Synergistic Antibiofilm Action of Cinnamomum verum and Brazilian Green Propolis Hydroethanolic Extracts against Multidrug-Resistant Strains of Acinetobacter baumannii and Pseudomonas aeruginosa and Their Biocompatibility on Human Keratinocytes
Abstract
:1. Introduction
2. Results
2.1. Content of Soluble Solids
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.3. Minimum Inhibitory (MIC) and Minimum Bactericidal (MBC) Values
2.4. Combined Extracts’ Synergistic Effect
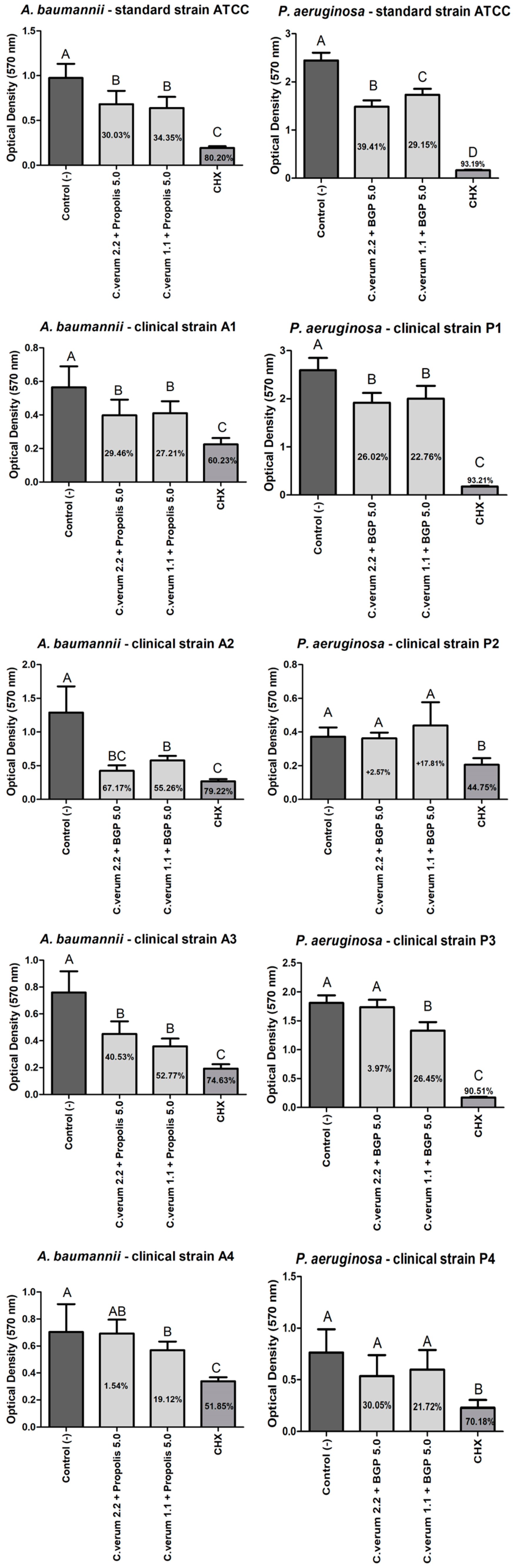
2.5. Antibiofilm Effect (Preventing Biofilms Formation)
2.6. Antibiofilm Effect (against Mature Biofilms)
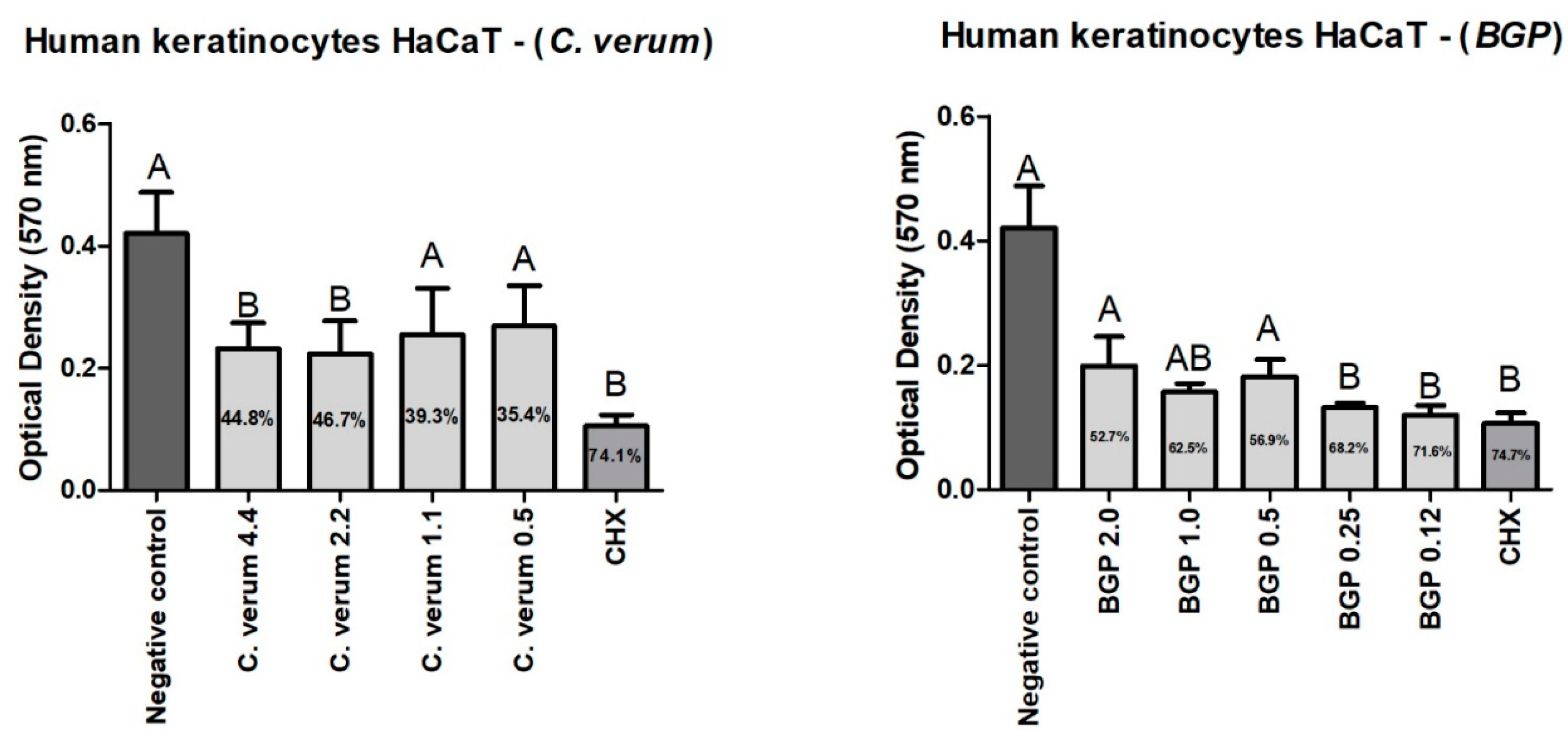
2.7. Cytotoxicity of Plant Extracts on Human Keratinocytes (HaCaT)
3. Discussion
4. Materials and Methods
4.1. Plants and Propolis Extracts
4.2. Content of Soluble Solids
4.3. High-Performance Liquid Chromatography Analysis of the Plant Extracts
4.4. Inoculum Preparation
4.5. Minimum Inhibitory (MIC) and Minimum Bactericidal (MBC) Concentrations
4.6. Combined Extracts Synergistic Effect
4.7. Antibiofilm Action
4.8. Cytotoxicity of Plant Extracts on Human Keratinocytes (HaCaT)
4.9. Statistical Analysis
5. Conclusions
- C. verum and BGP hydroethanolic extracts have bactericidal and antibiofilm action against multidrug resistant strains of A. baumannii and P. aeruginosa.
- The combined extracts were capable of expressively inhibiting the formation of A. baumannii and P. aeruginosa biofilms (prophylactic effect) acting similarly to 0.12% chlorhexidine gluconate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial stewardship: Fighting antimicrobial resistance and protecting global public health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Ramadan, R.A.; Gebriel, M.G.; Kadry, H.M.; Mosallem, A. Carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: Characterization of carbapenemase genes and E-test evaluation of colistin-based combinations. Infect. Drug Resist. 2018, 11, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Mea, H.J.; Yong, P.V.C.; Wong, E.H. An overview of Acinetobacter baumannii pathogenesis: Motility, adherence and biofilm formation. Microbiol. Res. 2021, 247, 126722. [Google Scholar] [CrossRef] [PubMed]
- Takahama, A.; de Sousa, V.I.; Tanaka, E.E.; Ono, E.; Ito, F.A.N.; Costa, P.P.; Pedriali, M.B.B.P.; de Lima, H.G.; Fornazieri, M.A.; Correia, L.S.; et al. Analysis of oral risk factors for ventilator-associated pneumonia in critically ill patients. Clin. Oral Investig. 2021, 25, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Jiang, Y.-Z.; Hsu, C.-M.; Chen, L.-W. Pseudomonas aeruginosa Ventilator-Associated Pneumonia Induces Lung Injury through TNF-α/c-Jun NH2-Terminal Kinase Pathways. PLoS ONE 2017, 12, e0169267. [Google Scholar] [CrossRef]
- Minkoff, J.M.; tenOever, B. Innate immune evasion strategies of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 178–194. [Google Scholar] [CrossRef]
- Deschepper, M.; Waegeman, W.; Eeckloo, K.; Vogelaers, D.; Blot, S. Effects of chlorhexidine gluconate oral care on hospital mortality: A hospital-wide, observational cohort study. Intensive Care Med. 2018, 44, 1017–1026. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Zanatta, F.B.; Antoniazzi, R.P.; Rösing, C.K. Staining and calculus formation after 0.12% chlorhexidine rinses in plaque-free and plaque covered surfaces: A randomized trial. J. Appl. Oral Sci. 2010, 18, 515–521. [Google Scholar] [CrossRef]
- Santos, P.B.d.R.E.D.; Ávila, D.d.S.; Ramos, L.d.P.; Yu, A.R.; Santos, C.E.d.R.; Berretta, A.A.; Camargo, S.E.A.; Oliveira, J.R.d.; Oliveira, L.D.d. Effects of Brazilian green propolis extract on planktonic cells and biofilms of multidrug-resistant strains of Klebsiella pneumoniae and Pseudomonas aeruginosa. Biofouling 2020, 36, 834–845. [Google Scholar] [CrossRef]
- Yu, A.R.; de Paula Ramos, L.; de Lima, P.M.N.; Hasna, A.A.; da Rocha Santos, C.E.; dos Santos, J.M.T.; Pereira, T.C.; de Oliveira, L.D. Punica granatum L. Extract Antibiofilm Action against Acinetobacter baumannii Carbapenem-Resistant and Biocompatibility over Human Keratinocytes. J. Health Sci. 2022, 24, 215–219. [Google Scholar]
- Domingues, N.; Ramos, L.D.P.; Pereira, L.M.; do Rosário Estevam Dos Santos, P.B.; Scorzoni, L.; Pereira, T.C.; Abu Hasna, A.; Carvalho, C.A.T.; de Oliveira, L.D. Antimicrobial action of four herbal plants over mixed-species biofilms of Candida albicans with four different microorganisms. Aust. Endod. J. 2023, 49, 262–271. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Pasiecznik, N. Cinnamomum verum (cinnamon). In CABI Compendium; CAB International: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.; Umsza-Guez, M.A.; Barbosa, J.D.; Portela, R.D.; Machado, B.A. Propolis: Types, composition, biological activities, and veterinary product patent prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- de Sá Assis, M.A.; de Paula Ramos, L.; Abu Hasna, A.; de Queiroz, T.S.; Pereira, T.C.; Nagai de Lima, P.M.; Berretta, A.A.; Marcucci, M.C.; Talge Carvalho, C.A.; de Oliveira, L.D. Antimicrobial and Antibiofilm Effect of Brazilian Green Propolis Aqueous Extract against Dental Anaerobic Bacteria. Molecules 2022, 27, 8128. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Meccatti, V.M.; Santos, L.F.; de Carvalho, L.S.; Souza, C.B.; Carvalho, C.A.T.; Marcucci, M.C.; Abu Hasna, A.; de Oliveira, L.D. Antifungal Action of Herbal Plants’ Glycolic Extracts against Candida Species. Molecules 2023, 28, 2857. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.D.; Ramos, L.P.; Silva, T.A.; Lapena, S.A.B.D.; Santos, C.E.R.; Hasna, A.A.; Bressane, A.; Oliveira, L.D.D. Effect of combining Zingiber officinale and Juglans regia extracts on Propionibacterium acnes, Staphylococcus aureus and Staphylococcus epidermidis: Antibiofilm action and low toxicity. An. Acad. Bras Cienc. 2022, 94, e20201133. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-Y.; Kim, S.; Park, H.-M.; Lim, C.-M.; Kim, J.; Park, J.-Y.; Jeon, K.-B.; Poudel, A.; Lee, H.P.; Oh, S.-R.; et al. Cinnamomum verum extract inhibits NOX2/ROS and PKCδ/JNK/AP-1/NF-κB pathway-mediated inflammatory response in PMA-stimulated THP-1 monocytes. Phytomedicine 2023, 112, 154685. [Google Scholar] [CrossRef] [PubMed]
- Šuran, J.; Cepanec, I.; Mašek, T.; Starčević, K.; Tlak Gajger, I.; Vranješ, M.; Radić, B.; Radić, S.; Kosalec, I.; Vlainić, J. Nonaqueous Polyethylene Glycol as a Safer Alternative to Ethanolic Propolis Extracts with Comparable Antioxidant and Antimicrobial Activity. Antioxidants 2021, 10, 978. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Gaber, S.N.; Hemeda, E.E.M.; Elsayeh, H.-A.S.; Protectionsititue, A.R.C.; Wahed, W.Y.A.; Khalil, M.A.F.; Ibrahim, E.G. Propolis Extract: A Possible Antiseptic Oral Care against Multidrug-Resistant Non-Fermenting Bacteria Isolated from Non-Ventilator Hospital-Acquired Pneumonia. J. Pure Appl. Microbio. 2020, 14, 123–131. [Google Scholar] [CrossRef]
- Intorasoot, A.; Chornchoem, P.; Sookkhee, S.; Intorasoot, S. Bactericidal activity of herbal volatile oil extracts against multidrug-resistant Acinetobacter baumannii. J. Intercult. Ethnopharmacol. 2017, 6, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ganić, T.; Vuletić, S.; Nikolić, B.; Stevanović, M.; Kuzmanović, M.; Kekić, D.; Đurović, S.; Cvetković, S.; Mitić-Ćulafić, D. Cinnamon essential oil and its emulsion as efficient antibiofilm agents to combat Acinetobacter baumannii. Front. Microbiol. 2022, 13, 989667. [Google Scholar] [CrossRef]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Pandey, H.; Narvi, S.S.; Agarwal, V. Effect of Cinnamon Oil on Quorum Sensing-Controlled Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa. PLoS ONE 2015, 10, e0135495. [Google Scholar] [CrossRef]
- Brackman, G.; Celen, S.; Hillaert, U.; Van Calenbergh, S.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS ONE 2011, 6, e16084. [Google Scholar] [CrossRef]
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097. [Google Scholar] [CrossRef]
- Meto, A.; Colombari, B.; Meto, A.; Boaretto, G.; Pinetti, D.; Marchetti, L.; Benvenuti, S.; Pellati, F.; Blasi, E. Propolis Affects Pseudomonas aeruginosa Growth, Biofilm Formation, eDNA Release and Phenazine Production: Potential Involvement of Polyphenols. Microorganisms 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, G.K.; de Oliveira, T.R.; Maia, F.C.; de Feiria, S.B.; Barbosa, J.P.; Joia, F.; Boni, G.C.; Höfling, J.F. Efficacy of true cinnamon (Cinnamomum verum) leaf essential oil as a therapeutic alternative for Candida biofilm infections. Iran. J. Basic Med. Sci. 2021, 24, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.A.; Ha, J.W.; Choi, J.Y.; Boo, Y.C. Antioxidant effects of korean propolis in hacat keratinocytes exposed to particulate matter 10. Antioxidants 2022, 11, 781. [Google Scholar] [CrossRef]
- Vrca, I.; Ramić, D.; Fredotović, Ž.; Smole Možina, S.; Blažević, I.; Bilušić, T. Chemical Composition and Biological Activity of Essential Oil and Extract from the Seeds of Tropaeolum majus L. var. altum. Food Technol. Biotechnol. 2022, 60, 533–542. [Google Scholar] [CrossRef]
- Vargas, R.E.S. Ministério da Agricultura e do Abastecimento Secretaria Dedefesa Agropecuária Instrução Normativa no 3. 2001. Available online: http://iberpharm.com.br/www/arquivos/IN03-19-01-2001.pdf (accessed on 10 June 2023).
- Marcucci, M.C.; Sawaya, A.; Custodio, A.R.; Paulino, N.; Eberlin, M.N. HPLC and ESI-MS typification: New approaches for natural therapy with Brazilian propolis. In Scientific Evidence of the Use of Propolis in Ethnomedicine; Transworld Research Network: Trivandrum, India, 2008; ISBN 978-81-7895-357-1. [Google Scholar]
- Cedeño-Pinos, C.; Marcucci, M.C.; Bañón, S. Contribution of Green Propolis to the Antioxidant, Physical, and Sensory Properties of Fruity Jelly Candies Made with Sugars or Fructans. Foods 2021, 10, 2586. [Google Scholar] [CrossRef]
- Moreno, M.A.; Zampini, I.C.; Isla, M.I. Antifungal, anti-inflammatory and antioxidant activity of bi-herbal mixtures with medicinal plants from Argentinean highlands. J. Ethnopharmacol. 2020, 253, 112642. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A novel interpretation of the Fractional Inhibitory Concentration Index: The case Origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef]
- Abu Hasna, A.; de Paula Ramos, L.; Campos, T.M.B.; de Castro Lopes, S.L.P.; Rachi, M.A.; de Oliveira, L.D.; Carvalho, C.A.T. Biological and chemical properties of five mineral oxides and of mineral trioxide aggregate repair high plasticity: An in vitro study. Sci. Rep. 2022, 12, 14123. [Google Scholar] [CrossRef]




| Bacterial Strains | Isolated Extract MBC Value (mg/mL) | Combined Concentrations (mg/mL) | FBC Index | Reduction in MBC | Effect | |||
|---|---|---|---|---|---|---|---|---|
| C. verum | BGP | C. verum | BGP | C. verum | BGP | |||
| A. baumannii ATCC | 2.2 | 10 | 1.1 | 5 | 1.00 | 2× | 2× | Add |
| 0.5 | 5 | 0.72 | 4× | 2× | Add | |||
| A1 | 4.4 | 5 | - | - | - | - | - | - |
| A2 | 4.4 | 10 | 2.2 | 5 | 1.00 | 2× | 2× | Add |
| 1.1 | 5 | 0.75 | 4× | 2× | Add | |||
| 0.5 | 5 | 0.61 | 8× | 2× | Add | |||
| 0.2 | 5 | 0.54 | 20× | 2× | Add | |||
| 0.1 | 5 | 0.53 | 40× | 2× | Add | |||
| 2.2 | 2.5 | 0.75 | 2× | 4× | Add | |||
| 1.1 | 2.5 | 0.50 | 4× | 4× | Syn | |||
| 2.2 | 1.2 | 0.62 | 2× | 8× | Add | |||
| A3 | 4.4 | 10 | 2.2 | 5 | 1.00 | 2× | 2× | Add |
| 1.1 | 5 | 0.75 | 4× | 2× | Add | |||
| A4 | 4.4 | 5 | - | - | - | - | - | - |
| P. aeruginosa ATCC | 0.5 | 1.2 | 0.2 | 0.6 | 0.90 | 2× | 2× | Add |
| P1 | 1.1 | 2.5 | 0.5 | 1.2 | 0.93 | 2× | 2× | Add |
| 0.2 | 1.2 | 0.66 | 5× | 2× | Add | |||
| 0.5 | 0.07 | 0.47 | 2× | 34× | Syn | |||
| P2 | 4.4 | 20 | 2.2 | 10 | 1.00 | 2× | 2× | Add |
| P3 | 0.5 | 1.2 | - | - | - | - | - | - |
| P4 | 4.4 | 20 | 2.2 | 10 | 1.00 | 2× | 2× | Add |
| Antibiotic | A. baumannii Clinical Strains | |||
|---|---|---|---|---|
| A1 | A2 | A3 | A4 | |
| Ertapenem | Res | Res | Res | Res |
| Imipenem | Res | Res | Res | Res |
| Meropenem | Res | Res | Res | Res |
| P. aeruginosa clinical strains | ||||
| P1 | P2 | P3 | P4 | |
| Amikacin | Res | Res | Sen | Res |
| Aztreonam | Res | Inter | Res | Res |
| Cefepime | Res | Res | Res | Sen |
| Ceftazidime | Res | Res | Res | Sen |
| Ciprofloxacin | Res | Sen | Res | Res |
| Colistin | Inter | Res | - | - |
| Gentamicin | Res | Res | Sen | Res |
| Imipenem | Res | Res | Inter | Res |
| Levofloxacin | Res | Sen | Res | Res |
| Meropenem | Res | Res | Res | Res |
| Piperacillin/Tazobactam | Res | Inter | Inter | Sen |
| Polymyxin B | - | - | - | Sen |
| Tobramycin | Res | - | - | Res |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meccatti, V.M.; Martins, K.M.C.; Ramos, L.d.P.; Pereira, T.C.; de Menezes, R.T.; Marcucci, M.C.; Abu Hasna, A.; de Oliveira, L.D. Synergistic Antibiofilm Action of Cinnamomum verum and Brazilian Green Propolis Hydroethanolic Extracts against Multidrug-Resistant Strains of Acinetobacter baumannii and Pseudomonas aeruginosa and Their Biocompatibility on Human Keratinocytes. Molecules 2023, 28, 6904. https://doi.org/10.3390/molecules28196904
Meccatti VM, Martins KMC, Ramos LdP, Pereira TC, de Menezes RT, Marcucci MC, Abu Hasna A, de Oliveira LD. Synergistic Antibiofilm Action of Cinnamomum verum and Brazilian Green Propolis Hydroethanolic Extracts against Multidrug-Resistant Strains of Acinetobacter baumannii and Pseudomonas aeruginosa and Their Biocompatibility on Human Keratinocytes. Molecules. 2023; 28(19):6904. https://doi.org/10.3390/molecules28196904
Chicago/Turabian StyleMeccatti, Vanessa Marques, Karoline Moura Chagas Martins, Lucas de Paula Ramos, Thaís Cristine Pereira, Raquel Teles de Menezes, Maria Cristina Marcucci, Amjad Abu Hasna, and Luciane Dias de Oliveira. 2023. "Synergistic Antibiofilm Action of Cinnamomum verum and Brazilian Green Propolis Hydroethanolic Extracts against Multidrug-Resistant Strains of Acinetobacter baumannii and Pseudomonas aeruginosa and Their Biocompatibility on Human Keratinocytes" Molecules 28, no. 19: 6904. https://doi.org/10.3390/molecules28196904
APA StyleMeccatti, V. M., Martins, K. M. C., Ramos, L. d. P., Pereira, T. C., de Menezes, R. T., Marcucci, M. C., Abu Hasna, A., & de Oliveira, L. D. (2023). Synergistic Antibiofilm Action of Cinnamomum verum and Brazilian Green Propolis Hydroethanolic Extracts against Multidrug-Resistant Strains of Acinetobacter baumannii and Pseudomonas aeruginosa and Their Biocompatibility on Human Keratinocytes. Molecules, 28(19), 6904. https://doi.org/10.3390/molecules28196904







