Abstract
At present, phenolic acid derivatives and triazole derivatives have a good antifungal effect, which has attracted widespread attention. A series of novel phenolic acid triazole derivatives were synthesized, and their structures were characterized by IR, MS, NMR, and X-ray crystal diffraction. Compound methyl 4-(2-bromoethoxy)benzoate, methyl 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy)benzoate, 4-(2-(1H-1,2,4-triazol-1-yl)ethoxy)benzoic acid and 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy)-3-methoxybenzoic acid crystallize in the monoclinic system with space group P21/n, the monoclinic system with space group P21, the monoclinic system with space group P21 and the orthorhombic system with space group Pca21, respectively. At a concentration of 100 μg/mL and 200 μg/mL, the antifungal activity against seven plant pathogen fungi was determined. Compound methyl 4-(2-bromoethoxy)benzoate has the best inhibitory effect on Rhizoctonia solani AG1, and the inhibitory rate reached 88.6% at 200 μg/mL. The inhibitory rates of compound methyl 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy)benzoate against Fusarium moniliforme and Sphaeropsis sapinea at a concentration of 200 μg/mL were 76.1% and 75.4%, respectively, which were better than that of carbendazim.
1. Introduction
Plant diseases are one of the most important natural disasters in agricultural production, and plant diseases caused by plant pathogenic fungi have always been the main cause of crop yield reduction [1]. The use of fungicides is the main control measure of agricultural crop diseases and has made great contributions to improving crop yields and quality benefit [2]. However, long-term use of existing traditional fungicides has caused environmental pollution, pesticide residue toxicity, and drug resistance to plant fungal diseases [3]. Therefore, it is of great significance to develop new and promising plant-protection antibacterial agents. A large number of derivatives can be obtained by structural modification of natural products with certain antibacterial activities. It is possible to screen antibacterial functional factors with high activity and little or no side effect, and it is a promising way to develop pesticides and fungicides.
Phenolic acids are substances containing polyphenol hydroxyl groups, including chlorogenic acid, ferulic acid, caffeic acid and rosmarinic acid, which are widely found in natural plants [4,5]. It has pharmacological effects such as anti-oxidative stress [6], improvement of microcirculation [7], improvement of cell energy metabolism [8], etc., and can fully protect the cranial nerves and significantly improve the quality of life of patients with cerebral infarction. Widely used in ischemic cerebrovascular diseases, it has anti-free-radicals and anti-tumor effects [9,10]. Phenolic acid also has a good antibacterial effect [11,12]. However, the further development and utilization of phenolic acids are limited due to their unstable structure, strong hydrophilicity, poor lipid solubility and low bioavailability.
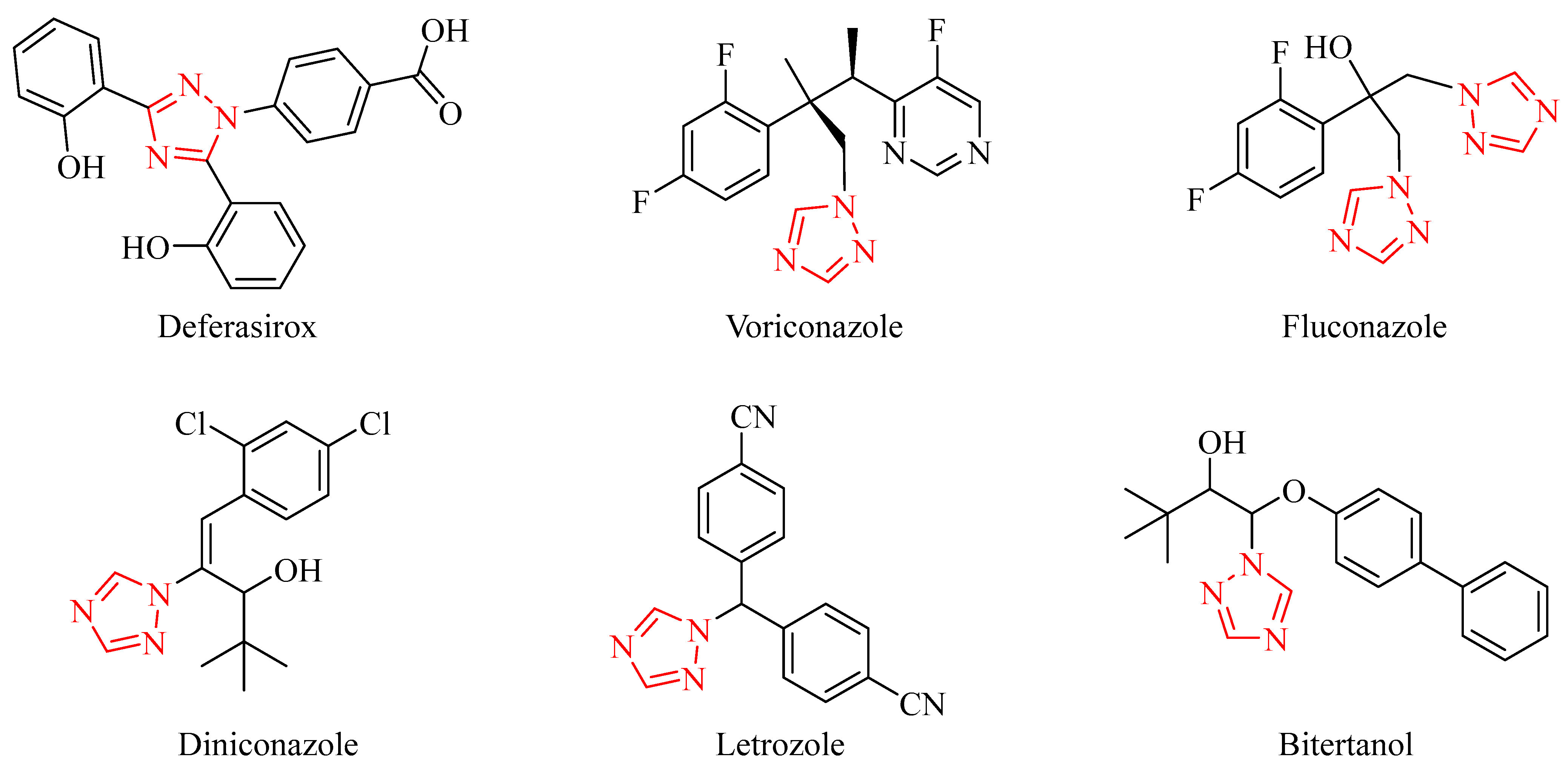
A triazole is a five-membered heterocyclic compound containing three nitrogen atoms. Triazole compounds, as an important part of nitrogen-containing heterocycles, are widely used because of their unique structural characteristics. Triazole compounds have unique biological activity, low toxicity and strong systemic properties. They are often used as structural units of drugs and pesticides, and they play an important role in their synthesis [13,14]. Triazole derivatives have anti-corrosion, antibacterial [15], weeding [16], antivirus [17], anti-influenza [18], anti-cancer [19,20] and anti-oxidation characteristics [21]. Among them, triazole derivatives have a long history of application in sterilization and are an important class of fungicides. Triazole fungicides can be regarded as five-membered heterocyclic derivatives and the structures and positions of the connected atomic groups are different, which leads many triazole fungicides to have different functions (Figure 1) [22,23,24]. Most triazole pesticides are derivatives of a triazole ring substituted by alkyl or aralkyl.

Figure 1.
Structural formulas of selected triazole fungicides.
It is known that the combination of two active groups can enhance its activity. For example, Li and Wu, who combined triazoles with pyrimidine, both obtained compounds with good antibacterial activity [25]. Slivka et al. synthesized several compounds with triazole, thiazole and phenol substituents and their copper complexes, and the synthesized compounds had good fungicidal activity [26]. We found that most triazole pesticides are derivatives in which the nitrogen atom of the triazole ring is substituted by alkyl or aralkyl. Because alkylating reagents can easily react with the nitrogen atoms of heterocyclic rings and phenolic hydroxyl groups of phenolic acid, it is very convenient and reasonable to connect triazole rings with phenolic fragments with alkyl chains. We have been committed to the synthesis of and antifungal activity research into natural product derivatives and their complexes, and we have reported a series of crystal structures and biological activity [27,28]. It is found that triazole pesticides have a good inhibitory effect on common crop pathogenic fungi. Therefore, we used 1,2-dibromoethane to introduce 1,2,4-triazole into the phenolic hydroxyl of hydroxybenzoate and used vanillic acid and syringic acid to synthesize a series of phenolic acid triazole derivatives, and we studied their antifungal activities. Herein, we report the synthesis, crystal structure, spectral characterization, and antifungal activity of novel phenolic acid triazole derivatives.
2. Results
2.1. Chemistry
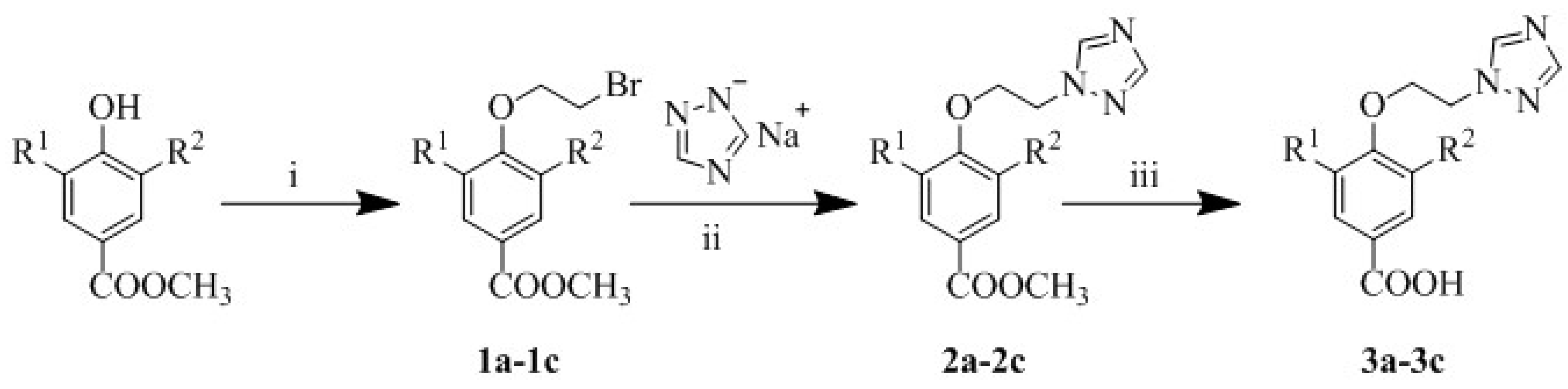
The synthetic route of compounds 3a–3c is summarized in Scheme 1. The structures of compounds 1a–3c are shown in Figure 2. All the target phenolic acid triazole derivatives were synthesized by a two-step reaction with methyl p-hydroxybenzoate, methyl 4-hydroxy-3-methoxybenzoate and methyl 4-hydroxy-3,5-dimethoxybenzoate as raw materials.

Scheme 1.
The synthetic route of compounds 3a–3c. Reagents and conditions. (i): Dibromoethane, K2CO3, acetonitrile, 85 °C, 10 h; (ii): acetonitrile, 85 °C, 5 h; (iii): 10% Sodium hydroxide, ethanol, room temperature, 3 h.

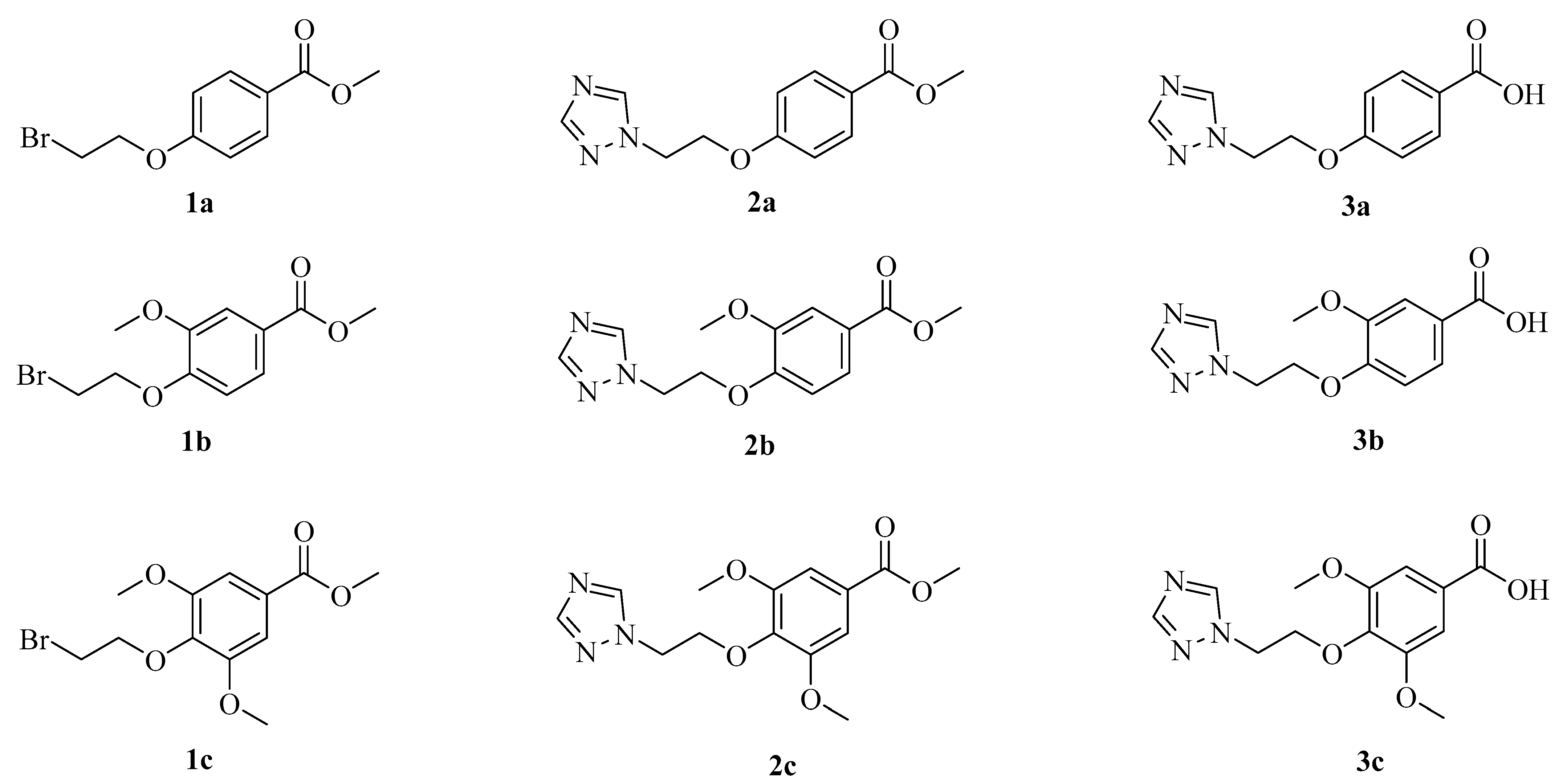
Figure 2.
Structures of compounds 1a–3c.
2.2. FT-IR Spectral Characterization
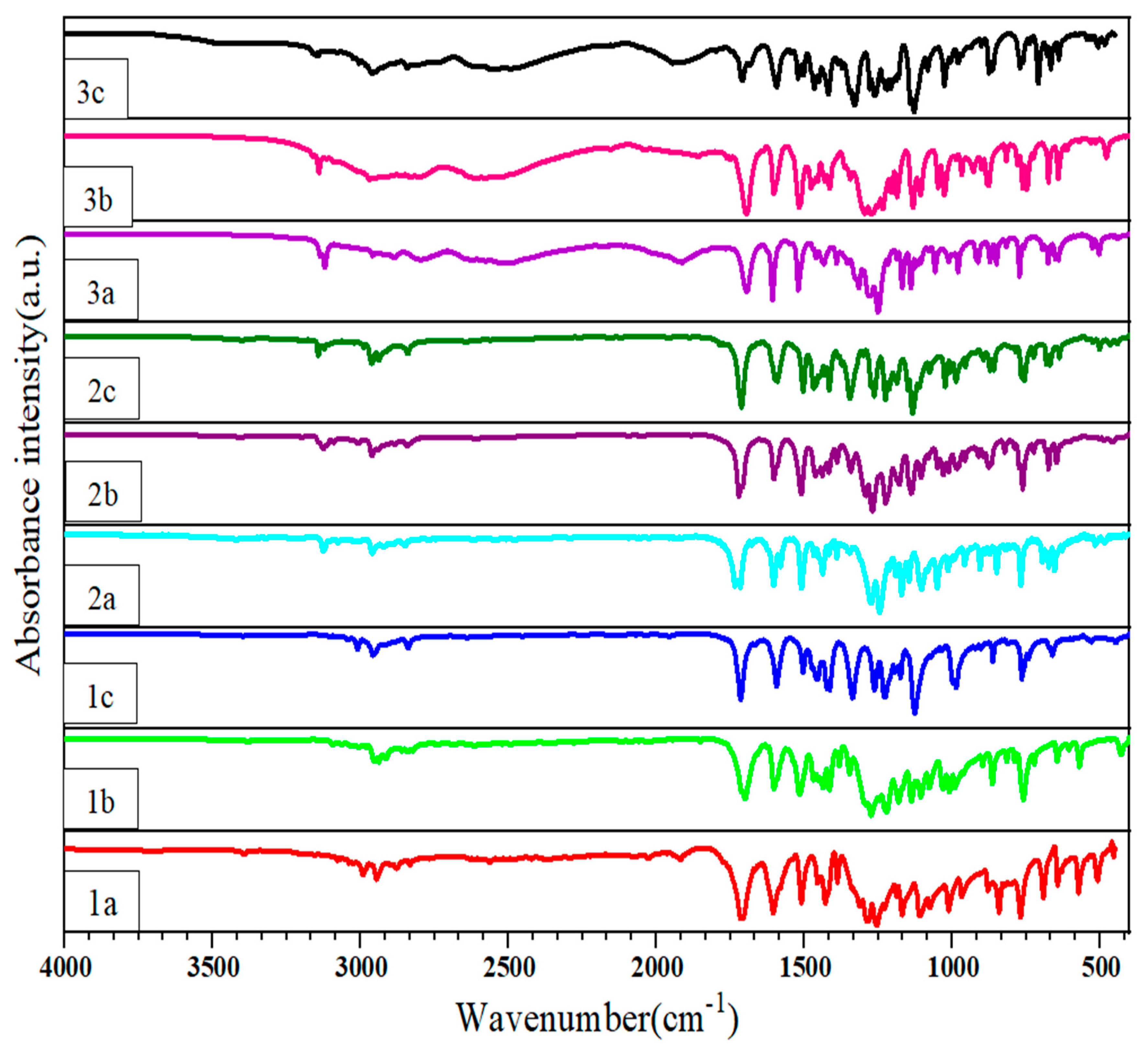
All compounds were characterized by an infrared spectrum in the range of 500–4000 cm−1. Compounds 1a–1c are structurally similar, differing only by methoxy in the neighboring position, which is difficult to distinguish in the IR spectrum (Figure 3). 2a–2c and 3a–3c are similar to 1a–1c, so we divided the synthesized compounds into three groups for infrared resolution. 2a–2c and 3a–3c show a peak at 3200 cm−1, which is consistent with the stretching vibration of N-H on the triazole ring. 3a–3c shows a broad and scattered peak at 2500–3300 cm−1, consistent with the O-H stretching vibration of the carboxyl group, and a peak near 1700 cm−1, consistent with the C=O absorption of the carboxyl group. 1a–1c and 2a–2c show a large and deep peak around 1715 cm−1, which is consistent with the C=O absorption of the ester group.

Figure 3.
FT-IR spectral characterization of compounds 1a–3c.
2.3. NMR Spectral Characterization
In the 1H NMR of these compounds, the chemical shifts of the benzene ring and triazole ring are 6.87–8.01 and 7.98–8.60, respectively. The two triplet peaks with the same coupling constants near 4.0 belong to the (-CH2CH2-) structure. The chemical shift of methyl hydrogen (-CH3), connected directly to the O atom, is about 3.85. A unimodal peak above 12.0 is the (-COOH) carboxyl hydrogen.
In the 13C NMR of these compounds, the chemical shifts of the carbonyl carbon are in the range of 165.71–166.92. The chemical shift of the triazole ring is in the range of 140.01–152.14. The chemical shifts of the methylene carbon (-CH2-) linked to the O atom is in the range of 65.64–72.56, and that of the methyl carbon (-CH3) linked to the O atom is in the range of 51.91–56.24. Comparing the (-OCH3) substitution of compound 2b with 2c in the fifth position, the chemical shifts of 2b and 2c in the fourth position are 151 and 140, respectively, the chemical shifts in the fifth position are 112 and 152 and the chemical shifts in the sixth position of the carbon are 122 and 106. The changes in the chemical shifts of the benzene ring carbons of compounds 2b and 2c are basically consistent with the above situation.
2.4. Crystal Structures of Compounds 1a–3a and 3b
2.4.1. Crystal Structure of Compound 1a
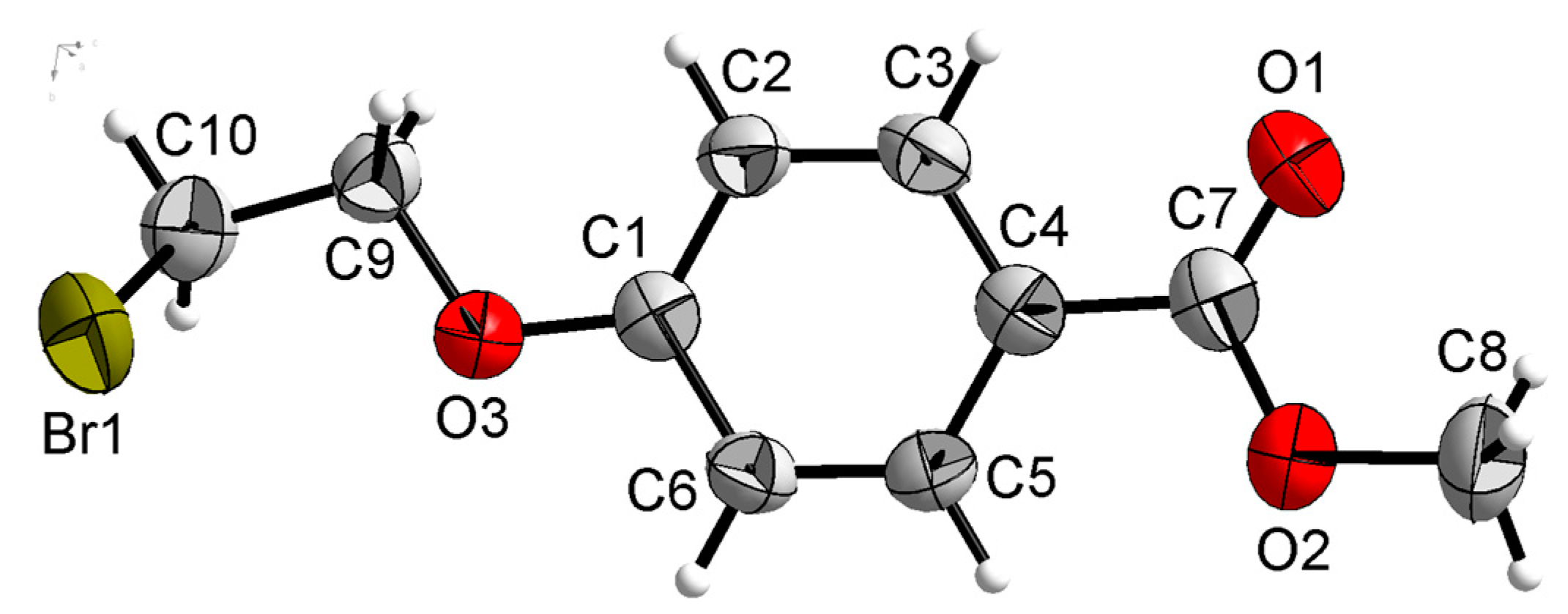
Compound 1a was crystallized in the monoclinic system with space group P21/n. In the molecule of 1a (as shown in Figure 4), the bond lengths and angles were very similar to those of methyl p-hydroxybenzoate derivatives [29,30]. The part of methyl p-hydroxybenzoate was approximately planar. The dihedral angles of the C1-C6 phenyl plane and the carboxylate group O1-C7-O2 plane were 4.031(187)°. The torsion angles of C4-C7-O2-C8, C1-O3-C9-C10 and O3-C9-C10-Br1 were 178.233(246)°, 144.470(224)° and 69.885(255)°, respectively.

Figure 4.
Crystal structure of compound 1a with 30% thermal ellipsoids.
2.4.2. Crystal Structure of Compound 2a
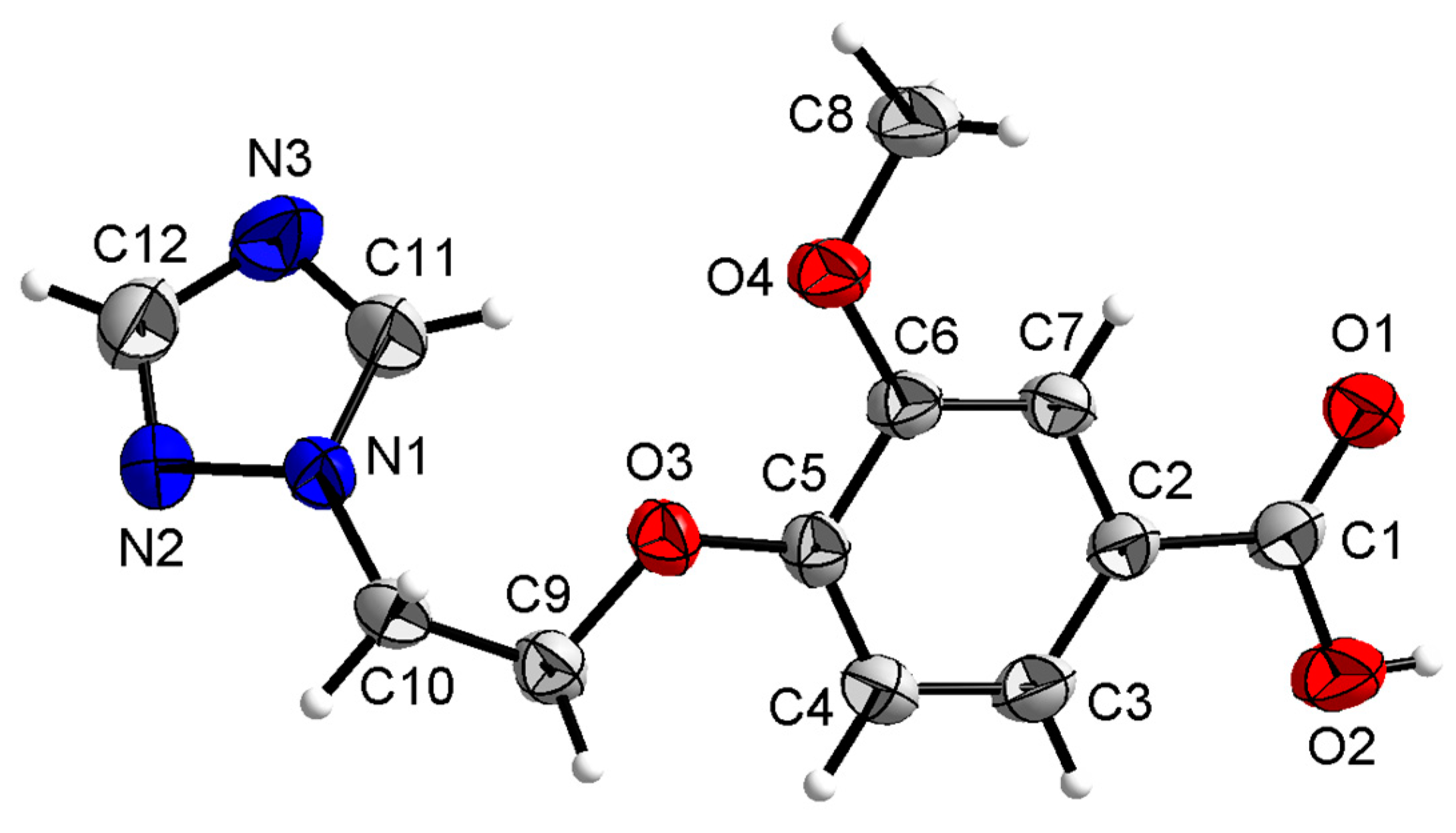
Compound 2a was crystallized in the monoclinic system with space group P21. In the molecule of 2a (Figure 5), the methyl p-hydroxybenzoate part was approximately planar. The dihedral angles of the C1-C6 phenyl plane, the triazole plane and the carboxylate group O1-C7-O2 plane were 1.190(152)°, 70.285(75)° and 70.283(143)°, respectively. The torsion angles of C6-C7-O2-C8, C3-O3-C9-C10 and O3-C9-C10-N1 were −179.767(185)°, 173.314(157)°, and 69.912(197)°, respectively.

Figure 5.
Crystal structure of compound 2a with 30% thermal ellipsoids.
2.4.3. Crystal Structure of Compound 3a
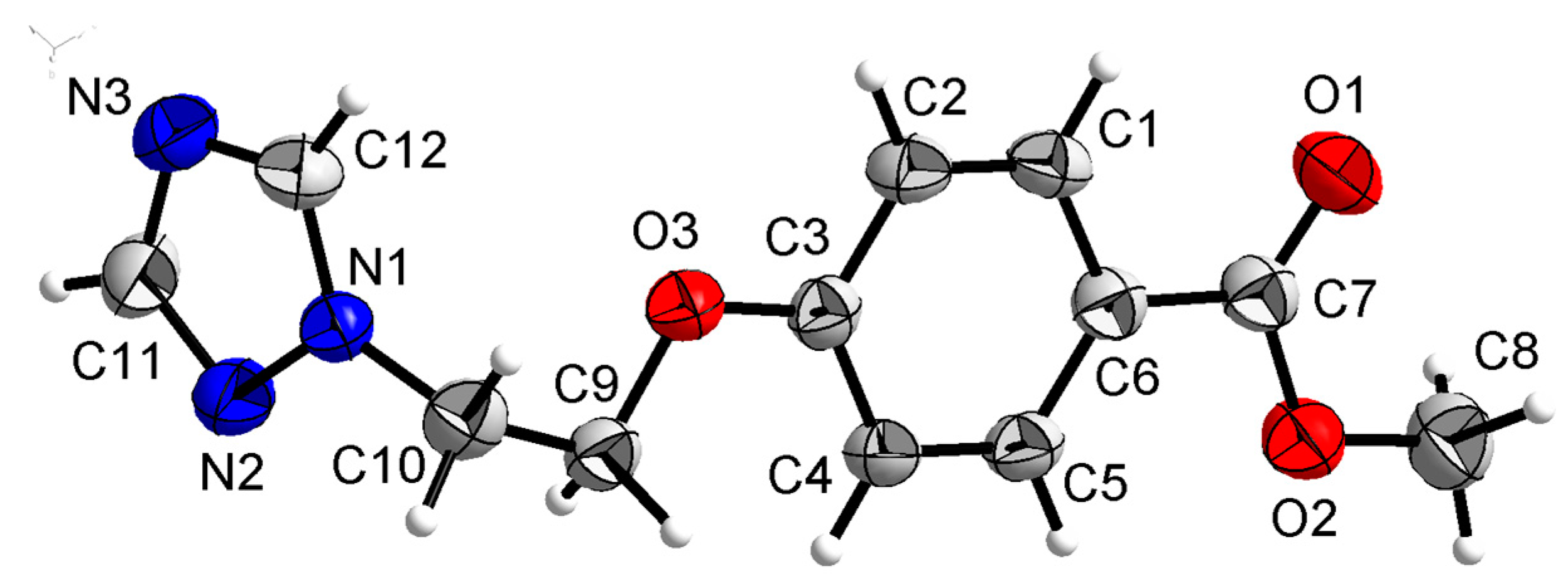
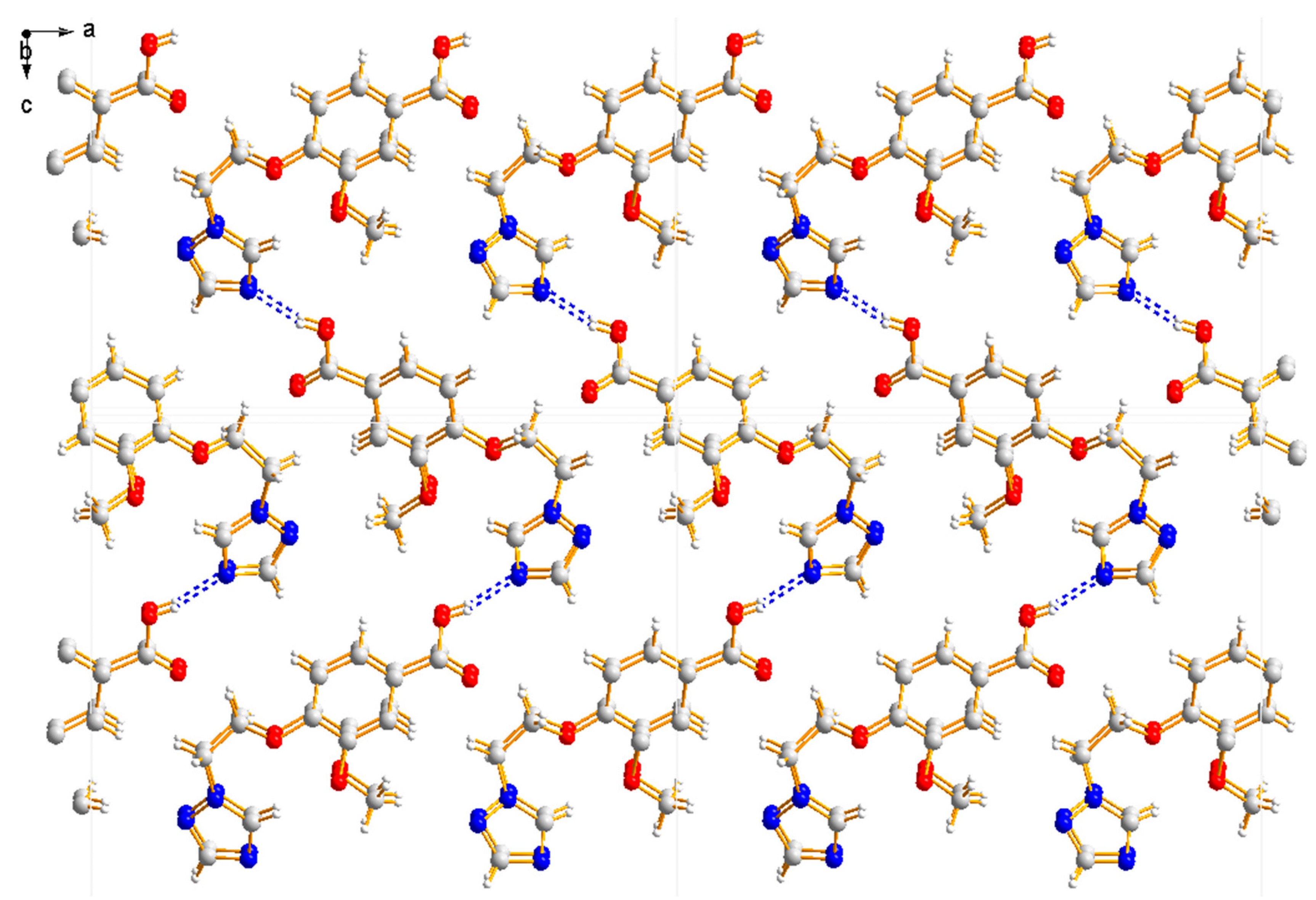
Compound 3a was crystallized in the monoclinic system with space group P21. In the molecule of 3a (Figure 6), the methyl p-hydroxybenzoate part was approximately planar. The dihedral angles of the C1-C6 phenyl plane, the triazole plane and the carboxylate group O1-C7-O2 plane were 4.767(187)°, 70.984(124)° and 70.009(221)°, respectively. The torsion angles of C3-O3-C9-C10 and O3-C9-C10-N1 were 173.098(219)° and 66.035(289)°, respectively. Intermolecular O–H…N hydrogen bonds between the oxygen atom (O2) of the carboxylate group and the nitrogen atom (N3) of the triazole ring linked the molecules in a one-dimensional chain structure (Figure 7).
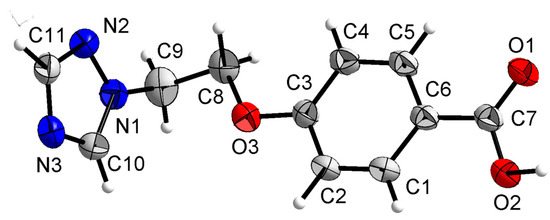
Figure 6.
Crystal structure of compound 3a with 30% thermal ellipsoids.
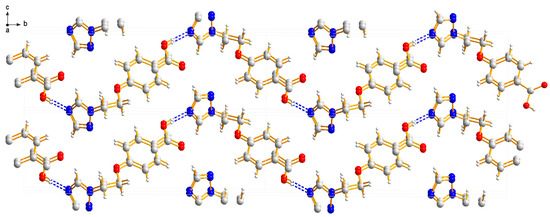
Figure 7.
One-dimensional chain structure of compound 3a.
2.4.4. Crystal Structure of Compound 3b
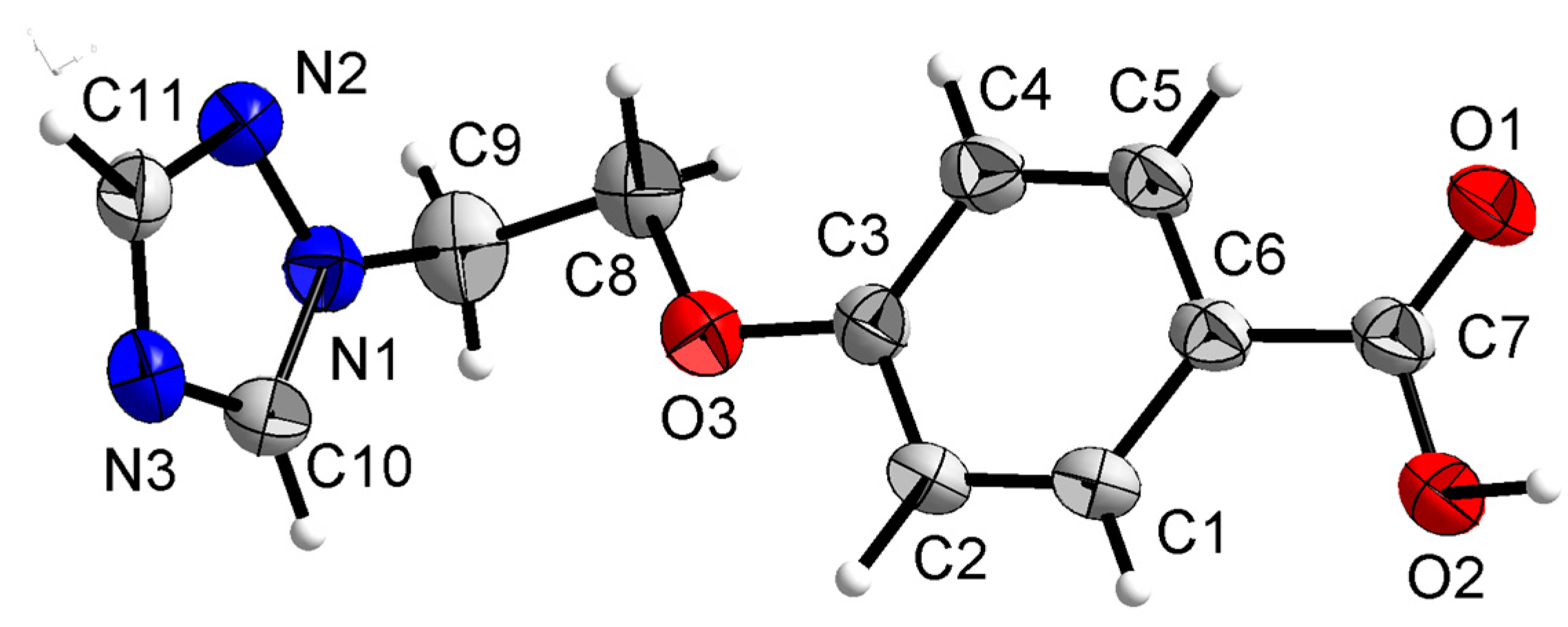
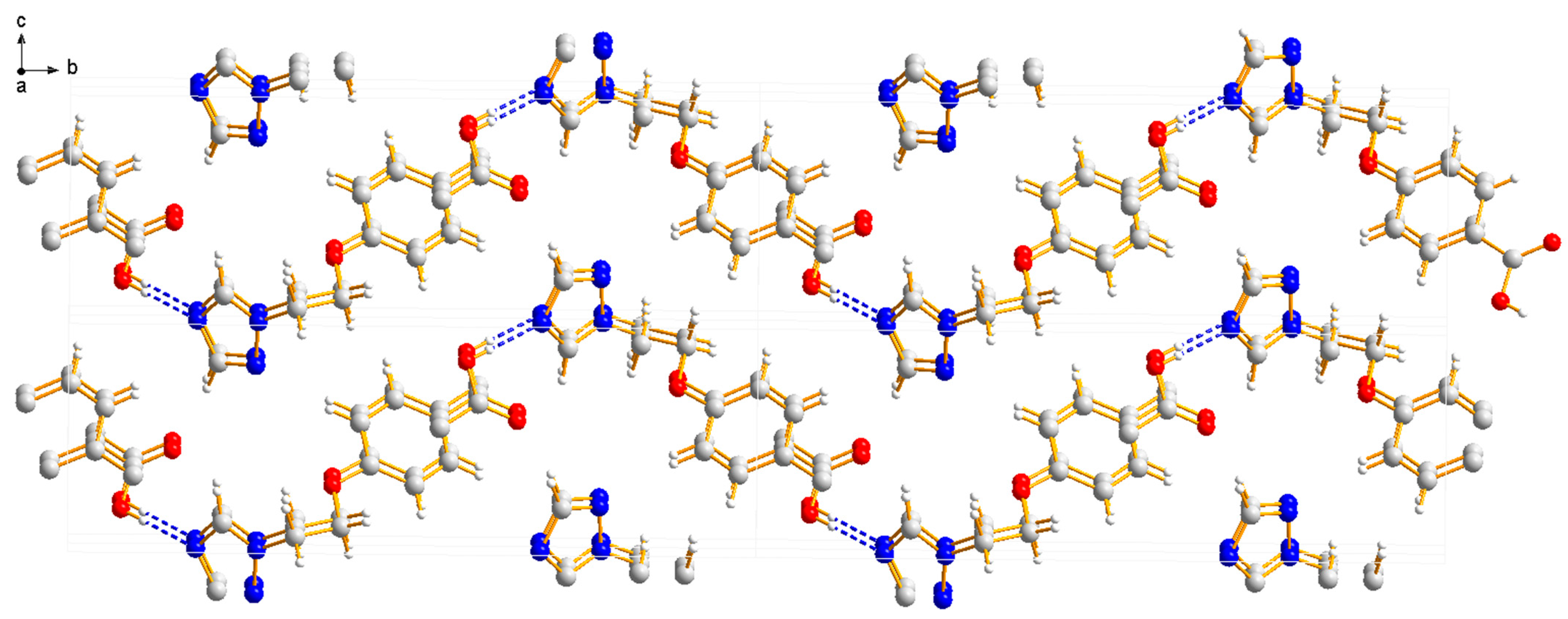
Compound 3b was crystallized in the orthorhombic system with space group Pca21. In the molecule of 3b (Figure 8), the methyl p-hydroxybenzoate part was approximately planar. The dihedral angles of the C2-C7 phenyl plane, the triazole plane and the carboxylate group O1-C1-O2 plane were 3.631(341), 49.059(106) and 50.307(210)°, respectively. The torsion angles of C5-O3-C9-C10, O3-C9-C10-N1 and C5-C6-O4-C8 were −174.442(232), 66.341(300) and −177.680(259)°, respectively. Intermolecular O–H…N hydrogen bonds between the oxygen atom (O2) of the carboxylate group and the nitrogen atom (N3) of the triazole ring linked the molecules into a one-dimensional chain structure (Figure 9).
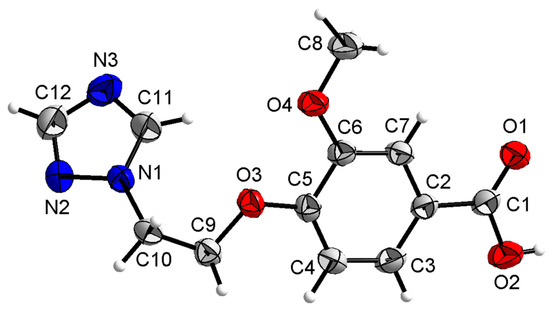
Figure 8.
Crystal structure of compound 3b with 30% thermal ellipsoids.
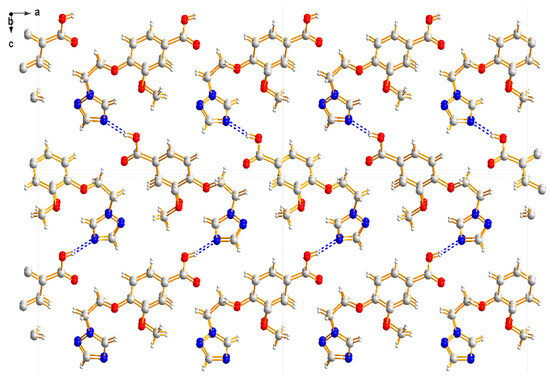
Figure 9.
One-dimensional chain structure of compound 3b.
2.5. Antifungal Activity Analysis
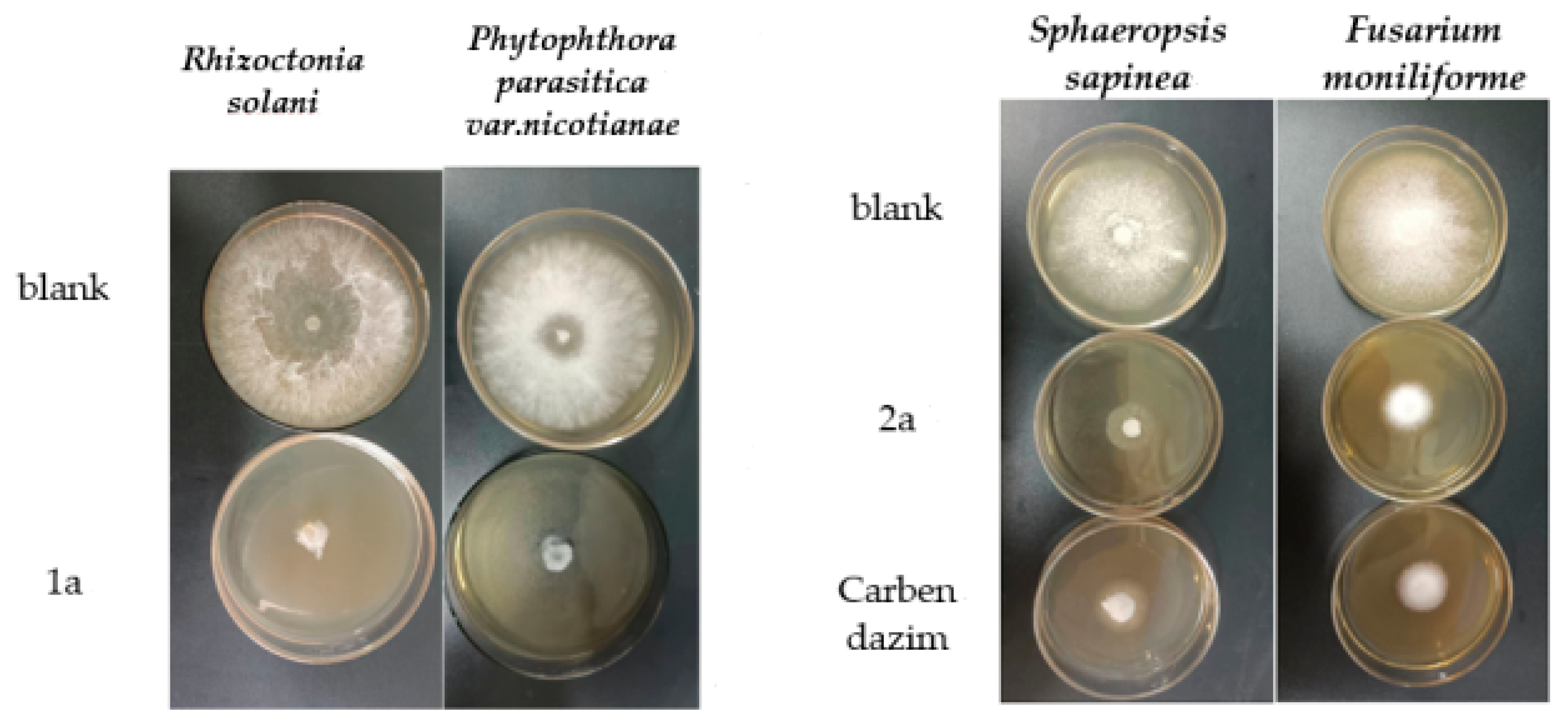
The diameter of each inhibition zone (IZ) was measured to calculate the inhibition rates of the compounds. The results listed in Table 1 show that compounds 1a–2c were effective against these seven plant pathogenic fungi: Rhizoctonia solani. AG1, Fusarium moniliforme, Colletotrichum fructicola, Phytophthora parasitica var. nicotianae, Fusarium oxysporum f. sp. niveum, Fusarium verticillioide and Sphaeropsis sapinea. Among them, compound 1a has the strongest inhibitory activity against Rhizoctonia solani. AG1, Phytophthora parasitica var., nicotine, Fusarium oxysporum f. sp. niveum and Fusarium verticillioide. At a concentration of 200 μg/mL, the inhibitory rate against Rhizoctonia solani. AG1 was the highest, reaching 88.6%. For structurally similar compounds, compounds 1a–1c showed similar inhibitory activity against Colletotrichum fructicola, with the remaining six fungi all following the 1a ˃ 1b ˃ 1c pattern. This may be due to the methoxylation (-OCH3) of the neighboring position, so we make a guess: for 1a, the electron-donating group in the neighboring position diminishes its inhibitory activity. Although compound 1a was less effective than the positive control carbendazim, we believe it has the potential to synthesize compounds with good inhibition. Specifically, the inhibitory rates of compound 1a against Rhizoctonia solani. AG1 and Phytophthora parasitica var. nicotianae were 88.6% and 80.7%, respectively (on the left of Figure 10). Among them, compound 2c has the highest inhibitory effect on Rhizoctonia solani. AG1, and the inhibitory rate was 56.8% at a concentration of 200 μg/mL. Compound 2b has the highest inhibitory effect on Colletotrichum fructicola and the inhibitory rate was 45.1% at a concentration of 200 μg/mL. Compound 2a has a better inhibitory effect on Fusarium moniliforme and Sphaeropsis sapinea than other compounds. The inhibitory rates of compound 2a against Fusarium moniliforme and Sphaeropsis sapinea at 200 μg/mL were as high as 76.1% and 75.4%, respectively (on the right of Figure 10), which was better than those of carbendazim. Compounds 3a–3c have poor antifungal activity against seven plant pathogenic fungi, which may be due to their high hydrophilicity and low lipid solubility.

Table 1.
The inhibition rates against fungi of 1a–3c.

Figure 10.
The antifungal effect of 1a, 2a and carbendazim. Left is the antifungal effect of 1a at 200 μg/mL; right is the antifungal effect of 2a and carbendazim at 200 μg/mL.
3. Discussion
From the perspective of inhibitory effects, we think that compounds 1a and 2a have good antifungal potential. Compound 1a showed good inhibitory activity against all seven tested fungi. It contained bromine and halogen, which are not uncommon in pesticide fungicides, such as iprodione, dimethachlon and bromonirol. In the future, we will consider adding some halogen to the compounds with better antifungal activity. In the analysis of our results against fungi, we compared 1a, 1b and 1c and found that the electron-donating group in the ortho position may reduce the activity of 1a. We will try to introduce an electron-withdrawing group in the ortho position to test whether this is consistent with our idea. Compound 2a has a very good inhibitory effect on Fusarium moniliforme and Sphaeropsis sapinea, even better than that of carbendazim. Compared with triazole fungicides such as triadimefon and phenoxymethoxazole, compound 2a has a simple structure and is easy to modify. We will also try to modify 2a in the future, possibly by introducing halogen or modifying the methyl ester into an amide structure.
4. Experimental Procedure
4.1. Materials and Methods
FT-IR spectrum at 500–4000 cm−1 was confirmed by potassium bromide compression method. 1H NMR and 13C were recorded on the NMR spectrum of Bruker Advance NEO 500 M instrument, TMS was used as the internal standard and CDCl3 or DMSO-d6 was used as the internal solvent. X-ray analysis of the crystal samples was measured by graphite monochromatic Mo Kα radiation (λ = 0.71073 Å) under the diffraction instrument of Bruker Apexii detector. Melting point is determined by X-4 microscopic melting point instrument (Shanghai Jingmi Co., Ltd., Shanghai, China) without correction.
Unless otherwise specified, all chemical reagents are commercially available and have been treated with standard methods before use. The progress of the chemical reactions and the purity of all products were monitored with thin-layer chromatography (TLC) on silica gel plates (silicone 60 GF254, ready-to-use). All strains of plant pathogenic fungi were provided by the Plant Pathology Laboratory of the College of Agricultural, Jiangxi Agricultural University. The cultivation bases come from Beijing Boxing Biotechnology Co., Ltd., Beijing, China.
4.2. Synthesis of 3a–3c
4.2.1. Synthesis of Compounds 1a–1c
Compounds 1a–1c were synthesized by the revised method of reference [28].
4.2.2. Synthesis of Compounds 2a–2c
In the synthesis example, compounds 2a, 2b and 2c were synthesized in the same way, except that 1a was replaced by 1b and 1c. Methyl 4-(2-bromoethoxy)benzoate (1a) (0.05 mol, 12.96 g) and sodium triazole (0.05 mol, 4.55 g) were dissolved in acetonitrile (30 mL). The reaction mixture was stirred well for about 5 h at 85 °C. Reaction was monitored by TLC. After the reaction was completed, the obtained solution was filtered and washed with water (10 mL) three times. The filtrate was extracted with Ethyl acetate and dried over anhydrous Na2SO4. The product was purified to obtain compounds 2a–2c by column chromatography on silica gel using ethyl acetate/petroleum ether (VEthyl acetate/Vpetroleum ether = 3:1) as eluent.
4.2.3. Syntheses of Compounds 3a–3c
For example, 3a was synthesized in the same way as 3b and 3c, except that 2b and 2c replaced 2a. The mixture of methyl 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy) benzoate (2a) (0.01 mol, 2.47 g), NaOH solution (10%, 40 mL) and ethanol (2 mL) was reacted under stirring at room temperature. Reaction was monitored by TLC. After the reaction was completed, the pH of the obtained solution was adjusted to 5–6 with diluted hydrochloric acid. A large amount of white solids was precipitated, filtered and dried, and the products 3a–3c were obtained. The crystals were obtained after one week of slow volatilization at room temperature.
- Methyl 4-(2-bromoethoxy) benzoate (1a): white solid; the yield is 35.8%, m. p: 63.4~65.0 °C. 1H NMR (500 MHz, CDCl3) δ 8.00 (d, J = 8.8 Hz, 2H), 6.93 (d, J = 8.8 Hz, 2H), 4.34 (t, J = 6.2 Hz, 2H), 3.89 (s, 3H), 3.66 (t, J = 6.2 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 166.91, 161.99, 131.90, 123.55, 114.43, 68.04, 52.13, 28.82. HRMS: C10H11BrO3 for [M + H]+, calculated 258.9925, found 258.9960.
- Methyl 4-(2-bromoethoxy)-3-methoxybenzoate (1b): white solid; the yield is 40.2%, m. p: 69.6~72.1 °C. 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 10.3 Hz, 1H), 7.57 (s, 1H), 6.90 (d, J = 8.4 Hz, 1H), 4.38 (t, J = 6.7 Hz, 2H), 3.92 (s, 3H), 3.90 (s, 3H), 3.68 (t, J = 6.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 166.68, 151.39, 149.13, 123.75, 123.26, 112.88, 112.63, 68.75, 56.14, 52.04, 28.29. HRMS: C11H13BrO4 for [M + H]+, calculated 289.0031, found 289.0072.
- Methyl 4-(2-bromoethoxy)-3,5-dimethoxybenzoate (1c): white solid; the yield is 36.7%, m. p: 102.1~103.5 °C. 1H NMR (500 MHz, CDCl3) δ 7.29 (s, 2H), 4.34–4.29 (m, 2H), 3.93 (s, 3H), 3.92 (s, 6H), 3.63–3.58 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 166.58, 152.91, 140.35, 125.69, 106.69, 72.56, 56.24, 52.25, 29.47. HRMS: C12H15BrO5 for [M + H]+, calculated 319.0136, found 319.0170.
- Methyl 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy) benzoate (2a): white solid; the yield is 62.5%, m. p: 113.5~116.1 °C. 1H NMR (500 MHz, CDCl3) δ 8.22 (d, J = 9.0 Hz, 2H), 7.98 (s, 1H), 7.97 (d, J = 3.2 Hz, 1H), 6.87 (d, J = 8.8 Hz, 2H), 4.60 (t, J = 5.0 Hz, 2H), 4.39 (t, J = 5.0 Hz, 2H), 3.88 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 166.82, 161.43, 152.14, 143.96, 131.69, 123.58, 114.06, 65.64, 51.95, 49.07. HRMS: C12H13N3O3 for [M + H]+, calculated 248.0990, found 248.1028.
- Methyl 4-(2-(1H-1,2,4-triazol-1-yl)ethoxy)-3-methoxybenzoate (2b): white solid; the yield is 65.6%, m. p:118.3~120.4 °C. 1H NMR (500 MHz, DMSO) δ 8.55 (s, 1H), 7.99 (s, 1H), 7.55 (d, J = 8.4 Hz, 1H), 7.45 (s, 1H), 7.08 (d, J = 8.5 Hz, 1H), 4.62 (t, J = 5.1 Hz, 2H), 4.43 (t, J = 5.1 Hz, 2H), 3.81 (s, 3H), 3.78 (s, 3H). 13C NMR (126 MHz, DMSO) δ 165.83, 151.50, 151.42, 148.57, 144.63, 122.99, 122.50, 112.70, 112.23, 66.54, 55.71, 51.91, 48.23. HRMS: C13H15N3O4 for [M + H]+, calculated 278.1096, found 278.1125.
- Methyl 4-(2-(1H-1,2,4-triazol-1-yl)ethoxy)-3,5-dimethoxybenzoate (2c): white solid; the yield is 59.8%, m. p: 88.1~89.6 °C. 1H NMR (500 MHz, DMSO) δ 8.60 (s, 1H), 8.04 (s, 1H), 7.27 (s, 2H), 4.54 (t, J = 5.0 Hz, 2H), 4.37 (t, J = 5.0 Hz, 2H), 3.91 (s, 3H), 3.84 (s, 6H). 13C NMR (126 MHz, DMSO) δ 165.71, 152.53, 151.15, 144.62, 140.01, 124.98, 106.22, 70.52, 55.95, 52.18, 49.24. HRMS: C14H17N3O5 for [M + 1], calculated 308.1202, found 308.1215.
- 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy) benzoic acid (3a): white solid; the yield is 84.8%, m. p: 194.3~197.5 °C. 1H NMR (500 MHz, DMSO) δ 12.64 (s, 1H), 8.58 (s, 1H), 7.99 (s, 1H), 7.87 (d, J = 8.7 Hz, 2H), 7.00 (d, J = 8.8 Hz, 2H), 4.61 (t, J = 5.0 Hz, 2H), 4.43 (t, J = 5.0 Hz, 2H). 13C NMR (126 MHz, DMSO) δ 166.84, 161.41, 151.49, 144.68, 131.31, 123.41, 114.31, 65.87, 48.18. HRMS: C11H11N3O3 for [M + H]+, calculated 234.0834, found 234.0859.
- 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy)-3-methoxybenzoic acid (3b): white solid; the yield is 82.1%, m. p: 178.3~180.2 °C. 1H NMR (500 MHz, DMSO) δ 12.70 (s, 1H), 8.54 (s, 1H), 7.98 (s, 1H), 7.52 (d, J = 10.2 Hz, 1H), 7.44 (s, 1H), 7.05 (d, J = 16.2 Hz, 1H), 4.62 (t, J = 5.1 Hz, 2H), 4.43 (t, J = 5.1 Hz, 2H), 3.77 (s, 3H). 13C NMR (126 MHz, DMSO) δ 166.92, 151.39, 151.15, 148.49, 144.60, 123.74, 123.01, 112.67, 112.53, 66.54, 55.64, 48.24. HRMS: C12H13N3O4 for [M + H]+, calculated 264.0940, found 264.0946.
- 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy)-3,5-dimethoxybenzoic acid (3c): white solid; the yield is 80.2%, m. p: 168.5~171.7 °C. 1H NMR (500 MHz, DMSO) δ 8.54 (s, 1H), 7.96 (s, 1H), 7.19 (s, 2H), 4.46 (t, J = 5.0 Hz, 2H), 4.28 (t, J = 5.0 Hz, 2H), 3.74 (s, 6H). 13C NMR (126 MHz, DMSO) δ 167.05, 152.42, 151.19, 144.70, 139.49, 127.01, 106.37, 70.55, 55.92, 49.34. HRMS: C13H15N3O5 for [M + H]+, calculated 294.1045, found 294.1055.
4.3. Crystal Structure Determination
The crystal structures of 1a–3a and 3b were determined by X-ray single-crystal diffraction (see Supplementary Materials). Reflectance data were collected at room temperature using Bruker APEX II area detector [31], which was equipped with a graphite-monochromatic MoKα radiation (λ = 0.71073 Å) at 296(2) K, and the scanning mode was ω-2θ. All data were corrected using SAD ABS by empirical adsorption. The structures were solved by direct methods and refined by full-matrix least squares on F2 using SHELXTL 97 software [32,33]. All non-hydrogen atoms were located by the direct method and subsequent differential Fourier synthesis method.
Crystallographic data for the structures described in this paper were stored in the Cambridge Crystallographic Data Centre (CCDC) with identification numbers 2214417 (1a), 2214418 (2a), 2214429 (3a) and 2214420 (3b).
4.4. Antifungal Activity Test
The antifungal activity of the target compounds and their intermediates against 7 plant pathogenic fungi were determined by the disk diffusion method [34,35] at 100 μg/mL and 200 μg/mL. We used Carbendazim as a bactericide comparison.
The preparation of the test sample: 20 mg of the test sample was dissolved in 1 mL of acetone, then the solution was transferred to a 10 mL volumetric flask, with 0.1% tween-80 aqueous solution used for constant volume. The prepared solution was poured into 90 mL (50~55 °C) potato glucose agar (PDA) medium, mixed evenly, and then quickly poured into 6 Petri dishes (90 mm in diameter) to detect 2 kinds of fungus. At the same time, a blank control group (0.1% tween-80 aqueous solution) without drugs was set up.
Inoculation of pathogenic fungus: the fungus was cut from the edge of the colony with a sterile drill (5 mm) and was inoculated in the center of the dish containing the medicine with an inoculation stick. Then, the Petri dishes were cultured in an illumination incubator (28 °C) for 5–7 days. When the hyphae of the fungi in blank control group grew to 7–8 cm, all experimental data of the fungi were recorded. The diameter of the mycelium was measured using the cross method. Each antifungal test was repeated separately three times. The inhibition ratio I (%) was calculated by the following formula, in which C represents the diameter of control group (untreated with compound) and T represents the diameter of treated group [36]:
5. Conclusions
Phenolic acid triazole derivatives were synthesized by introducing a triazole ring into the hydroxyl group of phenolic acid through the alkyl chain. The structures of all the target compounds were characterized by IR, MS and NMR. Single crystals were cultured with synthetic compounds, and compound methyl 4-(2-bromoethoxy) benzoate, methyl 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy) benzoate, 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy)benzoic acid and 4-(2-(1H-1,2,4-triazol-1-yl)ethoxy)-3-methoxybenzoic acid were cultured with X-ray crystal. The potential antifungal activity against seven plant pathogens at 100 μg/mL and 200 μg/mL were tested. Research has shown that the compounds have antifungal activity against plant pathogens. Compound methyl 4-(2-bromoethoxy) benzoate has a good inhibitory effect on the tested fungi. Among them, compound methyl 4-(2-bromoethoxy) benzoate has the highest inhibitory rate against Rhizoctonia solani AG1, which reaches 88.6% at 200 μg/mL. The inhibitory rates of compound methyl 4-(2-(1H-1,2,4-triazol-1-yl) ethoxy)benzoate against Fusarium moniliforme and Sphaeropsis sapinea at 200 μg/mL were 76.1% and 75.4%, respectively, which were superior to those of carbendazim.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28196970/s1, Table S1: Crystal and structure refinement data of 1a–3a and 3b; Table S2: Selected bond lengths (Å) and bond angles (°) of 1a–3a and 3b.
Author Contributions
Writing—original draft, P.-L.X.; data curation, X.-Y.S.; investigation, X.-Y.S.; methodology, X.-T.X.; supervision, D.-Y.P.; supervision, X.-L.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “13th Five-Year” National Key Research Program of China (2017YFD0301604), the National Natural Science Foundation of China (32160660, 21562022), the Natural Science Foundation of Jiangxi Province (Grant No. 20181BAB203015), the Key Research Foundation of Educational Department of Jiangxi Province of China (GJJ200404, GJJ160382), and the Research Foundation of Jiangxi Medical Products Administration (2022JS34).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
CCDC numbers 2214417 (1a), 2214418 (2a), 2214429 (3a), and 2214420 (3b) contain the supplementary crystallographic data for the structures reported in this paper. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: (+44) 1223 336-033; e-mail: deposit@ccdc.cam.ac.uk; or http://www.ccdc.cam.ac.uk, accessed on 1 August 2023) or also available from the author Xu-Liang Nie. accessed on 1 January 2025.
Acknowledgments
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang 330063, China.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the obtained compounds are not available from the authors.
References
- Yi, J.P.; He, J. Principle, Diagnosis and Treatment of Common Plant Diseases, 1st ed.; China Agricultural University Press: Beijing, China, 2012. [Google Scholar]
- Wang, H.; Gao, X.; Zhang, X.; Jin, H.; Tao, K.; Hou, T. Design, synthesis and antifungal activity of novel fenfuJram-diarylamine hybrids. Bioorg. Med. Chem. Lett. 2017, 27, 90–93. [Google Scholar] [CrossRef]
- Qi, L.; Li, M.C.; Bai, J.C.; Ren, Y.H.; Ma, H.X. In vitro antifungal activities, molecular docking, and DFT studies of 4-amine-3-hydrazino-5-mercapto-1,2,4-triazole derivatives. Bioorg. Med. Chem. Lett. 2021, 40, 127902. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liang, L.; Su, M.; Yang, T.; Mao, X.; Wang, Y. Variations in phenolic acids and antioxidant activity of navel orange at different growth stages. Food Chem. 2021, 360, 129980. [Google Scholar] [CrossRef] [PubMed]
- Hssaini, L.; Hernandez, F.; Viuda-Martos, M.; Charafi, J.; Razouk, R.; Houmanat, K.; Ouaabou, R.; Ennahli, S.; Elothmani, D.; Hmid, I.; et al. Survey of Phenolic Acids, Flavonoids and In Vitro Antioxidant Potency Between Fig Peels and Pulps: Chemical and Chemometric Approach. Molecules 2021, 26, 2574. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Kang, I.J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental Pretreatment with Chlorogenic Acid Prevents Transient Ischemia-Induced Cognitive Decline and Neuronal Damage in the Hippocampus through Anti-Oxidative and Anti-Inflammatory Effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lai, C.J.S.; Zhang, J.; Feng, Y.; Wen, Q.; Tan, T. Comprehensive metabolic profile of phenolic acids and flavonoids in Glechomae Herba using ultra-high-performance liquid chromatography coupled to quadrupole-time-of-flight tandem mass spectrometry with diagnostic ion filtering strategy. J. Pharm. Biomed. Anal. 2018, 164, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of Phenolic Acid Antimicrobial and Antioxidant Structure–Property Relationships. Pharmaceutical 2020, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Debjani, G.; Parnajyoti, K. Insight into anti-oxidative carbohydrate polymers from medicinal plants: Structure-activity relationships, mechanism of actions and interactions with bovine serum albumin. Int. J. Biol. Macromol. 2020, 166, 1022–1034. [Google Scholar] [CrossRef]
- Gu, J.; Feng, L.; Song, J.; Cui, L.; Liu, D.; Ma, L.; Jia, X. The effect and mechanism of combination of total paeony glycosides and total ligustici phenolic acids against focal cerebral ischemia. Sci. Rep. 2020, 10, 3689. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, J.; Zheng, B.; Sun, J.; Luo, D.; Li, P.; Fan, J. Diversity of phenolics including hydroxycinnamic acid amide derivatives, phenolic acids contribute to antioxidant properties of proso millet. LWT 2022, 154, 112611. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Cegielska-Radziejewska, R.; Goral, T.; Kurasiak-Popowska, D.; Stuper-Szablewska, K. Assessment of Antimicrobial Properties of Phenolic Acid Extracts from Grain Infected with Fungi from the Genus Fusarium. Molecules 2022, 27, 1741. [Google Scholar] [CrossRef]
- Hassan, M.; Vanjare, B.D.; Sim, K.Y.; Raza, H.; Lee, K.H.; Shahzadi, S.; Kloczkowski, A. Biological and Cheminformatics Studies of Newly Designed Triazole Based Derivatives as Potent Inhibitors against Mushroom Tyrosinase. Molecules 2022, 27, 1731. [Google Scholar] [CrossRef] [PubMed]
- Oukoloff, K.; Lucero, B.; Francisco, K.R.; Brunden, K.R.; Ballatore, C. 1,2,4-Triazolo[1,5-a]pyrimidines in drug design. Eur. J. Med. Chem. 2019, 165, 332–346. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Han, L.; Min, L.; Wang, H.; Yu, C. Synthesis, Cyrstal Structure and Fungicidal Activity of New Triazole Compounds Containing Trifluoromethylphenyl Moiety. Chin. J. Org. Chem. 2021, 41, 4498–4503. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Y.; Lu, Z.; Liang, J.; Wang, T.; Luo, J. Synthesis and herbicidal activity of pyrimidyl-1,2,4-triazole derivatives containing aryl sulfonyl moiety. J. Heterocycl. Chem. 2021, 59, 704–719. [Google Scholar]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef]
- Ji, K.; Zhang, G.N.; Zhao, J.Y.; Zhu, M.; Wang, M.H.; Wang, J.X.; Cen, S.; Wang, Y.C.; Li, W.Y. Design, synthesis, and anti-influenza A virus activity evaluation of novel indole containing derivatives of triazole. Bioorg. Med. Chem. Lett. 2022, 64, 128681. [Google Scholar] [CrossRef]
- Harikrishna, K.; Hua, G.; Fuad, N.; Yogindra, V.; Moonsoo, J.M.; Timothy, D.M.; Behfar, E.; Haluk, B.S.; Oguz, A.; Omer, A.; et al. 18F-Positron Emitting/Trimethine Cyanine-Fluorescent Contrast for Image-Guided Prostate Cancer Management. J. Med. Chem. 2018, 61, 4256–4262. [Google Scholar] [CrossRef]
- Guo, H.Y.; Chen, Z.A.; Shen, Q.K.; Quan, Z.S. Application of triazoles in the structural modification of natural products. J. Enzyme Inhib. Med. Chem. 2021, 36, 1115–1144. [Google Scholar] [CrossRef]
- Kannan, R.; Mamangam, S.; Perumal, R. Synthesis, optical properties, and antioxidant and anticancer activity of benzoheterazole dendrimers with triazole bridging unit. New J. Chem. 2018, 42, 3282–3292. [Google Scholar] [CrossRef]
- Kavita, B.; Vir, S.J.; Aakriti, S.; Arshmeet, K.; Nitish, K.; Kaur, G.H.; Atamjit, S.; Harbinder, S.; Singh, B.P.M. Novel series of triazole containing coumarin and isatin based hybrid molecules as acetylcholinesterase inhibitors. J. Mol. Struct. 2021, 1245, 131085. [Google Scholar] [CrossRef]
- Łukasz, L.; Michał, B.; Ewa, M.; Andrzej, Ł.; Radosław, P.; Jadwiga, T.G. Synthesis and characterization of triazole based nanocrystalline cellulose solid proton conductors. Eur. Polym. J. 2021, 161, 110825. [Google Scholar] [CrossRef]
- Mohamed, K.S.; Elbialy, E.; Fadda, A.A. Application of N-(Aryl)-2-oxo-2-(arylamino)acetohydrazonoyl Cyanide in Synthesis of Some Novel Triazole Derivatives and Their Biological Activity. Russ. J. Gen Chem. 2021, 91, 1592–1603. [Google Scholar] [CrossRef]
- Li, M.C.; Han, Y.Y.; Ren, Y.H.; Yang, B.; Qi, L.; Zhang, W.H. the Synthesis, Antibacterial Activity, and Molecular Docking of Pyrine Structures. Pesticides 2022, 61, 798–802+844. [Google Scholar] [CrossRef]
- Slivka, M.; Fizer, M.; Mariychuk, R.; Ostafin, M.; Moyzesh, O.; Koval, G.; Oksana, H.K.; Rusyn, I.; Lendel, V. Synthesis and Antimicrobial Activity of Functional Derivatives of thiazolo[2,3-c][1,2,4]triazoles. Lett. Drug. Des. Discov. 2022, 19, 791–799. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.L.; Wang, J.; Zhong, L.; Nie, X.L.; Peng, D.Y. Synthesis, Crystal Structure, Spectroscopic Characterization and Anti-fungal Activity of Ethyl 2-Oxo-2H-chromene-3-carboxylateDerivatives. J. Mol. Struct. 2022, 1257, 132576. [Google Scholar] [CrossRef]
- Huang, C.X.; Gu, J.; Xiong, W.M.; Chen, J.Z.; Nie, X.L.; Shang-Guan, X.C. Synthesis, Crystal Structures and Antibacterial Activities of Two Complexes of Zn(II)/Cd(II) Assembled by 4-Carboxymethoxycinnamic Acid Ligand. Chin. J. Inorg. Chem. 2021, 37, 1197–1203. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Miao, T.F.; Yang, Y.; Zhuang, D.Y.; Zheng, K.C.; Wong, W.T. Directionality and site selectivity of N⋯Cl halogen bonding in two azaaromatic chloride crystals. J. Mol. Struct. 2010, 975, 274–279. [Google Scholar] [CrossRef]
- Ma, Y.L.; Zhou, R.; Zeng, X.; An, Y.X.; Qiu, S.; Nie, L. Synthesis, DFT and antimicrobial activity assays in vitro for novel cis/trans-but-2-enedioic acid esters. J. Mol. Struct. 2014, 1063, 226–234. [Google Scholar] [CrossRef]
- Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2005. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crvstallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crvstal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Gen, Q.C.; Lina, Z.; Jiaxuan, H.; Song, Z.; Yuan, H.L.; Xiaolong, G.; Di, S.; Yuee, T.; Sheng, M.L.; Xiao, B.H.; et al. Combinatorial Synthesis of Novel 1-Sulfonyloxy/acyloxyeugenol Derivatives as Fungicidal Agents. Comb. Chem. High Throughput Screen. 2022, 25, 1545–1551. [Google Scholar] [CrossRef]
- Yao, C.Y.; Liu, C.; Li, X.J.; Bai, J.K.; Xu, M.; Qiu, R.; Chen, Y.G.; Kang, Y.B.; Li, S.J. The screening of the root rot prevention of tobacco sickle bacteria. Chin. J. Henan. Agric. Sci. 2022, 51, 87–94. [Google Scholar] [CrossRef]
- Lei, Y. Design, Synthesis, and antifungal activity of novel benzimidazole derivatives bearing thioether and carbamate moieties. J. Chem. 2022, 8646557. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).