar] [CrossRef]
- Bai, S.; Han, Y.-F. Metal-N-Heterocyclic Carbene Chemistry Directed toward Metallosupramolecular Synthesis and Beyond. Acc. Chem. Res. 2023, 56, 1213–1227. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.-G.; Ma, L.-L.; Li, M.; An, Y.-Y.; Han, Y.-F. Strategies for the construction of supramolecular assemblies from poly-NHC ligand precursors. Sci. China. Chem. 2021, 64, 701–718. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Bai, S.; Wang, Y.-Y.; Han, Y.-F. A strategy for the construction of triply interlocked organometallic cages by rational design of poly-NHC Precursors. J. Am. Chem. Soc. 2020, 142, 13614–13621. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Y.; Dang, L.-L.; Zhang, T.-T.; Lu, X.-L.; Chai, Y.-H.; Aznarez, F.; Ma, L.-F. Self-Assembly and Photothermal Conversion of MetallaRussian Doll and Metalla[2]catenanes Induced via Multiple Stacking Interactions. J. Am. Chem. Soc. 2023, 145, 18036–18047. [Google Scholar] [CrossRef]
- Li, G.F.; Zhou, Z.X.; Yuan, C.; Guo, Z.W.; Liu, Y.H.; Zhao, D.; Liu, K.; Zhao, J.; Tan, H.W.; Yan, X.Z. TrackableSupramolecular Fusion: Cage to Cage Transformation of Tetraphenylethylene-Based Metallo-assemblies. Angew. Chem. Int. Ed. 2020, 59, 10013–10017. [Google Scholar] [CrossRef]
- Mu, C.Q.; Zhang, Z.Y.; Hou, Y.L.; Liu, H.F.; Ma, L.Z.; Li, X.P.; Ling, S.L.; He, G.; Zhang, M.M. Tetraphenylethylene-Based Multicomponent Emissive Metallacages as Solid-State Fluorescent Materials. Angew. Chem. Int. Ed. 2021, 60, 12293–12297. [Google Scholar] [CrossRef]
- Zhang, H.-N.; Feng, H.-J.; Lin, Y.-J.; Jin, G.-X. Cation-Templated Assembly of 613 and 623 Metalla-Links. J. Am. Chem. Soc. 2023, 145, 4746–4756. [Google Scholar] [CrossRef]
- Yang, Y.; Ronson, T.K.; Lu, Z.; Zheng, J.; Vanthuyne, N.; Martinez, A.; Nitschke, J.R. A curved host and second guest cooperatively inhibit the dynamic motion of corannulene. Nat. Commun. 2021, 12, 4079. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhang, J.R.; Tian, X.K.; Qin, J.H.; Zhang, X.Y.; Ma, L.F. Enhanced Activity of Enzyme Immobilized on Hydrophobic ZIF-8 Modified by Ni2+ Ions. Angew. Chem. Int. Ed. 2023, 62, e202216699. [Google Scholar] [CrossRef]
- Zhang, Z.-E.; Zhang, Y.-F.; Zhang, Y.-Z.; Li, H.-L.; Sun, L.-Y.; Wang, L.-J.; Han, Y.-F. Construction and Hierarchical Self-Assembly of Multifunctional Coordination Cages with Triangular Metal-Metal-Bonded Units. J. Am. Chem. Soc. 2023, 145, 7446–7453. [Google Scholar] [CrossRef]
- Guo, S.-T.; Cui, P.-F.; Liu, X.-R.; Jin, G.-X. Synthesis of Carborane-Backbone Metallacycles for Highly Selective Capture of n-Pentane. J. Am. Chem. Soc. 2022, 144, 22221–22228. [Google Scholar] [CrossRef]
- Cui, P.-F.; Liu, X.-R.; Lin, Y.-J.; Li, Z.-H.; Jin, G.-X. Highly Selective Separation of Benzene and Cyclohexane in a Spatially Confined Carborane Metallacage. J. Am. Chem. Soc. 2022, 144, 6558–6565. [Google Scholar] [CrossRef]
- Chen, R.; Chen, G.; He, Y.; Zhang, J. Coordination Assembly of Tetrahedral Ti4 (embonate)6 Cages with Alkaline-Earth Metal Ions. Chin. J. Struct. Chem. 2022, 41, 2201001–2201006. [Google Scholar]
- Guo, J.; Fan, Y.Z.; Lu, Y.L.; Zheng, S.P.; Su, C.Y. Visible-light photocatalysis of asymmetric [2+2] cycloaddition in cage-confined nanospace merging chirality with triplet-state photosensitization. Angew. Chem. Int. Ed. 2020, 59, 8661–8669. [Google Scholar] [CrossRef]
- Cai, L.X.; Li, S.C.; Yan, D.N.; Zhou, L.P.; Guo, F.; Sun, Q.F. Water-Soluble Redox-Active Cage Hosting Polyoxometalates for Selective Desulfurization Catalysis. J. Am. Chem. Soc. 2018, 140, 4869–4876. [Google Scholar] [CrossRef]
- Wang, L.-J.; Li, X.; Bai, S.; Wang, Y.-Y.; Han, Y.-F. Self-Assembly, Structural Transformation, and Guest-Binding Properties of Supramolecular Assemblies with Triangular Metal-Metal Bonded Units. J. Am. Chem. Soc. 2020, 142, 2524–2531. [Google Scholar] [CrossRef]
- Wang, L.-J.; Bai, S.; Han, Y.-F. Water-Soluble Self-Assembled Cage with Triangular Metal–Metal-Bonded Units Enabling the Sequential Selective Separation of Alkanes and Isomeric Molecules. J. Am. Chem. Soc. 2022, 144, 16191–16198. [Google Scholar] [CrossRef]
- Liu, T.; Bai, S.; Zhang, L.; Hahn, F.E.; Han, Y.-F. N-Heterocyclic Carbene-Stabilized Metal Nanoparticles within Porous Organic Cages for Catalytic Applications. Natl. Sci. Rev. 2022, 9, nwac067. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, H.; Shao, Y.; Wang, P.; Yu, S.; Li, H.; Zhou, Y.; Zhou, Z.; Ma, L.; Tan, C. Silver nanoparticle-modified 2D MOF nanosheets for photothermally enhanced silver ion release antibacterial treatment. Acta Phys. Chim. Sin. 2023, 39, 2211043. [Google Scholar] [CrossRef]
- Liu, J.-C.; Wang, J.-F.; Han, Q.; Shangguan, P.; Liu, L.-L.; Chen, L.-J.; Zhao, J.-W.; Streb, C.; Song, Y.-F. Multicomponent Self-Assembly of a Giant Heterometallic Polyoxotungstate Supercluster with Antitumor Activity. Angew. Chem. Int. Ed. 2021, 60, 11153–11157. [Google Scholar] [CrossRef]
- Lu, H.-L.; Tong, J.; Ma, H.-W.; Yu, S.-Y. Iodine Adsorption Studies of Dipalladium-based Supramolecular Cages. Chin. J. Struct. Chem. 2021, 40, 1680–1686. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Huang, J.-J.; Chen, T.; Zheng, J.; Liu, M.; Wang, X.-Y.; Li, Y.-X.; Niu, X.; Dang, L.-L. A Coordination-Driven Self-Assembly and NIR Photothermal Conversion Study of Organometallic Handcuffs. Molecules 2023, 28, 6826. https://doi.org/10.3390/molecules28196826
Lu X, Huang J-J, Chen T, Zheng J, Liu M, Wang X-Y, Li Y-X, Niu X, Dang L-L. A Coordination-Driven Self-Assembly and NIR Photothermal Conversion Study of Organometallic Handcuffs. Molecules. 2023; 28(19):6826. https://doi.org/10.3390/molecules28196826
Chicago/Turabian StyleLu, Xiaoyan, Jing-Jing Huang, Tian Chen, Jie Zheng, Ming Liu, Xin-Yi Wang, Yu-Xin Li, Xinkai Niu, and Li-Long Dang. 2023. "A Coordination-Driven Self-Assembly and NIR Photothermal Conversion Study of Organometallic Handcuffs" Molecules 28, no. 19: 6826. https://doi.org/10.3390/molecules28196826
APA StyleLu, X., Huang, J.-J., Chen, T., Zheng, J., Liu, M., Wang, X.-Y., Li, Y.-X., Niu, X., & Dang, L.-L. (2023). A Coordination-Driven Self-Assembly and NIR Photothermal Conversion Study of Organometallic Handcuffs. Molecules, 28(19), 6826. https://doi.org/10.3390/molecules28196826