Synthesis of Triblock Polycarboxylate Superplasticizers with Well-Defined Structure and Its Dispersing Performance in β-Hemihydrate Gypsum
Abstract
:1. Introduction
2. Results
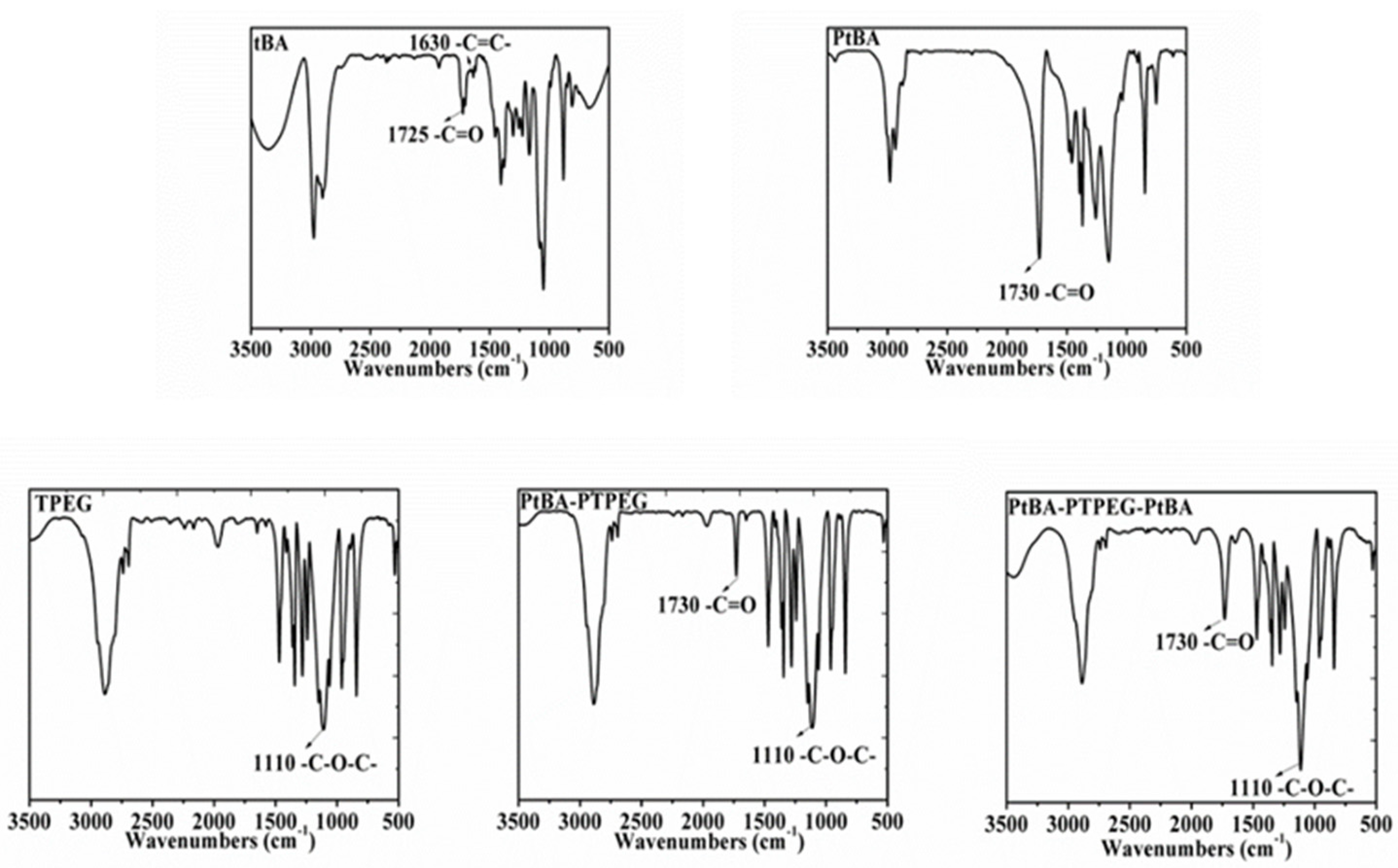
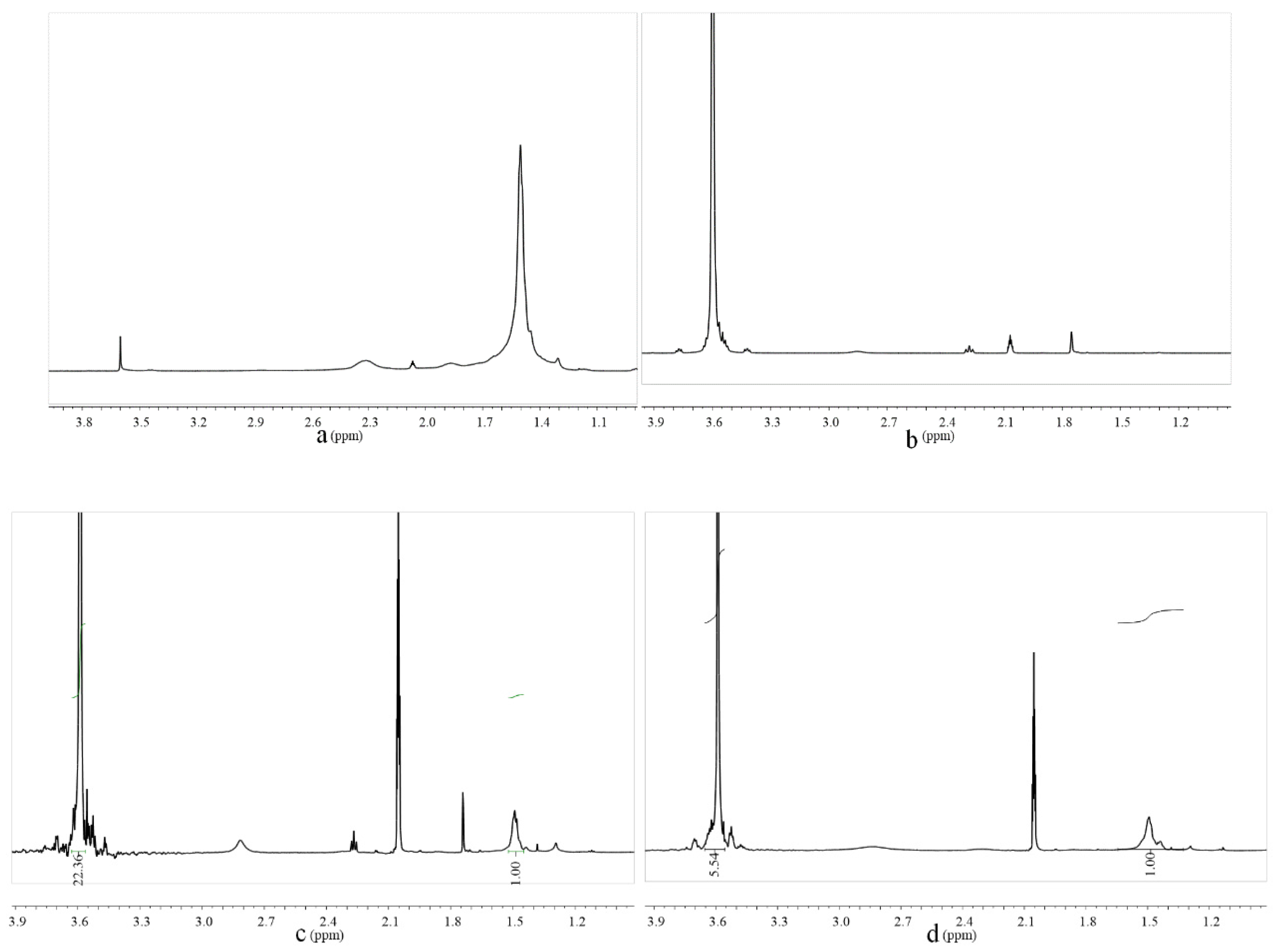
2.1. Structure Characterization of PCEs
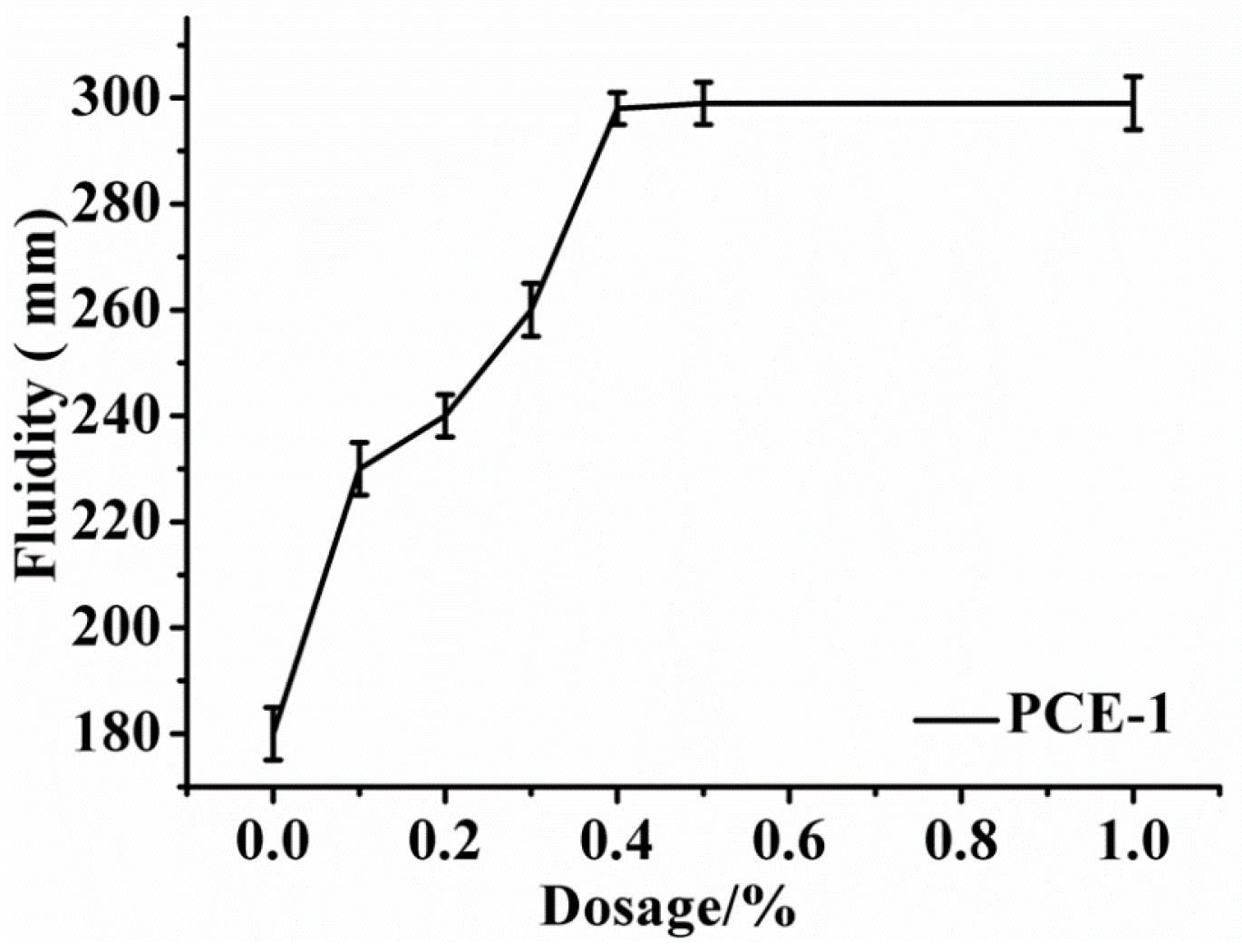
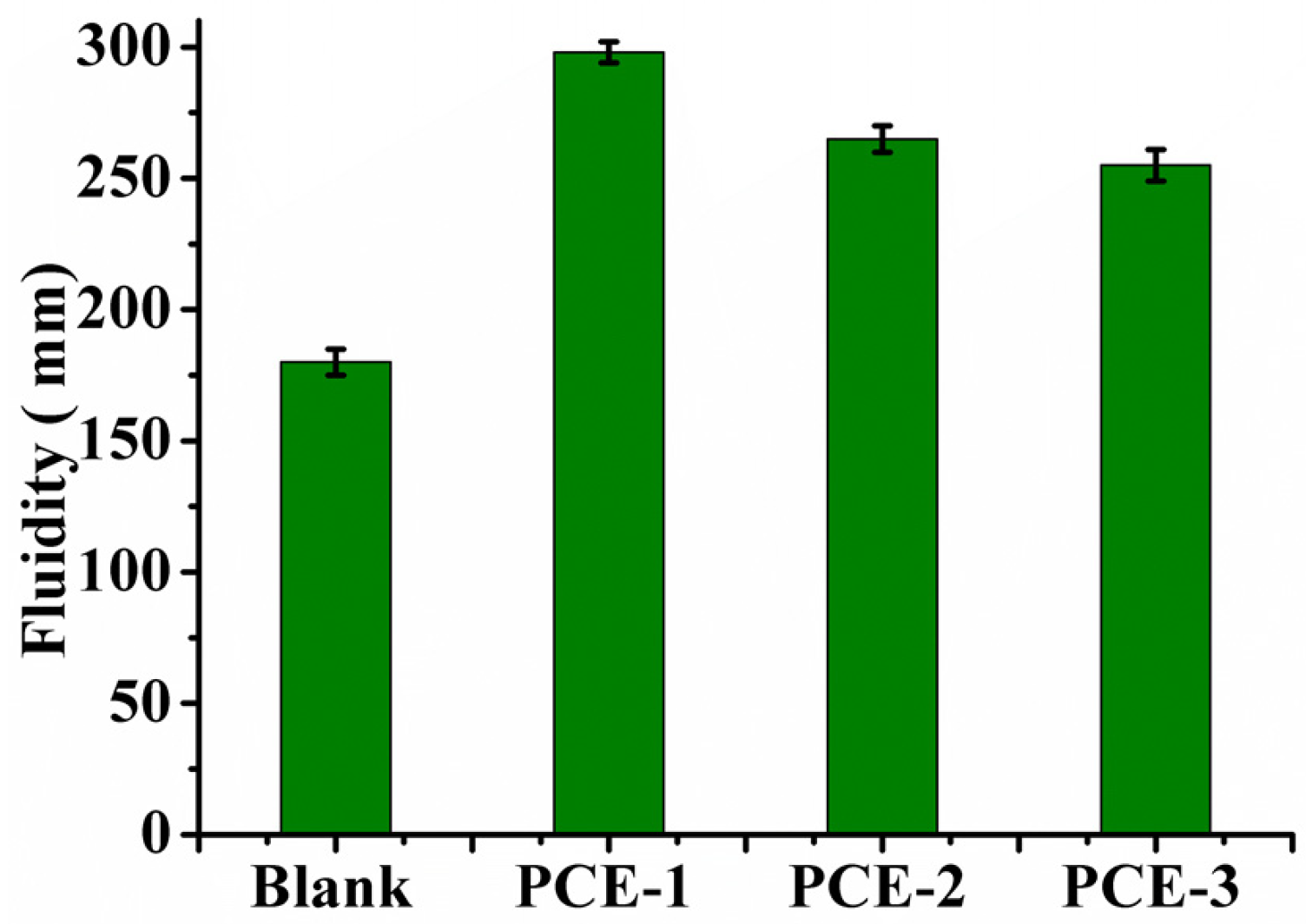
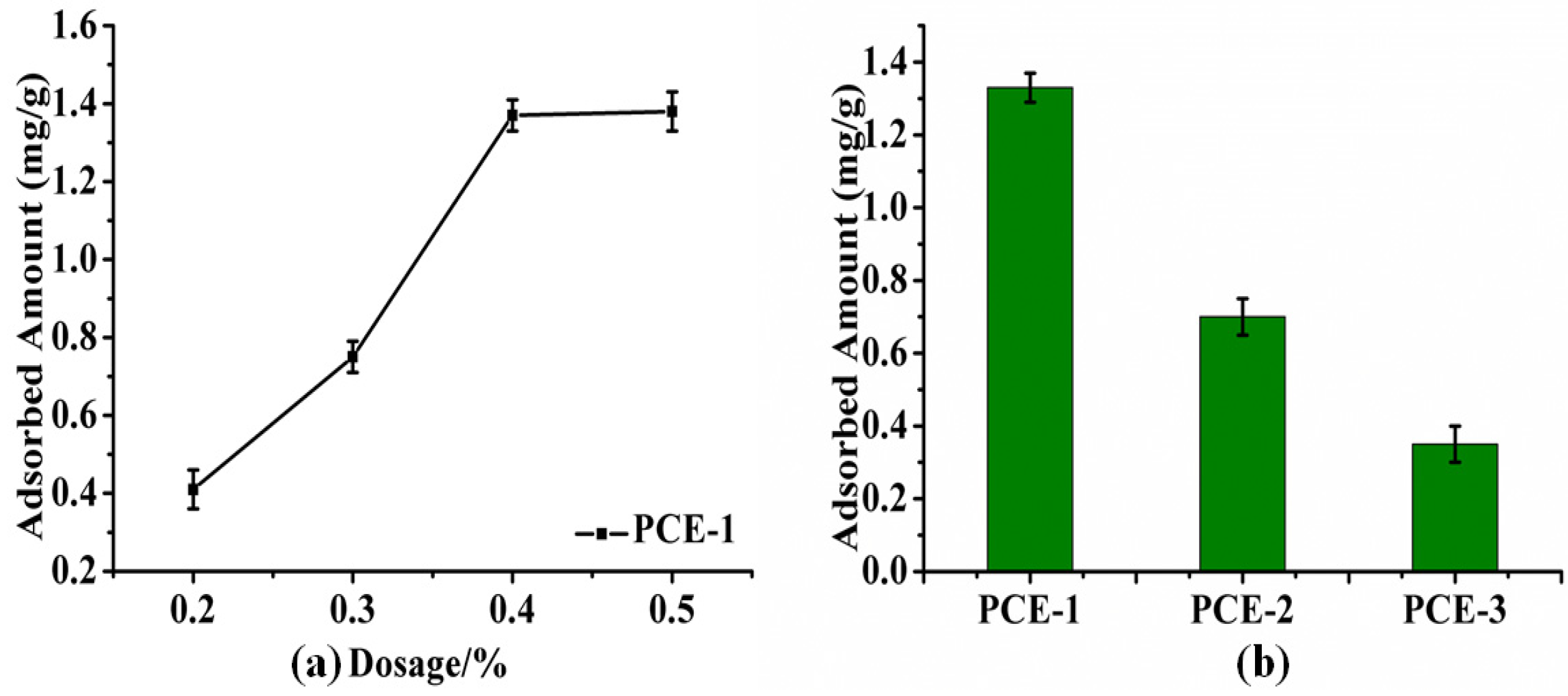
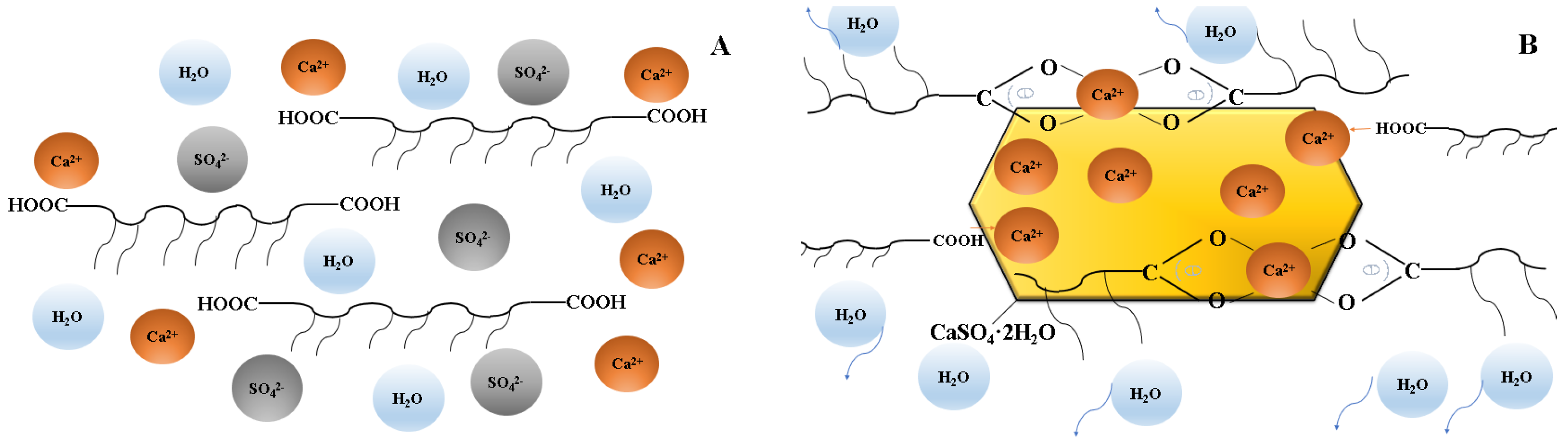
2.2. Dispersion Performance of PCEs
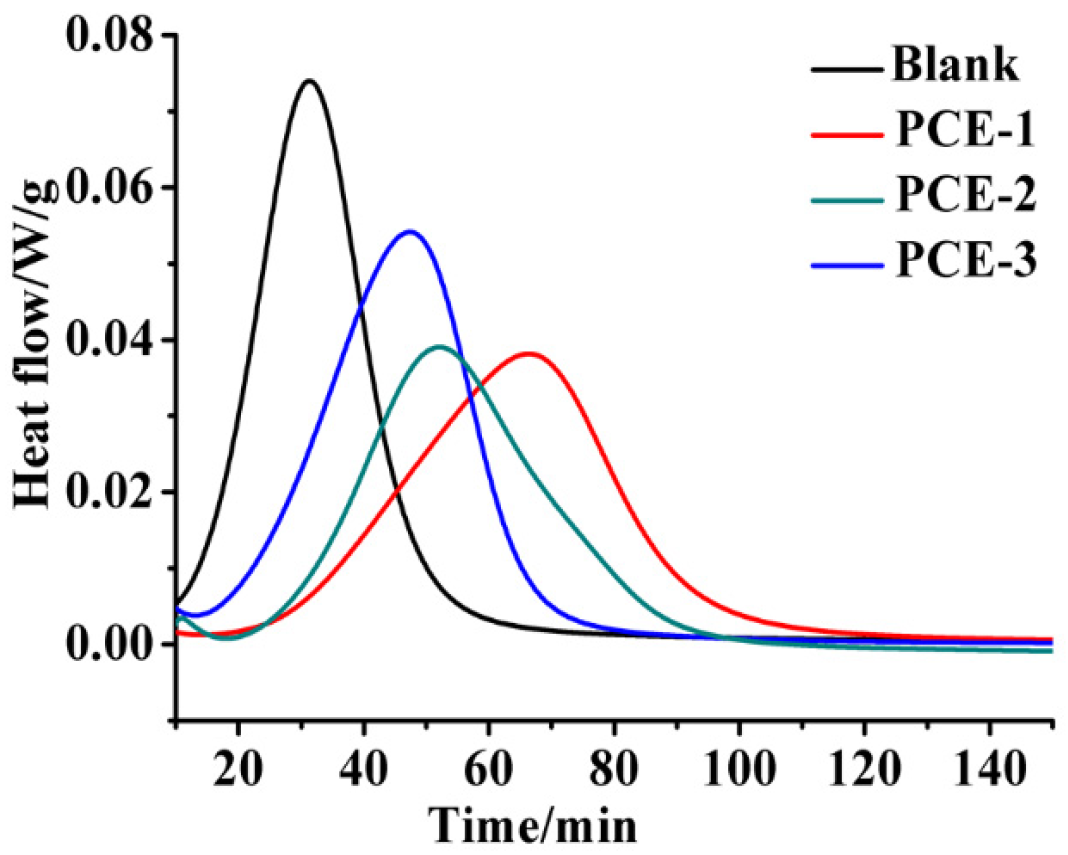
2.3. Heat Flow Calorimetry
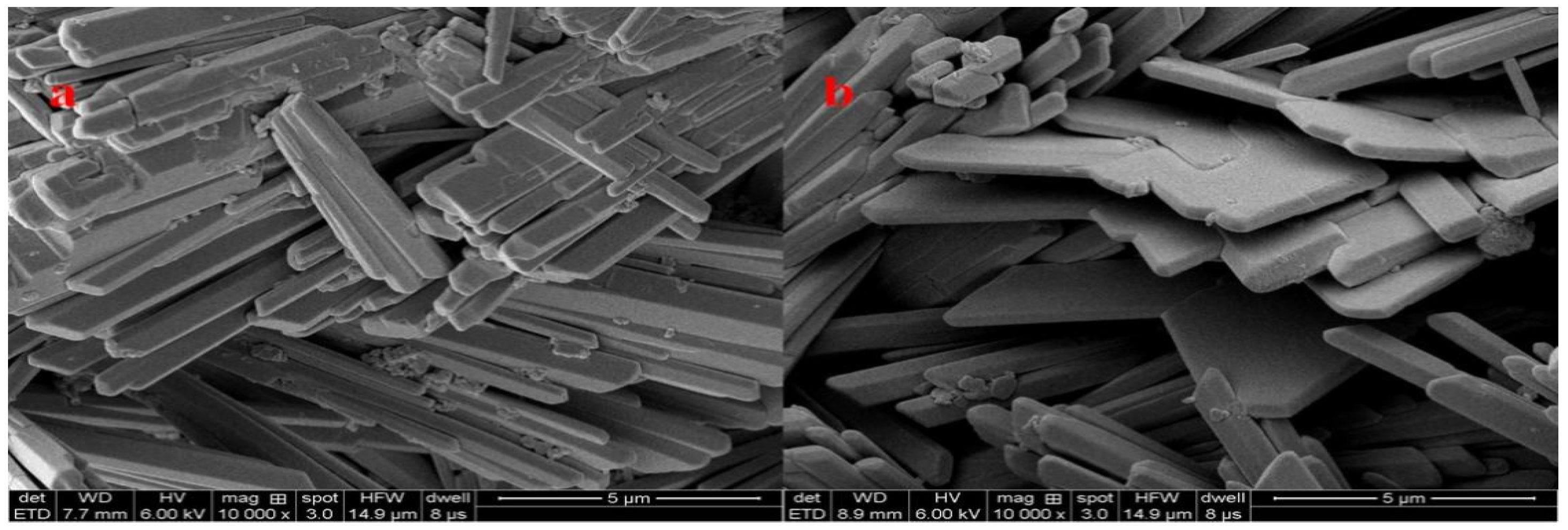
2.4. SEM
2.5. Mechanical Properties
3. Materials
3.1. Materials
3.2. RAFT Polymerization of PCEs
3.3. Measurement
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.2. 1H Nuclear Magnetic Resonance Spectroscopy (1HNMR)
3.3.3. Gel Permeation Chromatography
3.3.4. Fluidity of Gypsum Plaster
3.3.5. Adsorption Measurement
3.3.6. Heat Flow Calorimetry
3.3.7. Morphologycharacterization
3.3.8. Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Elsheikh, A. Bistable morphing composites for energy-harvesting applications. Polymers 2022, 14, 1893. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.H.; Panchal, H.; Shanmugan, S.; Muthuramalingam, T.; El-Kassas, A.M.; Ramesh, B. Recent progresses in wood-plastic composites: Pre-processing treatments, manufacturing techniques, recyclability and eco-friendly assessment. Clean. Eng. Technol. 2022, 8, 100450. [Google Scholar] [CrossRef]
- Raj, M.K.A.; Muthusamy, S.; Panchal, H.; Ibrahim, A.M.M.; Alsoufi, M.S.; Elsheikh, A.H. Investigation of mechanical properties of dual-fiber reinforcement in polymer composite. J. Mater. Res. Technol. 2022, 18, 3908–3915. [Google Scholar]
- Thangaraj, M.; Ahmadein, M.; Alsaleh, N.A.; Elsheikh, A.H. Optimization of abrasive water jet machining of SiC reinforced aluminum alloy based metal matrix composites using Taguchi-DEAR technique. Materials 2021, 14, 6250. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Yu, Q.L.; Brouwers, H.J.H. Microstructure and mechanical properties of β-hemihydrate produced gypsum: An insight from its hydration process. Constr. Build. Mater. 2011, 25, 3149–3157. [Google Scholar] [CrossRef]
- Yu, Q.L.; Brouwers, H.J.H. Thermal properties and microstructure of gypsum board and its dehydration products: A theoretical and experimental investigation. Fire Mater. 2012, 36, 575–589. [Google Scholar] [CrossRef]
- Lei, L.; Hirata, T.; Plank, J. 40 years of PCE superplasticizers—History, current state-of-the-art and an outlook. Cem. Concr. Res. 2022, 157, 106826. [Google Scholar] [CrossRef]
- Abile, R.; Russo, A.; Limone, C.; Montagnaro, F. Impact of the charge density on the behaviour of polycarboxylate ethers as cement dispersants. Constr. Build. Mater. 2018, 180, 477–490. [Google Scholar] [CrossRef]
- Dalas, F.; Pourchet, S.; Nonat, A.; Rinaldi, D.; Sabio, S.; Mosquet, M. Fluidizing efficiency of comb-like superplasticizers: The effect of the anionic function, the side chain length and the grafting degree. Cem. Concr. Res. 2015, 71, 115–123. [Google Scholar] [CrossRef]
- Huang, H.; Qian, C.; Zhao, F.; Qu, J.; Guo, J.; Danzinger, M. Improvement on microstructure of concrete by polycarboxylate superplasticizer (PCE) and its influence on durability of concrete. Constr. Build. Mater. 2016, 110, 293–299. [Google Scholar] [CrossRef]
- Kong, F.R.; Pan, L.S.; Wang, C.M.; Zhang, D.L.; Xu, N. Effects of polycarboxylate superplasticizers with different molecular structure on the hydration behavior of cement paste. Constr. Build. Mater. 2016, 105, 545–553. [Google Scholar] [CrossRef]
- Ran, Q.; Somasundaran, P.; Miao, C.; Liu, J.; Wu, S.; Shen, J. Adsorption mechanism of comb polymer dispersants at the cement/water interface. J. Dispers. Sci. Technol. 2010, 31, 790–798. [Google Scholar] [CrossRef]
- Tsubakimoto, T.; Hosoido, M.; Tawara, H.; Hirata, T. Cement Dispersants. JP Patent 19810172829(S59–018338), 30 October 1981. [Google Scholar]
- Hanehara, S.; Cement, K.Y.J.; Research, C. Rheology and early age properties of cement systems. Cem. Concr. Res. 2008, 38, 175–195. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F.; Bowen, P.; Houst, Y.F. Effect of PCs superplasticizers on the rheological properties and hydration process of slag-blended cement pastes. J. Mater. Sci. 2009, 44, 2714–2723. [Google Scholar] [CrossRef]
- Yamada, K.; Takahashi, T.; Hanehara, S.; Matsuhisa, M.J.C.; Research, C. Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- Qi, H.H.; Ma, B.G.; Tan, H.B.; Su, Y.; Jin, Z.H.; Li, C.B.; Liu, X.H.; Yang, Q.; Luo, Z.T. Polycarboxylate superplasticizer modified by phosphate ester in side chain and its basic properties in gypsum plaster. Constr. Build. Mater. 2021, 271, 121566. [Google Scholar] [CrossRef]
- Tan, H.B.; Deng, X.F.; Gu, B.Q.; Ma, B.G.; Luo, S.Q.; Zhi, Z.Z.; Guo, Y.L.; Zou, F.B. Effect of borax and sodium tripolyphosphate on fluidity of gypsum paste plasticized by polycarboxylate superplasticizer. Constr. Build. Mater. 2019, 176, 394–402. [Google Scholar] [CrossRef]
- Peng, J.H.; Qu, J.D.; Zhang, J.X.; Chen, M.F.; Wan, T.Z. Adsorption characteristics of water-reducing agents on gypsum surface and its effect on the rheology of gypsum plaster. Cem. Concr. Res. 2005, 35, 527–531. [Google Scholar] [CrossRef]
- Vo, M.L.; Plank, J. Dispersing effectiveness of a phosphated polycarboxylate in α- and β-calcium sulfate hemihydrate systems. Constr. Build. Mater. 2020, 237, 117731. [Google Scholar] [CrossRef]
- Guan, B.H.; Ye, Q.Q.; Zhang, J.L.; Lou, W.B.; Wu, Z.B. Interaction between α-calcium sulfate hemihydrate and superplasticizer from the point of adsorption characteristics, hydration and hardening process. Cem. Concr. Res. 2010, 40, 253–259. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, X.Z.; Mi, X.Y.; Zhang, M.; Guo, W.J.; Pei, M.S. Preparation and dispersion properties of polycarbonate superplasticizers based on RAFT polymerization. J. Polym. Res. 2021, 28, 105. [Google Scholar] [CrossRef]
- Metwally, E.; Xu, X.W.; Khadija, E.; Karel, L.; Richard, H.; Geert, D.S. Structure-property relationships for polycarboxylate ether superplasticizers by means of RAFT polymerization. J. Colloid Interface Sci. 2019, 553, 788–797. [Google Scholar]
- Stecher, J.; Plank, J. Novel concrete superplasticizers based on phosphate esters. Cem. Concr. Res. 2019, 119, 36–43. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Cai, X.P.; Kong, X.M.; Gao, L. Effects of comb-shaped superplasticizers with different charge characteristics on the microstructure and properties of fresh cement pastes. Constr. Build. Mater. 2017, 155, 441–450. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Experimental determination of the effective anionic charge density of polycarboxylate superplasticizers in cement pore solution. Cem. Concr. Res. 2009, 39, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Kong, X.M.; Lu, Z.B.; Lu, Z.C.; Hou, S.S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retarda-tion in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Ilg, M.; Plank, J. Non-adsorbing small molecules as auxiliary dispersants for polycarboxylate superplasticizers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124307. [Google Scholar] [CrossRef]
- Tan, H.; Zou, F.; Ma, B.; Liu, M.; Li, X.; Jian, S. Effect of sodium tripolyphosphate on adsorbing behavior of polycarboxylate superplasticizer. Constr. Build. Mater. 2016, 126, 617–623. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhang, Y.; Zheng, J.; Guo, H.; Lu, M. Study on dispersion, adsorption and flow retaining behaviors of coment motars with TPEG-type polyether kind polycarboxylate superplasticizers. Constr. Build. Mater. 2014, 64, 324–332. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Li, H.; Jiang, Z.; Gong, Z.; Yang, X.; Chen, Q. Utilization of the black tea powder as multifunctional admixture for the hemihydrate gypsum. J. Clean. Prod. 2019, 210, 231–237. [Google Scholar] [CrossRef]

| Sample | Theoretical-Mn (g/mol) | Mn (g/mol) | Mw (g/mol) |
|---|---|---|---|
| PtBA | 8421 | 11,900 | 12,700 |
| PAA-TPEG | 42,415 | 40,400 | 42,500 |
| PAA-TPEG-PAA | 45,375 | 44,800 | 49,000 |
| Sample | Flexural Strength (Mpa) | Compressive Strength (Mpa) |
|---|---|---|
| Blank | 2.9 | 7.0 |
| PCE-1 | 4.3 | 10.0 |
| PCE-2 | 3.8 | 9.6 |
| PCE-3 | 3.4 | 9.4 |
| Sample | tBA:TPEG | a:b | tBA/TPEG/CTA/AIBN Mole Ratio |
|---|---|---|---|
| PCE-1 | 5:1 | 1:1 | 5:1:0.061:0.06 |
| PCE-2 | 5:1 | 2:3 | 5:1:0.061:0.06 |
| PCE-3 | 5:1 | 4:1 | 5:1:0.061:0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Gao, G.; Jiang, W.; Wang, X.; Pei, M.; Wang, L. Synthesis of Triblock Polycarboxylate Superplasticizers with Well-Defined Structure and Its Dispersing Performance in β-Hemihydrate Gypsum. Molecules 2023, 28, 513. https://doi.org/10.3390/molecules28020513
Guo G, Gao G, Jiang W, Wang X, Pei M, Wang L. Synthesis of Triblock Polycarboxylate Superplasticizers with Well-Defined Structure and Its Dispersing Performance in β-Hemihydrate Gypsum. Molecules. 2023; 28(2):513. https://doi.org/10.3390/molecules28020513
Chicago/Turabian StyleGuo, Guangming, Guohua Gao, Weiliang Jiang, Xianglong Wang, Meishan Pei, and Luyan Wang. 2023. "Synthesis of Triblock Polycarboxylate Superplasticizers with Well-Defined Structure and Its Dispersing Performance in β-Hemihydrate Gypsum" Molecules 28, no. 2: 513. https://doi.org/10.3390/molecules28020513
APA StyleGuo, G., Gao, G., Jiang, W., Wang, X., Pei, M., & Wang, L. (2023). Synthesis of Triblock Polycarboxylate Superplasticizers with Well-Defined Structure and Its Dispersing Performance in β-Hemihydrate Gypsum. Molecules, 28(2), 513. https://doi.org/10.3390/molecules28020513







