Identification of Structural Determinants of the Transport of the Dehydroascorbic Acid Mediated by Glucose Transport GLUT1
Abstract
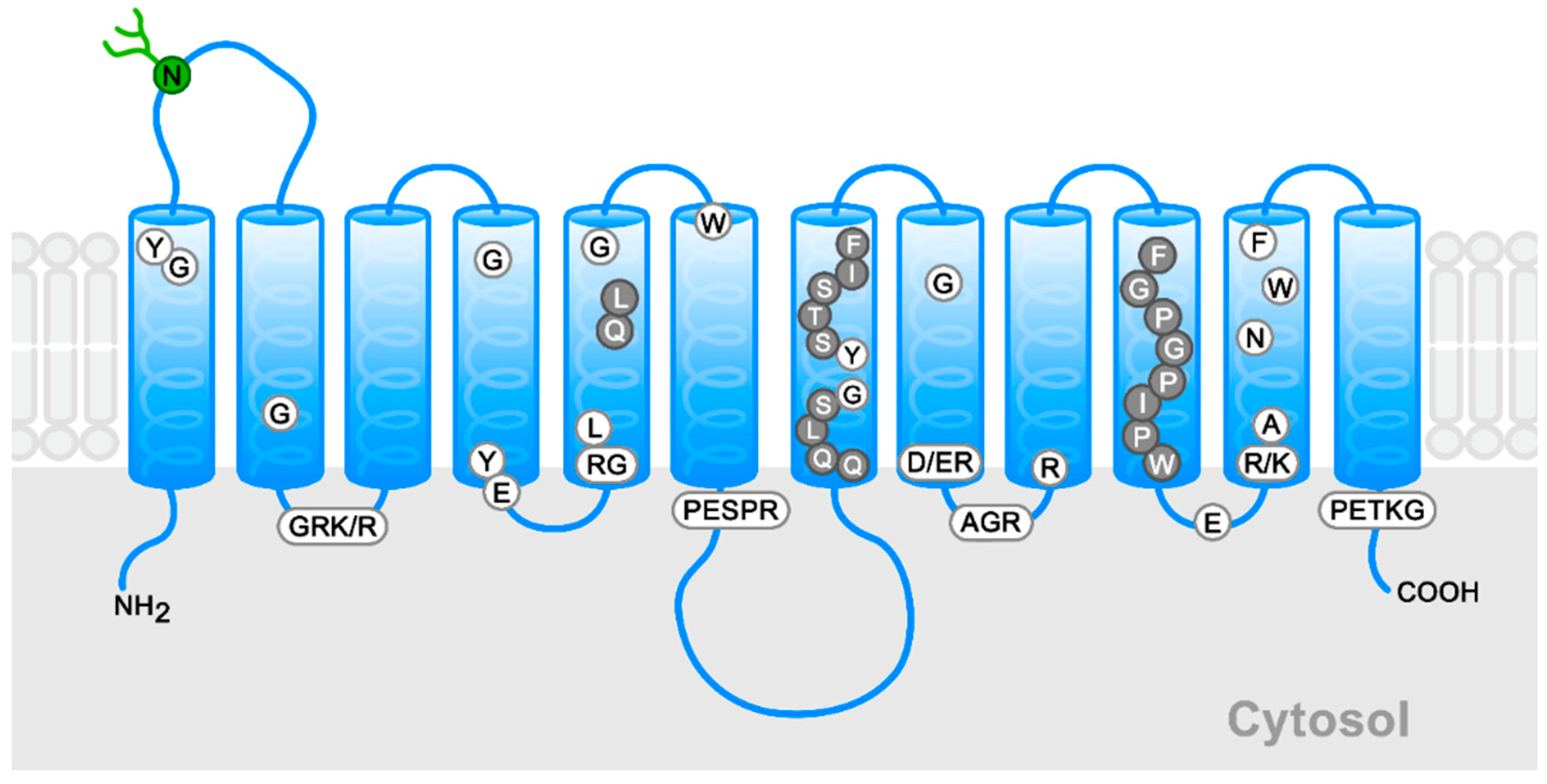
1. Introduction
2. Results
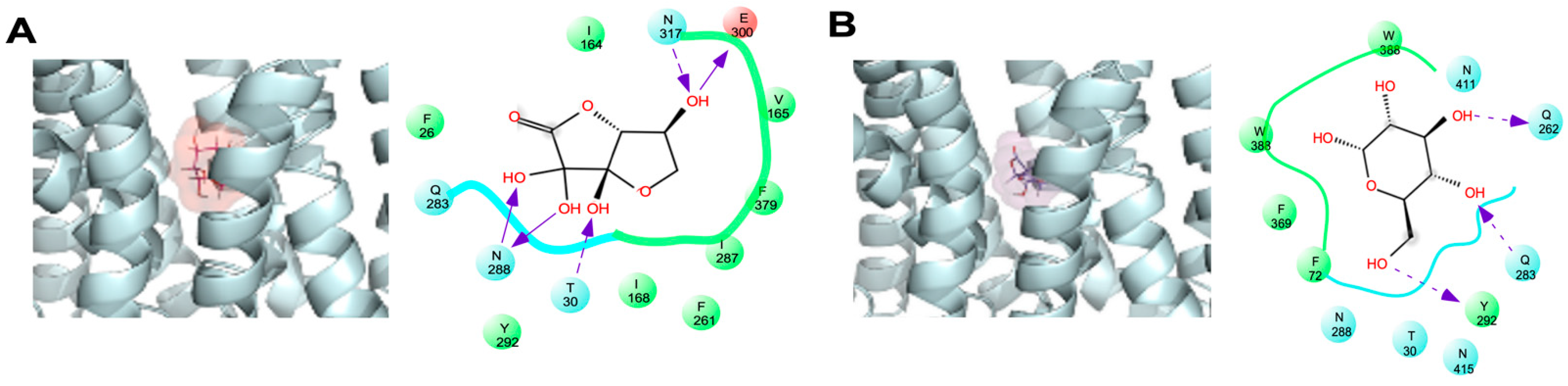
2.1. Docking Study of DHA in GLUT1
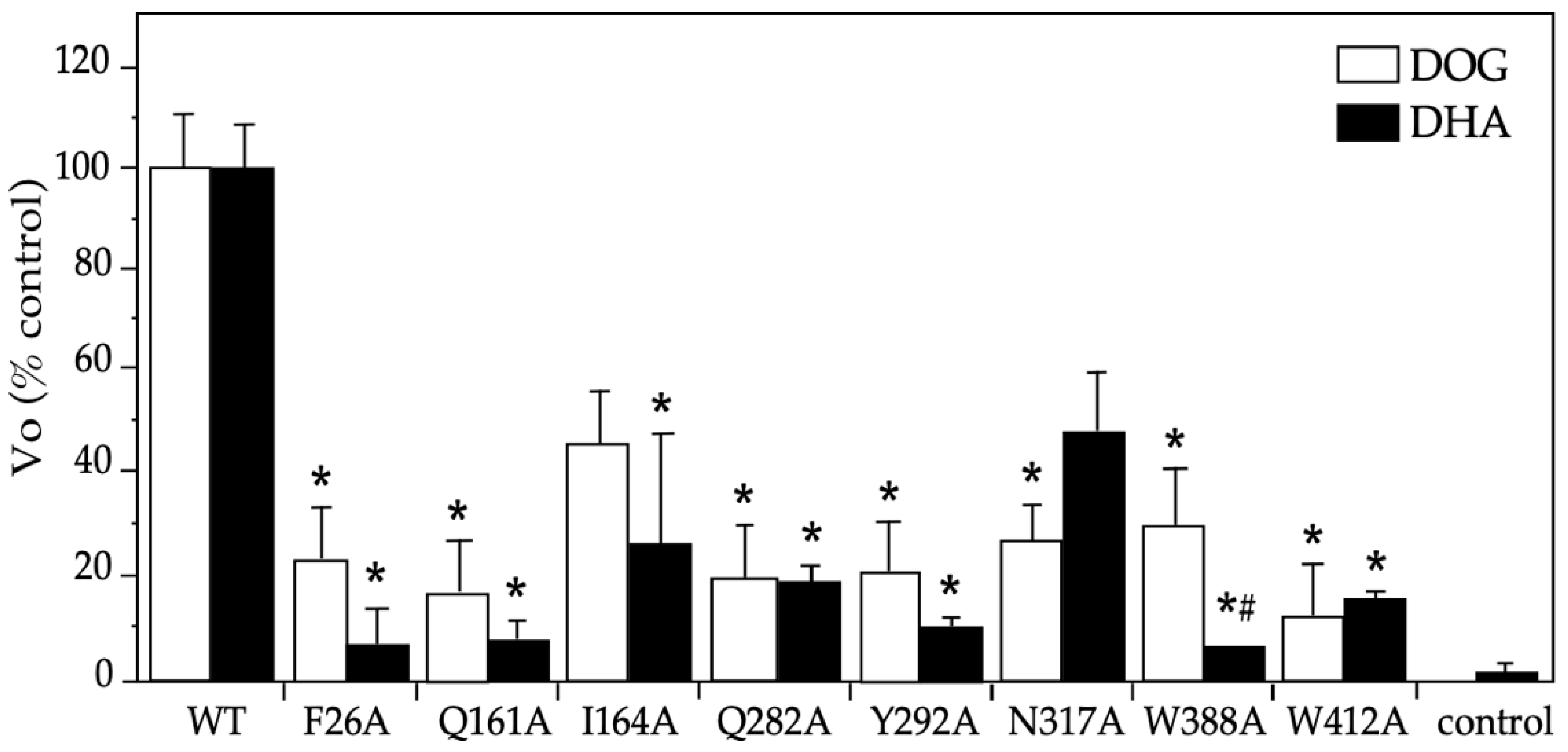
2.2. Functional Studies on DHA’s Transport via the Eight GLUT1 Mutants
2.3. Functional Characterization of the DHA Transport via the GLUT1 Mutant N317A
3. Discussion
4. Materials and Methods
4.1. Docking Simulation
4.2. GLUT1 Mutants
4.3. Animal Procedure
4.4. Transport Assays
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AA | ascorbic acid |
| DHA | dehydroascorbic acid |
| DHA(bi) | bicyclic hemiacetal dehydroascorbic acid |
| ∆Gbind | binding free energy |
| G-6-P | glucose-6-phosphate |
| GLUT | glucose transporter |
| IC50 | inhibitory concentration 50 |
| Km | Michaelis–Menten kinetic constant |
| TM | transmembrane helix |
| Vmax | maximum velocity |
| Vo | initial transport rate |
References
- Hruz, P.W.; Mueckler, M.M. Structural analysis of the GLUT1 facilitative glucose transporter (review). Mol. Membr. Biol. 2001, 18, 183–193. [Google Scholar] [CrossRef]
- Carruthers, A.; DeZutter, J.; Ganguly, A.; Devaskar, S.U. Will the original glucose transporter isoform please stand up! Am. J. Physiol. Endocrinol. Metab. 2009, 297, E836–E848. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Kinne, R.K. Pathogenic mutations causing transport defects in GLUT1 transporter: The role of intermolecular forces in protein structure-function. Biophys. Chem. 2015, 200–201, 9–17. [Google Scholar] [CrossRef]
- Klepper, J.; Akman, C.; Armeno, M.; Auvin, S.; Cervenka, M.; Cross, H.J.; De Giorgis, V.; Della Marina, A.; Engelstad, K.; Heussinger, N.; et al. Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open. 2020, 5, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Ancey, P.B.; Contat, C.; Meylan, E. Glucose transporters in cancer—From tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Sage, J.M.; Carruthers, A. Human erythrocytes transport dehydroascorbic acid and sugars using the same transporter complex. Am. J. Physiol. Cell Physiol. 2014, 306, C910–C917. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutterdge, J. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Rivas, C.I.; Zúñiga, F.A.; Salas-Burgos, A.; Mardones, L.; Ormazabal, V.; Vera, J.C. Vitamin C transporters. J. Physiol. Biochem. 2008, 64, 357–375. [Google Scholar] [CrossRef]
- Nualart, F.J.; Rivas, C.I.; Montecinos, V.P.; Godoy, A.S.; Guaiquil, V.H.; Golde, D.W.; Vera, J.C. Recycling of vitamin C by a bystander effect. J. Biol. Chem. 2003, 278, 10128–10133. [Google Scholar] [CrossRef]
- Hosoya, K.; Minamizono, A.; Katayama, K.; Terasaki, T.; Tomi, M. Vitamin C transport in oxidized form across the rat blood-retinal barrier. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1232–1239. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Kinet, S.; Manel, N.; Mongellaz, C.; Prohaska, R.; Battini, J.L.; Delaunay, J.; Sitbon, M.; Taylor, N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell 2008, 132, 1039–1048. [Google Scholar] [CrossRef]
- Rungaldier, S.; Oberwagner, W.; Salzer, U.; Csaszar, E.; Prohaska, R. Stomatin interacts with GLUT1/SLC2A1, band 3/SLC4A1, and aquaporin-1 in human erythrocyte membrane domains. Biochim. Biophys. Acta 2013, 1828, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Kinne, R.K.H. Mechanistic insights into protein stability and self-aggregation in GLUT1 genetic variants causing GLUT1-deficiency syndrome. J. Membr. Biol. 2020, 253, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, D.; Engelstad, K.; Bagay, L.; Wei, Y.; Rotstein, M.; Aggarwal, V.; Levy, B.; Ma, L.; Chung, W.K.; et al. Glut1 deficiency syndrome and erythrocyte glucose uptake assay. Ann. Neurol. 2011, 70, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Kc, S.; Cárcamo, J.M.; Golde, D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005, 19, 1657–1667. [Google Scholar] [CrossRef]
- Astuya, A.; Caprile, T.; Castro, M.; Salazar, K.; García, M.D.L.; Reinicke, K.; Rodríguez, F.; Vera, J.C.; Millán, C.; Ulloa, V.; et al. Vitamin C uptake and recycling among normal and tumor cells from the central nervous system. J. Neurosci. Res. 2005, 79, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Villagran, M.; Ferreira, J.; Martorell, M.; Mardones, L. The Role of Vitamin C in Cancer Prevention and Therapy: A Literature Review. Antioxidants 2021, 10, 1894. [Google Scholar] [CrossRef]
- Yi-Ching, L.; Hsun-Yi, H.; Chia-Jung, C.; Chao-Hung, C.; Yuan-Tsong, C. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: Mechanistic insight into arterial tortuosity syndrome. Hum. Mol. Genet. 2010, 19, 3721–3733. [Google Scholar] [CrossRef]
- Segade, F. Glucose transporter 10 and arterial tortuosity syndrome: The vitamin C connection. FEBS Lett. 2010, 584, 2990–2994. [Google Scholar] [CrossRef]
- Long, W.; Cheeseman, C.I. Structure of, and functional insight into the GLUT family of membrane transporters. Cell Health Cytoskelet. 2015, 7, 167–183. [Google Scholar] [CrossRef]
- Park, M.S. Molecular dynamics simulations of the human glucose transporter GLUT1. PLoS ONE 2015, 10, e0125361. [Google Scholar] [CrossRef]
- Joost, H.G.; Thorens, B. The extended GLUT-family of sugar/polyol transport facilitators: Nomenclature, sequence characteristics, and potential function of its novel members (review). Mol. Membr. Biol. 2001, 18, 247–256. [Google Scholar] [CrossRef]
- Ferreira, R.S.; Pons, R.L.; Labesse, G. Insights into substrate and inhibitor selectivity among human GLUT transporters through comparative modeling and molecular docking. ACS Omega 2019, 4, 4748–4760. [Google Scholar] [CrossRef]
- Deng, D.; Xu, C.; Sun, P.; Wu, J.; Yan, C.; Hu, M.; Yan, N. Crystal structure of the human glucose transporter 1. Nature 2014, 510, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Salas-Burgos, A.; Iserovich, P.; Zuniga, F.; Vera, J.C.; Fischbarg, J. Predicting the three-dimensional structure of the human facilitative glucose transporter glut1 by a novel evolutionary homology strategy: Insights on the molecular mechanism of substrate migration, and binding sites for glucose and inhibitory molecules. Biophys. J. 2004, 7, 2990–2999. [Google Scholar] [CrossRef]
- Zuniga, F.A.; Shi, G.; Haller, J.F.; Rubashkin, A.; Flynn, D.R.; Iserovich, P.; Fischbarg, J. A three-dimensional model of the human facilitative glucose transporter Glut1. J. Biol. Chem. 2001, 276, 44970–44975. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Weng, W.; Kruse, M. Glutamine 161 of Glut1 glucose transporter is critical for transport activity and exofacial ligand binding. J. Biol. Chem. 1994, 269, 20533–20538. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Makepeace, C. Analysis of transmembrane segment 8 of the GLUT1 glucose transporter by cysteine-scanning mutagenesis and substituted cysteine accessibility. J. Biol. Chem. 2004, 279, 10494–10499. [Google Scholar] [CrossRef]
- Kasahara, T.; Maeda, M.; Boles, E.; Kasahara, M. Identification of a key residue determining substrate affinity in the human glucose transporter GLUT1. Biochim. Biophys. Acta 2009, 1788, 1051–1055. [Google Scholar] [CrossRef]
- Galochkina, T.; Ng Fuk Chong, M.; Challali, L.; Abbar, S.; Etchebest, C. New insights into GLUT1 mechanics during glucose transfer. Sci. Rep. 2019, 9, 998. [Google Scholar] [CrossRef]
- Lloyd, K.P.; Ojelabi, O.A.; De Zutter, J.K.; Carruthers, A. Reconciling contradictory findings: Glucose transporter 1 (GLUT1) functions as an oligomer of allosteric, alternating access transporters. J. Biol. Chem. 2017, 292, 21035–21046. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, G.; Liu, R.; Wei, J.; Zhang-Negrerie, D.; Jian, X.; Gao, Q. Mechanistic study of human glucose transport mediated by GLUT1. J. Chem. Inf. Model 2016, 56, 517–526. [Google Scholar] [CrossRef]
- Custódio, T.F.; Paulsen, P.A.; Frain, K.M.; Pedersen, B.P. Structural comparison of GLUT1 to GLUT3 reveal transport regulation mechanism in sugar porter family. Life Sci. Alliance 2021, 4, e202000858. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Wang, Y.; Li, H.; Brinster, L.R.; Levine, M. Chemical transport knockout for oxidized vitamin C, dehydroascorbic acid, reveals its functions in vivo. E Bio. Med. 2017, 23, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hornung, T.C.; Biesalski, H.K. GLUT-1 explains the evolutionary advantage of the loss of endogenous vitamin C-synthesis: The electron transfer hypothesis. Evol. Med. Public Health 2019, 1, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Mardones, L.; Ormazabal, V.; Romo, X.; Jaña, C.; Binder, P.; Peña, E.; Vergara, M.; Zúñiga, F. The glucose transporter-2 (GLUT2) is a low affinity dehydroascorbic acid transporter. Biochem. Biophys. Res. Commun. 2011, 410, 7–12. [Google Scholar] [CrossRef]
- Tower, D.B. The effects of 2-deoxy-D-glucose on metabolism of slices of cerebral cortex incubated in vitro. J. Neurochem. 1958, 3, 185–205. [Google Scholar] [CrossRef]
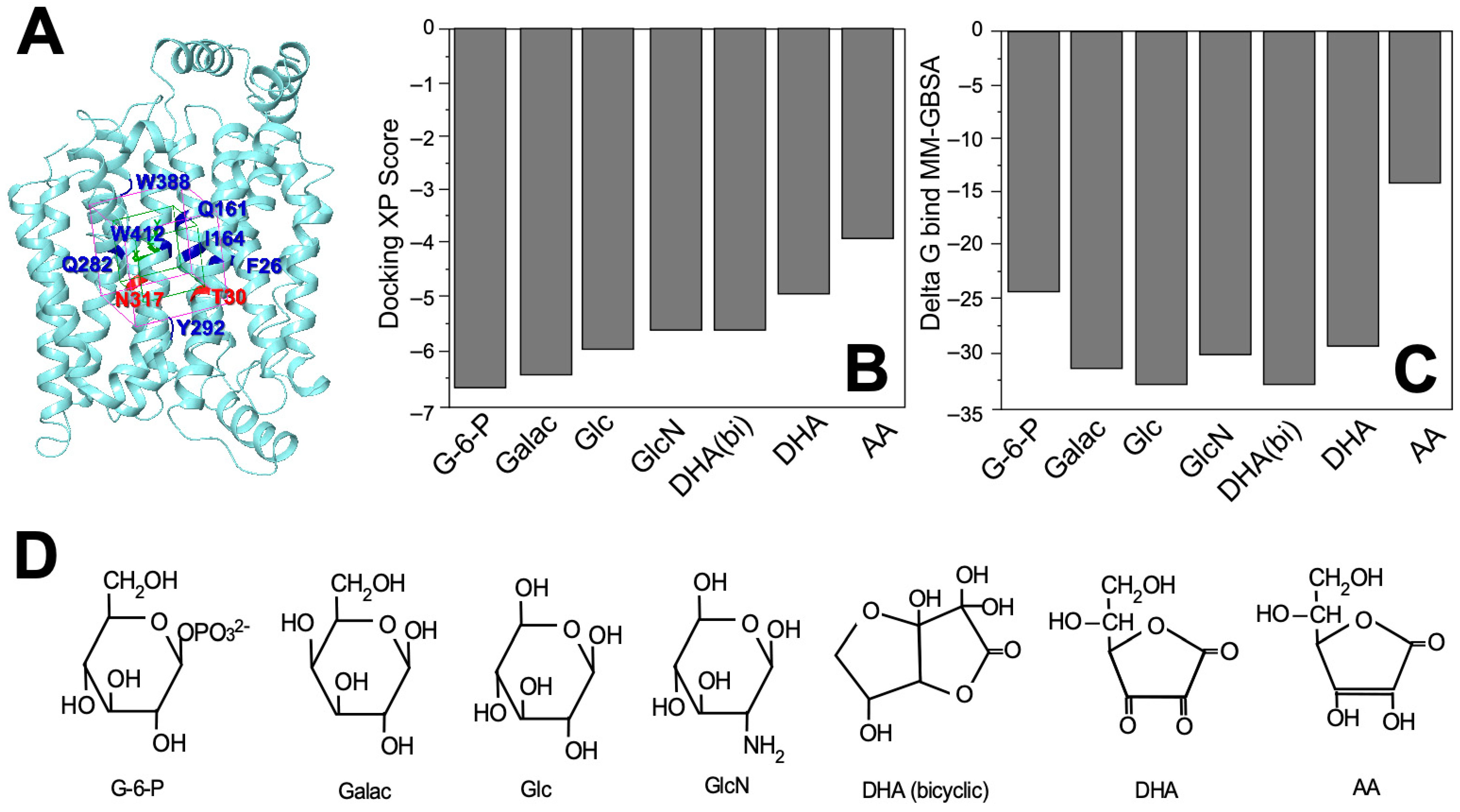


| Molecule | Docking Score | ∆Gbind (kcal/mol) |
|---|---|---|
| Glucose-6-phoshpate | −6.620 | −24.46 |
| D-Galactose | −6.469 | −31.58 |
| D-Glucose | −5.959 | −33.37 |
| D-Glucosamine | −5.628 | −30.53 |
| Dehydroascorbic acid (bicyclic form) | −5.585 | −33.12 |
| Dehydroascorbic acid | −4.874 | −29.99 |
| Ascorbic acid | −3.885 | −14.49 |
| Substrate | Amino Acid Residue |
|---|---|
| DHA(bi) Glucose | F26, R30, I164, V165, I168, F291, Q283, I287, N288, Y292, E300, N317, F379 F26, R30, F72, Q282, Q283, N288, Y292, W388, N411, W412, N415 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villagrán, M.; Burgos, C.F.; Rivas, C.I.; Mardones, L. Identification of Structural Determinants of the Transport of the Dehydroascorbic Acid Mediated by Glucose Transport GLUT1. Molecules 2023, 28, 521. https://doi.org/10.3390/molecules28020521
Villagrán M, Burgos CF, Rivas CI, Mardones L. Identification of Structural Determinants of the Transport of the Dehydroascorbic Acid Mediated by Glucose Transport GLUT1. Molecules. 2023; 28(2):521. https://doi.org/10.3390/molecules28020521
Chicago/Turabian StyleVillagrán, Marcelo, Carlos F. Burgos, Coralia I. Rivas, and Lorena Mardones. 2023. "Identification of Structural Determinants of the Transport of the Dehydroascorbic Acid Mediated by Glucose Transport GLUT1" Molecules 28, no. 2: 521. https://doi.org/10.3390/molecules28020521
APA StyleVillagrán, M., Burgos, C. F., Rivas, C. I., & Mardones, L. (2023). Identification of Structural Determinants of the Transport of the Dehydroascorbic Acid Mediated by Glucose Transport GLUT1. Molecules, 28(2), 521. https://doi.org/10.3390/molecules28020521







