Ni/Mn-Complex-Tethered Tetranuclear Polyoxovanadates: Crystal Structure and Inhibitory Activity on Human Hepatocellular Carcinoma (HepG-2)
Abstract
:1. Introduction
2. Results and Discussion
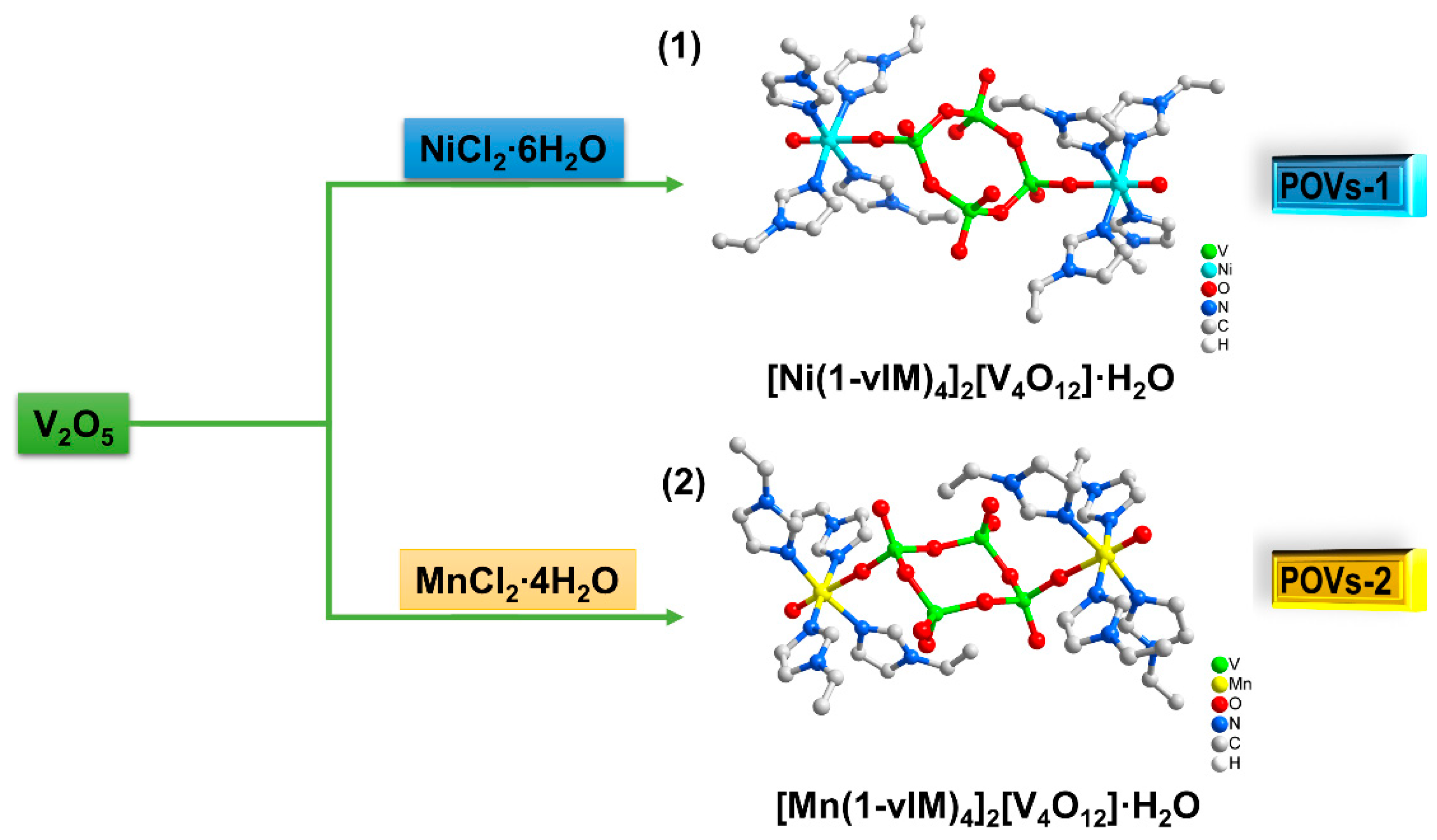
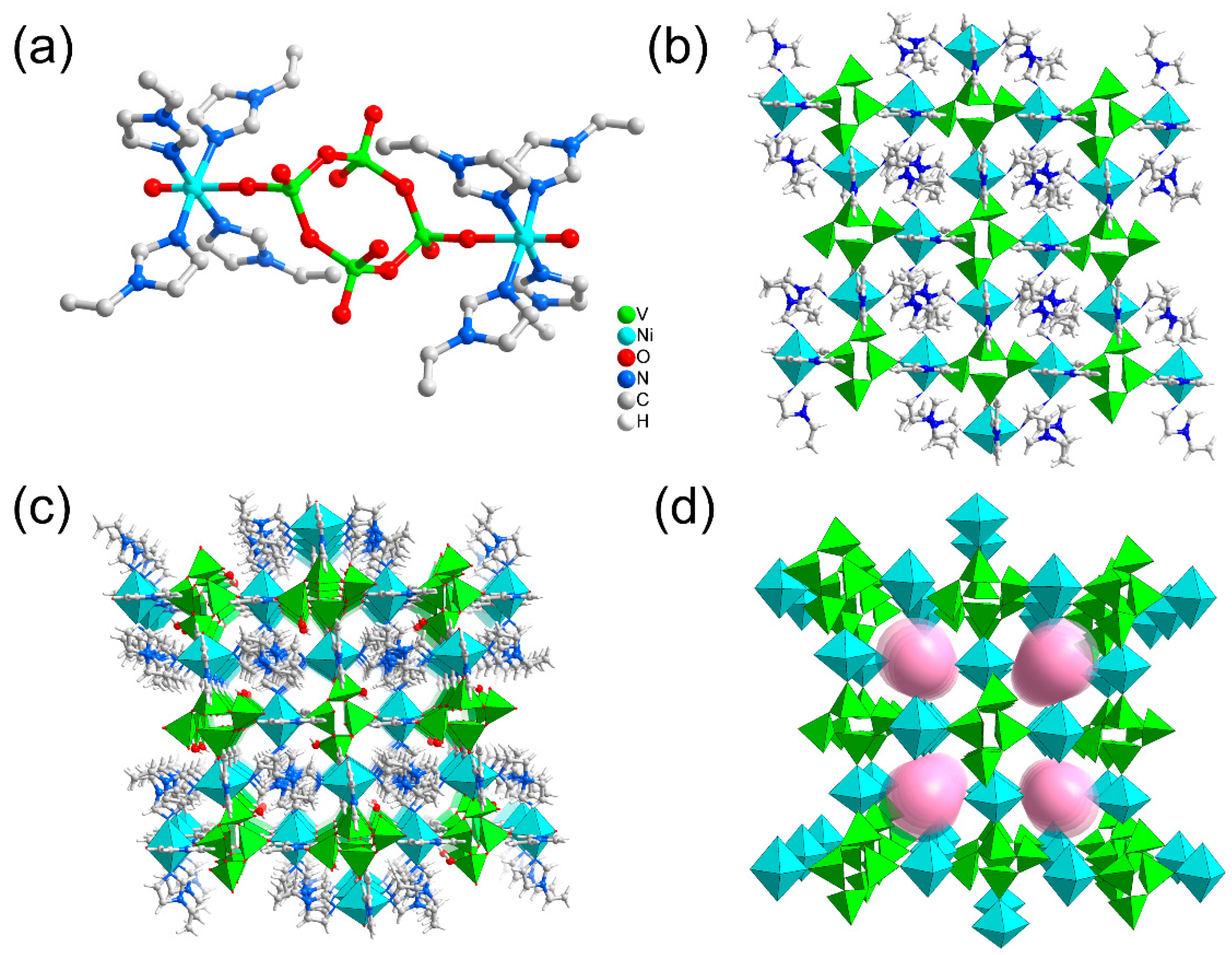
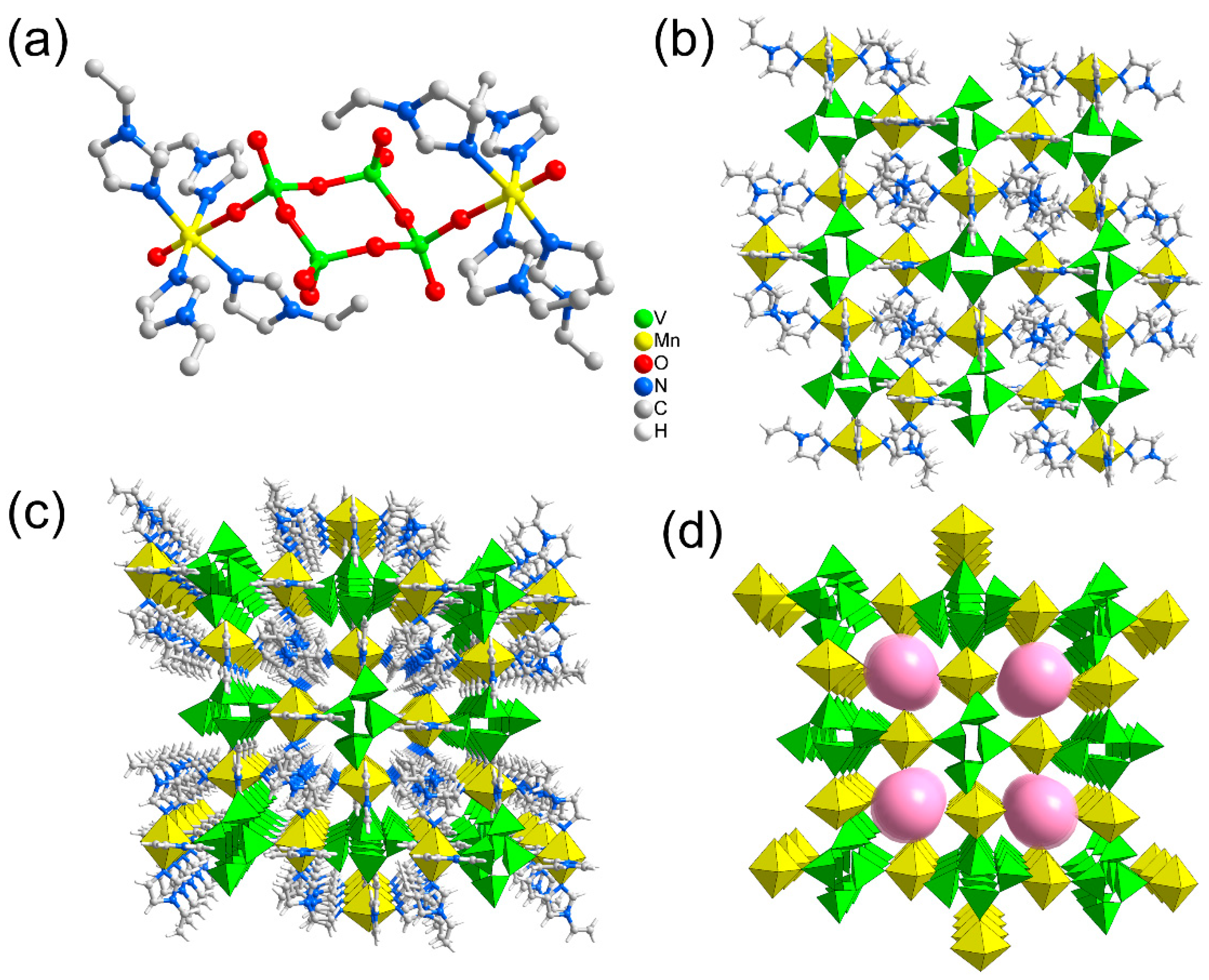
2.1. Structure Analysis
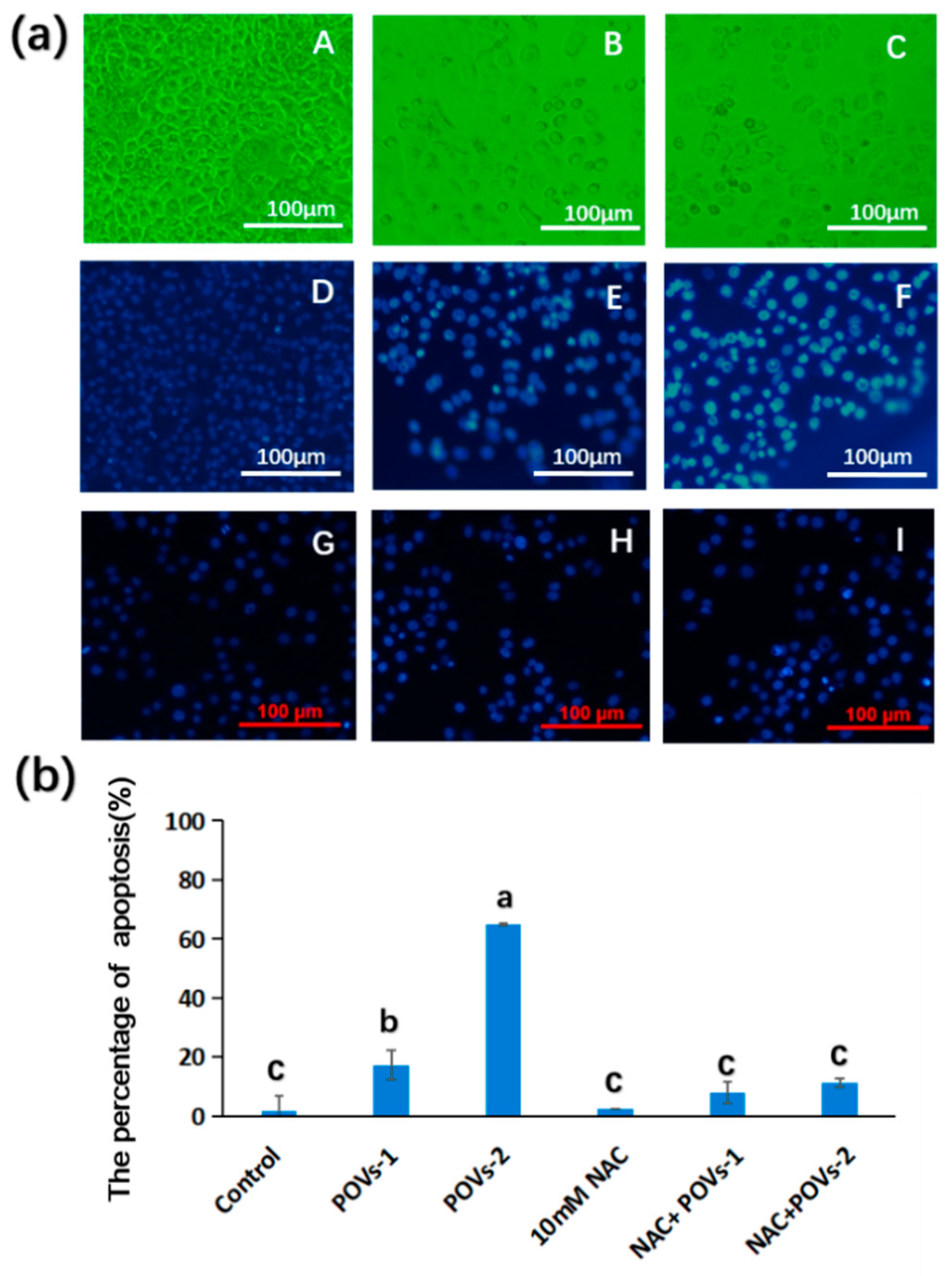
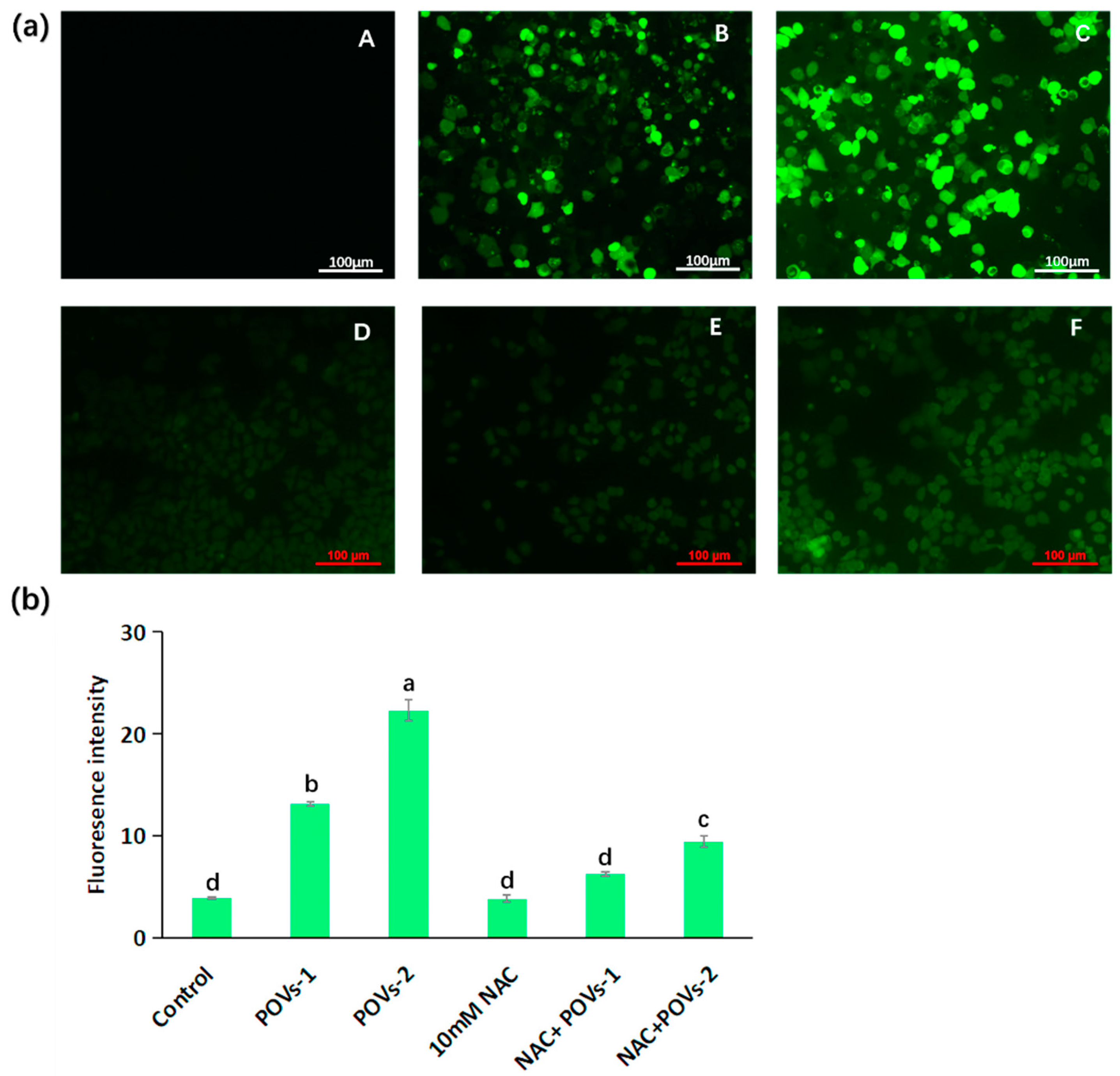
2.2. Pharmacology Evaluation
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of POVs-1 and POVs-2
3.3. Cell Culture
3.4. Determination of Cell Viability
3.5. DAPI Staining of Cells
3.6. Assessment of Mitochondrial Membrane Potential (Δψm)
3.7. Measurement of Intracellular Reactive Oxygen Species (ROS)
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-A Cancer. J. Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, Y.Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Antitumor agents based on Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 16763–16776. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Cui, Y.N.; Zhou, J.H.; Zhang, Y.L.; Shen, G.D.; Yao, Q.X.; Li, J.K.; Xue, Z.C.; Yang, G.P. Self-assembly of three Ag-polyoxovanadates frameworks for their efficient construction of CN bond and detoxification of simulant sulfur mustard. Chin. Chem. Lett. 2022, 33, 2605–2610. [Google Scholar] [CrossRef]
- Ji, Y.C.; Huang, L.J.; Hu, J.; Streb, C.; Song, Y.F. Polyoxometalate-functionalized nanocarbon materials for energy conversion, energy storage and sensor systems. Energy Environ. Sci. 2015, 8, 776–789. [Google Scholar] [CrossRef]
- Sun, T.D.; Cui, W.; Yan, M.; Qin, G.; Guo, W.; Gu, H.X.; Liu, S.Q. Target delivery of a novel antitumor organoplatinum(IV)-substituted polyoxometalate complex for safer and more effective colorectal cancer therapy in vivo. Adv. Mater. 2016, 28, 7397–7404. [Google Scholar] [CrossRef]
- Guo, L.; He, L.; Zhang, Q.H.; Li, B.L.; Wang, C.F.; Lv, Y.F.; Chu, J.F.; Song, Y.F. Recent advances in confining polyoxometalates and the applications. Small 2022, 19, 2207315. [Google Scholar] [CrossRef]
- Zhao, J.; Li, K.X.; Wan, K.W.; Sun, T.D.; Zhang, N.N.; Zhu, F.J.; Ma, J.C.; Jiao, J.; Li, T.C.; Ni, J.Y.; et al. Organoplatinum-substituted polyoxometalate inhibits β-amyloid aggregation for Alzheimer’s therapy. Angew. Chem. Int. Ed. 2019, 58, 18032–18039. [Google Scholar] [CrossRef]
- Liu, J.C.; Zhao, J.W.; Streb, C.; Song, Y.F. Recent advances on high-nuclear polyoxometalate clusters. Coord. Chem. Rev. 2022, 471, 214734. [Google Scholar] [CrossRef]
- Horn, M.; Singh, A.; Alomari, S.; Goberna-Ferrón, S.; Benages-Vilau, R.; Chodankar, N.; Motta, N.; Ostrikov, K.; Macleod, J.; Sonar, P.; et al. Polyoxometalates (POMs): From electroactive clusters to energy materials. Energy Environ. Sci. 2021, 14, 1652–1700. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Hayashi, Y. Hetero and lacunary polyoxovanadate chemistry: Synthesis, reactivity and structural aspects. Coord. Chem. Rev. 2011, 255, 2270–2280. [Google Scholar] [CrossRef]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Sun, H.J.; Dong, K.; Ren, J.S.; Duan, T.C.; Xu, C.; Qu, X.G. Transition-metal-substituted polyoxometalate derivatives as functional anti-amyloid agents for Alzheimer’s disease. Nat. Commun. 2014, 5, 3422. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, M.; Ren, J.S.; Wang, E.B.; Qu, X.G. Polyoxometalates as inhibitors of the aggregation of amyloid β Peptides associated with Alzheimer’s disease. Angew. Chem. Int. Ed. 2011, 50, 4184–4188. [Google Scholar] [CrossRef] [PubMed]
- Yamase, T. Anti-tumor, -viral, and -bacterial activities of polyoxometalates for realizing an inorganic drug. J. Mater. Chem. 2005, 15, 4773–4792. [Google Scholar] [CrossRef]
- Huang, X.Q.; Gu, X.Y.; Qi, Y.Q.; Zhang, Y.R.; Shen, G.D.; Yang, B.C.; Duan, W.Z.; Gong, S.W.; Xue, Z.C.; Chen, Y.F. Decavanadate-based transition metal hybrids as bifunctional catalysts for sulfide oxidation and C-C bond construction. Chin. J. Chem. 2021, 39, 2495–2503. [Google Scholar] [CrossRef]
- Vogelsberg, E.; Moors, M.; Sorokina, A.S.; Ryndyk, D.A.; Schmitz, S.; Freitag, J.S.; Subbotina, A.V.; Heine, T.; Abel, B. Solution-processed formation of DNA-origami-supported polyoxometalate multi-level switches with countercation-controlled conductance tunability. Chem. Mater. 2023, 35, 5447–5457. [Google Scholar] [CrossRef]
- Wang, J.J.; Mi, X.G.; Guan, H.Y.; Wang, X.H.; Wu, Y. Assembly of folate-polyoxometalate hybrid spheres for colorimetric immunoassay like oxidase. Chem. Commun. 2011, 47, 2940–2942. [Google Scholar] [CrossRef]
- Galani, A.; Tsitsias, V.; Stellas, D.; Psycharis, V.; Raptopoulou, C.P.; Karaliota, A. Two novel compounds of vanadium and molybdenum with carnitine exhibiting potential pharmacological use. J. Inorg. Biochem. 2015, 142, 109–117. [Google Scholar] [CrossRef]
- Li, J.K.; Wei, C.P.; Guo, D.G.J.; Wang, C.C.; Han, Y.F.; He, G.F.; Zhang, J.P.; Huang, X.Q.; Hu, C.W. Inorganic–organic hybrid polyoxovanadates based on [V4O12]4− or [VO3]22− clusters: Controllable synthesis, crystal structures and catalytic properties in selective oxidation of sulfides. Dalton Trans. 2020, 49, 14148–14157. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Liu, S.; Liu, G.; Tao, Y.W.; Wang, C.R.; Zhang, Y.L.; Li, Z.; Wang, H.W.; Zhou, Z.; Shen, G.D.; et al. An unprecedented 2-fold interpenetrated lvt open framework built from Zn6 ring seamed trivacant polyoxotungstates used for photocatalytic synthesis of pyridine derivatives. Appl. Catal. B Environ. 2023, 323, 122134. [Google Scholar] [CrossRef]
- Vaid, T.P.; Kelley, S.P.; Rogers, R.D. Metal carbonate complexes formed through the capture of ambient O2 and CO2 by elemental metals in 1-methylimidazole: Molecular Cu(CO3)(MeIm)3 and polymeric M(CO3)(MeIm)2·2H2O (M = Co, Zn). Dalton Trans. 2017, 46, 8920–8923. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, N.; Wang, N.; Hu, K.H.; Xiao, Z.C.; Wu, P.F.; Wei, Y.H. Synthesis, structure and antitumor studies of a novel decavanadate complex with a wavelike two-dimensional network. Polyhedron 2018, 155, 313–319. [Google Scholar] [CrossRef]
- Grzybowska-Szatkowska, L.; Slaska, B. Mitochondrial DNA and carcinogenesis (Review). Mol. Med. Rep. 2012, 6, 923–930. [Google Scholar] [CrossRef]
- Heinke, L. Mitochondrial ROS drive cell cycle progression. Nat. Rev. Mol. Cell. Biol. 2022, 23, 581. [Google Scholar] [CrossRef]
- Broker, L.E.; Kruyt, F.A.; Giaccone, G. Cell death independent of caspases: A Review. Clin. Cancer Res. 2005, 11, 3155–3162. [Google Scholar] [CrossRef]
- Pedersen, P.L. Mitochondria in relation to cancer metastasis: Introduction to a mini-review series. J. Bioenerg. Biomembr. 2012, 44, 615–617. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G.; Ralph, S.J. Targeting the redox imbalance in mitochondria: A novel mode for cancer therapy. Mitochondrion 2022, 62, 50–73. [Google Scholar] [CrossRef]
- MacDonald, J.A.; Kura, N.; Sussman, C.; Woods, D.C. Mitochondrial membrane depolarization enhances TRAIL-induced cell death in adult human granulosa tumor cells, KGN, through inhibition of BIRC5. J. Ovarian. Res. 2018, 11, 89. [Google Scholar] [CrossRef]
- Suzuki, Y.; Imai, Y.; Nakayama, H.; Takahashi, K.; Takio, K.; Takahashi, R.A. Serine Protease, HtrA2, Is Released from the Mitochondria and Interacts with XIAP, Inducing Cell Death. Mol. Cell 2001, 8, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, D.; Gaume, B.; Karbowski, M.; Sharpe, J.C.; Cecconi, F.; Youle, R.J. Mitochondrial release of AIF and EndoG requires caspase activation downstream of BAX/BAK-mediated permeabilization. EMBO J. 2003, 22, 4385–4399. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Ohsakaya, S.; Nagaura, Z.; Ishihara, N.; Mihara, K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005, 24, 1375–1386. [Google Scholar] [CrossRef]
- Uren, R.T.; Dewson, G.; Bonzon, C.; Lithgow, T.; Newmeyer, D.D.; Kluck, R.M. Mitochondrial release of pro-apoptotic proteins: Electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J. Biol. Chem. 2005, 280, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- David, L.V. Apoptogenic factors released from mitochondria. BBA-Mol. Cell Res. 2011, 1813, 546–550. [Google Scholar]
- Reichert, S.; Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 2017, 13, 20170463. [Google Scholar] [CrossRef]
- Finelli, R.; Leisegang, K.; Kandil, H.; Agarwal, A. Oxidative stress: A comprehensive review of biochemical, molecular, and genetic aspects in the pathogenesis and management of varicocele. World J. Mens. Health 2022, 40, 87–103. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Spector, A. Review: Oxidative Stress and Disease. J. Ocul. Pharmacol. Ther. 2000, 16, 193–201. [Google Scholar] [CrossRef]
- Prakash, C.; Soni, M.; Kumar, V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: A review. J. Appl. Toxicol. 2016, 36, 179–188. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta 2006, 1757, 509–517. [Google Scholar] [CrossRef]
- Guo, Z.P.; Wang, Z.A.; Liang, R.F.; Tian, H.Y.; Chen, X.S.; Chen, M.W. Reactive oxygen species activated by mitochondria-specific camptothecin prodrug for enhanced chemotherapy. Bosn. J. Basic Med. Sci. 2022, 22, 934–948. [Google Scholar] [CrossRef]
- Luo, X.J.; Chi, X.Q.; Lin, Y.Y.; Yang, Z.X.; Lin, H.Y.; Gao, J.H. A camptothecin prodrug induces mitochondria-mediated apoptosis in cancer cells with cascade activations. Chem. Commun. 2021, 57, 11033–11036. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Tu, Z.S.; Cui, H.M.; Yan, S.; Duan, D.C.; Tang, W.; Dai, F.; Zhou, B. Redox-based strategy for selectively inducing energy crisis inside cancer cells: An example of modifying dietary curcumin to target mitochondria. J. Agric. Food Chem. 2022, 70, 2898–2910. [Google Scholar] [CrossRef] [PubMed]
- Casares, C.; Ramirez-Camacho, R.; Trinidad, A.; Roldan, A.; Jorge, E.; García-Berrocal, J. R Reactive oxygen species in apoptosis induced by cisplatin: Review of physiopathological mechanisms in animal models. Eur. Arch. Oto-Rhino-L 2012, 269, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.D.; Orrenius, S.; Zhivotovsky, B. Review: Nuclear events in apoptosis. J. Struct. Biol. 2000, 129, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, F.; Chen, Y.; Dong, C.; Wang, J.; Song, C.; Zhang, Y.; Li, Z.; Huang, X. Ni/Mn-Complex-Tethered Tetranuclear Polyoxovanadates: Crystal Structure and Inhibitory Activity on Human Hepatocellular Carcinoma (HepG-2). Molecules 2023, 28, 6843. https://doi.org/10.3390/molecules28196843
Shi F, Chen Y, Dong C, Wang J, Song C, Zhang Y, Li Z, Huang X. Ni/Mn-Complex-Tethered Tetranuclear Polyoxovanadates: Crystal Structure and Inhibitory Activity on Human Hepatocellular Carcinoma (HepG-2). Molecules. 2023; 28(19):6843. https://doi.org/10.3390/molecules28196843
Chicago/Turabian StyleShi, Fumei, Yilan Chen, Chuanheng Dong, Jiajia Wang, Chunman Song, Yalin Zhang, Zhen Li, and Xianqiang Huang. 2023. "Ni/Mn-Complex-Tethered Tetranuclear Polyoxovanadates: Crystal Structure and Inhibitory Activity on Human Hepatocellular Carcinoma (HepG-2)" Molecules 28, no. 19: 6843. https://doi.org/10.3390/molecules28196843





