Unveiling the Antioxidant, Clinical Enzyme Inhibitory Properties and Cytotoxic Potential of Tambourissa peltata Baker—An Understudied Endemic Plant
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction of Phytochemicals
4.3. Phytochemical Composition
4.4. HPLC-MS Analysis
4.5. Evaluation of Biological Activities
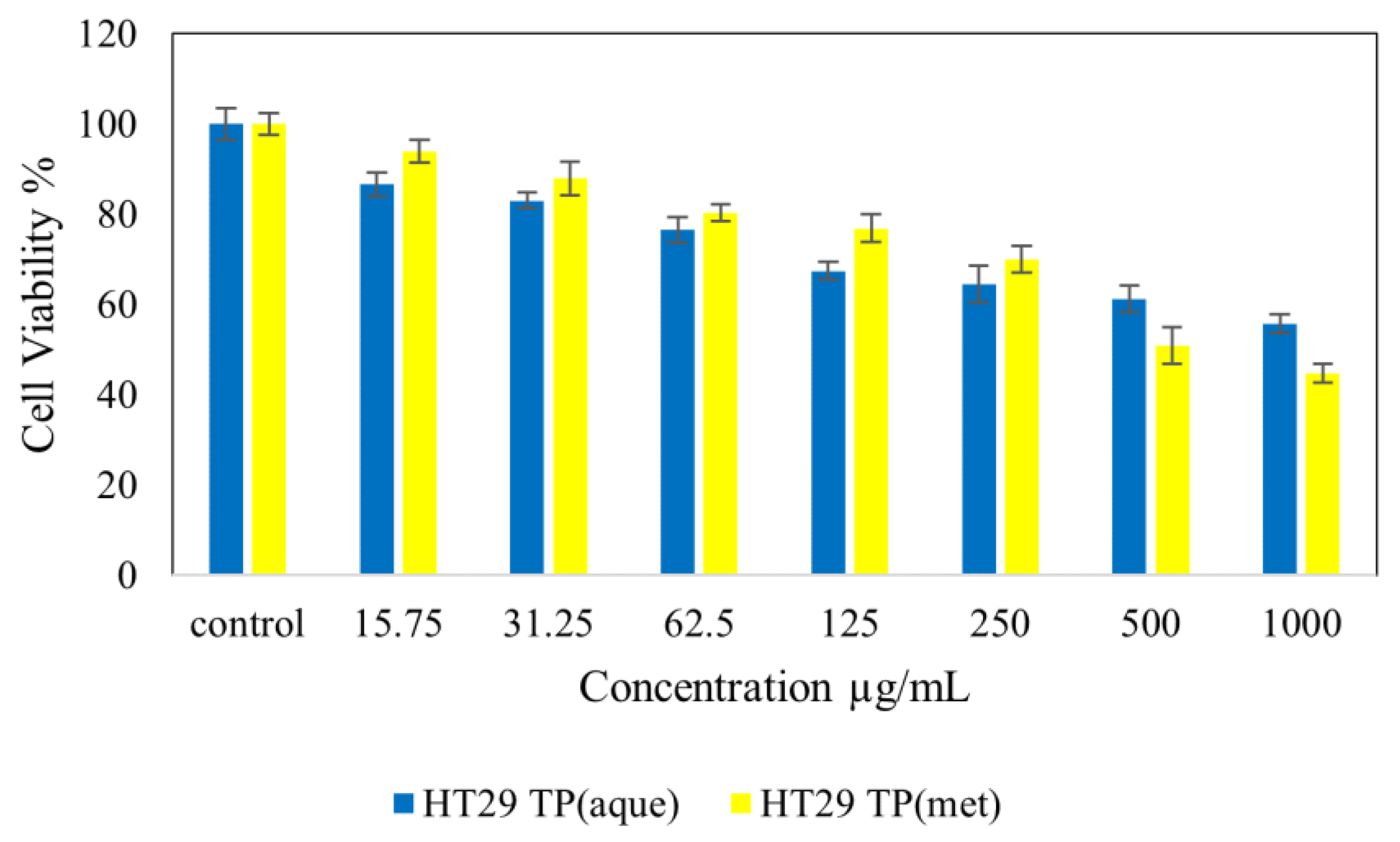
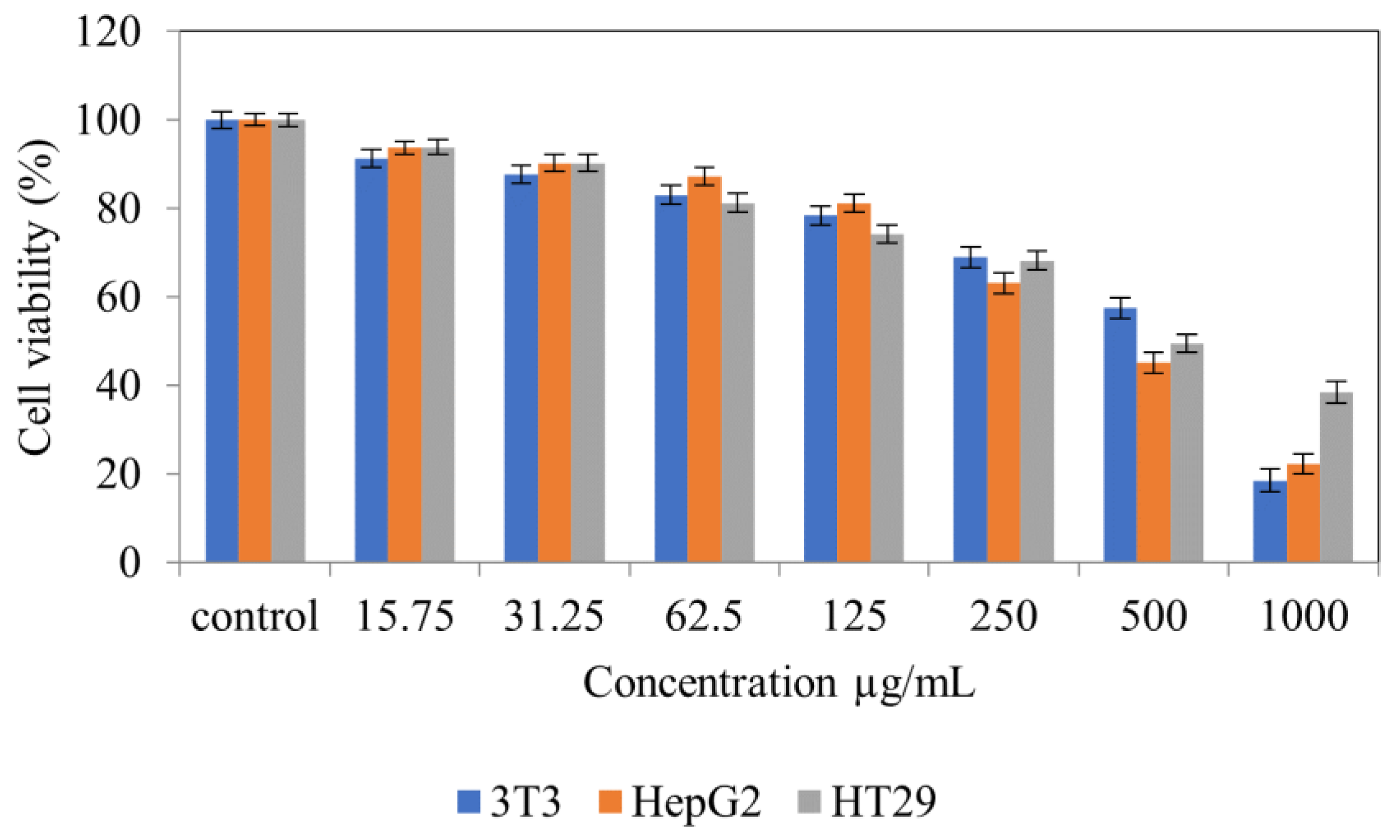
4.6. Cell Viability Assay
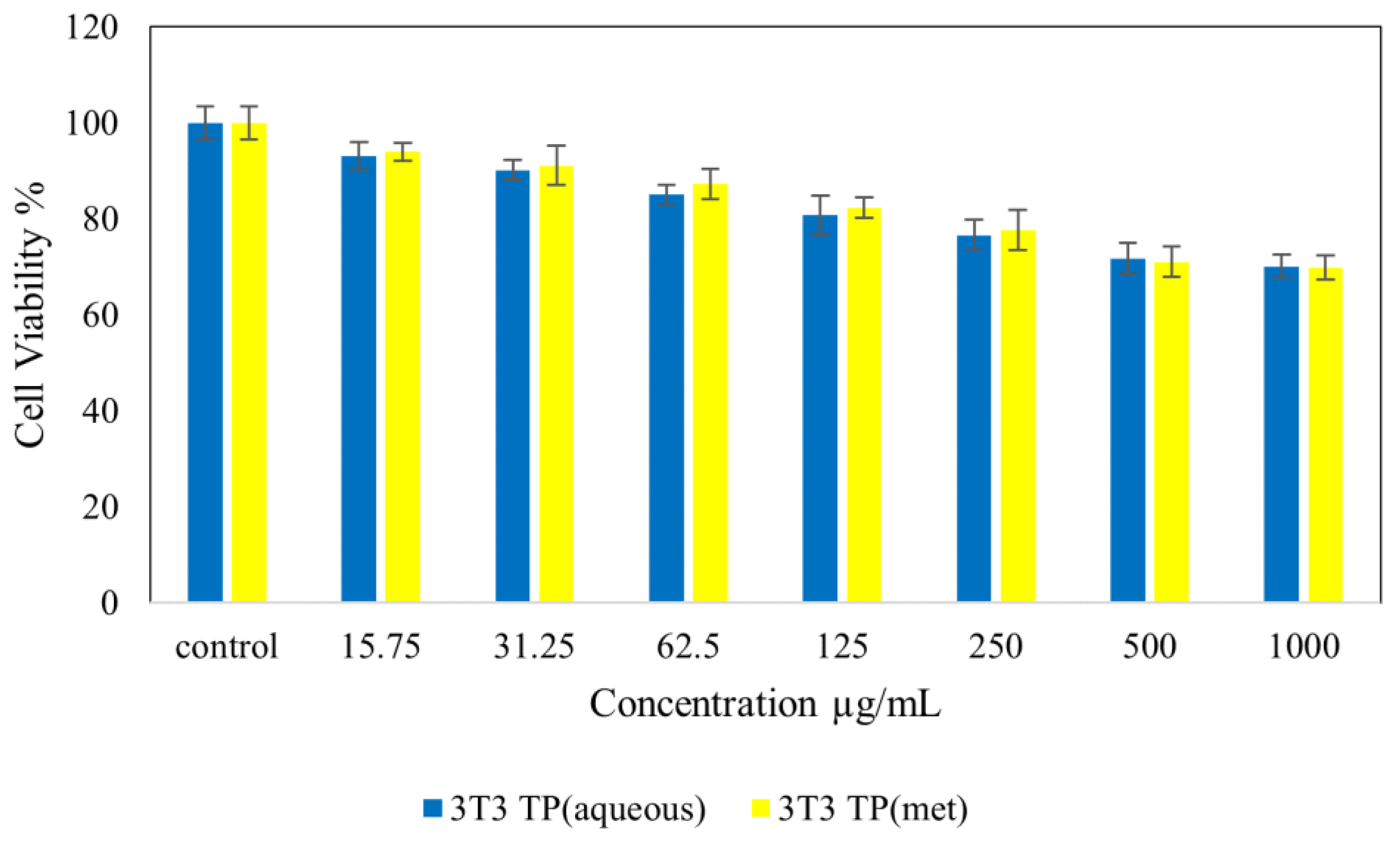
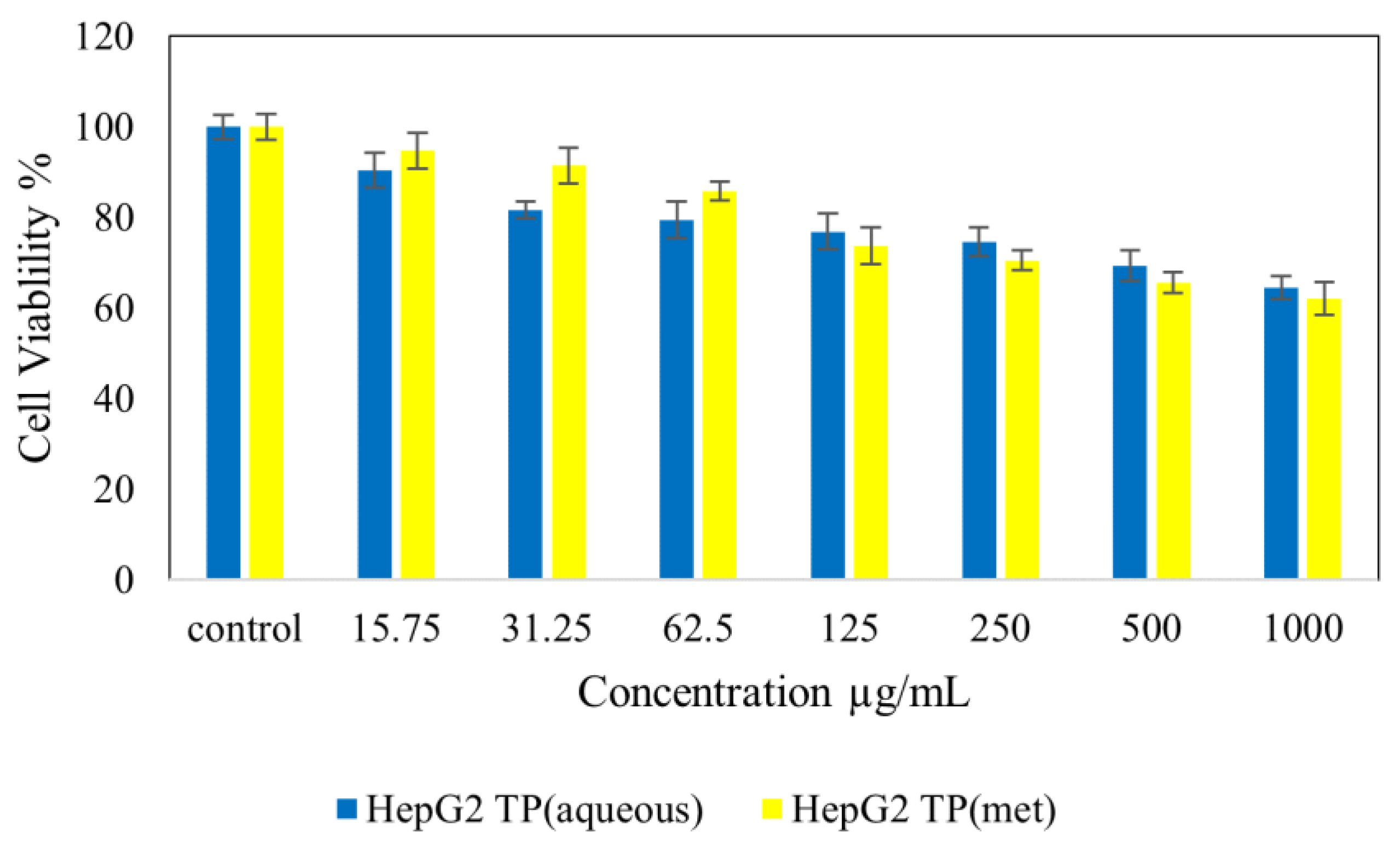
4.7. Cytotoxicity Studies on Normal (NIH 3T3) and Cancer (HepG2 and HT 29) Cell Lines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suroowan, S.; Pynee, K.; Mahomoodally, M. A comprehensive review of ethnopharmacologically important medicinal plant species from Mauritius. S. Afr. J. Bot. 2019, 122, 189–213. [Google Scholar] [CrossRef]
- Rangasamy, O. The Search for Anti-Infectives from Mauritian Flora. Ph.D. Thesis, University of Mauritius, Moka, Mauritius, 2014. [Google Scholar]
- El Mahdi, O.; Ouakil, A.; Lachkar, M. Non-volatile constituents from Monimiaceae, Siparunaceae and Atherospermataceae plant species and their bioactivities: An up-date covering 2000–2021. Phytochemistry 2022, 202, 113291. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.S.; Ijaz, M.; Buabeid, M.; Kharaba, Z.J.; Yaseen, H.S.; Murtaza, G. Therapeutic effects and safe uses of plant-derived polyphenolic compounds in cardiovascular diseases: A review. Drug Des. Dev. Ther. 2021, 15, 4713. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dementia. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 1 September 2022).
- World Health Organization. Diabetes Mellitus. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 1 September 2022).
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Tangau, M.J.; Chong, Y.K.; Yeong, K.Y. Advances in cosmeceutical nanotechnology for hyperpigmentation treatment. J. Nanopart. Res. 2022, 24, 155. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Nicoletti, M. New Solutions Using Natural Products. In Insect-Borne Diseases in the 21st Century; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–351. [Google Scholar]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Berida, T.I.; Habtemariam, S. Plants-derived neuroprotective agents: Cutting the cycle of cell death through multiple mechanisms. Evid.-Based Complement. Altern. Med. 2017, 2017, 3574012. [Google Scholar] [CrossRef] [Green Version]
- Pisani, L.; Catto, M.; Leonetti, F.; Nicolotti, O.; Stefanachi, A.; Campagna, F.; Carotti, A. Targeting monoamine oxidases with multipotent ligands: An emerging strategy in the search of new drugs against neurodegenerative diseases. Curr. Med. Chem. 2011, 18, 4568–4587. [Google Scholar] [CrossRef]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Bektaş, N.Y.; Ersoy, E.; Mehmet, B.; Boran, T.; Cinar, E.; Özhan, G.; Gören, A.C.; Özkan, E.E. Cytotoxic and apoptotic effects of Hypericum androsaemum on prostate adenocarcinoma (PC-3) and hepatocellular carcinoma (Hep G2) cell lines with identification of secondary metabolites by LC-HRMS. Turk. J. Chemistry 2021, 45, 1621. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Miotto, D. Honey melanoidins: Analysis of the compositions of the high molecular weight melanoidins exhibiting radical-scavenging activity. Food Chem. 2011, 127, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Calani, L.; Cossu, M.; Mena, P.; Sayegh, M.; Ray, S.; Del Rio, D. (Poly) phenolic characterization of three food supplements containing 36 different fruits, vegetables and berries. PharmaNutrition 2015, 3, 11–19. [Google Scholar] [CrossRef]
- Hamed, A.I.; Al-Ayed, A.S.; Moldoch, J.; Piacente, S.; Oleszek, W.; Stochmal, A. Profiles analysis of proanthocyanidins in the argun nut (Medemia argun—An ancient Egyptian palm) by LC–ESI–MS/MS. J. Mass Spectrom. 2014, 49, 306–315. [Google Scholar] [CrossRef]
- Kajdžanoska, M.; Gjamovski, V.; Stefova, M. HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Maced. J. Chem. Chem. Eng. 2010, 29, 181–194. [Google Scholar] [CrossRef] [Green Version]
- Sarnoski, J.; Johnson, J.V.; Reed, K.A.; Tanko, J.M.; O’Keefe, S.F. Separation and characterisation of proanthocyanidins in Virginia type peanut skins by LC–MSn. Food Chem. 2012, 131, 927–939. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.-r.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef]
- The Plant List. Tambourissa peltata Baker. Available online: http://www.theplantlist.org/tpl/record/tro-21200221 (accessed on 14 November 2022).
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Sharma, A.K.; Beniwal, V. Biosynthesis and medicinal applications of proanthocyanidins: A recent update. Biocatal. Agric. Biotechnol. 2022, 45, 102500. [Google Scholar]
- Abou Baker, D.H. An ethnopharmacological review on the therapeutical properties of flavonoids and their mechanisms of actions: A comprehensive review based on up to date knowledge. Toxicol. Rep. 2022, 9, 445–469. [Google Scholar] [CrossRef]
- Coelho dos Santos, T.; Gomes, T.; Pinto, B.; Camara, A.; Antonio de Andrade Paes, M. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease. Ther. Front. Pharm. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, A.; Faraoni, M.B.; Castro, M.J.; Alza, N.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Jin, W.; Nazir, Y.; Fercher, C.; Blaskovich, M.A.; Cooper, M.A.; Barnard, R.T.; Ziora, Z.M. Tyrosinase inhibitors as potential antibacterial agents. Eur. J. Med. Chem. 2020, 187, 111892. [Google Scholar] [CrossRef] [PubMed]
- Stapelberg, J.; Nqephe, M.; Lambrechts, I.; Crampton, B.; Lall, N. Selected South African plants with tyrosinase enzyme inhibition and their effect on gene expression. S. Afr. J. Bot. 2019, 120, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Benarba, B.; Pandiella, A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed. Pharmacother. 2018, 107, 408–423. [Google Scholar] [CrossRef]
- Forester, S.C.; Gu, Y.; Lambert, J.D. Inhibition of starch digestion by the green tea polyphenol, (−)-epigallocatechin-3-gallate. Mol. Nutr. Food Res. 2012, 56, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Kochi, T.; Ideta, T.; Miyazaki, T.; Moriwaki, H. Chemopreventive potential of green tea catechins in hepatocellular carcinoma. Int. J. Mol. Sci. 2015, 16, 6124–6139. [Google Scholar] [CrossRef] [PubMed]
- Baby, J.; Devan, A.R.; Kumar, A.R.; Gorantla, J.N.; Nair, B.; Aishwarya, T.S.; Nath, L.R. Cogent role of flavonoids as key orchestrators of chemoprevention of hepatocellular carcinoma: A review. J. Food Biochem. 2021, 45, e13761. [Google Scholar] [CrossRef]
- Picot, C.; Subratty, A.H.; Mahomoodally, M.F. Inhibitory potential of five traditionally used native antidiabetic medicinal plants on α-amylase, α-glucosidase, glucose entrapment, and amylolysis kinetics in vitro. Adv. Pharmacol. Sci. 2014, 2014, 739834. [Google Scholar]
- Sadeer, N.B.; Llorent-Martínez, E.J.; Bene, K.; Mahomoodally, M.F.; Mollica, A.; Sinan, K.I.; Stefanucci, A.; Ruiz-Riaguas, A.; Fernández-de Córdova, M.L.; Zengin, G. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal. 2019, 174, 19–33. [Google Scholar] [CrossRef]
- Uysal, S.; Aktumsek, A. A phytochemical study on Potentilla anatolica: An endemic Turkish plant. Ind. Crops Prod. 2015, 76, 1001–1007. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savran, A.; Zengin, G.; Aktumsek, A.; Mocan, A.; Glamoćlija, J.; Ćirić, A.; Soković, M. Phenolic compounds and biological effects of edible Rumex scutatus and Pseudosempervivum sempervivum: Potential sources of natural agents with health benefits. Food Funct. 2016, 7, 3252–3262. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.; Ortega-Barrales, P.; Zengin, G.; Uysal, S.; Ceylan, R.; Guler, G.; Mocan, A.; Aktumsek, A. Lathyrus aureus and Lathyrus pratensis: Characterization of phytochemical profiles by liquid chromatography-mass spectrometry, and evaluation of their enzyme inhibitory and antioxidant activities. RSC Adv. 2016, 6, 88996–89006. [Google Scholar] [CrossRef]

| Extract | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) | Total Phenolic Acid Content (mg CAE/g) | Total Flavonol Content (mg CE/g) |
|---|---|---|---|---|
| TP-Aq | 179.91 ± 0.67 a | 19.94 ± 0.34 c | 55.74 ± 1.43 a | 3.60 ± 0.08 c |
| TP-EA | 64.51 ± 0.56 c | 28.97 ± 0.46 a | 0 | 19.63 ± 0.42 b |
| TP-MEOH | 129.79 ± 0.72 b | 24.85 ± 0.45 b | 9.32 ± 1.45 b | 33.71 ± 0.13 a |
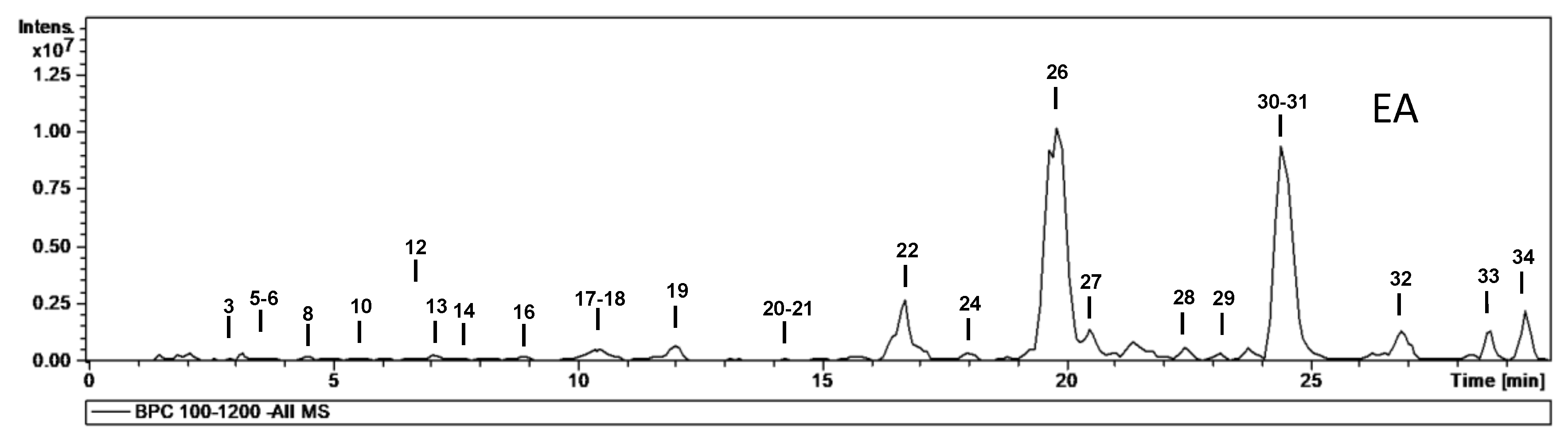
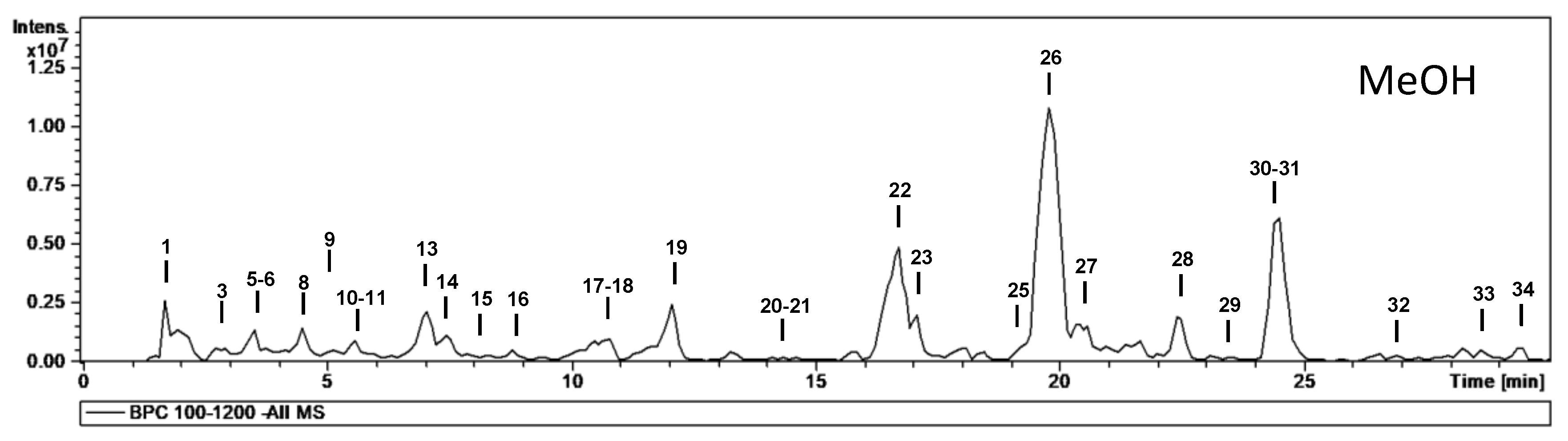
| No. | tR (min) | [M − H]− m/z | m/z (% Base Peak) | H2O | EA | MeOH | Assigned Identification |
|---|---|---|---|---|---|---|---|
| 1 | 1.7 | 377 | MS2 [377]: 341 (100), 179 (15) MS3 [377→341]: 179 (100), 143 (11), 131 (11) MS4 [377→341→179]: 161 (78), 149 (65), 119 (100), 113 (53) | ✓ | ✓ | Disaccharide (HCl adduct) | |
| 2 | 2.1 | 191 | MS2 [191]: 173 (48), 111 (100) | ✓ | Isocitric acid | ||
| 3 | 2.7 | 609 | MS2 [609]: 591 (27), 483 (22), 471 (17), 441 (84), 423 (100), 305 (38) | ✓ | ✓ | ✓ | Prodelphinidin dimer B (two units of (epi)GC) |
| 4 | 3.1 | 865 | MS2 [865]: 739 (12), 695 (100), 577 (18), 575 (31), 451 (16), 407 (22), 287 (19) MS3 [865→695]: 587 (35), 543 (100), 525 (37), 289 (32), 243 (59) | ✓ | Procyanidin trimer B-type | ||
| 5 | 3.5 | 609 | MS2 [609]: 591 (7), 483 (15), 441 (100), 423 (89), 305 (41) | ✓ | ✓ | ✓ | Prodelphinidin dimer B (two units of (epi)GC) |
| 6 | 3.6 | 593 | MS2 [593]: 467 (25), 441 (27), 425 (100), 423 (47), 407 (43), 305 (26), 289 (24) | ✓ | ✓ | ✓ | Prodelphinidin dimer B-type (one unit of (epi)GC) |
| 7 | 4.3 | 593 | MS2 [593]: 467 (53), 441 (79), 425 (35), 423 (100), 305 (74), 287 (8) | ✓ | Prodelphinidin dimer B-type (one unit of (epi)GC) | ||
| 8 | 4.5 | 305 | MS2 [305]: 261 (20), 221 (52), 219 (69), 179 (100), 165 (30), 125 (31) | ✓ | ✓ | ✓ | Gallocatechin |
| 9 | 5.3 | 365 | MS2 [365]: 321 (55), 211 (65), 153 (100) MS3 [365→153]: 109 (100) | ✓ | ✓ | Dihydroxybenzoic acid derivative | |
| 10 | 5.6 | 593 | MS2 [593]: 467 (11), 425 (100), 407 (57), 289 (20) | ✓ | ✓ | ✓ | Prodelphinidin dimer B-type (one unit of (epi)GC) |
| 11 | 5.7 | 761 | MS2 [761]: 609 (98), 591 (54), 465 (22), 441 (52), 423 (100), 305 (34) MS3 [761→609]: 591 (32), 483 (85), 423 (40), 305 (100), 177 (36) | ✓ | ✓ | Prodelphinidin dimer B-type gallate (two units (epi)GC) | |
| 12 | 6.8 | 577 | MS2 [577]: 451 (28), 425 (100), 407 (68), 289 (24), 287 (20), 245 (11) | ✓ | ✓ | Procyanidin dimer B-type | |
| 13 | 7.1 | 305 | MS2 [305]: 261 (21), 221 (45), 219 (75), 179 (100), 165 (27), 125 (42) | ✓ | ✓ | ✓ | Epigallocatechin |
| 14 | 7.5 | 593 | MS2 [593]: 575 (30), 467 (31), 441 (84), 425 (100), 423 (92), 305 (52), 289 (28) | ✓ | ✓ | ✓ | Prodelphinidin dimer B-type (one unit of (epi)GC) |
| 15 | 8.0 | 761 | MS2 [761]: 743 (30), 635 (38), 609 (65), 593 (72), 591 (43), 575 (70), 457 (30), 423 (100) MS3 [761→593]: 575 (26), 405 (19), 423 (100) | ✓ | ✓ | Prodelphinidin dimer B-type gallate (two units (epi)GC) | |
| 16 | 8.9 | 289 | MS2 [289]: 245 (100), 205 (34), 203 (15), 179 (11), 151 (9) | ✓ | ✓ | ✓ | Catechin |
| 17 | 10.6 | 431 | MS2 [431]: 385 (100), 223 (10), 153 (9) MS3 [431→385]: 223 (100), 205 (39), 161 (52), 153 (73), 138 (9) | ✓ | ✓ | ✓ | Roseoside (formate adduct) |
| 18 | 10.8 | 577 | MS2 [577]: 451 (10), 425 (100), 407 (95), 289 (26), 287 (8) | ✓ | ✓ | ✓ | Procyanidin dimer B-type |
| 19 | 12.0 | 289 | MS2 [289]: 245 (100), 205 (33), 203 (15), 179 (13) | ✓ | ✓ | ✓ | Epicatechin |
| 20 | 14.2 | 865 | MS2 [865]: 713 (33), 695 (100), 577 (45), 575 (36), 451 (33), 425 (30), 407 (53), 289 (41) | ✓ | ✓ | ✓ | Procyanidin trimer B-type |
| 21 | 14.3 | 593 | MS2 [593]: 575 (14), 467 (23), 441 (30), 425 (100), 423 (58), 407 (51), 305 (27) | ✓ | ✓ | ✓ | Prodelphinidin dimer B-type (one unit of (epi)GC) |
| 22 | 16.7 | 479 | MS2 [479]: 317 (79), 316 (100), 179 (9) MS3 [479→316]: 271 (100), 179 (40), 151 (17) | ✓ | ✓ | ✓ | Myricetin-O-hexoside |
| 23 | 17.1 | 479 | MS2 [479]: 317 (99), 316 (100), 179 (12) MS3 [479→316]: 271 (79), 179 (100), 151 (27) | ✓ | ✓ | Myricetin-O-hexoside | |
| 24 | 18.0 | 449 | MS2 [449]: 317 (100), 316 (94) MS3 [449→317]: 271 (100), 179 (86), 151 (41) | ✓ | ✓ | ✓ | Myricetin-O-pentoside |
| 25 | 19.3 | 449 | MS2 [449]: 317 (40), 316 (100) MS3 [449→316]: 271 (100), 179 (13), 151 (22) | ✓ | ✓ | Myricetin-O-pentoside | |
| 26 | 19.8 | 463 | MS2 [463]: 317 (70), 316 (100) MS3 [463→316]: 217 (100), 179 (29), 151 (17) | ✓ | ✓ | ✓ | Myricetin-O-deoxyhexoside |
| 27 | 20.5 | 463 | MS2 [463]: 301 (100) MS3 [463→301]: 179 (100), 151 (88) | ✓ | ✓ | ✓ | Quercetin-O-hexoside |
| 28 | 22.3 | 415 | MS2 [415]: 369 (21), 225 (21), 179 (100), 161 (10) | ✓ | ✓ | ✓ | Unknown |
| 29 | 23.2 | 433 | MS2 [433]: 301 (100) MS3 [433→301]: 271 (56), 255 (100), 179 (75), 151 (33) | ✓ | ✓ | ✓ | Quercetin-O-pentoside |
| 30 | 24.4 | 477 | MS2 [477]: 331 (100), 316 (34) MS3 [477→331]: 316 (100), 271 (2) MS4 [477→331→316]: 316 (100), 287 (30), 271 (50), 179 (43), 136 (60) | ✓ | ✓ | ✓ | Mearnsetin-O-deoxyhexoside |
| 31 | 24.7 | 447 | MS2 [447]: 301 (100) MS3 [447→301]: 179 (77), 151 (100) | ✓ | ✓ | ✓ | Quercetin-O-deoxyhexoside |
| 32 | 26.8 | 317 | MS2 [317]: 271 (5), 179 (100), 151 (46) | ✓ | ✓ | Myricetin | |
| 33 | 28.7 | 431 | MS2 [431]: 285 (100), 255 (8) MS3 [431→285]: 257 (55), 255 (100), 227 (24), 241 (17) | ✓ | ✓ | ✓ | Kaempferol-O-deoxyhexoside |
| 34 | 29.4 | 461 | MS2 [461]: 315 (100) MS3 [461→315]: 300 (100), 271 (6) | ✓ | ✓ | ✓ | Isorhamnetin-O-deoxyhexoside |
| No. | Assigned Identification | H2O | EA | MeOH |
|---|---|---|---|---|
| Proanthocyanidins | ||||
| 3 | Prodelphinidin dimer | 14.1 ± 0.7 | 1.13 ± 0.08 | -- |
| 4 | Procyanidin trimer | 2.4 ± 0.2 | -- | -- |
| 5 + 6 | Prodelphinidin dimers | 3.2 ± 0.2 | -- | 2.2 ± 0.1 |
| 10 + 11 | Prodelphinidin dimers | 3.0 ± 0.2 | 0.79 ± 0.05 | 0.78 ± 0.05 |
| 12 | Procyanidin dimer | 2.2 ± 0.1 | 0.17 ± 0.01 | -- |
| 14 | Prodelphinidin dimer | 1.4 ± 0.07 | 0.24 ± 0.02 | 0.36 ± 0.02 |
| 20 + 21 | Procyanidin trimer + prodelphinidin dimer | 1.38 ± 0.08 | -- | 0.59 ± 0.03 |
| Total | 27.7 ± 0.8 | 2.3 ± 0.1 | 3.9 ± 0.1 | |
| Flavonoids | ||||
| 8 | Gallocatechin | 4.4 ± 0.2 | 0.133 ± 0.009 | -- |
| 13 | Epigallocatechin | 4.3 ± 0.3 | 0.21 ± 0.01 | 1.17 ± 0.06 |
| 16 | Catechin | 0.066 ± 0.004 | 1.5 ± 0.1 | 2.2 ± 0.1 |
| 19 | Epicatechin | 7.7 ± 0.3 | 0.72 ± 0.05 | 4.1 ± 0.2 |
| 22 | Myricetin-O-hexoside | 2.1 ± 0.1 | 0.69 ± 0.04 | 2.5 ± 0.1 |
| 23 | Myricetin-O-hexoside | 0.47 ± 0.03 | -- | 0.28 ± 0.02 |
| 24 | Myricetin-O-pentoside | 0.135 ± 0.008 | -- | -- |
| 25 | Myricetin-O-pentoside | 0.121 ± 0.008 | -- | -- |
| 26 | Myricetin-O-deoxyhexoside | 13.4 ± 0.6 | 16.2 ± 0.8 | 12.2 ± 0.6 |
| 27 | Quercetin-O-hexoside | 0.21 ± 0.01 | 0.24 ± 0.02 | 0.27 ± 0.02 |
| 29 | Quercetin-O-pentoside | 0.0073 ± 0.0005 | 0.043 ± 0.003 | 0.023 ± 0.002 |
| 30 + 31 | Mearnsetin + quercetin glycosides | 3.8 ± 0.2 | 13.5 ± 0.8 | 4.4 ± 0.3 |
| 32 | Myricetin | -- | 0.55 ± 0.03 | -- |
| 33 | Kaempferol-O-deoxyhexoside | 0.137 ± 0.008 | 0.24 ± 0.02 | 0.130 ± 0.008 |
| 34 | Isorhamnetin-O-deoxyhexoside | 0.140 ± 0.008 | 0.41 ± 0.03 | 0.15 ± 0.01 |
| Total | 37.0 ± 0.8 | 34 ± 1 | 27.4 ± 0.8 | |
| Others | ||||
| 9 | Dihydroxybenzoic acid derivative | 0.29 ± 0.02 | -- | -- |
| TIPC | 65 ± 1 | 36 ± 1 | 31.3 ± 0.8 |
| Samples | DPPH (mg TE/g) | ABTS (mg TE/g) | FRAP (mg TE/g) | CUPRAC (mg TE/g) | Metal Chelating (mg EDTAE/g) | Phosphomolybdenum (mmol TE/g) |
|---|---|---|---|---|---|---|
| TP-Aq | 973.40 ± 5.65 a | 2030.37 ± 40.83 a | 1461.39 ± 5.95 a | 1940.99 ± 20.95 a | 4.75 ± 0.70 a | 8.37 ± 0.23 a |
| TP-EA | 186.80 ± 0.18 c | 482.13 ± 0.96 c | 357.69 ± 7.77 c | 548.62 ± 5.16 d | 4.88 ± 0.32 a | 3.64 ± 0.26 c |
| TP-MEOH | 399.47 ± 0.73 b | 973.23 ± 2.25 b | 835.27 ± 23.49 b | 1161.15 ± 10.47 c | 2.12 ± 0.26 b | 5.91 ± 0.41 b |
| Samples | AChE Inhibition (mg GALAE/g) | BChE Inhibition (mg GALAE/g) | Tyrosinase Inhibition (mg KAE/g) | Amylase Inhibition (mmol ACAE/g) | Glucosidase Inhibition (mmol ACAE/g) |
|---|---|---|---|---|---|
| TP-Aq | 3.49 ± 0.06 c | 2.83 ± 0.42 c | 124.27 ± 1.77 c | 1.37 ± 0.04 a | 1.82 ± 0.01 a |
| TP-EA | 3.98 ± 0.10 b | 9.76 ± 0.14 a | 132.77 ± 0.90 b | 0.72 ± 0.07 c | 1.70 ± 0.03 b |
| TP-MEOH | 4.87 ± 0.04 a | 5.80 ± 0.12 b | 155.30 ± 0.55 a | 0.92 ± 0.02 b | 1.80 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suroowan, S.; Llorent-Martínez, E.J.; Zengin, G.; Buskaran, K.; Fakurazi, S.; Abdalla, A.N.; Khalid, A.; Le Van, B.; Mahomoodally, M.F. Unveiling the Antioxidant, Clinical Enzyme Inhibitory Properties and Cytotoxic Potential of Tambourissa peltata Baker—An Understudied Endemic Plant. Molecules 2023, 28, 599. https://doi.org/10.3390/molecules28020599
Suroowan S, Llorent-Martínez EJ, Zengin G, Buskaran K, Fakurazi S, Abdalla AN, Khalid A, Le Van B, Mahomoodally MF. Unveiling the Antioxidant, Clinical Enzyme Inhibitory Properties and Cytotoxic Potential of Tambourissa peltata Baker—An Understudied Endemic Plant. Molecules. 2023; 28(2):599. https://doi.org/10.3390/molecules28020599
Chicago/Turabian StyleSuroowan, Shanoo, Eulogio J. Llorent-Martínez, Gokhan Zengin, Kalaivani Buskaran, Sharida Fakurazi, Ashraf N. Abdalla, Asaad Khalid, Bao Le Van, and Mohamad Fawzi Mahomoodally. 2023. "Unveiling the Antioxidant, Clinical Enzyme Inhibitory Properties and Cytotoxic Potential of Tambourissa peltata Baker—An Understudied Endemic Plant" Molecules 28, no. 2: 599. https://doi.org/10.3390/molecules28020599
APA StyleSuroowan, S., Llorent-Martínez, E. J., Zengin, G., Buskaran, K., Fakurazi, S., Abdalla, A. N., Khalid, A., Le Van, B., & Mahomoodally, M. F. (2023). Unveiling the Antioxidant, Clinical Enzyme Inhibitory Properties and Cytotoxic Potential of Tambourissa peltata Baker—An Understudied Endemic Plant. Molecules, 28(2), 599. https://doi.org/10.3390/molecules28020599











