Ternary Organic Solar Cells by Small Amount of Efficient Light Absorption Polymer PSEHTT as Third Component Materials
Abstract
:1. Introduction
2. Results and Discussions
3. Experimental Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhou, H.; Zhang, L.; Ma, X.; Xibei, Y.; Zheng, Y.; Liu, Z.; Gao, X.; Zhang, J.; Liu, Z.; Zhang, F. Approaching 18% efficiency of ternary layer-by-layer polymer solar cells with alloyed acceptors. Chem. Eng. J. 2023, 462, 142327. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Liang, Z.; Wang, L.; Han, S.; Dou, Z.; Lu, X.; Tong, J.; Liu, J.; Li, J. Efficient ternary organic photovoltaic device with a non-halogenated solvent via synergistic inhibiting charge recombination and regulating morphology. J. Mater. Chem. C 2023, 11, 2871–2879. [Google Scholar] [CrossRef]
- Abdullah; Lee, S.-J.; Park, J.B.; Kim, Y.S.; Shin, H.-S.; Kotta, A.; Siddiqui, Q.T.; Lee, Y.-S.; Seo, H.-K. Linear-Shaped Low-Bandgap Asymmetric Conjugated Donor Molecule for Fabrication of Bulk Heterojunction Small-Molecule Organic Solar Cells. Molecules 2023, 28, 1538. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhi, H.-F.; Zhang, B.; Yang, C.; Mahmood, A.; Zhang, M.; Woo, H.Y.; Zhang, F.; Wang, J.-L.; An, Q. Controlling Morphology and Voltage Loss with Ternary Strategy Triggers Efficient All-Small-Molecule Organic Solar Cells. ACS Energy Lett. 2023, 8, 1058–1067. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Z.; Sun, Y. Polymer Donors for High-Performance Non-Fullerene Organic Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 4442–4453. [Google Scholar] [CrossRef]
- Yang, M.; Fu, S.; Wang, L.; Ren, M.; Li, H.; Han, S.; Lu, X.; Lu, F.; Tong, J.; Li, J. Efficient organic solar cells by modulating photoactive layer morphology with halogen-free additives. Opt. Mater. 2023, 137, 113503. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Niu, L.; Jeong, S.Y.; Woo, H.Y.; Zhou, Z.; Zhang, F. 18.66% Efficiency of Polymer Solar Cells Employing Two Nonfullerene Acceptors with Fluorine or Chlorine Substitution. Sol. RRL 2023, 7, 2200957. [Google Scholar] [CrossRef]
- Jiang, K.; Wei, Q.; Lai, J.Y.L.; Peng, Z.; Kim, H.K.; Yuan, J.; Ye, L.; Ade, H.; Zou, Y.; Yan, H. Alkyl Chain Tuning of Small Molecule Acceptors for Efficient Organic Solar Cells. Joule 2019, 3, 3020–3033. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Bi, P.; Zhang, S.; Chen, Z.; Xu, Y.; Cui, Y.; Zhang, T.; Ren, J.; Qin, J.; Hong, L.; Hao, X.; et al. Reduced non-radiative charge recombination enables organic photovoltaic cell approaching 19% efficiency. Joule 2021, 5, 2408–2419. [Google Scholar] [CrossRef]
- Yan, T.T.; Song, W.; Huang, J.M.; Peng, R.X.; Huang, L.K.; Ge, Z.Y. 16.67% Rigid and 14.06% Flexible Organic Solar Cells Enabled by Ternary Heterojunction Strategy. Adv. Mater. 2019, 31, 1902210–1902217. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Fu, S.; Wang, L.; Du, S.; Dou, Z.; Yang, X.; Li, J. Enhanced performance of inverted polymer solar cells by adding benzyl viologen dichloride into ZnO electron transport layer. Opt. Mater. 2023, 139, 113782. [Google Scholar] [CrossRef]
- Xie, Y.; Li, T.; Guo, J.; Bi, P.; Xue, X.; Ryu, H.S.; Cai, Y.; Min, J.; Huo, L.; Hao, X.; et al. Ternary Organic Solar Cells with Small Nonradiative Recombination Loss. ACS Energy Lett. 2019, 4, 1196–1203. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, Z.; Wang, H.; Zhang, J.; Ma, X.; Zhang, F. Recent Progress of All Polymer Solar Cells with Efficiency Over 15%. Sol. RRL 2023, 7, 2300219. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Q.; Li, G.; Du, M.; Geng, Y.; Sun, X.; Tang, A.; Liu, Y.; Guo, Q.; Zhou, E. Side chain engineering of quinoxaline-based small molecular nonfullerene acceptors for high-performance poly(3-hexylthiophene)-based organic solar cells. Sci. China-Chem. 2019, 62, 254–264. [Google Scholar] [CrossRef]
- Alam, S.; Lee, J. Progress and Future Potential of All-Small-Molecule Organic Solar Cells Based on the Benzodithiophene Donor Material. Molecules 2023, 28, 3171. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, Z.; Du, S.; Niu, X.; Tong, J.; Yang, C.; Lu, X.; Bao, X.; Yan, L.; Li, J.; et al. Two Compatible Acceptors as an Alloy Model with a Halogen-Free Solvent for Efficient Ternary Polymer Solar Cells. Acs Appl. Mater. Interfaces 2022, 14, 9386–9397. [Google Scholar] [CrossRef]
- Dai, T.; Tang, A.; He, Z.; Du, M.; Lei, P.; Wang, Z.; Zeng, Q.; Wang, Y.; Lu, S.; Zhong, Y.; et al. Modulating intermolecular interactions by collaborative material design to realize THF-processed organic photovoltaic with 1.3 V open-circuit voltage. Energy Environ. Sci. 2023, 16, 2199–2211. [Google Scholar] [CrossRef]
- Kan, B.; Yi, Y.-Q.-Q.; Wan, X.; Feng, H.; Ke, X.; Wang, Y.; Li, C.; Chen, Y. Ternary Organic Solar Cells With 12.8% Efficiency Using Two Nonfullerene Acceptors With Complementary Absorptions. Adv. Energy Mater. 2018, 8, 1800424–1800430. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, M.; Ma, X.; Zhu, X.; Jeong, S.Y.; Woo, H.Y.; Zhang, J.; Du, W.; Wang, J.; Liu, X.; et al. Over 17.4% Efficiency of Layer-by-Layer All-Polymer Solar Cells by Improving Exciton Utilization in Acceptor Layer. Adv. Funct. Mater. 2023, 33, 2215204. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, Q.; Geng, Y.; Tang, A.; Zhang, M.; Du, M.; Sun, X.; Zhou, E. Recent advances in PM6:Y6-based organic solar cells. Mater. Chem. Front. 2021, 5, 3257–3280. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Li, H.; Courtright, B.A.E.; Subramaniyan, S.; Jenekhe, S.A. Nonfullerene Polymer Solar Cells with 8.5% Efficiency Enabled by a New Highly Twisted Electron Acceptor Dimer. Adv. Mater. 2016, 28, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Ma, R.; Yang, J.; Gao, J.; Bai, H.; Su, W.; Liang, Z.; Wu, Y.; Tang, L.; Li, Y.; et al. Unidirectional Sidechain Engineering to Construct Dual-Asymmetric Acceptors for 19.23 % Efficiency Organic Solar Cells with Low Energy Loss and Efficient Charge Transfer. Angew. Chem. Int. Ed. 2023, 62, e202308307. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Wang, Z.; Li, Y.; Zhu, H.; Lu, X.; Chen, W.; Qiu, H.; Zhang, Q. 18.02% Efficiency ternary organic solar cells with a small-molecular donor third component. Chem. Eng. J. 2021, 424, 130397. [Google Scholar] [CrossRef]
- Guan, M.; Tao, W.; Xu, L.; Qin, Y.; Zhang, J.; Tan, S.; Huang, M.; Zhao, B. An asymmetric small-molecule donor enables over 18% efficiency in ternary organic solar cells. J. Mater. Chem. A 2022, 10, 9746–9752. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Ji, L.; Hu, H.; Li, G.; Wang, K. A meta-alkylthio-phenyl chain–substituted small-molecule donor as the third component for high-efficiency organic solar cells. J. Mater. Chem. A 2022, 10, 22812–22818. [Google Scholar] [CrossRef]
- Peng, W.; Lin, Y.; Jeong, S.Y.; Genene, Z.; Magomedov, A.; Woo, H.Y.; Chen, C.; Wahyudi, W.; Tao, Q.; Deng, J.; et al. Over 18% Ternary Polymer Solar Cells Enabled by A Terpolymer as The Third Component. Nano Energy 2021, 92, 106681. [Google Scholar] [CrossRef]
- Chen, Z.; Song, W.; Yu, K.; Ge, J.; Zhang, J.; Xie, L.; Peng, R.; Ge, Z. Small-molecular donor guest achieves rigid 18.5% and flexible 15.9% efficiency organic photovoltaic via fine-tuning microstructure morphology. Joule 2021, 5, 2395–2407. [Google Scholar] [CrossRef]
- Zhang, T.; An, C.; Bi, P.; Lv, Q.; Qin, J.; Hong, L.; Cui, Y.; Zhang, S.; Hou, J. A Thiadiazole-Based Conjugated Polymer with Ultradeep HOMO Level and Strong Electroluminescence Enables 18.6% Efficiency in Organic Solar Cell. Adv. Energy Mater. 2021, 11, 2101705. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Liu, Y.; Shi, Y.; Qiu, D.; Deng, D.; Zhang, J.; Wang, B.; Adil, M.A.; Amin, K.; et al. Simultaneously Decreasing the Bandgap and Voc Loss in Efficient Ternary Organic Solar Cells. Adv. Energy Mater. 2022, 12, 2200129. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Xu, J.; Li, C.; Yan, J.; Zhou, G.; Zhong, W.; Hao, T.; Song, J.; Xue, X.; et al. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 2022, 21, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, N. Complementary Light Absorption and Efficient Exciton Dissociation Lead to Efficient and Excellent Ternary Polymer Solar Cells. J. Mater. Chem. A 2020, 8, 3211–3221. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, Z.; Liu, M.; Zhou, Z.; Wang, K.; Ma, X.; Wang, J.; He, Z.; Zhang, F. Employing liquid crystal material as regulator to enhance performance of photomultiplication type polymer photodetectors. Chem. Eng. J. 2022, 427, 131802. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, X.; Ma, X.; Zhou, H.; Li, X.; Jeong, S.Y.; Woo, H.Y.; Zhou, Z.; Sun, Q.; Zhang, F. Achieving 15.81% and 15.29% efficiency of all-polymer solar cells based on layer-by-layer or bulk heterojunction structure. J. Mater. Chem. A 2022, 10, 6291. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Zhang, H.; Zhu, L.; Xu, L.; Zhang, F.; Tsang, C.-S.; Lee, L.Y.S.; Woo, H.Y.; He, Z.; et al. Metallated terpolymer donors with strongly absorbing iridium complex enables polymer solar cells with 16.71% efficiency. Chem. Eng. J. 2022, 430, 132832. [Google Scholar] [CrossRef]
- Wang, J.-L.; Yang, C.; An, Q.; Bai, H.-R.; Zhi, H.-F.; Ryu, H.S.; Mahmood, A.; Zhao, X.; Zhang, S.; Woo, H.Y. Synergistic Strategy of Manipulating the Number of Selenophene Units and Asymmetric Central Core of Small Molecular Acceptors Enables Polymer Solar Cells with 17.5% Efficiency. Angew. Chem. Int. Ed. 2021, 60, 19241–19252. [Google Scholar] [CrossRef]
- Mikheeva, A.N.; Kuznetsov, I.E.; Tepliakova, M.M.; Elakshar, A.; Gapanovich, M.V.; Gladush, Y.G.; Perepelitsina, E.O.; Sideltsev, M.E.; Akhkiamova, A.F.; Piryazev, A.A.; et al. Novel Push-Pull Benzodithiophene-Containing Polymers as Hole-Transport Materials for Efficient Perovskite Solar Cells. Molecules 2022, 27, 8333. [Google Scholar] [CrossRef]
- Nie, Q.; Tang, A.; Guo, Q.; Zhou, E. Benzothiadiazole-based non-fullerene acceptors. Nano Energy 2021, 87, 106174. [Google Scholar] [CrossRef]
- Ge, J.; Xie, L.; Peng, R.; Fanady, B.; Huang, J.; Song, W.; Yan, T.; Zhang, W.; Ge, Z. 13.34 % Efficiency Non-Fullerene All-Small-Molecule Organic Solar Cells Enabled by Modulating the Crystallinity of Donors via a Fluorination Strategy. Angew. Chem. Int. Ed. Engl. 2020, 59, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shi, R.; Gui, R.; Hu, H.; Zhang, W.; Zhang, K.; Cui, B.; Yin, H.; Gao, K.; Liu, J. Fluorination of Terminal Groups Promoting Electron Transfer in Small Molecular Acceptors of Bulk Heterojunction Films. Molecules 2022, 27, 9037. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-W.; Guan, Z.; Cheng, Y.; Lui, T.; Yang, Q.; Lee, C.-S.; Chen, S.; Tsang, S.-W. On the Study of Exciton Binding Energy with Direct Charge Generation in Photovoltaic Polymers. Adv. Electron. Mater. 2016, 2, 1600200–1600209. [Google Scholar] [CrossRef]
- Narasimhan, V.; Jiang, D.; Park, S.-Y. Design and optical analyses of an arrayed microfluidic tunable prism panel for enhancing solar energy collection. Appl. Energy 2016, 162, 450–459. [Google Scholar] [CrossRef]
- An, Q.; Zhang, F.; Gao, W.; Sun, Q.; Zhang, M.; Yang, C.; Zhang, J. High-efficiency and air stable fullerene-free ternary organic solar cells. Nano Energy 2018, 45, 177–183. [Google Scholar] [CrossRef]
- Zhao, B.; Liang, Z.; Zhang, Y.; Sui, Y.; Shi, Y.; Zhang, X.; Li, M.; Deng, Y.; Geng, Y. Direct Arylation Polycondensation toward Water/Alcohol-Soluble Conjugated Polymers: Influence of Side Chain Functional Groups. ACS Macro Lett. 2021, 10, 419–425. [Google Scholar] [CrossRef]
- An, N.; Cai, Y.; Wu, H.; Tang, A.; Zhang, K.; Hao, X.; Ma, Z.; Guo, Q.; Ryu, H.S.; Woo, H.Y.; et al. Solution-Processed Organic Solar Cells with High Open-Circuit Voltage of 1.3 V and Low Non-Radiative Voltage Loss of 0.16 V. Adv. Mater. 2020, 32, 2002122. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, N.; Zhou, W.; Yu, Z.; Liu, F.; Chen, Y. Miscibility Tuning for Optimizing Phase Separation and Vertical Distribution toward Highly Efficient Organic Solar Cells. Adv. Sci. 2019, 6, 1900565. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, J.; Deng, W.; Luo, M.; Xie, Y.; Liang, Q.; Zou, Y.; He, Z.; Wu, H.; Cao, Y. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics 2020, 14, 300–305. [Google Scholar] [CrossRef]
- Yang, J.; Chen, F.; Cong, P.; Xiao, H.; Geng, Y.; Liao, Z.; Chen, L.; Zhang, B.; Zhou, E. Tuning the optoelectronic properties of vinylene linked perylenediimide dimer by ring annulation at the inside or outside bay positions for fullerene-free organic solar cells. J. Energy Chem. 2020, 40, 112–119. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, S.; Xu, Z.; Yang, Q.; Huang, D.; Xu, X. A simple method to adjust the morphology of gradient three-dimensional PTB7-Th:PC71BM polymer solar cells. Nanoscale 2015, 7, 5537–5544. [Google Scholar] [CrossRef]
- Nie, S.; Qin, F.; Liu, Y.; Qiu, C.; Jin, Y.; Wang, H.; Liu, L.; Hu, L.; Su, Z.; Song, J.; et al. High Conductivity, Semiconducting, and Metallic PEDOT:PSS Electrode for All-Plastic Solar Cells. Molecules 2023, 28, 2836. [Google Scholar] [CrossRef]
- Xu, W.; Ma, X.; Son, J.H.; Jeong, S.Y.; Niu, L.; Xu, C.; Zhang, S.; Zhou, Z.; Gao, J.; Woo, H.Y.; et al. Smart ternary strategy in promoting the performance of polymer solar cells based on bulk-heterojunction and layer-by-layer structures. Small 2022, 18, 2104215. [Google Scholar] [CrossRef]
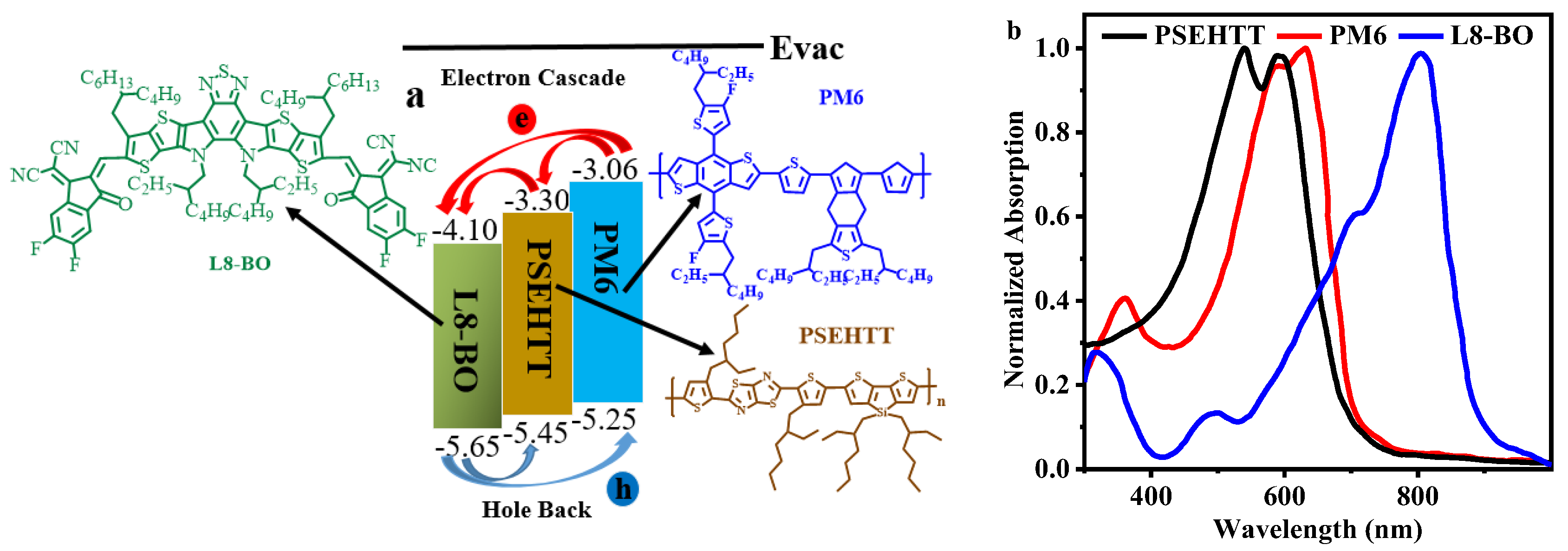

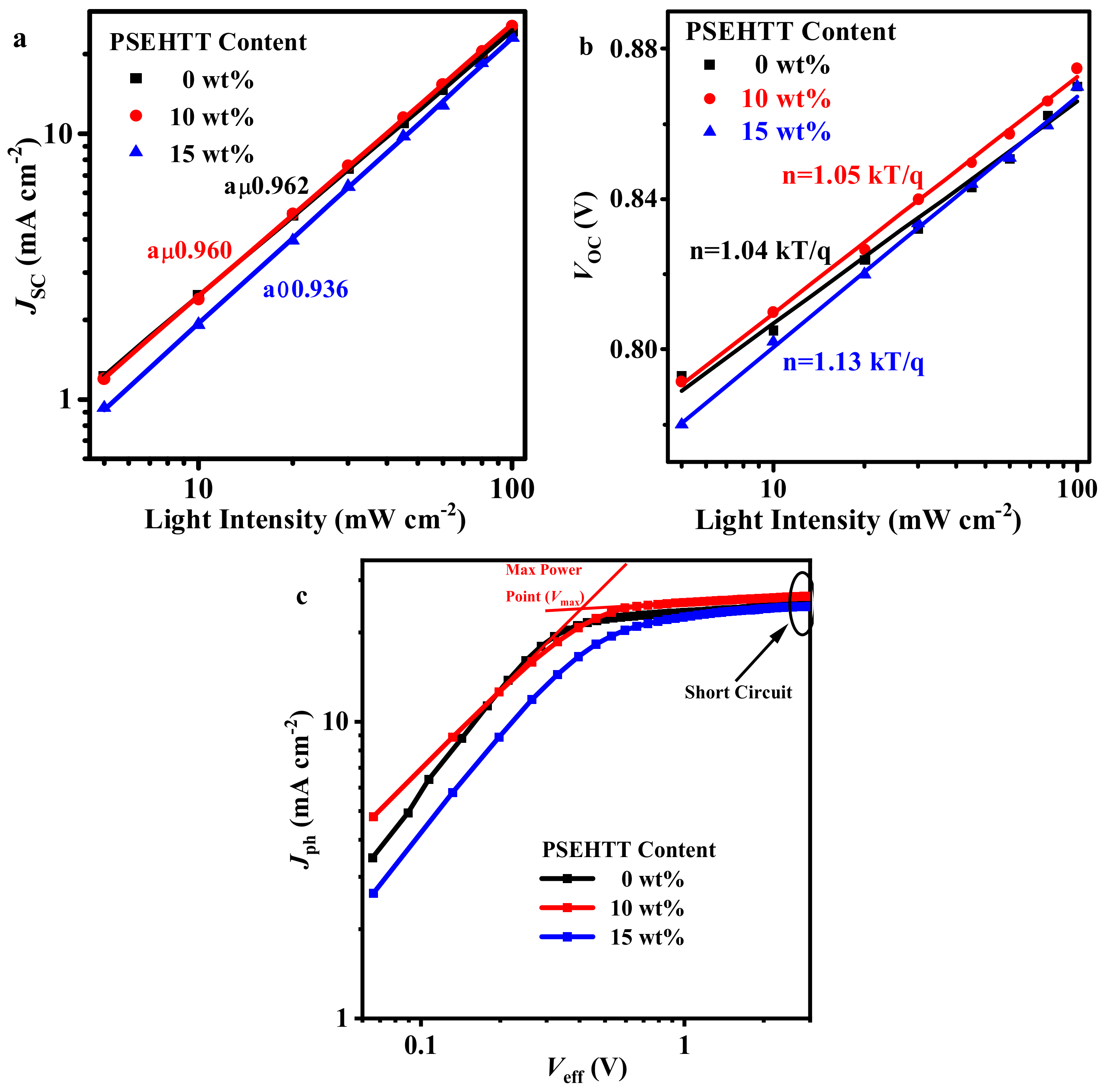

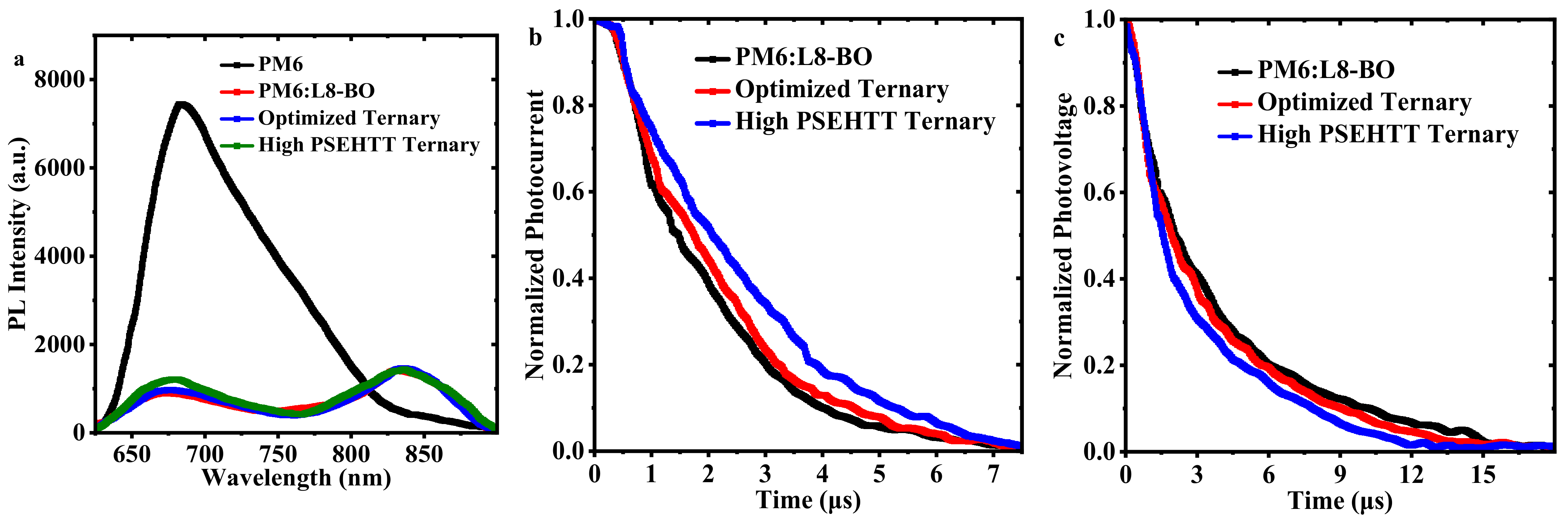
| PM6: PSEHTT | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 100:0 | 0.870 | 24.39 ± 0.13 | 74.6 ± 1.18 | 15.83 ± 0.12 |
| 95:5 | 0.872 | 25.09 ± 0.14 | 74.4 ± 1.22 | 16.28 ± 0.13 |
| 92.5:7.5 | 0.874 | 25.28 ± 0.14 | 74.3 ± 1.27 | 16.42 ± 0.12 |
| 90:10 | 0.875 | 25.66 ± 0.13 | 74.2 ± 1.21 | 16.66 ± 0.13 |
| 87.5:12.5 | 0.877 | 25.68 ± 0.14 | 73.3 ± 1.18 | 16.51 ± 0.15 |
| 85:15 | 0.870 | 23.10 ± 0.13 | 71.5 ± 1.17 | 14.37 ± 0.14 |
| Binary Hybrid (D:A) | PCE (%) | Third Component (%) | PCE (%) | Increased Ratio (%) | References |
|---|---|---|---|---|---|
| PM6:BTP-eC9 | 17.34 | BPR-SCl (20%) | 18.02 | 3.92% | [24] |
| PM6:Y6 | 16.47 | TTBT-R (10%) | 18.07 | 9.71% | [25] |
| PM6:L8-BO | 17.55 | BTC (15%) | 18.24 | 3.93% | [26] |
| PM6:C9 | 17.38 | PM6-Si30 (15%) | 18.27 | 5.12% | [27] |
| D18-Cl:Y6 | 17.35 | G19 (10%) | 18.53 | 6.80% | [28] |
| PM6:BTP-eC9 | 17.30 | PB2F (10%) | 18.60 | 7.51% | [29] |
| PM6:L8-BO | 17.63 | BTID-2F (10%) | 18.52 | 5.05% | [30] |
| PM6:L8-BO | 18.20 | D18 (20%) | 19.60 | 7.69% | [31] |
| PM6: PSEHTT | Jpha (mA cm−2) | Jphb (mA cm−2) | Jsat (mA cm−2) | Gmax (m−3s−1) | P (E, T) a (%) | P (E, T) b (%) |
|---|---|---|---|---|---|---|
| 100:0 | 24.39 | 22.55 | 25.14 | 1.571 × 1028 | 97.0 | 89.7 |
| 90:10 | 25.66 | 23.72 | 26.51 | 1.657 × 1028 | 96.8 | 89.5 |
| 85:15 | 23.10 | 21.14 | 24.47 | 1.530 × 1028 | 94.4 | 86.4 |
| PM6: PSEHTT | μe (cm2 V−1 s−1) | μh (cm2 V−1 s−1) |
|---|---|---|
| 100:0 | 3.87 × 10−4 | 4.45 × 10−4 |
| 90:10 | 3.82 × 10−4 | 4.41 × 10−4 |
| 85:15 | 3.34 × 10−4 | 3.94 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Jia, S.; Liu, Z.; Chen, Z. Ternary Organic Solar Cells by Small Amount of Efficient Light Absorption Polymer PSEHTT as Third Component Materials. Molecules 2023, 28, 6832. https://doi.org/10.3390/molecules28196832
Zhang H, Jia S, Liu Z, Chen Z. Ternary Organic Solar Cells by Small Amount of Efficient Light Absorption Polymer PSEHTT as Third Component Materials. Molecules. 2023; 28(19):6832. https://doi.org/10.3390/molecules28196832
Chicago/Turabian StyleZhang, Han, Songrui Jia, Zhiyong Liu, and Zheng Chen. 2023. "Ternary Organic Solar Cells by Small Amount of Efficient Light Absorption Polymer PSEHTT as Third Component Materials" Molecules 28, no. 19: 6832. https://doi.org/10.3390/molecules28196832
APA StyleZhang, H., Jia, S., Liu, Z., & Chen, Z. (2023). Ternary Organic Solar Cells by Small Amount of Efficient Light Absorption Polymer PSEHTT as Third Component Materials. Molecules, 28(19), 6832. https://doi.org/10.3390/molecules28196832






