Application of Gas-Liquid Microextraction (GLME)/GC-MS for Flavour and Fragrance in Ice Cream Detection and Composition Analysis
Abstract
1. Introduction
2. Results and Discussion
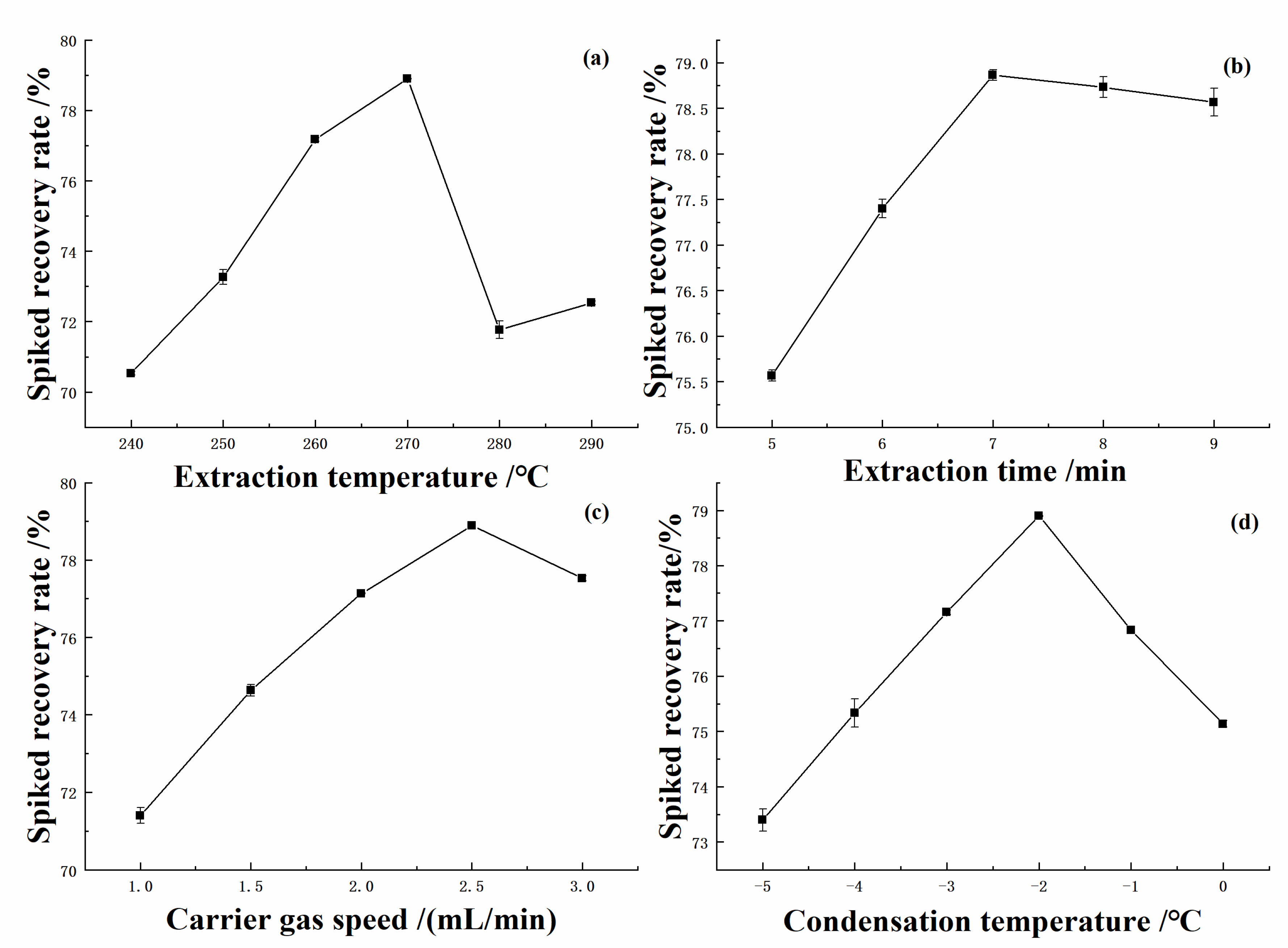
2.1. Optimization of GLME Operating Conditions
2.1.1. Extraction Temperature
2.1.2. Extraction Time
2.1.3. Carrier Gas Speed
2.1.4. Condensation Temperature
2.1.5. Response Surface Design Optimization
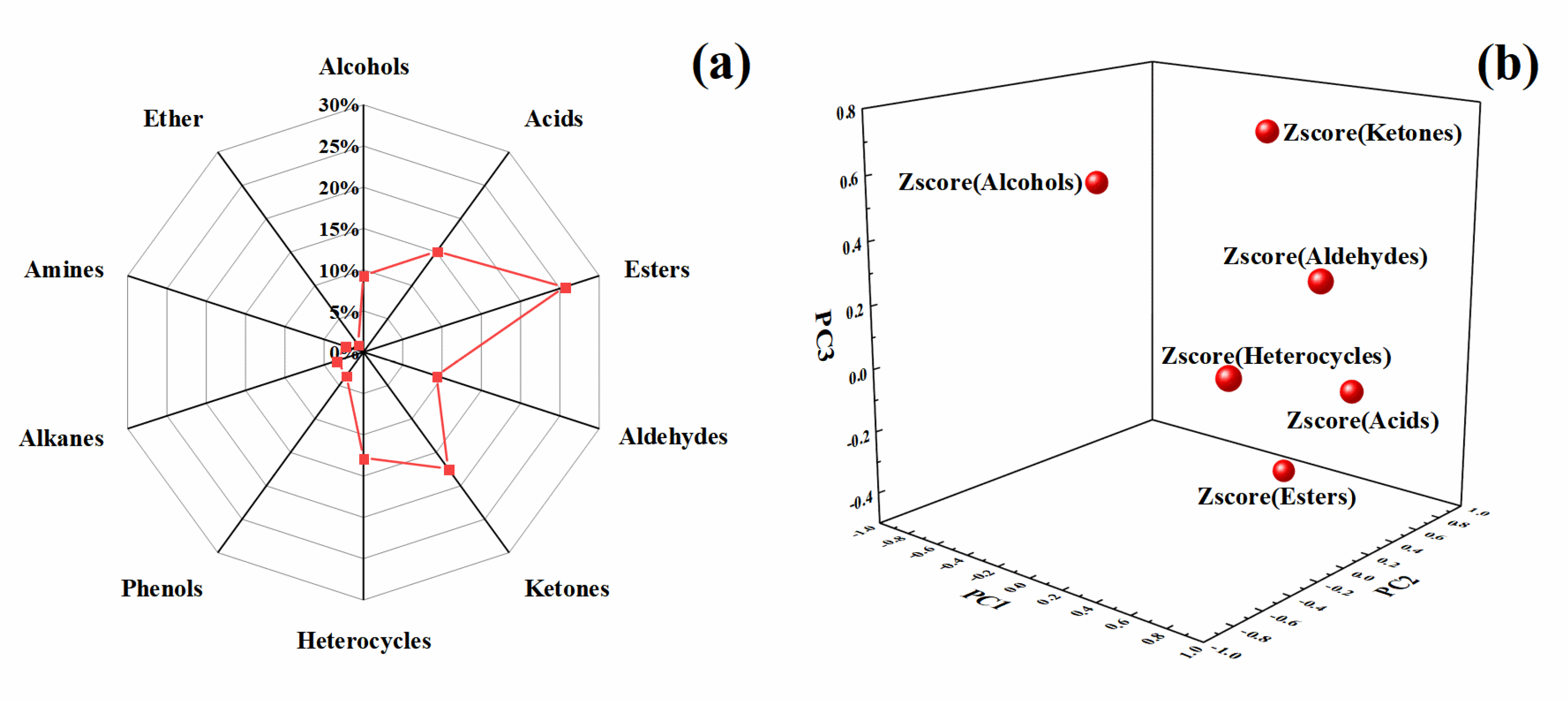
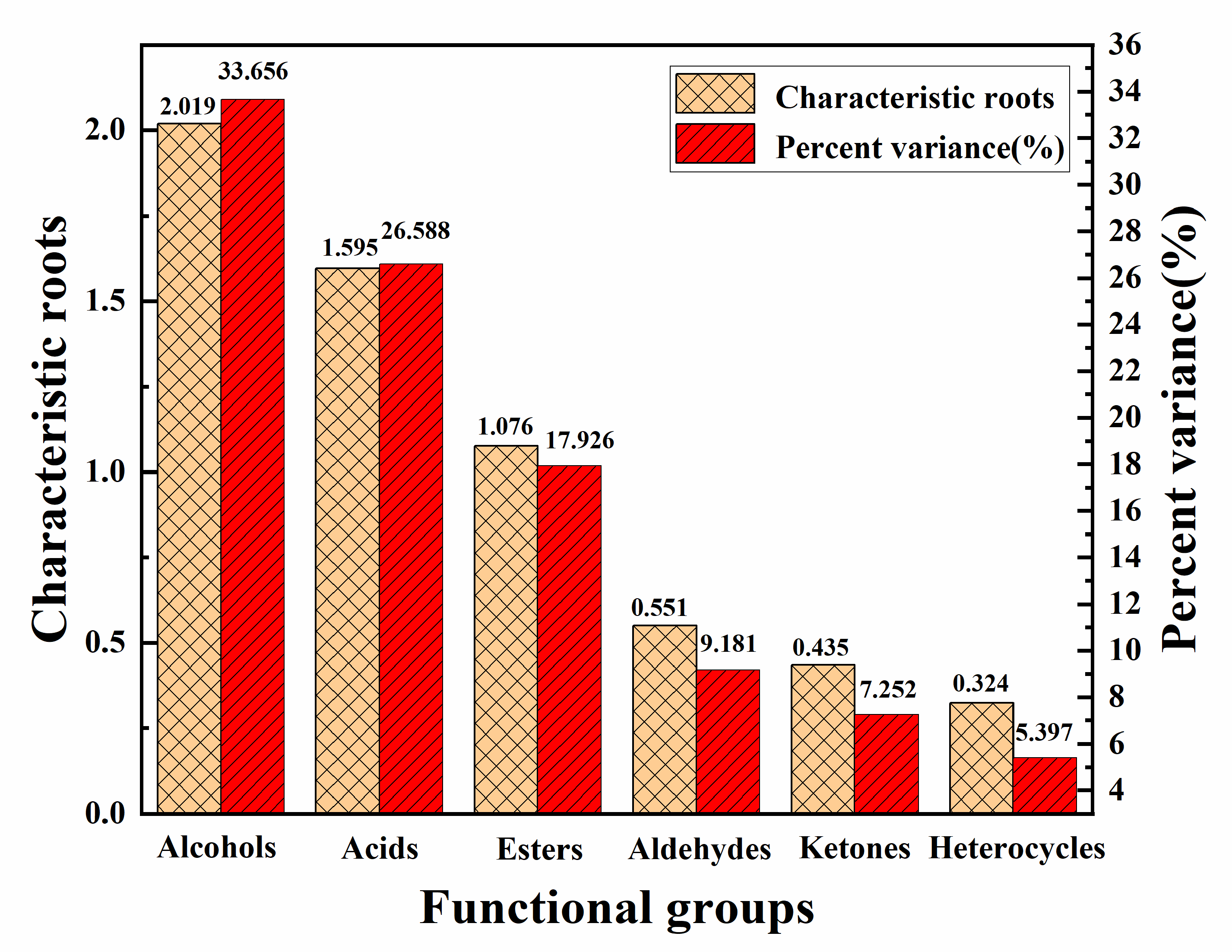
2.2. Principal Components(Pcs) Analysis of Ice Cream
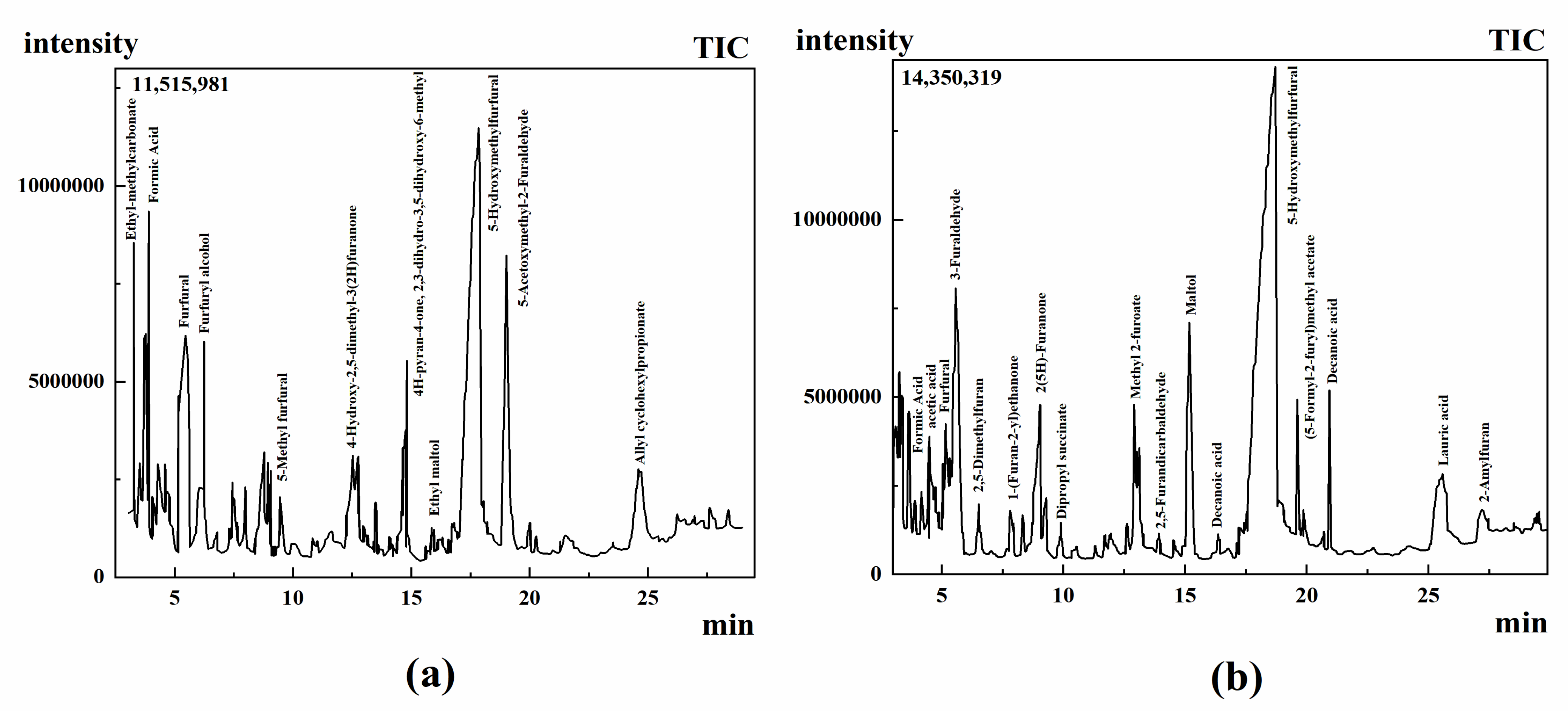
2.2.1. GC-MS Analysis
2.2.2. Analysis of Volatile Aroma Components
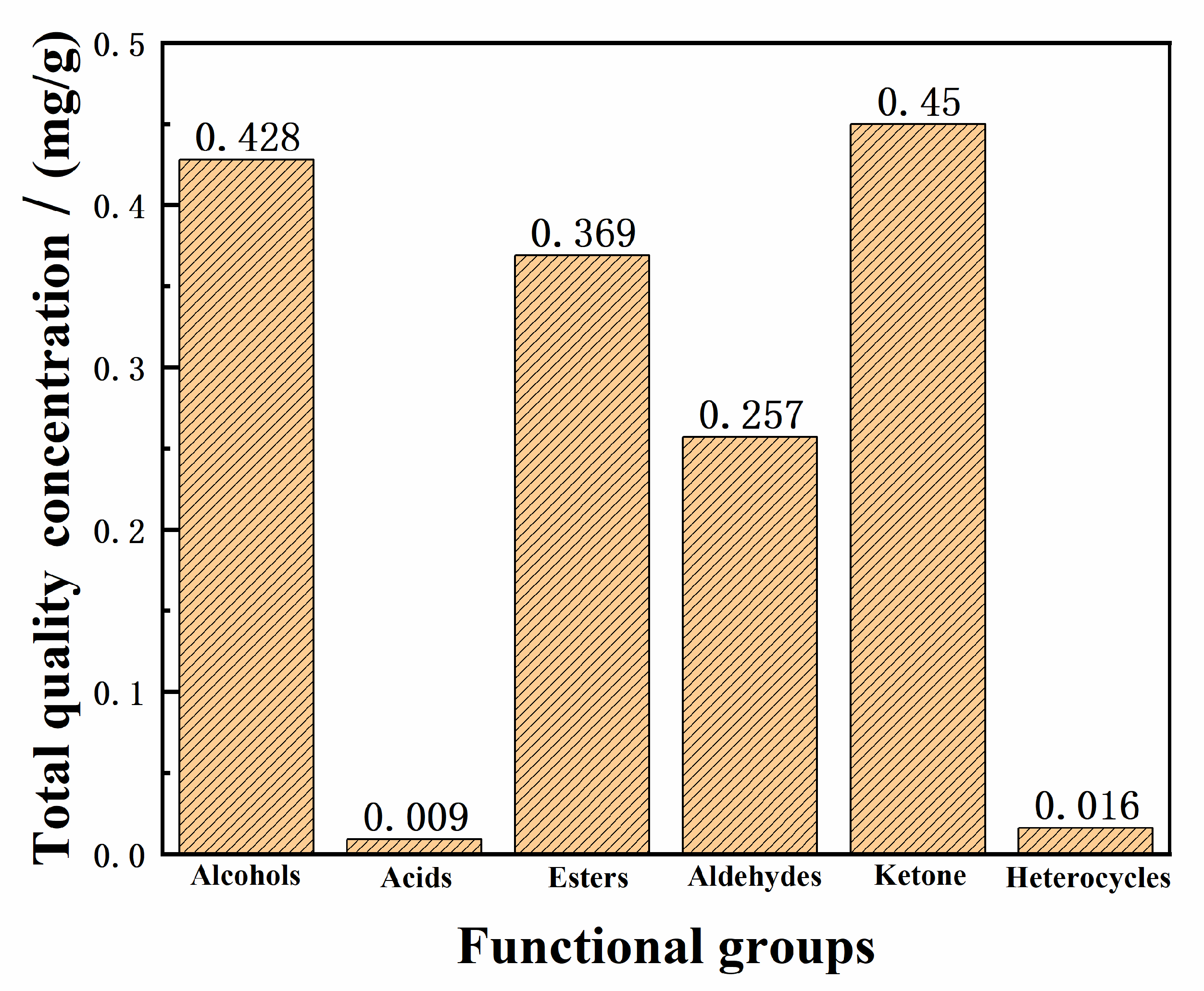
2.3. Quantitative Analysis of Samples
3. Materials and Methods
3.1. Experimental Materials and Reagents
3.2. Experimental Instruments
3.3. GC-MS Conditions
3.3.1. Gas Chromatography Conditions
3.3.2. Mass Spectroscopy Conditions
3.4. Sample Preparation
3.5. Preparation of Standard Solution
3.6. Glme Working Principle and Operation Conditions
3.7. Principal Component Analysis
3.8. Quantitative Calculation of Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shimoda, M.; Shiratsuchi, H.; Nakada, Y.; Wu, Y.; Osajima, Y. Identification and sensory characterization of volatile flavor compounds in sesame seed oil. J. Agric. Food Chem. 1996, 44, 3909–3912. [Google Scholar] [CrossRef]
- Schrankel, K.R. Safety evaluation of food flavorings. Toxicology 2004, 198, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, W.; Yang, Q.; Xu, X.; Jin, Y. Study on extraction process of hyssopus essential oil by hydro-distillation. Food Res. Dev. 2016, 37, 39–41. [Google Scholar]
- Cecati, F.M.; Cid, F.D.; Ardanaz, C.; Kurina-Sanz, M. Eupatorium buniifolium aroma profile assessment by HS-SPME, steam distillation and organic solvent extraction. J. Essent. Oil Res. 2021, 33, 80–93. [Google Scholar] [CrossRef]
- Wu, T.; Mao, L. Influences of hot air drying and microwave drying on nutritional and odorous properties of grass carp (Ctenopharyngodon idellus) fillets. Food Chem. 2008, 110, 647–653. [Google Scholar] [CrossRef]
- Long, B.; Wang, X.; Zhang, F.; Cao, J.; Liu, Y. Analysis of volatile flavor compounds of farmed silurus meridionalischen meat by simultaneous distillation extraction and gas chromatography-mass spectrometry distillation extraction and gas chromatography-mass spectrometry. Food Sci. 2013, 34, 188–192. [Google Scholar]
- Peng, W.U.; Zhou, T.; Kou, L.J.; Wang, C. Automatic Static Headspace GC-MS Analysis of Flavor Compounds in Two Jiaozhou Chinese Cabbage Varieties and Tai an Chinese Cabbage. Food Sci. 2009, 30, 215–218. [Google Scholar]
- Jeleń, H.; Gracka, A.; Myśków, B. Static headspace extraction with compounds trapping for the analysis of volatile lipid oxidation products. Food Anal. Methods 2017, 10, 2729–2734. [Google Scholar] [CrossRef][Green Version]
- Yatoo, G.N.; Wani, H.; Shah, S.A.; Zargar, M.I.; Rather, M.A.; Banday, J.A. Gas chromatographic-mass spectrometric analysis, antibacterial, antioxidant and antiproliferative activities of the needle essential oil of Abies pindrow growing wild in Kashmir, India. Microb. Pathog. 2021, 158, 105013. [Google Scholar]
- Kamimura, G.; Matsuura, H. Separation of ZnCl2 from less-volatile chlorides by vacuum distillation. Metall. Mater. Trans. B 2020, 51, 1395–1405. [Google Scholar] [CrossRef]
- Rong, L.I.; Jiang, Z.T. Analysis of chemical composition of volatile oil of natural spice, Origanum vulgare L.obtained by supercritical fluid extraction. China Condiment 2011, 36, 107–114. [Google Scholar]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Guerrero-Beltrán, J.Á. Supercritical extraction of essential oils of Piper auritum and Porophyllum ruderale. J. Supercrit. Fluids 2017, 127, 97–102. [Google Scholar] [CrossRef]
- Cao, Y.P.; Zhang, D. Analysis of Volatile Compounds from Baked and Hot Air-dried Sichuan Pepper (Zanthoxylum piperitum DC.) Using Solid-phase Micro-extraction Coupled with Gas Chromatography-mass Spectrometry. Food Sci. 2011, 32, 190–193. [Google Scholar]
- Zheng, Y.; Xiu-Li, X.U.; Shun-Li, J.I.; Yuan, F.; Huang, Z.Q.; Yang, B.C.; Zhang, F. Determination of Three Restricted Flavor Additives in Cigarettes by Solid-phase Extraction with Gas Chromatography-Tandem Mass Spectrometry. J. Instrum. Anal. 2016, 35, 987–992. [Google Scholar]
- Nandiwale, K.Y.; Galande, N.D.; Bokade, V.V. Process optimization by response surface methodology for transesterification of renewable ethyl acetate to butyl acetate biofuel additive over borated USY zeolite. RSC Adv. 2015, 5, 17109–17116. [Google Scholar] [CrossRef]
- Yang, C.; Piao, X.; Qiu, J.; Wang, X.; Ren, C.; Li, D. Gas purge microsyringe extraction for quantitative direct gas chromatographic-mass spectrometric analysis of volatile and semivolatile chemicals. J. Chromatogr. A 2011, 1218, 1549–1555. [Google Scholar] [CrossRef]
- Nan, J.; Wang, J.; Piao, X.; Yang, C.; Wu, X.; Quinto, M.; Li, D. Novel and rapid method for determination of organophosphorus pesticide residues in edible fungus using direct gas purge microsyringe extraction coupled on-line with gas chromatography–mass spectrometry. Talanta 2015, 142, 64–71. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Wei, Y.; Song, H. Dynamic Changes of the Total Content of Glycoside Aroma Components in Tobacco Leaves in Different Producing Areas During the Late Growth Period. J. Plant Sci. 2018, 6, 164–172. [Google Scholar]
- Legako, J.F.; Cramer, T.; Yardley, K.; Murphy, T.J.; Gardner, T.; Chail, A.; Pitcher, L.R.; MacAdam, J.W. Retail stability of three beef muscles from grass-, legume-, and feedlot-finished cattle. J. Anim. Sci. 2018, 96, 2238–2248. [Google Scholar] [CrossRef]
- Guo, B.B.; Zhang, X.; Meng, L.I.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T.; Tian, H.Y. Analysis of Volatile Flavor Constituents of Wuxi Sauce Sparerib by SPME-GC-MS and SDE-GC-MS. Fine Chem. 2014, 31, 732–733. [Google Scholar]
- Wojdylo, A.; Figureiel, A.; Legua, P.; Lech, K.; Carbonell-Barrachina, A.A.; Hernandez, F. Chemical composition, antioxidant capacity, and sensory quality of dried jujube fruits as affected by cultivar and drying method. Food Chem. 2016, 207, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Qian, M.C. Headspace solid phase microextraction and gas chromatography− olfactometry dilution analysis of young and aged Chinese “Yanghe Daqu” liquors. J. Agric. Food Chem. 2005, 53, 7931–7938. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zheng, F.P.; Wang, N.; Chen, H.T.; Huang, M.Q.; Sun, B.G. Analysis of Volatiles in Sauced Beef by Microwave-Assisted Extraction Coupled with Solvent-Assisted Flavor Evaporation and Gas Chromatography-Mass Spectrometry. Food Sci. 2013, 34, 250–254. [Google Scholar]
- Mayuoni-Kirshinbaum, L.; Tietel, Z.; Porat, R.; Ulrich, D. Identification of aroma-active compounds in ‘Wonderful’ pomegranate fruit using solvent-assisted flavour evaporation and headspace solid-phase micro-extraction methods. Eur. Food Res. Technol. 2012, 235, 277–283. [Google Scholar] [CrossRef]
- Api, A.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.; Dekant, W.; Fryer, A.; Kromidas, L.; La Cava, S. RIFM fragrance ingredient safety assessment, allyl (cyclohexyloxy) acetate, CAS registry number 68901-15-5. Food Chem. Toxicol. 2015, 82, S59–S65. [Google Scholar] [CrossRef]
- Beiranvand, M.; Ghiasvand, A. Simple, Low-Cost and Reliable Device for Vacuum-Assisted Headspace Solid-Phase Microextraction of Volatile and Semivolatile Compounds from Complex Solid Samples. Chromatographia 2017, 80, 1771–1780. [Google Scholar] [CrossRef]
- Xiao, Z.J.; Gen-Rong, L.I.; Duan, Y.P.; Hua, H.U.; Zheng, H.L.; Zhu, Y.H.J.F.C. Analysis of Volatile Components from Essence of Soy Sauces and Caramels. Fine Chem. 2009, 26, 1200–1205. [Google Scholar]
- Yang, C.; Zhao, J.; Wang, J.; Yu, H.; Piao, X.; Li, D. Water-based gas purge microsyringe extraction coupled with liquid chromatography for determination of alkylphenols from sea food Laminaria japonica Aresh. J. Chromatogr. A 2013, 1300, 38–42. [Google Scholar] [CrossRef]
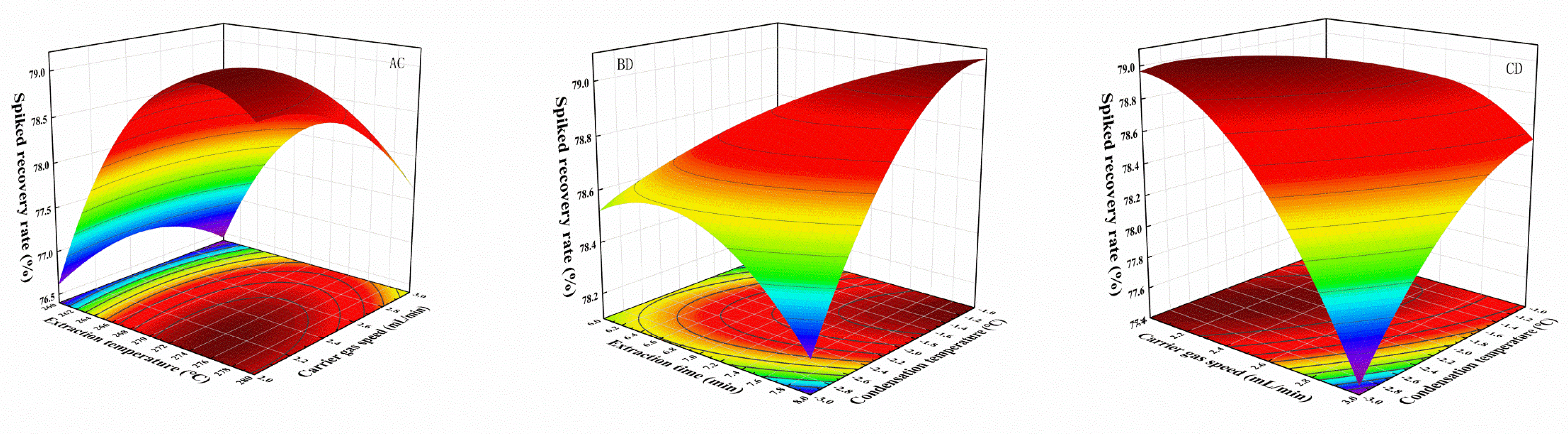

| Scheme | Sum of Squares | df | Mean SQUARE | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 20.28 | 14 | 1.45 | 97.37 | <0.0001 | significant |
| A-Extraction temperature | 8.82 | 1 | 8.82 | 593.04 | <0.0001 | |
| B-Extraction time | 0.2791 | 1 | 0.2791 | 18.76 | 0.0007 | |
| C-Carrier gas speed | 1.45 | 1 | 1.45 | 97.39 | <0.0001 | |
| D-Condensation temperature | 0.1704 | 1 | 0.1704 | 11.45 | 0.0045 | |
| AB | 0.0121 | 1 | 0.0121 | 0.8132 | 0.3824 | |
| AC | 0.2916 | 1 | 0.2916 | 19.60 | 0.0006 | |
| AD | 0.0042 | 1 | 0.0042 | 0.2840 | 0.6025 | |
| BC | 0.0420 | 1 | 0.0420 | 2.82 | 0.1150 | |
| BD | 0.3600 | 1 | 0.3600 | 24.20 | 0.0002 | |
| CD | 0.6724 | 1 | 0.6724 | 45.19 | <0.0001 | |
| A² | 8.01 | 1 | 8.01 | 538.13 | <0.0001 | |
| B² | 0.2687 | 1 | 0.2687 | 18.06 | 0.0008 | |
| C² | 0.8690 | 1 | 0.8690 | 58.40 | <0.0001 | |
| D² | 0.3097 | 1 | 0.3097 | 20.82 | 0.0004 | |
| Residual | 0.2083 | 14 | 0.0149 | |||
| Lack of Fit | 0.2083 | 10 | 0.0208 | 1.041 × 105 | <0.0001 | significant |
| Pure Error | 8.000 × 10−7 | 4 | 2.000 × 10−7 | |||
| Cor Total | 20.49 | 28 |
| Sample | 1 | Sample | 2 | ||||
|---|---|---|---|---|---|---|---|
| Retention time/min | CAS | Compound | FEMA | Retention time/min | CAS | Compound | FEMA |
| 3.395 | 623-53-0 | Ethyl-methylcarbonate | - | 3.55 | 64-18-6 | Formic Acid | 2487 |
| 3.55 | 64-18-6 | Formic Acid | 2487 | 3.825 | 64-19-7 | Acetic acid | 2006 |
| 3.68 | 56-81-5 | Glycerol | 2525 | 3.995 | 116-09-6 | Acetol | 4462 |
| 3.850 | 64-19-7 | Acetic acid | 2006 | 4.6 | 56-81-5 | Glycerol | 2525 |
| 3.99 | 57-55-6 | Propylene Glycol | 2940 | 4.935 | 57-55-6 | Propylene Glycol | 2940 |
| 4.1 | 116-09-6 | Acetol | 4462 | 5.35 | 625-86-5 | 2,5-Dimethylfuran | 4106 |
| 4.59 | 68-12-2 | N, N-Dimethylformamide | - | 5.75 | 498-60-2 | 3-Furaldehyde | 3737 |
| 4.975 | 107-92-6 | Butyric Acid | 2221 | 6.8 | 4412-91-3 | 3-Furancarbinol | - |
| 5.485 | 98-01-1 | Furfural | 2489 | 7 | 1759-71-3 | cis-1,2-Cyclohexanediol diacetate | - |
| 5.58 | 498-60-2 | Furan-3-carboxaldehyde | 3737 | 7.775 | 1192-62-7 | 1-(Furan-2-yl)ethanone | 3163 |
| 6.085 | 4412-91-3 | 3-Furanmethanol | - | 8.1 | 497-23-4 | 2(5H)-Furanone | 4138 |
| 6.24 | 98-00-0 | Furfuryl alcohol | 2491 | 9.305 | 620-02-0 | 5-Methyl furfural | 2702 |
| 6.86 | 606-45-1 | Methyl 2-methoxybenzoate | 2717 | 10.02 | 924-88-9 | Diisopropyl succinate | - |
| 7.195 | 108-94-1 | Cyclohexanone | 3909 | 11.45 | 80-71-7 | Methyl cyclopentenolone | 2700 |
| 7.64 | 1192-62-7 | 2-Acetylfuran | 3163 | 13.135 | 611-13-2 | Methyl 2-furoate | 2703 |
| 8.4 | 591-11-7 | BETA-ANGELICA LACTONE | 4438 | 14.14 | 118-71-8 | Maltol | 2656 |
| 8.96 | 16867-04-2 | 2,3-Dihydroxypyridine | - | 16.535 | 124-07-2 | Octanoic acid | 2799 |
| 9.175 | 620-02-0 | 5-Methyl furfural | 2702 | 17.6 | 4412-96-8 | 3-Methyl-2-furoic acid | - |
| 9.8 | 925-15-5 | Dipropyl succinate | - | 19.06 | 67-47-0 | 5-Hydroxymethylfurfural | - |
| 11.84 | 110-13-4 | Acetonyl acetone | - | 19.97 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - |
| 12.485 | 3658-77-3 | 4-Hydroxy-2,5-dimethyl-3(2H)furanone | 3174 | 20.625 | 4282-34-2 | 2,5-Thiophenedicarboxylic acid dimethyl ester | - |
| 12.835 | 504-15-4 | Orcinol | 3102 | 21.86 | 334-48-5 | Decanoic acid | 2364 |
| 13.07 | 696-11-7 | 1-methyl-1,3-diazinane-2,4-dione | - | 24.275 | 706-14-9 | γ-Decanolactone | 2360 |
| 15.025 | 28564-83-2 | 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | - | 26.275 | 143-07-7 | Lauric acid | 2614 |
| 16.245 | 1073-96-7 | 3,5-dihydroxy-2-methylpyran-4-one | - | 27.1 | 4437-22-3 | 2,2’-(Oxybis(methylene)) difuran | 3337 |
| 16.49 | 4940-11-8 | Ethyl maltol | 3487 | 27.395 | 77-93-0 | Triethyl citrate | 3083 |
| 17.845 | 67-47-0 | 5-Hydroxymethylfurfural | - | 28.635 | 544-63-8 | Myristic acid | 2764 |
| 19.83 | 10551-58-3 | 5-Acetoxymethyl-2-Furaldehyde | - | ||||
| 23.145 | 2705-87-5 | Allyl cyclohexylpropionate | 2026 | ||||
| Sample | 3 | Sample | 4 | ||||
| Retention time/min | CAS | Compound | FEMA | Retention time/min | CAS | Compound | FEMA |
| 3.57 | 64-18-6 | Formic Acid | 2487 | 3.415 | 64-18-6 | Formic Acid | 2487 |
| 3.855 | 64-19-7 | acetic acid | 2006 | 3.71 | 64-19-7 | acetic acid | 2006 |
| 4.1 | 116-09-6 | acetol | 4462 | 3.92 | 116-09-6 | acetol | 4462 |
| 4.61 | 56-81-5 | glycerol | 2525 | 4.46 | 56-81-5 | glycerol | 2525 |
| 5.13 | 617-35-6 | Ethylpyruvate | 2457 | 5.315 | 98-01-1 | Furfural | 2489 |
| 5.35 | 2041-15-8 | 1,3,5-Cyclohexanetriol | - | 5.77 | 498-60-2 | 3-Furaldehyde | 3737 |
| 5.75 | 498-60-2 | 3-Furaldehyde | 3737 | 6.7 | 4412-91-3 | 3-Furancarbinol | - |
| 6.75 | 4412-91-3 | 3-Furancarbinol | - | 7.75 | 1192-62-7 | 1-(Furan-2-yl)ethanone | 3163 |
| 8.05 | 497-23-4 | 2(5H)-Furanone | 4138 | 8.01 | 497-23-4 | 2(5H)-Furanone | 4138 |
| 8.96 | 16867-04-2 | hydroxypyridone | - | 8.985 | 2361-27-5 | 2-Thiophenecarbohydrazide | - |
| 9.255 | 620-02-0 | 5-Methyl furfural | 2702 | 9.285 | 620-02-0 | 5-Methyl furfural | 2702 |
| 10.025 | 924-88-9 | Diisopropyl succinate | - | 10 | 925-15-5 | Dipropyl succinate | - |
| 10.46 | 765-70-8 | Maple lactone | - | 10.475 | 637-88-7 | 1,4-Cyclohexanedione | - |
| 11.235 | 5989-27-5 | (+)-Limonene | 2633 | 11.445 | 80-71-7 | Methyl cyclopentenolone | 2700 |
| 11.405 | 80-71-7 | Methyl cyclopentenolone | 2700 | 11.975 | 110-13-4 | Acetonylacetone | - |
| 11.985 | 3128-07-2 | 6-Oxoheptanoic acid | - | 12.96 | 823-82-5 | 2,5-Furandicarbaldehyde | - |
| 12.46 | 1192-62-7 | 1-(Furan-2-yl)ethanone | 3163 | 13.115 | 611-13-2 | Methyl 2-furoate | 2703 |
| 12.975 | 3658-77-3 | 4-Hydroxy-2,5-dimethylfuran-3(2H)-one | 3174 | 14.11 | 118-71-8 | Maltol | 2656 |
| 13.1 | 611-13-2 | Methyl 2-furoate | 2703 | 16.19 | 1193-79-9 | 1-(5-Methylfuran-2-yl)ethanone | 3609 |
| 13.635 | 590-86-3 | Isovaleraldehyde | 2692 | 19.225 | 67-47-0 | 5-hydroxymethylfurfural | - |
| 13.92 | 98-00-0 | Furfuryl alcohol | 2491 | 20 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - |
| 14.055 | 118-71-8 | Maltol | 2656 | 20.99 | 102-76-1 | Triacetin | 2007 |
| 17.4 | 18720-62-2 | 2-methylheptan-3-ol | - | 25.855 | 498-07-7 | Levoglucosan | - |
| 17.56 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - | 28.605 | 544-63-8 | Myristic acid | 2764 |
| 18.85 | 67-47-0 | 5-hydroxymethylfurfural | - | 29.52 | 84-69-5 | Diisobutyl phthalate | - |
| 21.76 | 112-37-8 | Undecanoic acid | 3245 | ||||
| 23.16 | 2705-87-5 | Allyl 3-cyclohexylpropionate | 2026 | ||||
| 27.09 | 3777-69-3 | 2-Amylfuran | 3317 | ||||
| 28.605 | 544-63-8 | Myristic acid | 2764 | ||||
| Sample | 5 | Sample | 6 | ||||
| Retention time/min | CAS | Compound | FEMA | Retention time/min | CAS | Compound | FEMA |
| 3.155 | 64-18-6 | Formic Acid | 2487 | 3.055 | 64-18-6 | Formic Acid | 2487 |
| 3.43 | 64-19-7 | acetic acid | 2006 | 3.33 | 116-09-6 | acetol | 4462 |
| 3.635 | 116-09-6 | acetol | 4462 | 3.615 | 627-03-2 | Ethoxyacetic acid | - |
| 3.94 | 79-09-4 | Propionic acid | - | 3.94 | 56-81-5 | glycerol | 2525 |
| 4.19 | 3393-64-4 | 4-hydroxy-3-methylbutan-2-one | - | 4.22 | 3121-61-7 | 2-Methoxyethyl acrylate | - |
| 4.245 | 56-81-5 | glycerol | 2525 | 4.475 | 1117-97-1 | N-methoxymethanamine | - |
| 5.31 | 98-01-1 | Furfural | 2489 | 5.385 | 498-60-2 | 3-Furaldehyde | 3737 |
| 5.71 | 498-60-2 | 3-Furaldehyde | 3737 | 5.65 | 98-01-1 | Furfural | 2489 |
| 6.555 | 4412-91-3 | 3-Furancarbinol | - | 6.375 | 4412-91-3 | 3-Furancarbinol | - |
| 7.73 | 1192-62-7 | 1-(Furan-2-yl)ethanone | 3163 | 6.93 | 930-60-9 | 2-Cyclopentene-1,4-dione | - |
| 7.89 | 497-23-4 | 2(5H)-Furanone | 4138 | 7.73 | 497-23-4 | 2(5H)-Furanone | 4138 |
| 8.995 | 16867-04-2 | hydroxypyridone | - | 8.85 | 5380-42-7 | 2-(Carbomethoxy)thiophene | - |
| 9.27 | 620-02-0 | 5-Methyl furfural | 2702 | 8.975 | 16867-04-2 | hydroxypyridone | - |
| 9.81 | 53119-25-8 | 1-thiophen-2-ylpentan-1-one | - | 9.235 | 620-02-0 | 5-Methyl furfural | 2702 |
| 9.95 | 925-15-5 | Dipropyl succinate | - | 9.6 | 675-10-5 | triacetate lactone | - |
| 11.35 | 80-71-7 | Methyl cyclopentenolone | 2700 | 9.75 | 88-15-3 | 2-Acetylthiophene | - |
| 11.945 | 1117-31-3 | 1,3-butanediol diacetate | - | 11.275 | 80-71-7 | Methyl cyclopentenolone | 2656 |
| 12.965 | 504-15-4 | Orcinol | - | 11.885 | 110-13-4 | Acetonylacetone | - |
| 13.07 | 611-13-2 | Methyl 2-furoate | 2703 | 12.035 | 19432-69-0 | Methyl 5-methylthiophene-2-carboxylate | - |
| 13.9 | 98-00-0 | Furfuryl alcohol | 2491 | 12.755 | 3658-77-3 | 4-Hydroxy-2,5-dimethylfuran-3(2H)-one | 3174 |
| 14.11 | 118-71-8 | Maltol | 2656 | 12.855 | 823-82-5 | 2,5-Furandicarbaldehyde | - |
| 14.65 | 7492-38-8 | 2-methyloctan-4-one | - | 12.985 | 611-13-2 | Methyl 2-furoate | 2703 |
| 15.32 | 1540-29-0 | Ethyl 2-acetylhexanoate | 4452 | 13.44 | 110-62-3 | Valeraldehyde | 3098 |
| 18.9 | 67-47-0 | 5-hydroxymethylfurfural | - | 13.935 | 118-71-8 | Maltol | 2656 |
| 19.36 | 6434-78-2 | trans-2-nonene | - | 14.625 | 629-62-9 | pentadecane | - |
| 19.96 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - | 16.395 | 501-30-4 | kojic acid | - |
| 20.635 | 4282-34-2 | 2,5-Thiophenedicarboxylic acid dimethyl este | - | 17.505 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - |
| 26.215 | 498-07-7 | Levoglucosan | - | 18.38 | 67-47-0 | 5-hydroxymethylfurfural | - |
| 27.63 | 6968-62-3 | D-ERYTHRO-l-TALO-OCTONIC ACID, γ-LACTONE | - | 20.6 | 4282-34-2 | 2,5-Thiophenedicarboxylic acid dimethyl este | - |
| 29.525 | 84-69-5 | Diisobutyl phthalate | - | 20.905 | 102-76-1 | Triacetin | 2007 |
| 25.5 | 498-07-7 | Levoglucosan | - | ||||
| 25.89 | 2311-46-8 | propan-2-yl hexanoate | 2950 | ||||
| 26.15 | 143-07-7 | Lauric acid | 2614 | ||||
| 28.595 | 544-63-8 | Myristic acid | 2764 | ||||
| 29.52 | 84-69-5 | Diisobutyl phthalate | - | ||||
| Sample | 7 | Sample | 8 | ||||
| Retention time/min | CAS | Compound | FEMA | Retention time/min | CAS | Compound | FEMA |
| 3.25 | 64-18-6 | Formic Acid | 2487 | 3.305 | 64-18-6 | Formic Acid | 2487 |
| 3.38 | 141-46-8 | Glycolaldehyde | - | 3.52 | 64-19-7 | acetic acid | 2006 |
| 3.46 | 64-19-7 | acetic acid | 2006 | 3.81 | 116-09-6 | acetol | 4462 |
| 3.68 | 116-09-6 | acetol | 4462 | 4.04 | 623-53-0 | Ethyl-methylcarbonat | - |
| 3.945 | 623-53-0 | Ethyl-methylcarbonat | - | 4.305 | 56-81-5 | glycerol | 2525 |
| 4.235 | 56-81-5 | glycerol | 2525 | 4.6 | 4254-15-3 | (S)-(+)-1,2-Propanediol | - |
| 4.565 | 4254-15-3 | (S)-(+)-1,2-Propanediol | - | 5.3 | 98-01-1 | Furfural | 2489 |
| 5.3 | 98-01-1 | Furfural | 2489 | 5.71 | 498-60-2 | 3-Furaldehyde | 3737 |
| 5.675 | 498-60-2 | 3-Furaldehyde | 3737 | 6.6 | 4412-91-3 | 3-Furancarbinol | - |
| 6.575 | 4412-91-3 | 3-Furancarbinol | - | 7.915 | 497-23-4 | 2(5H)-Furanone | 4138 |
| 7.885 | 497-23-4 | 2(5H)-Furanone | 4138 | 8.995 | 16867-04-2 | hydroxypyridone | - |
| 8.85 | 22913-26-4 | Methyl thiophene-3-carboxylate | - | 9.26 | 620-02-0 | 5-Methyl furfural | 2702 |
| 8.985 | 16867-04-2 | hydroxypyridone | - | 9.965 | 924-88-9 | Diisopropyl succinate | - |
| 9.235 | 620-02-0 | 5-Methyl furfural | 2702 | 11.325 | 80-71-7 | Methyl cyclopentenolone | 2700 |
| 9.96 | 924-88-9 | Diisopropyl succinate | - | 12.94 | 823-82-5 | 2,5-Furandicarbaldehyde | - |
| 12.935 | 504-15-4 | Orcinol | - | 13.03 | 611-13-2 | Methyl 2-furoate | 2703 |
| 13.045 | 611-13-2 | Methyl 2-furoate | 2703 | 13.93 | 118-71-8 | Maltol | 2656 |
| 17.52 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - | 17.515 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - |
| 18.74 | 67-47-0 | 5-hydroxymethylfurfural | - | 18.695 | 67-47-0 | 5-hydroxymethylfurfural | - |
| 19.93 | 118-71-8 | Maltol | 2656 | 20.605 | 4282-34-2 | 2,5-Thiophenedicarboxylic acid dimethyl este | - |
| 20.945 | 102-76-1 | Triacetin | 2007 | 25.52 | 498-07-7 | Levoglucosan | - |
| 25.52 | 498-07-7 | Levoglucosan | - | 29.52 | 84-69-5 | Diisobutyl phthalate | - |
| 29.525 | 84-69-5 | Diisobutyl phthalate | - | ||||
| Sample | 9 | Sample | 10 | ||||
| Retention time/min | CAS | Compound | FEMA | Retention time/min | CAS | Compound | FEMA |
| 3.54 | 64-18-6 | Formic Acid | 2487 | 4.01 | 64-18-6 | Formic Acid | 2487 |
| 3.795 | 64-19-7 | acetic acid | 2006 | 4.205 | 64-19-7 | acetic acid | 2006 |
| 4.06 | 116-09-6 | acetol | 4462 | 4.46 | 116-09-6 | acetol | 4462 |
| 4.3 | 79-09-4 | Propionic acid | 2924 | 4.635 | 56-81-5 | glycerol | 2525 |
| 4.635 | 56-81-5 | glycerol | 2525 | 5.18 | 4254-15-3 | (S)-(+)-1,2-Propanediol | - |
| 4.935 | 4254-15-3 | (S)-(+)-1,2-Propanediol | - | 5.795 | 498-60-2 | 3-Furaldehyde | 3737 |
| 5.79 | 498-60-2 | 3-Furaldehyde | 3737 | 6.92 | 4412-91-3 | 3-Furancarbinol | - |
| 6.74 | 4412-91-3 | 3-Furancarbinol | - | 8.095 | 497-23-4 | 2(5H)-Furanone | 4138 |
| 7.78 | 1192-62-7 | 2-Acetylfuran | 3163 | 8.765 | 591-12-8 | a-Angelic lactone | 3293 |
| 8.04 | 497-23-4 | 2(5H)-Furanone | 4138 | 8.895 | 16867-04-2 | hydroxypyridone | - |
| 9.015 | 16867-04-2 | hydroxypyridone | - | 8.99 | 22913-26-4 | Methyl thiophene-3-carboxylate | - |
| 9.28 | 620-02-0 | 5-Methyl furfural | 2702 | 9.28 | 620-02-0 | 5-Methyl furfural | 2702 |
| 10.04 | 924-88-9 | Diisopropyl succinate | - | 10.025 | 925-15-5 | Dipropyl succinate | - |
| 11.4 | 80-71-7 | Methyl cyclopentenolone | 2700 | 11.95 | 110-13-4 | Acetonylacetone | - |
| 12.96 | 504-15-4 | Orcinol | - | 12.765 | 3658-77-3 | 4-Hydroxy-2,5-dimethylfuran-3(2H)-one | 3174 |
| 13.09 | 611-13-2 | Methyl 2-furoate | 2703 | 12.945 | 504-15-4 | Orcinol | - |
| 16.63 | 4940-11-8 | Ethyl maltol | 3487 | 13.07 | 611-13-2 | Methyl 2-furoate | 2703 |
| 17.55 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - | 13.885 | 98-00-0 | Furfuryl alcohol | 2491 |
| 19.1 | 67-47-0 | 5-hydroxymethylfurfural | - | 17.54 | 10551-58-3 | (5-Formyl-2-furyl)methyl acetate | - |
| 19.95 | 118-71-8 | Maltol | 2656 | 19.14 | 67-47-0 | 5-hydroxymethylfurfural | - |
| 25.57 | 498-07-7 | Levoglucosan | - | 25.715 | 498-07-7 | Levoglucosan | - |
| 29.52 | 84-69-5 | Diisobutyl phthalate | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, L.; Tong, Q.; Liu, Y.; Meng, X.; He, P.; Li, G.; Ye, L. Application of Gas-Liquid Microextraction (GLME)/GC-MS for Flavour and Fragrance in Ice Cream Detection and Composition Analysis. Molecules 2023, 28, 522. https://doi.org/10.3390/molecules28020522
Mu L, Tong Q, Liu Y, Meng X, He P, Li G, Ye L. Application of Gas-Liquid Microextraction (GLME)/GC-MS for Flavour and Fragrance in Ice Cream Detection and Composition Analysis. Molecules. 2023; 28(2):522. https://doi.org/10.3390/molecules28020522
Chicago/Turabian StyleMu, Li, Qi Tong, Yuhang Liu, Xianglong Meng, Peng He, Gang Li, and Linyang Ye. 2023. "Application of Gas-Liquid Microextraction (GLME)/GC-MS for Flavour and Fragrance in Ice Cream Detection and Composition Analysis" Molecules 28, no. 2: 522. https://doi.org/10.3390/molecules28020522
APA StyleMu, L., Tong, Q., Liu, Y., Meng, X., He, P., Li, G., & Ye, L. (2023). Application of Gas-Liquid Microextraction (GLME)/GC-MS for Flavour and Fragrance in Ice Cream Detection and Composition Analysis. Molecules, 28(2), 522. https://doi.org/10.3390/molecules28020522





