Synthesis of Liquid Hydrocarbon via Direct Hydrogenation of CO2 over FeCu-Based Bifunctional Catalyst Derived from Layered Double Hydroxides
Abstract
:1. Introduction
1.1. Background
1.2. Challenges
1.3. Catalyst Development
2. Results and Discussion
2.1. Chemical Phase of Catalyst
2.2. Textural Properties of Catalyst
2.3. Surface Morphology of Catalyst
2.4. Surface Composition Properties of Catalyst
2.5. Reducibility and Reaction Adsorption State of Catalyst
2.6. Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Su, X.; Zhang, J.; Fan, S.; Ma, Q.; Zhao, T.S. Effect of preparation of Fe-Zr-K catalyst on the product distribution of CO2 hydrogenation. RSC Adv. 2015, 98, 80196–80202. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Steam cracking and methane to olefins: Energy use, CO2 emissions and production costs. Energy 2008, 33, 817–833. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Wang, K.Z.; Wang, Y.; Liu, Z.H.; Gao, X.H.; Zhang, J.L.; Ma, Q.X.; Fan, S.B.; Zhao, T.S.; Yao, M. Recent advances in thermocatalytic hydrogenation of carbon dioxide to light olefins and liquid fuels via modified Fischer-Tropsch pathway. J. CO2 Util. 2023, 67, 102321. [Google Scholar] [CrossRef]
- Keim, W. Oligomerization of ethylene to α-olefins: Discovery and development of the shell higher olefin process (SHOP). Angew. Chem. Int. Ed. 2013, 48, 12492–12496. [Google Scholar] [CrossRef]
- Li, Z.L.; Wang, J.J.; Qu, Y.Z.; Liu, H.L.; Tang, C.Z.; Miao, S.; Feng, Z.C.; An, H.Y.; Li, C. Highly selective conversion of carbon dioxide to lower olefins. ACS Catal. 2017, 12, 8544–8548. [Google Scholar] [CrossRef]
- Guo, L.S.; Sun, J.; Ji, X.W.; Wei, J.; Wen, Z.Y.; Yao, R.W.; Xu, H.Y.; Ge, Q.J. Directly converting carbon dioxide to linear α-olefins on bio-promoted catalysts. Commun. Chem. 2018, 1, 11. [Google Scholar] [CrossRef]
- Lu, P.F.; Liang, J.; Wang, K.Z.; Liu, B.; Atchimarungsri, T.; Wang, Y.; Zhang, X.J.; Tian, J.M.; Jiang, Y.J.; Liu, Z.H.; et al. Boosting liquid hydrocarbons synthesis from CO2 hydrogenation via tailoring acid properties of HZSM-5 zeolite. Ind. Eng. Chem. Res. 2022, 61, 16393–16401. [Google Scholar] [CrossRef]
- Luque, R.; de la Osa, A.R.; Campelo, J.M.; Romero, A.A.; Valverde, J.L.; Sanchez, P. Design and development of catalysts for biomass-to-liquid-Fischer-Tropsch (BTL-FT) processes for biofuels production. Energy Environ. Sci. 2012, 1, 5186–5202. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Jacobs, G.; Davis, B.H.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L. Fischer-Tropsch synthesis: An in-situ TPR-EXAFS/XANES investigation of the influence of group I alkali promoters on the local atomic and electronic structure of carburized iron/silica catalysts. J. Phys. Chem. C 2010, 17, 7895–7903. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, H.W.; Xu, Y.Y.; Bai, L.; Li, Y.W. Effect of potassium promoter on precipitated iron-manganese catalyst for Fischer-Tropsch synthesis. Appl. Catal. A 2004, 266, 181–194. [Google Scholar] [CrossRef]
- Jiang, B.; Xia, D.; Yu, B.; Xiong, R.; Ao, W.; Zhang, P.; Cong, L. An environment-friendly process for limestone calcination with CO2 looping and recovery. J. Clean. Prod. 2019, 240, 118147. [Google Scholar] [CrossRef]
- Kang, S.C.; Jun, K.W.; Lee, Y.J. Effects of the CO/CO2 ratio in synthesis gas on the catalytic behavior in Fischer-Tropsch synthesis using K/Fe-Cu-Al catalysts. Energy Fuels 2013, 27, 6377–6387. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, L.; Li, S.; Zhou, Z.; Sun, Y. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels. ACS Cent. Sci. 2020, 6, 1657–1670. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green. Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Guo, L.S.; Cui, Y.; Li, H.J.; Fang, Y.; Prasert, R.; Wu, J.H.; Yang, G.H.; Yoneyama, Y.; Tsubaki, N. Selective formation of linear-alpha olefins (LAOs) by CO2 hydrogenation over bimetallic Fe/Co-Y catalyst. Catal. Commun. 2019, 130, 105759. [Google Scholar] [CrossRef]
- Han, Y.; Fang, C.Y.; Ji, X.W.; Wei, J.; Ge, Q.J.; Sun, J. Interfacing with carbonaceous potassium promoters boosts catalytic CO2 hydrogenation of iron. ACS Catal. 2020, 10, 12098–12108. [Google Scholar] [CrossRef]
- Orege, J.I.; Wei, J.; Han, Y.; Yang, M.; Sun, X.T.; Zhang, J.X.; Amoo, C.C.; Ge, Q.J.; Sun, J. Highly stable Sr and Na co-decorated Fe catalyst for high-valued olefin synthesis from CO2 hydrogenation. Appl. Catal. B 2022, 316, 121640. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Huang, G.X.; Tang, X.L.; Yin, H.R.; Kang, J.C. Zn and Na promoted Fe catalysts for sustainable production of high-valued olefins by CO2 hydrogenation. Fuel 2022, 309, 122105. [Google Scholar] [CrossRef]
- Numpilat, T.; Chanlek, N.; Poo-arporn, Y.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Witoon, T. Tuning interactions of surface-adsorbed species over Fe-Co/K-Al2O3 catalyst by different K contents: Selective CO2 hydrogenation to light olefins. ChemCatChem 2020, 12, 3306–3320. [Google Scholar] [CrossRef]
- Hu, B.X.; Frueh, S.; Garces, H.F.; Zhang, L.C.; Aindow, M.; Broooks, C.; Kreidler, E.; Suib, S.L. Selective hydrogenation of CO2 and CO to useful light olefins over octahedral molecular sieve manganese oxide supported iron catalysts. Appl. Catal. B 2013, 132–133, 54–61. [Google Scholar] [CrossRef]
- Dokania, A.; Chowdhury, A.D.; Ramirez, A.; Telalovic, S.; Abou-Hamad, E.; Gevers, L.; Gascon, J. Acidity modification of ZSM-5 for enhanced production of light olefins from CO2. J Catal. 2020, 381, 347–354. [Google Scholar] [CrossRef]
- Guo, L.S.; Sun, J.; Wei, J.; Wen, Z.Y.; Xu, H.Y.; Ge, Q.J. Fischer–Tropsch synthesis over iron catalysts with corncob-derived promoters. J. Energy Chem. 2017, 26, 632–638. [Google Scholar] [CrossRef]
- Gao, X.H.; Zhang, J.L.; Chen, N.; Ma, Q.X.; Fan, S.B.; Zhao, T.S.; Tsubaki, N. Effects of zinc on Fe-based catalysts during the synthesis of light olefins from the Fischer-Tropsch process. Chin. J. Catal. 2016, 37, 510–516. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Shafer, W.D.; Martinelli, M.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L.; Davis, B.H. Ga and In modified ceria as supports for cobalt-catalyzed Fischer-Tropsch synthesis. Appl. Catal. A 2017, 547, 115–123. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.F.; Bao, J.; Zhang, Y. Manganese-modified Fe3O4 microsphere catalyst with effective active phase of forming light olefins from syngas. ACS Catal. 2015, 5, 3905–3909. [Google Scholar] [CrossRef]
- Yao, B.; Xiao, T.; Makgae, O.A.; Jie, X.; Gonzalez-Cortes, S.; Guan, S.; Kirkland, A.I.; Dilworth, J.R.; Al-Megren, H.A.; Alshihri, S.M.; et al. Transforming carbon dioxide into jet fuel using an organic combustion-synthesized Fe-Mn-K catalyst. Nat. Commun. 2020, 11, 6395. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174–15181. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, S.; Bu, X.; Dang, S.; Liu, Z.; Wang, H.; Zhong, L.; Qiu, M.; Yang, C.; Cai, J.; et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Xu, J.; Wang, H.; Ding, M.; Li, Y. Study of bimetallic interactions and promoter effects of FeZn, FeMn and FeCr Fischer-Tropsch synthesis catalysts. J. Mol. Catal. A Chem. 2010, 326, 29–40. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Guo, X.; Song, C.; Guo, X. Recent advances in application of iron-based catalysts for COx hydrogenation to value-added hydrocarbons. Chin. J. Catal. 2022, 43, 731–754. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, P.P.; Xiao, L.W.; Liang, J.M.; Zhang, B.Z.; Zhao, H.; Kosol, R.; Ma, Q.X.; Chen, J.N.; Peng, X.B.; et al. Structure-Performance correlations over Cu/ZnO interface for low-temperature methanol synthesis from syngas containing CO2. ACS Appl. Mater. Interfaces 2021, 13, 8191–8205. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z.; Li, A.W.; Krishnamoorthy, S.; Iglesia, E. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer–Tropsch synthesis catalysts. Catal. Lett. 2001, 77, 197–205. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Liu, M.; Sun, Y.; Song, C.; Guo, X. Selective CO2 hydrogenation to hydrocarbons on Cu-promoted Fe-based catalysts: Dependence on Cu-Fe interaction. ACS Sustain. Chem. Eng. 2018, 6, 10182–10190. [Google Scholar] [CrossRef]
- Niu, J.T.; Guo, F.; Ran, J.Y.; Qi, W.J.; Yang, Z.Q. Methane dry (CO2) reforming to syngas (H2/CO) in catalytic process: From experimental study and DFT calculations. Int. J. Hydrog. Energy 2020, 45, 30267–30287. [Google Scholar] [CrossRef]
- Wang, W.J.; Jiang, X.; Wang, X.X.; Song, C.S. Fe–Cu bimetallic catalysts for selective CO2 hydrogenation to olefin-rich C2+ hydrocarbons. Ind. Eng. Chem. Rec. 2018, 57, 4535–4542. [Google Scholar] [CrossRef]
- Amoyal, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 2017, 348, 29–39. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Perathoner, S.; Abate, S.; Centi, G.; Palkovits, R. Hydrotalcite based Ni-Fe/(Mg, Al)Ox catalysts for CO2 methanation–tailoring Fe content for improved CO dissociation, basicity, and particle size. Catal. Sci. Technol. 2018, 4, 1016–1027. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, Y.; Zhou, X.; Gao, P.; van Bavel, A.P.; Wang, H.; Li, S.; Shi, L.; Yang, Y.; Vovk, E.I.; et al. Direct conversion of CO2 to a jet fuel over CoFe alloy catalysts. Innovation 2021, 2, 100170. [Google Scholar] [CrossRef]
- Wan, H.J.; Wu, B.S.; Zhang, C.H.; Xiang, H.W.; Li, Y.W.; Xu, B.F.; Yi, F. Study on Fe-Al2O3 interaction over precipitated iron catalyst for Fischer-Tropsch synthesis. Catal. Commun. 2007, 8, 1538–1545. [Google Scholar] [CrossRef]
- Lenglet, M.; Foulatier, P.; Düeb, J.; Arséne, J. Caractérisation de la liaison Cu-O dans les oxydes mixtes CuMM′ O4 (M= Fe, Cr; M′= Al, Ga, Mn). Corrélation avec l’effet Jahn-Teller. Phys. Status Solidi A. 1986, 94, 461–466. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.H.; Evans, D.G.; Duan, X. Structure and surface chemistry of manganese-doped copper-based mixed metal oxides derived from layered double hydroxides. Colloids Surf. A. 2004, 244, 169–177. [Google Scholar] [CrossRef]








| Samples | ABET (m2/g) a | Pore Volume (cm3/g) b | Pore Diameter (nm) c |
|---|---|---|---|
| Na-AlFeCu | 200 | 0.33 | 5.40 |
| Na-MgFeCu | 124 | 0.48 | 4.02 |
| Na-GaFeCu | 178 | 0.22 | 4.02 |
| Na-MnFeCu | 189 | 0.43 | 5.73 |
| Na-ZnFeCu | 110 | 0.41 | 4.03 |
| Samples | CO2 Conv. (%) | CO Sel. (%) | CO-Free Hydrocarbon Sel. (mol%) | C5+ Yield (%) | ||
|---|---|---|---|---|---|---|
| CH4 | C2-4 | C5+ | ||||
| Na-FeCu | 35.9 | 11.0 | 17.7 | 41.1 | 41.2 | 14.8 |
| Na-AlFeCu | 44.5 | 9.9 | 15.2 | 26.5 | 58.3 | 25.9 |
| Na-MgFeCu | 30.6 | 24.0 | 22.3 | 36.9 | 40.7 | 12.5 |
| Na-GaFeCu | 32.8 | 14.2 | 10.7 | 24.1 | 65.2 | 21.4 |
| Na-MnFeCu | 33.3 | 23.1 | 15.5 | 34.0 | 50.5 | 16.8 |
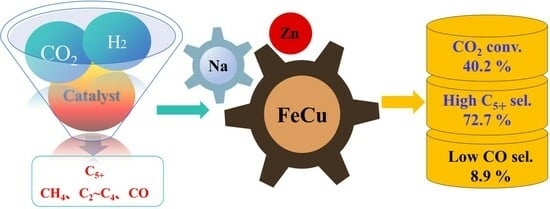
| Na-ZnFeCu | 40.2 | 8.9 | 7.1 | 20.7 | 72.2 | 29.0 |
| MFeCu | M (g) | ||||
|---|---|---|---|---|---|
| Al | Mg | Mn | Ga | Zn | |
| 4.26 | 5.12 | 5.02 | 5.11 | 5.96 | |
| Fe(NO3)3·9H2O (g) | 8.08 | 8.08 | 8.08 | 8.08 | 8.08 |
| Cu(NO3)2·3H2O (g) | 4.84 | 4.84 | 4.84 | 4.84 | 4.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, K.; Xing, Y.; Song, W.; Gao, X.; Ma, Q.; Zhao, T.; Zhang, J. Synthesis of Liquid Hydrocarbon via Direct Hydrogenation of CO2 over FeCu-Based Bifunctional Catalyst Derived from Layered Double Hydroxides. Molecules 2023, 28, 6920. https://doi.org/10.3390/molecules28196920
Li Z, Wang K, Xing Y, Song W, Gao X, Ma Q, Zhao T, Zhang J. Synthesis of Liquid Hydrocarbon via Direct Hydrogenation of CO2 over FeCu-Based Bifunctional Catalyst Derived from Layered Double Hydroxides. Molecules. 2023; 28(19):6920. https://doi.org/10.3390/molecules28196920
Chicago/Turabian StyleLi, Ziqin, Kangzhou Wang, Yaqin Xing, Wenlong Song, Xinhua Gao, Qingxiang Ma, Tiansheng Zhao, and Jianli Zhang. 2023. "Synthesis of Liquid Hydrocarbon via Direct Hydrogenation of CO2 over FeCu-Based Bifunctional Catalyst Derived from Layered Double Hydroxides" Molecules 28, no. 19: 6920. https://doi.org/10.3390/molecules28196920