Effective Removal of Metal ion and Organic Compounds by Non-Functionalized rGO
Abstract
:1. Introduction
2. Results and Discussion
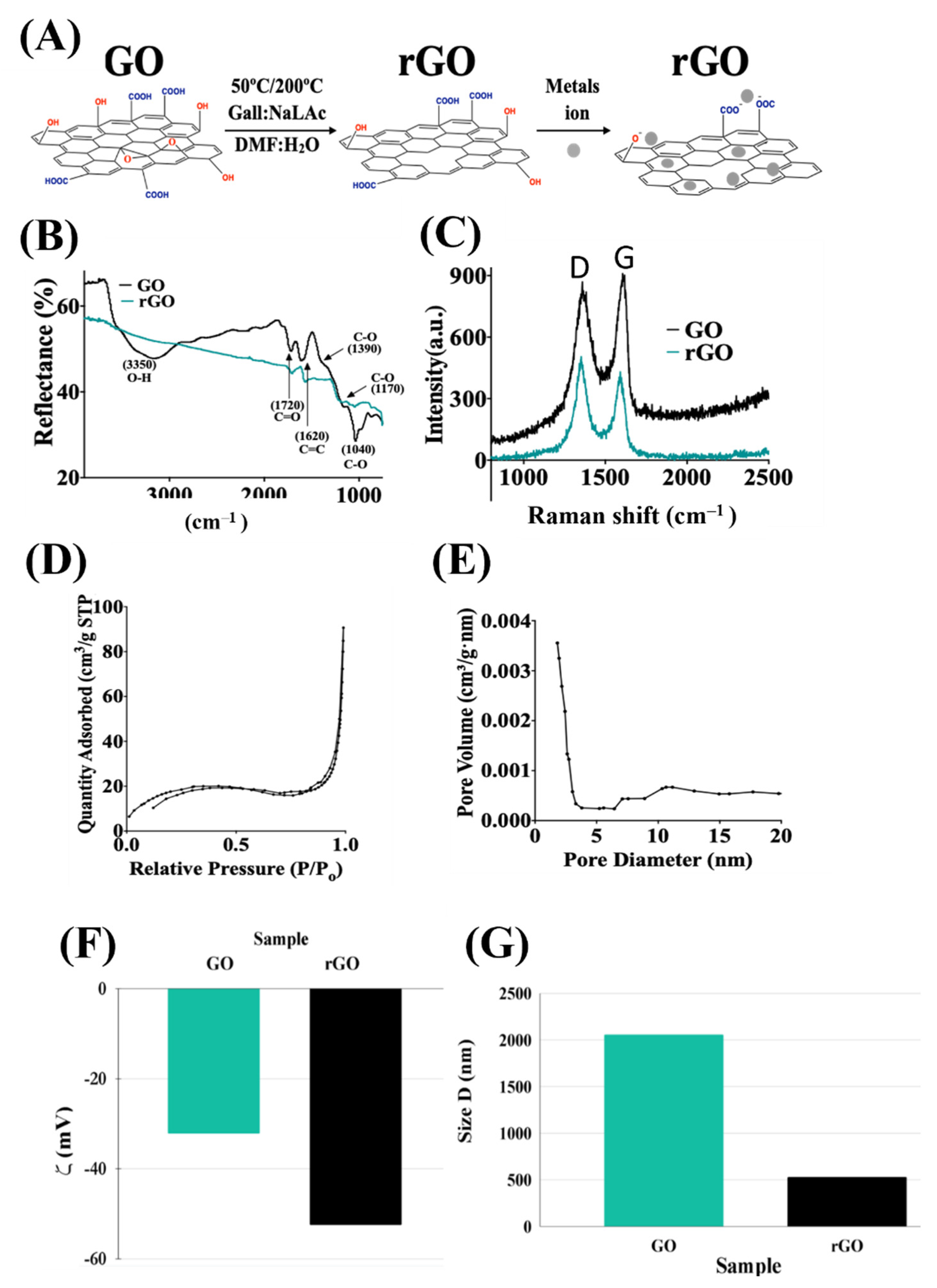
2.1. rGO Properties
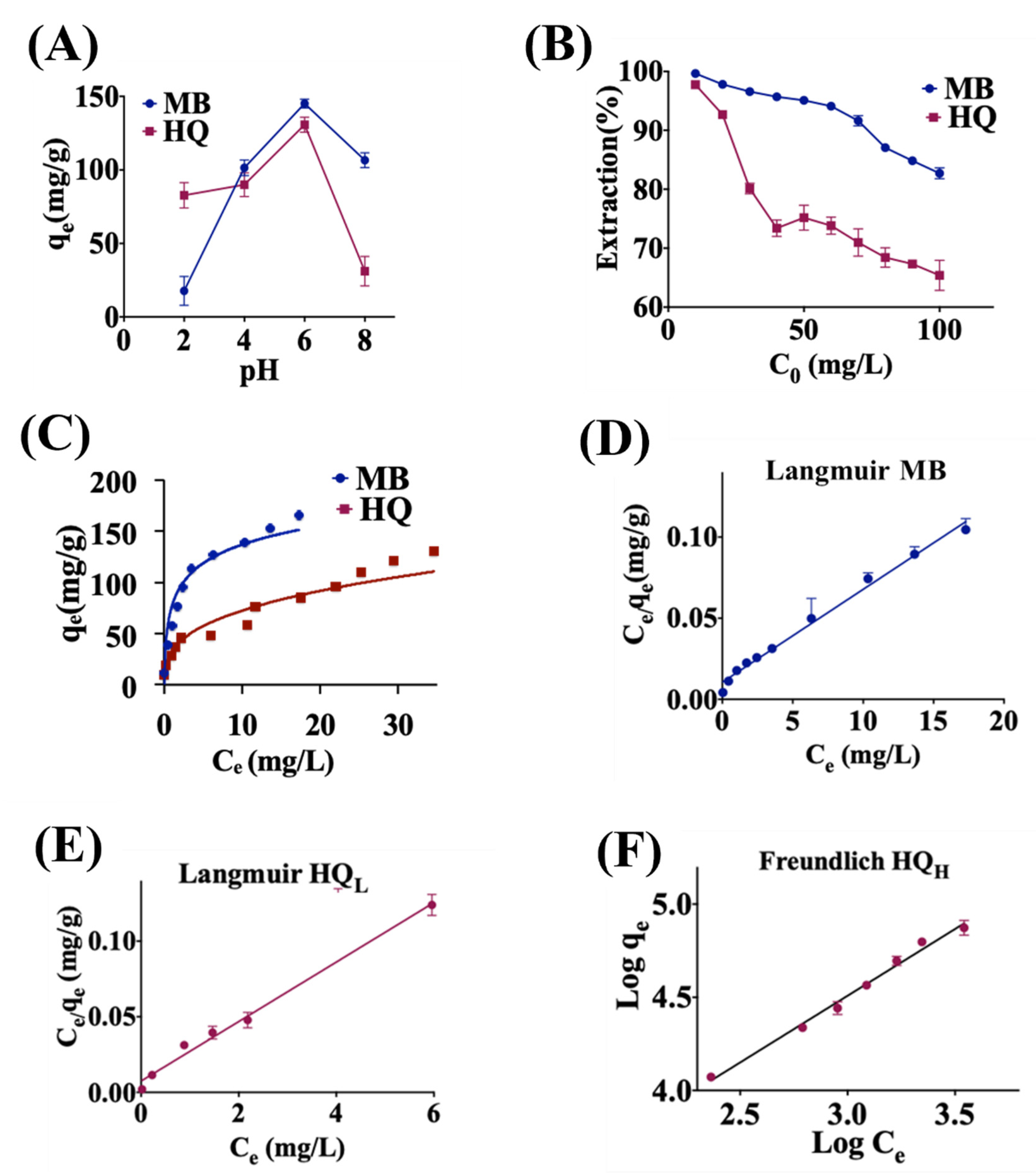
2.2. Adsorption of Metal Ions
2.3. Adsorption of Methylene Blue (MB) and Hydroquinone (HQ)
2.4. Cleaning Waste Water from Paper Photography Processing Operation
3. Experimental Section
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bellinger, D.C. Lead Contamination in Flint—An Abject Failure to Protect Public Health. N. Engl. J. Med. 2016, 374, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.V.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Gaur, N.; Bhardwaj, V.; Rathi, M. Heavy Metals and Their Effects. JPRBioMedRx An Int. J. 2013, 1, 928–933. [Google Scholar]
- Beckett, W.S. Human health effects of metals. Epidemiology 1998, 9, S22. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mainali, B.; Pham, T.T.N.; Ngo, H.H.; Guo, W. Maximum allowable values of the heavy metals in recycled water for household laundry. Sci. Total Environ. 2013, 452–453, 427–432. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, W.; Dang, Z.; Hu, Q.; Wu, F.; Feng, C.; Zhao, X.; Meng, W.; Xing, B.; Giesy, J.P. Refocusing on Nonpriority Toxic Metals in the Aquatic Environment in China. Environ. Sci. Technol. 2017, 51, 3117–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradham, K.; Wentsel, R. Scientific Issues in the U.S. EPA Framework for Metals Risk Assessment. J. Toxicol. Environ. Health Part A 2010, 73, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Nemery, B. Metal Toxicity and the Respiratory Tract. J. Occup. Environ. Med. 1990, 32, 1157–1158. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Nebert, D.W.; Woods, J.M.; Barchowsky, A.; Atchison, W.D. The Vascular System as a Target of Metal Toxicity. Toxicol. Sci. 2008, 102, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Heavy Metal Poisoning and Cardiovascular Disease. J. Toxicol. 2011, 2011, 870125. [Google Scholar] [CrossRef] [PubMed]
- Akther, J.; Nabi, A.H.M.N.; Ebihara, A. Heavy metals as environmental risk factors for cardiovascular diseases: From the perspective of the renin angiotensin aldosterone system and oxidative stress. Rev. Agric. Sci. 2019, 7, 68–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, O.; Jacquillet, G.; Tauc, M.; Cougnon, M.; Poujeol, P. Effect of Heavy Metals on, and Handling by, the Kidney. Nephron Physiol. 2005, 99, p105–p110. [Google Scholar] [CrossRef] [PubMed]
- de Burbure, C.; Buchet, J.-P.; Leroyer, A.; Nisse, C.; Haguenoer, J.-M.; Mutti, A.; Smerhovský, Z.; Cikrt, M.; Trzcinka-Ochocka, M.; Razniewska, G.; et al. Renal and Neurologic Effects of Cadmium, Lead, Mercury, and Arsenic in Children: Evidence of Early Effects and Multiple Interactions at Environmental Exposure Levels. Environ. Health Perspect. 2006, 114, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef]
- Sabra, S.; Malmqvist, E.; Saborit, A.; Gratacós, E.; Roig, M.D.G. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PLoS ONE 2017, 12, e0185645. [Google Scholar] [CrossRef]
- Wai, K.M.; Mar, O.; Kosaka, S.; Umemura, M.; Watanabe, C. Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int. J. Environ. Res. Public Health 2017, 14, 1339. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Gillman, P.K. CNS toxicity involving methylene blue: The exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity. J. Psychopharmacol. 2011, 25, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Schwiebert, C.; Irving, C.; Gillman, P.K. Small doses of methylene blue, previously considered safe, can precipitate serotonin toxicity. Anaesthesia 2009, 64, 924. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.C.; Bernard, L.G.; O’Donoghue, J.L.; English, J.C. Hydroquinone: Acute and subchronic toxicity studies with emphasis on neurobehavioral and nephrotoxic effects. Food Chem. Toxicol. 2007, 45, 70–78. [Google Scholar] [CrossRef]
- Kooyers, T.; Westerhof, W. Toxicology and health risks of hydroquinone in skin lightening formulations. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 777–780. [Google Scholar] [CrossRef]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental Pollution, Toxicity, and Microbial Answers. BioMed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- de Oliveira, G.A.R.; Leme, D.M.; de Lapuente, J.; Brito, L.B.; Porredón, C.; de Brito Rodrigues, L.; Brull, N.; Serret, J.T.; Borràs, M.; Disner, G.R.; et al. A test battery for assessing the ecotoxic effects of textile dyes. Chem. Biol. Interac. 2018, 291, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Alzaydien, A.S. Adsorption of Methylene Blue from Aqueous Solution onto a Low-Cost Natural Jordanian Tripoli. Am. J. Environ. Sci. 2009, 5, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Guerra, R. Ecotoxicological and chemical evaluation of phenolic compounds in industrial effluents. Chemosphere 2001, 44, 1737–1747. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Borbély, G.; Nagy, E. Removal of zinc and nickel ions by complexation–membrane filtration process from industrial wastewater. Desalination 2009, 240, 218–226. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Haralambous, K.J. Industrial wastewater pre-treatment for heavy metal reduction by employing a sorbent-assisted ultrafiltration system. Chemosphere 2011, 82, 557–564. [Google Scholar] [CrossRef]
- Ozaki, H.; Sharma, K.; Saktaywin, W. Performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) for separating heavy metal: Effects of interference parameters. Desalination 2002, 144, 287–294. [Google Scholar] [CrossRef]
- Özverdi, A.; Erdem, M. Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J. Hazard. Mater. 2006, 137, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalination 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Abu-Nada, A.; McKay, G.; Abdala, A. Recent Advances in Applications of Hybrid Graphene Materials for Metals Removal from Wastewater. Nanomaterials 2020, 10, 595. [Google Scholar] [CrossRef] [Green Version]
- Ince, M.; Ince, O.K. An Overview of Adsorption Technique for Heavy Metal Removal from Water/Wastewater: A Critical Review. Int. J. Pure Appl. Sci. 2017, 3, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Arora, R. Adsorption of Heavy Metals—A Review. Mater. Today: Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Alene, A.N. A comparative study of acidic, basic, and reactive dyes adsorption from aqueous solution onto kaolin adsorbent: Effect of operating parameters, isotherms, kinetics, and thermodynamics. Emerg. Contam. 2022, 8, 59–74. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Kashi, E.; Yaseen, Z.M.; Alothman, Z.A.; Khan, M.R. Cross-Linked Chitosan-Glyoxal/Kaolin Clay Composite: Parametric Optimization for Color Removal and COD Reduction of Remazol Brilliant Blue R Dye. J. Polym. Environ. 2022, 30, 164–178. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef] [Green Version]
- Kamali, M.; Esmaeili, H.; Tamjidi, S. Synthesis of Zeolite Clay/Fe-Al Hydrotalcite Composite as a Reusable Adsorbent for Adsorption/Desorption of Cationic Dyes. Arab. J. Sci. Eng. 2022, 47, 6651–6665. [Google Scholar] [CrossRef]
- Loutfi, M.; Mariouch, R.; Mariouch, I.; Belfaquir, M.; ElYoubi, M. Adsorption of methylene blue dye from aqueous solutions onto natural clay: Equilibrium and kinetic studies. Mater. Today Proc. 2022; in press, corrected proof. [Google Scholar] [CrossRef]
- Rahimi, A.A.; Alihosseini, F. Application of Dye Saturated Clay Adsorbent from Dyeing Wastewater as Textile Printing Pigment. J. Chem. Technol. Biotechnol. 2022, 97, 3152–3162. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.M.; Jalab, R.; Benamor, A.; Nasser, M.S.; Ba-Abbad, M.M.; El-Naas, M.; Mohammad, A.W. Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. J. Mol. Liq. 2020, 301, 112335. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-Ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostructure Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’Homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2009, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A review on the mechanical and thermal properties of graphene and graphene-based polymer nanocomposites: Understanding of modelling and MD simulation. Mol. Simul. 2020, 46, 136–154. [Google Scholar] [CrossRef]
- Lee, G.-H.; Cooper, R.C.; An, S.J.; Lee, S.; van der Zande, A.; Petrone, N.; Hammerberg, A.G.; Lee, C.; Crawford, B.; Oliver, W.; et al. High-Strength Chemical-Vapor–Deposited Graphene and Grain Boundaries. Science 2013, 340, 1073–1076. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Tian, Z.; Simon, G.P.; Li, D. Scalable production of graphene via wet chemistry: Progress and challenges. Mater. Today 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Parvez, K.; Yang, S.; Feng, X.; Müllen, K. Exfoliation of graphene via wet chemical routes. Synth. Met. 2015, 210, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Jiang, Y.; Biswas, P.; Fortner, J.D. A review of recent developments in graphene-enabled membranes for water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 915–922. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to improve the adsorption properties of graphene-based adsorbent towards heavy metal ions and their compound pollutants: A review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hou, Q.; Huang, G.; Fan, Q. Removal of heavy metals in aquatic environment by graphene oxide composites: A review. Environ. Sci. Pollut. Res. 2020, 27, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.M.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Gohel, V.D.; Rajput, A.; Gahlot, S.; Kulshrestha, V. Removal of Toxic Metal Ions From Potable Water by Graphene Oxide Composites. Macromol. Symp. 2017, 376, 1700050. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; Sobrinho, M.A.D.M.; Carvalho, M.N.; Ghislandi, M.G. State of the art: Synthesis and characterization of functionalized graphene nanomaterials. Nano Express 2020, 1, 022002. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; Ghislandi, M.G.; Carvalho, M.N.; Sobrinho, M.A.D.M. One step forward: How can functionalization enhance the adsorptive properties of graphene towards metallic ions and dyes? Environ. Res. 2020, 184, 109362. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Pang, L.; Li, M.; Song, X.; Wen, J.; Zhao, H. Preparation of Reduced Graphene Oxide/Poly(acrylamide) Nanocomposite and Its Adsorption of Pb(II) and Methylene Blue. Langmuir 2013, 29, 10727–10736. [Google Scholar] [CrossRef]
- Awad, F.S.; AbouZeid, K.M.; El-Maaty, W.M.A.; El-Wakil, A.M.; El-Shall, M.S. Efficient Removal of Heavy Metals from Polluted Water with High Selectivity for Mercury(II) by 2-Imino-4-thiobiuret–Partially Reduced Graphene Oxide (IT-PRGO). ACS Appl. Mater. Interfaces 2017, 9, 34230–34242. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.; He, S.; Huang, F.; Peng, Z. Adsorption Behaviour of Reduced Graphene Oxide for Removal of Heavy Metal Ions. Asian J. Chem. 2014, 26, 4901–4906. [Google Scholar] [CrossRef]
- Xu, M.; Chai, J.; Hu, N.; Huang, D.; Wang, Y.; Huang, X.; Wei, H.; Yang, Z.; Zhang, Y. Facile synthesis of soluble functional graphene by reduction of graphene oxide via acetylacetone and its adsorption of heavy metal ions. Nanotechnology 2014, 25, 395602. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Dong, Z.; Hao, X.; Yao, Y.; Guo, S. Preparation of Reduced Graphene Oxide Aerogel and Its Adsorption for Pb(II). ACS Omega 2020, 5, 9903–9911. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jhi, S.-H.; Lim, S.; Park, N. Effect of vacancy defects in graphene on metal anchoring and hydrogen adsorption. Appl. Phys. Lett. 2009, 94, 173102. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Yang, Z.; Dai, X. Trapping of metal atoms in the defects on graphene. J. Chem. Phys. 2011, 135, 224704. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Caffrey, N.M.; Iakimov, T.; Khranovskyy, V.; Abrikosov, I.A.; Yakimova, R. On the interaction of toxic Heavy Metals (Cd, Hg, Pb) with graphene quantum dots and infinite graphene. Sci. Rep. 2017, 7, 3934. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’Ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Andrijanto, E.; Shoelarta, S.; Subiyanto, G.; Rifki, S. Facile synthesis of graphene from graphite using ascorbic acid as reducing agent. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1725. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xiao, G.; Chen, C.; Li, R.; Yan, D. Superior dispersions of reduced graphene oxide synthesized by using gallic acid as a reductant and stabilizer. J. Mater. Chem. A 2013, 1, 1481–1487. [Google Scholar] [CrossRef]
- Aunkor, M.T.H.; Mahbubul, I.M.; Saidur, R.; Metselaar, H.S.C. The green reduction of graphene oxide. RSC Adv. 2016, 6, 27807–27828. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lu, M.; Wang, H.; Pei, Y.; Rao, H.; Du, X. Three-dimensional graphene aerogels–mesoporous silica frameworks for superior adsorption capability of phenols. Sep. Purif. Technol. 2015, 153, 7–13. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.S.; Derbalah, A.S.; Masoud, M.S. Fabrication and characterization of graphene oxide–titanium dioxide nanocomposite for degradation of some toxic insecticides. J. Ind. Eng. Chem. 2019, 69, 315–323. [Google Scholar] [CrossRef]
- Chen, S.; Chen, D.; Wang, W.; Quan, H.; Luo, X.; Guo, L. rGO-stabilized MnO/N-doped carbon nanofibers for efficient removal of Pb(II) ion and catalytic degradation of methylene blue. J. Mater. Sci. 2017, 52, 5117–5132. [Google Scholar] [CrossRef]
- Prasad, C.; Murthy, P.K.; Krishna, R.H.; Rao, R.S.; Suneetha, V.; Venkateswarlu, P. Bio-inspired green synthesis of RGO/Fe3O4 magnetic nanoparticles using Murrayakoenigii leaves extract and its application for removal of Pb(II) from aqueous solution. J. Environ. Chem. Eng. 2017, 5, 4374–4380. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Li, Y.-H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.; Fang, Y. Using graphene oxide as a superior adsorbent for the highly efficient immobilization of Cu(II) from aqueous solution. J. Mol. Liq. 2014, 199, 237–243. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H. Adsorption of zinc(II) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145. [Google Scholar] [CrossRef]
- Shanmugavalli, R.; Shabudeen, P.S.S.; Venckatesh, R.; Kadirvelu, K.; Madhavakrishnan, S.; Pattabhi, S. Uptake of Pb(II) ion From Aqueous Solution Using Silk Cotton Hull Carbon: An Agricultural Waste Biomass. E-Journal Chem. 2006, 3, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Sar, P.; Kazy, S.; Asthana, R.; Singh, S. Metal adsorption and desorption by lyophilized Pseudomonas aeruginosa. Int. Biodeterior. Biodegradation 1999, 44, 101–110. [Google Scholar] [CrossRef]
- Namasivayam, C.; Ranganathan, K. Removal of Cd(II) from wastewater by adsorption on “waste” Fe(III)Cr(III) hydroxide. Water Res. 1995, 29, 1737–1744. [Google Scholar] [CrossRef]
- Rao, M.M.; Ramana, D.; Seshaiah, K.; Wang, M.; Chien, S.C. Removal of some metal ions by activated carbon prepared from Phaseolus aureus hulls. J. Hazard. Mater. 2009, 166, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Ma, Y.; Zhou, W.; Guo, L. One-Pot Hydrothermal Synthesis of RGO-Fe3O4 Hybrid Nanocomposite for Removal of Pb (II) via Magnetic Separation. Chem. Res. Chin. Univ. 2015, 31, 508–513. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Chang, Y.-Y.; Karri, R.R. Process optimization and adsorption modeling of Pb(II) on nickel ferrite-reduced graphene oxide nano-composite. J. Mol. Liq. 2018, 250, 202–211. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Kim, I.-S.; Ha, J.-H.; Chang, Y.-Y.; Koduru, J.R.; Yang, J.-K. Enhanced Adsorption Removal of Pb(II) and Cr(III) by Using Nickel Ferrite-Reduced Graphene Oxide Nanocomposite. Metals 2017, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Ahmad, A.; Khan, S.; Ahmad, S.; Khan, I.; Zada, S.; Fu, P. Algal extracts based biogenic synthesis of reduced graphene oxides (rGO) with enhanced heavy metals adsorption capability. J. Ind. Eng. Chem. 2019, 72, 117–124. [Google Scholar] [CrossRef]
- Najafabadi, H.H.; Irani, M.; Rad, L.R.; Haratameh, A.H.; Haririan, I. Removal of Cu2+, Pb2+ and Cr6+ from Aqueous Solutions Using a Chitosan/Graphene Oxide Composite Nanofibrous Adsorbent. Rsc Adv. 2015, 5, 16532–16539. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Bao, C.; Jia, Q.; Xiao, P.; Liu, X.; Zhang, Q. Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au(III) and Pd(II). Talanta 2012, 93, 350–357. [Google Scholar] [CrossRef]
- Eslami, P.A.; Kamboh, M.A.; Nodeh, H.R.; Ibrahim, W.A.W. Equilibrium and kinetic study of novel methyltrimethoxysilane magnetic titanium dioxide nanocomposite for methylene blue adsorption from aqueous media. Appl. Organomet. Chem. 2018, 32, e4331. [Google Scholar] [CrossRef]
- Özdemir, Y.; Doğan, M.; Alkan, M. Adsorption of cationic dyes from aqueous solutions by sepiolite. Microporous Mesoporous Mater. 2006, 96, 419–427. [Google Scholar] [CrossRef]
- Blanco-Martínez, D.; Giraldo, L.; Moreno-Piraján, J. Effect of the pH in the adsorption and in the immersion enthalpy of monohydroxylated phenols from aqueous solutions on activated carbons. J. Hazard. Mater. 2009, 169, 291–296. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Adsorption of Phenolic Compounds by Carbon Nanotubes: Role of Aromaticity and Substitution of Hydroxyl Groups. Environ. Sci. Technol. 2008, 42, 7254–7259. [Google Scholar] [CrossRef]
- Suresh, S.; Srivastava, V.C.; Mishra, I.M. Adsorption of Hydroquinone in Aqueous Solution by Granulated Activated Carbon. J. Environ. Eng. 2011, 137, 1145–1157. [Google Scholar] [CrossRef]
- Firdaus, R.M.; Rosli, N.I.; Ghanbaja, J.; Vigolo, B.; Mohamed, A.R. Enhanced adsorption of methylene blue on chemically modified graphene nanoplatelets thanks to favorable interactions. J. Nanoparticle Res. 2019, 21, 257. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; Xia, L. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr. Polym. 2013, 95, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, A.; Dan, S.; Hashemipour, H. Preparation and optimization of novel graphene oxide and adsorption isotherm study of methylene blue. Arab. J. Chem. 2021, 14, 103003. [Google Scholar] [CrossRef]
- Yan, H.; Tao, X.; Yang, Z.; Li, K.; Yang, H.; Li, A.; Cheng, R. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J. Hazard. Mater. 2014, 268, 191–198. [Google Scholar] [CrossRef]
- Yıldız, N.; Gönülşen, R.; Koyuncu, H.; Çalımlı, A. Adsorption of Benzoic Acid and Hydroquinone by Organically Modified Bentonites. Colloids Surf. A Physicochem. Eng. Asp. 2005, 260, 87–94. [Google Scholar] [CrossRef]
- Tyagi, A.; Das, S.; Srivastava, V.C. Removal of toxic hydroquinone: Comparative studies on use of iron impregnated granular activated carbon as an adsorbent and catalyst. Environ. Eng. Res. 2019, 24, 474–483. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S.; Srivastava, V.C.; Mishra, I.M. Isotherm, Thermodynamics, Desorption, and Disposal Study for the Adsorption of Catechol and Resorcinol onto Granular Activated Carbon. J. Chem. Eng. Data 2011, 56, 811–818. [Google Scholar] [CrossRef]
- Shengli, S.; Junping, L.; Qi, L.; Fangru, N.; Jia, F.; Shulian, X. Optimized preparation of Phragmites australis activated carbon using the Box-Behnken method and desirability function to remove hydroquinone. Ecotoxicol. Environ. Saf. 2018, 165, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, J.; Xiao, Y.; Zhao, Z.; Zhang, M.; Wang, L.; Zhang, X.; Zhou, C. High-efficiency adsorption of and competition between phenol and hydroquinone in aqueous solution on highly cationic amino-poly(vinylamine)-functionalized GO-(o-MWCNTs) magnetic nanohybrids. Chem. Eng. J. 2020, 389, 124223. [Google Scholar] [CrossRef]
- Li, L.; Fan, L.; Sun, M.; Qiu, H.; Li, X.; Duan, H.; Luo, C. Adsorbent for hydroquinone removal based on graphene oxide functionalized with magnetic cyclodextrin–chitosan. Int. J. Biol. Macromol. 2013, 58, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Jiang, J. Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene–carbon nanotube hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Arias, F.A.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Gomez, C.V.; Arias, M.; Caputi, L.S. The Adsorption of Methylene Blue on Eco-Friendly Reduced Graphene Oxide. Nanomaterials 2020, 10, 681. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Zhang, Y.; Ma, L.; Zhang, H.; Huang, H. Synthesis and Response of Pineapple Peel Carboxymethyl Cellulose-g-Poly (Acrylic Acid-Co-Acrylamide)/Graphene Oxide Hydrogels. Carbohydr. Polym. 2019, 215, 366–376. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Fadillah, G.; Saleh, T.A.; Wahyuningsih, S.; Putri, E.N.K.; Febrianastuti, S. Electrochemical removal of methylene blue using alginate-modified graphene adsorbents. Chem. Eng. J. 2019, 378, 122140. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Elgarhy, G.S.; El-Subruiti, G.M.; Omer, A.M. Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int. J. Biol. Macromol. 2020, 154, 307–318. [Google Scholar] [CrossRef] [PubMed]


| Metal | qmax (mg/g) | KL (mg/L) | RL | R2 |
|---|---|---|---|---|
| Mn(II) | 57.80 | 0.0232 | 0.1773 | 0.9984 |
| Cu(II) | 78.12 | 0.0505 | 0.0900 | 0.9965 |
| Pb(II) | 243.90 | 0.0086 | 0.3676 | 0.9942 |
| Ag(I) | 63.29 | 0.0593 | 0.0777 | 0.9970 |
| Fe(II) | 80.64 | 0.0302 | 0.1420 | 0.9983 |
| Material | Metal ion | qmax (mg/g) | Reference |
|---|---|---|---|
| RGO/Fe3O4 Magnetic Nanoparticles [86] | Pb (II) | 30 | [86] |
| RGO-Fe3O4 Hybrid Nanocomposite [97] | Pb (II) | 50 | [97] |
| Nickel Ferrite-Reduced Graphene Oxide Nanocomposite [98,99] | Pb (II) | 120−150 | [98,99] |
| Reduced Graphene Oxide aerogel [73] | Pb (II) | 58.04 | [73] |
| 2-Imino-4-Thiobiuret-Partially Reduced Graphene Oxide (IT-PRGO) [70] | Pb (II) | 102.2 | [70] |
| Cu (II) | 37.9 | ||
| rGO from algal extracts [100] | Pb (II) | 95 | [100] |
| Cu (II) | 90 | ||
| rGO (with ethylenediamine) [71] | Pb (II) | 413.34 | [71] |
| Cu (II) | 55.34 | ||
| Mn (II) | 42.46 | ||
| rGO by by Gallic Acid and Sodium Ascorbate | Pb (II) | 243.90 | Present study |
| Cu (II) | 78.12 | ||
| Mn (II) | 57.83 | ||
| Ag (I) | 63.29 | ||
| Fe (II) | 80.64 | ||
| Chitosan/Graphene Oxide Composite [102] | Au (III) | 1076.65 | [102] |
| Pd (II) | 216.92 | ||
| Chitosan/Graphene Oxide Composite Nanofibrous [101] | Pb (II) | 461.3 | [101] |
| Cu (II) | 423.8 | ||
| Cr (VI) | 310 |
| Methylene Blue | ||||
|---|---|---|---|---|
| Langmuir | qmax (mg/g) | KL (mg/L) | RL | R2 |
| MB | 238.45 | 0.0164 | 0.3787 | 0.9878 |
| Freundlich | Kf | 1/nF | nF | R2 |
| MB | 1.381 | 2.19 | 0.4556 | 0.973 |
| Hydroquinone at low (HQL) and High (HQH) Ce | ||||
| Langmuir | qmax (mg/g) | KL (mg/L) | RL | R2 |
| HQL | 51.020 | 0.0067 | 0.5988 | 0.9937 |
| HQH | 238.09 | 0.0959 | 0.0944 | 0.7235 |
| Freundlich | Kf | 1/nF | nF | R2 |
| HQL | 1.229 | 0.277 | 3.607 | 0.9780 |
| HQH | 0.834 | 0.709 | 1.408 | 0.9923 |
| Adsorbent Material | qmax (mg/g) | Reference |
|---|---|---|
| Self-assembled graphene-carbon nanotube hybrid [118] | 81.97 | [118] |
| Eco-friendly rGO [119] | 121.95 | [119] |
| Pineapple peel carboxy methylcellulose-g-poly (acryliccid-co-acrylamide)/graphene oxide hydrogels [120] | 133.32 | [120] |
| rGO by hydrazine reduction of GO [121] | 153.85 | [121] |
| Alginate modified graphene [122] | 159 | [122] |
| Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads [123] | 180.23 | [123] |
| Graphene oxide/calcium alginate composites [109] | 181.81 | [109] |
| Graphene nanoplatelets [108] | 225 | [108] |
| rGO by Gallic Acid and Sodium Ascorbate | 238.45 | Present Study |
| GO with high-oxidation degree [110,111] | 600–1635 | [110,111] |
| Adsorbent Material | qmax (mg/g) | Reference |
|---|---|---|
| Organobentonites (ODTMA-B, HDTMA-B) [112] | 12.05–21.55 | [112] |
| Iron (Fe) impregnated granular activated carbon (Fe-GAC) [113] | 26.65 | [113] |
| Graphene aerogels–mesoporous silica (GAs–MS) [83] | 67 | [83] |
| Granular activated carbon (GAC) [114] | 102.3–135.3 | [114] |
| rGO by Gallic Acid and Sodium Ascorbate | ~150 | Present Study |
| Phragmites australis activated carbon (PAAC) [115] | 156.25 | [115] |
| Cationic amino-poly(vinylamine) (PVAm)-functionalized GO-(o-MWCNTs)-Fe3O4 [116] | 293.25 | [116] |
| Magnetic cyclodextrin chitosan/graphene oxide (CCGO) [117] | 428.72 | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmiento, V.; Lockett, M.; Sumbarda-Ramos, E.G.; Vázquez-Mena, O. Effective Removal of Metal ion and Organic Compounds by Non-Functionalized rGO. Molecules 2023, 28, 649. https://doi.org/10.3390/molecules28020649
Sarmiento V, Lockett M, Sumbarda-Ramos EG, Vázquez-Mena O. Effective Removal of Metal ion and Organic Compounds by Non-Functionalized rGO. Molecules. 2023; 28(2):649. https://doi.org/10.3390/molecules28020649
Chicago/Turabian StyleSarmiento, Viviana, Malcolm Lockett, Emigdia Guadalupe Sumbarda-Ramos, and Oscar Vázquez-Mena. 2023. "Effective Removal of Metal ion and Organic Compounds by Non-Functionalized rGO" Molecules 28, no. 2: 649. https://doi.org/10.3390/molecules28020649
APA StyleSarmiento, V., Lockett, M., Sumbarda-Ramos, E. G., & Vázquez-Mena, O. (2023). Effective Removal of Metal ion and Organic Compounds by Non-Functionalized rGO. Molecules, 28(2), 649. https://doi.org/10.3390/molecules28020649





