High Rate Performance Supercapacitors Based on N, O Co-Doped Hierarchical Porous Carbon Foams Synthesized via Chemical Blowing and Dual Templates
Abstract
:1. Introduction
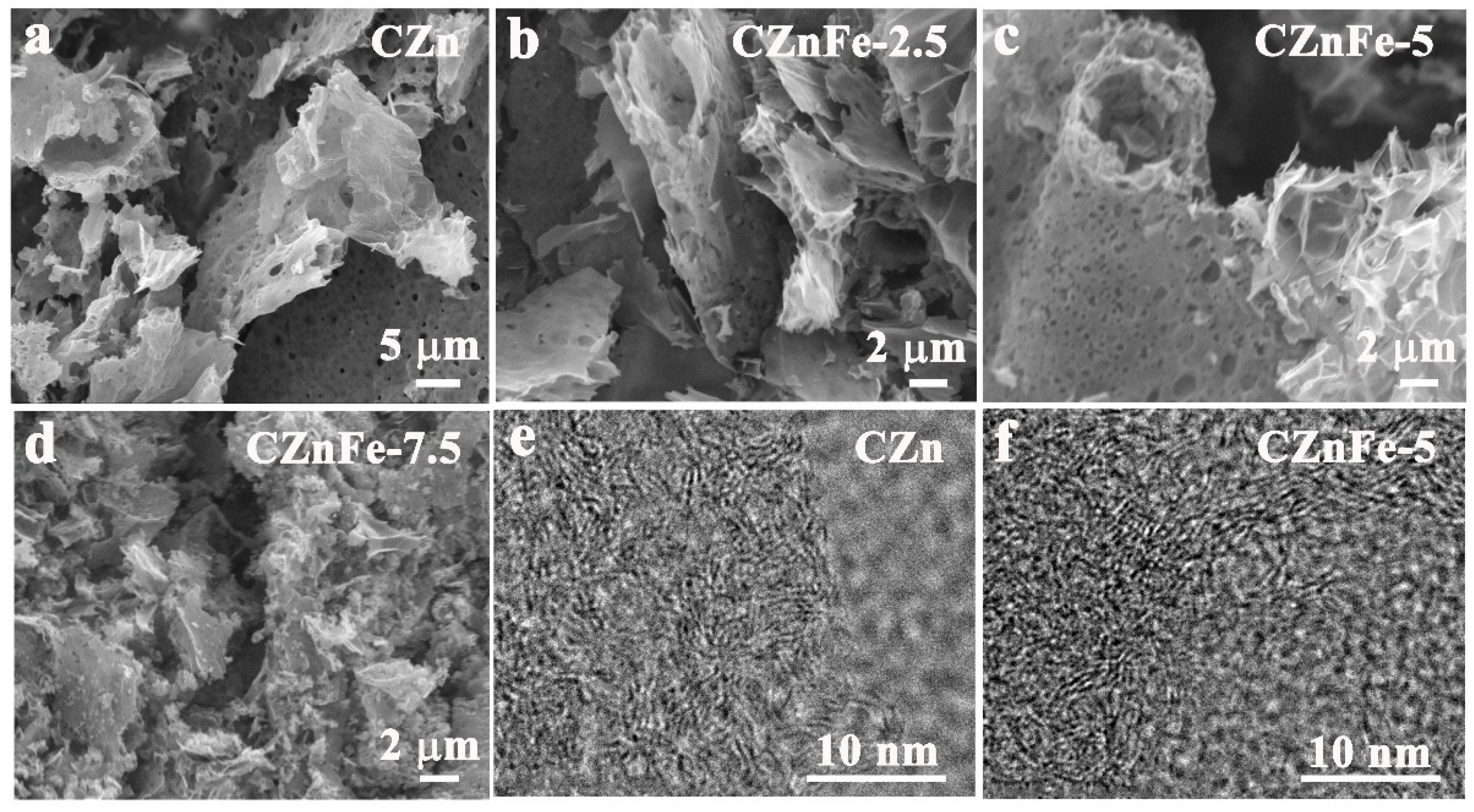
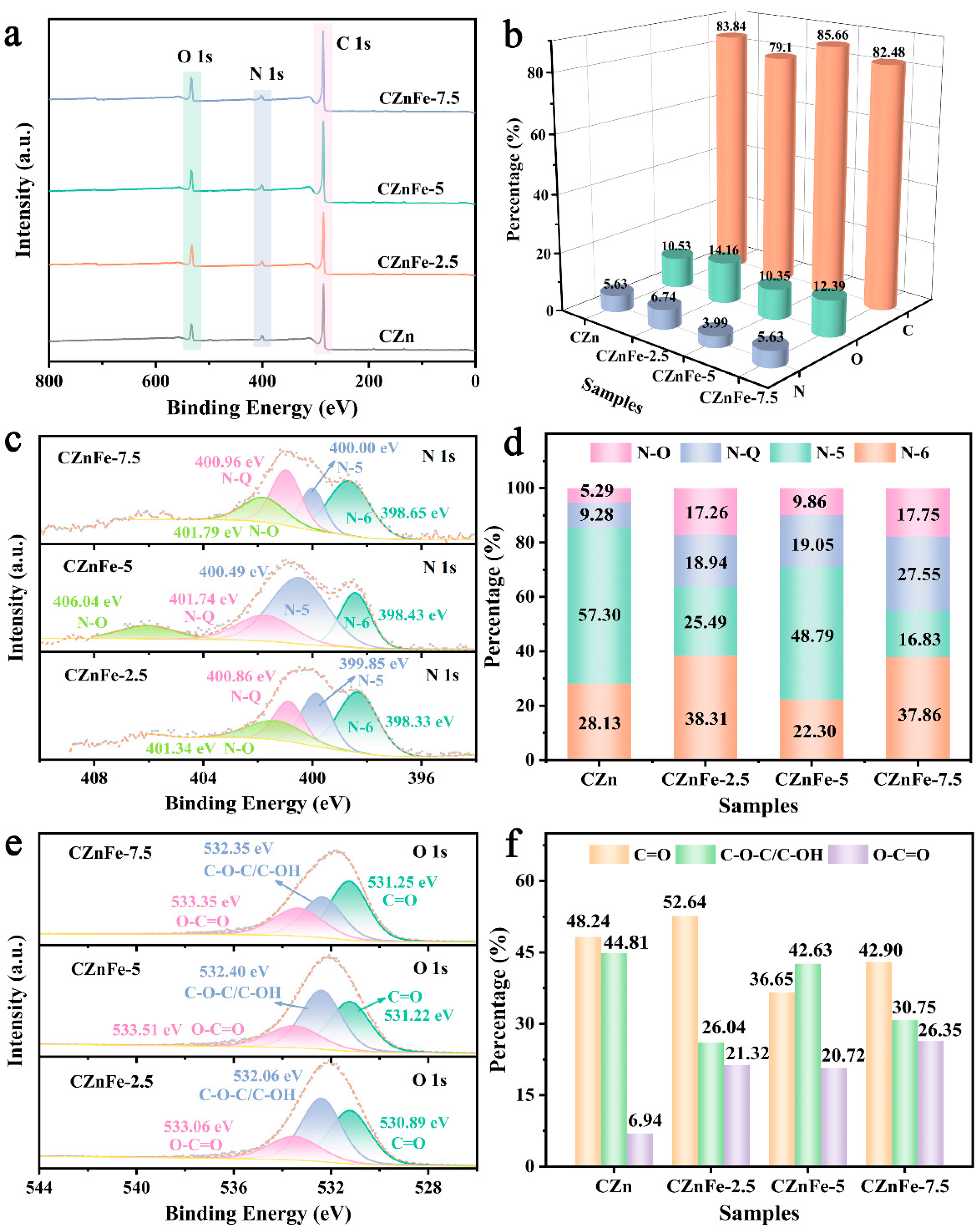
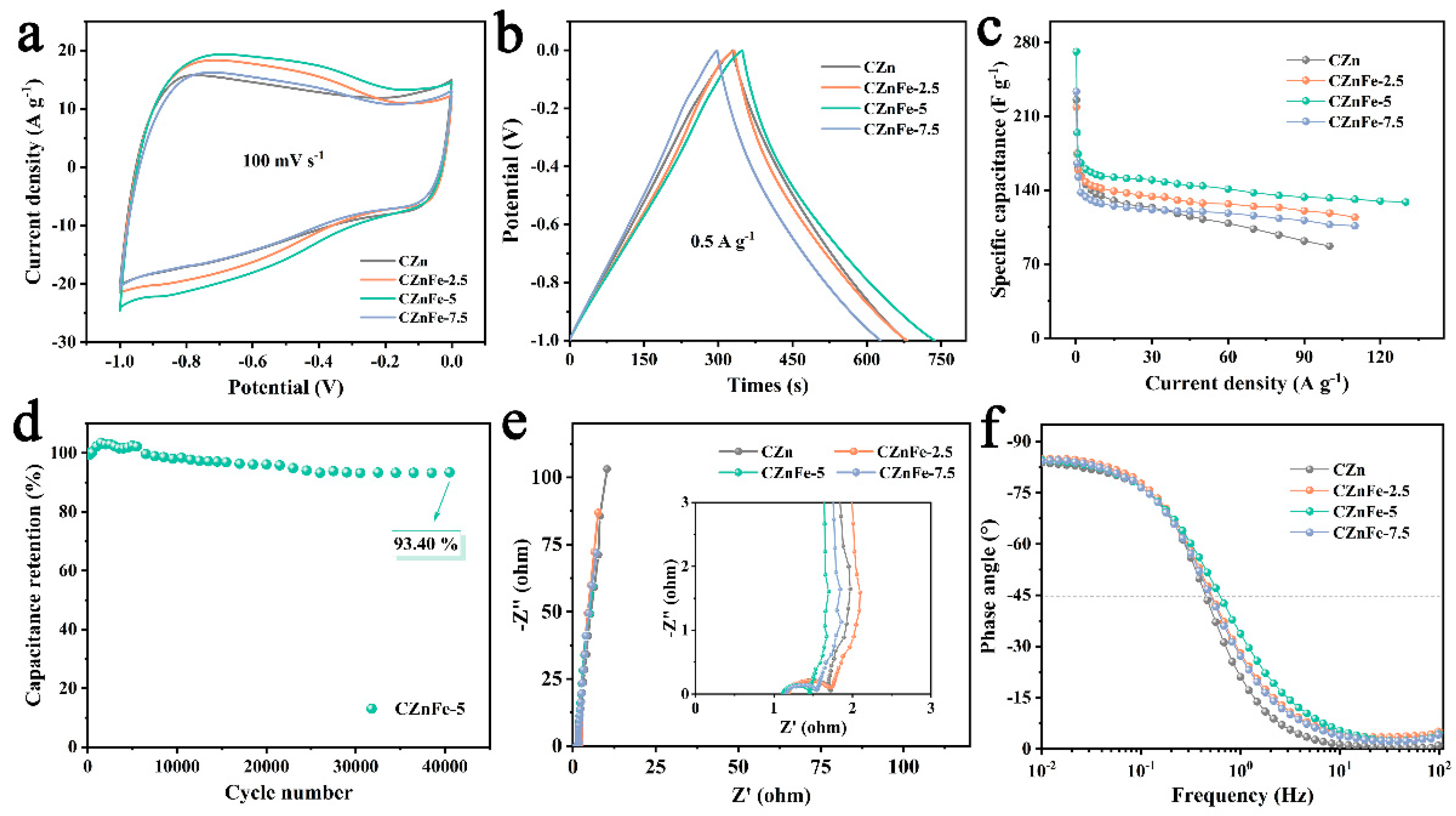
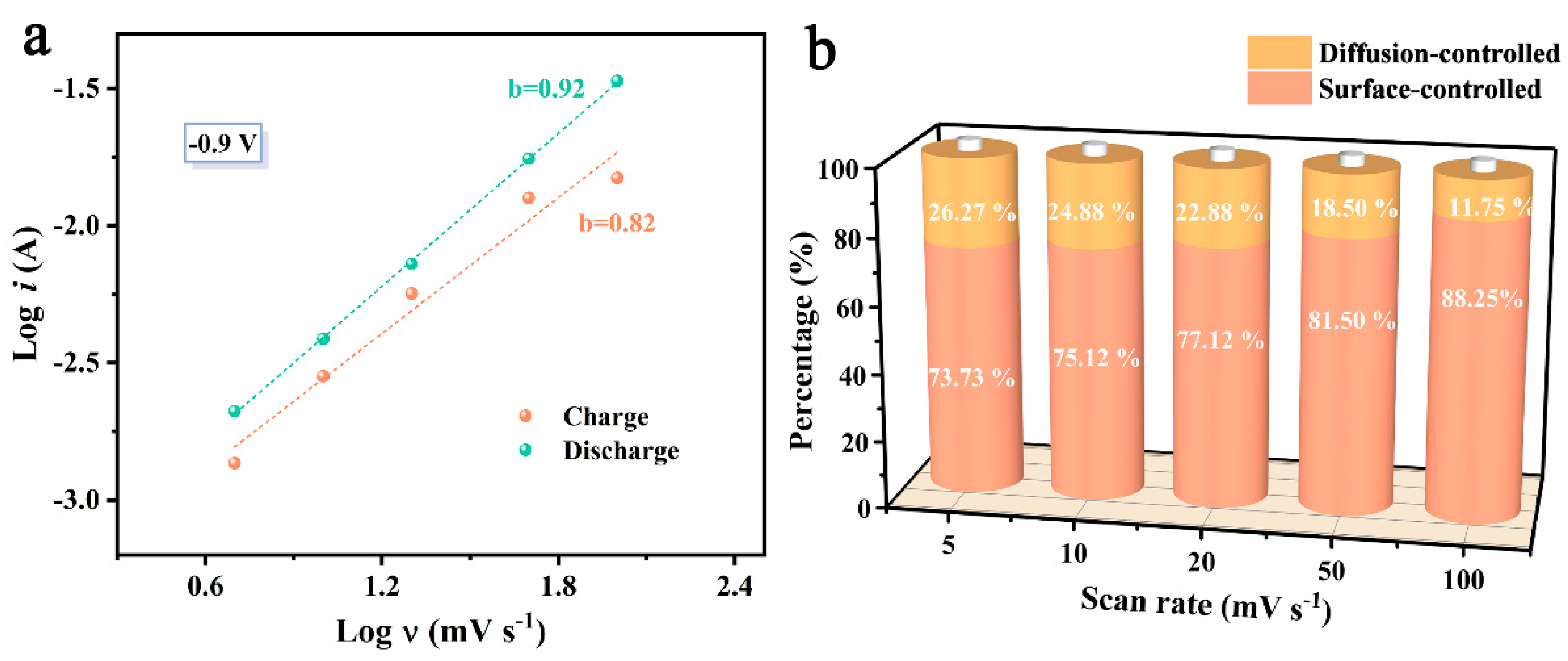
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
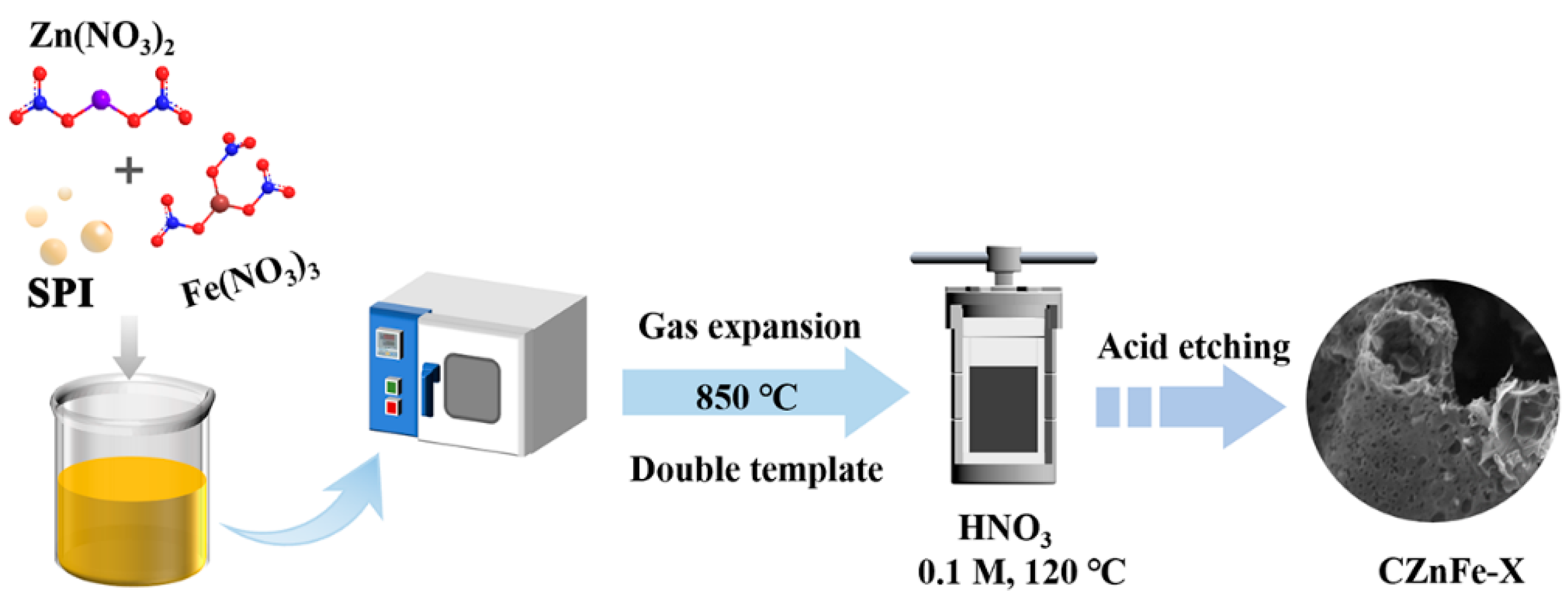
3.2. Synthesis of CZnFe-X Hierarchical Porous Carbon
3.3. Characterization Technique
3.4. Electrochemical Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, X.; Han, R.; Liu, Y.; Li, H.; Shi, W.; Yan, X.; Zhao, X.; Li, Y.; Liu, B. Porous and graphitic structure optimization of biomass-based carbon materials from 0D to 3D for supercapacitors: A review. Chem. Eng. J. 2023, 460, 141607. [Google Scholar]
- Lenders, J.; Cook, J.; Liang, D.; Mostaghaci, B.; Perets, E.; Shi, L.; Levy, E. Opening the Doors to Advanced Materials in 2022. Adv. Mater. 2022, 34, 2109845. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, L.; Koh, S.W.; Ge, J.; Fei, J.; Yao, M.; Hong, W.; Liu, S.; Yamauchi, Y.; Li, H. Photovoltaic-powered supercapacitors for driving overall water splitting: A dual-modulated 3D architecture. Carbon Energy 2022, 4, 1262–1273. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, J.; Zou, Z.; Li, J.; Wu, C.; Jiang, Y.; Chen, Y.; Zeng, Q.; Wu, X.; Sun, W.; et al. Labyrinth maze-like long travel-reduction of sulfur and polysulfides in micropores of a spherical honeycomb carbon to greatly confine shuttle effects in lithium-sulfur batteries. Mater. Rep. Energy 2022, 2, 100159. [Google Scholar] [CrossRef]
- Deng, W.; Liu, W.; Zhu, H.; Chen, L.; Liao, H.; Chen, H. Click-chemistry and ionic cross-linking induced double cross-linking ionogel electrolyte for flexible lithium-ion batteries. J. Energy Storage 2023, 72, 108509. [Google Scholar] [CrossRef]
- Zhao, X.; He, D.; You, B. Laser engraving and punching of graphene films as flexible all-solid-state planar micro-supercapacitor electrodes. Mater. Today Sustain. 2022, 17, 100096. [Google Scholar]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar]
- Yan, B.; Zhao, W.; Zhang, Q.; Kong, Q.; Chen, G.; Zhang, C.; Han, J.; Jiang, S.; He, S. One stone for four birds: A “chemical blowing” strategy to synthesis wood-derived carbon monoliths for high-mass loading capacitive energy storage in low temperature. J. Colloid Interface Sci. 2023, 653, 1526–1538. [Google Scholar]
- Wu, Y.; Xu, G.; Zhang, W.; Song, C.; Wang, L.; Fang, X.; Xu, L.; Han, S.; Cui, J.; Gan, L. Construction of ZIF@electrospun cellulose nanofiber derived N doped metallic cobalt embedded carbon nanofiber composite as binder-free supercapacitance electrode. Carbohydr. Polym. 2021, 267, 118166. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Feng, X.; Ma, H.; Li, X.; Zhou, J. Sustainable Lignin-Derived Hierarchical Porous Carbon for Supercapacitors: A Novel Approach for Holding Electrochemical Attraction Natural Texture Property of Precursor. ACS Omega 2021, 6, 33171–33179. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Li, Y.; Liu, X.; Xia, Z.; Yang, Y. A facile preparation of polyaniline/cellulose hydrogels for all-in-one flexible supercapacitor with remarkable enhanced performance. Carbohydr. Polym. 2020, 245, 116611. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zheng, J.; Feng, L.; Zhang, Q.; Zhang, C.; Ding, Y.; Han, J.; Jiang, S.; He, S. Pore engineering: Structure-capacitance correlations for biomass-derived porous carbon materials. Mater. Des. 2023, 229, 111904. [Google Scholar]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar]
- Wu, C.W.; Li, P.H.; Wei, Y.M.; Yang, C.; Wu, W.J. Review on the preparation and application of lignin-based carbon aerogels. RSC Adv. 2022, 12, 10755–10765. [Google Scholar] [CrossRef]
- Liu, C.; Hou, Y.; Li, Y.; Xiao, H. Heteroatom-doped porous carbon microspheres derived from ionic liquid-lignin solution for high performance supercapacitors. J. Colloid Interface Sci. 2022, 614, 566–573. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Q.; Li, H.; Wang, J.; Tai, G.; Wang, F.; Han, J.; Zhu, Y.; Wu, G. Waste-to-Resource Strategy to Fabricate Functionalized MOFs Composite Material Based on Durian Shell Biomass Carbon Fiber and Fe3O4 for Highly Efficient and Recyclable Dye Adsorption. Int. J. Mol. Sci. 2022, 23, 5900. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Cao, M.; Yang, L.; Yao, J. Direct Coating Pen Ink Carbon on a Carbonized Melamine Sponge as a Flexible Free-Standing Electrode. Ind. Eng. Chem. Res. 2021, 60, 3597–3604. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Qing, Y.; Liao, Y.; Liu, j.; Tian, C.; Xu, H.; Wu, Y. Research progress of wood-derived energy storage materials. J. For. Eng. 2021, 6, 1–13. [Google Scholar]
- Wang, R.; Zhou, X.; Xu, T.; Bian, H.; Dai, H. Research progress on the preparation of lignin-derived carbon dots and graphene quantum dots. J. For. Eng. 2021, 6, 29–37. [Google Scholar]
- Li, P.H.; Wei, Y.M.; Wu, C.W.; Yang, C.; Jiang, B.; Wu, W.J. Lignin-based composites for high-performance supercapacitor electrode materials. RSC Adv. 2022, 12, 19485–19494. [Google Scholar]
- Bassey, E.; Yang, L.; Cao, M.; Feng, Y.; Yao, J. Molten salt synthesis of capacitive porous carbon from Allium cepa (onion) for supercapacitor application. J. Electroanal. Chem. 2021, 881, 114972. [Google Scholar] [CrossRef]
- Yangyang, C.; Qingtong, Z.; Mingchao, C.H.I.; Chenyan, G.U.O.; Shuangfei, W.; Douyong, M.I.N. Preparation and performance of different carbonized wood electrodes. J. For. Eng. 2022, 7, 127–135. [Google Scholar]
- Zuo, S.; Zhang, W.; Wang, Y.; Xia, H. Low-Cost Preparation of High-Surface-Area Nitrogen-Containing Activated Carbons from Biomass-Based Chars by Ammonia Activation. Ind. Eng. Chem. Res. 2020, 59, 7527–7537. [Google Scholar] [CrossRef]
- Feng, L.; Yan, B.; Zheng, J.; Chen, J.; Wei, R.; Jiang, S.; Yang, W.; Zhang, Q.; He, S. Soybean protein-derived N, O co-doped porous carbon sheets for supercapacitor applications. New J. Chem. 2022, 46, 10844–10853. [Google Scholar]
- Feng, L.; Yan, B.; Zheng, J.; Zhang, Q.; Wei, R.; Zhang, C.; Han, J.; Jiang, S.; He, S. Chemical foaming-assisted synthesis of N, O co-doped hierarchical porous carbon from soybean protein for high rate performance supercapacitors. Diam. Relat. Mater. 2023, 133, 109767. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Wang, F.; Zhao, L.; Zhang, Q.; Xu, W.; He, S. Review on porous carbon materials engineered by ZnO templates: Design, synthesis and capacitance performance. Mater. Des. 2021, 201, 109518. [Google Scholar]
- Tang, L.; Mao, Q.; You, Z.; Yao, Z.; Zhu, X.; Zhong, Q.; Xiao, J. Catalytic graphitization in anthracite by reduced iron particles and investigating the mechanism of catalytic transformation via molecular dynamics. Carbon 2022, 188, 336–348. [Google Scholar]
- Guo, Y.; Wang, T.; Chen, X.; Wu, D. Agar-based porous electrode and electrolyte for flexible symmetric supercapacitors with ultrahigh energy density. J. Power Sources 2021, 507, 230252. [Google Scholar]
- Kim, M.; Lim, H.; Xu, X.; Hossain, M.S.A.; Na, J.; Awaludin, N.N.; Shah, J.; Shrestha, L.K.; Ariga, K.; Nanjundan, A.K.; et al. Sorghum biomass-derived porous carbon electrodes for capacitive deionization and energy storage. Microporous Mesoporous Mater. 2021, 312, 110757. [Google Scholar] [CrossRef]
- Kong, L.; Su, L.; Hao, S.; Yang, W.; Shao, G.; Qin, X. Graphene-like nitrogen-doped porous carbon nanosheets as both cathode and anode for high energy density lithium-ion capacitor. Electrochim. Acta 2020, 349, 136303. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Zhang, H.; Jin, L.; Chu, X.; Gu, B.; Huang, H.; Yang, W. Nitrogen, oxygen and sulfur co-doped hierarchical porous carbons toward high-performance supercapacitors by direct pyrolysis of kraft lignin. Carbon 2019, 149, 105–116. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Y.; Chen, Y.; Tan, F.; Cao, Y.; Cai, W. Hierarchically porous carbon derived from renewable Chingma Abutilon Seeds for high-energy supercapacitors. Adv. Powder Technol. 2021, 32, 718–727. [Google Scholar] [CrossRef]
- Luo, X.; Li, S.; Xu, H.; Zou, X.; Wang, Y.; Cheng, J.; Li, X.; Shen, Z.; Wang, Y.; Cui, L. Hierarchically porous carbon derived from potassium-citrate-loaded poplar catkin for high performance supercapacitors. J. Colloid Interface Sci. 2021, 582, 940–949. [Google Scholar] [PubMed]
- Wang, Q.; Qin, B.; Li, H.-X.; Zhang, X.-H.; Tian, X.; Jin, L.e.; Cao, Q. Honeycomb-like carbon with tunable pore size from bio-oil for supercapacitor. Microporous Mesoporous Mater. 2020, 309, 110551. [Google Scholar] [CrossRef]
- Pokharel, J.; Gurung, A.; Baniya, A.; He, W.; Chen, K.; Pathak, R.; Lamsal, B.S.; Ghimire, N.; Zhou, Y. MOF-derived hierarchical carbon network as an extremely-high-performance supercapacitor electrode. Electrochim. Acta 2021, 394, 139058. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, S.; Xu, X.; Zhu, J. In-situ template formation method to synthesize hierarchically porous carbon for electrocatalytic reduction of 4-nitrophenol. Carbon 2021, 184, 596–608. [Google Scholar]
- Yang, J.; Zuo, S. Facile synthesis of graphitic mesoporous carbon materials from sucrose. Diam. Relat. Mater. 2019, 95, 1–4. [Google Scholar] [CrossRef]
- Pang, Z.; Li, G.; Zou, X.; Sun, C.; Hu, C.; Tang, W.; Ji, L.; Hsu, H.-Y.; Xu, Q.; Lu, X. An integrated strategy towards the facile synthesis of core-shell SiC-derived carbon@N-doped carbon for high-performance supercapacitors. J. Energy Chem. 2021, 56, 512–521. [Google Scholar] [CrossRef]
- Liu, K.; Yu, C.; Xie, Y.; Guo, W.; Yu, J.; Ni, L.; Wang, Z.; Fu, R.; Qiu, J. Correlation between self-discharge behavior and heteroatoms over doped carbon sheets for enhanced pseudocapacitance. J. Energy Chem. 2022, 72, 291–298. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Lee, J.; An, G.-H. Surface Engineering of Carbon via Coupled Porosity Tuning and Heteroatom-Doping for High-Performance Flexible Fibrous Supercapacitors. Adv. Funct. Mater. 2021, 31, 2104256. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Qian, F.; Qing, Z.; Xie, X.; Song, Y. Ferric nitrate/dopamine/melamine-derived nitrogen doped carbon material as the activator of peroxymonosulfate to degrade sulfamethoxazole. Sep. Purif. Technol. 2022, 281, 119844. [Google Scholar] [CrossRef]
- Li, D.; Gao, J.; Du, Q.; Zhao, Z.; Dong, H.; Cui, Z. Influence of an iron compound added to coal on soot formation. Energy 2023, 266, 126259. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Yang, C.; Wu, S.; Fu, X.; Li, X. Calcination temperature regulates non-radical pathways of peroxymonosulfate activation via carbon catalysts doped by iron and nitrogen. Chem. Eng. J. 2023, 451, 138468. [Google Scholar]
- Ran, F.; Yang, X.; Xu, X.; Li, S.; Liu, Y.; Shao, L. Green activation of sustainable resources to synthesize nitrogen-doped oxygen-riched porous carbon nanosheets towards high-performance supercapacitor. Chem. Eng. J. 2021, 412, 128673. [Google Scholar]
- Li, D.; Guo, Y.; Li, Y.; Liu, Z.; Chen, Z. Waste-biomass tar functionalized carbon spheres with N/P Co-doping and hierarchical pores as sustainable low-cost energy storage materials. Renew. Energy 2022, 188, 61–69. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhao, J.; Zhang, X.; Zhang, C.; Zhang, G.; Liu, Q.; Duan, H. Enhancement of charge transport in porous carbon nanofiber networks via ZIF-8-enabled welding for flexible supercapacitors. Chem. Eng. J. 2020, 382, 122979. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar]
- Li, Y.; Zhou, M.; Xia, Z.; Gong, Q.; Liu, X.; Yang, Y.; Gao, Q. Facile preparation of polyaniline covalently grafted to isocyanate functionalized reduced graphene oxide nanocomposite for high performance flexible supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125172. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, D.; Yang, K.; Li, Z.; Hu, Y.; Xia, S.; Chen, W.; Zhou, X. An energy-efficient method inspired by nutrient transport during the growth of tree to prepare free-standing wood/MXene electrodes. Fuel Process. Technol. 2022, 238, 107496. [Google Scholar] [CrossRef]
- Liu, X.; Du, S.; Zuo, X.; Zhang, X.; Jiang, Y. Facile synthesis of Ni(OH)2 nanoarrays on graphene@carbon fabric as dual-functional electrochemical materials for supercapacitors and capacitive desalination. RSC Adv. 2021, 12, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, A.F.M.; Yu, T.C.; Kuo, S.-W. Synthesis of multiple heteroatom–doped mesoporous carbon/silica composites for supercapacitors. Chem. Eng. J. 2021, 414, 128796. [Google Scholar] [CrossRef]
- Leng, C.; Zhao, Z.; Song, Y.; Sun, L.; Fan, Z.; Yang, Y.; Liu, X.; Wang, X.; Qiu, J. 3D Carbon Frameworks for Ultrafast Charge/Discharge Rate Supercapacitors with High Energy-Power Density. Nano Micro Lett. 2020, 13, 8. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Dong, Y.; Ding, Y.; Yang, W.; Han, J.; Jiang, S.; He, S. Nitrogen-doped carbon layer on cellulose derived free-standing carbon paper for high-rate supercapacitors. Appl. Surf. Sci. 2023, 608, 155144. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Zhang, C.; Ding, Y.; Han, J.; Jiang, S.; He, S. In situ growth of N/O-codoped carbon nanotubes in wood-derived thick carbon scaffold to boost the capacitive performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131018. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Pham, H.D.; Nanjundan, A.K.; Fernando, J.F.S.; Jayaramulu, K.; Golberg, D.; Han, Y.-K.; Dubal, D.P. True Meaning of Pseudocapacitors and Their Performance Metrics: Asymmetric versus Hybrid Supercapacitors. Small 2020, 16, 2002806. [Google Scholar]
- Lv, H.; Wang, S.; Xiao, Z.; Qin, C.; Zhai, S.; Wang, G.; Zhao, Z.; An, Q. N-doped hollow carbon spheres with controllable shell numbers for high-performance electrical double-layer capacitors. J. Power Sources 2021, 493, 229679. [Google Scholar] [CrossRef]
- Shang, P.; Liu, M.; Mei, Y.; Liu, Y.; Wu, L.; Dong, Y.; Zhao, Z.; Qiu, J. Urea-Mediated Monoliths Made of Nitrogen-Enriched Mesoporous Carbon Nanosheets for High-Performance Aqueous Zinc Ion Hybrid Capacitors. Small 2022, 18, 2108057. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, H.; Duan, G.; He, S.; Zhang, L.; Zhang, G.; Zhou, Z.; Jiang, S. N-doped honeycomb-like porous carbon towards high-performance supercapacitor. Chin. Chem. Lett. 2020, 31, 1986–1990. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Shen, F.; Tian, D.; Huang, M.; He, J.; Zou, J.; Zhao, L.; Zeng, Y. Trichoderma bridges waste biomass and ultra-high specific surface area carbon to achieve a high-performance supercapacitor. J. Power Sources 2021, 497, 229880. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, B.; Wang, Q.; Zhao, X.; Wu, D.; Wu, H.; Sun, X.; Hou, C.; Yang, X.; Yu, R.; et al. Efficient microwave absorber and supercapacitors derived from puffed-rice-based biomass carbon: Effects of activating temperature. J. Colloid Interface Sci. 2021, 594, 290–303. [Google Scholar] [CrossRef]
- Li, J.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L.; Li, B.; Zhang, J. Osmanthus fragrans-derived N-doped porous carbon for supercapacitor applications. J. Energy Storage 2021, 42, 103017. [Google Scholar] [CrossRef]
- Lei, X.; Pan, F.; Hua, C.; Wang, S.; Xiong, B.; Liu, Y.; Fu, Z.; Xiang, B.; Lu, Y. Oxide-doped hierarchically porous carbon for high-performance supercapacitor. J. Alloys Compd. 2022, 901, 163624. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, Z.; He, L.; Li, H. Three-dimensional high graphitic porous biomass carbon from dandelion flower activated by K2FeO4 for supercapacitor electrode. J. Energy Storage 2022, 52, 104889. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, C.; Zhang, S.; Yu, S.; Huang, L. Self-assembly of biomass-based hybrid hydrogel electrode for an additive-free flexible supercapacitor. J. Mater. Chem. A 2022, 10, 16853–16865. [Google Scholar] [CrossRef]
- He, L.; Pan, L.; Zhou, W.; Niu, Z.; Chen, X.; Chen, M.; Zhang, Q.; Pan, W.; Xiao, P.; Li, Y. Thermal corrosion behavior of Yb4Hf3O12 ceramics exposed to calcium-ferrum-alumina-silicate (CFAS) at 1400 °C. J. Eur. Ceram. Soc. 2023, 43, 4114–4123. [Google Scholar] [CrossRef]
- Pan, L.; He, L.; Niu, Z.; Xiao, P.; Zhou, W.; Li, Y. Corrosion behavior of ytterbium hafnate exposed to water-vapor with Al(OH)3 impurities. J. Eur. Ceram. Soc. 2023, 43, 612–620. [Google Scholar] [CrossRef]
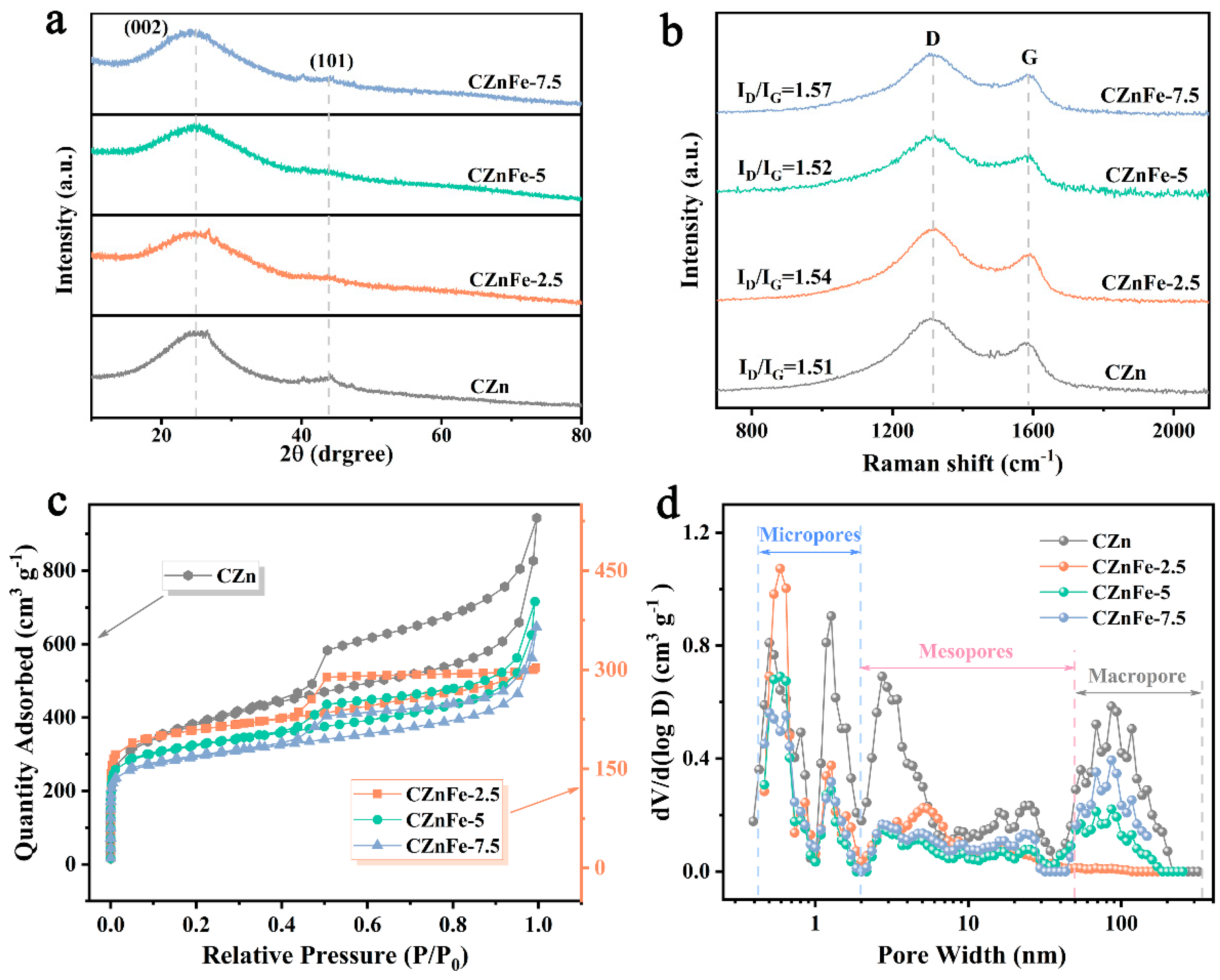

| Sample | SBET (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) | Da (nm) | Vt (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Vmicro /Vt | Vmeso /Vt |
|---|---|---|---|---|---|---|---|---|---|
| CZn | 1350.34 | 491.66 | 580.53 | 2.99 | 1.01 | 0.21 | 0.64 | 0.21 | 0.63 |
| CZnFe-2.5 | 775.43 | 538.25 | 171.11 | 2.32 | 0.45 | 0.22 | 0.19 | 0.49 | 0.42 |
| CZnFe-5 | 616.46 | 377.04 | 169.46 | 2.64 | 0.41 | 0.16 | 0.21 | 0.39 | 0.51 |
| CZnFe-7.5 | 679.70 | 417.74 | 184.41 | 2.69 | 0.46 | 0.17 | 0.23 | 0.37 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Feng, L.; Liu, Z.; Jiang, L.; Lan, T.; Zhang, C.; Liu, K.; He, S. High Rate Performance Supercapacitors Based on N, O Co-Doped Hierarchical Porous Carbon Foams Synthesized via Chemical Blowing and Dual Templates. Molecules 2023, 28, 6994. https://doi.org/10.3390/molecules28196994
Zhang Q, Feng L, Liu Z, Jiang L, Lan T, Zhang C, Liu K, He S. High Rate Performance Supercapacitors Based on N, O Co-Doped Hierarchical Porous Carbon Foams Synthesized via Chemical Blowing and Dual Templates. Molecules. 2023; 28(19):6994. https://doi.org/10.3390/molecules28196994
Chicago/Turabian StyleZhang, Qian, Li Feng, Zhenlu Liu, Longjun Jiang, Tiancheng Lan, Chunmei Zhang, Kunming Liu, and Shuijian He. 2023. "High Rate Performance Supercapacitors Based on N, O Co-Doped Hierarchical Porous Carbon Foams Synthesized via Chemical Blowing and Dual Templates" Molecules 28, no. 19: 6994. https://doi.org/10.3390/molecules28196994
APA StyleZhang, Q., Feng, L., Liu, Z., Jiang, L., Lan, T., Zhang, C., Liu, K., & He, S. (2023). High Rate Performance Supercapacitors Based on N, O Co-Doped Hierarchical Porous Carbon Foams Synthesized via Chemical Blowing and Dual Templates. Molecules, 28(19), 6994. https://doi.org/10.3390/molecules28196994









