Can DFT Calculations Provide Useful Information for SERS Applications?
Abstract
1. Introduction
2. Results
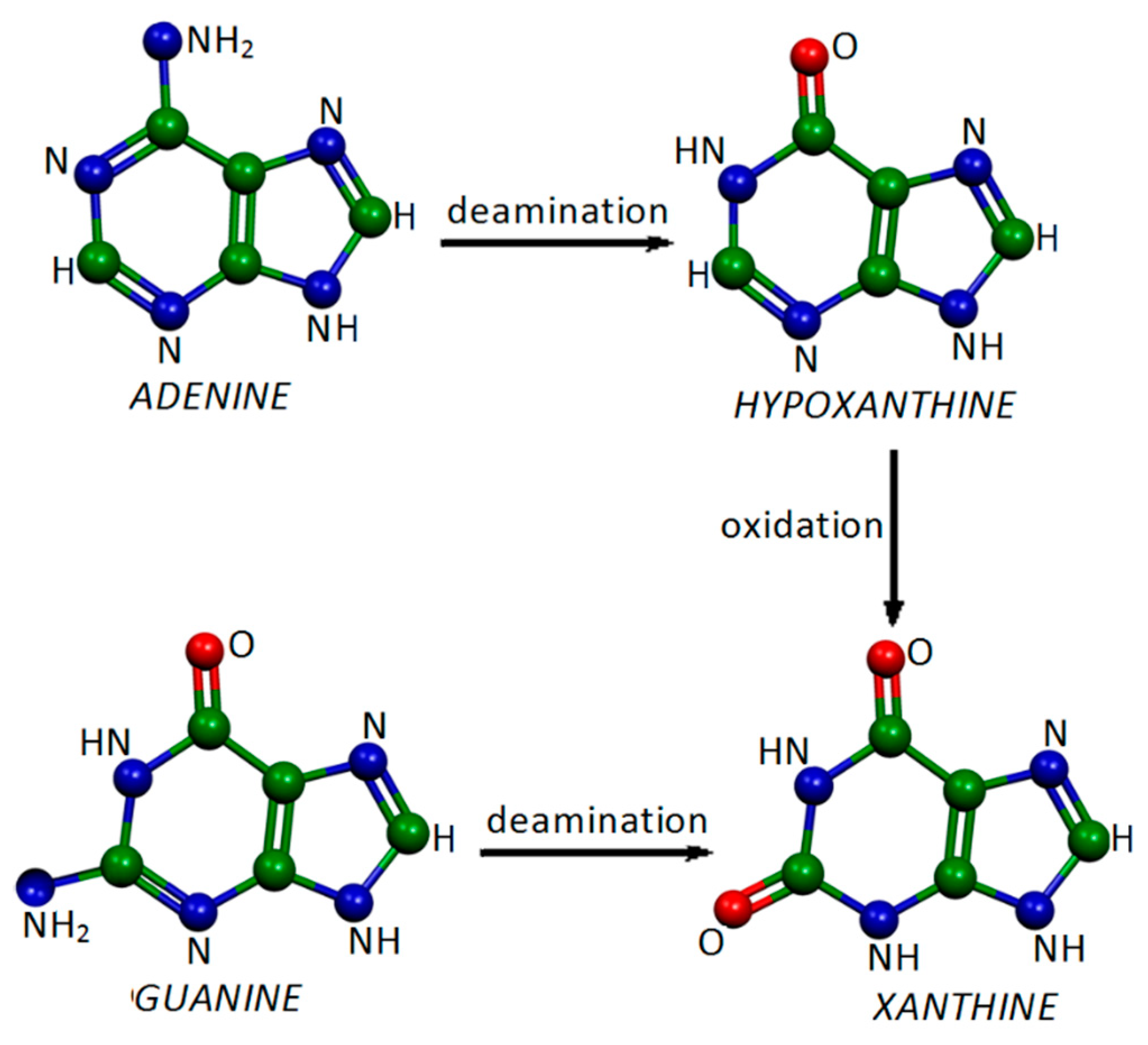
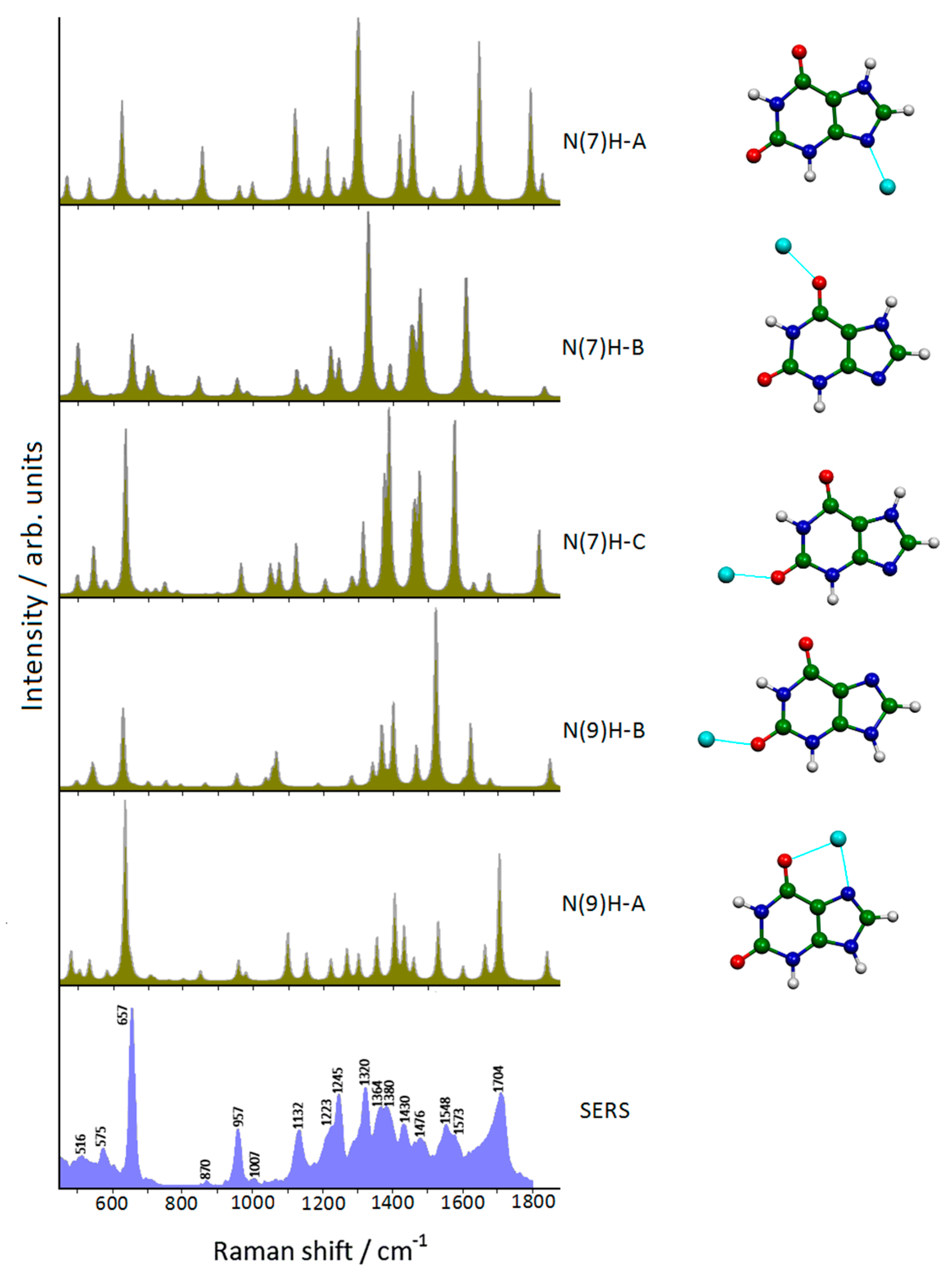
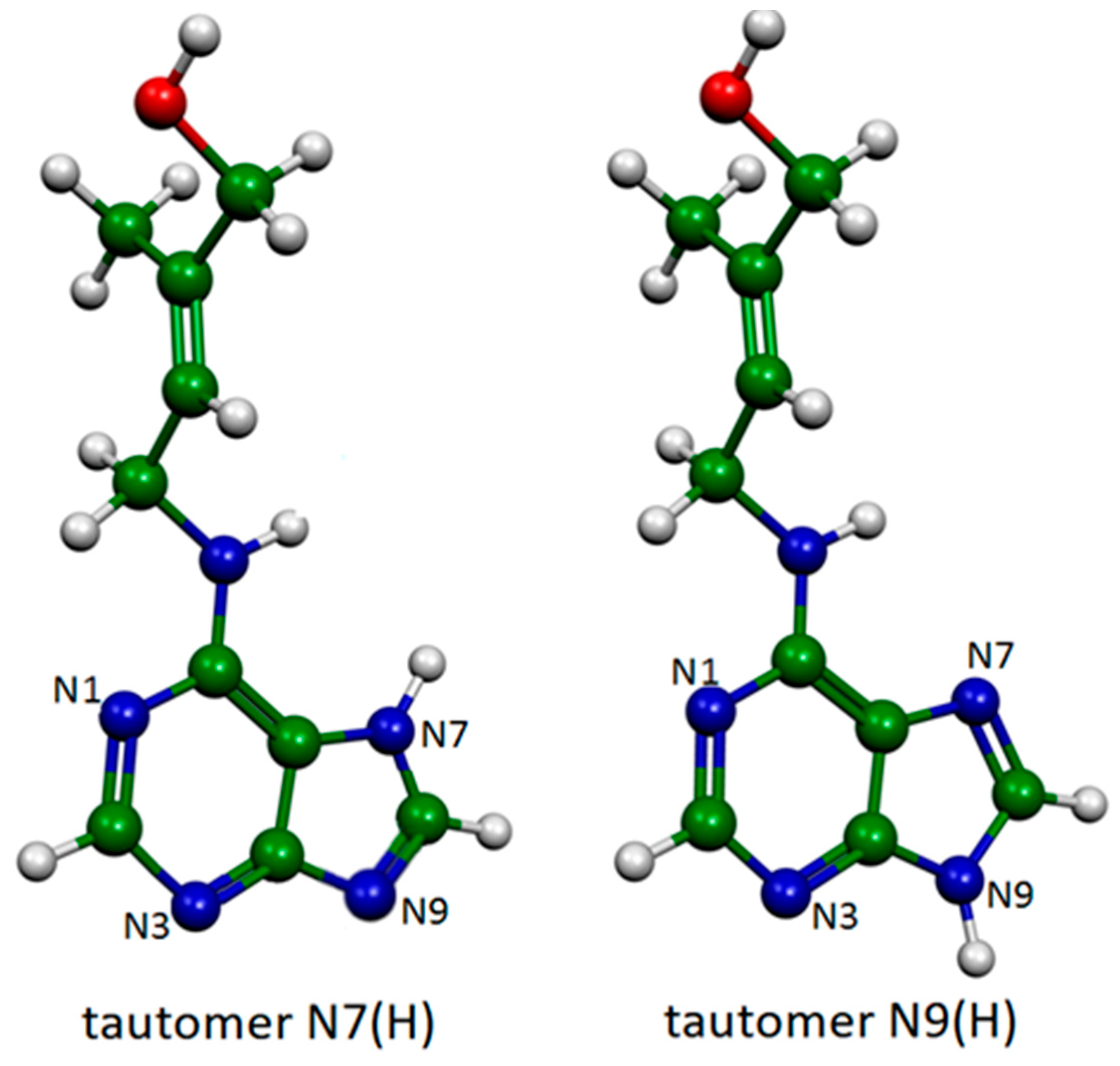
2.1. Xanthine Adsorbed on Silver Colloidal Nanoparticles
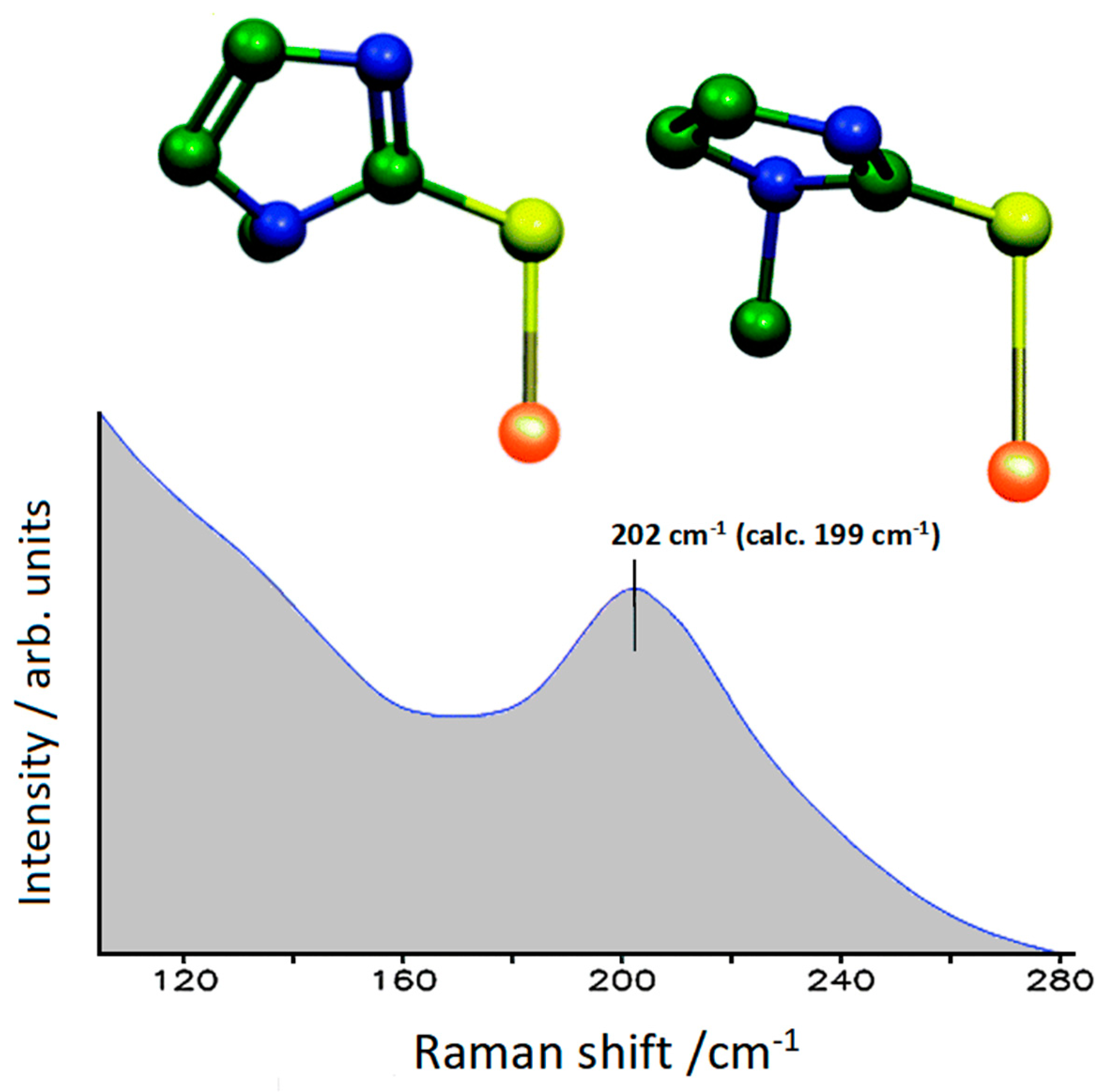
2.2. Tapazole Adsorbed on Gold Colloidal Nanoparticles
2.3. Zeatin Adsorbed on Gold Colloidal Nanoparticles
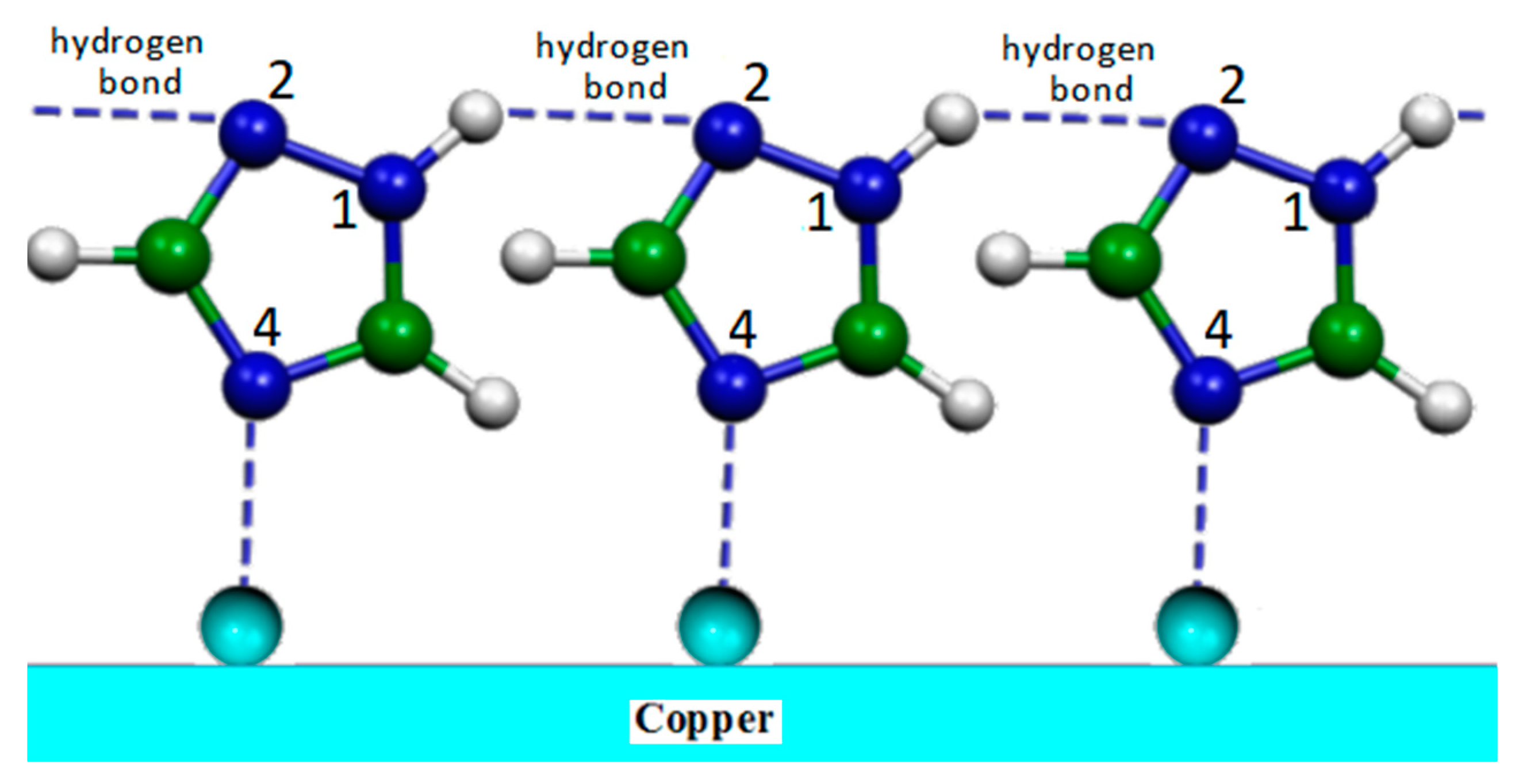
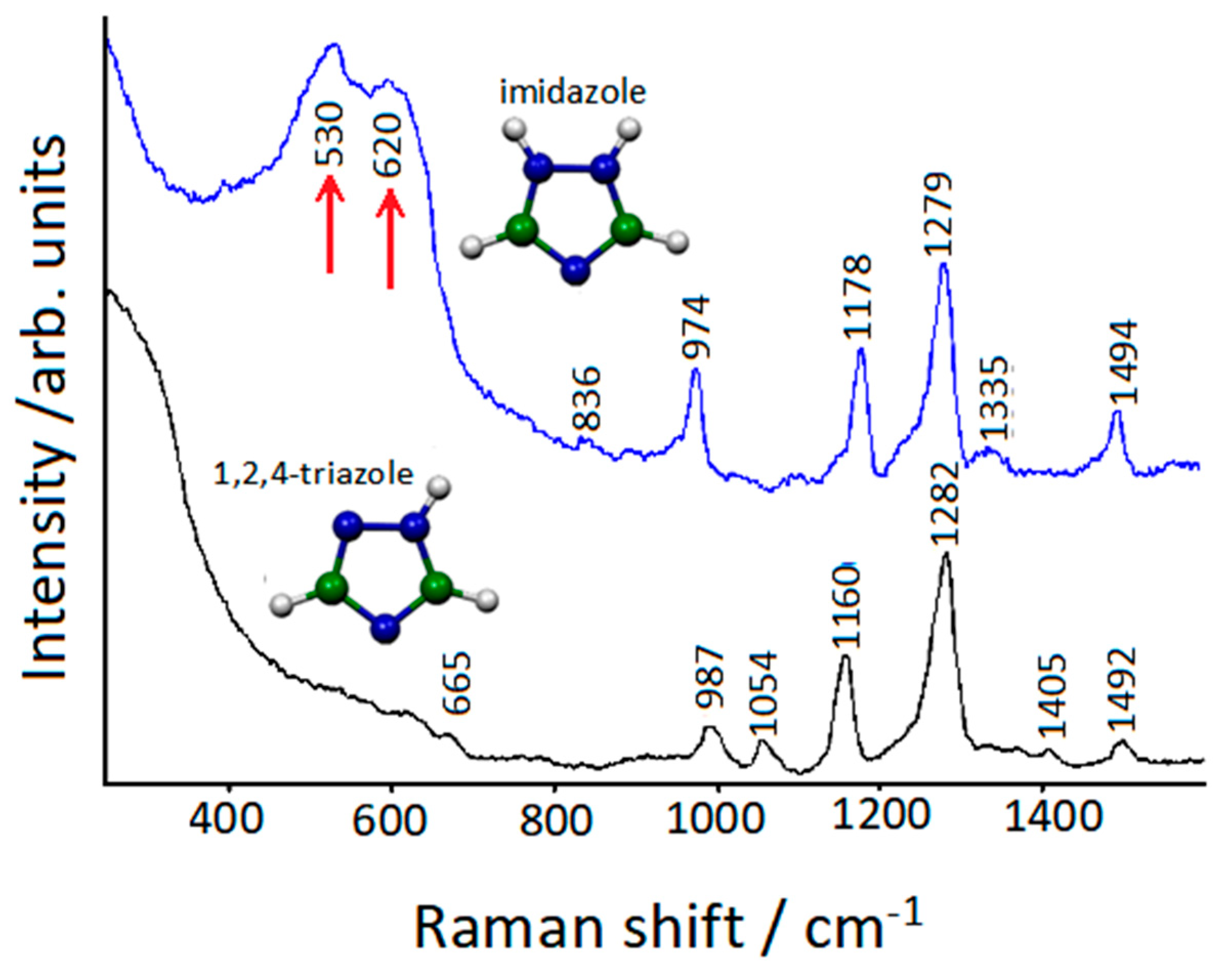
2.4. Corrosion Inhibitors on Copper Rough Surfaces
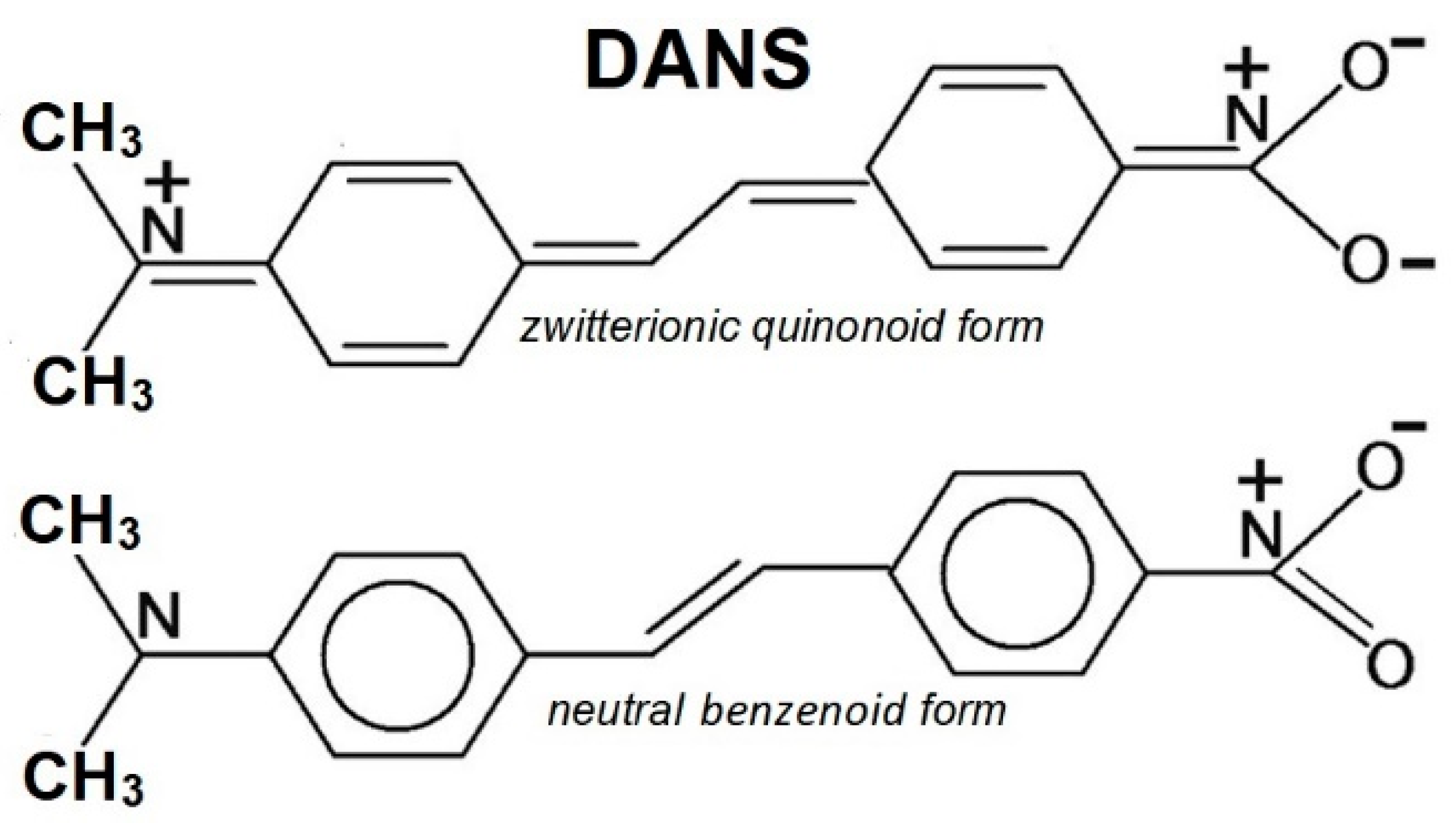
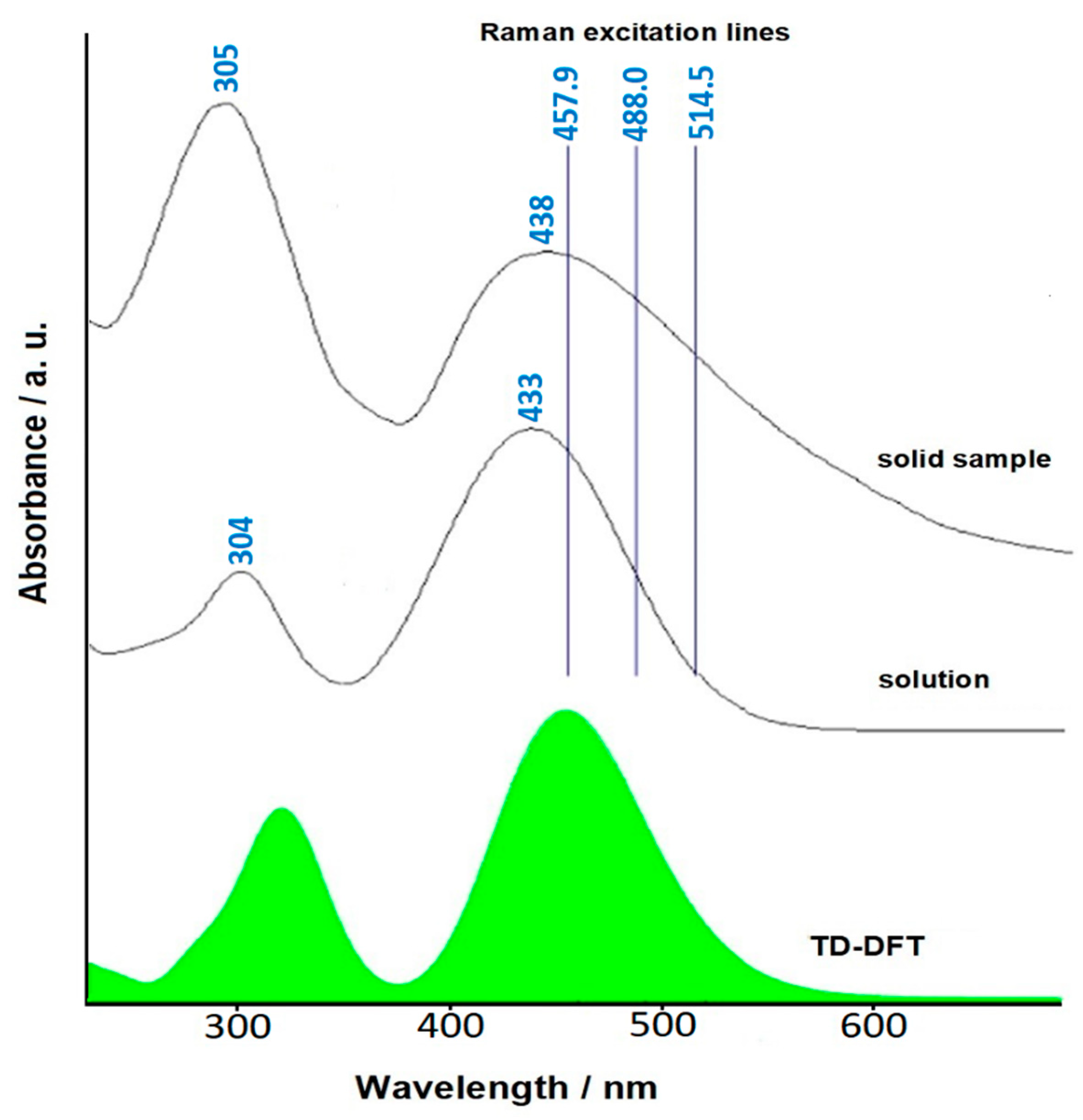
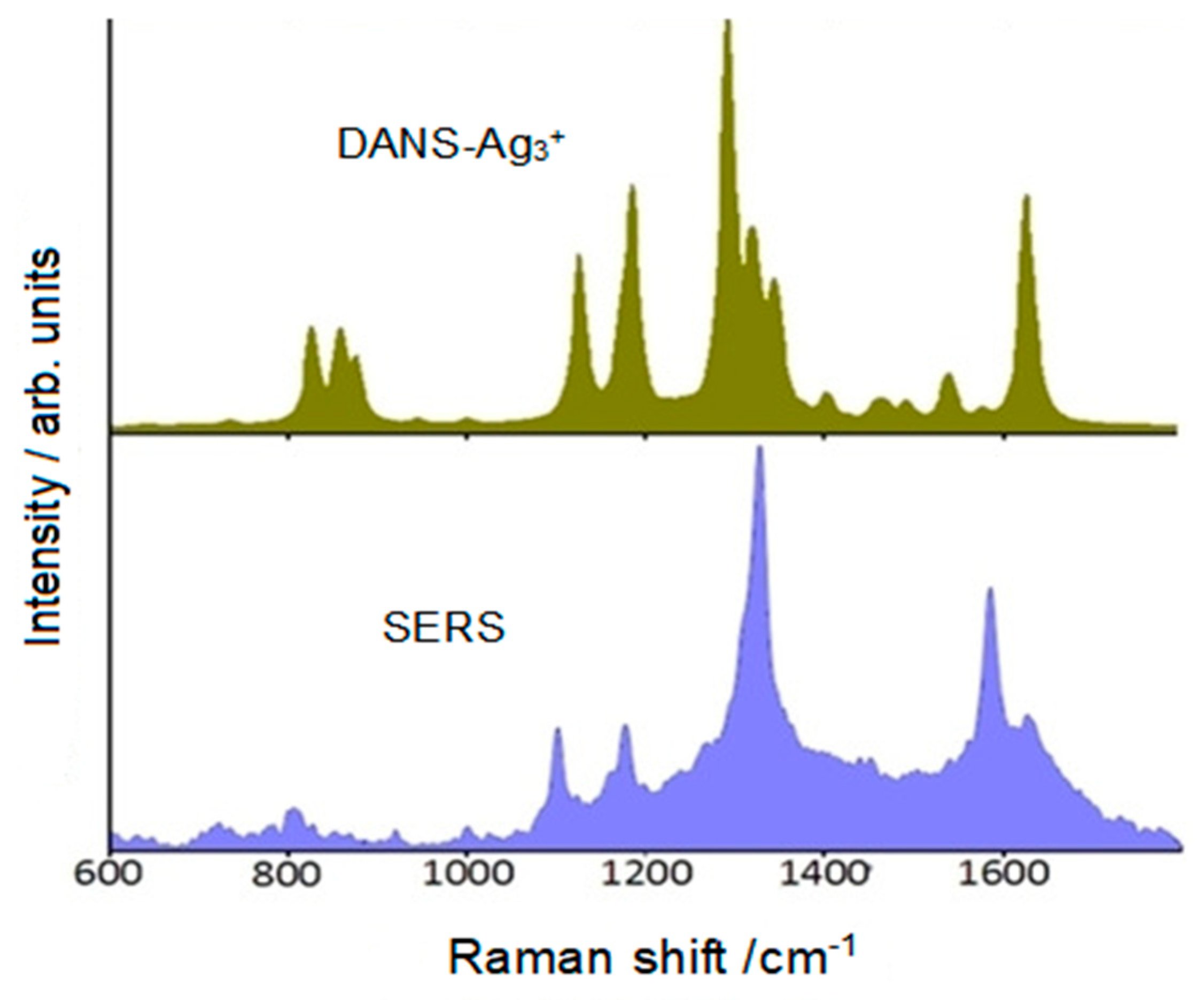
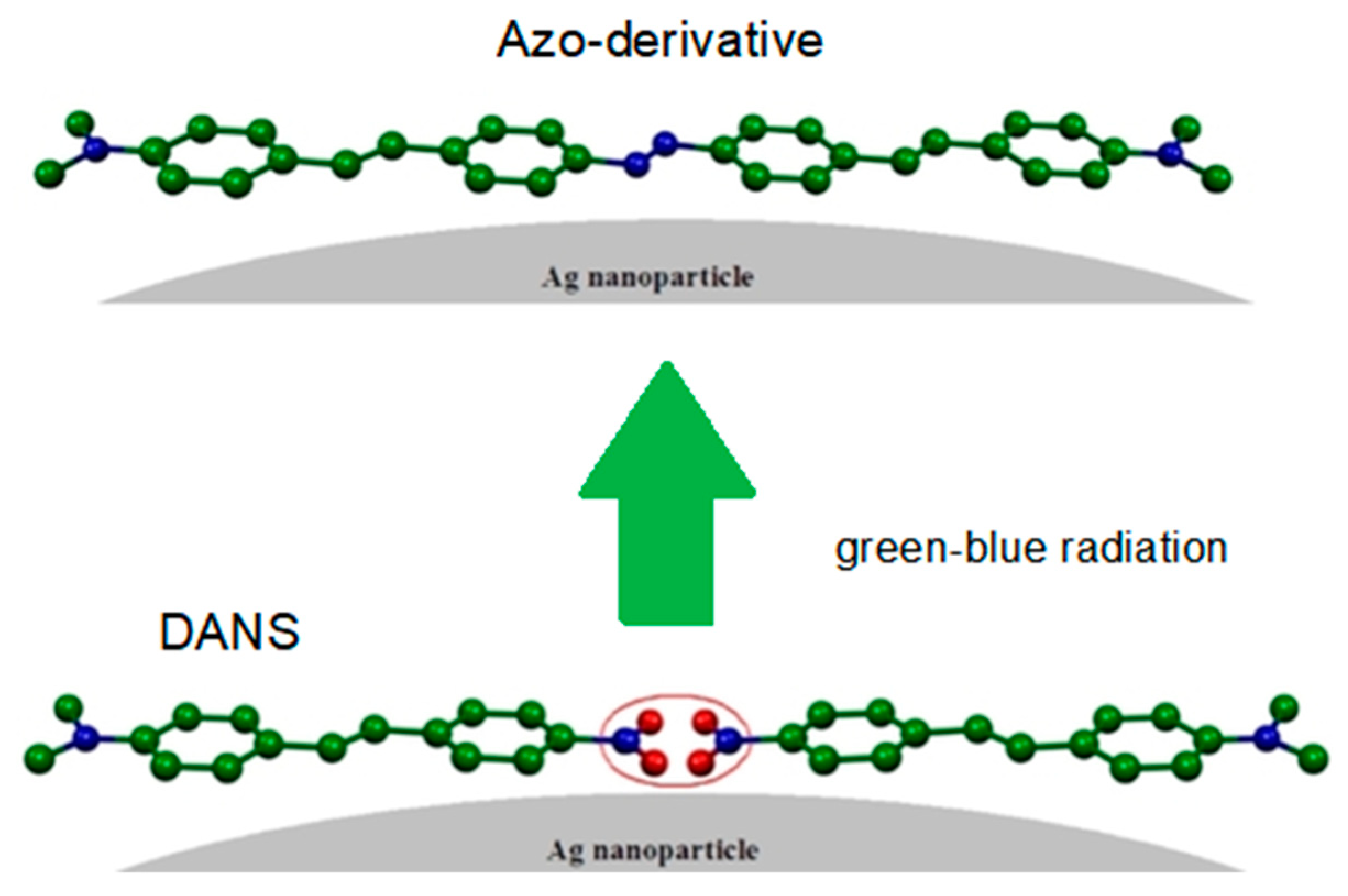
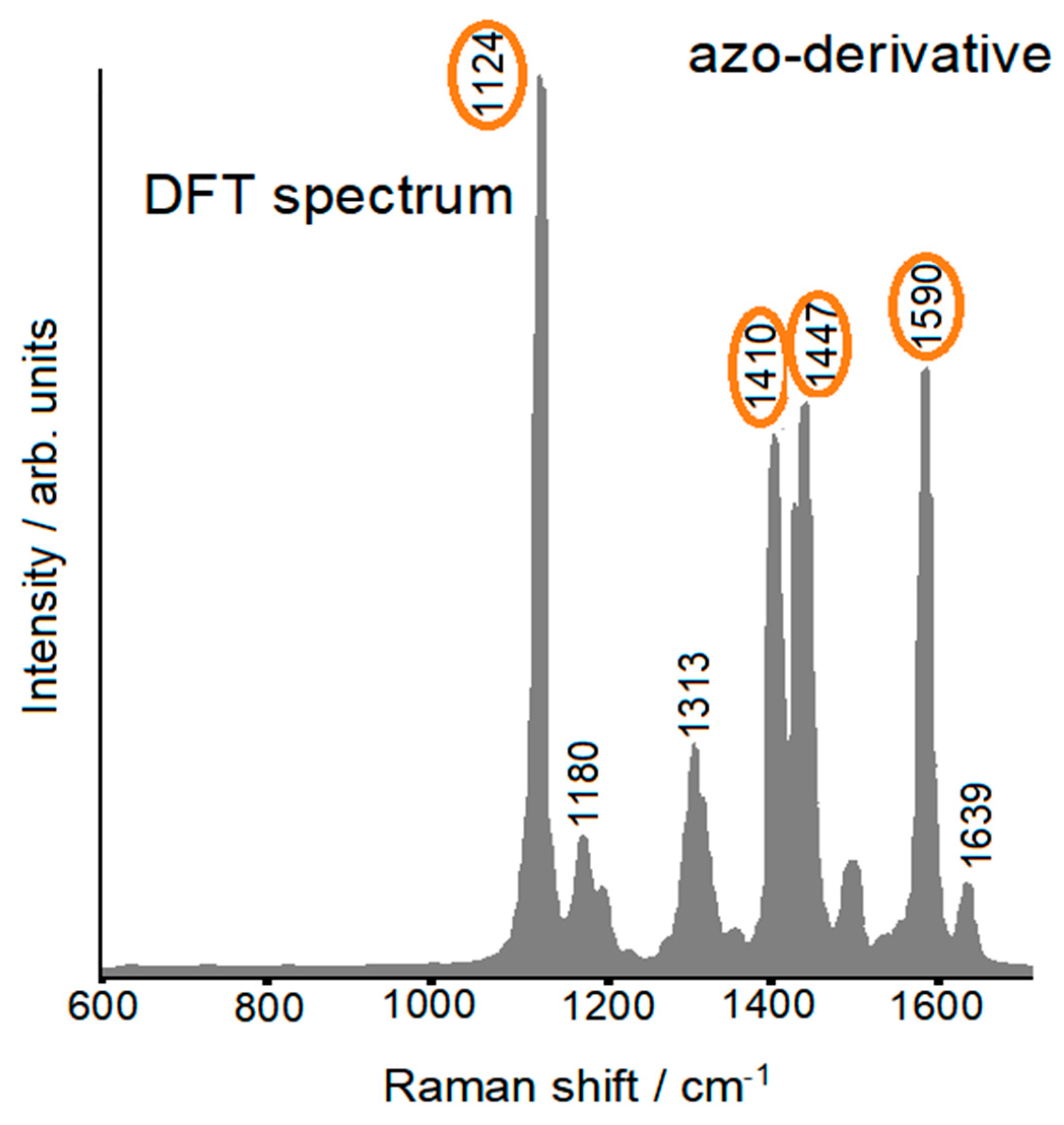
2.5. Push-Pull Molecule Adsorbed on Silver Colloidal Nanoparticles
3. Computational Details
4. Recent Developments
4.1. Sensors
4.2. Biomedicine
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le Ru, E.C.; Etchegoin, P.G. Principles of Surface-Enhanced Raman Spectroscopy and Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Schlücker, S. Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Procházka, M. Surface-Enhanced Raman Spectroscopy, Bioanalytical, Biomolecular and Medical Applications; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Szaniawska, A.; Kudelski, A. Applications of Surface-Enhanced Raman Scattering in Biochemical and Medical Analysis. Front. Chem. 2021, 9, 664134. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; de Aberasturi, D.J.; Aizpurura, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Amaya, Y.; Kamatani, N.; Nishino, T.; Hosoya, T.; Sakai, O. Identification of two mutations in human xanthine dehydrogenase gene responsible for classical type I xanthinuria. J. Clin. Investig. 1997, 99, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Raman and computational study on the adsorption of xanthine on silver nanocolloids. ACS Omega 2018, 3, 13530–13537. [Google Scholar] [CrossRef]
- Woeber, K.A. Methimazole-induced hepatotoxicity. Endocr. Pract. 2002, 8, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.; Armstrong, D.R.; Reglinski, J.; Smith, W.E.; Wilson., R.; McKillop, J.H. The structure of methimazole and its consequences for current therapeutic models of graves’ disease. Bioorg. Med. Chem. Lett. 1994, 4, 1357–1360. [Google Scholar] [CrossRef]
- Heidari, R.; Babaei, H.; Roshangar, L.; Eghbal, M.A. Effects of Enzyme Induction and/or Glutathione Depletion on Methimazole-Induced Hepatotoxicity in Mice and the Protective Role of N-Acetylcysteine. Adv. Pharm. Bull. 2014, 4, 21–28. [Google Scholar]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Pedone, A. Raman and DFT study of methimazole chemisorbed on gold colloidal nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 5974–5980. [Google Scholar] [CrossRef]
- Zalabák, D.; Pospíšilová, H.; Šmehilová, M.; Mrízová, K.; Frébort, I.; Galuszka, P. Genetic engineering of cytokinin metabolism: Prospective way to improve agricultural traits of crop plants. Biotechnol. Adv. 2013, 31, 97. [Google Scholar] [CrossRef]
- Kokina, I.; Gerbreders, V.; Sledevskis, E.; Bulanovs, A. Penetration of nanoparticles in flax (Linum usitatissimum L) calli and regenerants. J. Biotechnol. 2013, 165, 127–132. [Google Scholar] [CrossRef]
- Zhai, G.; Walters, K.S.; Peate, D.W.; Alvarez, P.J.J.; Schnoor, J.L. Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ. Sci. Technol. Lett. 2014, 1, 146–151. [Google Scholar] [CrossRef]
- Zoppi, A.; Caporali, S.; Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Adsorption of Trans-Zeatin on Laser-Ablated Gold Nanoparticles for Transport into Plant Cells and Growth Stimulation. ACS Appl. Nano Mater. 2019, 2, 7319–7327. [Google Scholar] [CrossRef]
- Sherif, E.M.; Erasmus, R.M.; Comins, J.D. Corrosion of copper in aerated synthetic sea water solutions and its inhibition by 3-amino-1,2,4-triazole. J. Colloid Interface Sci. 2007, 309, 470–477. [Google Scholar] [CrossRef]
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1,2,3-benzotriazole: A review. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Fox, P.G.; Bradley, P.A. 1:2:4-triazole as a corrosion inhibitor for copper. Corros. Sci. 1980, 20, 643–649. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Caporali, S. SERS and DFT study of copper surfaces coated with corrosion inhibitor. Beilstein J. Nanotechnol. 2014, 5, 2489–2497. [Google Scholar] [CrossRef]
- Niaura, G. Surface-enhanced Raman spectroscopic observation of two kinds of adsorbed OH− ions at copper electrode. Electrochim. Acta 2000, 45, 3507–3519. [Google Scholar] [CrossRef]
- Shoute, L.C.T.; Woo, H.Y.; Vak, D.; Bazan, G.C.; Kelley, A.M. Solvent effects on resonant first hyperpolarizabilities and Raman and hyper-Raman spectra of DANS and a water-soluble analog. J. Chem. Phys. 2006, 125, 054506. [Google Scholar] [CrossRef]
- Lapouyade, R.; Kuhn, A.; Letard, J.F.; Rettig, W. Multiple relaxation pathways in photoexcited dimethylaminonitro- and dimethylaminocyano-stilbenes. Chem. Phys. Lett. 1993, 208, 48–58. [Google Scholar] [CrossRef]
- Oberlé, J.; Abraham, E.; Jonususkas, G.; Rulliere, C. Study of the intramolecular charge-transfer (ICT) process in 4-dimethylamino-4′-nitrostilbene by picosecond time-resolved CARS. J. Raman Spectrosc. 2000, 31, 311–317. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Pedone, A. SERS and DFT investigation on push-pull molecules: 4-Dimethylamino- 4′-nitrostilbene adsorbed on silver colloidal nanoparticles. ChemistrySelect 2018, 3, 8698–8702. [Google Scholar] [CrossRef]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Spectroscopic and DFT investigation on the photo-chemical properties of a push-pull chromophore: 4-Dimethylamino-4′-nitrostilbene. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 190, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Plenum: New York, NY, USA, 1976; Volume 3. [Google Scholar]
- Jensen, F. Introduction to Computational Chemistry; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Abdulazeez, I.; Popoola, S.A.; Saleh, T.A.; Al-Saadi, A.A. Spectroscopic, DFT and trace detection study of procaine using surface enhanced Raman scattering technique. Chem. Phys. Lett. 2019, 730, 617–622. [Google Scholar] [CrossRef]
- Rusu, E.A.; Magyari, K.; Baia, L.; Baia, M. Vibrational analysis of α-lipoic acid and its adsorption behavior study by SERS. J. Mol. Struct. 2022, 1248, 131501. [Google Scholar] [CrossRef]
- Mary, Y.S.; Mary, Y.S.; Armaković, S.; Armaković, S.J.; Krátký, M.; Vinsova, J.; Baraldi, C.; Gamberini, M.C. Concentration and solvent dependent SERS, DFT, MD simulations and molecular docking studies of a thioxothiazolidine derivative with antimicrobial properties. J. Mol. Liq. 2021, 329, 115582. [Google Scholar] [CrossRef]
- Mary, Y.S.; Mary, Y.S.; Krátký, M.; Vinsova, J.; Baraldi, C.; Gamberini, M.C. DFT, SERS-concentration and solvent dependent and docking studies of a bioactive benzenesulfonamide derivative. J. Mol. Struct. 2021, 1228, 129680. [Google Scholar] [CrossRef]
- Mary, Y.S.; Mary, Y.S.; Krátký, M.; Vinsova, J.; Baraldi, C.; Gamberini, M.C. DFT, molecular docking and SERS (concentration and solvent dependant) investigations of a methylisoxazole derivative with potential antimicrobial activity. J. Mol. Struct. 2021, 1232, 130034. [Google Scholar] [CrossRef]
- Premkumar, R.; Hussain, S.; Koyambo-Konzapa, S.-J.; Dhanpal Jayram, N.; Mathavan, T.; Franklin Benial, A.M. SERS and DFT investigations of methyl 4–bromo-1H-pyrrole-2-carboxylate adsorbed on silver and gold substrates: In perspective of biosensor applications. J. Mol. Struct. 2021, 1236, 130272. [Google Scholar] [CrossRef]
- Tiwari, M.; Singh, A.; Dureja, S.; Basu, S.; Pattanayek, S.K. Au nanoparticles decorated ZnO/ZnFe2O4 composite SERS-active substrate for melamine detection. Talanta 2022, 236, 122819. [Google Scholar] [CrossRef]
- Teixeira, R.A.R.; Lima, F.R.A.; Campos Silva, P.; Costa, L.A.S.; Sant’Ana, A.C. Tracking chemical interactions of folic acid on gold surface by SERS spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117305. [Google Scholar] [CrossRef]
- Nie, X.; Chen, Z.; Tian, Y.; Chen, S.; Qu, L.; Fan, M. Rapid detection of trace formaldehyde in food based on surface-enhanced Raman scattering coupled with assembled purge trap. Food Chem. 2021, 340, 127930. [Google Scholar] [CrossRef]
- Botta, R.; Eiamchai, P.; Horprathum, M.; Limwichean, S.; Chananonnawathorn, C.; Patthanasettakul, V.; Maezono, R.; Jomphoak, A.; Nuntawong, N. 3D structured laser engraves decorated with gold nanoparticle SERS chips or paraquat herbicide detection in environments. Sens. Actuators B Chem. 2020, 304, 127327. [Google Scholar] [CrossRef]
- Onawole, A.T.; Popoola, S.A.; Saleh, T.A.; Abdulaziz, A.; Al-Saadi, A.A. Silver-loaded graphene as an effective SERS substrate for clotrimazole detection: DFT and spectroscopic studies. Spectrochim. Acta A Mol. Biomol. Spectrosc 2018, 201, 354–361. [Google Scholar] [CrossRef]
- Hariharan, A.; Kurnoothala, R.; Chinthakayala, S.K.; Vishnubhatla, K.C.; Vadlamudi, P. SERS of Dopamine: Computational and experimental studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 260, 119962. [Google Scholar] [CrossRef]
- Ngo, T.C.; Trinh, Q.T.; Thi Thai An, N.; Tri, N.N.; Trung, N.T.; Truong, D.H.; Huy, B.T.; Nguyen, M.T.; Dao, D.Q. SERS Spectra of the Pesticide Chlorpyrifos Adsorbed on Silver Nanosurface: The Ag20 Cluster Model. J. Phys. Chem. C 2020, 124, 21702–21716. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Pozzi, F.; Lombardi, J.R. Raman, SERS, and DFT Analysis of the Main Alkaloids Contained in Syrian Rue. J. Phys. Chem. C 2019, 123, 9262–9271. [Google Scholar] [CrossRef]
- Fraser, J.P.; Postnikov, P.; Miliutina, E.; Kolska, Z.; Valiev, R.; Švorcĩk, V.; Lyutakov, O.; Ganin, A.Y.; Guselnikova, O. Application of a 2D Molybdenum Telluride in SERS Detection of Biorelevant Molecules. ACS Appl. Mater. Interfaces 2020, 12, 47774–47783. [Google Scholar] [CrossRef] [PubMed]
- Shaine, M.L.; Premasiri, W.R.; Ingraham, H.M.; Andino, R.; Lemler, P.; Brodeur, A.N.; Ziegler, L.D. Surface enhanced Raman scattering for robust, sensitive detection and confirmatory identification of dried bloodstains. Analyst 2020, 145, 6097. [Google Scholar] [CrossRef]
- Perumal, J.; Wang, Y.; Attia, A.B.E.; Dinish, U.S.; Olivo, M. Towards a point-of-care SERS sensor for biomedical and agri-food analysis applications: A review of recent advancements. Nanoscale 2021, 13, 553. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2021, 1–22. [Google Scholar] [CrossRef]
- Premkumar, R.; Hussain, S.; Koyambo-Konzapa, S.-J.; Jayram, N.D.; Meera, M.R.; Mathavan, T.; Benial, A.M.F. SERS and DFT studies of 2-(trichloroacetyl) pyrrole chemisorbed on the surface of silver and gold coated thin films: In perspective of biosensor applications. J. Mol. Recognit. 2021, 34, 22921. [Google Scholar] [CrossRef] [PubMed]
- Roschi, E.; Gellini, C.; Ricci, M.; Sanchez-Cortes, S.; Focardi, C.; Neri, B.; Otero, J.C.; López-Tocón, I.; Smulevich, G.; Becucci, M. Surface-Enhanced Raman Spectroscopy for Bisphenols Detection: Toward a Better Understanding of the Analyte–Nanosystem Interactions. Nanomaterials 2021, 11, 881. [Google Scholar] [CrossRef]
- Ly, N.H.; Nguyen, T.H.; Nghi, N.D.; Kim, Y.-H.; Joo, S.W. Surface-Enhanced Raman Scattering Detection of Fipronil Pesticide Adsorbed on Silver Nanoparticles. Sensors 2019, 19, 1355. [Google Scholar] [CrossRef]
- Sheena Mary, Y.; Shyma Mary, Y.; Temiz-Arpaci, O.; Yadav, R.; Celik, I. DFT, docking, MD simulation, and vibrational spectra with SERS analysis of a benzoxazole derivative: An anti-cancerous drug. Chem. Pap. 2021, 75, 4269–4284. [Google Scholar] [CrossRef]
- Song, H.; Yan, X.; Zhao, H.; Zhang, H.; Shi, X.; Ma, J. Adsorption of polychlorinated biphenyls on gold colloid by surface-enhanced Raman spectroscopy and density functional theory. Vib. Spectrosc. 2022, 120, 103369. [Google Scholar] [CrossRef]
- Verma, A.K.; Soni, R.K. Ultrasensitive surface-enhanced Raman spectroscopy detection of explosive molecules with multibranched silver nanostructures. J. Raman Spectrosc. 2022, 53, 694–708. [Google Scholar] [CrossRef]
- Ly, N.H.; Nguyen, P.-D.; Son, S.J.; Lee, C.; Jang, S.; Joo, S.-W.; Kim, K.; Choi, J.; Joo, Y.-e.; Lee, J.I. Detection of benzalkonium chloride on glass surfaces using silver nanoparticles. Bull. Korean Chem. Soc. 2022, 43, 246–254. [Google Scholar] [CrossRef]
- Wang, Q.; Lian, S.; Guo, C.; Gao, X.; Dou, Y.; Song, C.; Lin, J. The chemical adsorption effect of surface enhanced Raman spectroscopy of nitrobenzene and aniline using the density functional theory. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121428. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Lv, M.; Bai, Y.; Liu, Z.; Weng, X.; You, C. Determination of 5-Hydroxymethylfurfural (5-HMF) in milk products by surface-enhanced Raman spectroscopy and its simulation analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121393. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ze, H.; Zhang, X.-G.; Zhang, Y.-J.; Song, J.; Zhang, H.; Zhong, H.-L.; Yang, Z.-L.; Yang, C.; Li, J.-F.; et al. Direct and Simultaneous Identification of Multiple Mitochondrial Reactive Oxygen Species in Living Cells Using a SERS Borrowing Strategy. Angew. Chem. Int. Ed. 2022, 61, e202203511. [Google Scholar]
- Wu, X.; Canñamares, M.V.; Kakoulli, I.; Sanchez-Cortes, S. Chemical Characterization and Molecular Dynamics Simulations of Bufotenine by Surface-Enhanced Raman Scattering (SERS) and Density Functional Theory (DFT). J. Phys. Chem. Lett. 2022, 13, 5831–5837. [Google Scholar] [CrossRef]
- Gellini, C.; Muniz-Miranda, M.; Pagliai, M.; Salvi, P.R. Spectroscopic studies on antimalarian Artesunate: Raman and Surface-enhanced Raman scattering and adsorption geometries of Artesunate on silver nanoparticles. J. Mol. Struct. 2021, 1224, 129020. [Google Scholar] [CrossRef]
- Dina, N.E.; Tahir, M.A.; Bajwa, S.Z.; Amin, I.; Valev, V.K.; Zhang, L. SERS-based antibiotic susceptibility testing: Towards point-of-care clinical diagnosis. Biosens. Bioelectron. 2023, 219, 114843. [Google Scholar] [CrossRef]
- Mohammadi, M.; Antoine, D.; Vitt, M.; Dickie, J.M.; Jyoti, S.S.; Wall, J.G.; Johnson, P.A.; Wawrousek, K.E. A fast, ultrasensitive SERS immunoassay to detect SARS-CoV-2 in saliva. Anal. Chim. Acta 2022, 1229, 340290. [Google Scholar] [CrossRef]
- Ramirez-Perez, J.C.; Durigo, D. Surface-Enhanced Raman Spectroscopy (SERS) for characterization SARS-CoV-2. J. Saudi Chem. Soc. 2022, 26, 101531. [Google Scholar] [CrossRef]
- Fornasaro, S.; Cialla-May, D.; Sergo, V.; Bonifacio, A. The Role of Surface Enhanced Raman Scattering for Therapeutic Drug Monitoring of Antimicrobial Agents. Chemosensors 2022, 10, 128. [Google Scholar] [CrossRef]
- Hassanain, W.A.; Johnson, C.L.; Faulds, K.; Graham, D.; Keegan, N. Recent advances in antibiotic resistance diagnosis using SERS: Focus on the “Big 5” challenges. Analyst 2022, 147, 4674–4700. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniz-Miranda, M.; Muniz-Miranda, F.; Menziani, M.C.; Pedone, A. Can DFT Calculations Provide Useful Information for SERS Applications? Molecules 2023, 28, 573. https://doi.org/10.3390/molecules28020573
Muniz-Miranda M, Muniz-Miranda F, Menziani MC, Pedone A. Can DFT Calculations Provide Useful Information for SERS Applications? Molecules. 2023; 28(2):573. https://doi.org/10.3390/molecules28020573
Chicago/Turabian StyleMuniz-Miranda, Maurizio, Francesco Muniz-Miranda, Maria Cristina Menziani, and Alfonso Pedone. 2023. "Can DFT Calculations Provide Useful Information for SERS Applications?" Molecules 28, no. 2: 573. https://doi.org/10.3390/molecules28020573
APA StyleMuniz-Miranda, M., Muniz-Miranda, F., Menziani, M. C., & Pedone, A. (2023). Can DFT Calculations Provide Useful Information for SERS Applications? Molecules, 28(2), 573. https://doi.org/10.3390/molecules28020573





