Combination of Enzymes and Deep Eutectic Solvents as Powerful Toolbox for Organic Synthesis
Abstract
:1. Introduction
2. Reductions
2.1. Reductions with Isolated Enzymes
2.2. Whole-Cells Catalysed Reductions
3. Oxidations
3.1. Oxidationss with Isolated Enzymes
3.1.1. Oxidations Catalyzed by Laccases
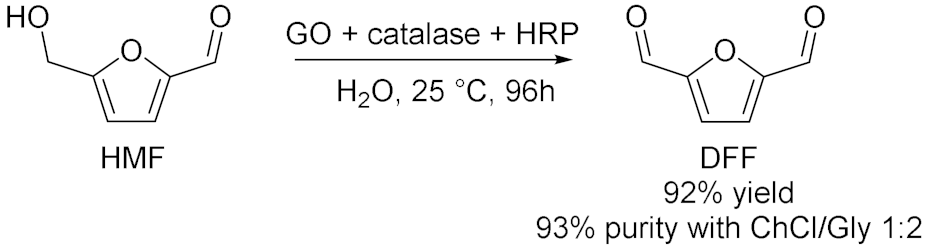
3.1.2. Oxidations Catalyzed by Peroxygenases
3.1.3. Oxidations Catalyzed by Peroxidases
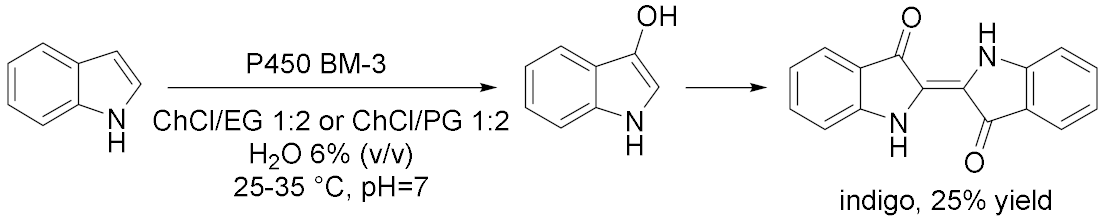
3.1.4. Oxidations Catalyzed by Cytochromes
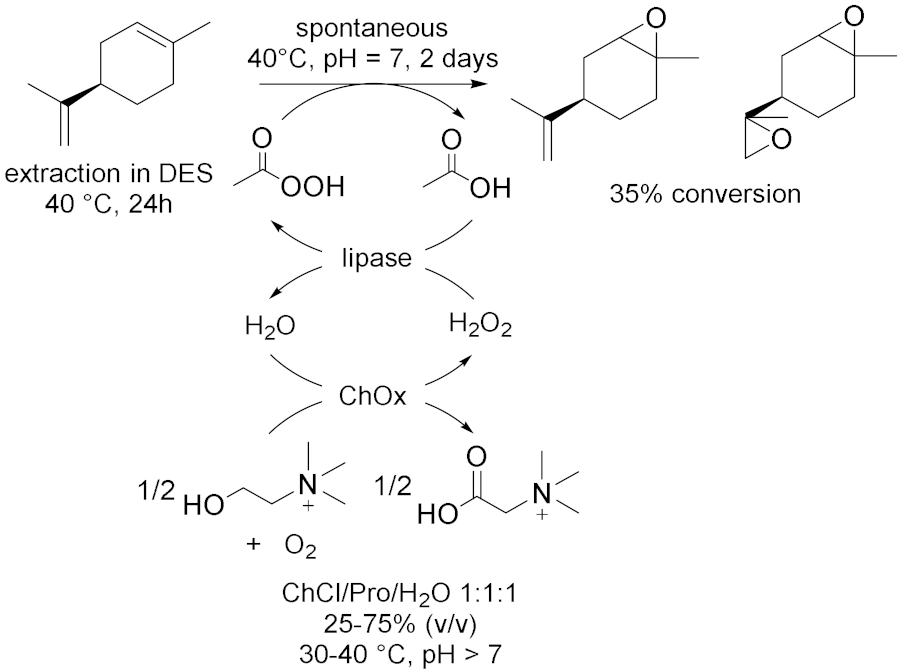
3.1.5. Oxidations Catalyzed by Other Enzymes
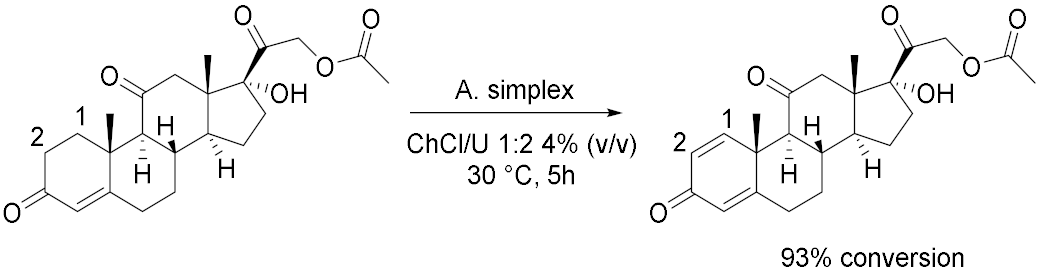
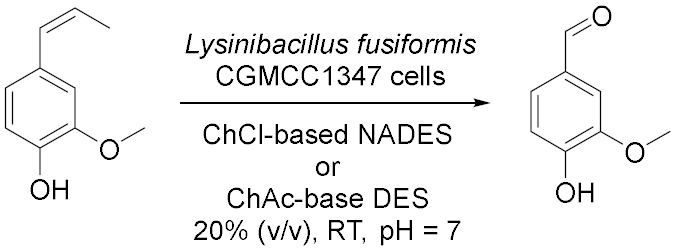
3.2. Whole-Cells Catalysed Oxidations
4. Hydrolysis
4.1. Hydrolyses with Isolated Enzymes
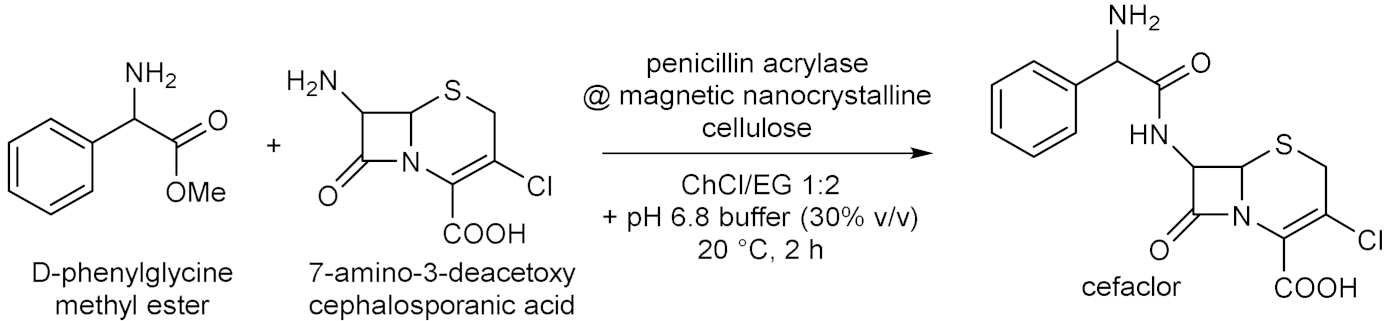
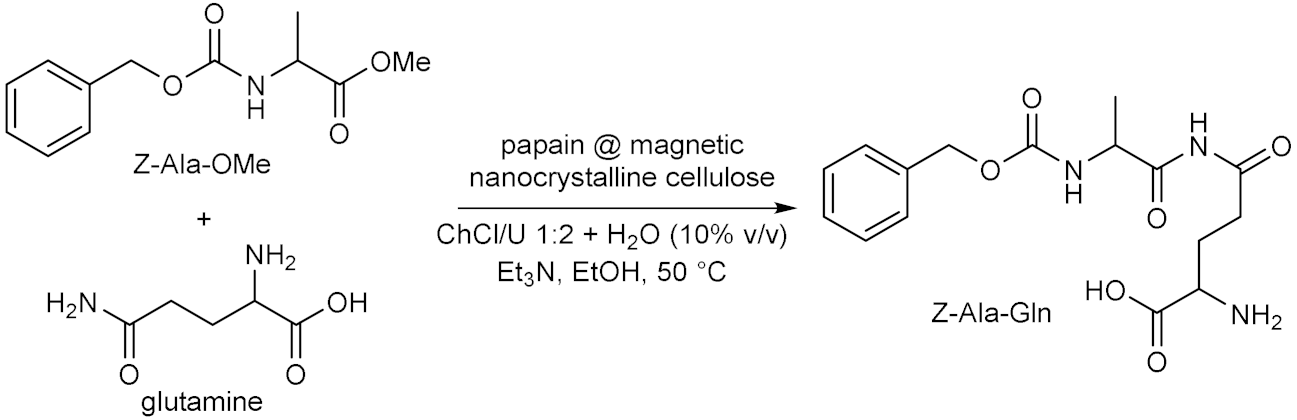
4.2. Whole-Cells Catalysed Hydrolyses
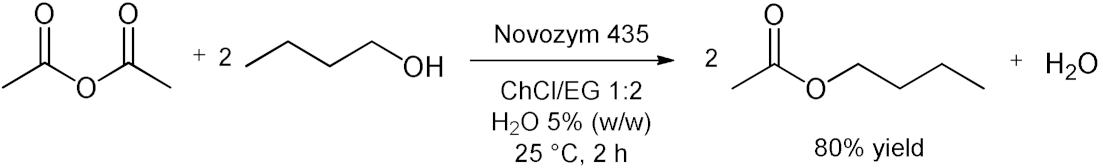
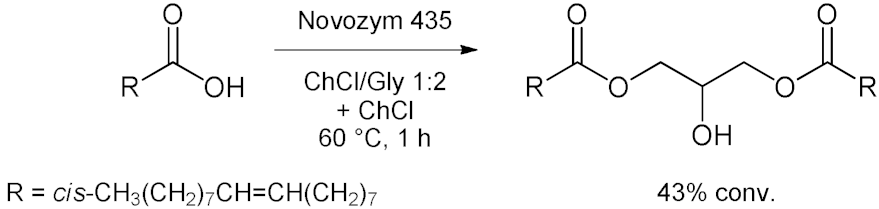
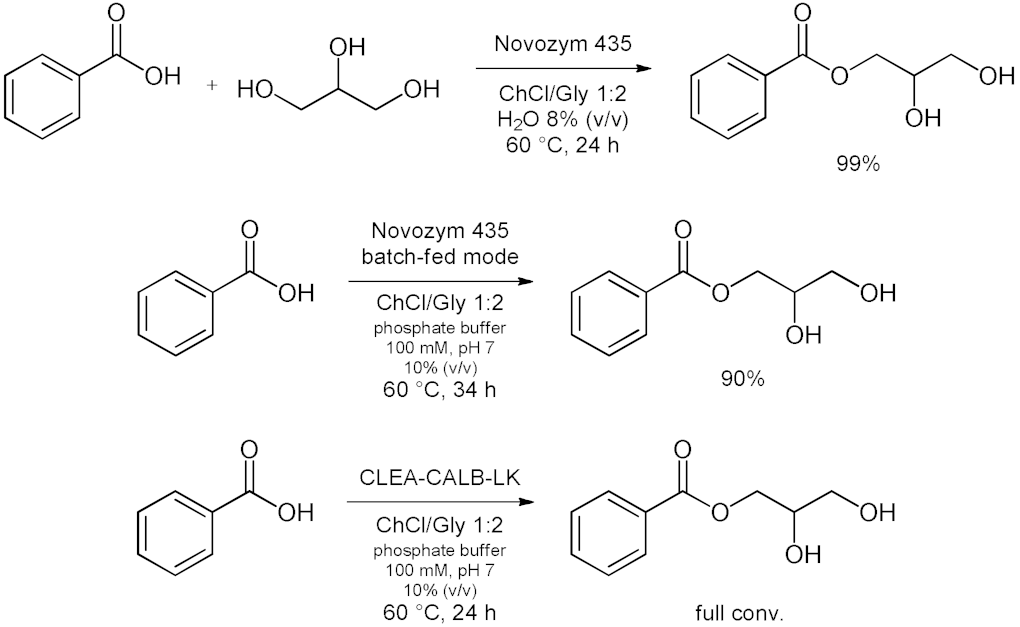
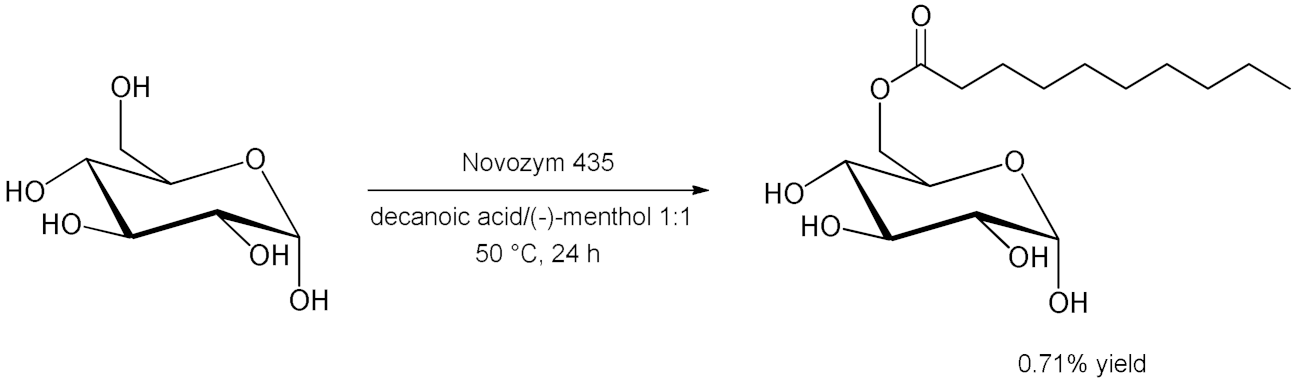
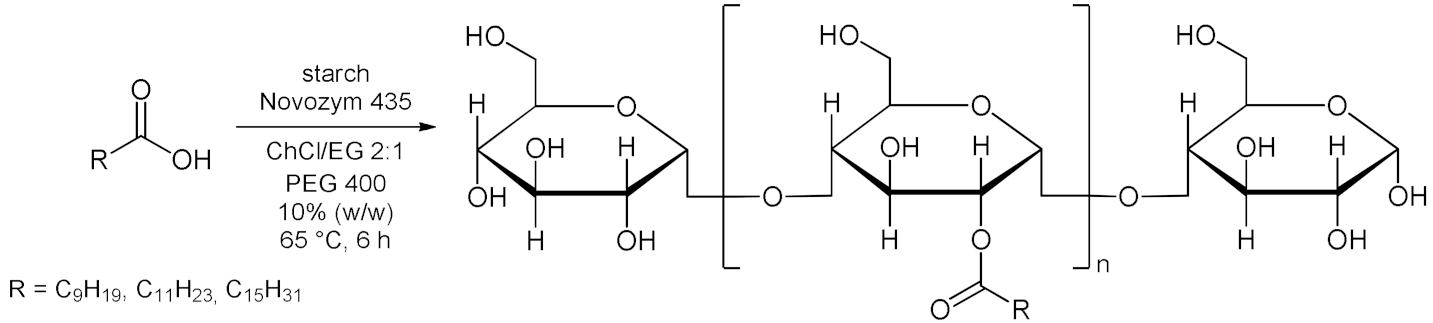
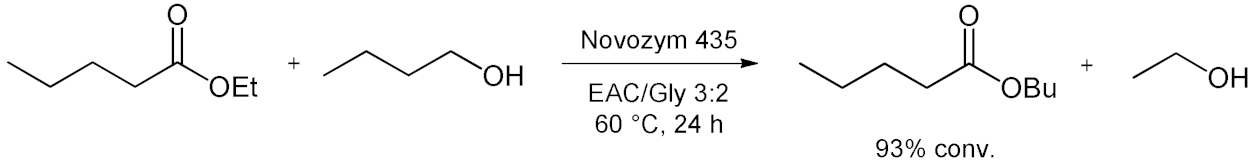
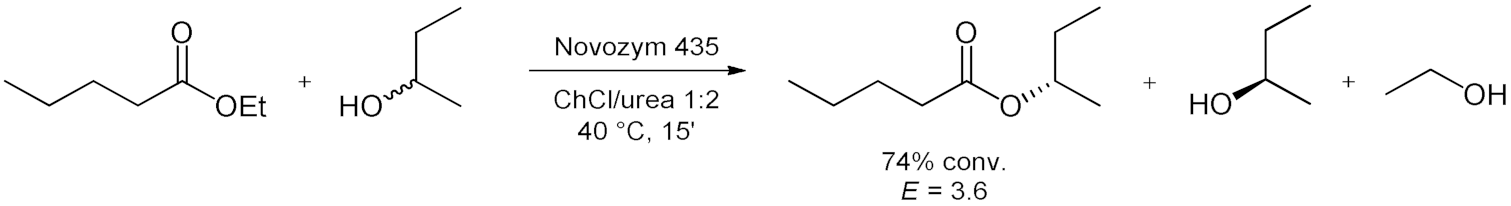
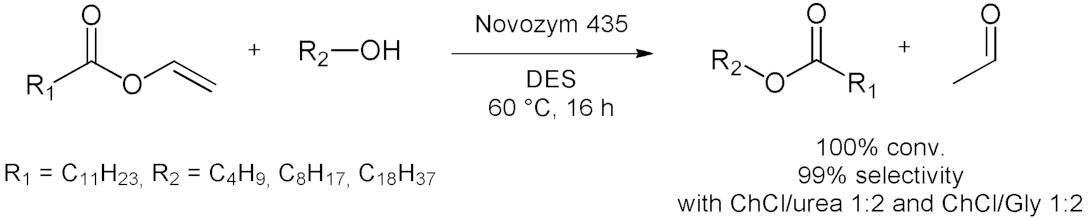
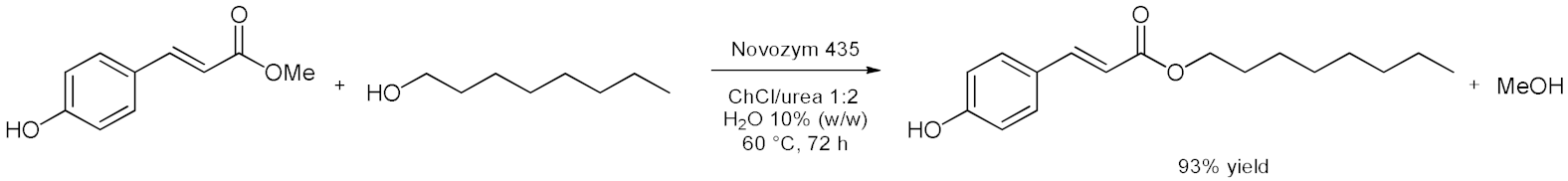
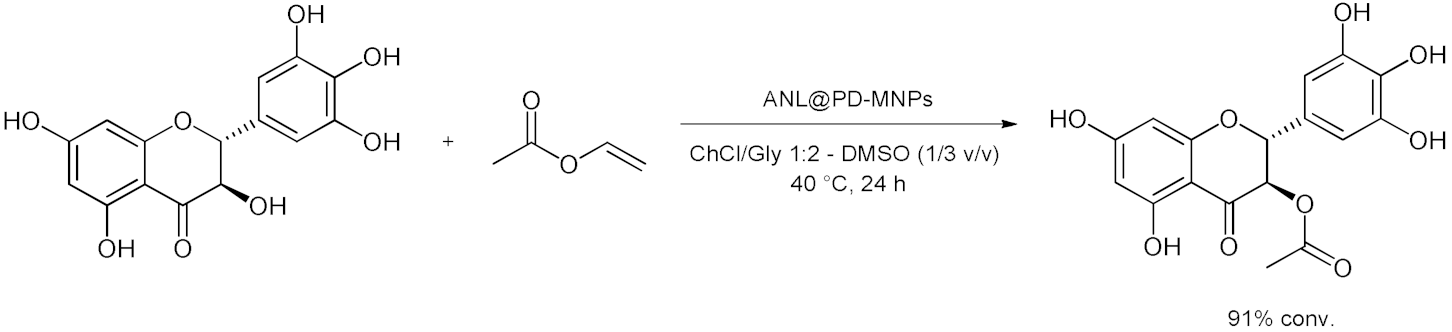
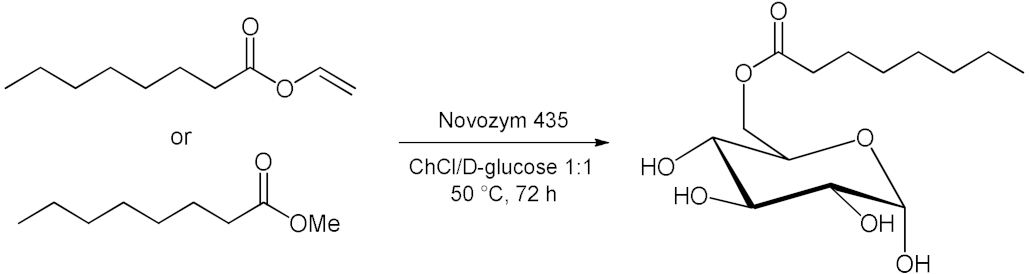
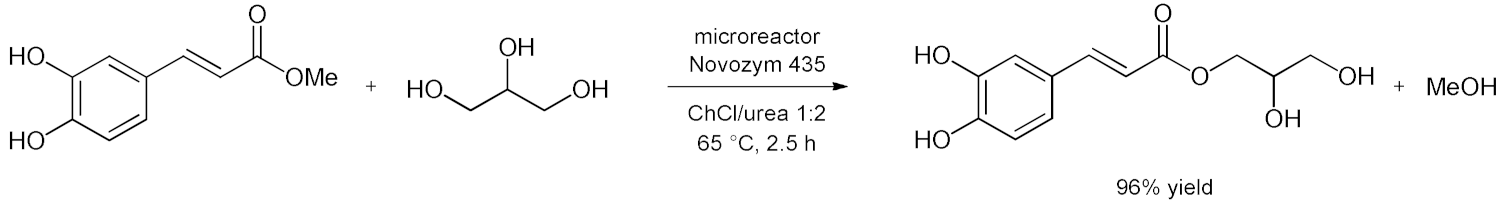
5. Esterifications and Transesterification
5.1. Esterification Reactions with Isolated Enzymes
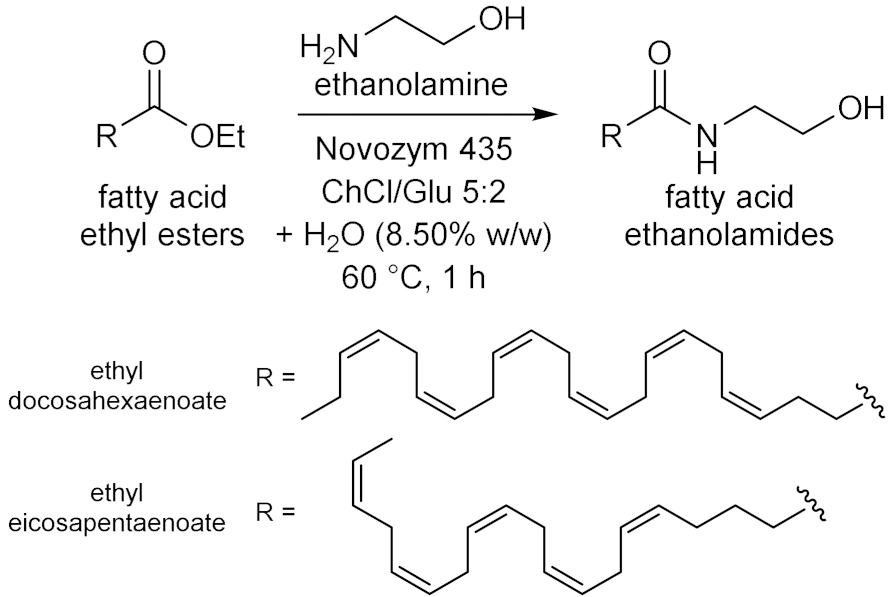
5.2. Transesterifications Reactions with Isolated Enzymes
6. Miscellaneous Enzymatic Reactions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panić, M.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Designing a biocatalytic process involving deep eutectic solvents. J. Chem. Technol. Biotechnol. 2021, 96, 14–30. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Paul, C.E. Deep eutectic solvents for redox biocatalysis. J. Biotechnol. 2019, 293, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Intensification of biotransformations using deep eutectic solvents: Overview and outlook. Process Biochem. 2018, 66, 33–60. [Google Scholar] [CrossRef]
- Ibn Majdoub Hassani, F.Z.; Amzazi, S.; Lavandera, I. The versatile applications of DES and their influence on oxidoreductase-mediated transformations. Molecules 2019, 24, 2190. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Zheng, G.-W.; Zong, M.-H.; Li, N.; Lou, W.-Y. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.-N.; Dou, Y. Deep eutectic solvents for biocatalytic transformations: Focused lipase-catalyzed organic reactions. Appl. Microbiol. Biotechnol. 2020, 104, 1481–1496. [Google Scholar] [CrossRef]
- Chenault, H.K.; Simon, E.S.; Whitesides, G.M. Cofactor Regeneration for Enzyme-Catalysed Synthesis. Biotechnol. Genet. Eng. Rev. 1988, 6, 221–270. [Google Scholar] [CrossRef] [Green Version]
- Van der Donk, W.A.; Zhao, H. Recent developments in pyridine nucleotide regeneration. Curr. Opin. Biotechnol. 2003, 14, 421–426. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L.; Antiochia, R. Direct Electron Transfer of Dehydrogenases for Development of 3rd Generation Biosensors and Enzymatic Fuel Cells. Sensors 2018, 18, 1319. [Google Scholar] [CrossRef]
- Betancor, L.; Berne, C.; Luckarift, H.R.; Spain, J.C. Coimmobilization of a redox enzyme and a cofactor regeneration system. Chem. Commun. 2006, 34, 3640–3642. [Google Scholar] [CrossRef]
- Cicco, L.; Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Morís, F.; Perna, F.M.; Capriati, V.; García-Álvarez, J.; González-Sabín, J. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media. Green Chem. 2018, 20, 3468–3475. [Google Scholar] [CrossRef]
- Chanquia, S.N.; Huang, L.; García Liñares, G.; Domínguez de María, P.; Kara, S. Deep Eutectic Solvents as Smart Cosubstrate in Alcohol Dehydrogenase-Catalyzed Reductions. Catalysts 2020, 10, 1013. [Google Scholar] [CrossRef]
- Bittner, J.P.; Zhang, N.; Huang, L.; Domínguez de María, P.; Jakobtorweihen, S.; Kara, S. Impact of deep eutectic solvents (DESs) and individual DES components on alcohol dehydrogenase catalysis: Connecting experimental data and molecular dynamics simulations. Green Chem. 2022, 24, 1120–1131. [Google Scholar] [CrossRef]
- Meyer, L.-E.; Andersen, M.B.; Kara, S.A. Deep Eutectic Solvent Thermomorphic Multiphasic System for Biocatalytic Applications. Angew. Chem. Int. Ed. 2022, 61, e202203823. [Google Scholar] [CrossRef]
- Panić, M.; Elenkov, M.M.; Roje, M.; Bubalo, M.C.; Redovniković, I.R. Plant-mediated stereoselective biotransformations in natural deep eutectic solvents. Process Biochem. 2018, 66, 133–139. [Google Scholar] [CrossRef]
- Vitale, P.; Perna, F.; Agrimi, G.; Pisano, I.; Mirizzi, F.; Capobianco, R.; Capriati, V. Whole-Cell Biocatalyst for Chemoenzymatic Total Synthesis of Rivastigmine. Catalysts 2018, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, P.; He, Y.-S.; Zhu, Z.-R.; Huang, J. Toward Designing a Novel Oligopeptide-Based Deep Eutectic Solvent: Applied in Biocatalytic Reduction. ACS Sustain. Chem. Eng. 2019, 7, 1318–1326. [Google Scholar] [CrossRef]
- Panić, M.; Delac, D.; Roje, M.; Radojcic Redovniković, I.; Cvjetko Bubalo, M. Green asymmetric reduction of acetophenone derivatives: Saccharomyces cerevisiae and aqueous natural deep eutectic solvent. Biotechnol. Lett. 2019, 41, 253–262. [Google Scholar] [CrossRef]
- He, Y.; Huang, Q.; Wang, P. Design and evaluation of novel bio-based deep eutectic solvents for highly efficient bioproduction of chiral aryl alcohol. J. Chem. Technol. Biotechnol. 2020, 95, 1980–1988. [Google Scholar] [CrossRef]
- Pavoković, D.; Košpić, K.; Panić, M.; Radojčić Redovniković, I.; Cvjetko Bubalo, M. Natural deep eutectic solvents are viable solvents for plant cell culture-assisted stereoselective biocatalysis. Process Biochem. 2020, 93, 69–76. [Google Scholar] [CrossRef]
- Peng, F.; Chen, Q.-S.; Li, F.-Z.; Ou, X.-Y.; Zong, M.-H.; Lou, W.-Y. Using deep eutectic solvents to improve the biocatalytic reduction of 2-hydroxyacetophenone to (R)-1-phenyl-1,2-ethanediol by Kurthia gibsonii SC0312. Mol. Catal. 2020, 484, 110773. [Google Scholar] [CrossRef]
- Xia, N.; Xiong, L.; Bi, S.; Qian, F.; Wang, P. Development of biocompatible DES/NADES as co-solvents for efficient biosynthesis of chiral alcohols. Bioprocess Biosyst. Eng. 2020, 43, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Liu, H.; Lin, H.; Wang, P. Integration of natural deep-eutectic solvent and surfactant for efficient synthesis of chiral aromatic alcohol mediated by Cyberlindnera saturnus whole cells. Biochem. Eng. J. 2021, 172, 108053. [Google Scholar] [CrossRef]
- Xiong, L.; Kong, X.; Liu, H.; Wang, P. Efficient biosynthesis of (S)-1-[2-(trifluoromethyl)phenyl]ethanol by a novel isolate Geotrichum silvicola ZJPH1811 in deep eutectic solvent/cyclodextrin-containing system. Bioresour. Technol. 2021, 329, 124832. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, L.; Di, J.; Ma, C.; Li, Q.; He, Y. A Hybrid Process for Valorization of d-Fructose to 2,5-Bis(hydroxymethyl)furan by Bridging Chemocatalysis and Biocatalysis in a Betaine:Benzenesulfonic Acid System. ACS Sustain. Chem. Eng. 2022, 10, 12165–12176. [Google Scholar] [CrossRef]
- Khlupova, M.; Vasil’eva, I.; Shumakovich, G.; Zaitseva, E.; Chertkov, V.; Shestakova, A.; Morozova, O.; Yaropolov, A. Enzymatic Polymerization of Dihydroquercetin (Taxifolin) in Betaine-Based Deep Eutectic Solvent and Product Characterization. Catalysts 2021, 11, 639. [Google Scholar] [CrossRef]
- Toledo, M.L.; Pereira, M.M.; Freire, M.G.; Silva, J.P.; Coutinho, J.O.A.; Tavares, A.P. Laccase activation in deep eutectic solvents. ACS Sustain. Chem. Eng. 2019, 7, 11806–11814. [Google Scholar] [CrossRef]
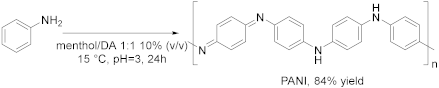
- Altundağ, A.; Ünlü, A.E.; Takaç, S. Deep eutectic solvent-assisted synthesis of polyaniline by laccase enzyme. J. Chem. Technol. Biotechnol. 2021, 96, 1107–1115. [Google Scholar] [CrossRef]
- Vasil’eva, I.; Morozova, O.; Shumakovich, G.; Yaropolov, A. Betaine-Based Deep Eutectic Solvent as a New Media for Laccase-Catalyzed Template-Guided Polymerization/Copolymerization of Aniline and 3-Aminobenzoic Acid. Int. J. Mol. Sci. 2022, 23, 11409. [Google Scholar] [CrossRef]
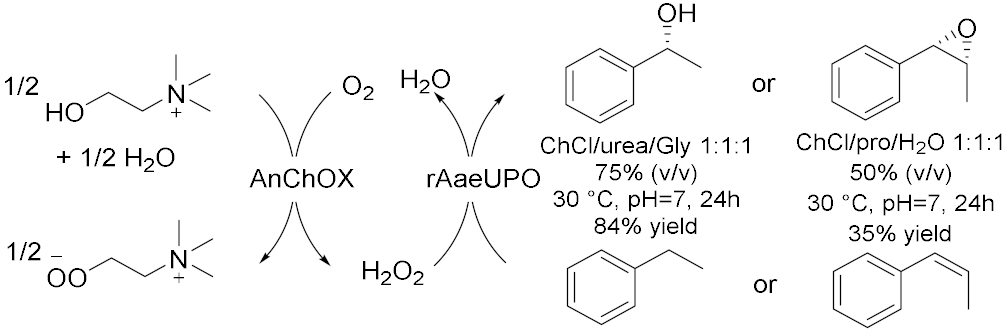
- Ma, Y.; Li, Y.; Ali, S.; Li, P.; Zhang, W.; Rauch, M.; Willot, S.; Ribitsch, D.; Choi, Y.H.; Alcalde, M. Natural deep eutectic solvents as performance additives for peroxygenase catalysis. ChemCatChem 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Li, P.; Zhang, X.; Ribitsch, D.; Alcalde, M.; Hollmann, F.; Wang, Y. Enantioselective sulfoxidation of thioanisole by cascading a choline oxidase and a peroxygenase in the presence of natural deep eutectic solvents. ChemPlusChem 2020, 85, 254–257. [Google Scholar] [CrossRef]
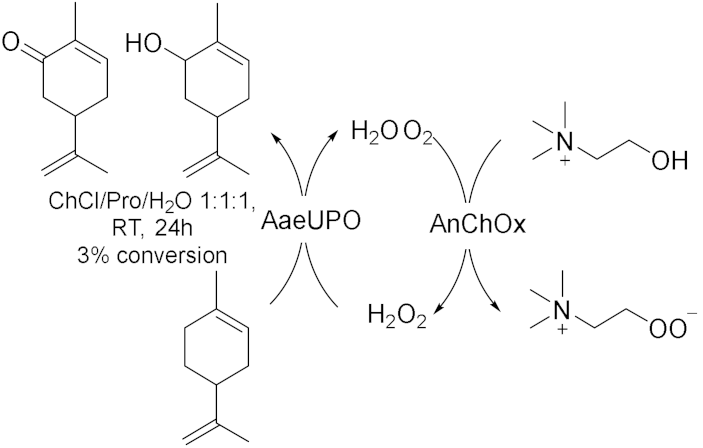
- Li, Z.; Ma, Y.; Hollmann, F.; Wang, Y. Study on green extraction of limonene from orange peel and cascade catalysis to produce carvol and carvone in deep eutectic solvents. Flavour Fragr. J. 2022, 37, 254–261. [Google Scholar] [CrossRef]
- Wu, B.-P.; Wen, Q.; Xu, H.; Yang, Z. Insights into the impact of deep eutectic solvents on horseradish peroxidase: Activity, stability and structure. J. Mol. Catal. B Enzym. 2014, 101, 101–107. [Google Scholar] [CrossRef]
- Sánchez-Leija, R.; Torres-Lubián, J.; Reséndiz-Rubio, A.; Luna-Bárcenas, G.; Mota-Morales, J. Enzyme-mediated free radical polymerization of acrylamide in deep eutectic solvents. RSC Adv. 2016, 6, 13072–13079. [Google Scholar] [CrossRef]
- Sun, H.; Xin, R.; Qu, D.; Yao, F. Mechanism of deep eutectic solvents enhancing catalytic function of cytochrome P450 enzymes in biosynthesis and organic synthesis. J. Biotechnol. 2020, 323, 264–273. [Google Scholar] [CrossRef]
- Qin, Y.-Z.; Li, Y.-M.; Zong, M.-H.; Wu, H.; Li, N. Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2, 5-diformylfuran using deep eutectic solvents. Green Chem. 2015, 17, 3718–3722. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Li, Y.; Willot, S.J.; Zhang, W.; Ribitsch, D.; Choi, Y.H.; Verpoorte, R.; Zhang, T.; Hollmann, F. Natural Deep Eutectic Solvents as Multifunctional Media for the Valorization of Agricultural Wastes. ChemSusChem 2019, 12, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Mourelle-Insua, Á.; Aalbers, F.S.; Lavandera, I.; Gotor-Fernández, V.; Fraaije, M.W. What to sacrifice? Fusions of cofactor regenerating enzymes with Baeyer-Villiger monooxygenases and alcohol dehydrogenases for self-sufficient redox biocatalysis. Tetrahedron 2019, 75, 1832–1839. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Martin, C.; Fraaije, M.W. Positive impact of natural deep eutectic solvents on the biocatalytic performance of 5-hydroxymethyl-furfural oxidase. Catalysts 2020, 10, 447. [Google Scholar] [CrossRef]
- Mazur, M.; Janeczko, T.; Gładkowski, W. Lipase-mediated Baeyer–Villiger oxidation of benzylcyclopentanones in ester solvents and deep eutectic solvents. Sci. Rep. 2022, 12, 14795. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-X.; Zhao, L.-Q.; Wang, J.; Song, G.-L.; Liu, H.-M.; Cheng, H.; Yang, Z. Improving whole-cell biocatalysis by addition of deep eutectic solvents and natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722. [Google Scholar] [CrossRef]
- Xu, P.; Cheng, J.; Lou, W.-Y.; Zong, M.-H. Using deep eutectic solvents to improve the resolution of racemic 1-(4-methoxyphenyl) ethanol through Acetobacter sp. CCTCC M209061 cell-mediated asymmetric oxidation. RSC Adv. 2015, 5, 6357–6364. [Google Scholar] [CrossRef]
- Wei, P.; Liang, J.; Cheng, J.; Zong, M.-H.; Lou, W.-Y. Markedly improving asymmetric oxidation of 1-(4-methoxyphenyl) ethanol with Acetobacter sp. CCTCC M209061 cells by adding deep eutectic solvent in a two-phase system. Microb. Cell Factories 2016, 15, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, S.; Yu, L.; Ji, S.; Liu, X.; Lu, F. Evaluation of deep eutectic solvents as co-solvent for steroids 1-en-dehydrogenation biotransformation by Arthrobacter simplex. J. Chem. Technol. Biotechnol. 2016, 91, 1099–1104. [Google Scholar] [CrossRef]
- Mao, S.; Li, K.; Hou, Y.; Liu, Y.; Ji, S.; Qin, H.; Lu, F. Synergistic effects of components in deep eutectic solvents relieve toxicity and improve the performance of steroid biotransformation catalyzed by Arthrobacter simplex. J. Chem. Technol. Biotechnol. 2018, 93, 2729–2736. [Google Scholar] [CrossRef]
- Mao, S.; Wang, X.; Zhang, Z.; Wang, S.; Li, K.; Lu, F.; Qin, H. 15α-hydroxylation of D-ethylgonendione by Penicillium raistrickii in deep eutectic solvents DESs containing system. Biochem. Eng. J. 2020, 164, 107781. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, N.; Tang, S.; Zhu, X.; Ma, C.; Fan, B.; Liang, J.; Yu, B.; Yang, L.; He, Y.-C. A hybrid strategy for efficient valorization of bulrush into furoic acid in water–ChCl-based deep eutectic solvent. Ind. Crops Prod. 2022, 177, 114434. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Kazlauskas, R.J. Hydrolases in Organic Synthesis: Regio- and Stereoselective Biotransformations; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Zhu, D.; Wu, Q.; Wang, N. 3.02—Industrial Enzymes. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 3–13. [Google Scholar]
- Hollands, T.R.; Fruton, J.S. On the mechanism of pepsin action. Proc. Natl. Acad. Sci. USA 1969, 62, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Brady, L.; Brzozowski, A.M.; Derewenda, Z.S.; Dodson, E.; Dodson, G.; Tolley, S.; Turkenburg, J.P.; Christiansen, L.; Huge-Jensen, B.; Norskov, L.; et al. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature 1990, 343, 767–770. [Google Scholar] [CrossRef]
- Harrison, M.J.; Burton, N.A.; Hillier, I.H.; Gould, I.R. Mechanism and transition state structure for papain catalysed amide hydrolysis, using a hybrid QM/MM potential. Chem. Commun. 1996, 24, 2769–2770. [Google Scholar] [CrossRef]
- Faber, K. Biocatalytic Applications. In Biotransformations in Organic Chemistry: A Textbook; Faber, K., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 31–313. [Google Scholar]
- Wang, W.; Lee, D.-J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Cao, S.-L.; Yue, D.-M.; Li, X.-H.; Smith, T.J.; Li, N.; Zong, M.-H.; Wu, H.; Ma, Y.-Z.; Lou, W.-Y. Novel Nano-/Micro-Biocatalyst: Soybean Epoxide Hydrolase Immobilized on UiO-66-NH2 MOF for Efficient Biosynthesis of Enantiopure (R)-1,2-Octanediol in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2016, 4, 3586–3595. [Google Scholar] [CrossRef]
- Weiz, G.; Braun, L.; Lopez, R.; de María, P.D.; Breccia, J.D. Enzymatic deglycosylation of flavonoids in deep eutectic solvents-aqueous mixtures: Paving the way for sustainable flavonoid chemistry. J. Mol. Catal. B Enzym. 2016, 130, 70–73. [Google Scholar] [CrossRef]
- Peng, F.; Zhao, Y.; Li, F.-Z.; Zong, M.-H.; Lou, W.-Y. The effect of deep eutectic solvents on the asymmetric hydrolysis of styrene oxide by mung bean epoxide hydrolases. Bioresour. Bioprocess. 2018, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.-J.; Huang, Y.-K.; Li, F.; Wang, D.-D.; Yin, M.-N.; Wang, M.; Xia, Z.-N. Improving β-glucosidase biocatalysis with deep eutectic solvents based on choline chloride. Biochem. Eng. J. 2018, 138, 37–46. [Google Scholar] [CrossRef]
- Yang, G.; Tong, T.; Yang, Y.; Liu, W.; Wang, X. Amano Lipase PS-catalyzed Hydrolysis of Pine Nut Oil for the Fatty Acids Production Using Deep Eutectic Solvent as Co-solvent. J. Oleo Sci. 2019, 68, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Li, W.; Duan, Z.; Ma, X.; Fan, D. Biocatalytic production of compound K in a deep eutectic solvent based on choline chloride using a substrate fed-batch strategy. Bioresour. Technol. 2020, 305, 123039. [Google Scholar] [CrossRef]
- Zang, Y.-Y.; Yang, X.; Chen, Z.-G.; Wu, T. One-pot preparation of quercetin using natural deep eutectic solvents. Process Biochem. 2020, 89, 193–198. [Google Scholar] [CrossRef]
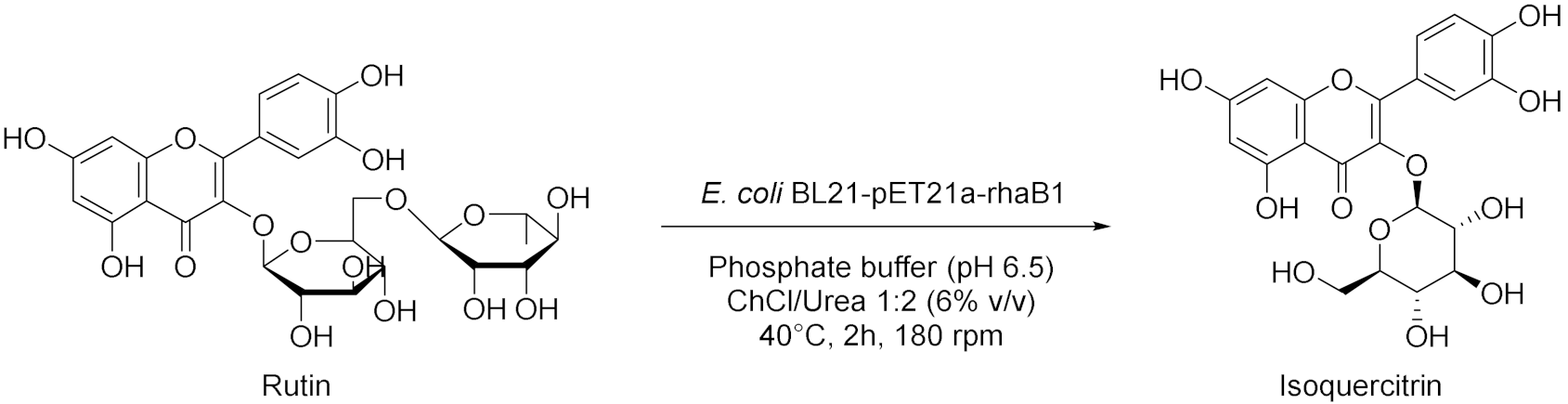
- Zhang, F.; Zhu, C.T.; Peng, Q.M.; Wang, F.Q.; Sheng, S.; Wu, Q.Y.; Wang, J. Enhanced permeability of recombinant E. coli cells with deep eutectic solvent for transformation of rutin. J. Chem. Technol. Biotechnol. 2019, 95, 384–393. [Google Scholar] [CrossRef]
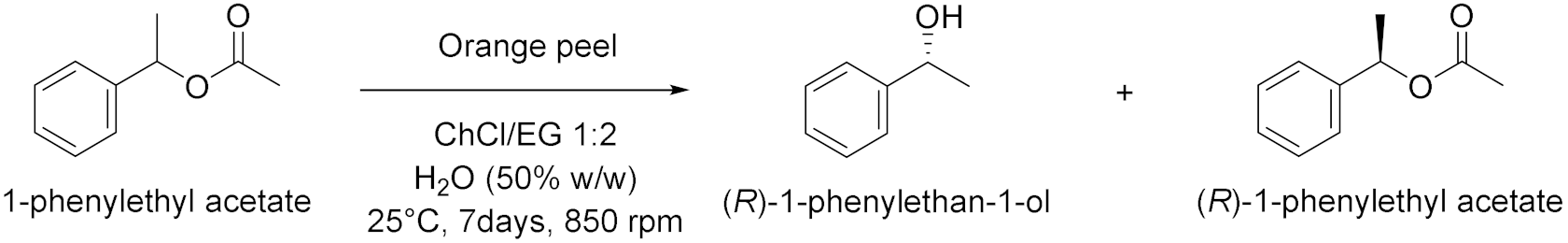
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Otera, J.; Nishikido, J. Esterification: Methods, Reactions, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chen, C.-C.; Reddy, P.M.; Devi, C.S.; Chang, P.-C.; Ho, Y.-P. Study of microwave effects on the lipase-catalyzed hydrolysis. Enzym. Microb. Technol. 2016, 82, 164–172. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.O.M.; Antunes, O.A.; Kroutil, W.; Kappe, C.O. Kinetic resolution of rac-1-phenylethanol with immobilized lipases: A critical comparison of microwave and conventional heating protocols. J. Org. Chem. 2009, 74, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Galletti, P.; Moretti, F.; Samorì, C.; Tagliavini, E. Enzymatic acylation of levoglucosan in acetonitrile and ionic liquids. Green Chem. 2007, 9, 987–991. [Google Scholar] [CrossRef]
- Izquierdo, D.F.; Bernal, J.M.; Burguete, M.I.; García-Verdugo, E.; Lozano, P.; Luis, S.V. An efficient microwave-assisted enzymatic resolution of alcohols using a lipase immobilised on supported ionic liquid-like phases (SILLPs). RSC Adv. 2013, 3, 13123–13126. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, H.M.; Kim, D.; Ahn, Y.; Park, J. Dynamic kinetic resolution of secondary alcohols by enzyme–metal combinations in ionic liquid. Green Chem. 2004, 6, 471–474. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T. Lipase-catalyzed syntheses of sugar esters in non-aqueous media. Biotechnol. Lett. 2011, 33, 1911–1919. [Google Scholar] [CrossRef] [Green Version]
- Magrone, P.; Cavallo, F.; Panzeri, W.; Passarella, D.; Riva, S. Exploiting enzymatic regioselectivity: A facile methodology for the synthesis of polyhydroxylated hybrid compounds. Org. Biomol. Chem. 2010, 8, 5583–5590. [Google Scholar] [CrossRef]
- Prabhakar, S.; Vivès, T.; Ferrières, V.; Benvegnu, T.; Legentil, L.; Lemiègre, L. A fully enzymatic esterification/transesterification sequence for the preparation of symmetrical and unsymmetrical trehalose diacyl conjugates. Green Chem. 2017, 19, 987–995. [Google Scholar] [CrossRef]
- Shi, Y.G.; Li, J.R.; Chu, Y.H. Enzyme-catalyzed regioselective synthesis of sucrose-based esters. J. Chem. Technol. Biotechnol. 2011, 86, 1457–1468. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Ma, D.; Wu, H.; Wang, Z.; Wang, L.; Fang, X. Microwave-assisted fatty acid methyl ester production from soybean oil by Novozym 435. Green Chem. 2010, 12, 844–850. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Tušek, A.J.; VinkoviĿ, M.; RadoševiĿ, K.; SrĿek, V.G.; RedovnikoviĿ, I.R. Cholinium-based deep eutectic solvents and ionic liquids for lipase-catalyzed synthesis of butyl acetate. J. Mol. Catal. B Enzym. 2015, 122, 188–198. [Google Scholar] [CrossRef]
- Zeng, C.-X.; Qi, S.-J.; Xin, R.-P.; Yang, B.; Wang, Y.-H. Enzymatic selective synthesis of 1, 3-DAG based on deep eutectic solvent acting as substrate and solvent. Bioprocess Biosyst. Eng. 2015, 38, 2053–2061. [Google Scholar] [CrossRef]
- Guajardo, N.; Müller, C.R.; Schrebler, R.; Carlesi, C.; Dominguez de Maria, P. Deep eutectic solvents for organocatalysis, biotransformations, and multistep organocatalyst/enzyme combinations. ChemCatChem 2016, 8, 1020–1027. [Google Scholar] [CrossRef]
- Guajardo, N.; Schrebler, R.A.; de María, P.D. From batch to fed-batch and to continuous packed-bed reactors: Lipase-catalyzed esterifications in low viscous deep-eutectic-solvents with buffer as cosolvent. Bioresour. Technol. 2019, 273, 320–325. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; de María, P.D. Immobilized lipase-CLEA aggregates encapsulated in lentikats® as robust biocatalysts for continuous processes in deep eutectic solvents. J. Biotechnol. 2020, 310, 97–102. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Li, D.; Liu, P.; Tan, C.P.; Wang, W.; Liu, X.; Yang, B.; Lan, D.; Wang, Y. Deep Eutectic Solvents Enable the Enhanced Production of n-3 PUFA-Enriched Triacylglycerols. Eur. J. Lipid Sci. Technol. 2017, 119, 1700300. [Google Scholar] [CrossRef]
- Hümmer, M.; Kara, S.; Liese, A.; Huth, I.; Schrader, J.; Holtmann, D. Synthesis of (-)-menthol fatty acid esters in and from (-)-menthol and fatty acids–novel concept for lipase catalyzed esterification based on eutectic solvents. Mol. Catal. 2018, 458, 67–72. [Google Scholar] [CrossRef]
- Craveiro, R.; Meneses, L.; Durazzo, L.; Rocha, A.N.; Silva, J.M.; Reis, R.L.; Barreiros, S.; Duarte, A.R.C.; Paiva, A. Deep eutectic solvents for enzymatic esterification of racemic menthol. ACS Sustain. Chem. Eng. 2019, 7, 19943–19950. [Google Scholar] [CrossRef]
- Hollenbach, R.; Ochsenreither, K.; Syldatk, C. Enzymatic synthesis of glucose monodecanoate in a hydrophobic deep eutectic solvent. Int. J. Mol. Sci. 2020, 21, 4342. [Google Scholar] [CrossRef]
- Lozano, P.; Alvarez, E.; Nieto, S.; Villa, R.; Bernal, J.M.; Donaire, A. Biocatalytic synthesis of panthenyl monoacyl esters in ionic liquids and deep eutectic solvents. Green Chem. 2019, 21, 3353–3361. [Google Scholar] [CrossRef]
- Deng, X.; Han, X.; Hu, X.; Zheng, S.; Liu, K. Enzyme-Catalyzed Starch Esterification in Deep Eutectic Solvent. ChemistrySelect 2019, 4, 565–569. [Google Scholar] [CrossRef]
- Arıkaya, A.; Ünlü, A.E.; Takaç, S. Use of deep eutectic solvents in the enzyme catalysed production of ethyl lactate. Process Biochem. 2019, 84, 53–59. [Google Scholar] [CrossRef]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 10, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M. Ionic liquid applications: Pharmaceuticals, therapeutics, and biotechnology. J. Am. Chem. Soc. 2010, 132, 17975. [Google Scholar]
- Durand, E.; Lecomte, J.; Baréa, B.; Piombo, G.; Dubreucq, E.; Villeneuve, P. Evaluation of deep eutectic solvents as new media for Candida antarctica B lipase catalyzed reactions. Process Biochem. 2012, 47, 2081–2089. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Baréa, B.; Dubreucq, E.; Lortie, R.; Villeneuve, P. Evaluation of deep eutectic solvent–water binary mixtures for lipase-catalyzed lipophilization of phenolic acids. Green Chem. 2013, 15, 2275–2282. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Baréa, B.; Villeneuve, P. Towards a better understanding of how to improve lipase-catalyzed reactions using deep eutectic solvents based on choline chloride. Eur. J. Lipid Sci. Technol. 2014, 116, 16–23. [Google Scholar] [CrossRef]
- Cao, S.-L.; Deng, X.; Xu, P.; Huang, Z.-X.; Zhou, J.; Li, X.-H.; Zong, M.-H.; Lou, W.-Y. Highly efficient enzymatic acylation of dihydromyricetin by the immobilized lipase with deep eutectic solvents as cosolvent. J. Agric. Food. Chem. 2017, 65, 2084–2088. [Google Scholar] [CrossRef]
- Siebenhaller, S.; Hajek, T.; Muhle-Goll, C.; Himmelsbach, M.; Luy, B.; Kirschhöfer, F.; Brenner-Weiß, G.; Hahn, T.; Zibek, S.; Syldatk, C. Beechwood carbohydrates for enzymatic synthesis of sustainable glycolipids. Bioresour. Bioprocess. 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Meng, X.-Y.; Xu, Y.; Dong, T.; Zhang, D.-Y.; Guan, H.-X.; Zhuang, Y.; Wang, J. Enzymatic synthesis of 1-caffeoylglycerol with deep eutectic solvent under continuous microflow conditions. Biochem. Eng. J. 2019, 142, 41–49. [Google Scholar] [CrossRef]
- Fotiadou, R.; Patila, M.; Hammami, M.A.; Enotiadis, A.; Moschovas, D.; Tsirka, K.; Spyrou, K.; Giannelis, E.P.; Avgeropoulos, A.; Paipetis, A. Development of effective lipase-hybrid nanoflowers enriched with carbon and magnetic nanomaterials for biocatalytic transformations. Nanomaterials 2019, 9, 808. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhang, C.; Crittle, T.D. Choline-based deep eutectic solvents for enzymatic preparation of biodiesel from soybean oil. J. Mol. Catal. B Enzym. 2013, 85, 243–247. [Google Scholar] [CrossRef]
- Hao, X.; Suo, H.; Zhang, G.; Xu, P.; Gao, X.; Du, S. Ultrasound-assisted enzymatic preparation of fatty acid ethyl ester in deep eutectic solvent. Renew. Energy 2021, 164, 937–947. [Google Scholar] [CrossRef]
- Delavault, A.; Opochenska, O.; Laneque, L.; Soergel, H.; Muhle-Goll, C.; Ochsenreither, K.; Syldatk, C. Lipase-Catalyzed Production of Sorbitol Laurate in a “2-in-1” Deep Eutectic System: Factors Affecting the Synthesis and Scalability. Molecules 2021, 26, 2759. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Pätzold, M.; Burek, B.; Liese, A.; Bloh, J.; Holtmann, D. Product recovery of an enzymatically synthesized (−)-menthol ester in a deep eutectic solvent. Bioprocess Biosyst. Eng. 2019, 42, 1385–1389. [Google Scholar] [CrossRef]
- Pavlačková, J.; Egner, P.; Sedláček, T.; Mokrejš, P.; Sedlaříková, J.; Polášková, J. In vivo efficacy and properties of semisolid formulations containing panthenol. J. Cosmet. Dermatol. 2019, 18, 346–354. [Google Scholar] [CrossRef] [Green Version]
- Ülger, C.; Takaç, S. Kinetics of lipase-catalysed methyl gallate production in the presence of deep eutectic solvent. Biocatal. Biotransform. 2017, 35, 407–416. [Google Scholar] [CrossRef]
- Siebenhaller, S.; Gentes, J.; Infantes, A.; Muhle-Goll, C.; Kirschhöfer, F.; Brenner-Weiß, G.; Ochsenreither, K.; Syldatk, C. Lipase-catalyzed synthesis of sugar esters in honey and agave syrup. Front. Chem. 2018, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Siebenhaller, S.; Kirchhoff, J.; Kirschhöfer, F.; Brenner-Weiß, G.; Muhle-Goll, C.; Luy, B.; Haitz, F.; Hahn, T.; Zibek, S.; Syldatk, C. Integrated process for the enzymatic production of fatty acid sugar esters completely based on lignocellulosic substrates. Front. Chem. 2018, 6, 421. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Qin, X.; Tan, C.P.; Li, D.; Wang, Y. Choline-chloride-based eutectic solvent for the efficient production of docosahexaenoyl and eicosapentaenoyl ethanolamides via an enzymatic process. J. Agric. Food Chem. 2018, 66, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiong, J.; Huang, Z.; Cao, S.; Zong, M.; Lou, W. Improving biocatalysis of cefaclor with penicillin acylase immobilized on magnetic nanocrystalline cellulose in deep eutectic solvent based co-solvent. Bioresour. Technol. 2019, 288, 121548. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-X.; Cao, S.-L.; Xu, P.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Preparation of a novel nanobiocatalyst by immobilizing penicillin acylase onto magnetic nanocrystalline cellulose and its use for efficient synthesis of cefaclor. Chem. Eng. J. 2018, 346, 361–368. [Google Scholar] [CrossRef]
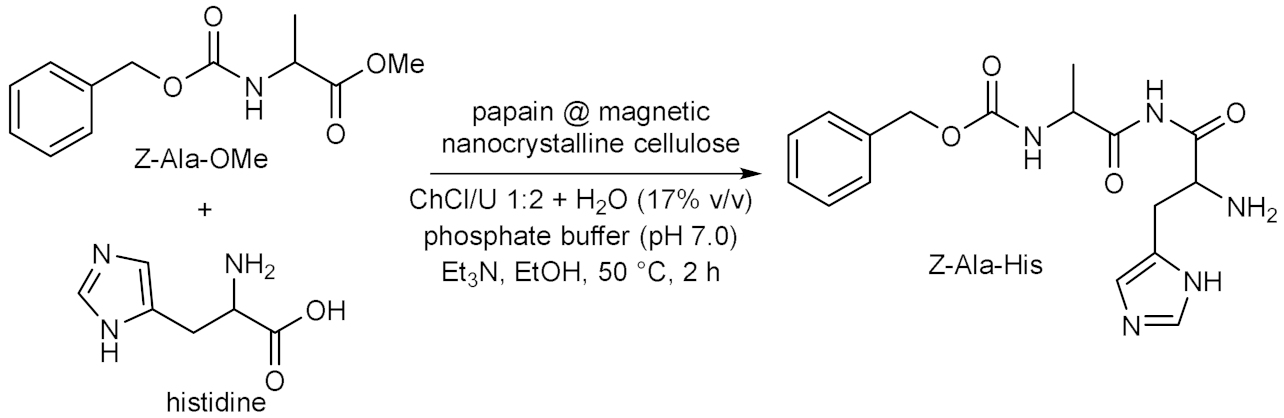
- Cao, S.-L.; Xu, H.; Li, X.-H.; Lou, W.-Y.; Zong, M.-H. Papain@ magnetic nanocrystalline cellulose nanobiocatalyst: A highly efficient biocatalyst for dipeptide biosynthesis in deep eutectic solvents. ACS Sustain. Chem. Eng. 2015, 3, 1589–1599. [Google Scholar] [CrossRef]
- Xiong, J.; Cao, S.-L.; Zong, M.-H.; Lou, W.-Y.; Wu, X.-L. Biosynthesis of alanyl-histidine dipeptide catalyzed by papain immobilized on magnetic nanocrystalline cellulose in deep eutectic solvents. Appl. Biochem. Biotechnol. 2020, 192, 573–584. [Google Scholar] [CrossRef]
- Li, H.; Ni, Y.; Cao, X.; He, X.; Li, G.; Chen, K.; Ouyang, P.; Yang, J.; Tan, W. Highly active nanobiocatalysis in deep eutectic solvents via metal-driven enzyme-surfactant nanocomposite. J. Biotechnol. 2019, 292, 39–49. [Google Scholar] [CrossRef]
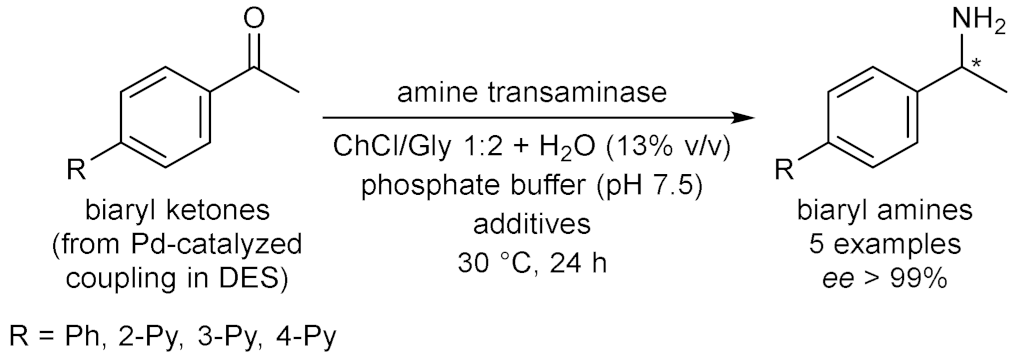
- Paris, J.; Telzerow, A.; Ríos-Lombardía, N.; Steiner, K.; Schwab, H.; Morís, F.; Gröger, H.; González-Sabín, J. Enantioselective one-pot synthesis of biaryl-substituted amines by combining palladium and enzyme catalysis in deep eutectic solvents. ACS Sustain. Chem. Eng. 2019, 7, 5486–5493. [Google Scholar] [CrossRef]
- Li, Q.; Di, J.; Liao, X.; Ni, J.; Li, Q.; He, Y.-C.; Ma, C. Exploration of benign deep eutectic solvent–water systems for the highly efficient production of furfurylamine from sugarcane bagasse via chemoenzymatic cascade catalysis. Green Chem. 2021, 23, 8154–8168. [Google Scholar] [CrossRef]
- Ni, J.; Li, Q.; Gong, L.; Liao, X.-L.; Zhang, Z.-J.; Ma, C.; He, Y. Highly efficient chemoenzymatic cascade catalysis of biomass into furfurylamine by a heterogeneous shrimp shell-based chemocatalyst and an ω-transaminase biocatalyst in deep eutectic solvent–water. ACS Sustain. Chem. Eng. 2021, 9, 13084–13095. [Google Scholar] [CrossRef]
- Wang, Z.; Chai, H.; Ren, J.; Tao, Y.; Li, Q.; Ma, C.; Ai, Y.; He, Y. Biocatalytic valorization of biobased 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furfurylamine in a three-constituent deep eutectic solvent–water system. ACS Sustain. Chem. Eng. 2022, 10, 8452–8463. [Google Scholar] [CrossRef]
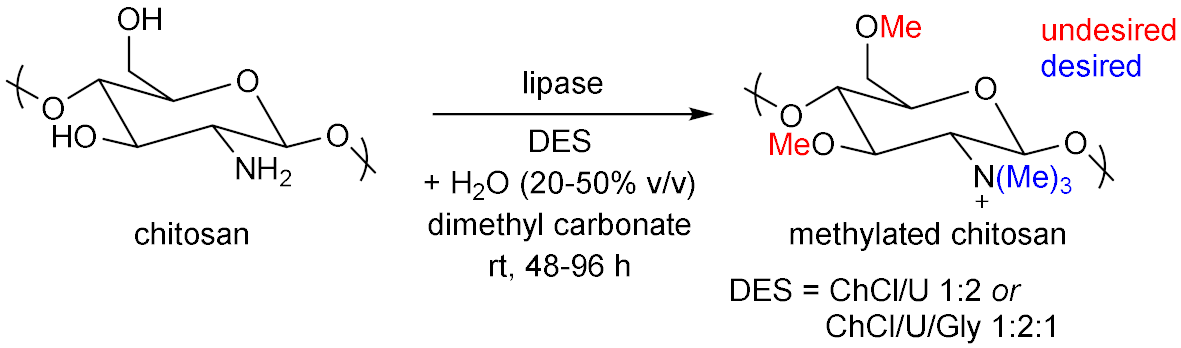
- Bangde, P.S.; Jain, R.; Dandekar, P. Alternative approach to synthesize methylated chitosan using deep eutectic solvents, biocatalyst and “green” methylating agents. ACS Sustain. Chem. Eng. 2016, 4, 3552–3557. [Google Scholar] [CrossRef]
- Mahajan, T.; Bangde, P.; Dandekar, P.; Jain, R. Greener approach for synthesis of N,N,N-trimethyl chitosan (TMC) using ternary deep eutectic solvents (TDESs). Carbohydr. Res. 2020, 493, 108033. [Google Scholar] [CrossRef]
- Morley, K.L.; Grosse, S.; Leisch, H.; Lau, P.C. Antioxidant canolol production from a renewable feedstock via an engineered decarboxylase. Green Chem. 2013, 15, 3312–3317. [Google Scholar] [CrossRef]
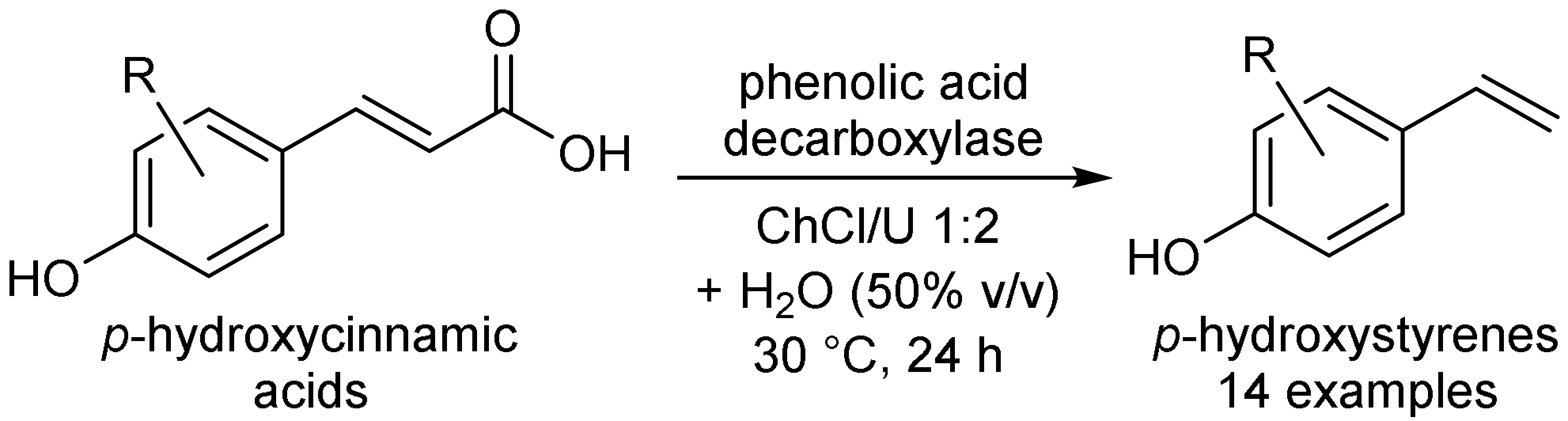
- Schweiger, A.K.; Ríos-Lombardía, N.; Winkler, C.K.; Schmidt, S.; Morís, F.; Kroutil, W.; Gonzalez-Sabin, J.; Kourist, R. Using deep eutectic solvents to overcome limited substrate solubility in the enzymatic decarboxylation of bio-based phenolic acids. ACS Sustain. Chem. Eng. 2019, 7, 16364–16370. [Google Scholar] [CrossRef]
- Grabner, B.; Schweiger, A.K.; Gavric, K.; Kourist, R.; Gruber-Woelfler, H. A chemo-enzymatic tandem reaction in a mixture of deep eutectic solvent and water in continuous flow. React. Chem. Eng. 2020, 5, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Morís, F.; Kourist, R.; Comino, N.; López-Gallego, F.; González-Sabín, J.; García-Álvarez, J. DES ign of Sustainable One-Pot Chemoenzymatic Organic Transformations in Deep Eutectic Solvents for the Synthesis of 1, 2-Disubstituted Aromatic Olefins. Front. Chem. 2020, 8, 139. [Google Scholar] [CrossRef]
- Yang, S.-L.; Duan, Z.-Q. Insight into enzymatic synthesis of phosphatidylserine in deep eutectic solvents. Catal. Commun. 2016, 82, 16–19. [Google Scholar] [CrossRef]
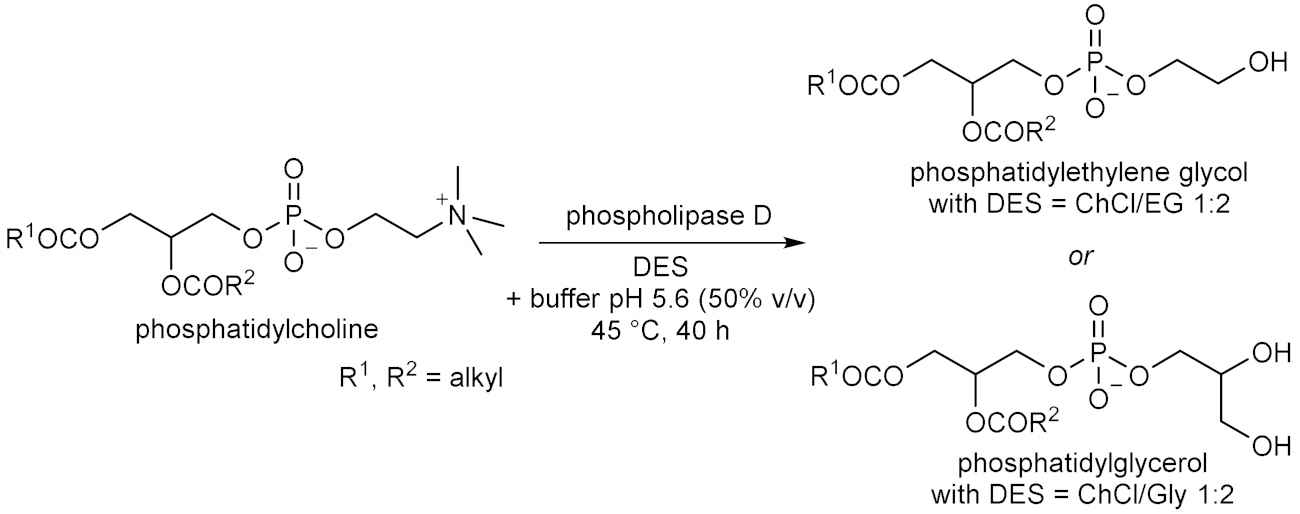
- Allegretti, C.; Gatti, F.G.; Marzorati, S.; Rossato, L.A.M.; Serra, S.; Strini, A.; D’Arrigo, P. Reactive Deep Eutectic Solvents (RDESs): A new tool for phospholipase D-catalyzed preparation of phospholipids. Catalysts 2021, 11, 655. [Google Scholar] [CrossRef]
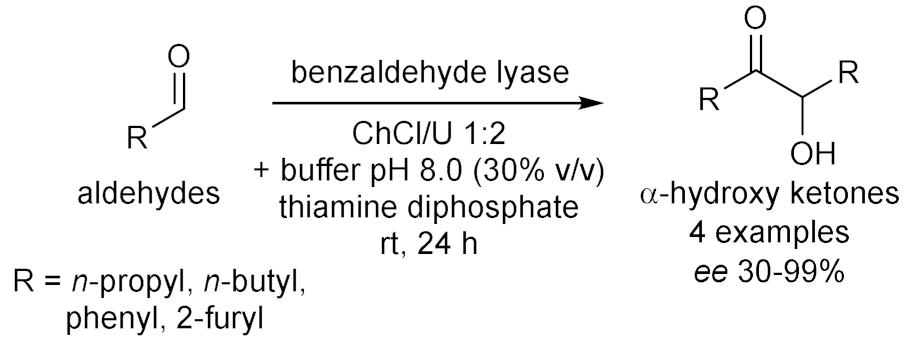
- Maugeri, Z.; de María, P.D. Benzaldehyde lyase (BAL)-catalyzed enantioselective CC bond formation in deep-eutectic-solvents–buffer mixtures. J. Mol. Catal. B Enzym. 2014, 107, 120–123. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.-Y.; Liang, X.-Y.; Liang, Z.-H.; Cheng, H.; Li, X.; Li, L.-L. Pepsin-Catalyzed Asymmetric Cross Aldol Reaction Promoted by Ionic Liquids and Deep Eutectic Solvents. Catal. Lett. 2020, 150, 2549–2557. [Google Scholar] [CrossRef]
- Nejrotti, S.; Antenucci, A.; Pontremoli, C.; Gontrani, L.; Barbero, N.; Carbone, M.; Bonomo, M. Critical Assessment of the Sustainability of Deep Eutectic Solvents: A Case Study on Six Choline Chloride-Based Mixtures. ACS Omega 2022, 7, 47449–47461. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Trivedi, S.; Rai, R.; Pandey, S. Densities and dynamic viscosities of (choline chloride + glycerol) deep eutectic solvent and its aqueous mixtures in the temperature range (283.15–363.15) K. Fluid Phase Equilibria 2014, 367, 135–142. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, S. Densities and viscosities of (choline chloride + urea) deep eutectic solvent and its aqueous mixtures in the temperature range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Mannu, A.; Blangetti, M.; Baldino, S.; Prandi, C. Promising Technological and Industrial Applications of Deep Eutectic Systems. Materials 2021, 14, 2494. [Google Scholar] [CrossRef]
| Entry | Reductions with Isolated Enzymes | Ref. |
|---|---|---|
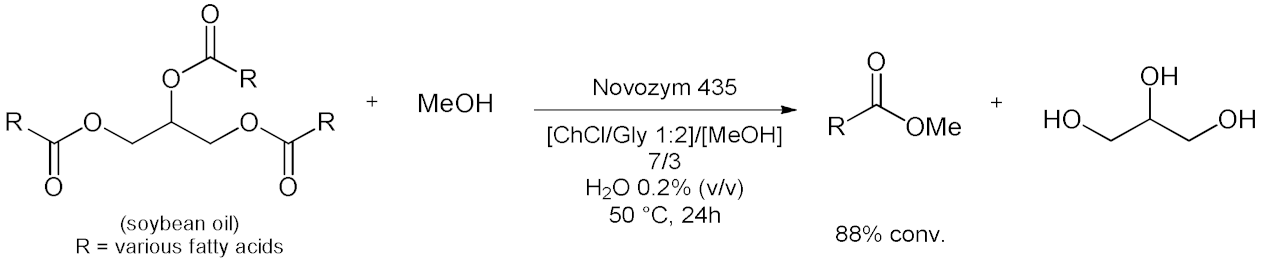
| 1 | 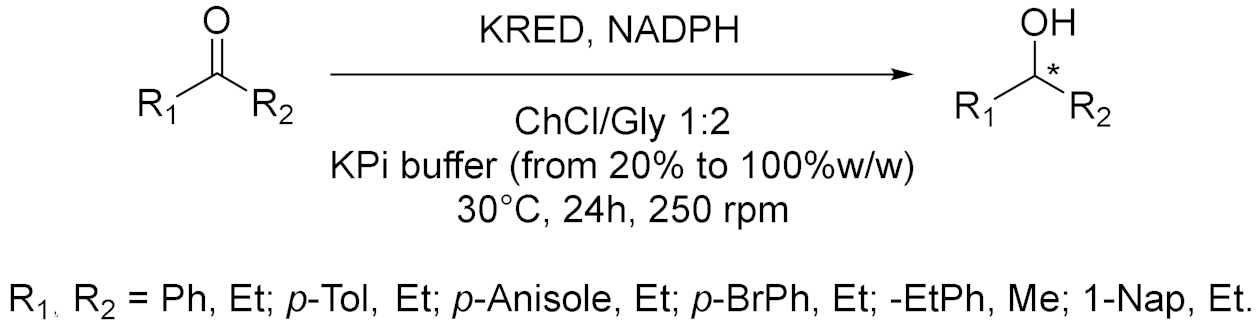 | [12] |
| 2 |  | [13] |
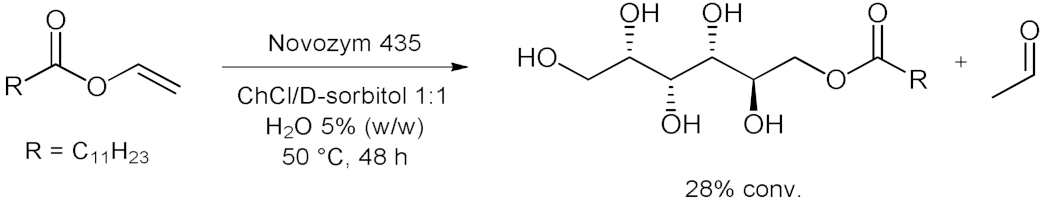
| 3 | 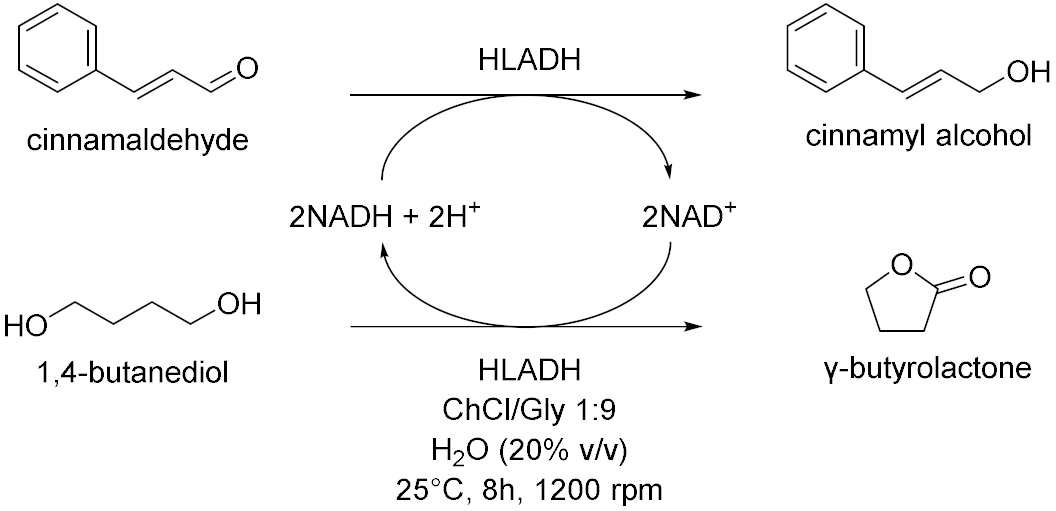 | [14] |
| 4 | 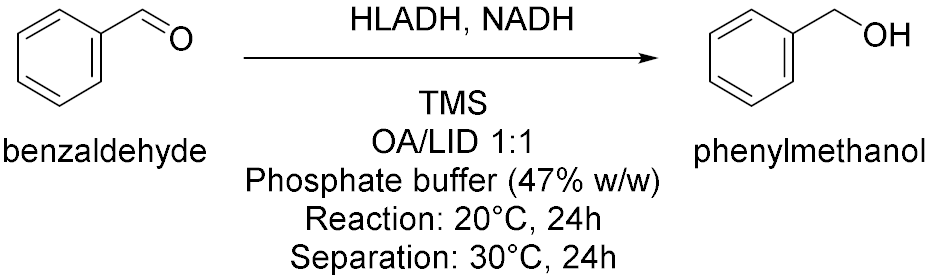 | [15] |
| Entry | Whole-Cells Catalysed Reductions | Ref. |
|---|---|---|
| 1 | 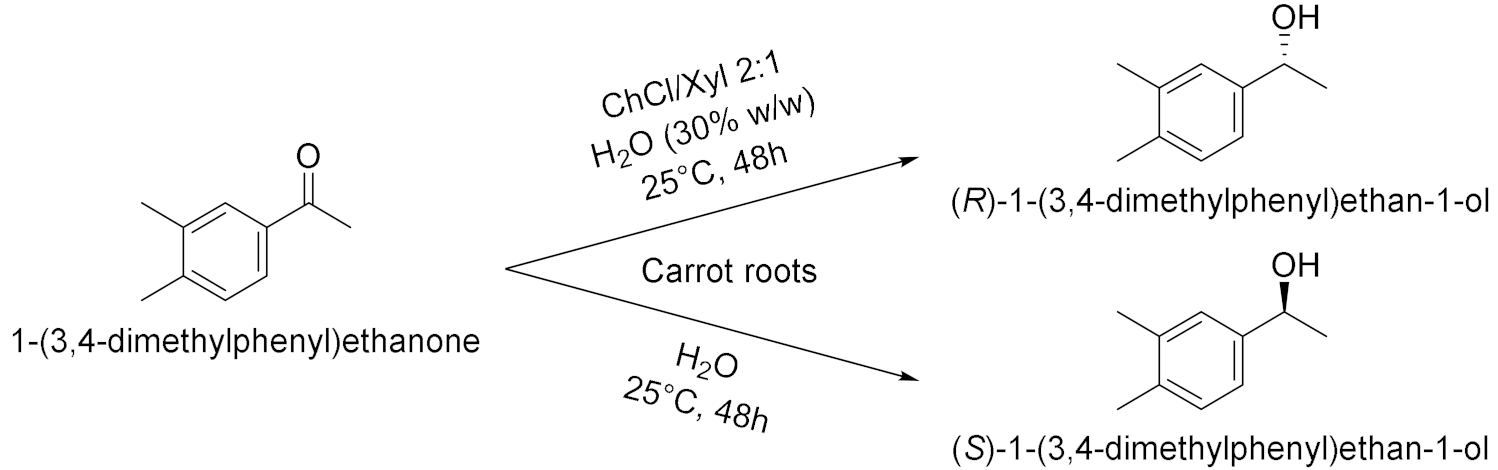 | [16] |
| 2 | 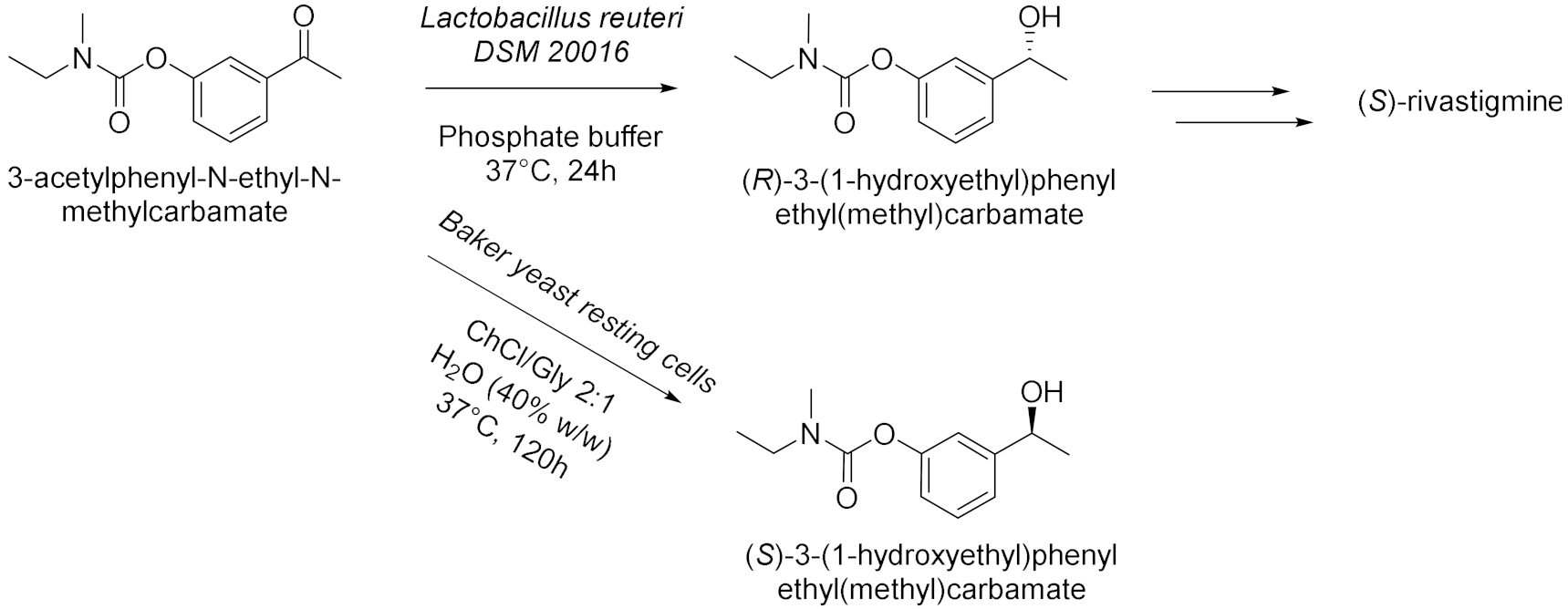 | [17] |
| 3 | 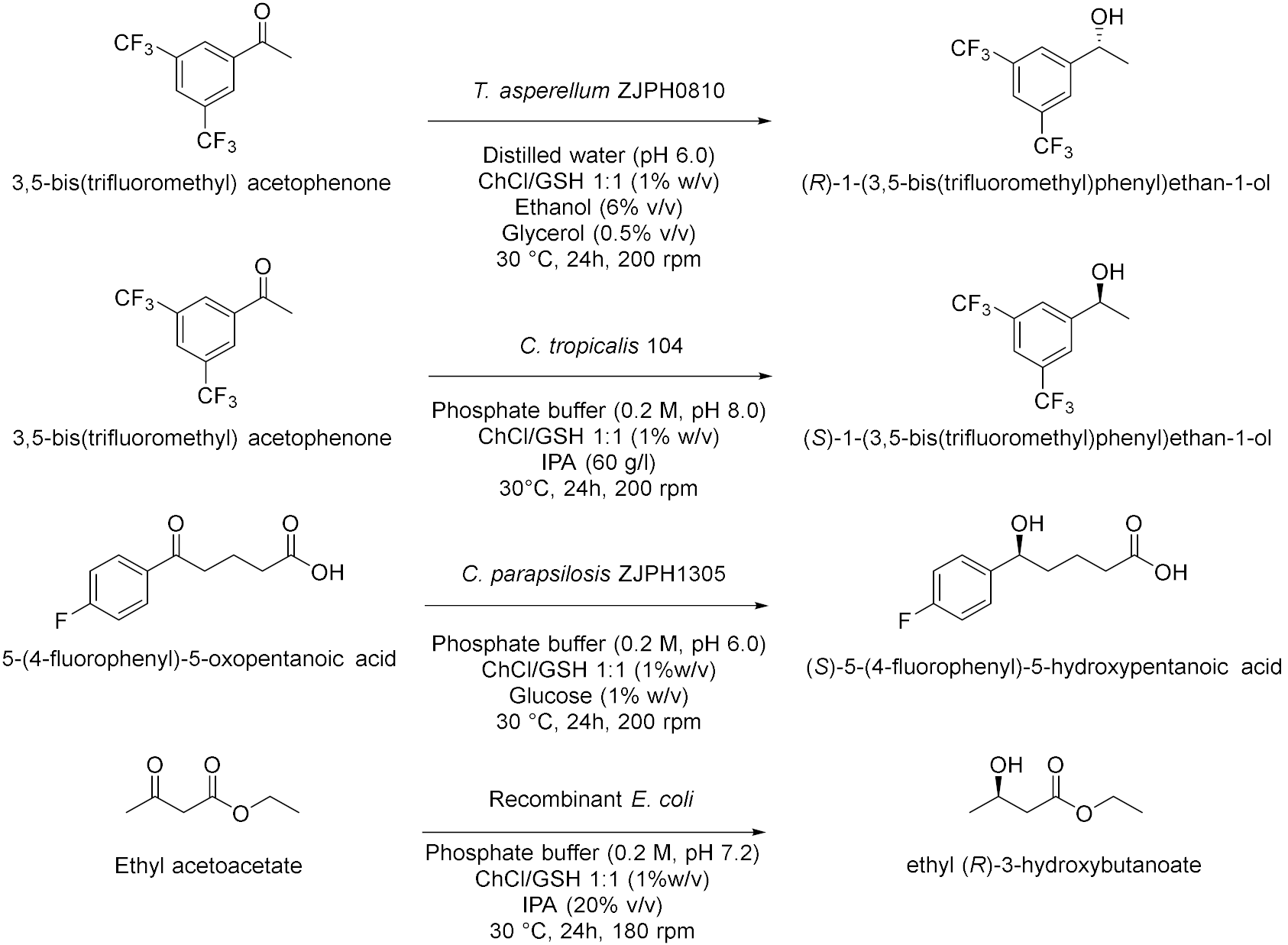 | [18] |
| 4 | 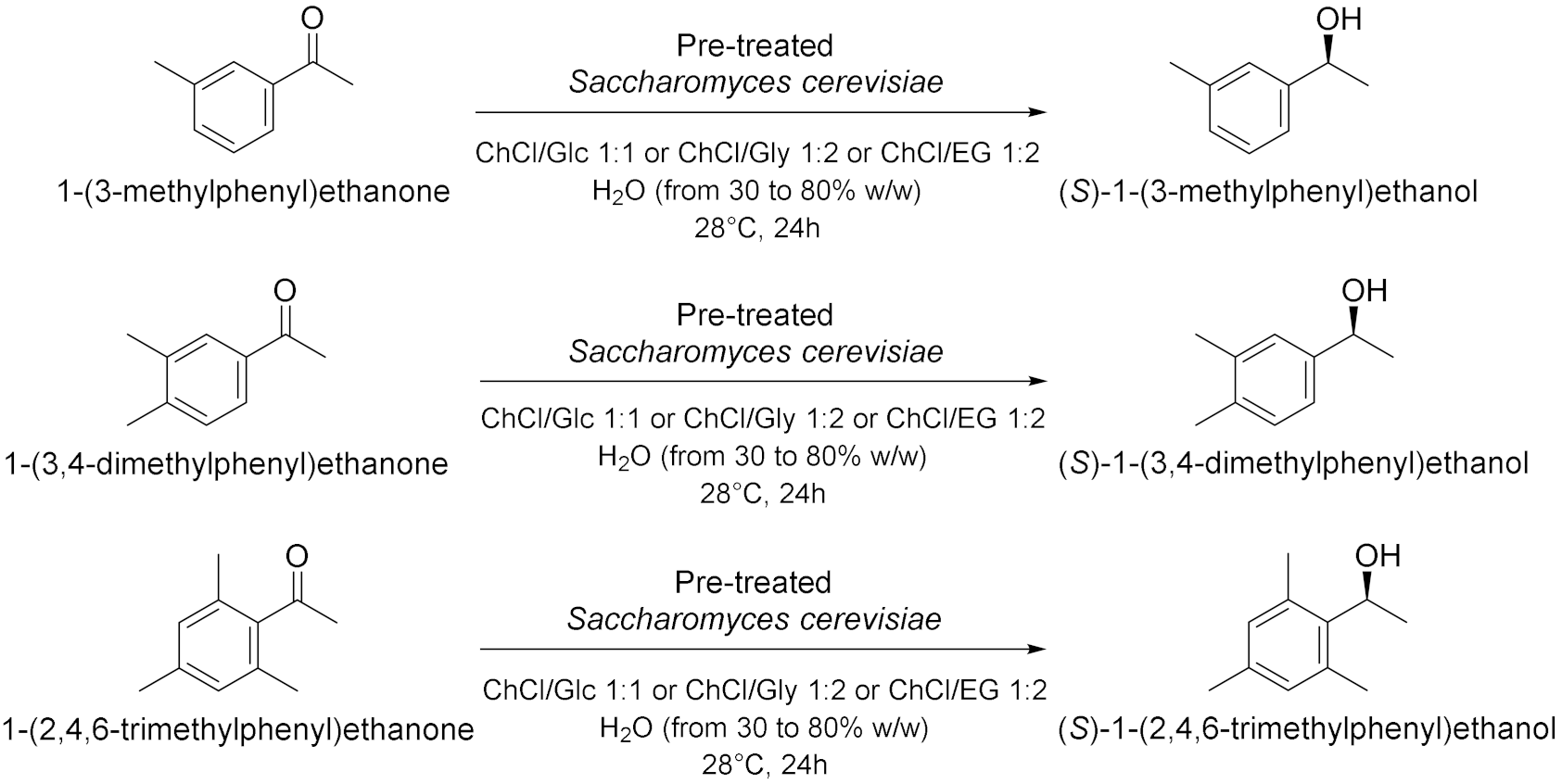 | [19] |
| 5 | 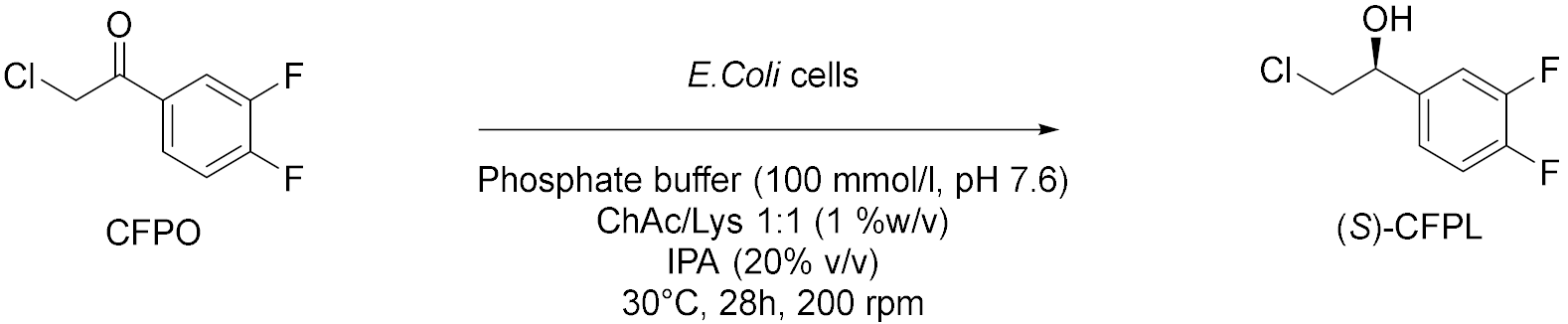 | [20] |
| 6 |  | [21] |
| 7 | 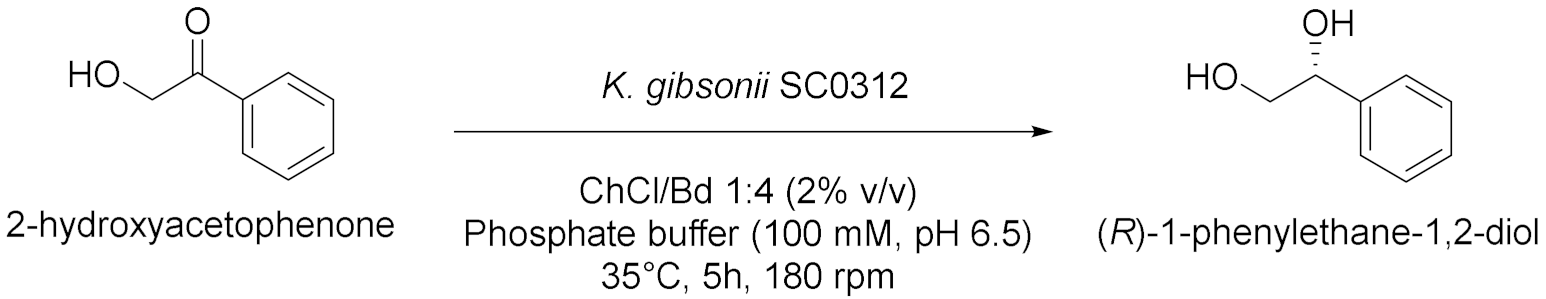 | [22] |
| 8 |  | [23] |
| 9 | 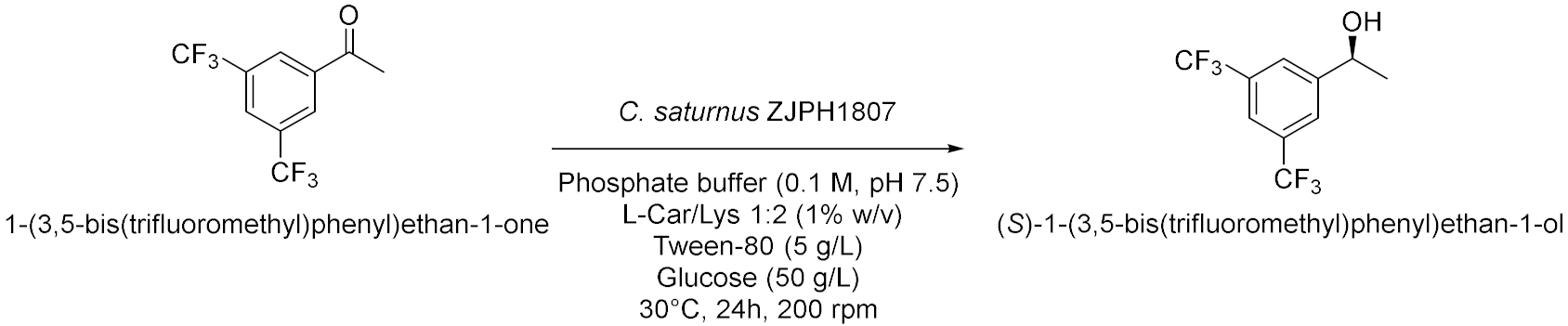 | [24] |
| 10 | 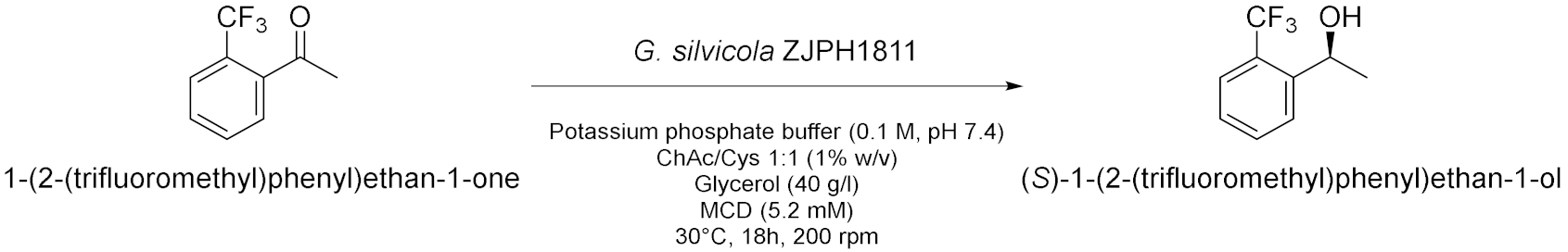 | [25] |
| 11 | 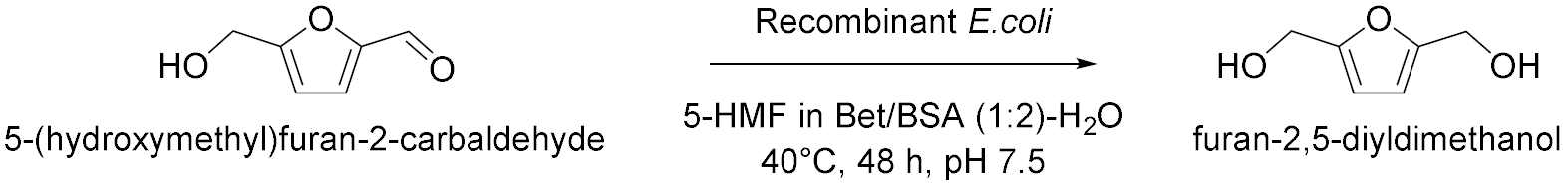 | [26] |
| Entry | Hydrolyses with Isolated Enzymes | Ref. |
|---|---|---|
| 1 | 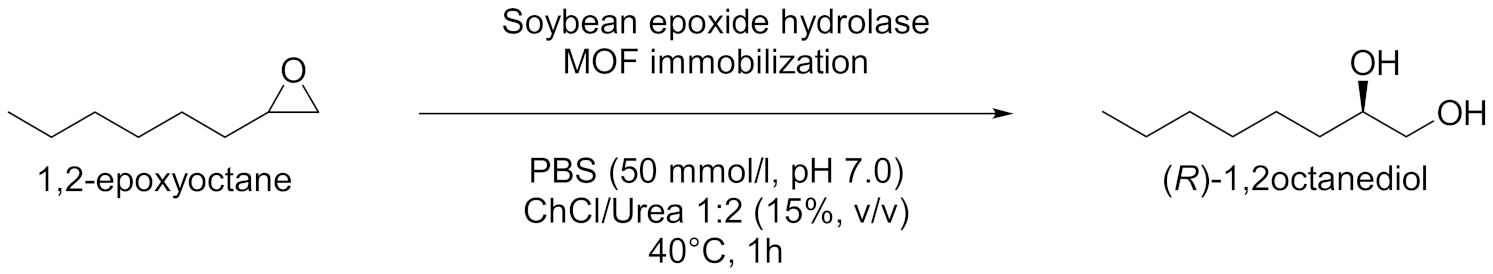 | [56] |
| 2 | 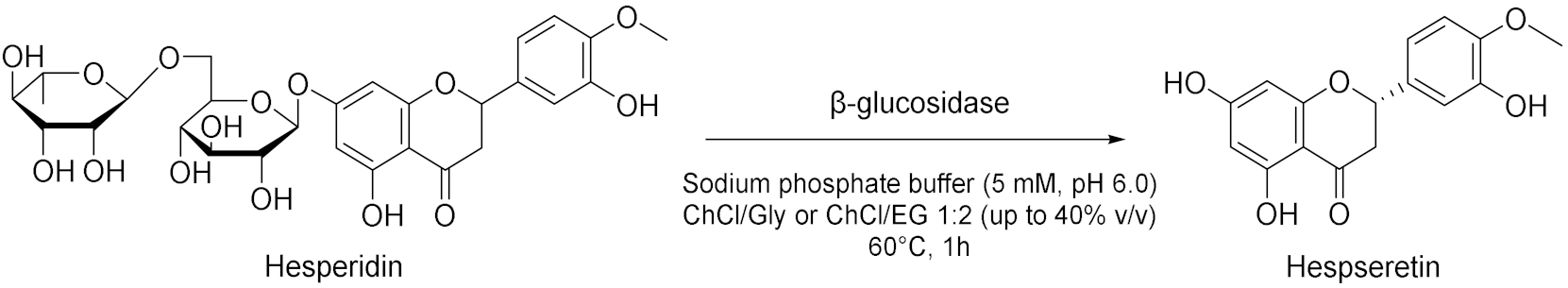 | [57] |
| 3 |  | [58] |
| 4 |  | [59] |
| 5 |  | [60] |
| 6 | 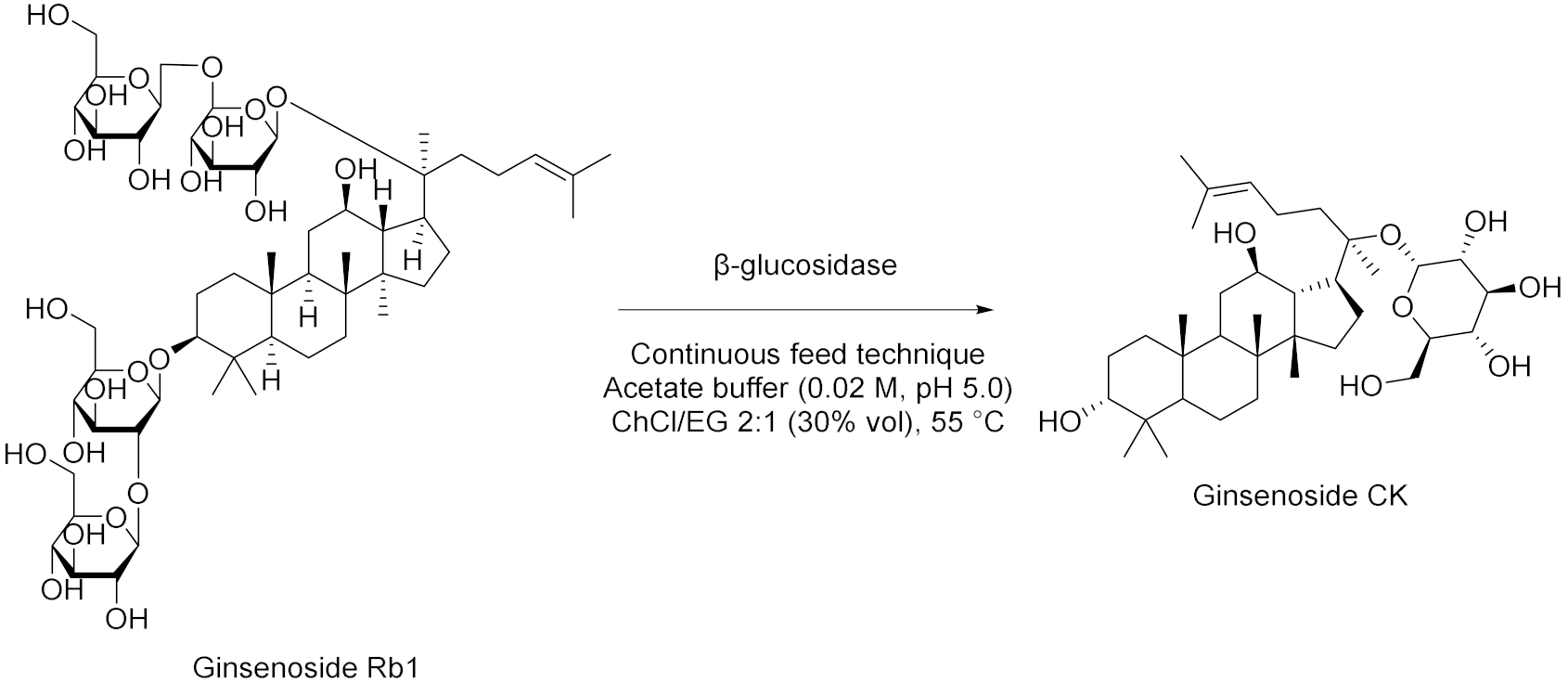 | [61] |
| 7 |  | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnodo, D.; Maffeis, E.; Marra, F.; Nejrotti, S.; Prandi, C. Combination of Enzymes and Deep Eutectic Solvents as Powerful Toolbox for Organic Synthesis. Molecules 2023, 28, 516. https://doi.org/10.3390/molecules28020516
Arnodo D, Maffeis E, Marra F, Nejrotti S, Prandi C. Combination of Enzymes and Deep Eutectic Solvents as Powerful Toolbox for Organic Synthesis. Molecules. 2023; 28(2):516. https://doi.org/10.3390/molecules28020516
Chicago/Turabian StyleArnodo, Davide, Elia Maffeis, Francesco Marra, Stefano Nejrotti, and Cristina Prandi. 2023. "Combination of Enzymes and Deep Eutectic Solvents as Powerful Toolbox for Organic Synthesis" Molecules 28, no. 2: 516. https://doi.org/10.3390/molecules28020516
APA StyleArnodo, D., Maffeis, E., Marra, F., Nejrotti, S., & Prandi, C. (2023). Combination of Enzymes and Deep Eutectic Solvents as Powerful Toolbox for Organic Synthesis. Molecules, 28(2), 516. https://doi.org/10.3390/molecules28020516