Advances in the Chemistry, Analysis and Adulteration of Anthocyanin Rich-Berries and Fruits: 2000–2022
Abstract
1. Introduction
2. Occurrence, Chemistry, and Stability of Anthocyanins
Stability Considerations
3. Preparation of Anthocyanin Samples for Analysis
4. Analytical Techniques including Identification and Quantification of Anthocyanins
4.1. Nuclear Magnetic Resonance (NMR)
4.2. Infrared Spectroscopy (IR)
4.3. High Performance Thin Layer Chromatography (HPTLC) Using UV and MS
| No. | Identification Technique (Year) | Source | Extraction Solvent | Compounds | Activity | Ref |
|---|---|---|---|---|---|---|
| 1 | HPTLC (2003) | Skin of V. vinifera | MeOH-HCl (99.1:0.1) for 16 h agitation at 25 °C | Glycosides, acetylglycosides and coumaroylglycosides of 5 (Dp, Mv, Cy, Pn, Pg) main anthocyanins | - | [57] |
| 2 | HPTLC fingerprinting (2003) | Red fruits and teas (raspberry, straw-berry, blueberry, rose hip, wild berry) | acidified ethanol (HCl 0.1% v/v) | - | - | [61] |
| 3 | HPTLC (2012) | Açai berry and supplements | Methanol-10% aq. formic acid (9:1) | Cy-3-glc, Cy-3-rut | - | [58] |
| 4 | HPTLC-ESI-MS (2013) | Grape skin, pomace, Grape juice, wine | Mv 3-glc, Cy 3-glc, Pn 3-glc, Dp 3-glc, Pg-3-glc and diglc of Mv | - | [59] | |
| 5 | HPTLC–Vis–MS (2014) | bilberry, blueberry, chokeberry, açai berry and cranberry | mixture of methanol and hydrochloric acid, 25%, 4:1, v/v | Mv 3-glc, Cy 3-glc, Pn 3-glc, Dp 3-glc | - | [60] |
| 6 | HPTLC-UV/Vis and HPTLC-ESI-MS (2015) | Fresh and dried elderberry | Acidified methanol | Cy-3-sam and Cy-3-glc | - | [63] |
| 7 | HPTLC (2015) | cranberry, blueberry, bilberry, chokeberry and açai berry | Acidified methanol (4:1) | Mv 3-glc, Cy 3-glc, Pn 3-glc, Dp 3-glc | Antioxidant activity | [62] |
| 8 | CE-UV (2003) | Grapes skin and wine | 5% v/v of formic acid in methanol | My-3, 5-diglc, My-3-glc, My-3-gal, Pg-3-glc, Cy-3, 5-diglc, Cy-3-gal | - | [66] |
| 9 | CE-UV (2000) | Wild-type blueberry (bilberry) | 3% aq.TFA | Mv-3-glc, Pn-3-glc, Pt-3-glc, Cy-3-glc, Mv-3-gal, Pt-3-gal, Dp-3-glc, Cy-3-gal, Dp-3-gal, Cy-3-ara, unknown | - | [67] |
| 10 | CE-UV (2001) | Wild-type blueberry (bilberry) | 1% TFA | Mv-3-glc, Pn-3-glc, Pt-3-glc, Cy-3-glc, Mv-3-gal, Pt-3-gal, Dp-3-glc, Cy-3-gal, Dp-3-gal, Cy-3-ara, unknown 1-2 | - | [68] |
| 11 | CE-UV (2004) | Wild-type blueberry (bilberry) | 3% aq.TFA | Mv-3-glc, Pn-3-glc, Pt-3-glc, Cy-3-glc, Mv-3-gal, Pt-3-gal, Dp-3-glc, Cy-3-gal, Dp-3-gal, Cy-3-ara, Mv-3-ara, Pn-3-ara, Pn-3-gal, Pt-3-ara | - | [69] |
| 12 | CE-UV (2004) | Wild-type blueberry (bilberry) | [1] 100 mM of AAPH at pH 5.6 (0.1 M phosphate buffer) [2] H2O2 and t-BuOOH | Mv-3-glc, Pn-3-glc, Mv-3-gal, Pt-3-glc, Pn-3-gal, Cy-3-glc, Dp-3-glc, Pt-3-gal, Cy-3-gal, Dp-3-gal, Cy-3-ara, Dp-3-ara | - | [70] |
| 13 | CE-UV (2004) | Wild-type blueberry (bilberry) | 1% aq.TFA | Mv-3-glc, Pn-3-glc, Pt-3-glc, Cy-3-glc, Mv-3-gal, Pt-3-gal, Dp-3-glc, Cy-3-gal, Dp-3-gal, Cy-3-ara, Mv-3-ara, Pn-3-ara, Pn-3-gal, Pt-3-ara, Dp-3-ara | - | [71] |
| 14 | CE-UV (2004) | Cranberry | 95% ethanol:1.5 M HCl 85:15 v/v | Pn, Cy | - | [72] |
| 15 | CE-UV/Fluo (2007) | Grape skin and commercial extracts | 0.8% HCl in ethanol–water mixture | Mv-3-glc, Pn-3-glc, Cn-3-glc, Dp-3-glc | - | [73] |
| 16 | CE-UV (2008) | Strawberries (cv. Camarosa) | acidified water (3% formic acid) | Pg-3-glu, Pg-3-rut and Cy-3-glc | - | [74] |
4.4. Capillary Zone Electrophoresis (CZE) Using UV/Vis and Mass Spectrometry
4.5. CE with MS Detection
4.6. Liquid Chromatography Using UV and MS
| Açai (E. oleracea) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Source (Year) | Anthocyanins | Extraction Solvent | % Yield | Conditions | Detection Method | Purpose of Analysis | Chemometrics | Pharmacological Activity | [Ref] | |
| Stationary Phase | Mobile Phase | ||||||||||
| 1 | Açai berries (2005) | Cy-3-glc; Cy-3-rut; Pn-3-rut; | Millipore water | 13–463 mg/L | HPLC-Vis Max-RP 80A column (150 × 4.6 mm, 4 µm) | Gradient program | HPLC-MS and HPLC-UV 525 nm | Antioxidant capacities of açai fruits | - | Antioxidant activity | [84] |
| 2 | Açai berries (2008) | Cy-3,5-hex-pent; Cy-3-glc; Cy-3-rut; Pg-3-glc; Pn-3-glc; Pn-3-rut; Cy-3-Ac-hex; | 95% ethanol/1.5 N HCl | 282.5–303.7 mg/100 g FW | Shim-pack CLC-ODS column (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS/MS 520 nm | Determination of anthocyanins | - | - | [88] |
| 3 | Açai fruit pulp (2009) | Cy-3-glc; Cy-3-rut; Pn-3-rut; | Aqueous solution adjusted to pH to 3.5 with citric acid | 224.7 mg/100 g FW | Synergi Hydro-RP 80 OA (150 × 2 mm, 4 µm) | Gradient program | HPLC-PDA-ESI-MSn | Phytochemical composition and thermal stability of açai species | - | Antioxidant activity | [91] |
| 4 | Açai fruit (pulp, mesocarp, and Endocarp) (2011) | Cy-3-glc; Cy-3-rut; | 80% ethanol containing 0.5% acetic acid | Pulp: 34.1 mg/g DEW; Mesocarp: 18.5 mg/g DEW; Endocarp: 1.64 mg/g DEW | Capcell Pak ACR (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD | Anthocyanins in different parts of açai fruit | - | Antioxidant activity | [93] |
| 5 | Açai berries pulp (2012) | Cy-3-glc; Cy-3-rut; Dp-3-glc; Mv-3-glc; Pg-3-glc; Pn-3-glc; | 400 mL of hexane followed by 400 mL of chloroform for 36 h. The leftover pulp extracted with ethyl acetate for 36 h at room temp. | EtOAc fraction: 0.12 μg/mg DEW Acetonitrile fraction: 2.3 μg/mg DEW Methanol fraction; 11.3 μg/mg DEW Ethanol fraction: 8.9 μg/mg DEW | Inertsil ODS-2 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | Attenuation of inflammatory stress signaling in mouse brain BV-2 microglial cells | - | Effect on signaling pathways linked to inflammation in microglial cells/Neuroinflammation | [96] |
| 6 | Açai fruit (2012) | Cy-3,5-diglc, Cy-3-glc, Cy-3-rut, Pg-3-glc, Pn-3-glc, Pn-3-rut | Methanol (0.1% HCl) | Major anthocyanins (Cy-glc and Cy-rut) 489–584 mg/kg FW | HSS C18 (100 × 2.1 mm, 1.8 µm) | Gradient program | UHPLC-PDA | Validated UHPLC-PDA method for anthocyanin quantification | - | - | [95] |
| Apples-Red peel (M. domestica) | |||||||||||
| 7 | Red apples Peel and Whole (2006) | Cy-3-gal; Cy-3-glc; Cy-pent-rha; Cy-3-arab; Pn-3-gal; Cy-3-maloyl-gal; 5-carboxy-pyrano-Cy-hex; Cy-7-arab; Cy-3-xyl; Cy-3-pent; | Aq. acetone (pH 1.0 water acidified with TFA/acetone 30/70 v/v) | Apple with peel: 10.8 mg/100 g FW; Apple flesh: 8.1 mg/100 g FW; Apple peel: 20.6 mg/100 g FW; | Sunfire C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Anthocyanins pattern in red fleshed Weirouge apples | - | Antioxidant property | [85] |
| 8 | Fuji apples Peel (2009) | Cy-3-gal; Cy-3-glc; Cy-3-arab; Cy-pent; | Methanol containing 1% v/v HCl and 1% w/v BHT | Normal: 11–24.8 mg/100 g FW; Hailnet: 18.2–26.1 mg/100 g FW | Gemini C18 (150 × 4.6 mm, 3 µm) | Gradient program | HPLC-PDA-MS 520 nm | Influence of light exposure on phenolic content | - | - | [90] |
| 9 | Red apples Peel (2012) | Cy-hex; Mv-pent; Cy-pentoxide; Cy-pent; | - | - | Suplelco ODS Hypersil (150 × 2.1 mm, 5 µm) | Gradient program | HPLC-DAD-MS/MS 530 nm | Investigation of phyto components in red apples by LC-MS and GC-MS | - | - | [94] |
| 10 | Apples Whole (2020) | Cy-3-gal; | 10 mL of mixture containing methanol (30 mL/100 mL), ascorbic acid (2 g/100 mL) and acetic acid (1 mL/100 mL) | Cy-3-gal; 0–41 mg/100 g DW | Acquity BEH C18 (100 × 2.1, 1.7 µm) | Gradient program | LC-QToF-MS | Phytochemical analysis of old apple varieties | Hierarchical cluster analysis and Principle component analysis (PCA) | Antioxidative activity | [103] |
| Bilberry (V. myrtillus) | |||||||||||
| 11 | Bilberry (2001) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; | Aliquot of 5 mL of extract from fresh berries | - | Restek Pinnacle ODS (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-ESI-MS | Identification of anthocyanins in different berries | - | - | [82] |
| 12 | Bilberry (2003) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; | acetonitrile/TFA/water (49.5:0.5:50 v/v/v) | 472.3 mg/100 g FW | Zorbax SB-C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm HPLC-DAD-MS | Isolation and identification of anthocyanins from berries | - | Antioxidant activity | [107] |
| 13 | Bilberries (2008) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | 10%A and 90%B solvents. Solvent A: acetonitrile/methanol (85/15 v/v) Solvent B: 8.5% aq. Formic acid | 350–525 mg/100 g FW (Quant based on Aglycones content) | Phenomenex gemini C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Anthocyanins analysis in wild bilberries | - | - | [204] |
| 14 | Bilberries (2008) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | - | Peel: 2025.6 mg/100 g Pulp: 104 mg/g FW | Lichrocart Purospher RP-18e (125 × 3 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Organ specific distribution of phenolics in bilberries | - | - | [87] |
| 15 | Bilberries (2010) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | SEP-PAK C18 cartridge. The cartridge was previously conditioned with 3 mL methanol and 5 mL 5 mM H2SO4 and washed with 5 mL of 5 mM H2SO4 and dried with nitrogen before eluted with 4 mL of methanol. | 967.8 mg/100 g FW | Phenomenex gemini C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Pharmacological effects of bilberry anthocyanins | - | Antioxidant property, Acute Cardioprotective and Cardiotoxic effects | [92] |
| 16 | Bilberries (2012) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | acetonitrile/water/formic acid (87:3:10 v/v) | 6102–7465 mg/100 g DW | Phenomenex Luna C18(2) (250 × 4.6 mm, 3.0 µm) | Gradient program | HPLC-UV/Vis 520 nm | Analysis of anthocyanins in bilberries and their commercial products | - | - | [120] |
| 17 | Bilberries (2018) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | 2% HCl and methanol (5:95 v/v) | 582–795 mg/100 g FW | Zorbax SB-C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD-MS 520 nm | HPLC analysis of anthocyanins in bilberries and their food products | Principle component analysis (PCA) | - | [100] |
| Blackberry (R. fruiticosus) | |||||||||||
| 18 | Blackberry (Rubus sp.) (2001) | Cy-glycosides; Pg-glycosides; Mv-glycosides; | Aliquot of 5 mL of extract from fresh berries was taken and centrifuged | - | Restek Pinnacle ODS (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-ESI-MS | Identification of anthocyanins in different berries | - | - | [82] |
| 19 | Blackberry genotypes (2004) | Cy-3-glc; Cy-3-rut; Cy-3-xyl; Cy-3-malonyl-glc; Cy-3-dioxaloyl-glc; | methanol/water/FA (60:37:3 v/v) | Blackberry: 1.1–2.4 g/kg FW | Symmetry C18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-PDA-MS PDA at 520 nm | Flavonoid glycosides estimation in various genotypes of red wine grapes, blackberry and blueberries | - | Antioxidant properties | [83] |
| 20 | Blackberries (Rubus sp.) (2007) | Cy-3-glc; Cy-3-rut; Cy-3-malonyl-glc; | 70% aq. Acetone containing 2% formic acid | 720–1010 mg/100 g DW | Lichrospher ODS-2 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD-MS 520 nm | Analysis of phenolic compounds in blackberry sp. | - | - | [86] |
| 21 | Blackberries (2009) | Cy-3-glc; | after ethyl acetate extraction sample was acidified with 1 mL of 2 M HCl and extracted with 5 mL of methanol for 4–8 times. | 97.7 mg/100 g FW | Omnispher C18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-PDA 520 nm | Phenolic composition of berries | - | Antioxidant activity | [89] |
| 22 | Blackberries (2011) | Cy-3-glc; Cy-3-rut; Cy-3-xyl; Cy-3-(6-malonyl)-glc; Cy-3-(6-(3-OH-3-methylglutaroyl)-glc) | 0.5% TFA acidified methanol. | 323.3 mg/100 g FW | Hypersil ODS (200 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Identification of anthocyanins from wild and cultivated blueberries | - | - | [202] |
| 23 | Blackberries (2016) | Dp-3-glc; Cy-3-glc; Cy-3-rut; Cy-3-xyl; | - | 647 mg/100 g FW | Lichrocart RP-18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Phenolic composition and antioxidant activity of different berries | - | Antioxidant activity | [97] |
| 24 | Blackberries (2017) | Cy-3-glc; Cy-3-xyl; Cy-3-malonyl-glc; Cy-3-dioxaloyl-glc; | 1% TFA in ethanol | 27230 mg/100 g DEW | TSK-GEL ODS-100 V (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD-MS 520 nm | Anthocyanin content in blackberries | - | - | [99] |
| 25 | Blackberries (2017) | Cy-3-gal; Cy-3-glc; Cy-3-rut; Cy-3-arab; Cy-3-xyl; Cy-3-malonyl-glc; Cy-3-dioxaloyl-glc; | 0.1% HCl in 15 mL of ethanol | 0.17 mg/mL | Zorbax Eclipse plus C18 (100 × 4.5 mm, 3.5 μm) | Ethanol and α-hydroxy acid aqueous solution | HPLC-DAD 520 nm HPLC-DAD-MS | Green chromatography for anthocyanins determination in berries | - | - | [98] |
| 26 | Blackberries (2018) | Cy-3-glc; Cy-3-xyl; Cy-3-(6-malonyl)-glc; Cy-3-(6-(3-OH-3-methylglutaroyl)-glc; | 80:20 v/v methanol and water with 0.5% acetic acid | 222 mg/100 g FW | Phenomenex gemini C18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Anthocyanins and ellagitannins from blackberries | - | Bioaccessability studies in GI tract and colonic levels | [101] |
| 27 | Blackberries (2018) | Cy-3-glc; Cy-3-rut; | 1 N HCl in 75% methanol | 192.4 mg/100 g DW | Zorbax SB-C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 nm | Comparative study of red berry pomaces | stepwise linear discriminant analysis (SLDA) | Antioxidant activity | [102] |
| 28 | Blackberries (2021) | Cy-3-glc; Cy-3-rut; Cy-3-xylosyl-rut; | 80:20 ethanol/water | 847–3465 mg/100 g DEW | - | - | HPLC-MS | Antibacterial effects of berries against H. pylori infection | - | Antibacterial activity | [132] |
| Blackcurrant (R. nigrum) | |||||||||||
| 29 | Blackcurrant (2003) | Dp-3-glc; Dp-3-rut; Cy-3-glc; Cy-3-rut; Pt-3-rut | acetonitrile/TFA/water (49.5:0.5:50 v/v) | 213.7 mg/100 g FW | Zorbax SB-C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm HPLC-DAD-MS | Isolation and identification of anthocyanins from berries | - | Antioxidant activity | [107] |
| 30 | Black currant (2011) | Dp-3-glc; Dp-3-rut; Cy-3-glc; Cy-3-rut; Pt-3-(6-coumaroyl)-glc; | 5 g of frozen material extracted with 10 mL of extraction solvent., i.e., acetone/acetic acid (99:1) | 162.8–180.4 mg/100 g FW | Zorbax SB C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD-MS 520 nm | Characterization and quantification of phenolic compounds in blueberries, black and red currants | - | [118] | |
| Blueberries (V. corymbosum) | |||||||||||
| 31 | Blueberries (2000) | Pg-3-glc; Cy-3-glc; Pn-3-glc; Mv-3-glc | acetone/methanol/water/FA (40:40:20:0.1 v/v) | HPLC: 0.08–0.64 mg/mL MALDI-ToF-MS: 0.08–0.8 mg/mL | Zorbax SB-C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-PDA 520 nm MALDI-ToF-MS | Comparison of HPLC and MALDI-ToF-MS for anthocyanins analysis in blueberries | - | - | [104] |
| 32 | Blueberries (2001) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | Acetone/water/acetic acid (70:29.5:0.5 v/v) | Lowbush blueberries: 1.7 mg/g FW Highbush blueberries: 2.2–2.8 mg/g FW | Zorbax C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-MS/MS | Identification of anthocyanins and procyanidins in blueberries and cranberries | - | - | [106] |
| 33 | Blueberry genotypes (2004) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | methanol/water/FA (60:37:3 v/v) | Blueberries: 1.43–8.2 g/kg FW | Symmetry C18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-PDA-MS PDA at 520 nm | Flavonoid glycosides estimation in various genotypes of red wine grapes, blackberry and blueberries | - | Antioxidant properties | [83] |
| 34 | Lowbush blueberry (V. angustifolium) (2006) | - | 0.1% HCl in methanol | V. angustifolium: 350–725 mg/100 g FW | Zorbax C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD 517 nm, 520 nm, 525 nm, 530 nm | Anthocyanin content in different vaccinium types | - | - | [109] |
| 35 | Wild blueberries (2007) | - | Ethanol at 77 °C, 26 °C, or 79 °C without acid (pH 5.4) or acidified with hydrochloric (pH 4.1), citric (pH 4.9), tartaric (pH 5.0), lactic (pH 4.8), or phosphoric acid (pH 4.6; 0.02% v/v) | 24 mg/g DEW | Supelco C18 (250 × 4.6 mm, 4 µm) | Gradient program | HPLC-PDA 520 nm | Anthocyanins in wild blueberries | - | - | [110] |
| 36 | Blueberries (2007) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | 1 N HCl acidified methanol (85:15 v/v)—pH to 1.0 | 558.3 mg/100 g DW | Luna C18 (150 × 3 mm, 3 µm) | Gradient program | HPLC-PDA 520 nm and UPLC-ESI-MS | Anthocyanin content determination in berries | - | - | [153] |
| 37 | Blueberries (2008) | Dp-glycosides; Pt-glycosides; Mv-glycosides; | methanol/acetic acid/water (25:1:24 v/v) | - | Zorbax Eclipse XDB C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-ESI-MS 520 nm | Identification of scavenging compounds in blueberry extract by HPLC coupled to online ABTS based assay | - | Antioxidant activity | [111] |
| 38 | Highbush blueberries (V. corymbosum) (2008) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | MeOH/Water/Acetic acid (25:24:1 v/v) | 5.8–9.6 g/kg DW | Xterra MS C18 (150 × 2.1 mm) | Gradient program | LC-MS (SQD) PDA at 520 nm | Determination of anthocyanins content in various cultivars | - | - | [112] |
| 39 | Blueberries (V. corymbosum) (2009) | List of aglycones provided | 0.1% HCl in methanol | Blueberry fruit skin: 6.2 mg/g FW; Blueberry pulp: 0.02 mg/g FW | Spherisorb ODS C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD | Degradation of anthocyanins and anthocyanidins in blueberry jams/stuffed fish | - | - | [115] |
| 40 | Blueberries (2009) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | MeOH: Water: TFA (70:30:1 v/v) | - | Gemini C18 (100 × 2.0 mm, 3 μm) | Gradient program | LC-MS-IT-ToF-MS(PDA 520 nm) | Characterization/Identification of anthocyanins | - | - | [114] |
| 41 | Highbush blueberries (V. corymbosum) (2009) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | methanol/acetone/water/acetic acid (30:30:35:0.1 v/v) | Blueberries: 78.5 mg/100 g FW | Phenomenex Luna C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | Effect of processing and storage conditions on phenolic compounds | - | Antioxidant activity | [116] |
| 42 | lowbush blueberries (V. angustifolium) (2010) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | methanol with 0.1% HCl | Qualitative | Synergi RP-Max (250 × 2 mm, 4 µm) | Gradient program | HPLC-DAD-MS/MS | Anthocyanins analysis in berries | - | - | [117] |
| 43 | Different cultivars of blueberries (2011) | Dp-3-gal; Dp-3-glc; Dp-3-arab; Pt-3-gal; Pt-3-glc | methanol/acetic acid/water (25:1:24 v/v) | - | Zorbax Eclipse XDB C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-ESI/MS and online HPLC-ABTS 520 nm | Evaluation of antioxidant activity in Blueberry cultivars by HPLC-ESI/MS and HPLC-ABTS system | - | Antioxidant activity | [119] |
| 44 | Blueberry (V. corymbosum), (2011) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | acetone/acetic acid (99:1 v/v) | 42–83.6 mg/100g FW | Zorbax SB C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD-MS 520 nm | Characterization and quantification of phenolic compounds in blueberries, black and red currants | - | - | [118] |
| 45 | Highbush blueberries (V. corymbosum) (2012) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | acetonitrile/water/formic acid (87:3:10 v/v) | Highbush blueberries: 1.6–2.8 g/100 g DW | Phenomenex Luna C18(2) (250 × 4.6 mm, 3.0 µm) | Gradient program | HPLC-UV/Vis 520 nm | Analysis of anthocyanins in bilberries and blueberries | - | - | [120] |
| 46 | Blueberries (2016) | Dp-3-glc; Cy-3-glc; Pt-3-glc; Pn-3-glc; Mv-3-glc; | - | 77.5 mg/100 g FW | Lichrocart RP-18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Phenolic composition and antioxidant activity of different berries | - | Antioxidant activity | [97] |
| 47 | Blueberries of different varieties (2016) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | methanol with 0.1% HCl v/v | Total Anthocyanidins: 108.1–300.6 mg/100 g FW | Zorbax Eclipse XDB C18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-PDA-MS | Chemical profiling of different anthocyanins using HPLC-PDA-MS | PCA | - | [124] |
| 48 | Fresh skin of blueberry (2016) | - | 0.1 M HCl solution 90:10 (v/v) | - | Analytical C18 | Gradient program | HPLC-PDA 525 nm | Mobile phase variation studies using fruit and rose extract using HPLC method | - | - | [123] |
| 49 | Blueberries (2016) | Dp-3-glc; Mv-3-gal; Cy-3-gal; Dp-3-gal; Cy-3-rut; Mv-3-glc | Microwave assisted extraction: methanol/chloroform (2:1) Irradiation time: 20 min at 40 OC. | Hydroalcoholic extract: 9.9–45.5 µg/g DEW Organic extract: nd–31.65 µg/g DEW | GraceSmart RP 18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | Microwave assisted extraction on Italian blueberry varieties | - | Carbonic anhydrase inhibition | [125] |
| 50 | Blueberry wine lees (2017) | Dp-3-glc; Pt-3-glc; Mv-3-gal; Mv-3-glc; Mv-3-arab; Mv-3-Ac-gal; Mv-3-Ac-glc; | 0.1% HCl acidified 70% (v/v) ethanol | - | TSK Gel ODS-100 Z (150 × 4.6 mm, 5 µm) | Gradient program | LC-MS DAD at 520 nm | Identification of anthocyanins from Blueberry wine lees | - | - | [127] |
| 51 | Bluehaven Highbush blueberries (2017) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; Acylated anthocyanins | MeOH with 1.3%FA (v/v) | - | Kinetex PFP (150 × 4.6 mm, 2.6 μm) | Gradient program | PDA at 520 nm | Development of fast chromatography with unique separation using PFP stationary phase | - | - | [126] |
| 52 | Highbush blueberries (V. corymbosum) (2018) | Dp-glycosides; Cy-glycosides; Mv-glycosides; Pn-glycosides; | - | Blueberry fruit skin: 672 mg/g FW; Blueberry pulp: 18.1 mg/g FW | Waters C18 column (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | Comparison of phytochemical profiles of highbush blueberries at different developmental stages | - | Antioxidant activity | [129] |
| 53 | Blueberry anthocyanin extract (2018) | Dp-3-glc; Cy-3-glc; Mv-3-glc; Pg-3-glc; Cy-3-arab; Pn-3-glc; | 60% ethanol | - | Innoval ODS-2 C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS | Antioxidant activity evaluation of anthocyanin extracts and their protective effect against acrylamide induced toxicity in HepG2 cells | - | Antioxidant properties | [128] |
| 54 | Blueberries (2018) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | 1 N HCl in 75% methanol | 1188.3 mg/100 g DW | Zorbax SB-C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 nm | Comparative study of red berry pomaces | stepwise linear discriminant analysis (SLDA) | Antioxidant activity | [102] |
| 55 | Ripened blueberries (2021) | Dp-3-gal; Dp-3-glc; Cy-3-gal; Cy-3-glc; Cy-3-ara; Pt-3-ara; Mv-3-gal; Mv-3-glc; Mv-3-ara; | 60% EtOH at 1:15 (Solid to liquid) ratio | - | Waters C18 column (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-PDA-MS PDA at 520 nm | Antioxidant and bioaccessability of blueberry anthocyanins under in vitro simulated digestion | - | Antioxidant activity | [133] |
| 56 | Dried blueberries (2021) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | Water as solvent (25 g/2 L) | 147.59 mg/L | Zorbax C18 (250 × 4.6 mm, 5 μm) | Gradient program | PDA at 520 nm | Optimization of membrane filtrations for aqueous blueberry extracts | - | - | [131] |
| 57 | Blueberries (2021) | Dp-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | Solvent extraction: acidic methanol with a concentration of 80% v/v Enzymatic extraction | - | Kromasil C18 | Gradient program | HPLC-PDA 525 nm | Efficiency of different extraction methods on blueberry anthocyanins antioxidant properties | - | Antioxidant properties | [134] |
| 58 | Blueberry extracts (2021) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | Millipore water | - | Zorbax C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-ESI-MS PDA 520 nm | Anthocyanins identification and their fouling mechanisms in non-thermal nanofiltration of blueberry aqueous extracts | - | - | [130] |
| 59 | Blueberries (2021) | Dp-3-glc; Cy-3-glc; Pt-3-glc; Pg-3-glc; Pn-3-glc; Mv-3-glc | 1% citric acid -acidified 75% ethanol | - | Zorbax SB-C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | Application of HPLC for simultaneous separation of six major anthocyanins in blueberries | - | - | [135] |
| 60 | Fresh fruits of blueberries (2022) | Dp-3,5-diglc; Dp-3-gal; Cy-3-glc; Pt-3-glc; Pg-3-glu; Mv-3-gal; | EtOH: water (7:3 v/v) mixture acidified with 1.5% HCl | Blueberries: 1107 mg/kg FW | Synergi Polar RP-18 (250 × 4.6 mm,4 µm) | Gradient program | LC-ESI-MS/MS | Simultaneous determination of anthocyanins in blueberry, strawberry and their commercial products using HPLC-MS/MS analysis | - | - | [136] |
| 61 | Highbush blueberries cultivars (2022) | Del-3-gal; Del-3-glu; Cya-3-glu; Pet-3-glu; Peo-3-glu; Mal-3-gal; Mal-3-glu; | 80% MeOH containing 0.1%FA | 0.3–3.2 g/kg DW | Zorbax SB-C18 (50 × 4.6 mm, 5 μm) | Gradient program | HPLC-QToF-MS | Quantification of free and bound phenolics in northern highbush blueberries | - | - | [138] |
| Sweet cherry (P. avium) | |||||||||||
| 62 | Sweet cherry (2002) | Cy-3-glc; Cy-3-rut; Pn-3-glc; Pg-3-rut; Pn-3-rut; | Methanol | 29–62 mg/100 g FW | Hypersil PEP 300 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA PDA at 520 nm | Quantitation of anthocyanins in different cultivars of sweet cherries | - | - | [140] |
| 63 | Sweet cherry (2004) | Cy-3-glc; Cy-3-rut; Pn-3-glc; Pg-3-rut; Pn-3-rut; | 60% methanol | 4.9–230.3 mg/100 g FW | Novapak C18 (150 × 3.9 mm, 5 µm) | - | HPLC-PDA PDA at 520 nm | Effect of ripeness and postharvest storage on phenolic content of cherries | - | - | [141] |
| 64 | Sweet cherry (2004) | Cy-3-glc; Cy-3-rut; Pn-3-glc; Pg-3-rut; Pn-3-rut; | Methanol | 0.3–116.1 mg/100 g FW | Hypersil PEP 300 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA PDA at 520 nm | Changes of anthocyanins affecting skin color of sweet cherries | - | - | [142] |
| 65 | Sweet cherry (2007) | Cy-3-glc; Cy-3-rut; Pn-3-glc; Pg-3-rut; Pn-3-rut; | 60% methanol | - | Novapak C18 (150 × 3.9 mm, 5 µm) | - | HPLC-PDA PDA at 520 nm | Effect of ripeness and postharvest storage on the evaluation of anthocyanins in cherries | - | - | [143] |
| 66 | Sweet cherry (2008) | Cy-3-glc; Cy-3-rut; Pg-3-rut; Pn-3-rut; | Methanol containing 1% HCl and 1% BHT | 1.1–16.2 mg/100 g FW | Gemini C18 (150 × 4.6 mm, 3.0 µm) | Gradient program | HPLC-DAD-MS 520 nm | Phenolic compounds from sweet cherry | - | Antioxidant activity | [144] |
| 67 | Sweet cherry (2009) | Cy-3-glc; Cy-3-rut; | acidified methanol containing 2M HCl | 26.0 mg/100 g FW | Omnispher C18 (250 × 4.6 mm, 5μm) | Gradient program | HPLC-PDA 520 nm | Phenolic composition of berries | - | Antioxidant activity | [89] |
| 68 | Sweet cherry (2014) | Cy-3-sop; Cy-3-rut; Cy-3-glc; Pg-3-rut; Pn-3-rut; | Methanol containing 1%BHA | 642.5 mg/100 g FW | Luna C18 (150 × 2.0 mm, 3 µm) | Gradient program | HPLC-DAD-MS 520 nm | Metabolomic profiling of flavonoids in sweet cherry | - | - | [145] |
| 69 | Sweet cherry (2021) | Pt-3-(6-acetyl)-glc; | 70% ethanol in water | Qualitative | Synergi Hydro-RP (250 × 4.6 mm, 4 µm) | Gradient program | HPLC-ESI-MS | Characterization of phenolic compounds from sweet cherry | - | Antioxidant activity | [181] |
| Black Chokeberry (A. melanocarpa) | |||||||||||
| 70 | Chokeberry (2004) | Cy-3-gal; Cy-3-glc; Pg-3-gal; Cy-3-arab; Pg-3-arab; Cy-3-xyl; | Acetone/water/acetic acid (70:29.5:0.5 v/v) | - | Zorbax SB-C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 nm | Characterization of anthocyanins | - | Antioxidant property | [154] |
| 71 | Chokeberry (2016) | Cy-3-gal; Cy-3-glc; Cy-3-arab; Cy-3-xyl; | 80% ethanol in water | 249–737 mg/100 g FW | Chromalith Performance RP18e (100 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD 520 nm | Anthocyanins content variation different Aronia cultivars | - | Antioxidant activity | [155] |
| 72 | Chokeberry (2020) | Cy-3-arab; Cy-3-gal; Cy-3-glc; | Methanol containing 6% formic acid | - | Purosphere STAR RP-18e (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD 520 nm | Chemometric studies of A. melanocarpa fruits | PCA | - | [40] |
| American large cranberry (V. macrocarpon) | |||||||||||
| 73 | Cranberries extract (V. macrocarpon) (2004) | Cy-3-gal; Cy-3-arab; Pn-3-gal; Pn-3-arab; | Extract powder dissolved in water | - | Nova-Pak C18 (150 × 3.9 mm, 4 μm) | Gradient program | HPLC-PDA-MS 520 nm | Phytochemical constituents from total cranberry extract | - | Inhibitory effect against Hep-G2 liver cancer cells, | [188] |
| 74 | Cranberries (V. macrocarpon) (2011) | Cy-3-gal; Cy-3-glc; Cy-3-arab; Pn-3-gal; Pn-3-arab; | 98% methanol in 2% HCl (v/v) | 4.3 mg/g DW | Cosmosil 5C18-PAW (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-UV/Vis 520 nm | Determination of anthocyanins in cranberry fruit | - | - | [189] |
| European small cranberry (V. oxycoccus) | |||||||||||
| 75 | Cranberries (V. oxycoccus) (2001) | Cy-3-gal; Cy-3-glc; Pt-3-gal; Cy-3-arab; Pn-3-gal; Pn-3-arab; | Acetone/water/acetic acid (70:29.5:0.5 v/v) | Cranberries: 360 mg/100 g FW | Zorbax C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-MS/MS | Identification of anthocyanins and procyanidins in blueberries and cranberries | - | - | [106] |
| 76 | Cranberries (V. oxycoccus) (2009) | Cy-3-gal; Cy-3-glc; Cy-3-arab; Pn-3-glc; Pn-3-gal; Pn-3-arab; | Acidified 95% ethanol containing 0.1 N HCl (v/v) | 40.7–207.3 mg/100 g FW | Lichrosphere C18 (125 × 4.0 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Anthocyanins in berries of European cranberry | - | Antibacterial activity | [162] |
| 77 | Fresh skin of cranberry (2016) | - | 0.1 M HCl solution 90:10 (v/v) | - | Analytical C18 | Gradient program | HPLC-PDA 525 nm | Mobile phase variation studies using fruit and rose extract using HPLC method | - | [123] | |
| 78 | Cranberries (V. oxycoccus) (2021) | Cy-3-glc; Cy-3-rut; Pt-3-glc; Pn-3,5-dihex; Pg-3-glc; Pg-3-(6-malonyl)-glc; | 60% ethanol at solid to liquid ration of 1:30 g/mL | - | Zorbax Eclipse XDB C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 nm | Optimization of ultrasound-assisted extraction of anthocyanins from cranberries. | - | - | [190] |
| Black Crowberry (E. nigrum) | |||||||||||
| 79 | Crowberry (E. nigrum) (2010) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | 70% aq. Acetone containing 1% formic acid | 401–768.2 mg/100 g FW | XTrerra Phenyl (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Variation of anthocyanins in wild populations of crowberries | Hierarchical cluster analysis (HCA) | - | [156] |
| Elderberry (S. nigra) | |||||||||||
| 80 | American and European elderberry (2007) | Cy-glycosides; Cy-acyl-glycosides; | acidified methanol (0.1% FA) | S. canadensis: 207.1–1005.2 mg/100 g S. nigra: 656.5–806.1 mg/100 g fresh weight | Synergi Hydro RP (150 × 2 mm, 4 µm) | Gradient program | HPLC-DAD-MS 520 nm | Distribution of anthocyanins in American and European elderberry varieties | - | - | [147] |
| 81 | Elderberry fruit cultivars (2009) | Cy 3-sam-5-glc; Cy 3,5-diglc; Cy 3-sam; Cy 3-glc; Cy 3-rut; | Acidified methanol containing 1% HCL and 1% 2,6-di-tert-butyl-4-methylphenol (BHT) | 603–1265 mg/100 g FW | Gemini C18 (150 × 4.6 mm, 3 µm) | Gradient program | HPLC-DAD | Anthocyanin content | - | - | [216] |
| 82 | Elderberry species and hybrids (2014) | Cy-glycosides; Cy-acyl-glycosides; Pg-glycosides; | Methanol with 3% formic acid and 1% 2,6-di-tert-butyl-4-methylphenol (BHT) | 560 mg/100 g FW | Gemini C18 (150 × 4.6 mm, 3 µm) | Gradient program | HPLC-DAD-ESI-MS | Anthocyanin analysis in different accessions of elderberry fruits | - | - | [163] |
| 83 | Elderberry fruit, other species (2022) | Cy-3-sam-5-glc; Cy-3-sam; Cy-3-glc | acidified methanol (1% formic acid) | 0.08–5.3 mg/g DW | HSS C18 (100 × 2.1 mm, 1.8 µm) | Gradient program | UHPLC-PDA-MSUHPLC-QToF-MS | Authentication, characterization of anthocyanins and other polyphenolic compounds | - | - | [182] |
| Goji berry/Wolf berry (L. ruthenicum) | |||||||||||
| 84 | Black Goji berry (2003) | - | Various solvents such as petroleum ether, ethyl acetate, methanol and n-butanol inn individual | Tr-3.8 mg/g DEW | Discovery C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA | Antioxidant activity of Lycium extracts | - | Antioxidant activity | [148] |
| 85 | Black Goji berry (2011) | Pt-glycosides; Pt-acyl-glycosides; | Methanol containing 2% formic acid | 465–525 mg/100 g FW | ODS 80Ts QA (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD and HPLC-ESI-MS 520 nm | Anthocyanin composition of goji berries from Qinghai-Tibet Plateau | - | Antioxidant activity | [149] |
| 86 | Black Goji berry (2015) | Pt-acyl-glycosides; Dp-acyl-glycosides; Mv-acyl-glycosides; | 70% ethanol pH 2.5 adjusted with HCl | Qualitative | Xterra MS C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD 520 nm LC-QToF-MS | Characterization of anthocyanins from black goji berry | - | - | [212] |
| 87 | Black Goji berry (2016) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pg-glycosides; Acyl-glycosides | 2% formic acid in aq. Solution | 9.3–37.8 mg/g DW | ODS 80Ts QA (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Constituent analysis and quality control of anthocyanins in dried goji berries | - | - | [198] |
| 88 | Black Goji berry (2018) | Dp-glycosides; Pt-glycosides; Mv-glycosides; Pg-glycosides; Acyl-glycosides | Deep eutectic solvent (DES) extraction: various molar ratios of choline chloride: 1,2-propanediol (mol/mol) | 3.43–3.83 mg/g FW | Zorbax SB-C18 (100 × 4.5 mm, 3.5 µm) | Gradient program | 2D HPLC-DAD 520 nm LC-QToF-MS | DES based extraction for the determination of anthocyanins in goji berry using 2D LC-DAD-ESI-MS/MS | - | - | [184] |
| 89 | Black Goji berry (2022) | Dp-glycosides; Pt-glycosides; Mv-glycosides; Acyl-glycosides | 2% formic acid in methanol | 1485.2–5826.0 mg/100 g DW | Zorbax SB-C18 (250 × 4.5 mm, 5 µm) | Gradient program | HPLC-PDA 525 nm LC-QToF-MS | Anthocyanin fingerprint of black wolfberry fruit | PCA | - | [183] |
| Red grapes Peel (V. vinifera) | |||||||||||
| 90 | Red grapes skin (2001) | Dp-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | - | Relative quantification (%) | Waters Novapak (150 × 3.9 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | HPLC analysis of anthocyanins in different red grape cultivars | - | - | [213] |
| 91 | Red grapes skin (2002) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | - | Relative quantification (%) | Waters Novapak (150 × 3.9 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | Anthocyanin patterns of red grape cultivars | - | - | [211] |
| 92 | Red grapes skin (2004) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | Methanol | - | Supersphere 100 RP 18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Anthocyanins identification in red grapes skin by LC-MS and NMR | HCA and RDA | - | [197] |
| 93 | Red wine grapes genotypes (2004) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | methanol/water/FA (60:37:3 v/v) | Red wine grapes: 0.38–7.9 g/kg FW | Symmetry C18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-PDA-MS PDA at 520 nm | Flavonoid glycosides estimation in various genotypes of red wine grapes, blackberry and blueberries | - | Antioxidant properties | [83] |
| 94 | Red grapes skin/peel (2005) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | 20% formic acid in methanol | - | Kromasil 100 C18 (250 × 4.0 mm, 4 µm) | Gradient program | HPLC-PDA 546 nm | Varietal difference among anthocyanins in red grape cultivars | PCA | - | [214] |
| 95 | Red grapes skin (2008) | Cy-3,5-diglc; Cy-3-glc; Mv-3-glc; Mv-3,5-diglc; Dp-3-glc; Pn-3-glc; Pg-3-diglc; Pt; Pg; | Acidified methanol (1% HCl v/v) | 3.4–7.1 mg/g FW | Inertsil ODS-3V (150 × 4.6 mm, 5 µm) | Gradient program | RP-HPLC-ED | Anthocyanin monitoring in red grape skin extracts | - | - | [185] |
| 96 | Red grapes skin (2008) | Dp-3-glc; Cy-3-glc; Pn-3-glc; Mv-3-glc; Pt-3-glc; | Pressurized fluid extraction: methanol | 564.7 mg/g DW | Synergi C12 Max-RP (250 × 4.6 mm, 4 µm) | Gradient program | HPLC-UV/Vis 520 nm | Determination of anthocyanins in red grape skin | - | - | [79] |
| 97 | Red grapes skin (2008) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | Acidic ethanol (0.1% HCl) | 1.7–4.1 mg/g FW | Eurosphere C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 530 nm | Extraction methods for separation of anthocyanins from red grape skin | - | - | [186] |
| 98 | Red grapes skin (2012) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | Methanol/water/formic acid (50:48.5:0.5 v/v) | 2.4–25.3 mg/100 g FW | Zorbax XDB-C18 (250 × 4.6 mm,5 µm) | Gradient program | HPLC-DAD | Phenolic composition of red grape varieties from Spanish region | - | - | [157] |
| 99 | Red grapes skin (2013) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | Buffer composition (1 L): 200 mL deionized water, 5 g tartaric acid, 120 mL of 95 % ethanol, 2 g sodium metabisulfite, 22 mL of a 1 N sodium hydroxide solution, and then, deionized water up to the 1 L volume. | 6.9–45.0 mg/100 g FW | Hypersil ODS (200 × 2.1 mm, 5 µm) | Gradient program | HPLC-DAD 520 nm | Changes in phenolic content during ripening | PCA | - | [165] |
| 100 | Red grapes skin (2014) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; acyl-glycosides; | Methanol containing 0.5% 12N HCl | - | Phenomenex Jupiter C18 (250 × 2.1 mm, 4 µm) | Gradient program | HPLC-DAD 520 nm MALDI-Tof-MS LC-QToF-MS | Profiling of anthocyanins in grape varieties | - | - | [215] |
| 101 | Red grapes skin (2015) | Dp-3-glc; Cy-3-glc; Pt-3-glc; Pg-3-glc; Mv-3-glc; Pn-3-acetyl-glc; Cy; Pn-3-coumaroyl-glc; Pn; Mv; | Distilled water | 7–24.3 mg/g DEW | Zorbax Eclipse plus C18 (150 × 4.6 mm, 3.5 µm) | Gradient program | HPLC-DAD 520 nm | Anthocyanins and total phenolics profile of red grape varieties. | - | Antioxidant activity | [187] |
| Black Mulberry (M. nigra) | |||||||||||
| 102 | Mulberry (2001) | Cy-3-sop; Cy-3-glc; Cy-3-rut; Pg-3-glc; Pg-3-rut; | Aliquot of 5 mL of extract from fresh berries was taken and centrifuged | - | Restek Pinnacle ODS (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-ESI-MS | Identification of anthocyanins in different berries | - | - | [82] |
| 103 | Mulberry (2010) | Cy-3-rut; Cy-3-glc; Pg-3-glc; Pg-3-rut; Cy; Pg; | - | Qualitative | Waters RP C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 nm | Analysis and characterization of anthocyanins from mulberry | - | - | [209] |
| 104 | Mulberry (2011) | Cy-3-(2-glucosyl)-rut; Dp-3-rut-5-glc; Cy-3,5-diglc; Cy-3-glc; Cy-3-rut; Pg-3-glc; Pg-3-rut; Dp-3-rut; | Acidified methanol concentration varied from 10–70% containing 1%TFA under microwave assisted extraction conditions | - | Zorbax SB-C18 (50 × 2.1 mm, 1.8 µm) | Gradient program | HPLC-ESI-MS | Optimization of microwave extraction for anthocyanins from mulberry and analysis using HPLC-ESI-MS | - | - | [164] |
| 105 | Mulberry (2017) | Dp-3-rut; Cy-3-glc; Cy-3-rut; Cy-3-rut-5-glc; | 0.1%HCl in ethanol | 0.49 mg/mL | Zorbax Eclipse plus C18 (100 × 4.5 mm, 3.5μm) | Ethanol and α-hydroxy acid aqueous solution | HPLC-DAD 520 nm HPLC-DAD-MS | Green chromatography for anthocyanins determination in berries | - | - | [98] |
| 106 | Mulberry (2020) | Dp-pent; Pt-pent; Pn-hex; Cy-pent-hex; Cy-rha-hex; Cy-sambu-glc; Dp-dirha-hex; | 1% formic acid in methanol | - | Varian pursuit C18 (150 × 2.0 mm, 3 µm | Gradient program | HPLC-DAD-MS 520 nm | Identification of phenolic compounds in edible wild fruits using HPLC-DAD-ESI-HRMS | - | - | [210] |
| 107 | Mulberry (2020) | Cy-3-glc; Cy-3-rut; Pg-3-glc; Pg-3-rut; | acetone containing 1% HCl | 125.3 mg/100 g DEW | Zorbax Eclipse plus C18 (150 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Comparative HPLC analysis of Morus species | - | - | [146] |
| Peach (yellow peel) (P. persica) | |||||||||||
| 108 | Peaches (2001) | Cy-3-glc; Cy-3-rut; | water: methanol (20:80) containing sodium formate | 6.9–33.6 mg/100 g FW | Nucleosil C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 510 nm | Phenolic compounds from peel of peaches and plums | - | - | [199] |
| 109 | Peaches (2021) | Cy-3-glc; Cy-3-rut; | methanol/water/0.1 M HCl (6:3:1 v/v) | 0.5–2.8 mg/100 g FW | Inertsil ODS-3 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD 520 nm | Polyphenols content in peaches and plums | PCA | Antioxidant activity | [205] |
| Plum (red and black peel) (P. domestica) | |||||||||||
| 110 | Plums peel (2001) | Cy-3-glc; Cy-3-rut; Cy-3-acetyl-glc; Cy-3-gal; | water: methanol (20:80) containing sodium formate | 12.9–161.4 mg/100 g FW | Nucleosil C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 510 nm | Phenolic compounds from peel of peaches and plums | - | - | [199] |
| 111 | Plums (2006) | Dp-3-sambu; Cy-3-sambu; Pn-3-sambu; Pt-3-sambu; | 80% methanol containing 0.1% HCl (v/v) | Davidson’s plum: 1.27 µM/g FW Lllawarra plum: 19.4 µM/g FW Burdekin plum: 6.0 µM/g FW | Luna C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Identification and quantification of anthocyanins in Australian native fruits | - | Antioxidant activity | [203] |
| 112 | Plums (2009) | Cy-3-xyl; Cy-3-glc; Cy-3-rut; Pn-3-rut; Pn-3-glc; | methanol containing 1% HCl and 1% BHT | 5.3–18.7 mg/100 g FW | Phenomenex gemini C18 (150 × 4.6 mm, 3 µm) | Gradient program | HPLC-PDA 530 nm | Anthocyanins and fruit color in plums | - | - | [200] |
| 113 | Plums (2021) | Cy-3-glc; Cy-3-rut; Pn-3-rut; | methanol/water/0.1 M HCl (6:3:1 v/v) | 1.2–16.4 mg/100 g FW | Inertsil ODS-3 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD 520 nm | Polyphenols content in peaches and plums | PCA | Antioxidant activity | [205] |
| Pomegranate (P. granatum) | |||||||||||
| 114 | Pomegranate peel (2011) | Dp-3,5-diglc; Cy-3,5-diglc; Pg-3,5-diglc; Dp-3-glc; Cy-pent-hex; Cy-3-glc; Cy-3-rut; Pg-3-glc; Cy-pent; | aqueous methanol (80% v/v; 0.15 M HCl) | Peel: 44.7 mg/100 g DW | Synergi Hydro-RP (150 × 3.0 mm, 4 µm) | Gradient program | HPLC-DAD-MS 520 nm | Identification and quantification of phenolic compounds from pomegranate | - | Antioxidant activity | [175] |
| 115 | Pomegranate peel (2013) | Dp-3-glc; Cy-3-glc; Pg-3-glc; Pn-hex; Cy-pent; | Methanol (0.1% HCl v/v) | 5.3–102.9 mg/100 g FW | Zorbax SB-C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Characterization and evaluation of major anthocyanins in pomegranate peel from different cultivars | - | - | [201] |
| 116 | Pomegranate peel (2015) | Dp-3,5-diglc; Dp-3-glc; Cy-3,5-diglc; Cy-3-glc; Pg-3,5-diglc; Pg-3-glc; | Methanol (0.1% HCl) | 45.2–344.1 mg/100 g FW | Zorbax SB-C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD 520 nm | Composition and content of anthocyanins in pomegranate cultivars | - | - | [207] |
| 117 | Pomegranate peel (2015) | Dp-3,5-diglc; Dp-3-glc; Cy-3,5-diglc; Cy-3-glc; Pg-3,5-diglc; Pg-3-glc; | Methanol (0.1% HCl) | 3.7 mg/100 g FW | Zorbax SB-C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD 520 nm | Patterns of pigment changes in pomegranate peel during fruit ripening | - | - | [208] |
| 118 | Pomegranate peel (2016) | - | Peel extraction (Soxhlet): Ethyl acetate | - | Lichrosorb RP 18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD | Evaluation of extraction methods from pomegranate whole fruit or peel | - | Antioxidant, antiproliferative activity and cytotoxicity assay | [176] |
| 119 | Pomegranate peel (2017) | Cy-3,5-diglc; Cy-3-glc; Pg-3-glc; | different concentration of ethanol. | - | BDS Hypersil (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | High-Pressure-Assisted Extraction of Bioactive Compounds from Pomegranate Peel | Response surface methodology (RSM) | Antioxidant activity | [177] |
| 120 | Pomegranate peel (2017) | Dp-3,5-diglc; Dp-3-glc; Cy-3,5-diglc; Cy-3-glc; Pg-3,5-diglc; Pg-3-glc; | water/methanol (80:20 v/v) containing 0.1% HCl. | - | Ascentis express C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 and 540 nm | Metabolite fingerprinting of pomegranate polyphenols using HPLC-DAD-MS | PCA | - | [178] |
| 121 | Pomegranate peel (2017) | Dp-3,5-diglc; Cy-3,5-diglc; Cy-3-glc; Pg-3,5-diglc; Pg-3-glc; | Soxhlet condition using ethanol as solvent | 7.9–10.3 mg/100 g DW | μBondapack (200 × 4.6 mm, 10 µm) | Gradient program | HPLC-PDA 520 nm | Phenolic compounds from pomegranate peel | - | Antioxidant activity | [179] |
| 122 | Pomegranate peel (2020) | Dp-3,5-diglc; Cy-3,5-diglc; Dp-3-glc; Cy-3-glc; Pg-3-glc; | Millipore water | Peel: nd–68 mg/100 g FW | Kinetex EC-C18 (30 × 3 mm, 2.6 µm) | Gradient program | HPLC-PDA-MS 520 nm | Characterization of peel from pomegranate | - | Antioxidant activity, α-amylase inhibitory activity and tyrosine inhibitory activity | [180] |
| Raspberry (red) (R. idaeus) | |||||||||||
| 123 | Red raspberries (2002) | Cy-3,5-diglc; Cy-3-glc | 0.1%HCl acidified methanol | - | Phenomenex Phenyl-hexyl (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-MS | Phenolic content of berries (not LC based) | - | Antioxidant activity | [15] |
| 124 | Red raspberries (2002) | Cy-glycosides; Pg-glycosides; acyl-glycosides; | 0.1% HCl in methanol | - | RP-MAX (250 × 4.6 mm, 4 µm) | Gradient program | HPLC-MS | Chemical composition of red raspberries | - | Antioxidant and Vasorelaxation activity | [169] |
| 125 | Red raspberries (2002) | Cy-3-sop; Cy-3-(2-glucosyl)-rut; Cy-3-glc; Pg-3-sop; Cy-3-rut; Pg-3-(2-glucosyl)-rut; Pg-3-glc; Pg-3-rut; | 0.1% HCl in methanol | - | NovaPac C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 nm | Characterization of anthocyanins in red raspberries | - | - | [170] |
| 126 | Red raspberries (2004) | Cy-3-hex; Cy-3-sop; Cy-3-(2-glucosyl)-rut; Cy-3-glc; Cy-3-rut; Pg-3-glc; Pg-3-rut; Cy; | ethyl acetate followed by acidified methanol | 53.6–88.8 mg/100 g FW | Lichrocart Purospher RP-18e (125 × 3 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Identification and quantification of phenolic compounds | - | - | [152] |
| 127 | Red raspberries (2007) | Cy-3-sop; Cy-3-(2-glucosyl)-rut; Cy-3-sambu; Cy-3-glc; Cy-3-xylosyl-rut; Cy-3-rut; Pg-3-rut; | 1.5 M HCl/95% ethanol (15:85 v/v) | - | Kromasil C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-MS | Optimization of ultrasound assisted extraction for anthocyanin extraction and their identification | - | - | [158] |
| 128 | Raspberry (2007) | Dp-glycosides; Cy-glycosides; Pt-glycosides; Mv-glycosides; Pn-glycosides; | 1 N HCl acidified methanol (85:15 v/v)—pH to 1.0 | 365.2 mg/100 g DW | Luna C18 (150 × 3 mm, 3 µm) | Gradient program | HPLC-PDA 520 nm and UPLC-ESI-MS | Anthocyanin content determination in berries | - | - | [153] |
| 129 | Red Raspberry (2009) | Cy-3-sop; Cy-3-glc; | Acidified methanol containing 2 M HCl | 77.2 mg/100 g FW | Omnispher C18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-PDA 520 nm | Phenolic composition of berries | - | Antioxidant activity | [89] |
| 130 | Raspberries (2010) | Cy-glycosides; Pg-glycosides; acyl-glycosides; | methanol with 0.1% HCl | Qualitative | Synergi RP-Max (250 × 2 mm, 4 µm) | Gradient program | HPLC-DAD-MS/MS | Anthocyanins analysis in berries | - | - | [117] |
| 131 | Raspberries (2010) | Cy-3-sop; Cy-3-(2-glc)-rut; Cy-3-glc; Cy-3-rut; Cy-3,5-diglc; | 0.1% formic acid in methanol | 76.2–277.0 mg/100 g DW | Nova-Pak C18 (150 × 3.9 mm, 4 µm) | Gradient program | HPLC-DAD 520 nm | HPLC analysis of polyphenols from Red raspberries | - | - | [159] |
| 132 | Raspberry (2016) | Cy-3-glc; Pt-3-glc; Cy-3-sop; Cy-3-glucosyl-rut; Cy-3-rut; | - | 133.9 mg/100 g FW | Lichrocart RP-18 (250 × 4.6 mm, 5 μm) | Gradient program | HPLC-DAD 520 nm | Phenolic composition and antioxidant activity of different berries | - | Antioxidant activity | [97] |
| 133 | Red raspberry (2018) | Cy-3-sop; Cy-3-(2-glucosyl)-rut; Cy-3-glc; Cy-3-rut; | 1 N HCl in 75% methanol | 188.0 mg/100 g DW | Zorbax SB-C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 nm | Comparative study of red berry pomaces | stepwise linear discriminant analysis (SLDA) | Antioxidant activity | [102] |
| 134 | Red raspberry (2020) | Cy-3-sop; Cy-3-glc; | ethanol/water mixtures acidified with citric acid until pH 3 | 613–1000 mg/100 g DEW | Phenomenex Aqua RP-C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Anthocyanins extraction from red raspberry and their activities | Response surface graphs | Antioxidant activity and Antibacterial effect | [161] |
| 135 | Red raspberry (2020) | Cy-3-sop; Cy-3-glc; Cy-3-glucosyl-rut; Cy-3-rut; Pg-3-glc; | 95% acidified ethanol (pH 3.0) | 1.7–102.5 mg/100 g FW | Thermo Hypersil Gold C18 (100 × 2.1 mm, 3 µm) | Gradient program | HPLC-DAD-MS 520 nm | Physicochemical characteristic evaluation for Red raspberries | PCA(Principle component analysis) | - | [160] |
| 136 | Red raspberry (2021) | Cy-3-glc; Cy-3-rut; Cy-3-xylosyl-rut; | 80:20 ethanol/water (v/v) | 336–554 mg/100 g DEW | - | - | HPLC-MS | Antibacterial effects of berries against H. pylori infection | - | Antibacterial activity | [132] |
| Raspberry (black) (R. occidentalis) | |||||||||||
| 137 | Black raspberries (2002) | Cy-3-(6-p-coumaroyl)-sambu; Cy-3-(6-p-coumaroyl)-glc; | 0.1%HCl acidified methanol | - | Phenomenex Phenyl-hexyl (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-MS | Phenolic content of berries (not LC based) | - | Antioxidant activity | [15] |
| 138 | Black raspberries (2005) | Cy-3-glc; Cy-3-sambu; Cy-3-(2-xylosyl)-rut; Cy-3-rut | Methanol | - | Symmetry C18 (75 × 4.6 mm, 3.5 µm) | Gradient program | LC-MS/MS | Anthocyanins determination in black raspberries | - | - | [206] |
| 139 | Black raspberry (2021) | Cy-3-glc; Cy-3-rut; Cy-3-xylosyl-rut; | 80:20 ethanol/water mixture | 2885–11109 mg/100 g DEW | - | - | HPLC-MS | Antibacterial effects of berries against H. pylori infection | - | Antibacterial activity | [132] |
| Strawberry (Fragaria × ananassa) | |||||||||||
| 140 | Strawberry (2002) | Cy-glycosides; Pg-glycosides; acyl-glycosides; | 0.1%HCl in methanol | - | Phenomenex Aqua C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA-MS 520 nm | Identification of anthocyanins in strawberry | - | - | [77] |
| 141 | Strawberry (2004) | Cy-3-glc; Cy-glc; Pg-3-glc; Pg-3-rut; Cy; Pg-3-malonyl-glc; Pg-3-succinyl-glc; | acidified methanol | 31.4–36.5 mg/100 g FW | Lichrocart Purospher RP-18e (125 × 3 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Identification and quantification of phenolic compounds | - | - | [152] |
| 142 | Strawberry (2004) | Cy-3-glc; Pg-3-glc; Pg-3-ara; Pg | acidified methanol | 25.3–39.8 mg/100 g FW | Lichrocart Purospher RP-18 (125 × 3 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | Comparison of different strawberry cultivars in Poland | - | Antioxidant activity | [151] |
| 143 | Strawberries (2007) | Dp-3-glc; Dp-3-rut; Dp-3-gal; Cy-3-glc; Mv-3-glc; Pn-3-glc; Pn-3-gal; Mv-3-gal; Mv-3-ara; | 1 N HCl acidified methanol (85:15 v/v)—pH to 1.0 | 97.5 mg/100 g DW | Luna C18 (150 × 3 mm, 3 µm) | Gradient program | HPLC-PDA 520 nm and UPLC-ESI-MS | Anthocyanin content determination in berries | - | - | [153] |
| 144 | Strawberry (2008) | Cy-glycosides; Pg-glycosides; acyl-glycosides; | 1% acetic acid in acetone/1% acetic acid in methanol (1:1 v/v) | Qualitative | Eclipse XDB C18 (150 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Identification of phenolic compounds in cultivated strawberries from Macedonia | - | - | [174] |
| 145 | Strawberry (2011) | Cy-3-glc; Cy-3-rut; Pg-3-glc; Pg-3-rut; Pg-3-malonyl-glc; Pg-3-acetyl-glc | acetone/water/acetic acid-70:29.5:0.5 v/v | 6.6–45.3 mg/100 g FW | Ultrasphere ODS (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-PDA 520 nm | Characterization of phenolic compounds in strawberry fruits | - | Antioxidant activity | [173] |
| 146 | Strawberry (2012) | Cy-3-glc; Pg-3-glc; Pg-3-rut; Pg-3-malonyl-glc; Pg-derivative; | acidified methanol (0.1% HCl v/v) | 269.2–559.4 mg/100 g DW | Sunfire C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Evaluation of freezing and thawing methods on strawberry anthocyanin and color stability | - | Antioxidant activity | [172] |
| 147 | Strawberry (2013) | Cy-3-glc; Pg-3-glc; Pg-3-rut; Pg-3-malonyl-glc; | acetone/water/acetic acid-70:29.5:0.5 v/v | 14.6–34.3 mg/100 g FW | Lichrocart C18 (250 × 4 mm, 5 μm) | Gradient program | HPLC-DAD-MS 520 nm | Bioactive compounds from strawberries | - | Antioxidant activity | [166] |
| 148 | Strawberry (2013) | Cy-3-glc; Pg-3-glc; Pg-3-rut; Pg-3-malonyl-glc; Pg-derivative; | acidified methanol (0.1% HCl v/v) | 4.7–31.7 mg/100 g FW | Sunfire C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Influence of PPO inhibitors on strawberry anthocyanin and color stability | - | Antioxidant activity | [167] |
| 149 | Strawberry (2015) | Cy-glycosides; Pg-glycosides; acyl-glycosides; | methanol containing 1%HCl v/v | 212.2–758.6 mg/100 g DW | Zorbax SB-C18 (250 × 4.6 mm, 5 µm) | Gradient program | HPLC-DAD-MS 520 nm | Assessment of differences in phenolic compositions of strawberry cultivars | Forward Linear Discriminant Analysis (LDA) | - | [171] |
| 150 | Strawberry (2016) | Cy-3-rut; Pg-3-glc; pg-3-rut; Pn-3-rut; | - | 407.8 mg/100 g DW | Lichrocart RP-18 (250 × 4.6 mm, 5μm) | Gradient program | HPLC-DAD 520 nm | Phenolic composition and antioxidant activity of different berries | - | Antioxidant activity | [97] |
| 151 | Strawberry (2020) | Cy-3-glc; Pg-3-glc; | acidified methanol (0.1% HCl v/v) | 11–49.3 mg/100 g FW | Inertsil ODS-3 (250 × 4.6 m, 5 µm) | Gradient program | HPLC-DAD 520 nm | Anthocyanin content and physicochemical properties of strawberry cultivars in anthocyanin content rich | - | - | [168] |
| 152 | Fresh fruits of strawberries (2022) | Strawberries: Cya-3-glu; Pg-3-rut; Pg-3-glu; | EtOH: water (7:3 v/v) mixture acidified with 1.5% HCl | Strawberries: 20 mg/100 g FW | Synergi Polar RP-18 (250 × 4.6 mm, 4 μm) | Gradient program | LC-ESI-MS/MS | Simultaneous determination of anthocyanins in blueberry, strawberry and their commercial products using HPLC-MS/MS analysis | - | - | [136] |
5. Adulteration Issues of Processed Food or Dietary Supplements
6. Health-Promoting Effects of Anthocyanins
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cásedas, G.; Les, F.; López, V. Anthocyanins: Plant pigments, food ingredients or therapeutic agents for the CNS? A mini-review focused on clinical trials. Curr. Pharm. Des. 2020, 26, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Indo, H.P.; Yen, H.-C.; Nakanishi, I.; Matsumoto, K.-i.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Islam, P.; Subhan, N.; Rahman, M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential health benefits of anthocyanins in oxidative stress related disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Shimizu, S.; Matsushita, H.; Morii, Y.; Ohyama, Y.; Morita, N.; Tachibana, R.; Watanabe, K.; Wakatsuki, A. Effect of anthocyanin-rich bilberry extract on bone metabolism in ovariectomized rats. Biomed. Rep. 2018, 8, 198–204. [Google Scholar] [CrossRef]
- Ye, J.; Meng, X.; Yan, C.; Wang, C. Effect of purple sweet potato anthocyanins on β-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochem. Res. 2010, 35, 357–365. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Anwar, S.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol. Nutr. Food Res. 2013, 57, 1979–1987. [Google Scholar] [CrossRef]
- Parrado-Fernández, C.; Sandebring-Matton, A.; Rodriguez-Rodriguez, P.; Aarsland, D.; Cedazo-Mínguez, A. Anthocyanins protect from complex I inhibition and APPswe mutation through modulation of the mitochondrial fission/fusion pathways. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 2110–2118. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Bergland, A.K.; Soennesyn, H.; Dalen, I.; Rodriguez-Mateos, A.; Berge, R.K.; Giil, L.M.; Rajendran, L.; Siow, R.; Tassotti, M.; Larsen, A.I. Corrigendum: Effects of Anthocyanin Supplementation on Serum Lipids, Glucose, Markers of Inflammation and Cognition in Adults With Increased Risk of Dementia–A Pilot Study. Front. Genet. 2021, 12, 678504. [Google Scholar] [CrossRef]
- Zhang, P.-W.; Chen, F.-X.; Li, D.; Ling, W.-H.; Guo, H.-H. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e758. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant Activity and Phenolic Content of Oregon Caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X. Analysis Methods of Anthocyanins. In Analysis of Antioxidant-Rich Phytochemicals; John Wiley & Sons Ltd.: West Sussex, UK, 2012; pp. 149–180. [Google Scholar]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Borkowski, T.; Szymusiak, H.; Gliszczyńska-Swigło, A.; Tyrakowska, B. The effect of 3-O-β-glucosylation on structural transformations of anthocyanidins. Food Res. Int. 2005, 38, 1031–1037. [Google Scholar] [CrossRef]
- Wang, B.; He, R.; Li, Z. The Stability and Antioxidant Activity of Anthocyanins from Blueberry. Food Technol. Biotechnol. 2010, 48, 42–49. [Google Scholar]
- Weber, F.; Larsen, L.R. Influence of fruit juice processing on anthocyanin stability. Food Res. Int. 2017, 100, 354–365. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Gradinaru, G.; Biliaderis, C.G.; Kallithraka, S.; Kefalas, P.; Garcia-Viguera, C. Thermal stability of Hibiscus sabdariffa L. anthocyanins in solution and in solid state: Effects of copigmentation and glass transition. Food Chem. 2003, 83, 423–436. [Google Scholar] [CrossRef]
- Markakis, P.; Jurd, L. Anthocyanins and their stability in foods. C R C Crit. Rev. Food Technol. 1974, 4, 437–456. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of variousdietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef]
- Seeram, N.P.; Bourquin, L.D.; Nair, M.G. Degradation Products of Cyanidin Glycosides from Tart Cherries and Their Bioactivities. J. Agric. Food Chem. 2001, 49, 4924–4929. [Google Scholar] [CrossRef]
- Quina, F.; Moreira, P.; Giongo, C.; Rettori, D.; Rodrigues, R.; Freitas, A.; Silva, P.; Macanita, A. Photochemistry of anthocyanins and their biological role in plant tissues. Pure Appl. Chem 2009, 81, 1687–1694. [Google Scholar] [CrossRef]
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef]
- Julia, M.; María José, N.; Ana María, J.-M.; Agustín, G.A. Anthocyanin Pigments: Importance, Sample Preparation and Extraction. In Phenolic Compounds; Marcos, S.-H., Mariana, P.-T., Maria del Rosario, G.-M., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Heffels, P.; Weber, F.; Schieber, A. Influence of Accelerated Solvent Extraction and Ultrasound-Assisted Extraction on the Anthocyanin Profile of Different Vaccinium Species in the Context of Statistical Models for Authentication. J. Agric. Food Chem. 2015, 63, 7532–7538. [Google Scholar] [CrossRef]
- Mazza, G.; Cacace, J.E.; Kay, C.D. Methods of analysis for anthocyanins in plants and biological fluids. J. AOAC Int. 2004, 87, 129–145. [Google Scholar] [CrossRef]
- Kirby, C.W.; Wu, T.; Tsao, R.; McCallum, J.L. Isolation and structural characterization of unusual pyranoanthocyanins and related anthocyanins from Staghorn sumac (Rhus typhina L.) via UPLC–ESI-MS, 1H, 13C, and 2D NMR spectroscopy. Phytochemistry 2013, 94, 284–293. [Google Scholar] [CrossRef]
- Wyzgoski, F.J.; Paudel, L.; Rinaldi, P.L.; Reese, R.N.; Ozgen, M.; Tulio, A.Z., Jr.; Miller, A.R.; Scheerens, J.C.; Hardy, J.K. Modeling Relationships among Active Components in Black Raspberry (Rubus occidentalis L.) Fruit Extracts Using High-Resolution 1H Nuclear Magnetic Resonance (NMR) Spectroscopy and Multivariate Statistical Analysis. J. Agric. Food Chem. 2010, 58, 3407–3414. [Google Scholar] [CrossRef]
- Acevedo De la Cruz, A.; Hilbert, G.; Rivière, C.; Mengin, V.; Ollat, N.; Bordenave, L.; Decroocq, S.; Delaunay, J.-C.; Delrot, S.; Mérillon, J.-M.; et al. Anthocyanin identification and composition of wild Vitis spp. accessions by using LC–MS and LC–NMR. Anal. Chim. Acta 2012, 732, 145–152. [Google Scholar] [CrossRef]
- Park, S.J.; Hyun, S.-H.; Suh, H.W.; Lee, S.-Y.; Min, T.-S.; Auh, J.-H.; Lee, H.-J.; Kim, J.-H.; Cho, S.-M.; Choi, H.-K. Differentiation of black raspberry fruits according to species and geographic origins by genomic analysis and 1H-NMR-based metabolic profiling. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 633–642. [Google Scholar] [CrossRef]
- Capitani, D.; Sobolev, A.P.; Delfini, M.; Vista, S.; Antiochia, R.; Proietti, N.; Bubici, S.; Ferrante, G.; Carradori, S.; Salvador, F.R.D.; et al. NMR methodologies in the analysis of blueberries. Electrophoresis 2014, 35, 1615–1626. [Google Scholar] [CrossRef]
- Goulas, V.; Minas, I.S.; Kourdoulas, P.M.; Lazaridou, A.; Molassiotis, A.N.; Gerothanassis, I.P.; Manganaris, G.A. 1H NMR Metabolic Fingerprinting to Probe Temporal Postharvest Changes on Qualitative Attributes and Phytochemical Profile of Sweet Cherry Fruit. Front. Plant Sci. 2015, 6, 959. [Google Scholar] [CrossRef]
- Hosoya, T.; Kubota, M.; Kumazawa, S. Analysis of Anthocyanins Using NMR and Antioxidant Activity in Berries. Bunseki Kagaku 2016, 65, 321–329. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.-L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa Fruits as a Rich Dietary Source of Chlorogenic Acids and Anthocyanins: 1H-NMR, HPLC-DAD, and Chemometric Studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef]
- Turbitt, J.R.; Colson, K.L.; Killday, K.B.; Milstead, A.; Neto, C.C. Application of 1H-NMR-based metabolomics to the analysis of cranberry (Vaccinium macrocarpon) supplements. Phytochem. Anal. 2020, 31, 68–80. [Google Scholar] [CrossRef]
- Hasanpour, M.; Saberi, S.; Iranshahi, M. Metabolic Profiling and Untargeted 1H-NMR-Based Metabolomics Study of Different Iranian Pomegranate (Punica granatum) Ecotypes. Planta Med. 2020, 86, 212–219. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Geng, Y.; Liu, F.; Guo, L.; Wang, X. Convenient use of low field nuclear magnetic resonance to determine the drying kinetics and predict the quality properties of mulberries dried in hot-blast air. LWT 2021, 137, 110402. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Silva, S.H.; Figueiredo, J.d.A.; Norcino, L.B.; Resende, J.V.d. Infrared-assisted freeze-drying (IRFD) of açai puree: Effects on the drying kinetics, microstructure and bioactive compounds. Innov. Food Sci. Emerg. Technol. 2021, 74, 102843. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Xie, L.; Zielinska, M.; Pan, Z.; Deng, L.-Z.; Zhang, J.-S.; Gao, L.; Wang, S.-Y.; Zheng, Z.-A.; Xiao, H.-W. Improvement of drying efficiency and quality attributes of blueberries using innovative far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD). Innov. Food Sci. Emerg. Technol. 2022, 77, 102948. [Google Scholar] [CrossRef]
- Rodríguez-Pulido, F.J.; Gil-Vicente, M.; Gordillo, B.; Heredia, F.J.; González-Miret, M.L. Measurement of ripening of raspberries (Rubus idaeus L) by near infrared and colorimetric imaging techniques. J. Food Sci. Technol. 2017, 54, 2797–2803. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Prieto-Perea, N.; Romera-Fernández, M.; Berrueta, L.A.; Gallo, B. Fast determination of anthocyanins in red grape musts by Fourier transform mid-infrared spectroscopy and partial least squares regression. Eur. Food Res. Technol. 2015, 240, 897–908. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Nogales-Bueno, J.; Rodríguez-Pulido, F.J.; Heredia, F.J. Feasibility Study on the Use of Near-Infrared Hyperspectral Imaging for the Screening of Anthocyanins in Intact Grapes during Ripening. J. Agric. Food Chem. 2013, 61, 9804–9809. [Google Scholar] [CrossRef]
- Inácio, M.R.C.; de Lima, K.M.G.; Lopes, V.G.; Pessoa, J.D.C.; de Almeida Teixeira, G.H. Total anthocyanin content determination in intact açaí (Euterpe oleracea Mart.) and palmitero-juçara (Euterpe edulis Mart.) fruit using near infrared spectroscopy (NIR) and multivariate calibration. Food Chem. 2013, 136, 1160–1164. [Google Scholar] [CrossRef]
- Beghi, R.; Spinardi, A.; Bodria, L.; Mignani, I.; Guidetti, R. Apples Nutraceutic Properties Evaluation Through a Visible and Near-Infrared Portable System. Food Bioprocess Technol. 2013, 6, 2547–2554. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, F.; Ning, J.; Liu, X.; Zhang, Z.; Yang, S. Predicting the anthocyanin content of wine grapes by NIR hyperspectral imaging. Food Chem. 2015, 172, 788–793. [Google Scholar] [CrossRef]
- Martínez-Sandoval, J.R.; Nogales-Bueno, J.; Rodríguez-Pulido, F.J.; Hernández-Hierro, J.M.; Segovia-Quintero, M.A.; Martínez-Rosas, M.E.; Heredia, F.J. Screening of anthocyanins in single red grapes using a non-destructive method based on the near infrared hyperspectral technology and chemometrics. J. Sci. Food Agric. 2016, 96, 1643–1647. [Google Scholar] [CrossRef]
- Yahui, L.; Xiaobo, Z.; Tingting, S.; Jiyong, S.; Jiewen, Z.; Holmes, M. Determination of Geographical Origin and Anthocyanin Content of Black Goji Berry (Lycium ruthenicum Murr.) Using Near-Infrared Spectroscopy and Chemometrics. Food Anal. Methods 2017, 10, 1034–1044. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Jin, X.; Li, C.; Wu, X.; Yang, S.; Ning, J.; Yanne, P. Determination of total iron-reactive phenolics, anthocyanins and tannins in wine grapes of skins and seeds based on near-infrared hyperspectral imaging. Food Chem. 2017, 237, 811–817. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Zhou, L.; Cheng, H.; Ye, X.; He, Y. Developing deep learning based regression approaches for determination of chemical compositions in dry black goji berries (Lycium ruthenicum Murr.) using near-infrared hyperspectral imaging. Food Chem. 2020, 319, 126536. [Google Scholar] [CrossRef]
- Gales, O.; Rodemann, T.; Jones, J.; Swarts, N. Application of near infra-red spectroscopy as an instantaneous and simultaneous prediction tool for anthocyanins and sugar in whole fresh raspberry. J. Sci. Food Agric. 2021, 101, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Lambri, M.; Jourdes, M.; Glories, Y.; Saucier, C. High Performance Thin Layer Chromatography (HPTLC) Analysis of Red Wine Pigments. JPC—J. Planar Chromatogr.—Mod. TLC 2003, 16, 88–94. [Google Scholar] [CrossRef]
- Rumalla, C.S.; Avula, B.; Wang, Y.-H.; Smillie, T.J.; Khan, I.A. Densitometric—HPTLC Method Development and Analysis of Anthocyanins from Acai (Euterpe oleracea Mart.) Berries and Commercial Products. JPC—J. Planar Chromatogr.—Mod. TLC 2012, 25, 409–414. [Google Scholar] [CrossRef]
- Krüger, S.; Urmann, O.; Morlock, G.E. Development of a planar chromatographic method for quantitation of anthocyanes in pomace, feed, juice and wine. J. Chromatogr. A 2013, 1289, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Cretu, G.C.; Morlock, G.E. Analysis of anthocyanins in powdered berry extracts by planar chromatography linked with bioassay and mass spectrometry. Food Chem. 2014, 146, 104–112. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Hosu, A.; Moldovan, B.; Cimpoiu, C. Evaluation and Authentication of Red Fruits Teas by High Performance Thin-Layer Chromatographic Fingerprinting. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1644–1653. [Google Scholar] [CrossRef]
- Craciun, M.E.; Cretu, G.; Mirea, C.M.; Tanczos, S.K.; Popa, A.G.; Miron, A.R. Anthocyanins Identification, Separation and Measurement from Cranberry, Blueberry, Bilberry, Chokeberry and Acai Berry Extracts by HPTLC. Rev. Chim. 2015, 66, 929–936. [Google Scholar]
- Krüger, S.; Mirgos, M.; Morlock, G.E. Effect-directed analysis of fresh and dried elderberry (Sambucus nigra L.) via hyphenated planar chromatography. J. Chromatogr. A 2015, 1426, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Koss-Mikołajczyk, I.; Lewandowska, A.; Pilipczuk, T.; Kusznierewicz, B.; Bartoszek, A. Composition of bioactive secondary metabolites and mutagenicity of Sambucus nigra L. Fruit at different stages of ripeness. Acta Aliment. 2016, 45, 442–451. [Google Scholar] [CrossRef]
- Bernardi, T.; Bortolini, O.; Massi, A.; Sacchetti, G.; Tacchini, M.; De Risi, C. Exploring the Synergy between HPTLC and HPLC-DAD for the Investigation of Wine-Making By-Products. Molecules 2019, 24, 3416. [Google Scholar] [CrossRef] [PubMed]
- Bednar, P.; Tomassi, A.V.; Presutti, C.; Pavlikova, M.; Lemr, K.; Fanali, S. Separation of Structurally Related Anthocyanins by MEKC. Chromatographia 2003, 58, 283–287. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Tateyama, C.; Oikawa, K.; Konishi, T. Comparison of Anthocyanin Distribution in Different Blueberry Sources by Capillary Zone Electrophoresis. Biol. Pharm. Bull. 2000, 23, 492–497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ichiyanagi, T.; Oikawa, K.; Tateyama, C.; Konishi, T. Acid Mediated Hydrolysis of Blueberry Anthocyanins. Chem. Pharm. Bull. 2001, 49, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Hatano, Y.; Matsugo, S.; Konishi, T. Kinetic Comparisons of Anthocyanin Reactivities towards 2,2′-Azobis(2-amidinopropane) (AAPH) Radicals, Hydrogen Peroxide and tert-Buthylhydroperoxide by Capillary Zone Electrophoresis. Chem. Pharm. Bull. 2004, 52, 434–438. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Hatano, Y.; Matsuo, S.; Konishi, T. Simultaneous Comparison of Relative Reactivities of Twelve Major Anthocyanins in Bilberry towards Reactive Nitrogen Species. Chem. Pharm. Bull. 2004, 52, 1312–1315. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Kashiwada, Y.; Ikeshiro, Y.; Hatano, Y.; Shida, Y.; Horie, M.; Matsugo, S.; Konishi, T. Complete Assignment of Bilberry (Vaccinium myrtillus L.) Anthocyanins Separated by Capillary Zone Electrophoresis. Chem. Pharm. Bull. 2004, 52, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.J.; Bushway, A.A.; Bushway, R.J. Separation of Peonidin and Cyanidin, Two Anthocyanidins, in Cranberries by Capillary Electrophoresis. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 113–121. [Google Scholar] [CrossRef]
- Priego Capote, F.; Rodríguez, J.M.L.; Luque de Castro, M.D. Determination of phenolic compounds in grape skin by capillary electrophoresis with simultaneous dual fluorescence and diode array absorption detection after dynamic superheated liquid leaching. J. Chromatogr. A 2007, 1139, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Comandini, P.; Blanda, G.; Cardinali, A.; Cerretani, L.; Bendini, A.; Caboni, M.F. CZE separation of strawberry anthocyanins with acidic buffer and comparison with HPLC. J. Sep. Sci. 2008, 31, 3257–3264. [Google Scholar] [CrossRef]
- Bednář, P.; Papoušková, B.; Müller, L.; Barták, P.; Stávek, J.; Pavloušek, P.; Lemr, K. Utilization of capillary electrophoresis/mass spectrometry (CE/MSn) for the study of anthocyanin dyes. J. Sep. Sci. 2005, 28, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Lopes-da-Silva, F.; de Pascual-Teresa, S.; Rivas-Gonzalo, J.; Santos-Buelga, C. Identification of anthocyanin pigments in strawberry (cv Camarosa) by LC using DAD and ESI-MS detection. Eur. Food Res. Technol. 2002, 214, 248–253. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Hatano, Y.; Matsugo, S.; Konishi, T. Structural Dependence of HPLC Separation Pattern of Anthocyanins from Bilberry (Vaccinium myrtillus L.). Chem. Pharm. Bull. 2004, 52, 628–630. [Google Scholar] [CrossRef]
- Hohnová, B.; Šťavíková, L.; Karasek, P. Determination of anthocyanins in red grape skin by pressurised fluid extraction and HPLC. Czech J. Food Sci. 2008, 26, 39–42. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, B.; Williams, P.; Pace, R.D. Identification of anthocyanins in muscadine grapes with HPLC-ESI-MS. LWT—Food Sci. Technol. 2009, 42, 819–824. [Google Scholar] [CrossRef]
- Benmeziane, F.; Cadot, Y.; Djamai, R.; Djermoun, L. Determination of major anthocyanin pigments and flavonols in red grape skin of some table grape varieties (Vitis vinifera sp.) by high-performance liquid chromatography–photodiode array detection (HPLC-DAD). OENO One 2016, 50, 125–135. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Errante, G.; Zappia, G.; Dugo, G. Identification of Anthocyanins in Berries by Narrow-Bore High-Performance Liquid Chromatography with Electrospray Ionization Detection. J. Agric. Food Chem. 2001, 49, 3987–3992. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Lichtenthäler, R.; Rodrigues, R.B.; Maia, J.G.S.; Papagiannopoulos, M.; Fabricius, H.; Marx, F. Total oxidant scavenging capacities of Euterpe oleracea Mart. (Açaí) fruits. Int. J. Food Sci. Nutr. 2005, 56, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Sadilova, E.; Stintzing, F.; Carle, R. Chemical quality parameters and anthocyanin pattern of red-fleshed Weirouge apples. J. Appl. Bot. Food Qual. 2006, 80, 82–87. [Google Scholar]
- Mertz, C.; Cheynier, V.; Günata, Z.; Brat, P. Analysis of Phenolic Compounds in Two Blackberry Species (Rubus glaucus and Rubus adenotrichus) by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ion Trap Mass Spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’ blueberry (Vaccinium corymbosum × V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Vera de Rosso, V.; Hillebrand, S.; Cuevas Montilla, E.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Šeruga, B.; Novak, I.; Medvidović-Kosanović, M. Phenolic compound composition and antioxidant activity of fruits of Rubus and Prunus species from Croatia. Int. J. Food Sci. Technol. 2009, 44, 860–868. [Google Scholar] [CrossRef]
- Jakopic, J.; Stampar, F.; Veberic, R. The influence of exposure to light on the phenolic content of ‘Fuji’ apple. Sci. Hortic. 2009, 123, 234–239. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Ziberna, L.; Lunder, M.; Moze, S.; Vanzo, A.; Tramer, F.; Passamonti, S.; Drevensek, G. Acute Cardioprotective and Cardiotoxic Effects of Bilberry Anthocyanins in Ischemia–Reperfusion Injury: Beyond Concentration-Dependent Antioxidant Activity. Cardiovasc. Toxicol. 2010, 10, 283–294. [Google Scholar] [CrossRef]
- Agawa, S.; Sakakibara, H.; Iwata, R.; Shimoi, K.; Hergesheimer, A.; Kumazawa, S. Anthocyanins in Mesocarp/Epicarp and Endocarp of Fresh Açai (Euterpe oleracea Mart.) and their Antioxidant Activities and Bioavailability. Food Sci. Technol. Res. 2011, 17, 327–334. [Google Scholar] [CrossRef]
- Balázs, A.; Tóth, M.; Blazics, B.; Héthelyi, É.; Szarka, S.; Ficsor, E.; Ficzek, G.; Lemberkovics, É.; Blázovics, A. Investigation of dietary important components in selected red fleshed apples by GC–MS and LC–MS. Fitoterapia 2012, 83, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.S.; Rozet, E.; Chataigné, G.; Oliveira, A.C.; Rabelo, C.A.S.; Hubert, P.; Rogez, H.; Quetin-Leclercq, J. A rapid validated UHPLC–PDA method for anthocyanins quantification from Euterpe oleracea fruits. J. Chromatogr. B 2012, 907, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich Açai (Euterpe oleracea Mart.) Fruit Pulp Fractions Attenuate Inflammatory Stress Signaling in Mouse Brain BV-2 Microglial Cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Alemán, M.D.; Gironés-Vilaplana, A.; Pérez, A.; Caravaca, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic Composition, Antioxidant Activity, and In Vitro Availability of Four Different Berries. J. Chem. 2016, 2016, 5194901. [Google Scholar] [CrossRef]
- Sang, J.; Ma, Q.; Li, C.-q. Development and validation of green chromatography for the determination of anthocyanins in haskap berry, mulberry and blackberry. Anal. Methods 2017, 9, 2535–2545. [Google Scholar] [CrossRef]
- Won Ryu, H.; Cho, B.O.; Ryu, J.; Jin, C.H.; Kim, J.-B.; Kang, S.Y.; Han, A.-R. Anthocyanin Contents Enhancement with Gamma Irradiated Mutagenesis in Blackberry (Rubus fructicosus). Nat. Prod. Commun. 2017, 12, 1451–1454. [Google Scholar] [CrossRef]
- Benvenuti, S.; Brighenti, V.; Pellati, F. High-performance liquid chromatography for the analytical characterization of anthocyanins in Vaccinium myrtillus L. (bilberry) fruit and food products. Anal. Bioanal. Chem. 2018, 410, 3559–3571. [Google Scholar] [CrossRef]
- Van de Velde, F.; Pirovani, M.E.; Drago, S.R. Bioaccessibility analysis of anthocyanins and ellagitannins from blackberry at simulated gastrointestinal and colonic levels. J. Food Compos. Anal. 2018, 72, 22–31. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F.J.; Escudero-Gilete, M.L. Comparative study of red berry pomaces (blueberry, red raspberry, red currant and blackberry) as source of antioxidants and pigments. Eur. Food Res. Technol. 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gamsjäger, H. Phytochemical analysis by liquid chromatography of ten old apple varieties grown in Austria and their antioxidative activity. Eur. Food Res. Technol. 2020, 246, 437–448. [Google Scholar] [CrossRef]
- Wang, J.; Kalt, W.; Sporns, P. Comparison between HPLC and MALDI-TOF MS Analysis of Anthocyanins in Highbush Blueberries. J. Agric. Food Chem. 2000, 48, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of Procyanidins and Anthocyanins in Blueberries and Cranberries (Vaccinium Spp.) Using High-Performance Liquid Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Hamamatsu, S.; Yabe, K.; Nawa, Y. Compositions of Anthocyanin and Other Flavonoids in Cultured Cells of Rabbiteye Blueberry (Vaccinium ashei Reade cv. Tifhlue). Food Sci. Technol. Res. 2004, 10, 239–246. [Google Scholar] [CrossRef]
- Li, Y.; Fanli, M.; Yinan, Z. Study of Anthocyanins in Fruit of Different Vaccinium Genotypes; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2006; pp. 589–594. [Google Scholar]
- Nicoué, E.É.; Savard, S.; Belkacemi, K. Anthocyanins in Wild Blueberries of Quebec: Extraction and Identification. J. Agric. Food Chem. 2007, 55, 5626–5635. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, H.J.; Lee, E.H.; Jung, S.H.; Lee, D.U.; Kang, S.W.; Hong, S.J.; Um, B.H. Rapid identification of radical scavenging compounds in blueberry extract by HPLC coupled to an on-line ABTS based assay and HPLC-ESI/MS. Korean Soc. Food Sci. Technol. 2008, 17, 846–849. [Google Scholar]
- Lohachoompol, V.; Mulholland, M.; Srzednicki, G.; Craske, J. Determination of anthocyanins in various cultivars of highbush and rabbiteye blueberries. Food Chem. 2008, 111, 249–254. [Google Scholar] [CrossRef]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Shimoi, K.; Kumazawa, S. Anthocyanin Composition and Antioxidant Activity of the Crowberry (Empetrum nigrum) and Other Berries. J. Agric. Food Chem. 2008, 56, 4457–4462. [Google Scholar] [CrossRef]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography–electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, F.; Oliveira, C.; Pinho, O.; Ferreira, I.M.P.L.V.O. Degradation of Anthocyanins and Anthocyanidins in Blueberry Jams/Stuffed Fish. J. Agric. Food Chem. 2009, 57, 10712–10717. [Google Scholar] [CrossRef] [PubMed]
- Ścibisz, I.; Mitek, M. Effect of Processing and Storage Conditions on Phenolic Compounds and Antioxidant Capacity of Highbush Blueberry Jams. Pol. J. Food Nutr. Sci. 2009, 59, 45–52. [Google Scholar]
- Mullen, W.; Larcombe, S.; Arnold, K.; Welchman, H.; Crozier, A. Use of Accurate Mass Full Scan Mass Spectrometry for the Analysis of Anthocyanins in Berries and Berry-Fed Tissues. J. Agric. Food Chem. 2010, 58, 3910–3915. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, V.; Kajdžanoska, M.; Gjamovski, V.; Stefova, M. Separation, Characterization and Quantification of Phenolic Compounds in Blueberries and Red and Black Currants by HPLC−DAD−ESI-MSn. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef]
- Kim, S.; Um, A.B.-H. Evaluation of the antioxidant activity of phenolic compounds among blueberry cultivars by HPLC-ESI/MS and on-line HPLC-ABTS system. J. Med. Plant Res. 2011, 5, 5008–5016. [Google Scholar]
- Müller, D.; Schantz, M.; Richling, E. High Performance Liquid Chromatography Analysis of Anthocyanins in Bilberries (Vaccinium myrtillus L.), Blueberries (Vaccinium corymbosum L.), and Corresponding Juices. J. Food Sci. 2012, 77, C340–C345. [Google Scholar] [CrossRef]
- Sun, L.-Q.; Ding, X.-P.; Qi, J.; Yu, H.; He, S.-a.; Zhang, J.; Ge, H.-X.; Yu, B.-Y. Antioxidant anthocyanins screening through spectrum–effect relationships and DPPH-HPLC-DAD analysis on nine cultivars of introduced rabbiteye blueberry in China. Food Chem. 2012, 132, 759–765. [Google Scholar] [CrossRef]
- Jo, Y.N.; Jin, D.E.; Jeong, J.H.; Kim, H.J.; Kim, D.-O.; Heo, H.J. Effect of anthocyanins from rabbit-eye blueberry (Vaccinium virgatum) on cognitive function in mice under trimethyltin-induced neurotoxicity. Food Sci. Biotechnol. 2015, 24, 1077–1085. [Google Scholar] [CrossRef]
- Cetin, G.; Turak, F. Effect of Mobile Phase Variation and Purification on Chromatogram View by Using Fruits and Rose Extracts and HPLC Method. Int. J. Chem. 2016, 8, 25. [Google Scholar] [CrossRef]
- Li, D.; Meng, X.; Li, B. Profiling of anthocyanins from blueberries produced in China using HPLC-DAD-MS and exploratory analysis by principal component analysis. J. Food Compos. Anal. 2016, 47, 1–7. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Macedonio, G.; Carradori, S.; Sobolev, A.P.; De Salvador, R.F.; Monti, S.M.; Buonanno, M.; Zengin, G.; Angeli, A.; et al. Microwave-assisted extraction, HPLC analysis, and inhibitory effects on carbonic anhydrase I, II, VA, and VII isoforms of 14 blueberry Italian cultivars. J. Enzym. Inhib. Med. Chem. 2016, 31, 1–6. [Google Scholar] [CrossRef]
- Šmídová, B.; Šatínský, D.; Dostálová, K.; Solich, P. The pentafluorophenyl stationary phase shows a unique separation efficiency for performing fast chromatography determination of highbush blueberry anthocyanins. Talanta 2017, 166, 249–254. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Tang, H.; Chen, Y.; Xie, B.; Wang, C.; Sun, Z. Separation and Identification of Anthocyanins Extracted from Blueberry Wine Lees and Pigment Binding Properties toward β-Glucosidase. J. Agric. Food Chem. 2017, 65, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Lv, L.; Yan, H.; Yuan, Y. Antioxidant activity of blueberry anthocyanin extracts and their protective effects against acrylamide-induced toxicity in HepG2 cells. Int. J. Food Sci. Technol. 2018, 53, 147–155. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Mitra, S.; Hafiz Muhammad, R.; Debnath, B.; Lu, X.; Jian, H.; Qiu, D. Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium corymbosum) at Different Developmental Stages. Molecules 2018, 23, 2209. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Xie, C.; Zhong, H.; Tian, B.; Yang, K. Identification of Anthocyanins and Their Fouling Mechanisms during Non-Thermal Nanofiltration of Blueberry Aqueous Extracts. Membranes 2021, 11, 200. [Google Scholar] [CrossRef]
- Cai, M.; Xie, C.; Zhong, H.; Yang, K.; Sun, P. Insights into changes of anthocyanins-rich blueberry extracts concentrated by different nanofiltrations and their storage stability. LWT 2021, 144, 111196. [Google Scholar] [CrossRef]
- Goodman, C.; Lyon, K.N.; Scotto, A.; Smith, C.; Sebrell, T.A.; Gentry, A.B.; Bala, G.; Stoner, G.D.; Bimczok, D. A High-Throughput Metabolic Microarray Assay Reveals Antibacterial Effects of Black and Red Raspberries and Blackberries against Helicobacter pylori Infection. Antibiotics 2021, 10, 845. [Google Scholar] [CrossRef]
- Lang, Y.; Li, B.; Gong, E.; Shu, C.; Si, X.; Gao, N.; Zhang, W.; Cui, H.; Meng, X. Effects of α-casein and β-casein on the stability, antioxidant activity and bioaccessibility of blueberry anthocyanins with an in vitro simulated digestion. Food Chem. 2021, 334, 127526. [Google Scholar] [CrossRef]
- Li, X.; Zhu, F.; Zeng, Z. Effects of different extraction methods on antioxidant properties of blueberry anthocyanins. Open Chem. 2021, 19, 138–148. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, S.; Xu, Q.; Yan, C.; Yang, J.; Zhou, Y. Optimization and application of HPLC for simultaneous separation of six well-known major anthocyanins in blueberry. Prep. Biochem. Biotechnol. 2021, 51, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-H.; Chen, L.-P.; Wu, C.-L.; Wang, J.-F.; Luo, S.-Z.; Luo, J.-P.; Zheng, Z. Microencapsulation of blueberry anthocyanins by spray drying with soy protein isolates/high methyl pectin combination: Physicochemical properties, release behavior in vitro and storage stability. Food Chem. 2022, 395, 133626. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Yan, Y.; Gerbrandt, E.M.; Castellarin, S.D. Determination of free and bound phenolics in northern highbush blueberries by a validated HPLC/QTOF methodology. J. Food Compos. Anal. 2022, 108, 104412. [Google Scholar] [CrossRef]
- Garcıia-Viguera, C.; Zafrilla, P.; Tomás-Barberán, F.A. Determination of Authenticity of Fruit Jams by HPLC Analysis of Anthocyanins. J. Sci. Food Agric. 1997, 73, 207–213. [Google Scholar] [CrossRef]
- Vodopivec, B.; Trebse, P.; Hribar, J. Determination and quantitation of anthocyanins and hydroxycinnamic acids in different cultivars of sweet cherries (Prunus avium L.) from Nova Gorica Region (Slovenia). Food Technol. Biotechnol. 2002, 40, 207–212. [Google Scholar]
- Gonçalves, B.; Landbo, A.-K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of Ripeness and Postharvest Storage on the Phenolic Profiles of Cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef]
- Mozetič, B.; Trebše, P.; Simčič, M.; Hribar, J. Changes of anthocyanins and hydroxycinnamic acids affecting the skin colour during maturation of sweet cherries (Prunus avium L.). LWT—Food Sci. Technol. 2004, 37, 123–128. [Google Scholar] [CrossRef]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Crupi, P.; Genghi, R.; Antonacci, D. In-time and in-space tandem mass spectrometry to determine the metabolic profiling of flavonoids in a typical sweet cherry (Prunus avium L.) cultivar from Southern Italy. J. Mass Spectrom. 2014, 49, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, D.; Kostic, D.; Mitić, M.; Paunović, D.; Stojanović, B.; Krstić, J.; Stevanovic, S.; Veličković, J. The comparative overview of HPLC analysis of different extracts from morus species from southeast Serbia. Stud. Univ. Babeș-Bolyai Chem. 2020, 65, 85–93. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Kosar, M.; Altintas, A.; Kirimer, N.; Baser, K.H.C. Determination of the Free Radical Scavenging Activity of Lycium Extracts. Chem. Nat. Compd. 2003, 39, 531–535. [Google Scholar] [CrossRef]
- Zheng, J.; Ding, C.; Wang, L.; Li, G.; Shi, J.; Li, H.; Wang, H.; Suo, Y. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Dossett, M.; Lee, J.; Finn, C.E. Variation in anthocyanins and total phenolics of black raspberry populations. J. Funct. Foods 2010, 2, 292–297. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmiański, J. Comparison of six cultivars of strawberries (Fragaria x ananassa Duch.) grown in northwest Poland. Eur. Food Res. Technol. 2004, 219, 66–70. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and Quantification of Phenolic Compounds in Berries of Fragaria and Rubus Species (Family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Beta, T. Saskatoon and Wild Blueberries Have Higher Anthocyanin Contents than Other Manitoba Berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia, and Sambucus and Their Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Koskela, A.K.J.; Anttonen, M.J.; Soininen, T.H.; Saviranta, N.M.M.; Auriola, S.; Julkunen-Tiitto, R.; Karjalainen, R.O. Variation in the Anthocyanin Concentration of Wild Populations of Crowberries (Empetrum nigrum L subsp. hermaphroditum). J. Agric. Food Chem. 2010, 58, 12286–12291. [Google Scholar] [CrossRef] [PubMed]
- Gómez Gallego, M.A.; Gómez García-Carpintero, E.; Sánchez-Palomo, E.; Hermosín-Gutiérrez, I.; González Viñas, M.A. Study of phenolic composition and sensory properties of red grape varieties in danger of extinction from the Spanish region of Castilla-La Mancha. Eur. Food Res. Technol. 2012, 234, 295–303. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J.; Wang, Z. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography–mass spectrometry. Ultrason. Sonochemistry 2007, 14, 767–778. [Google Scholar] [CrossRef]
- Sparzak, B.; Merino-Arevalo, M.; Vander Heyden, Y.; Krauze-Baranowska, M.; Majdan, M.; Fecka, I.; Głód, D.; Bączek, T. HPLC analysis of polyphenols in the fruits of Rubus idaeus L. (Rosaceae). Nat. Prod. Res. 2010, 24, 1811–1822. [Google Scholar] [CrossRef]
- Yang, J.; Cui, J.; Chen, J.; Yao, J.; Hao, Y.; Fan, Y.; Liu, Y. Evaluation of physicochemical properties in three raspberries (Rubus idaeus) at five ripening stages in northern China. Sci. Hortic. 2020, 263, 109146. [Google Scholar] [CrossRef]
- Rocha, R.; Pinela, J.; Abreu, R.M.V.; Añibarro-Ortega, M.; Pires, T.C.S.P.; Saldanha, A.L.; Alves, M.J.; Nogueira, A.; Ferreira, I.C.F.R.; Barros, L. Extraction of Anthocyanins from Red Raspberry for Natural Food Colorants Development: Processes Optimization and In Vitro Bioactivity. Processes 2020, 8, 1447. [Google Scholar] [CrossRef]
- Cesonienė, L.; Jasutiene, I.; Sarkinas, A. Phenolics and anthocyanins in berries of European cranberry and their antimicrobial activity. Medicina 2009, 45, 992–999. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of Anthocyanin Profile of Four Elderberry Species and Interspecific Hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef]
- Zou, T.; Wang, D.; Guo, H.; Zhu, Y.; Luo, X.; Liu, F.; Ling, W. Optimization of Microwave-Assisted Extraction of Anthocyanins from Mulberry and Identification of Anthocyanins in Extract Using HPLC-ESI-MS. J. Food Sci. 2012, 77, C46–C50. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M. HPLC-DAD detection of changes in phenol content of red berry skins during grape ripening. Eur. Food Res. Technol. 2013, 237, 555–564. [Google Scholar] [CrossRef]
- Donno, D.; Cavanna, M.; Beccaro, G.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.; Bounous, G. Currants and strawberries as bioactive compound sources: Determination of antioxidant profiles with HPLC-DAD/MS. J. Appl. Bot. Food Qual. 2013, 86, 1–10. [Google Scholar] [CrossRef]
- Holzwarth, M.; Wittig, J.; Carle, R.; Kammerer, D.R. Influence of putative polyphenoloxidase (PPO) inhibitors on strawberry (Fragaria x ananassa Duch.) PPO, anthocyanin and color stability of stored purées. LWT—Food Sci. Technol. 2013, 52, 116–122. [Google Scholar] [CrossRef]
- Sirijan, M.; Pipattanawong, N.; Saeng-on, B.; Chaiprasart, P. Anthocyanin content, bioactive compounds and physico-chemical characteristics of potential new strawberry cultivars rich in-anthocyanins. J. Berry Res. 2020, 10, 397–410. [Google Scholar] [CrossRef]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, Flavonoids, and Other Phenolics in Red Raspberries and Their Contribution to Antioxidant Capacity and Vasorelaxation Properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Mullen, W.; Lean, M.E.J.; Crozier, A. Rapid characterization of anthocyanins in red raspberry fruit by high-performance liquid chromatography coupled to single quadrupole mass spectrometry. J. Chromatogr. A 2002, 966, 63–70. [Google Scholar] [CrossRef]
- Fernández-Lara, R.; Gordillo, B.; Rodríguez-Pulido, F.J.; Lourdes González-Miret, M.; del Villar-Martínez, A.A.; Dávila-Ortiz, G.; Heredia, F.J. Assessment of the differences in the phenolic composition and color characteristics of new strawberry (Fragaria x ananassa Duch.) cultivars by HPLC–MS and Imaging Tristimulus Colorimetry. Food Res. Int. 2015, 76, 645–653. [Google Scholar] [CrossRef]
- Holzwarth, M.; Korhummel, S.; Carle, R.; Kammerer, D.R. Evaluation of the effects of different freezing and thawing methods on color, polyphenol and ascorbic acid retention in strawberries (Fragaria × ananassa Duch.). Food Res. Int. 2012, 48, 241–248. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Characterization of Phenolic Compounds in Strawberry Fruits By RP-HPLC-DAD and Investigation of their Antioxidant Capacity. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2495–2504. [Google Scholar] [CrossRef]
- Kajdžanoska, M.; Gjamovski, V.; Stefova, M. HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Maced. J. Chem. Chem. Eng. 2010, 29, 181–194. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Araújo, P.; Duarte, M.F.; de Freitas, V.; Pintado, M.; Saraiva, J.A. Experimental Design, Modeling, and Optimization of High-Pressure-Assisted Extraction of Bioactive Compounds from Pomegranate Peel. Food Bioprocess Technol. 2017, 10, 886–900. [Google Scholar] [CrossRef]
- Brighenti, V.; Groothuis, S.F.; Prencipe, F.P.; Amir, R.; Benvenuti, S.; Pellati, F. Metabolite fingerprinting of Punica granatum L. (pomegranate) polyphenols by means of high-performance liquid chromatography with diode array and electrospray ionization-mass spectrometry detection. J. Chromatogr. A 2017, 1480, 20–31. [Google Scholar] [CrossRef]
- Zarezadeh Mehrizi, R.; Emam-Djomeh, Z.; Shahedi, M.; Keramat, J.; Rezaei, K.; Loni, E. Phenolic Compounds and Antioxidant Activity of Dried Peel of Iranian Pomegranate. J. Food Qual. Hazards Control 2017, 4, 103–108. [Google Scholar]
- Balli, D.; Cecchi, L.; Khatib, M.; Bellumori, M.; Cairone, F.; Carradori, S.; Zengin, G.; Cesa, S.; Innocenti, M.; Mulinacci, N. Characterization of Arils Juice and Peel Decoction of Fifteen Varieties of Punica granatum L.: A Focus on Anthocyanins, Ellagitannins and Polysaccharides. Antioxidants 2020, 9, 238. [Google Scholar] [CrossRef]
- Hu, T.; Subbiah, V.; Wu, H.; Bk, A.; Rauf, A.; Alhumaydhi, F.A.; Suleria, H.A.R. Determination and Characterization of Phenolic Compounds from Australia-Grown Sweet Cherries (Prunus avium L.) and Their Potential Antioxidant Properties. ACS Omega 2021, 6, 34687–34699. [Google Scholar] [CrossRef]
- Avula, B.; Katragunta, K.; Wang, Y.-H.; Ali, Z.; Srivedavyasasri, R.; Gafner, S.; Slimestad, R.; Khan, I.A. Chemical profiling and UHPLC-QToF analysis for the simultaneous determination of anthocyanins and flavonoids in Sambucus berries and authentication and detection of adulteration in elderberry dietary supplements using UHPLC-PDA-MS. J. Food Compos. Anal. 2022, 110, 104584. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, W.; Chen, J.; Pan, H.; Xu, E.; Chen, S.; Ye, X.; Chen, J. Establishment of anthocyanin fingerprint in black wolfberry fruit for quality and geographical origin identification. LWT 2022, 157, 113080. [Google Scholar] [CrossRef]
- Sang, J.; Li, B.; Huang, Y.-y.; Ma, Q.; Liu, K.; Li, C.-q. Deep eutectic solvent-based extraction coupled with green two-dimensional HPLC-DAD-ESI-MS/MS for the determination of anthocyanins from Lycium ruthenicum Murr. fruit. Anal. Methods 2018, 10, 1247–1257. [Google Scholar] [CrossRef]
- Koźmiński, P.; Oliveira-Brett, A.M. Anthocyanin Monitoring in Four Red Grape Skin Extract Varieties Using RP-HPLC-ED. Anal. Lett. 2008, 41, 662–675. [Google Scholar] [CrossRef]
- Ghassempour, A.; Heydari, R.; Talebpour, Z.; Fakhari, A.R.; Rassouli, A.; Davies, N.; Aboul-Enein, H.Y. Study of New Extraction Methods for Separation of Anthocyanins from Red Grape Skins: Analysis by HPLC and LC-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 2686–2703. [Google Scholar] [CrossRef]
- Constantin, O.E.; Skrt, M.; Poklar Ulrih, N.; Râpeanu, G. Anthocyanins profile, total phenolics and antioxidant activity of two Romanian red grape varieties: Feteascǎ neagrǎ and Bǎbeascǎ neagrǎ (Vitis vinifera). Chem. Papers 2015, 69, 1573–1581. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total Cranberry Extract versus Its Phytochemical Constituents: Antiproliferative and Synergistic Effects against Human Tumor Cell Lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Shipley, P.R. Determination of anthocyanins in cranberry fruit and cranberry fruit products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation. J. AOAC Int. 2011, 94, 459–466. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Li, Q.; Cai, X.; Tang, J. Optimization ultrasound-assisted extraction of anthocyanins from cranberry using response surface methodology coupled with genetic algorithm and identification anthocyanins with HPLC-MS2. J. Food Process. Preserv. 2021, 45, e15378. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Braga, F.G.; Carvalho, L.M.; Carvalho, M.J.; Guedes-Pinto, H.; Torres-Pereira, J.M.; Neto, M.F.; Monteiro, A. Variation of the Anthocyanin Content in Sambucus nigra L. Populations Growing in Portugal. J. Herbs Spices Med. Plants 2002, 9, 289–295. [Google Scholar] [CrossRef]
- Kaack, K.; Fretté, X.C.; Christensen, L.P.; Landbo, A.-K.; Meyer, A.S. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of juice. Eur. Food Res. Technol. 2008, 226, 843–855. [Google Scholar] [CrossRef]
- Anton, A.M.; Pintea, A.; Rugină, D.; Diaconeasa, Z.; Hanganu, D.; Vlase, L.; Benedec, D. Preliminary studies on the chemical characterization and antioxidant capacity of polyphenols from Sambucus sp. Dig. J. Nanomater. Biostructures 2013, 8, 973–980. [Google Scholar]
- European Elder Berry Dry Extract. USP43-NF38; United States Pharmacopeial Convention: Rockville, MD, USA, 2021.
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [PubMed]
- Košir, I.J.; Lapornik, B.; Andrenšek, S.; Wondra, A.G.; Vrhovšek, U.; Kidrič, J. Identification of anthocyanins in wines by liquid chromatography, liquid chromatography-mass spectrometry and nuclear magnetic resonance. Anal. Chim. Acta 2004, 513, 277–282. [Google Scholar] [CrossRef]
- Tian, Z.; Aierken, A.; Pang, H.; Du, S.; Feng, M.; Ma, K.; Gao, S.; Bai, G.; Ma, C. Constituent analysis and quality control of anthocyanin constituents of dried Lycium ruthenicum Murray fruits by HPLC–MS and HPLC–DAD. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 453–458. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC−DAD−ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Z.; Fang, Y.; Yin, Y.; Feng, L. Characterization and evaluation of major anthocyanins in pomegranate (Punica granatum L.) peel of different cultivars and their development phases. Eur. Food Res. Technol. 2013, 236, 109–117. [Google Scholar] [CrossRef]
- Jordheim, M.; Enerstvedt, K.H.; Andersen, Ø.M. Identification of Cyanidin 3-O-β-(6″-(3-Hydroxy-3-methylglutaroyl)glucoside) and Other Anthocyanins from Wild and Cultivated Blackberries. J. Agric. Food Chem. 2011, 59, 7436–7440. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Sources of Antioxidant Activity in Australian Native Fruits. Identification and Quantification of Anthocyanins. J. Agric. Food Chem. 2006, 54, 9820–9826. [Google Scholar] [CrossRef]
- Lätti, A.K.; Riihinen, K.R.; Kainulainen, P.S. Analysis of Anthocyanin Variation in Wild Populations of Bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008, 56, 190–196. [Google Scholar] [CrossRef]
- Goto, T.; Obara, M.; Aoki, S.; Okazawa, K.; Konisho, K.; Osakabe, N.; Shoji, T. Evaluation of Polyphenolic Content and Potential Antioxidant Activity of Japanese Cultivars of Peaches, Prunes, and Plums Based on Reversed- and Normal-Phase HPLC and Principal Component Analyses. ACS Food Sci. Technol. 2021, 1, 2019–2029. [Google Scholar] [CrossRef]
- Tian, Q.; Aziz, R.M.; Stoner, G.D.; Schwartz, T.J. Anthocyanin Determination in Black Raspberry (Rubus occidentalis) and Biological Specimens Using Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. J. Food Sci. 2005, 70, C43–C47. [Google Scholar] [CrossRef]
- Feng, Z.; Zhaohe, Y.; Xueqing, Z.; Yanlei, Y.; Lijuan, F. Composition and Contents of Anthocyanins in Different Pomegranate Cultivars; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2015; pp. 35–41. [Google Scholar]
- Xueqing, Z.; Zhaohe, Y.; Yanlei, Y.; Lijuan, F. Patterns of Pigment Changes in Pomegranate (Punica granatum L.) Peel During Fruit Ripening; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2015; pp. 83–89. [Google Scholar]
- Qin, C.; Li, Y.; Niu, W.; Ding, Y.; Zhang, R.; Shang, X.; Shang, Z. Analysis and Characterisation of Anthocyanins in Mulberry Fruit. Czech J. Food Sci. 2009, 28, 117–126. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Peiretti, P.G. Identification of Polyphenolic Compounds in Edible Wild Fruits Grown in the North-West of Italy by Means of HPLC-DAD-ESI HRMS. Plant Foods Hum. Nutr. 2020, 75, 420–426. [Google Scholar] [CrossRef] [PubMed]
- García-Beneytez, E.; Revilla, E.; Cabello, F. Anthocyanin pattern of several red grape cultivars and wines made from them. Eur. Food Res. Technol. 2002, 215, 32–37. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Yang, F.; Wang, J.; Fu, D.; Zhang, X.; Peng, X.; Liang, X. Characterization of anthocyanins in wild Lycium ruthenicum Murray by HPLC-DAD/QTOF-MS/MS. Anal. Methods 2015, 7, 4947–4956. [Google Scholar] [CrossRef]
- Revilla, E.; García-Beneytez, E.; Cabello, F.; Martín-Ortega, G.; Ryan, J.-M.a. Value of high-performance liquid chromatographic analysis of anthocyanins in the differentiation of red grape cultivars and red wines made from them. J. Chromatogr. A 2001, 915, 53–60. [Google Scholar] [CrossRef]
- Pomar, F.; Novo, M.; Masa, A. Varietal differences among the anthocyanin profiles of 50 red table grape cultivars studied by high performance liquid chromatography. J. Chromatogr. A 2005, 1094, 34–41. [Google Scholar] [CrossRef]
- Picariello, G.; Ferranti, P.; Garro, G.; Manganiello, G.; Chianese, L.; Coppola, R.; Addeo, F. Profiling of anthocyanins for the taxonomic assessment of ancient purebred V. vinifera red grape varieties. Food Chem. 2014, 146, 15–22. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F. Flavonols and Anthocyanins of Elderberry Fruits (Sambucus nigra L.); International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; pp. 611–614. [Google Scholar]
- Gao, L.; Mazza, G. Quantitation and Distribution of Simple and Acylated Anthocyanins and Other Phenolics in Blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Kalt, W.; Ryan, D.A.J.; Duy, J.C.; Prior, R.L.; Ehlenfeldt, M.K.; Vander Kloet, S.P. Interspecific Variation in Anthocyanins, Phenolics, and Antioxidant Capacity among Genotypes of Highbush and Lowbush Blueberries (Vaccinium Section cyanococcus spp.). J. Agric. Food Chem. 2001, 49, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Impact of Juice Processing on Blueberry Anthocyanins and Polyphenolics: Comparison of Two Pretreatments. J. Food Sci. 2002, 67, 1660–1667. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Color and Pigment Analyses in Fruit Products; Agricultural Experiment Station, Oregon State University: Corvallis, OR, USA, 11 May 2010. [Google Scholar]
- Chandra, A.; Rana, J.; Li, Y. Separation, Identification, Quantification, and Method Validation of Anthocyanins in Botanical Supplement Raw Materials by HPLC and HPLC−MS. J. Agric. Food Chem. 2001, 49, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Wrolstad, R.E. Comparison of the Stability of Pelargonidin-based Anthocyanins in Strawberry Juice and Concentrate. J. Food Sci. 2002, 67, 1288–1299. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in Anthocyanins and Polyphenolics During Juice Processing of Highbush Blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Schieber, A.; Carle, R. A Novel Process for the Recovery of Polyphenols from Grape (Vitis vinifera L.) Pomace. J. Food Sci. 2005, 70, C157–C163. [Google Scholar] [CrossRef]
- Penman, K.G.; Halstead, C.W.; Matthias, A.; De Voss, J.J.; Stuthe, J.M.U.; Bone, K.M.; Lehmann, R.P. Bilberry Adulteration Using the Food Dye Amaranth. J. Agric. Food Chem. 2006, 54, 7378–7382. [Google Scholar] [CrossRef]
- Cassinese, C.; Combarieu, E.D.; Falzoni, M.; Fuzzati, N.; Pace, R.; Sardone, N. New Liquid Chromatography Method with Ultraviolet Detection for Analysis of Anthocyanins and Anthocyanidins in Vaccinium myrtillus Fruit Dry Extracts and Commercial Preparations. J. AOAC Int. 2007, 90, 911–919. [Google Scholar] [CrossRef]
- Artaria, C.; Pace, R.; Maramaldi, G.; Appendino, G. Different brands of bilberry extract: A comparison of selected components. Nutrafood 2007, 6, 13–18. [Google Scholar]
- Gardana, C.; Ciappellano, S.; Marinoni, L.; Fachechi, C.; Simonetti, P. Bilberry Adulteration: Identification and Chemical Profiling of Anthocyanins by Different Analytical Methods. J. Agric. Food Chem. 2014, 62, 10998–11004. [Google Scholar] [CrossRef]
- Gardana, C.; Scialpi, A.; Fachechi, C.; Simonetti, P. Near-Infrared Spectroscopy and Chemometrics for the Routine Detection of Bilberry Extract Adulteration and Quantitative Determination of the Anthocyanins. J. Spectrosc. 2018, 2018, 4751247. [Google Scholar] [CrossRef]
- Gardana, C.; Scialpi, A.; Fachechi, C.; Simonetti, P. Identification of markers for the authentication of cranberry extract and cranberry-based food supplements. Heliyon 2020, 6, e03863. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.P.; Lechtenberg, M.; Hensel, A. Quality Assessment of Bilberry Fruits (Vaccinium myrtillus) and Bilberry-Containing Dietary Supplements. J. Agric. Food Chem. 2021, 69, 2213–2225. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium macrocarpon (Cranberry)-Based Dietary Supplements: Variation in Mass Uniformity, Proanthocyanidin Dosage and Anthocyanin Profile Demonstrates Quality Control Standard Needed. Nutrients 2020, 12, 992. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Q.; Cheng, H.; Ge, Y.; Liu, H.; Ye, X.; Chen, Y. Metabolomic Approach for the Authentication of Berry Fruit Juice by Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry Coupled to Chemometrics. J. Agric. Food Chem. 2018, 66, 8199–8208. [Google Scholar] [CrossRef]
- Lee, J. Anthocyanin analyses of Vaccinium fruit dietary supplements. Food Sci Nutr 2016, 4, 742–752. [Google Scholar] [CrossRef]
- Lee, J. Rosaceae products: Anthocyanin quality and comparisons between dietary supplements and foods. NFS J. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Lee, J. Marketplace Analysis Demonstrates Quality Control Standards Needed for Black Raspberry Dietary Supplements. Plant Foods Hum. Nutr. 2014, 69, 161–167. [Google Scholar] [CrossRef]
- Gafner, S.; Bush, M.; Sudberg, S.; Feuillere, N.; Tenon, M.; Jolibois, J.; Bellenger, P.; You, H.; Adams, R.; Stewart, J.; et al. Tales from the elder: Adulteration issues of elder berry. HerbalGram 2021, 130, 24–32. [Google Scholar]
- Csapó, J.; Albert, C. Wine adulteration and its detection based on the rate and the concentration of free amino acids. Acta Agrar. Debr. 2018, 150, 139–151. [Google Scholar] [CrossRef]
- Giacomelli, L.; Appendino, G.; Franceschi, F.; Togni, S.; Pace, R. Omne Ignotum pro Magnifico: Characterization of commercial Bilberry extracts to fight adulteration. Eur. Rev. Med. Pharm. Sci. 2014, 18, 3948–3953. [Google Scholar]
- Dasenaki, M.E.; Drakopoulou, S.K.; Aalizadeh, R.; Thomaidis, N.S. Targeted and Untargeted Metabolomics as an Enhanced Tool for the Detection of Pomegranate Juice Adulteration. Foods 2019, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Krueger, D.; Durst, R.; Lee, R.; Wang, D.; Seeram, N.; Heber, D. International Multidimensional Authenticity Specification (IMAS) Algorithm for Detection of Commercial Pomegranate Juice Adulteration. J. Agric. Food Chem. 2009, 57, 2550–2557. [Google Scholar] [CrossRef]
- Krueger, D.A. Composition of Pomegranate Juice. J. AOAC Int. 2012, 95, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Vardin, H.; Tay, A.; Ozen, B.; Mauer, L. Authentication of pomegranate juice concentrate using FTIR spectroscopy and chemometrics. Food Chem. 2008, 108, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, L. Pomegranate fruit juice adulteration with apple juice: Detection by UV–visible spectroscopy combined with multivariate statistical analysis. Sci. Rep. 2022, 12, 5151. [Google Scholar] [CrossRef]
- Türkyılmaz, M. Anthocyanin and organic acid profiles of pomegranate (Punica granatum L.) juices from registered varieties in Turkey. Int. J. Food Sci. Technol. 2013, 48, 2086–2095. [Google Scholar] [CrossRef]
- Stój, A.; Malik, A.; Targoński, Z. Comparative Analysis of Anthocyanin Composition of Juices obtained from Selected Species of Berry Fruits. Pol. J. Food Nutr. Sci. 2006, 56, 401–407. [Google Scholar]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary Cyanidin 3-O-β-D-Glucoside-Rich Purple Corn Color Prevents Obesity and Ameliorates Hyperglycemia in Mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Guo, H.; Ling, W. The update of anthocyanins on obesity and type 2 diabetes: Experimental evidence and clinical perspectives. Rev. Endocr. Metab. Disord. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole Berries versus Berry Anthocyanins: Interactions with Dietary Fat Levels in the C57BL/6J Mouse Model of Obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.E.; Rogers, T.R.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified Blueberry Anthocyanins and Blueberry Juice Alter Development of Obesity in Mice Fed an Obesogenic High-Fat Diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.; Steward, W.P.; Gescher, A.J.; Marczylo, T. Anthocyans from fruits and vegetables—Does bright colour signal cancer chemopreventive activity? Eur. J. Cancer 2005, 41, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.; Teller, N.; Cai, H.; Marko, D.; Berry, D.P.; Steward, W.P.; Gescher, A.J. Do anthocyanins and anthocyanidins, cancer chemopreventive pigments in the diet, merit development as potential drugs? Cancer Chemother. Pharmacol. 2009, 64, 201–211. [Google Scholar] [CrossRef]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2018, 61, 76–82. [Google Scholar] [CrossRef]
- Amouretti, M. Therapeutic value of Vaccinium myrtillus anthocyanosides in an internal medicine department. Therapeutique 1972, 48, 579–581. [Google Scholar]
- Huang, W.; Hutabarat, R.P.; Chai, Z.; Zheng, T.; Zhang, W.; Li, D. Antioxidant blueberry anthocyanins induce vasodilation via PI3K/Akt signaling pathway in high-glucose-induced human umbilical vein endothelial cells. Int. J. Mol. Sci. 2020, 21, 1575. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef]
- Peng, J.-R.; Lu, T.-T.; Chang, H.-T.; Ge, X.; Huang, B.; Li, W.-M. Elevated levels of plasma superoxide dismutases 1 and 2 in patients with coronary artery disease. BioMed Res. Int. 2016, 2016, 3708905. [Google Scholar] [CrossRef]
- Lau, H.; Mat Ludin, A.F.; Rajab, N.F.; Shahar, S. Identification of neuroprotective factors associated with successful ageing and risk of cognitive impairment among Malaysia older adults. Curr. Gerontol. Geriatr. Res. 2017, 2017, 4218756. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Cui, H.; Tian, H.; Zhang, X.; Ma, L.; Ramassamy, C.; Li, J. Isolation of neuroprotective anthocyanins from black chokeberry (Aronia melanocarpa) against amyloid-β-induced cognitive impairment. Foods 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]

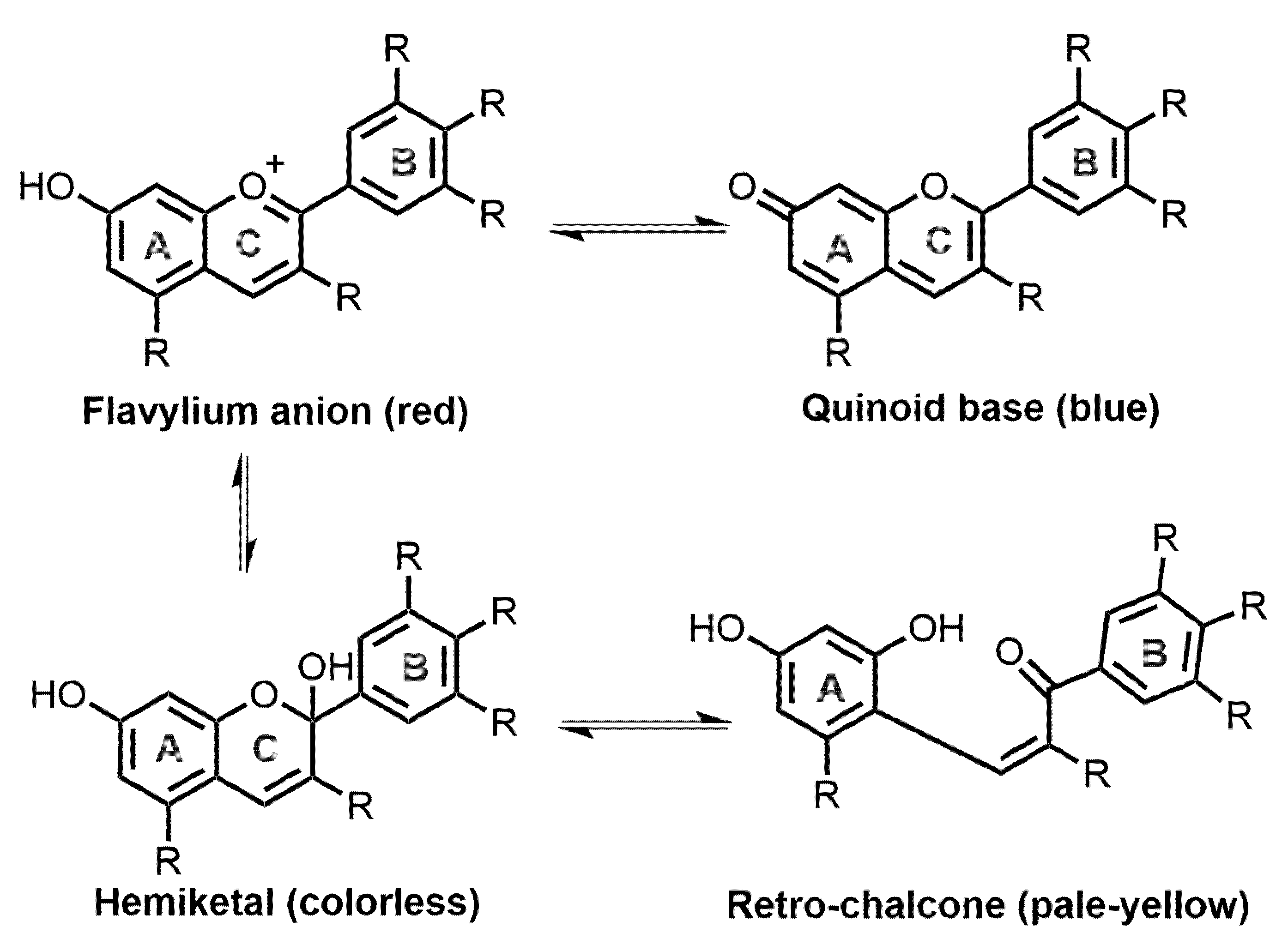
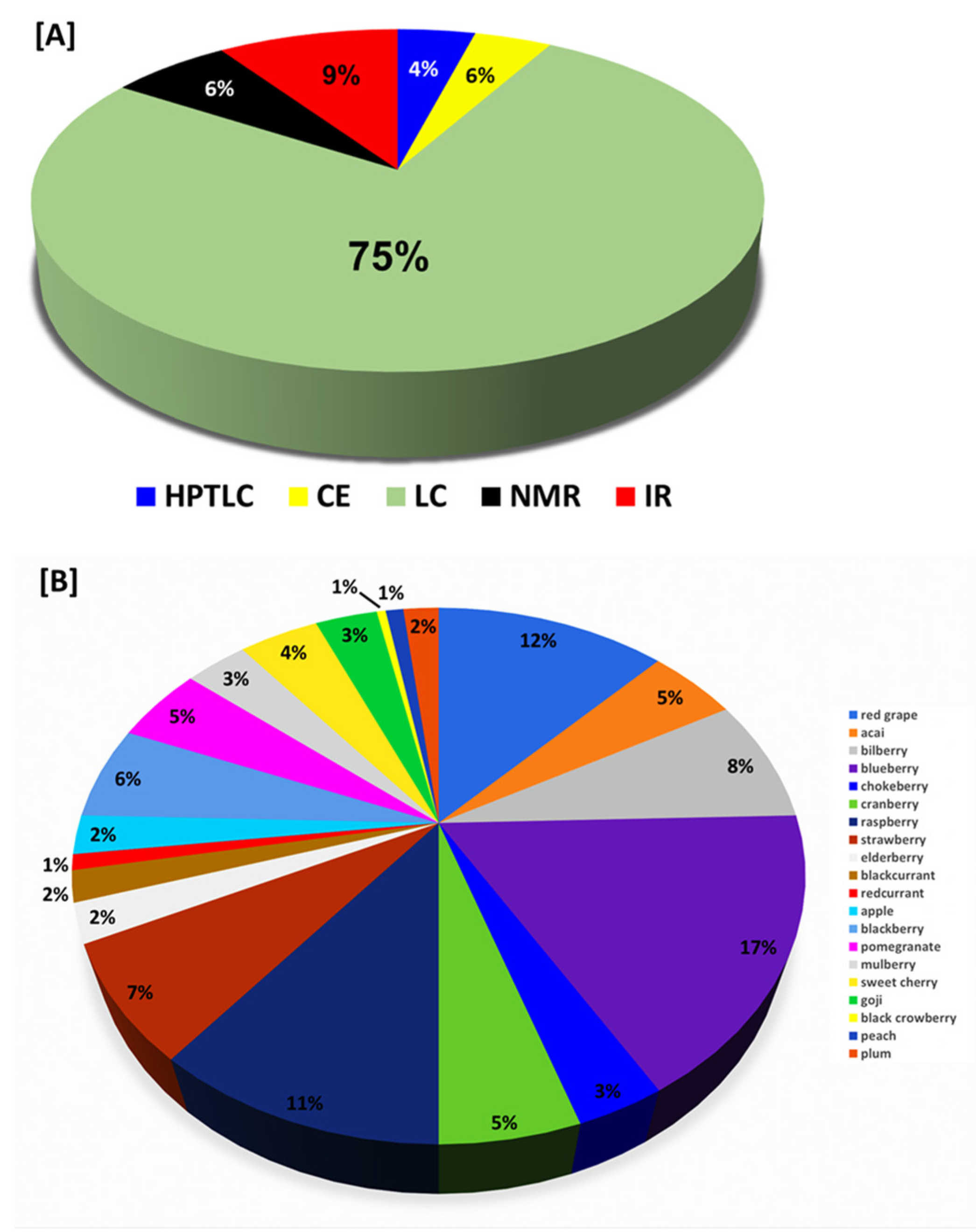
| No. | Botanical Name | Common Name | Family | Type |
|---|---|---|---|---|
| 1 | Aronia melanocarpa (Michx.) Elliott | Black Chokeberry | Rosaceae | Berry |
| 2 | Euterpe oleracea Mart. | Açaí Palm | Arecaceae | Drupe |
| 3 | Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier | Strawberry | Rosaceae | Aggregate drupelets |
| 4 | Lycium ruthenicum Murray | Black Goji Berry/Wolfberry | Solanaceae | Drupe |
| 5 | Malus domestica (Suckow) Borkh. | Apple | Rosaceae | Pome |
| 6 | Morus nigra L. | Black Mulberry | Moraceae | Aggregate drupelets |
| 7 | Prunus avium (L.) L. | Sweet Cherry | Rosaceae | Drupe |
| 8 | Prunus domestica L. | Plum | Rosaceae | Drupe |
| 9 | Prunus persica (L.) Batsch | Peach | Rosaceae | Drupe |
| 10 | Punica granatum L. | Pomegranate | Lythraceae | Berry |
| 11 | Ribes nigrum L. | Blackcurrant | Grossulariaceae | Berry |
| 12 | Rubus fruticosus Marshall | Blackberry | Rosaceae | Aggregate drupelets |
| 13 | Rubus idaeus L. | Raspberry (Red) | Rosaceae | Aggregate drupelets |
| 14 | Rubus occidentalis L. | Raspberry (Black) | Rosaceae | Aggregate drupelets |
| 15 | Sambucus nigra L. | Black Elderberry | Viburnaceae | Berry |
| 16 | Vaccinium angustifolium Aiton | Lowbush Blueberry | Ericaceae | Berry |
| 17 | Vaccinium corymbosum L. | Highbush Blueberry | Ericaceae | Berry |
| 18 | Vaccinium macrocarpon Aiton | Large Cranberry | Ericaceae | False berry |
| 19 | Vaccinium oxycoccos L. | Small Cranberry | Ericaceae | False berry |
| 20 | Vaccinium myrtillus L. | Bilberry | Ericaceae | False berry |
| 21 | Vitis labrusca L. | Concord Grapes | Vitaceae | Berry |
| 22 | Vitis vinifera L. | Grapes | Vitaceae | Berry |
| Compound | Açai | Goji Berry (Black) or Black wolfberry | Raspberry (Black and Red) | Strawberry | Cranberry (Small and Large) | Bilberry or Whortleberry | Blueberry (Highbush and Low Bush) | Black Elderberry | Mulberry (Black) | Blackberry | Chokeberry (Black) | Blackcurrant | Crowberry (Black) | Grapes (Red Peel) | Concord Grapes | Apples (Red Peel) | Sweet Cherry | Plum (Red and Black Peel) | Pomegranate Peel | Peach (Yellow Peel) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cy- | ||||||||||||||||||||
| 3-O-gal | + | + | + | + | + | + | + | + | ||||||||||||
| 3-O-glc | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 3-O-ara | + | + | + | + | + | + | + | + | ||||||||||||
| 7-O-ara | + | |||||||||||||||||||
| 3-O-rut | + | + | + | + | + | + | + | + | + | + | + | |||||||||
| 3-O-sop | + | |||||||||||||||||||
| 3-O-xyl | + | + | + | + | + | |||||||||||||||
| 3-O-sam | + | + | + | |||||||||||||||||
| 3,5-O-diglc | + | + | + | |||||||||||||||||
| 3-O-sam-5-O-rham | + | |||||||||||||||||||
| 3-O-sam-5-O-glc | + | |||||||||||||||||||
| 3-O-malglc | + | + | ||||||||||||||||||
| 3-O-dioxlglc | + | |||||||||||||||||||
| 3-(6″-ace)glc | + | + | ||||||||||||||||||
| 3-(6″-p-cou)-5-diglc | + | |||||||||||||||||||
| 3-O-(6″-p-couglc) | + | + | + | |||||||||||||||||
| 3-O-malglc-5-O-glc | + | |||||||||||||||||||
| Dp- | ||||||||||||||||||||
| 3-O-gal | + | + | + | + | ||||||||||||||||
| 3-O-glc | + | + | + | + | + | + | + | + | + | |||||||||||
| 3-O-ara | + | + | + | + | ||||||||||||||||
| 3-O-rut | + | |||||||||||||||||||
| 3,5-O-diglc | + | + | ||||||||||||||||||
| 3-O-aceglc | + | + | ||||||||||||||||||
| 3-O-(6″-p-couglc) | + | + | ||||||||||||||||||
| 3-O-rut-(p-cou)-5-O-glc | + | |||||||||||||||||||
| Mv- | ||||||||||||||||||||
| 3-O-gal | + | + | + | + | ||||||||||||||||
| 3-O-glc | + | + | + | + | + | + | + | + | ||||||||||||
| 3-O-ara | + | + | + | + | ||||||||||||||||
| 3-O-rut | + | |||||||||||||||||||
| 3,5-O-diglc | + | |||||||||||||||||||
| 3-O-aceglc | + | + | ||||||||||||||||||
| 3-O-p-couglc | + | + | + | |||||||||||||||||
| 3-O-rut(p-cou)-5-O-glc | + | |||||||||||||||||||
| Pn- | ||||||||||||||||||||
| 3-O-gal | + | + | + | + | + | |||||||||||||||
| 3-O-glc | + | + | + | + | + | + | + | + | + | + | + | |||||||||
| 3-O-ara | + | + | + | + | ||||||||||||||||
| 3-O-rut | + | + | + | + | + | + | ||||||||||||||
| 3,5-O-diglc | + | |||||||||||||||||||
| 3-O-aceglc | + | + | ||||||||||||||||||
| 3-O-p-couglc | + | + | ||||||||||||||||||
| Pt- | ||||||||||||||||||||
| 3-O-gal | + | + | + | + | ||||||||||||||||
| 3-O-glc | + | + | + | + | + | + | + | + | + | |||||||||||
| 3-O-ara | + | + | + | |||||||||||||||||
| 3-O-rut | + | |||||||||||||||||||
| 3,5-O-diglc | + | |||||||||||||||||||
| 3-O-rut-5-O-glc | + | |||||||||||||||||||
| 3-O-aceglc | + | + | ||||||||||||||||||
| 3-O-p-couglc | + | + | ||||||||||||||||||
| 3-O-rut (p-cou)-5-O-glc | + | |||||||||||||||||||
| 3-O-rut(fer)-5-O-glc | + | |||||||||||||||||||
| 3-O-(p-cou)-rut | + | |||||||||||||||||||
| 3-O-rut(caf)-5-O-glc | + | |||||||||||||||||||
| Pg- | ||||||||||||||||||||
| 3-O-gal | + | + | + | |||||||||||||||||
| 3-O-glc | + | + | + | + | + | + | + | + | + | + | + | |||||||||
| 3-O-ara | + | + | + | |||||||||||||||||
| 3-O-rut | + | + | + | + | + | |||||||||||||||
| 3-O-sam | + | |||||||||||||||||||
| 3-O-soph | + | |||||||||||||||||||
| 3,5-O-diglc | + | + | ||||||||||||||||||
| 3-O-aceglc | + | |||||||||||||||||||
| 3-O-malglc | + |
| Source | Content * (mg/100 g) | References |
|---|---|---|
| Açai (Euterpe oleracea Mart.) | 48.9–303.7 (FW) 10–3410 (DEW) | [84,88,91,93,95,96] |
| Black Goji berry or black wolfberry (Lycium ruthenicum Murray) | 930–5826 (DW) 343–525 (FW) tr–380 (DEW) | [148,149,183,184,198] |
| Raspberry (red) (Rubus idaeus) | 0.1–133.9 (FW) 76.2–365.2 (DW) 336–1030 (DEW) | [89,97,102,105,113,132,152,153,159,160,161] |
| Raspberry (black) (Rubus occidentalis) | 381–541.3 mg/100 mL puree 2885–11109 (DEW) | [132,150] |
| Strawberry (Fragaria × ananassa) | 0.4–84 (FW) 97.5–758.6 (DW) 520 (DEW) | [97,105,113,136,151,152,153,166,167,168,171,172,173] |
| Cranberry (Small and large) | ||
| American large cranberry (Vaccinium macrocarpon Aiton) | 78–430 (DW) | [189] |
| European small cranberry (Vaccinium oxycoccus L) | 40.7–360 (FW) 397 (DW) | [105,106,162] |
| Bilberry (Vaccinium myrtillus) | 104–2025.6 (FW) 2298–7465 (DW) | [87,92,100,105,107,113,120,204] |
| Blueberry (highbush and low bush) | 77.5–820 (FW) 558.3–1188.3 (DW) 1020 (DEW) | [83,97,102,113,136,153] |
| lowbush blueberry (Vaccinium angustifolium Aiton) | 110–725 (FW) 2400 (DEW) | [106,109,110] |
| highbush blueberry (Vaccinium corymbosum L.) | 42–6720 (FW) 30–2800 (DEW) | [108,112,115,116,118,120,129,138] |
| Black Elderberry (Sambucus nigra L.) | 391–1816 (FW) | [147,163,182,216] |
| Black Mulberry (Morus nigra) | 125.3–1610 (DEW) | [113,146] |
| Blackberry (Rubus fruticosus L.) | 97.7–647 (FW) 192.4–1010 (DW) 547–27230 (DEW) | [83,86,89,97,99,101,102,113,132,202] |
| Black Chokeberry (Aronia melanocarpa Elliott) | 249–737 (FW) 1041 (DW) | [105,155] |
| Blackcurrant (Ribes nigrum) | 130–586.6 (FW) 756–1064 (DW) 2100 (DEW) | [105,107,113,118] |
| Black crowberry (Empetrum nigrum) | 401–768.2 (FW) 2473–4180 (DW) | [105,113,156] |
| Grapes (red peel) (Vitis vinifera L.) | 2.4–710 (FW) 700–56470 (DEW) | [79,157,165,185,186,187] |
| Apples (red peel) (Malus domestica) | 11–26.1 (FW) | [85,90] |
| Sweet cherry (Prunus avium L.) | 0.3–642.5 (FW) | [140,141,142,144,145,202] |
| Plum (red and black peel) (Prunus domestica) | 12.9–161.4 (FW) | [199] |
| Pomegranate (Punica granatum L. Cv Ermioni) | nd–344.1 (FW) 7.9–44.7 (DW) | [175,179,180,201,207,208] |
| Peach (yellow peel) (Prunus persica) | 0.5–33.6 (FW) | [199,205] |
| No. | Fruit/Berry | Type | Analytical Method | Chemometrics | Adulterants/Contaminants | Ref |
|---|---|---|---|---|---|---|
| 1 | Bilberry | Extract | HPLC-MS, NMR | − | Amaranth/Red dye#2 | [225] |
| 2 | Bilberry | Supplements | HPLC-UV-MS | − | - | [226] |
| 3 | Bilberry | Supplements | HPLC-UV-MS | − | - | [227] |
| 4 | Bilberry | Supplements | HPLC-DAD, FT-NIR/PCA | + | Black mulberry, chokeberry, blackberry | [229] |
| 5 | Cranberry | Extracts and supplements | UPLC-DAD-MS | + | Black mulberry, Hibiscus | [230] |
| 6 | Bilberry | Fresh/Dried and Dietary supplements | LC-MS and TLC | − | - | [231] |
| 7 | Cranberry | Supplements | 1H-NMR | − | - | [41] |
| 8 | Blueberry/ cranberry | Juices | LC-QToF-MS | + | Apple and grape juices | [233] |
| 9 | Bilberry | Supplements | HPLC-UV | − | Andean blueberry fruit | [234] |
| 10 | Strawberry, Cherry, Blackberry, Red raspberry | Supplements | HPLC-UV | − | - | [235] |
| 11 | Black raspberry | Supplements | HPLC-DAD-MS | − | Blackberry | [236] |
| 12 | Black elderberry | Supplements | UHPLC-PDA-MS | − | Black rice, purple carrot and flower of S. nigra | [182,237] |
| 13 | Red grape | Wine | HPLC | − | Elderberry | [238] |
| 14 | Pomegranate | Juice | UPLC-QToF | + | Apple and red grape juice | [240] |
| 15 | Pomegranate | Juice | HPLC-PDA | − | Apple, pear, grape, sour cherry, plum, or Aronia juice | [241] |
| 16 | Pomegranate | Juice | HPLC-PDA | − | Apple, Pear, Cherry, Grape, Blackberry, Grape skin or Aronia | [242] |
| 17 | Pomegranate | Juice | FTIR | + | Grape juice | [243] |
| 18 | Pomegranate | Juice | UV-Vis | + | Apple juice | [244] |
| 19 | Pomegranate | Juice | HPLC | − | Grape, Sour cherry and apple juice | [245] |
| 20 | Raspberry and Blackcurrant | Juice | HPLC-DAD | − | Strawberry and Redcurrant juices | [246] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avula, B.; Katragunta, K.; Osman, A.G.; Ali, Z.; John Adams, S.; Chittiboyina, A.G.; Khan, I.A. Advances in the Chemistry, Analysis and Adulteration of Anthocyanin Rich-Berries and Fruits: 2000–2022. Molecules 2023, 28, 560. https://doi.org/10.3390/molecules28020560
Avula B, Katragunta K, Osman AG, Ali Z, John Adams S, Chittiboyina AG, Khan IA. Advances in the Chemistry, Analysis and Adulteration of Anthocyanin Rich-Berries and Fruits: 2000–2022. Molecules. 2023; 28(2):560. https://doi.org/10.3390/molecules28020560
Chicago/Turabian StyleAvula, Bharathi, Kumar Katragunta, Ahmed G. Osman, Zulfiqar Ali, Sebastian John Adams, Amar G. Chittiboyina, and Ikhlas A. Khan. 2023. "Advances in the Chemistry, Analysis and Adulteration of Anthocyanin Rich-Berries and Fruits: 2000–2022" Molecules 28, no. 2: 560. https://doi.org/10.3390/molecules28020560
APA StyleAvula, B., Katragunta, K., Osman, A. G., Ali, Z., John Adams, S., Chittiboyina, A. G., & Khan, I. A. (2023). Advances in the Chemistry, Analysis and Adulteration of Anthocyanin Rich-Berries and Fruits: 2000–2022. Molecules, 28(2), 560. https://doi.org/10.3390/molecules28020560









