Antidepressant-like Effects of Representative Types of Food and Their Possible Mechanisms
Abstract
:1. Introduction
2. The Pathogenesis of Depression
3. Role of Neurotransmitter Systems in Depression
3.1. Serotonin (5-hydroxytryptamine, 5-HT)
3.2. Norepinephrine (NE)
3.3. Dopamine (DA)
3.4. Glutamate (Glu)
3.5. Gamma-Aminobutyl Acid (GABA)
4. Other Hypotheses
4.1. Brain-Derived Neurotrophic Factor (BDNF)
4.2. Glial Cells
4.3. Inflammation Hypothesis
4.4. Neuroendocrine Systems
5. Antidepressant-like Effects of Dietary Manipulations
5.1. Fruits and Vegetables
5.2. Fish
5.3. Drinks
5.4. Vitamins
5.5. Homology of Medicine and Food
5.6. Other Foods
5.7. Dietary Treatments for Depression
6. Limitations
7. Overview
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kruizinga, J.; Liemburg, E.; Burger, H.; Cipriani, A.; Geddes, J.; Robertson, L.; Vogelaar, B.; Nolen, W.A. Pharmacological treatment for psychotic depression. Cochrane Database Syst. Rev. 2021, 12, CD004044. [Google Scholar] [CrossRef] [PubMed]
- van Bronswijk, S.; Moopen, N.; Beijers, L.; Ruhe, H.G.; Peeters, F. Effectiveness of psychotherapy for treatment-resistant depression: A meta-analysis and meta-regression. Psychol. Med. 2019, 49, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S.; Streim, J.; Carpenter, D.; Docherty, J.P.; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J. Clin. Psychiatry 2004, 65 (Suppl. S2), 5–99; discussion 100–102; quiz 103–104. [Google Scholar] [PubMed]
- Khawam, E.A.; Laurencic, G.; Malone, D.A., Jr. Side effects of antidepressants: An overview. Clevel. Clin. J. Med. 2006, 73, 351–353, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef]
- Manosso, L.M.; Arent, C.O.; Borba, L.A.; Abelaira, H.M.; Reus, G.Z. Natural Phytochemicals for the Treatment of Major Depressive Disorder: A Mini-Review of Pre- and Clinical Studies. CNS Neurol. Disord. Drug Targets 2022, 22, 237–254. [Google Scholar] [CrossRef]
- Donoso, F.; Cryan, J.F.; Olavarria-Ramirez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2022, 113, 246–259. [Google Scholar] [CrossRef]
- Masand, P.S.; Gupta, S. Selective serotonin-reuptake inhibitors: An update. Harv. Rev. Psychiatry 1999, 7, 69–84. [Google Scholar] [CrossRef]
- Ho, D. Antidepressants and the FDA’s black-box warning: Determining a rational public policy in the absence of sufficient evidence. AMA J. Ethics 2012, 14, 483–488. [Google Scholar] [CrossRef]
- Lambert, O.; Bourin, M. SNRIs: Mechanism of action and clinical features. Expert Rev. Neurother. 2002, 2, 849–858. [Google Scholar] [CrossRef]
- Sanchez, C.; Asin, K.E.; Artigas, F. Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol. Ther. 2015, 145, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Burch, R. Antidepressants for Preventive Treatment of Migraine. Curr. Treat. Options Neurol. 2019, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, K.Y.; Chang, T.; Vanle, B.; Dang, J.; Steiner, A.J.; Loera, N.; Abdelmesseh, M.; Danovitch, I.; Ishak, W.W. Trazodone for Insomnia: A Systematic Review. Innov. Clin. Neurosci. 2017, 14, 24–34. [Google Scholar] [PubMed]
- Davis, R.; Whittington, R.; Bryson, H.M. Nefazodone. A review of its pharmacology and clinical efficacy in the management of major depression. Drugs 1997, 53, 608–636. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, Ł. Monoamine oxidase inhibitors (MAOI): Pharmacology, metabolism and application in the treatment of depression. Postep. Biochem. 2021, 67, 130–140. [Google Scholar] [CrossRef]
- Hyman Rapaport, M. Translating the evidence on atypical depression into clinical practice. J. Clin. Psychiatry 2007, 68 (Suppl. S3), 31–36. [Google Scholar]
- Feighner, J.P. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999, 60 (Suppl. S4), 4–11; discussion 12–13. [Google Scholar]
- Wang, S.M.; Han, C.; Bahk, W.M.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Anttila, S.A.; Leinonen, E.V. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001, 7, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Protti, M.; Mandrioli, R.; Marasca, C.; Cavalli, A.; Serretti, A.; Mercolini, L. New-generation, non-SSRI antidepressants: Drug-drug interactions and therapeutic drug monitoring. Part 2: NaSSAs, NRIs, SNDRIs, MASSAs, NDRIs, and others. Med. Res. Rev. 2020, 40, 1794–1832. [Google Scholar] [CrossRef]
- Wang, P.; Jing, C.; Yu, P.; Lu, M.; Xu, X.; Pei, Q.; Yan, F. Profiling the structural determinants of aminoketone derivatives as hNET and hDAT reuptake inhibitors by field-based QSAR based on molecular docking. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2021, 29, 257–273. [Google Scholar] [CrossRef]
- Hu, L.Y.; Liu, C.J.; Lu, T.; Hu, T.M.; Tsai, C.F.; Hu, Y.W.; Shen, C.C.; Chang, Y.S.; Chen, M.H.; Teng, C.J.; et al. Delayed onset urticaria in depressive patients with bupropion prescription: A nationwide population-based study. PLoS ONE 2013, 8, e80064. [Google Scholar] [CrossRef]
- Bunney, W.E., Jr.; Davis, J.M. Norepinephrine in depressive reactions. A review. Arch. Gen. Psychiatry 1965, 13, 483–494. [Google Scholar] [CrossRef]
- Kohler, S.; Cierpinsky, K.; Kronenberg, G.; Adli, M. The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. J. Psychopharmacol. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Blier, P. Neurobiology of depression and mechanism of action of depression treatments. J. Clin. Psychiatry 2016, 77, e319. [Google Scholar] [CrossRef]
- Gu, S.; Wang, F.; Patel, N.P.; Bourgeois, J.A.; Huang, J.H. A Model for Basic Emotions Using Observations of Behavior in Drosophila. Front. Psychol. 2019, 10, 781. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A., Jr.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef] [PubMed]
- Kucukibrahimoglu, E.; Saygin, M.Z.; Caliskan, M.; Kaplan, O.K.; Unsal, C.; Goren, M.Z. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur. J. Clin. Pharmacol. 2009, 65, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Lepack, A.E.; Bang, E.; Lee, B.; Dwyer, J.M.; Duman, R.S. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 2016, 111, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef]
- Fang, Y.; Ding, X.; Zhang, Y.; Cai, L.; Ge, Y.; Ma, K.; Xu, R.; Li, S.; Song, M.; Zhu, H.; et al. Fluoxetine inhibited the activation of A1 reactive astrocyte in a mouse model of major depressive disorder through astrocytic 5-HT(2B)R/beta-arrestin2 pathway. J. Neuroinflamm. 2022, 19, 23. [Google Scholar] [CrossRef]
- Peng, C.H.; Chiou, S.H.; Chen, S.J.; Chou, Y.C.; Ku, H.H.; Cheng, C.K.; Yen, C.J.; Tsai, T.H.; Chang, Y.L.; Kao, C.L. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur. Neuropsychopharmacol. 2008, 18, 128–140. [Google Scholar] [CrossRef]
- Bjorkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef]
- Arauchi, R.; Hashioka, S.; Tsuchie, K.; Miyaoka, T.; Tsumori, T.; Limoa, E.; Azis, I.A.; Oh-Nishi, A.; Miura, S.; Otsuki, K.; et al. Gunn rats with glial activation in the hippocampus show prolonged immobility time in the forced swimming test and tail suspension test. Brain Behav. 2018, 8, e01028. [Google Scholar] [CrossRef]
- Maes, M.; Vandoolaeghe, E.; Ranjan, R.; Bosmans, E.; Bergmans, R.; Desnyder, R. Increased serum interleukin-1-receptor-antagonist concentrations in major depression. J. Affect. Disord. 1995, 36, 29–36. [Google Scholar] [CrossRef]
- Halbreich, U.; Asnis, G.M.; Shindledecker, R.; Zumoff, B.; Nathan, R.S. Cortisol secretion in endogenous depression. I. Basal plasma levels. Arch. Gen. Psychiatry 1985, 42, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Feinberg, M.; Greden, J.F.; Tarika, J.; Albala, A.A.; Haskett, R.F.; James, N.M.; Kronfol, Z.; Lohr, N.; Steiner, M.; et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry 1981, 38, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jacan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Frohlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, P.F. Tetrahydropterin-dependent amino acid hydroxylases. Annu. Rev. Biochem. 1999, 68, 355–381. [Google Scholar] [CrossRef]
- Kim, D.Y.; Camilleri, M. Serotonin: A mediator of the brain-gut connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Z.; Hou, W.; Zhang, X.; He, Z.; Yuan, W.; Yang, Y.; Zhang, S.; Jia, R.; Tai, F. Serotonin Signaling Trough Prelimbic 5-HT1A Receptors Modulates CSDS-Induced Behavioral Changes in Adult Female Voles. Int. J. Neuropsychopharmacol. 2019, 22, 208–220. [Google Scholar] [CrossRef]
- Goda, R.; Otsuka, T.; Iwamoto, A.; Kawai, M.; Shibata, S.; Furuse, M.; Yasuo, S. Serotonin levels in the dorsal raphe nuclei of both chipmunks and mice are enhanced by long photoperiod, but brain dopamine level response to photoperiod is species-specific. Neurosci. Lett. 2015, 593, 95–100. [Google Scholar] [CrossRef]
- Whitney, M.S.; Shemery, A.M.; Yaw, A.M.; Donovan, L.J.; Glass, J.D.; Deneris, E.S. Adult Brain Serotonin Deficiency Causes Hyperactivity, Circadian Disruption, and Elimination of Siestas. J. Neurosci. 2016, 36, 9828–9842. [Google Scholar] [CrossRef]
- David, D.J.; Gardier, A.M. The pharmacological basis of the serotonin system: Application to antidepressant response. Encephale 2016, 42, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Leonard, B.E. 5-HT1A and beyond: The role of serotonin and its receptors in depression and the antidepressant response. Hum. Psychopharmacol. 2000, 15, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Wieland, S.; Lucki, I. Blockade of the antidepressant-like effects of 8-OH-DPAT, buspirone and desipramine in the rat forced swim test by 5HT1A receptor antagonists. Psychopharmacology 1995, 119, 47–54. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.L.; Newman-Tancredi, A.; Leonardo, E.D. P5-HT1A receptors in mood and anxiety: Recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology 2014, 231, 623–636. [Google Scholar] [CrossRef]
- Cheetham, S.C.; Crompton, M.R.; Katona, C.L.; Horton, R.W. Brain 5-HT1 binding sites in depressed suicides. Psychopharmacology 1990, 102, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Leem, Y.H. Chronic exercise improves repeated restraint stress-induced anxiety and depression through 5HT1A receptor and cAMP signaling in hippocampus. J. Exerc. Nutr. Biochem. 2014, 18, 97–104. [Google Scholar] [CrossRef]
- Kennett, G.A.; Dourish, C.T.; Curzon, G. Antidepressant-like action of 5-HT1A agonists and conventional antidepressants in an animal model of depression. Eur. J. Pharmacol. 1987, 134, 265–274. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Du, X.; Ding, T.; Gong, W.; Liu, F. Review of antidepressants in clinic and active ingredients of traditional Chinese medicine targeting 5-HT1A receptors. Biomed. Pharmacother. 2019, 120, 109408. [Google Scholar] [CrossRef]
- Jones, M.D.; Lucki, I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology 2005, 30, 1039–1047. [Google Scholar] [CrossRef]
- Tatarczynska, E.; Klodzinska, A.; Stachowicz, K.; Chojnacka-Wojcik, E. Effects of a selective 5-HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav. Pharmacol. 2004, 15, 523–534. [Google Scholar] [CrossRef]
- Hasegawa, S.; Nishi, K.; Watanabe, A.; Overstreet, D.H.; Diksic, M. Brain 5-HT synthesis in the Flinders Sensitive Line rat model of depression: An autoradiographic study. Neurochem. Int. 2006, 48, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, T.; Zhao, Y.; Cai, E.; Zhu, H.; Liu, S. Panaxynol attenuates CUMS-induced anxiety and depressive-like behaviors via regulating neurotransmitters, synapses and the HPA axis in mice. Food Funct. 2020, 11, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Aznar, S.; Hervig, M.E.-S. The 5-HT2A serotonin receptor in executive function: Implications for neuropsychiatric and neurodegenerative diseases. Neurosci. Biobehav. Rev. 2016, 64, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, N.; Akamatsu, Y.; Nishida, M.M.; Hayashi, T.; Moritani, T. Effect of Tryptophan, Vitamin B6, and Nicotinamide-Containing Supplement Loading between Meals on Mood and Autonomic Nervous System Activity in Young Adults with Subclinical Depression: A Randomized, Double-Blind, and Placebo-Controlled Study. J. Nutr. Sci. Vitaminol. 2019, 65, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Tanke, M.A.; Alserda, E.; Doornbos, B.; van der Most, P.J.; Goeman, K.; Postema, F.; Korf, J. Low tryptophan diet increases stress-sensitivity, but does not affect habituation in rats. Neurochem. Int. 2008, 52, 272–281. [Google Scholar] [CrossRef]
- Mayerhofer, A.; Smith, G.D.; Danilchik, M.; Levine, J.E.; Wolf, D.P.; Dissen, G.A.; Ojeda, S.R. Oocytes are a source of catecholamines in the primate ovary: Evidence for a cell-cell regulatory loop. Proc. Natl. Acad. Sci. USA 1998, 95, 10990–10995. [Google Scholar] [CrossRef]
- Nagatsu, T.; Levitt, M.; Udenfriend, S. Tyrosine Hydroxylase: The Initial Step in Norepinephrine Biosynthesis. J. Biol. Chem. 1964, 239, 2910–2917. [Google Scholar] [CrossRef]
- Martin-Hernandez, D.; Pereira, M.P.; Tendilla-Beltran, H.; Madrigal, J.L.M.; Garcia-Bueno, B.; Leza, J.C.; Caso, J.R. Modulation of Monoaminergic Systems by Antidepressants in the Frontal Cortex of Rats after Chronic Mild Stress Exposure. Mol. Neurobiol. 2019, 56, 7522–7533. [Google Scholar] [CrossRef]
- Kremer, M.; Salvat, E.; Muller, A.; Yalcin, I.; Barrot, M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience 2016, 338, 183–206. [Google Scholar] [CrossRef]
- Brunello, N.; Mendlewicz, J.; Kasper, S.; Leonard, B.; Montgomery, S.; Nelson, J.C.; Paykel, E.; Versiani, M.; Racagni, G. The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur. Neuropsychopharmacol. 2002, 12, 461–475. [Google Scholar] [CrossRef]
- Li, X.R.; Sun, N.; Xu, Y.; Wang, Y.F.; Li, S.P.; Du, Q.R.; Peng, J.Y.; Luo, J.X.; Zhang, K.R. The norepinephrine transporter gene is associated with the retardation symptoms of major depressive disorder in the Han Chinese population. Neural Regen. Res. 2012, 7, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, X.Y.; Xia, H.J.; Wang, L.J.; Shi, P.; Chen, X.P.; Zhou, Q.X. Antidepressant effect of venlafaxine in chronic unpredictable stress: Evidence of the involvement of key enzymes responsible for monoamine neurotransmitter synthesis and metabolism. Mol. Med. Rep. 2019, 20, 2954–2962. [Google Scholar] [CrossRef]
- Alfinito, P.D.; Huselton, C.; Chen, X.; Deecher, D.C. Pharmacokinetic and pharmacodynamic profiles of the novel serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized Sprague-Dawley rats. Brain Res. 2006, 1098, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liebe, T.; Li, M.; Colic, L.; Munk, M.H.J.; Sweeney-Reed, C.M.; Woelfer, M.; Kretzschmar, M.A.; Steiner, J.; von During, F.; Behnisch, G.; et al. Ketamine influences the locus coeruleus norepinephrine network, with a dependency on norepinephrine transporter genotype—A placebo controlled fMRI study. NeuroImage Clin. 2018, 20, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Bui, B.V.; Fortune, B. Ganglion cell contributions to the rat full-field electroretinogram. J. Physiol. 2004, 555, 153–173. [Google Scholar] [CrossRef]
- Iro, C.M.; Hamati, R.; El Mansari, M.; Blier, P. Repeated but Not Single Administration of Ketamine Prolongs Increases of the Firing Activity of Norepinephrine and Dopamine Neurons. Int. J. Neuropsychopharmacol. 2021, 24, 570–579. [Google Scholar] [CrossRef]
- Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines 2021, 9, 109. [Google Scholar] [CrossRef]
- Hoefig, C.S.; Renko, K.; Piehl, S.; Scanlan, T.S.; Bertoldi, M.; Opladen, T.; Hoffmann, G.F.; Klein, J.; Blankenstein, O.; Schweizer, U.; et al. Does the aromatic L-amino acid decarboxylase contribute to thyronamine biosynthesis? Mol. Cell Endocrinol. 2012, 349, 195–201. [Google Scholar] [CrossRef]
- Pendleton, R.G.; Rasheed, A.; Paluru, P.; Joyner, J.; Jerome, N.; Meyers, R.D.; Hillman, R. A developmental role for catecholamines in Drosophila behavior. Pharmacol. Biochem. Behav. 2005, 81, 849–853. [Google Scholar] [CrossRef]
- Ishikawa, T.; Okano, M.; Minami, A.; Tsunekawa, H.; Satoyoshi, H.; Tsukamoto, Y.; Takahata, K.; Muraoka, S. Selegiline ameliorates depression-like behaviors in rodents and modulates hippocampal dopaminergic transmission and synaptic plasticity. Behav. Brain Res. 2019, 359, 353–361. [Google Scholar] [CrossRef]
- Camardese, G.; Di Giuda, D.; Di Nicola, M.; Cocciolillo, F.; Giordano, A.; Janiri, L.; Guglielmo, R. Imaging studies on dopamine transporter and depression: A review of literature and suggestions for future research. J. Psychiatr. Res. 2014, 51, 7–18. [Google Scholar] [CrossRef]
- Nikolaus, S.; Mamlins, E.; Hautzel, H.; Muller, H.W. Acute anxiety disorder, major depressive disorder, bipolar disorder and schizophrenia are related to different patterns of nigrostriatal and mesolimbic dopamine dysfunction. Rev. Neurosci. 2019, 30, 381–426. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Perreault, M.L.; Bambico, F.R.; Jones-Tabah, J.; Cheung, M.; Fan, T.; Nobrega, J.N.; George, S.R. Rapid anti-depressant and anxiolytic actions following dopamine D1–D2 receptor heteromer inactivation. Eur. Neuropsychopharmacol. 2015, 25, 2437–2448. [Google Scholar] [CrossRef]
- Hasbi, A.; Nguyen, T.; Rahal, H.; Manduca, J.D.; Miksys, S.; Tyndale, R.F.; Madras, B.K.; Perreault, M.L.; George, S.R. Sex difference in dopamine D1–D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol. Sex Differ. 2020, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Desormeaux, C.; Demars, F.; Davenas, E.; Jay, T.M.; Lavergne, F. Selective activation of D1 dopamine receptors exerts antidepressant-like activity in rats. J. Psychopharmacol. 2020, 34, 1443–1448. [Google Scholar] [CrossRef]
- Rocchetti, J.; Isingrini, E.; Dal Bo, G.; Sagheby, S.; Menegaux, A.; Tronche, F.; Levesque, D.; Moquin, L.; Gratton, A.; Wong, T.P.; et al. Presynaptic D2 dopamine receptors control long-term depression expression and memory processes in the temporal hippocampus. Biol. Psychiatry 2015, 77, 513–525. [Google Scholar] [CrossRef]
- Moraga-Amaro, R.; Gonzalez, H.; Pacheco, R.; Stehberg, J. Dopamine receptor D3 deficiency results in chronic depression and anxiety. Behav. Brain Res. 2014, 274, 186–193. [Google Scholar] [CrossRef]
- Perona, M.T.; Waters, S.; Hall, F.S.; Sora, I.; Lesch, K.P.; Murphy, D.L.; Caron, M.; Uhl, G.R. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: Prominent effects of dopamine transporter deletions. Behav. Pharmacol. 2008, 19, 566–574. [Google Scholar] [CrossRef]
- Romeo, B.; Blecha, L.; Locatelli, K.; Benyamina, A.; Martelli, C. Meta-analysis and review of dopamine agonists in acute episodes of mood disorder: Efficacy and safety. J. Psychopharmacol. 2018, 32, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Conway, C.R.; Chibnall, J.T.; Cumming, P.; Mintun, M.A.; Gebara, M.A.; Perantie, D.C.; Price, J.L.; Cornell, M.E.; McConathy, J.E.; Gangwani, S.; et al. Antidepressant response to aripiprazole augmentation associated with enhanced FDOPA utilization in striatum: A preliminary PET study. Psychiatry Res. 2014, 221, 231–239. [Google Scholar] [CrossRef]
- Moriguchi, S.; Takamiya, A.; Noda, Y.; Horita, N.; Wada, M.; Tsugawa, S.; Plitman, E.; Sano, Y.; Tarumi, R.; ElSalhy, M.; et al. Glutamatergic neurometabolite levels in major depressive disorder: A systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol. Psychiatry 2019, 24, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Iijima, M.; Funakoshi, T.; Chaki, S. 5-HT1A receptor stimulation in the medial prefrontal cortex mediates the antidepressant effects of mGlu2/3 receptor antagonist in mice. Neuropharmacology 2018, 137, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Palucha-Poniewiera, A.; Branski, P.; Wieronska, J.M.; Stachowicz, K.; Slawinska, A.; Pilc, A. The antidepressant-like action of mGlu5 receptor antagonist, MTEP, in the tail suspension test in mice is serotonin dependent. Psychopharmacology 2014, 231, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Podkowa, K.; Pilc, A.; Podkowa, A.; Sałat, K.; Marciniak, M.; Pałucha-Poniewiera, A. The potential antidepressant action and adverse effects profile of scopolamine co-administered with the mGlu7 receptor allosteric agonist AMN082 in mice. Neuropharmacology 2018, 141, 214–222. [Google Scholar] [CrossRef]
- Chen, Y.P.; Wang, C.; Xu, J.P. Chronic unpredictable mild stress induced depression-like behaviours and glutamate-glutamine cycling dysfunctions in both blood and brain of mice. Pharm. Biol. 2019, 57, 280–286. [Google Scholar] [CrossRef]
- de Lima, D.S.; Francisco, E.D.; Lima, C.B.; Guedes, R.C. Neonatal L-glutamine modulates anxiety-like behavior, cortical spreading depression, and microglial immunoreactivity: Analysis in developing rats suckled on normal size- and large size litters. Amino Acids 2017, 49, 337–346. [Google Scholar] [CrossRef]
- Greger, I.H.; Watson, J.F.; Cull-Candy, S.G. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 2017, 94, 713–730. [Google Scholar] [CrossRef]
- Gerhard, D.M.; Pothula, S.; Liu, R.J.; Wu, M.; Li, X.Y.; Girgenti, M.J.; Taylor, S.R.; Duman, C.H.; Delpire, E.; Picciotto, M.; et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J. Clin. Investig. 2020, 130, 1336–1349. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Dong, Z.; Milak, M.S.; Rashid, R.; Kegeles, L.S.; Javitt, D.C.; Lieberman, J.A.; John Mann, J. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl. Psychiatry 2021, 11, 419. [Google Scholar] [CrossRef]
- Milak, M.S.; Proper, C.J.; Mulhern, S.T.; Parter, A.L.; Kegeles, L.S.; Ogden, R.T.; Mao, X.; Rodriguez, C.I.; Oquendo, M.A.; Suckow, R.F.; et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol. Psychiatry 2016, 21, 320–327. [Google Scholar] [CrossRef]
- Petroff, O.A. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, H.; Wang, S.; Wang, H.; Wang, Y.; Liu, J.; Song, X.; Dong, Z.; Han, X.; Zhang, Y.; et al. The molecular mechanism underlying GABAergic dysfunction in nucleus accumbens of depression-like behaviours in mice. J. Cell Mol. Med. 2019, 23, 7021–7028. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Gueorguieva, R.; Epperson, C.N.; Wu, Y.T.; Appel, M.; Rothman, D.L.; Krystal, J.H.; Mason, G.F. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry 2004, 61, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhong, H.Q.; Rong, J.; Li, Y.C.; Zhu, C.T.; Zhou, L.; Zhou, R. Postnatal Lipopolysaccharide Exposure Impairs Adult Neurogenesis and Causes Depression-like Behaviors through Astrocytes Activation Triggering GABAA Receptor Downregulation. Neuroscience 2019, 422, 21–31. [Google Scholar] [CrossRef]
- Dafre, A.L.; Rosa, J.M.; Rodrigues, A.L.S.; Cunha, M.P. Multiple cellular targets involved in the antidepressant-like effect of glutathione. Chem. Biol. Interact. 2020, 328, 109195. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Odisho, A.S.; Lewis, K.; Kaskas, A.; Hunt, G.; Cornett, E.M.; Kaye, A.D.; Kaye, A.; Morgan, J.; Barrilleaux, P.S.; et al. Brexanolone, a GABA(A) Modulator, in the Treatment of Postpartum Depression in Adults: A Comprehensive Review. Front. Psychiatry 2021, 12, 699740. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Shi, Y.C.; You, H.P.; Lo, Y.H.; Pan, T.M. Antidepressant effect of GABA-rich monascus-fermented product on forced swimming rat model. J. Agric. Food Chem. 2011, 59, 3027–3034. [Google Scholar] [CrossRef]
- Simpson, S.M.; Hickey, A.J.; Baker, G.B.; Reynolds, J.N.; Beninger, R.J. The antidepressant phenelzine enhances memory in the double Y-maze and increases GABA levels in the hippocampus and frontal cortex of rats. Pharmacol. Biochem. Behav. 2012, 102, 109–117. [Google Scholar] [CrossRef]
- Esvald, E.E.; Tuvikene, J.; Sirp, A.; Patil, S.; Bramham, C.R.; Timmusk, T. CREB Family Transcription Factors Are Major Mediators of BDNF Transcriptional Autoregulation in Cortical Neurons. J. Neurosci. 2020, 40, 1405–1426. [Google Scholar] [CrossRef]
- Taliaz, D.; Stall, N.; Dar, D.E.; Zangen, A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry 2010, 15, 80–92. [Google Scholar] [CrossRef]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar]
- Fan, J.F.; Tang, Z.H.; Wang, S.Y.; Lei, S.; Zhang, B.; Tian, S.W. Ketamine enhances novel object recognition memory reconsolidation via the BDNF/TrkB pathway in mice. Physiol. Behav. 2021, 242, 113626. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Molteni, R.; Cattaneo, A.; Macchi, F.; Racagni, G.; Gennarelli, M.; Ellenbroek, B.A.; Riva, M.A. Long-Term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Mol. Pharmacol. 2010, 77, 846–853. [Google Scholar] [CrossRef]
- Wook Koo, J.; Labonte, B.; Engmann, O.; Calipari, E.S.; Juarez, B.; Lorsch, Z.; Walsh, J.J.; Friedman, A.K.; Yorgason, J.T.; Han, M.H.; et al. Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress-Induced Depressive Behaviors. Biol. Psychiatry 2016, 80, 469–478. [Google Scholar] [CrossRef]
- Taliaz, D.; Nagaraj, V.; Haramati, S.; Chen, A.; Zangen, A. Altered brain-derived neurotrophic factor expression in the ventral tegmental area, but not in the hippocampus, is essential for antidepressant-like effects of electroconvulsive therapy. Biol. Psychiatry 2013, 74, 305–312. [Google Scholar] [CrossRef]
- Lu, C.L.; Ren, J.; Mo, J.W.; Fan, J.; Guo, F.; Chen, L.Y.; Wen, Y.L.; Li, S.J.; Fang, Y.Y.; Wu, Z.F.; et al. Glucocorticoid Receptor-Dependent Astrocytes Mediate Stress Vulnerability. Biol. Psychiatry 2022, 92, 204–215. [Google Scholar] [CrossRef]
- Bender, C.L.; Sun, X.; Farooq, M.; Yang, Q.; Davison, C.; Maroteaux, M.; Huang, Y.S.; Ishikawa, Y.; Liu, S.J. Emotional Stress Induces Structural Plasticity in Bergmann Glial Cells via an AC5-CPEB3-GluA1 Pathway. J. Neurosci. 2020, 40, 3374–3384. [Google Scholar] [CrossRef]
- Liu, Q.O.; Li, B.; Zhu, H.Y.; Wang, Y.Q.; Yu, J.; Wu, G.C. Glia atrophy in the hippocampus of chronic unpredictable stress-induced depression model rats is reversed by electroacupuncture treatment. J. Affect. Disorders. 2011, 128, 309–313. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, Y.; Lin, S.; Wu, D.Y.; Hu, J.; Huang, L.; Zang, W.S.; Li, X.W.; Yang, J.M.; Gao, T.M. The ATP Level in the mPFC Mediates the Antidepressant Effect of Calorie Restriction. Neurosci. Bull. 2021, 37, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, Y.; Tang, J.; Liang, X.; Wang, J.; Xiao, Q.; Zhu, P.; Xiao, K.; Jiang, L.; Dou, X.; et al. The positive effects of running exercise on hippocampal astrocytes in a rat model of depression. Transl. Psychiatry 2021, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiao, Q.; Wang, J.; Jiang, L.; Hu, M.; Jiang, Y.; Tang, J.; Liang, X.; Qi, Y.; Dou, X.; et al. Running exercise protects oligodendrocytes in the medial prefrontal cortex in chronic unpredictable stress rat model. Transl. Psychiatry 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liang, X.; Zhang, Y.; Chen, L.; Wang, F.; Tan, C.; Luo, Y.; Xiao, Q.; Chao, F.; Zhang, L.; et al. The effects of running exercise on oligodendrocytes in the hippocampus of rats with depression induced by chronic unpredictable stress. Brain Res. Bull. 2019, 149, 1–10. [Google Scholar] [CrossRef]
- Dionisie, V.; Ciobanu, A.M.; Toma, V.A.; Manea, M.C.; Baldea, I.; Olteanu, D.; Sevastre-Berghian, A.; Clichici, S.; Manea, M.; Riga, S.; et al. Escitalopram Targets Oxidative Stress, Caspase-3, BDNF and MeCP2 in the Hippocampus and Frontal Cortex of a Rat Model of Depression Induced by Chronic Unpredictable Mild Stress. Int. J. Mol. Sci. 2021, 22, 7483. [Google Scholar] [CrossRef]
- Lago, N.; Kaufmann, F.N.; Negro-Demontel, M.L.; Ali-Ruiz, D.; Ghisleni, G.; Rego, N.; Arcas-Garcia, A.; Vitureira, N.; Jansen, K.; Souza, L.M.; et al. CD300f immunoreceptor is associated with major depressive disorder and decreased microglial metabolic fitness. Proc. Natl. Acad. Sci. USA 2020, 117, 6651–6662. [Google Scholar] [CrossRef]
- Kwon, S.H.; Han, J.K.; Choi, M.; Kwon, Y.J.; Kim, S.J.; Yi, E.H.; Shin, J.C.; Cho, I.H.; Kim, B.H.; Jeong Kim, S.; et al. Dysfunction of Microglial STAT3 Alleviates Depressive Behavior via Neuron-Microglia Interactions. Neuropsychopharmacology 2017, 42, 2072–2086. [Google Scholar] [CrossRef]
- Zhu, H.X.; Cheng, L.J.; Ou Yang, R.W.; Li, Y.Y.; Liu, J.; Dai, D.; Wang, W.; Yang, N.; Li, Y. Reduced Amygdala Microglial Expression of Brain-Derived Neurotrophic Factor and Tyrosine Kinase Receptor B (TrkB) in a Rat Model of Poststroke Depression. Med. Sci. Monit. 2020, 26, e926323. [Google Scholar] [CrossRef]
- Yue, N.; Huang, H.; Zhu, X.; Han, Q.; Wang, Y.; Li, B.; Liu, Q.; Wu, G.; Zhang, Y.; Yu, J. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J. Neuroinflamm. 2017, 14, 102. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef]
- Li, W.; Ali, T.; He, K.; Liu, Z.; Shah, F.A.; Ren, Q.; Liu, Y.; Jiang, A.; Li, S. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav. Immun. 2021, 92, 10–24. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Zhang, T.; Liu, S.; Cai, E.; Zhu, H. Study on the antidepressant effect of panaxynol through the IkappaB-alpha/NF-kappaB signaling pathway to inhibit the excessive activation of BV-2 microglia. Biomed. Pharmacother. 2021, 138, 111387. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xin, Y.; Zhang, J.; Wang, S.; Yang, Z.; Liu, C. Hydrogen sulfide alleviates the anxiety-like and depressive-like behaviors of type 1 diabetic mice via inhibiting inflammation and ferroptosis. Life Sci. 2021, 278, 119551. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, K.; Tchekalarova, J.; Atanasova, D.; Kortenska, L.; Atanasova, M. Antidepressant agomelatine attenuates behavioral deficits and concomitant pathology observed in streptozotocin-induced model of Alzheimer’s disease in male rats. Horm. Behav. 2019, 107, 11–19. [Google Scholar] [CrossRef]
- Rebai, R.; Jasmin, L.; Boudah, A. Agomelatine effects on fat-enriched diet induced neuroinflammation and depression-like behavior in rats. Biomed. Pharmacother. 2021, 135, 111246. [Google Scholar] [CrossRef]
- Demuyser, T.; Bentea, E.; Deneyer, L.; Albertini, G.; Massie, A.; Smolders, I. Disruption of the HPA-axis through corticosterone-release pellets induces robust depressive-like behavior and reduced BDNF levels in mice. Neurosci. Lett. 2016, 626, 119–125. [Google Scholar] [CrossRef]
- Camargo, A.; Dalmagro, A.P.; Rikel, L.; da Silva, E.B.; Simao da Silva, K.A.B.; Zeni, A.L.B. Cholecalciferol counteracts depressive-like behavior and oxidative stress induced by repeated corticosterone treatment in mice. Eur. J. Pharmacol. 2018, 833, 451–461. [Google Scholar] [CrossRef]
- Mukherjee, K.; Knisely, A.; Jacobson, L. Partial glucocorticoid agonist-like effects of imipramine on hypothalamic-pituitary-adrenocortical activity, thymus weight, and hippocampal glucocorticoid receptors in male C57BL/6 mice. Endocrinology 2004, 145, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zou, Y.; He, M.; Yang, P.; Qu, X.; Xu, L. Hydroxysafflor yellow A can improve depressive behavior by inhibiting hippocampal inflammation and oxidative stress through regulating HPA axis. J. Biosci. 2022, 47, 7. [Google Scholar] [CrossRef]
- Schule, C. Neuroendocrinological mechanisms of actions of antidepressant drugs. J. Neuroendocrinol. 2007, 19, 213–226. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264.e119. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, X.; Xu, S.; Hu, S.; Wang, S.; Shi, D.; Wang, K.; Wang, Z.; Lin, Q.; Li, S.; et al. Prevotella histicola Mitigated Estrogen Deficiency-Induced Depression via Gut Microbiota-Dependent Modulation of Inflammation in Ovariectomized Mice. Front. Nutr. 2021, 8, 805465. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Kaluzna-Czaplinska, J.; Gatarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjorklund, G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef]
- Jia, W.; Zhen, J.; Liu, A.; Yuan, J.; Wu, X.; Zhao, P.; Zhao, L.; Li, X.; Liu, Q.; Huang, G.; et al. Long-Term Vegan Meditation Improved Human Gut Microbiota. Evid. Based Complement. Altern. Med. 2020, 2020, 9517897. [Google Scholar]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jiang, M.; Gu, S.; Zhang, X.; Feng, G.; Ma, X.; Xu, S.; Wu, E.; Huang, J.H.; Wang, F. Metabolomics changes in brain-gut axis after unpredictable chronic mild stress. Psychopharmacology 2022, 239, 729–743. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, M.; Gazzin, S.; Zoratti, C.; Llido, J.P.; Lanza, G.; Tiribelli, C.; Moretti, R. Celiac Disease and Neurological Manifestations: From Gluten to Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 15564. [Google Scholar] [CrossRef]
- Veauthier, B.; Hornecker, J.R. Crohn’s Disease: Diagnosis and Management. Am. Fam. Physician 2018, 98, 661–669. [Google Scholar]
- Sharma, N.; Singh, K.; Senapati, S. Celiac disease poses significant risk in developing depression, anxiety, headache, epilepsy, panic disorder, dysthymia: A meta-analysis. Indian J. Gastroenterol. 2021, 40, 453–462. [Google Scholar] [CrossRef]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 359–370. [Google Scholar] [CrossRef]
- Hargreaves, D.; Mates, E.; Menon, P.; Alderman, H.; Devakumar, D.; Fawzi, W.; Greenfield, G.; Hammoudeh, W.; He, S.; Lahiri, A.; et al. Strategies and interventions for healthy adolescent growth, nutrition, and development. Lancet 2022, 399, 198–210. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef]
- Peng, X.P.; Nie, C.; Guan, W.Y.; Qiao, L.D.; Lu, L.; Cao, S.J. Regulation of Probiotics on Metabolism of Dietary Protein in Intestine. Curr. Protein Pept. Sci. 2020, 21, 766–771. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, N.; Lu, L.; Ma, X. Fermentation and Metabolism of Dietary Protein by Intestinal Microorganisms. Curr. Protein Pept. Sci. 2020, 21, 807–811. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl. Environ. Microbiol. 2012, 78, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Saqib, H.S.A.; Chen, J.H.; Ruan, Q.Q.; Vasseur, L.; He, W.Y.; You, M.S. Differential Profiles of Gut Microbiota and Metabolites Associated with Host Shift of Plutella xylostella. Int. J. Mol. Sci. 2020, 21, 6283. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.A.; Mehak, F.; Khan, Z.M.; Ahmed, W.; Haq, S.; Khan, M.R.; Bhat, Z.F.; Aadil, R.M. Delving the role of nutritional psychiatry to mitigate the COVID-19 pandemic induced stress, anxiety and depression. Trends Food Sci. Technol. 2022, 120, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Haridas, B.; Kossoff, E.H. Dietary Treatments for Epilepsy. Neurol. Clin. 2022, 40, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Kramer, H. Diet and Chronic Kidney Disease. Adv. Nutr. 2019, 10, S367–S379. [Google Scholar] [CrossRef]
- Paglia, L. WHO: Healthy diet to prevent chronic diseases and caries. Eur. J. Paediatr. Dent. 2018, 19, 5. [Google Scholar] [CrossRef]
- Gazerani, P. Migraine and Diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef]
- Opie, R.S.; Itsiopoulos, C.; Parletta, N.; Sanchez-Villegas, A.; Akbaraly, T.N.; Ruusunen, A.; Jacka, F.N. Dietary recommendations for the prevention of depression. Nutr. Neurosci. 2017, 20, 161–171. [Google Scholar] [CrossRef]
- Chatterton, M.L.; Mihalopoulos, C.; O’Neil, A.; Itsiopoulos, C.; Opie, R.; Castle, D.; Dash, S.; Brazionis, L.; Berk, M.; Jacka, F. Economic evaluation of a dietary intervention for adults with major depression (the “SMILES” trial). BMC Public Health 2018, 18, 599. [Google Scholar] [CrossRef]
- Stevenson, R.J. Psychological correlates of habitual diet in healthy adults. Psychol. Bull. 2017, 143, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Olivan-Blazquez, B.; Montero-Marin, J.; Garcia-Toro, M.; Vicens-Pons, E.; Serrano-Ripoll, M.J.; Castro-Gracia, A.; Sarasa-Bosque, M.C.; Mendive-Arbeloa, J.M.; Lopez-Del-Hoyo, Y.; Garcia-Campayo, J. Facilitators and barriers to modifying dietary and hygiene behaviours as adjuvant treatment in patients with depression in primary care: A qualitative study. BMC Psychiatry 2018, 18, 205. [Google Scholar] [CrossRef]
- Konttinen, H.; Silventoinen, K.; Sarlio-Lahteenkorva, S.; Mannisto, S.; Haukkala, A. Emotional eating and physical activity self-efficacy as pathways in the association between depressive symptoms and adiposity indicators. Am. J. Clin. Nutr. 2010, 92, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Hu, B.; Das, P.; Lv, X.; Shi, M.; Aa, J.; Wang, K.; Duan, L.; Gilbert, J.A.; Nie, Y.; Wu, X.L. Effects of ‘Healthy’ Fecal Microbiota Transplantation against the Deterioration of Depression in Fawn-Hooded Rats. mSystems 2022, 7, e0021822. [Google Scholar] [CrossRef] [PubMed]
- Kronsten, V.T.; Tranah, T.H.; Pariante, C.; Shawcross, D.L. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J. Hepatol. 2022, 76, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Samad, N.; Muneer, A.; Ullah, N.; Zaman, A.; Ayaz, M.M.; Ahmad, I. Banana fruit pulp and peel involved in antianxiety and antidepressant effects while invigorate memory performance in male mice: Possible role of potential antioxidants. Pak. J. Pharm. Sci. 2017, 30, 989–995. [Google Scholar] [PubMed]
- Valdes-Sustaita, B.; Lopez-Rubalcava, C.; Gonzalez-Trujano, M.E.; Garcia-Viguera, C.; Estrada-Camarena, E. Aqueous Extract of Pomegranate Alone or in Combination with Citalopram Produces Antidepressant-Like Effects in an Animal Model of Menopause: Participation of Estrogen Receptors. Int. J. Mol. Sci. 2017, 18, 2643. [Google Scholar] [CrossRef]
- Zhang, J.; Ning, L.; Wang, J. Dietary quercetin attenuates depressive-like behaviors by inhibiting astrocyte reactivation in response to stress. Biochem. Biophys. Res. Commun. 2020, 533, 1338–1346. [Google Scholar] [CrossRef]
- Hu, P.; Ma, L.; Wang, Y.G.; Ye, F.; Wang, C.; Zhou, W.H.; Zhao, X. Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of serotonergic system. Neurochem. Int. 2017, 108, 426–435. [Google Scholar] [CrossRef]
- Dang, R.; Zhou, X.; Tang, M.; Xu, P.; Gong, X.; Liu, Y.; Jiao, H.; Jiang, P. Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur. J. Nutr. 2018, 57, 893–906. [Google Scholar] [CrossRef]
- Zemdegs, J.; Rainer, Q.; Grossmann, C.P.; Rousseau-Ralliard, D.; Grynberg, A.; Ribeiro, E.; Guiard, B.P. Anxiolytic- and Antidepressant-Like Effects of Fish Oil-Enriched Diet in Brain-Derived Neurotrophic Factor Deficient Mice. Front. Neurosci. 2018, 12, 974. [Google Scholar] [CrossRef]
- Saleem, A.M.; Taufik Hidayat, M.; Jais, A.M.; Fakurazi, S.; Moklas, M.A.; Sulaiman, M.R.; Amom, Z.; Basir, R. Involvement of monoaminergic system in the antidepressant-like effect of aqueous extract of Channa striatus in mice. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2019–2022. [Google Scholar]
- Pechlivanova, D.M.; Tchekalarova, J.D.; Alova, L.H.; Petkov, V.V.; Nikolov, R.P.; Yakimova, K.S. Effect of long-term caffeine administration on depressive-like behavior in rats exposed to chronic unpredictable stress. Behav. Pharmacol. 2012, 23, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, S.; Wegener, G. Probiotics reduce risk-taking behavior in the Elevated Plus Maze in the Flinders Sensitive Line rat model of depression. Behav. Brain Res. 2019, 359, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, C.; Bock, A.; Urbach, A.; Hubner, C.A.; Engmann, O. Acute vitamin B12 supplementation evokes antidepressant response and alters Ntrk-2. Neuropharmacology 2020, 171, 108112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cong, Y.; Liu, H. Folic acid ameliorates depression-like behaviour in a rat model of chronic unpredictable mild stress. BMC Neurosci. 2020, 21, 1. [Google Scholar] [CrossRef]
- Moretti, M.; Budni, J.; Ribeiro, C.M.; Rodrigues, A.L. Involvement of different types of potassium channels in the antidepressant-like effect of ascorbic acid in the mouse tail suspension test. Eur. J. Pharmacol. 2012, 687, 21–27. [Google Scholar] [CrossRef]
- Xu, Y.X.; Liang, L.Y. Vitamin D3/vitamin D receptor signaling mitigates symptoms of post-stroke depression in mice by upregulating hippocampal BDNF expression. Neurosci. Res. 2021, 170, 306–313. [Google Scholar] [CrossRef]
- Koshkina, A.; Dudnichenko, T.; Baranenko, D.; Fedotova, J.; Drago, F. Effects of Vitamin D3 in Long-Term Ovariectomized Rats Subjected to Chronic Unpredictable Mild Stress: BDNF, NT-3, and NT-4 Implications. Nutrients 2019, 11, 1726. [Google Scholar] [CrossRef]
- Wang, G.L.; He, Z.M.; Zhu, H.Y.; Gao, Y.G.; Zhao, Y.; Yang, H.; Zhang, L.X. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng C.A. Meyer. J. Ethnopharmacol. 2017, 204, 118–124. [Google Scholar] [CrossRef]
- Choi, G.Y.; Kim, H.B.; Hwang, E.S.; Lee, S.; Kim, M.J.; Choi, J.Y.; Lee, S.O.; Kim, S.S.; Park, J.H. Curcumin Alters Neural Plasticity and Viability of Intact Hippocampal Circuits and Attenuates Behavioral Despair and COX-2 Expression in Chronically Stressed Rats. Mediat. Inflamm. 2017, 2017, 6280925. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xian, Y.F.; Ip, S.P.; Che, C.T. Involvement of serotonergic system in the antidepressant-like effect of piperine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1144–1147. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Huang, Z.; Zhong, X.M.; Xian, Y.F.; Ip, S.P. Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav. Brain Res. 2014, 261, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Q.; Jiang, S.; Xu, Z.; Jiang, Y.; Liu, L.; Jiang, J.; Tong, Y.; Wang, P. Crocetin ameliorates chronic restraint stress-induced depression-like behaviors in mice by regulating MEK/ERK pathways and gut microbiota. J. Ethnopharmacol. 2021, 268, 113608. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Xu, M.; Wu, B.; Liao, Z.; Liu, Z.; Zhao, X.; Bi, K.; Jia, Y. The effect of Schisandra chinensis extracts on depression by noradrenergic, dopaminergic, GABAergic and glutamatergic systems in the forced swim test in mice. Food Funct. 2016, 7, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Gao, Y.; Nie, K.; Wang, H.; Su, H.; Wang, Z.; Lu, F.; Huang, W.; Dong, H. Antidepressant Potential of Quercetin and its Glycoside Derivatives: A Comprehensive Review and Update. Front. Pharmacol. 2022, 13, 865376. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Immunoregulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Braz. J. Med. Biol. Res. 1998, 31, 467–490. [Google Scholar] [CrossRef]
- Miralles-Perez, B.; Mendez, L.; Nogues, M.R.; Sanchez-Martos, V.; Fortuno-Mar, A.; Ramos-Romero, S.; Hereu, M.; Medina, I.; Romeu, M. Effects of a Fish Oil Rich in Docosahexaenoic Acid on Cardiometabolic Risk Factors and Oxidative Stress in Healthy Rats. Mar. Drugs 2021, 19, 555. [Google Scholar] [CrossRef]
- Vines, A.; Delattre, A.M.; Lima, M.M.; Rodrigues, L.S.; Suchecki, D.; Machado, R.B.; Tufik, S.; Pereira, S.I.; Zanata, S.M.; Ferraz, A.C. The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism. Neuropharmacology 2012, 62, 184–191. [Google Scholar] [CrossRef]
- Pardo Lozano, R.; Alvarez Garcia, Y.; Barral Tafalla, D.; Farre Albaladejo, M. Caffeine: A nutrient, a drug or a drug of abuse. Adicciones 2007, 19, 225–238. [Google Scholar] [CrossRef]
- Faudone, G.; Arifi, S.; Merk, D. The Medicinal Chemistry of Caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef]
- Lopez-Cruz, L.; Salamone, J.D.; Correa, M. Caffeine and Selective Adenosine Receptor Antagonists as New Therapeutic Tools for the Motivational Symptoms of Depression. Front. Pharmacol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Boros, L.G. Population thiamine status and varying cancer rates between western, Asian and African countries. Anticancer Res. 2000, 20, 2245–2248. [Google Scholar] [PubMed]
- Sambon, M.; Wins, P.; Bettendorff, L. Neuroprotective Effects of Thiamine and Precursors with Higher Bioavailability: Focus on Benfotiamine and Dibenzoylthiamine. Int. J. Mol. Sci. 2021, 22, 5418. [Google Scholar] [CrossRef]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency-Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef]
- Huang, S.K.; Lu, C.W.; Lin, T.Y.; Wang, S.J. Neuroprotective Role of the B Vitamins in the Modulation of the Central Glutamatergic Neurotransmission. CNS Neurol. Disord. Drug Targets 2022, 21, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Dosedel, M.; Jirkovsky, E.; Macakova, K.; Krcmova, L.K.; Javorska, L.; Pourova, J.; Mercolini, L.; Remiao, F.; Novakova, L.; Mladenka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Urena-Torres, P.; Souberbielle, J.C. Pharmacologic role of vitamin D natural products. Curr. Vasc. Pharmacol. 2014, 12, 278–285. [Google Scholar] [CrossRef]
- Kouba, B.R.; Camargo, A.; Gil-Mohapel, J.; Rodrigues, A.L.S. Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. Int. J. Mol. Sci. 2022, 23, 7077. [Google Scholar] [CrossRef]
- Ni, X.C.; Wang, H.F.; Cai, Y.Y.; Yang, D.; Alolga, R.N.; Liu, B.; Li, J.; Huang, F.Q. Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol. 2022, 54, 102363. [Google Scholar] [CrossRef]
- Gong, L.; Yin, J.; Zhang, Y.; Huang, R.; Lou, Y.; Jiang, H.; Sun, L.; Jia, J.; Zeng, X. Neuroprotective Mechanisms of Ginsenoside Rb1 in Central Nervous System Diseases. Front. Pharmacol. 2022, 13, 914352. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, J.; Zhang, L.; Yang, L.; Ma, J.; Bai, Y.; Ma, W.; Wang, L.; Yu, H.; Zeng, Y.; et al. Ginsenoside Rb1 exerts antidepressant-like effects via suppression inflammation and activation of AKT pathway. Neurosci. Lett. 2021, 744, 135561. [Google Scholar] [CrossRef] [PubMed]
- Matias, J.N.; Achete, G.; Campanari, G.; Guiguer, E.L.; Araujo, A.C.; Buglio, D.S.; Barbalho, S.M. A systematic review of the antidepressant effects of curcumin: Beyond monoamines theory. Aust. N. Z. J. Psychiatry 2021, 55, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini, E.S.L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef]
- Imran, M.; Samal, M.; Qadir, A.; Ali, A.; Mir, S.R. A critical review on the extraction and pharmacotherapeutic activity of piperine. Polim. Med. 2022, 52, 31–36. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Wang, M.; Li, W.; Matsumoto, K.; Tang, Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007, 80, 1373–1381. [Google Scholar] [CrossRef]
- Glabska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef]
- McCarty, M.F. Scavenging of peroxynitrite-derived radicals by flavonoids may support endothelial NO synthase activity, contributing to the vascular protection associated with high fruit and vegetable intakes. Med. Hypotheses 2008, 70, 170–181. [Google Scholar] [CrossRef]
- Bishwajit, G.; O’Leary, D.P.; Ghosh, S.; Sanni, Y.; Shangfeng, T.; Zhanchun, F. Association between depression and fruit and vegetable consumption among adults in South Asia. BMC Psychiatry 2017, 17, 15. [Google Scholar] [CrossRef]
- Richard, A.; Rohrmann, S.; Vandeleur, C.L.; Mohler-Kuo, M.; Eichholzer, M. Associations between fruit and vegetable consumption and psychological distress: Results from a population-based study. BMC Psychiatry 2015, 15, 213. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Chu, Q.; Zheng, X. Vaccinium bracteatum Thunb. fruit extract reduces high-fat diet-induced obesity with modulation of the gut microbiota in obese mice. J. Food Biochem. 2021, 45, e13808. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.J.; Handu, S.S.; Dubey, A.K.; Mediratta, P.K.; Shukla, R.; Ahmed, Q.M. Effect of Musa sapientum Stem Extract on Animal Models of Depression. Pharmacogn. Res. 2016, 8, 249–252. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Hu, Y.; Xie, J. Nutritional component changes in Xiangfen 1 banana at different developmental stages. Food Funct. 2020, 11, 8286–8296. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Sustaita, B.; Estrada-Camarena, E.; Gonzalez-Trujano, M.E.; Lopez-Rubalcava, C. Estrogen receptors-beta and serotonin mediate the antidepressant-like effect of an aqueous extract of pomegranate in ovariectomized rats. Neurochem. Int. 2021, 142, 104904. [Google Scholar] [CrossRef]
- Mori-Okamoto, J.; Otawara-Hamamoto, Y.; Yamato, H.; Yoshimura, H. Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model ovariectomized mice. J. Ethnopharmacol. 2004, 92, 93–101. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Han, X.J.; Xu, T.S.; Fang, Q.J.; Zhang, H.J.; Yue, L.J.; Hu, G.; Sun, L.Y. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Kohno, M.; Hirotsuka, M.; Kito, M.; Matsuzawa, Y. Decreases in serum triacylglycerol and visceral fat mediated by dietary soybean beta-conglycinin. J. Atheroscler. Thromb. 2006, 13, 247–255. [Google Scholar] [CrossRef]
- Wu, S.J.; Chang, C.Y.; Lai, Y.T.; Shyu, Y.T. Increasing gamma-Aminobutyric Acid Content in Vegetable Soybeans via High-Pressure Processing and Efficacy of Their Antidepressant-Like Activity in Mice. Foods 2020, 9, 1673. [Google Scholar] [CrossRef]
- Ji, W.W.; Li, R.P.; Li, M.; Wang, S.Y.; Zhang, X.; Niu, X.X.; Li, W.; Yan, L.; Wang, Y.; Fu, Q.; et al. Antidepressant-like effect of essential oil of Perilla frutescens in a chronic, unpredictable, mild stress-induced depression model mice. Chin. J. Nat. Med. 2014, 12, 753–759. [Google Scholar] [CrossRef]
- Wang, A.; Wan, X.; Zhuang, P.; Jia, W.; Ao, Y.; Liu, X.; Tian, Y.; Zhu, L.; Huang, Y.; Yao, J.; et al. High fried food consumption impacts anxiety and depression due to lipid metabolism disturbance and neuroinflammation. Proc. Natl. Acad. Sci. USA 2023, 120, e2221097120. [Google Scholar] [CrossRef] [PubMed]
- Philippou, E.; Nikiphorou, E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun. Rev. 2018, 17, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Sharifan, P.; Hosseini, M.S.; Sharifan, A. The interventional relationship between frequent fish consumption and depression symptoms in aging adults: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2017, 32, E116–E122. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Chang, C.M.; Yang, C.C.; Pariante, C.M.; Su, K.P. Long COVID and long chain fatty acids (LCFAs): Psychoneuroimmunity implication of omega-3 LCFAs in delayed consequences of COVID-19. Brain Behav. Immun. 2022, 103, 19–27. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef]
- Nasehi, M.; Mosavi-Nezhad, S.M.; Khakpai, F.; Zarrindast, M.R. The role of omega-3 on modulation of cognitive deficiency induced by REM sleep deprivation in rats. Behav. Brain Res. 2018, 351, 152–160. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Whiting, C.V.; Bland, P.W.; Tarlton, J.F. Dietary n-3 polyunsaturated fatty acids reduce disease and colonic proinflammatory cytokines in a mouse model of colitis. Inflamm. Bowel Dis. 2005, 11, 340–349. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Naliwaiko, K.; Araujo, R.L.; da Fonseca, R.V.; Castilho, J.C.; Andreatini, R.; Bellissimo, M.I.; Oliveira, B.H.; Martins, E.F.; Curi, R.; Fernandes, L.C.; et al. Effects of fish oil on the central nervous system: A new potential antidepressant? Nutr. Neurosci. 2004, 7, 91–99. [Google Scholar] [CrossRef]
- Davis, D.J.; Hecht, P.M.; Jasarevic, E.; Beversdorf, D.Q.; Will, M.J.; Fritsche, K.; Gillespie, C.H. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain Behav. Immun. 2017, 59, 38–48. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; El Aidy, S.; Crispie, F.; O’Sullivan, O.; Cotter, P.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. N-3 Polyunsaturated Fatty Acids (PUFAs) Reverse the Impact of Early-Life Stress on the Gut Microbiota. PLoS ONE 2015, 10, e0139721. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef]
- Caroprese, M.; Ciliberti, M.G.; Annicchiarico, G.; Albenzio, M.; Muscio, A.; Sevi, A. Hypothalamic-pituitary-adrenal axis activation and immune regulation in heat-stressed sheep after supplementation with polyunsaturated fatty acids. J. Dairy Sci. 2014, 97, 4247–4258. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Q.; Yang, W.; Liu, J.; Gao, M.Q. Omega-3 polyunsaturated fatty acids ameliorated inflammatory response of mammary epithelial cells and mammary gland induced by lipopolysaccharide. Acta Biochim. Biophys. Sin. 2021, 53, 1142–1153. [Google Scholar] [CrossRef]
- Takeuchi, E.; Yamada, D.; Suzuki, S.; Saitoh, A.; Itoh, M.; Hayashi, T.; Yamada, M.; Wada, K.; Sekiguchi, M. Participation of the nucleus accumbens dopaminergic system in the antidepressant-like actions of a diet rich in omega-3 polyunsaturated fatty acids. PLoS ONE 2020, 15, e0230647. [Google Scholar] [CrossRef]
- Naeem, S.; Ali, L.; Rizwani, G.H.; Ikram, R.; Khan, S.S.; Shareef, H.; Younus, I.; Malick, T.Z.; Aleem, U. A comparative neurobehavioral study of sesame oil and fish oil on experimental animals. Pak. J. Pharm. Sci. 2020, 33, 511–521. [Google Scholar]
- Ferraz, A.C.; Kiss, A.; Araujo, R.L.; Salles, H.M.; Naliwaiko, K.; Pamplona, J.; Matheussi, F. The antidepressant role of dietary long-chain polyunsaturated n-3 fatty acids in two phases in the developing brain. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 183–188. [Google Scholar] [CrossRef]
- Wu, B.; Song, Q.; Zhang, Y.; Wang, C.; Yang, M.; Zhang, J.; Han, W.; Jiang, P. Antidepressant activity of omega-3 polyunsaturated fatty acids in ovariectomized rats: Role of neuroinflammation and microglial polarization. Lipids Health Dis. 2020, 19, 4. [Google Scholar] [CrossRef]
- Correa, C.R.; Schena, C.; Lopes, S.C.; Prediger, R.D.; Silva, E.L.; Venske, D.K.R.; Ribeiro, L.C.; Moreira, J.D. Combined effects of caloric restriction and fish oil attenuated anti-depressant and anxiolytic-like effects of fish oil: Association with hippocampal BDNF concentrations. Behav. Brain Res. 2020, 393, 112770. [Google Scholar] [CrossRef]
- Carabelli, B.; Delattre, A.M.; Pudell, C.; Mori, M.A.; Suchecki, D.; Machado, R.B.; Venancio, D.P.; Piazzetta, S.R.; Hammerschmidt, I.; Zanata, S.M.; et al. The Antidepressant-Like Effect of Fish Oil: Possible Role of Ventral Hippocampal 5-HT1A Post-synaptic Receptor. Mol. Neurobiol. 2015, 52, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Voyias, P.D.; Sallis, H.M.; Dawson, S.; Ness, A.R.; Churchill, R.; Perry, R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst. Rev. 2021, 11, CD004692. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Liang, S.; Chen, J.; Yan, X.; Duan, Z.; Zhou, D.; Li, Z. Evaluation of the Antidepressant Effect of the Functional Beverage Containing Active Peptides, Menthol and Eleutheroside and Investigation of Its Mechanism of Action in Mice. Food Technol. Biotechnol. 2020, 58, 295–302. [Google Scholar] [CrossRef]
- Yin, Y.Q.; Zhang, C.; Wang, J.X.; Hou, J.; Yang, X.; Qin, J. Chronic caffeine treatment enhances the resilience to social defeat stress in mice. Food Funct. 2015, 6, 479–491. [Google Scholar] [CrossRef]
- Szopa, A.; Doboszewska, U.; Herbet, M.; Wosko, S.; Wyska, E.; Swiader, K.; Serefko, A.; Korga, A.; Wlaz, A.; Wrobel, A.; et al. Chronic treatment with caffeine and its withdrawal modify the antidepressant-like activity of selective serotonin reuptake inhibitors in the forced swim and tail suspension tests in mice. Effects on Comt, Slc6a15 and Adora1 gene expression. Toxicol. Appl. Pharmacol. 2017, 337, 95–103. [Google Scholar] [CrossRef]
- Kale, P.P.; Addepalli, V. Augmentation of antidepressant effects of duloxetine and bupropion by caffeine in mice. Pharmacol. Biochem. Behav. 2014, 124, 238–244. [Google Scholar] [CrossRef]
- Liu, Q.S.; Deng, R.; Fan, Y.; Li, K.; Meng, F.; Li, X.; Liu, R. Low dose of caffeine enhances the efficacy of antidepressants in major depressive disorder and the underlying neural substrates. Mol. Nutr. Food Res. 2017, 61, 1600910. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Battig, K.; Holmen, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Serefko, A.; Szopa, A.; Wlaz, A.; Wosko, S.; Wlaz, P.; Poleszak, E. Synergistic antidepressant-like effect of the joint administration of caffeine and NMDA receptor ligands in the forced swim test in mice. J. Neural Transm. 2016, 123, 463–472. [Google Scholar] [CrossRef]
- Espinosa Jovel, C.A.; Sobrino Mejia, F.E. Caffeine and headache: Specific remarks. Neurologia 2017, 32, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Meller, F.O.; Manosso, L.M.; Schafer, A.A. The influence of diet quality on depression among adults and elderly: A population-based study. J. Affect. Disord. 2021, 282, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, D.O.; Zhang, L. Mechanisms Underlying the Anti-Depressive Effects of Regular Tea Consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wei, Y.; Wei, X. A comprehensive review on bioavailability, safety and antidepressant potential of natural bioactive components from tea. Food Res. Int. 2022, 158, 111540. [Google Scholar] [CrossRef]
- Voskoboinik, A.; Koh, Y.; Kistler, P.M. Cardiovascular effects of caffeinated beverages. Trends Cardiovasc. Med. 2019, 29, 345–350. [Google Scholar] [CrossRef]
- Teng, J.; Zhou, W.; Zeng, Z.; Zhao, W.; Huang, Y.; Zhang, X. Quality components and antidepressant-like effects of GABA green tea. Food Funct. 2017, 8, 3311–3318. [Google Scholar] [CrossRef]
- Zhu, W.L.; Shi, H.S.; Wei, Y.M.; Wang, S.J.; Sun, C.Y.; Ding, Z.B.; Lu, L. Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol. Res. 2012, 65, 74–80. [Google Scholar] [CrossRef]
- Linnoila, M.I. Anxiety and alcoholism. J. Clin. Psychiatry 1989, 50 (Suppl. S11), 26–29. [Google Scholar]
- Ciccocioppo, R.; Panocka, I.; Froldi, R.; Colombo, G.; Gessa, G.L.; Massi, M. Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology 1999, 144, 151–157. [Google Scholar] [CrossRef]
- Gea, A.; Beunza, J.J.; Estruch, R.; Sanchez-Villegas, A.; Salas-Salvado, J.; Buil-Cosiales, P.; Gomez-Gracia, E.; Covas, M.I.; Corella, D.; Fiol, M.; et al. Alcohol intake, wine consumption and the development of depression: The PREDIMED study. BMC Med. 2013, 11, 192. [Google Scholar] [CrossRef]
- Bonnet, U. How much alcohol is in ketamine’s antidepressant action? Life Sci. 2017, 168, 54–57. [Google Scholar] [CrossRef]
- Leclercq, S.; Le Roy, T.; Furgiuele, S.; Coste, V.; Bindels, L.B.; Leyrolle, Q.; Neyrinck, A.M.; Quoilin, C.; Amadieu, C.; Petit, G.; et al. Gut Microbiota-Induced Changes in beta-Hydroxybutyrate Metabolism Are Linked to Altered Sociability and Depression in Alcohol Use Disorder. Cell Rep. 2020, 33, 108238. [Google Scholar] [CrossRef]
- Morkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef]
- Silva, L.C.; de Souza Lago, H.; Rocha, M.O.T.; de Oliveira, V.S.; Laureano-Melo, R.; Stutz, E.T.G.; de Paula, B.P.; Martins, J.F.P.; Luchese, R.H.; Guerra, A.F.; et al. Craft Beers Fermented by Potential Probiotic Yeast or Lacticaseibacilli Strains Promote Antidepressant-Like Behavior in Swiss Webster Mice. Probiotics Antimicrob. Proteins 2021, 13, 698–708. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lan, Y.-W.; Tu, M.-Y.; Tung, Y.-T.; Chan, M.N.-Y.; Wu, H.-S.; Yen, C.-C.; Chen, C.-M. Kefir peptides exhibit antidepressant-like activity in mice through the BDNF/TrkB pathway. J. Dairy Sci. 2021, 104, 6415–6430. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Abildgaard, A.; Solskov, L.; Volke, V.; Harvey, B.H.; Lund, S.; Wegener, G. A high-fat diet exacerbates depressive-like behavior in the Flinders Sensitive Line (FSL) rat, a genetic model of depression. Psychoneuroendocrinology 2011, 36, 623–633. [Google Scholar] [CrossRef]
- Abildgaard, A.; Kern, T.; Pedersen, O.; Hansen, T.; Lund, S.; Wegener, G. A diet-induced gut microbiota component and related plasma metabolites are associated with depressive-like behaviour in rats. Eur. Neuropsychopharmacol. 2021, 43, 10–21. [Google Scholar] [CrossRef]
- Tillmann, S.; Awwad, H.M.; Eskelund, A.R.; Treccani, G.; Geisel, J.; Wegener, G.; Obeid, R. Probiotics Affect One-Carbon Metabolites and Catecholamines in a Genetic Rat Model of Depression. Mol. Nutr. Food Res. 2018, 62, e1701070. [Google Scholar] [CrossRef]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Miller, J.W. Vitamin B12 deficiency. Vitam. Horm. 2022, 119, 405–439. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Sun, L.; Zhang, H.; Liu, C.; Zhang, C.; Yu, L. B-vitamin supplementation ameliorates anxiety- and depression-like behavior induced by gestational urban PM2.5 exposure through suppressing neuroinflammation in mice offspring. Environ. Pollut. 2020, 266, 115146. [Google Scholar] [CrossRef] [PubMed]
- Mesripour, A.; Alhimma, F.; Hajhashemi, V. The effect of vitamin B6 on dexamethasone-induced depression in mice model of despair. Nutr. Neurosci. 2019, 22, 744–749. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-containing plant food sources for vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Coppen, A.; Bolander-Gouaille, C. Treatment of depression: Time to consider folic acid and vitamin B12. J. Psychopharmacol. 2005, 19, 59–65. [Google Scholar]
- Brocardo, P.S.; Budni, J.; Kaster, M.P.; Santos, A.R.; Rodrigues, A.L. Folic acid administration produces an antidepressant-like effect in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology 2008, 54, 464–473. [Google Scholar] [CrossRef]
- Rosa, P.B.; Ribeiro, C.M.; Bettio, L.E.; Colla, A.; Lieberknecht, V.; Moretti, M.; Rodrigues, A.L. Folic acid prevents depressive-like behavior induced by chronic corticosterone treatment in mice. Pharmacol. Biochem. Behav. 2014, 127, 1–6. [Google Scholar] [CrossRef]
- Thomas, J.; Khanam, R.; Vohora, D. Augmentation of effect of venlafaxine by folic acid in behavioral paradigms of depression in mice: Evidence of serotonergic and pro-inflammatory cytokine pathways. Pharmacol. Rep. 2016, 68, 396–403. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Riaz, A.; Khan, R.A. Behavioral effects of Citrus limon and Punica granatum combinations in rats. Metab. Brain Dis. 2017, 32, 123–131. [Google Scholar] [CrossRef]
- Binfare, R.W.; Rosa, A.O.; Lobato, K.R.; Santos, A.R.; Rodrigues, A.L. Ascorbic acid administration produces an antidepressant-like effect: Evidence for the involvement of monoaminergic neurotransmission. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 530–540. [Google Scholar] [CrossRef]
- Rosa, P.B.; Neis, V.B.; Ribeiro, C.M.; Moretti, M.; Rodrigues, A.L. Antidepressant-like effects of ascorbic acid and ketamine involve modulation of GABAA and GABAB receptors. Pharmacol. Rep. 2016, 68, 996–1001. [Google Scholar] [CrossRef]
- Fraga, D.B.; Olescowicz, G.; Moretti, M.; Siteneski, A.; Tavares, M.K.; Azevedo, D.; Colla, A.R.S.; Rodrigues, A.L.S. Anxiolytic effects of ascorbic acid and ketamine in mice. J. Psychiatr. Res. 2018, 100, 16–23. [Google Scholar] [CrossRef]
- Meredith, M.E.; May, J.M. Regulation of embryonic neurotransmitter and tyrosine hydroxylase protein levels by ascorbic acid. Brain Res. 2013, 1539, 7–14. [Google Scholar] [CrossRef]
- Zech, L.D.; Scherf-Clavel, M.; Daniels, C.; Schwab, M.; Deckert, J.; Unterecker, S.; Herr, A.S. Patients with higher vitamin D levels show stronger improvement of self-reported depressive symptoms in psychogeriatric day-care setting. J. Neural Transm. 2021, 128, 1233–1238. [Google Scholar] [CrossRef]
- Wyskida, M.; Wieczorowska-Tobis, K.; Chudek, J. Prevalence and factors promoting the occurrence of vitamin D deficiency in the elderly. Postep. Hig. I Med. Dosw. Online 2017, 71, 198–204. [Google Scholar] [CrossRef]
- Glade, M.J. Vitamin D: Health panacea or false prophet? Nutrition 2013, 29, 37–41. [Google Scholar] [CrossRef]
- Chuang, H.W.; Wei, I.H.; Lin, F.Y.; Li, C.T.; Chen, K.T.; Tsai, M.H.; Huang, C.C. Roles of Akt and ERK in mTOR-Dependent Antidepressant Effects of Vanillic Acid. ACS Omega 2020, 5, 3709–3716. [Google Scholar] [CrossRef]
- Moradi, O. A review on nanomaterial-based electrochemical sensors for determination of vanillin in food samples. Food Chem. Toxicol. 2022, 168, 113391. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, X.; Wu, Q.; Chang, F.; Zhou, T.; Zhou, M.; Pei, J. Active constituents of saffron (Crocus sativus L.) and their prospects in treating neurodegenerative diseases (Review). Exp. Ther. Med. 2023, 25, 235. [Google Scholar] [CrossRef]
- Sahib, N.G.; Anwar, F.; Gilani, A.H.; Hamid, A.A.; Saari, N.; Alkharfy, K.M. Coriander (Coriandrum sativum L.): A potential source of high-value components for functional foods and nutraceuticals—A review. Phytother. Res. 2013, 27, 1439–1456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Shen, C.Y.; Jiang, J.G. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: Pharmacological mechanisms and prospects for drug discovery. Pharmacol. Res. 2019, 150, 104520. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cui, R.; Zhao, L.; Fan, J.; Li, B. Mechanisms of Panax ginseng action as an antidepressant. Cell Prolif. 2019, 52, e12696. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, H.; Li, C.; Zeng, G.; Lv, J.; Wang, Q.; Chen, Y.; Liu, X. The antidepressant-like effects of the water extract of Panax ginseng and Polygala tenuifolia are mediated via the BDNF-TrkB signaling pathway and neurogenesis in the hippocampus. J. Ethnopharmacol. 2021, 267, 113625. [Google Scholar] [CrossRef]
- Yamada, N.; Araki, H.; Yoshimura, H. Identification of antidepressant-like ingredients in ginseng root (Panax ginseng C.A. Meyer) using a menopausal depressive-like state in female mice: Participation of 5-HT2A receptors. Psychopharmacology 2011, 216, 589–599. [Google Scholar] [CrossRef]
- Sanmukhani, J.; Anovadiya, A.; Tripathi, C.B. Evaluation of antidepressant like activity of curcumin and its combination with fluoxetine and imipramine: An acute and chronic study. Acta Pol. Pharm. 2011, 68, 769–775. [Google Scholar]
- Abd-Rabo, M.M.; Georgy, G.S.; Saied, N.M.; Hassan, W.A. Involvement of the serotonergic system and neuroplasticity in the antidepressant effect of curcumin in ovariectomized rats: Comparison with oestradiol and fluoxetine. Phytother. Res. 2019, 33, 387–396. [Google Scholar] [CrossRef]
- Lian, L.; Xu, Y.; Zhang, J.; Yu, Y.; Zhu, N.; Guan, X.; Huang, H.; Chen, R.; Chen, J.; Shi, G.; et al. Antidepressant-like effects of a novel curcumin derivative J147: Involvement of 5-HT1A receptor. Neuropharmacology 2018, 135, 506–513. [Google Scholar] [CrossRef]
- Quijia, C.R.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Piperine: A Review. Crit. Rev. Anal. Chem. 2020, 50, 62–77. [Google Scholar] [CrossRef]
- Rybnikar, M.; Smejkal, K.; Zemlicka, M. Schisandra chinensis and its phytotherapeutical applications. Ceska A Slov. Farm. 2019, 68, 95–118. [Google Scholar]
- Yan, T.; He, B.; Wan, S.; Xu, M.; Yang, H.; Xiao, F.; Bi, K.; Jia, Y. Antidepressant-like effects and cognitive enhancement of Schisandra chinensis in chronic unpredictable mild stress mice and its related mechanism. Sci. Rep. 2017, 7, 6903. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shan, B.; Zeng, S.; Zhang, J.; Jin, C.; Liao, Z.; Wang, T.; Zeng, Q.; He, H.; Wei, F.; et al. Raw and wine processed Schisandra chinensis attenuate anxiety like behavior via modulating gut microbiota and lipid metabolism pathway. J. Ethnopharmacol. 2021, 266, 113426. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, S.; Yamane, H.; Onitsuka, E.; Yamada, S.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Carnosine-induced antidepressant-like activity in rats. Pharmacol. Biochem. Behav. 2008, 89, 627–632. [Google Scholar] [CrossRef]
- Jacka, F.N.; Mykletun, A.; Berk, M.; Bjelland, I.; Tell, G.S. The association between habitual diet quality and the common mental disorders in community-dwelling adults: The Hordaland Health study. Psychosom. Med. 2011, 73, 483–490. [Google Scholar] [CrossRef]
- Nagasawa, M.; Murakami, T.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Dietary animal proteins alter monoamine metabolism in the brain. Anim. Sci. J. 2012, 83, 493–498. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, J.; Jia, X.; Huang, X.; Wu, Z.; Shi, X.; Zhao, J.; Liu, S.; Ding, G. Association between dietary patterns and depressive symptom based on reduced rank regression in people aged 55 and above in 4 provinces of China. Wei Sheng Yan Jiu 2021, 50, 29–36. [Google Scholar] [CrossRef]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame Lignans Suppress Age-Related Cognitive Decline in Senescence-Accelerated Mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of Sesamin Alleviates Stress-Induced Behavioral and Psychological Disorders via Reshaping the Gut Microbiota Structure. J. Agric. Food Chem. 2019, 67, 12441–12451. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Jia, M.; Fu, S.; Pan, J.; Chu, C.; Liu, X.; Liu, X.; Liu, Z. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019, 64, 61–71. [Google Scholar] [CrossRef]
- Lee, H.C.; Ko, H.K.; Huang, B.E.; Chu, Y.H.; Huang, S.Y. Antidepressant-like effects of Perilla frutescens seed oil during a forced swimming test. Food Funct. 2014, 5, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ce, Q.; Jin, L.; Zheng, J.; Sun, M.; Tang, X.; Li, D.; Sun, J. Deoiled sunflower seeds ameliorate depression by promoting the production of monoamine neurotransmitters and inhibiting oxidative stress. Food Funct. 2021, 12, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, P.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Effect of adzuki bean sprout fermented milk enriched in gamma-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ohya, R.; Kondo, K. Antidepressant-Like Effect of beta-Lactolin, a Glycine-Threonine-Tryptophan-Tyrosine Peptide. J. Nutr. Sci. Vitaminol. 2019, 65, 430–434. [Google Scholar] [CrossRef]
- Yun, B.; Yoo, J.Y.; Park, M.R.; Ryu, S.; Lee, W.J.; Choi, H.J.; Kang, M.K.; Kim, Y.; Oh, S. Ingestion of Gouda Cheese Ameliorates the Chronic Unpredictable Mild Stress in Mice. Food Sci. Anim. Resour. 2020, 40, 145–153. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, C.; Momma, H.; Ren, Z.; Sugiyama, S.; Guan, L.; Niu, K.; Nagatomi, R. Consumption of low-fat dairy, but not whole-fat dairy, is inversely associated with depressive symptoms in Japanese adults. Soc. Psychiatry Psychiatr. Epidemiol. 2017, 52, 847–853. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Zhang, D. Associations of different types of dairy intakes with depressive symptoms in adults. J. Affect. Disord. 2020, 274, 326–333. [Google Scholar] [CrossRef]
- Mahdavifar, B.; Hosseinzadeh, M.; Salehi-Abargouei, A.; Mirzaei, M.; Vafa, M. The association between dairy products and psychological disorders in a large sample of Iranian adults. Nutr. Neurosci. 2022, 25, 2379–2389. [Google Scholar] [CrossRef]
- Qian, J.; Fang, Y.; Yuan, N.; Gao, X.; Lv, Y.; Zhao, C.; Zhang, S.; Li, Q.; Li, L.; Xu, L.; et al. Innate immune remodeling by short-term intensive fasting. Aging Cell 2021, 20, e13507. [Google Scholar] [CrossRef]
- Spanaki, C.; Rodopaios, N.E.; Koulouri, A.; Pliakas, T.; Papadopoulou, S.K.; Vasara, E.; Skepastianos, P.; Serafeim, T.; Boura, I.; Dermitzakis, E.; et al. The Christian Orthodox Church Fasting Diet Is Associated with Lower Levels of Depression and Anxiety and a Better Cognitive Performance in Middle Life. Nutrients 2021, 13, 627. [Google Scholar] [CrossRef]
- Gomes, A.P.; Oliveira Bierhals, I.; Goncalves Soares, A.L.; Hellwig, N.; Tomasi, E.; Formoso Assuncao, M.C.; Goncalves, H. Interrelatioship between Diet Quality and Depressive Symptoms in Elderly. J. Nutr. Health Aging 2018, 22, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Rudzinska, A.; Perera, I.; Gryglewska, B.; Gasowski, J.; Piotrowicz, K. Can the Mediterranean diet decrease the risk of depression in older persons—A systematic review. Psychiatr. Pol. 2022, 57, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.J.; Ferreira, A.V.M.; Oliveira, A.L.; Oliveira, M.C.; Gomes, J.S.; Aguiar, D.C. Carbohydrate-enriched diet predispose to anxiety and depression-like behavior after stress in mice. Nutr. Neurosci. 2018, 21, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care 2008, 31, 2383–2390. [Google Scholar] [CrossRef]
- Linehan, V.; Fang, L.Z.; Hirasawa, M. Short-term high-fat diet primes excitatory synapses for long-term depression in orexin neurons. J. Physiol. 2018, 596, 305–316. [Google Scholar] [CrossRef]
- Dutheil, S.; Ota, K.T.; Wohleb, E.S.; Rasmussen, K.; Duman, R.S. High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology 2015, 41, 1874–1887. [Google Scholar] [CrossRef]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The Association of Emotional Eating with Overweight/Obesity, Depression, Anxiety/Stress, and Dietary Patterns: A Review of the Current Clinical Evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef]
- Schachter, J.; Martel, J.; Lin, C.S.; Chang, C.J.; Wu, T.R.; Lu, C.C.; Ko, Y.F.; Lai, H.C.; Ojcius, D.M.; Young, J.D. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav. Immun. 2018, 69, 1–8. [Google Scholar] [CrossRef]
- Martins, L.B.; Monteze, N.M.; Calarge, C.; Ferreira, A.V.M.; Teixeira, A.L. Pathways linking obesity to neuropsychiatric disorders. Nutrition 2019, 66, 16–21. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. CMLS 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Nedic Erjavec, G.; Sagud, M.; Nikolac Perkovic, M.; Svob Strac, D.; Konjevod, M.; Tudor, L.; Uzun, S.; Pivac, N. Depression: Biological markers and treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110139. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Lv, J.; Tang, F.; Liu, L.; Sun, Z.; Wang, L.; Siwela, S.P.; Wang, Y.; Song, Y.; et al. Additive antidepressant-like effects of fasting with imipramine via modulation of 5-HT2 receptors in the mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 199–206. [Google Scholar] [CrossRef]
- Wang, P.; Li, B.; Fan, J.; Zhang, K.; Yang, W.; Ren, B.; Cui, R. Additive antidepressant-like effects of fasting with beta-estradiol in mice. J. Cell Mol. Med. 2019, 23, 5508–5517. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
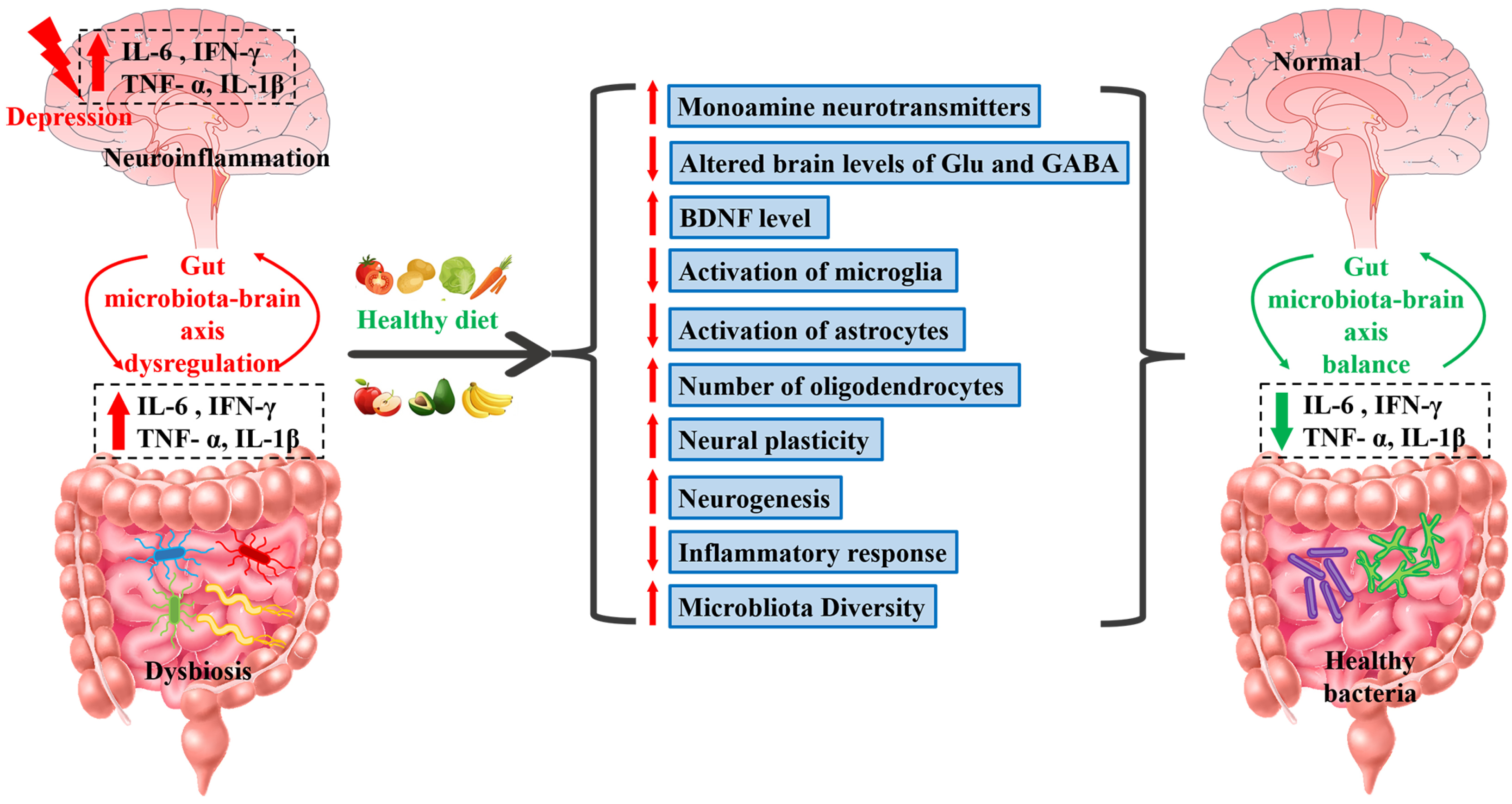


| NO. | Antidepressant Class | Representative Medications | Putative Antidepressant Mechanisms | Common Side Effects | Limitations | References |
|---|---|---|---|---|---|---|
| 1 | SSRIs | Paroxetine, sertraline, and fluoxetine | Enhancement of synaptic 5-HT levels via reuptake inhibition | Gastrointestinal disturbances, insomnia, impaired memory, and sexual dysfunction | Potential risk of suicidality in individuals aged 18–24 | [10,11] |
| 2 | SNRIs | Venlafaxine and duloxetine | Inhibition of 5-HT and NE reuptake, increasing their levels in the synaptic cleft | Gastrointestinal symptoms, sleep disturbances, and sexual dysfunction | Potential dependence issues during extended use | [12,13,14] |
| 3 | SARIs | Trazodone and nefazodone | Inhibition of 5-HT reuptake and antagonism of 5-HT2A receptors, resulting in elevated synaptic 5-HT levels | Dizziness, constipation, and drowsiness | Dose-dependency and substantial individual variability | [15,16] |
| 4 | MAOIs | Phenelzine and isocarboxazid | Inhibition of monoamine oxidase, leading to increased concentrations of neurotransmitters (5-HT, NE, and DA) in the brain synapses | Gastrointestinal reactions, dizziness, insomnia, orthostatic hypotension | Due to tyramine sensitivity, dietary restrictions required; potential drug interactions necessitate blood pressure monitoring | [17,18] |
| 5 | TCAs | Amitriptyline and doxepin | Elevation of 5-HT and NE levels in the synaptic cleft | Weight gain, constipation, dizziness, and cardiac side effects | Due to its elevated anticholinergic side effects and propensity for arrhythmias, it may not be appropriate for certain individuals with heart conditions | [19,20] |
| 6 | AAPs | Olanzapine and quetiapine | DA and 5-HT receptor antagonism, and modulation of other neurotransmitters | Weight gain and metabolic disturbances | Potential for obesity and related health issues | [21] |
| 7 | NaSSAs | Mirtazapine | Enhancement of NE release and 5-HT synaptic concentrations | Weight gain, dry mouth, and drowsiness | Potential for obesity and related health issues | [22] |
| 8 | NDRIs | Bupropion | Inhibition of NE and DA reuptake, leading to increased synaptic concentrations | Headache, insomnia, nausea, and loss of appetite | Occurrence of low tolerance or allergic reactions in some patient | [23,24,25] |
| NO. | Food Type | Food Name | Subjects | Depression Model | Ingredients | Period | Putative Antidepressant Mechanisms | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Fruit | Bananas | Locally bred albino Wistar mice | FST | Banana fruit pulp and peel | 14 days | Increase in antioxidant enzymes (e.g., CAT, SOD) levels and reduction in GSH levels | [184] |
| 2 | Fruit | Pomegranates | Female Wistar mice | OVX | Pomegranate extract | 14 days | Enhancement of central adrenergic function; activation of estrogen receptors and serotonergic systems | [185] |
| 3 | Fruit | Apple peels and citrus fruits | Male wildtype mice | CSDS | Rich in quercetin | 30 days | Inhibition of astrocyte activation and neuroprotection | [186] |
| 4 | Vegetable | Soybean | Male ICR mice | FST, TST | Genistein | 3 weeks | Regulation of brain 5-HT levels | [187] |
| 5 | Fish | Fish oil | Male Sprague–Dawley rats | LPS | ω-3PUFA | 21 days | Inhibition of activation of inflammatory NLRP3 and ionic purine receptor P2 × 7R | [188] |
| 6 | Fish | Fish oil | Mixed S129/Sv x C57BL/6 genetic mice | BDNF +/− mice and their wild-type | ω-3PUFA | 3 months | Reduction in hippocampal extracellular 5-HT levels and increase in Erk activation | [189] |
| 7 | Fish | Fish fillets | Male ICR mice | TST | AECSF | 4 consecutive days | Regulation of serotonergic and norepinephrine systems | [190] |
| 8 | Drinks | Coke or coffee | Male Wistar rats | CUS | Caffeine | 4 weeks | Increase in DA and serotonin levels | [191] |
| 9 | Drinks | Yogurt or beer | Male adult FSL and FRL rats | FSL | Probiotics | 9-week period | Normalization of microbiome function | [192] |
| 10 | Vitamins | Vitamin B | C57BL/6 mice of either sex | CMS | VB12 | 10 weeks | Reversion of NTRK-2 gene expression | [193] |
| 11 | Vitamins | Vitamin B | Male SD rats | CUMS | Folic acid | 6 weeks | Increase in monoamine neurotransmitter levels, BDNF, β-endorphins, and interleukin 6 | [194] |
| 12 | Vitamins | Vitamin C | Adult Swiss mice of either sex | FST, TST | Ascorbic acid | 1 day | Interaction of ascorbic acid with monoaminergic system | [195] |
| 13 | Vitamins | Vitamin D | Male C57BL/6 mice | PSD | VD3 | 4 weeks | Upregulation of the expression of BDNF in the HP | [196] |
| 14 | Vitamins | Vitamin D | Adult female Wistar rats | CUMS and OVX | VD3 | 28 days and 3 months | Reduction in serum corticosterone/ACTH levels; the increase in BDNF and NT-3/NT-4 levels | [197] |
| 15 | Homology of medicine and food | Ginseng | Male ICR mice and Wistar rats | CUMS | Ginsenoside Rb1 | 21 days | Activation of 5-HT, NE, and DA systems | [198] |
| 16 | Homology of medicine and food | Curcumin | Male SD rats | Chronically Stressed Rats | Diarylheptanoid component | 18 days | Upregulation of BDNF and decreased brain inflammatory factors | [199] |
| 17 | Homology of medicine and food | Piperine | Male ICR mice | CUMS | Major alkaloids of black pepper and long pepper | 3 weeks | Enhancement of 5-HT content and BDNF protein expression | [200,201] |
| 18 | Homology of medicine and food | Saffron | Male ICR mice | Chronic constraint pressure, TST | Saffron extract | 28 days | Increase in DA and CREB serum levels | [202] |
| 19 | Homology of medicine and food | Schisandra Chinensis | Male Kunming mice | FST | SCE | 4 consecutive days | Effects on NE, DA, GABA, and Glu systems | [203] |
| NO. | Food Effective Constituent | Molecular Formula | Structural Formula | Source | Putative Antidepressant Mechanisms | References |
|---|---|---|---|---|---|---|
| 1 | Quercetin | C15H10O7 |  | A variety of vegetables and fruits, such as apple peel and citrus fruits | Improvement of HPA axis dysfunction and reduction in inflammation | [204] |
| 2 | Eicosapentaenoic (EPA) and docosahexaenoic (DHA) | C20H30O2 | EPA | Fish oil | Increase in serotonergic neurotransmission | [205,206,207] |
 | ||||||
| C22H32O2 | DHA | |||||
 | ||||||
| 3 | Caffeine | C8H10N4O2 | 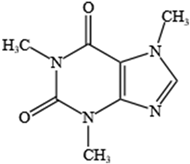 | Coffee, tea, chocolate, and caffeinated beverages | Enhancement of extracellular levels of DA and NE | [208,209,210] |
| 4 | Vitamin B | C12H17ClN4OS | Vitamin B1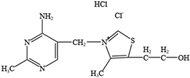 | Raw and fermented fish sauce and certain vegetables consumed primarily in Africa and Asia | Regulation of oxidative stress and inflammation | [211,212] |
| 5 | Vitamin B | C17H20N4O6 | Vitamin B2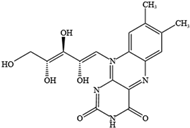 | Meat, milk, fish, nuts, eggs, vegetables, and some fruits | Regulation of oxidative stress and inflammation | [213,214] |
| 6 | Vitamin C | C6H8O6 |  | Fruits, vegetables, potatoes, and soft drinks (fruit juices) | Regulation of monoamine and glutamate neurotransmitter systems | [215,216] |
| 7 | Vitamin D | Vitamin D2 | Vitamin D2 | Many products of animal origin, such as deep-sea fish and animal liver | Regulation of monoamine and glutamate neurotransmitter systems | [217,218,219] |
| C28H44O |  | |||||
| Vitamin D3 | Vitamin D3 | |||||
| C27H44O |  | |||||
| 8 | Ginsenoside Rb1 | C54H92O23 |  | Ginseng | Inhibition of oxidation, apoptosis, inflammation, and autophagy | [220,221,222] |
| 9 | Curcumin | C21H20O6 |  | Turmeric plant | Increase in monoamines and BDNF levels and inhibition of pro-inflammatory factors and neuronal apoptosis in the brain | [223,224] |
| 10 | Piperine | C17H19NO3 |  | Pepper | Regulation of oxidative stress and inflammation | [225,226] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, J.; Wang, Y.; Zhang, T.; Zhao, J.; Lv, Q.; Ruan, M.; Yu, Q.; Li, B. Antidepressant-like Effects of Representative Types of Food and Their Possible Mechanisms. Molecules 2023, 28, 6992. https://doi.org/10.3390/molecules28196992
Piao J, Wang Y, Zhang T, Zhao J, Lv Q, Ruan M, Yu Q, Li B. Antidepressant-like Effects of Representative Types of Food and Their Possible Mechanisms. Molecules. 2023; 28(19):6992. https://doi.org/10.3390/molecules28196992
Chicago/Turabian StylePiao, Jingjing, Yingwei Wang, Tianqi Zhang, Jiayu Zhao, Qianyu Lv, Mengyu Ruan, Qin Yu, and Bingjin Li. 2023. "Antidepressant-like Effects of Representative Types of Food and Their Possible Mechanisms" Molecules 28, no. 19: 6992. https://doi.org/10.3390/molecules28196992
APA StylePiao, J., Wang, Y., Zhang, T., Zhao, J., Lv, Q., Ruan, M., Yu, Q., & Li, B. (2023). Antidepressant-like Effects of Representative Types of Food and Their Possible Mechanisms. Molecules, 28(19), 6992. https://doi.org/10.3390/molecules28196992





