Alkaline Modification of Arabica-Coffee and Theobroma-Cocoa Agroindustrial Waste for Effective Removal of Pb(II) from Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Alkaline Treatment
2.2. SEM/EDX and FTIR Analysis
2.3. Adsorption Experiments
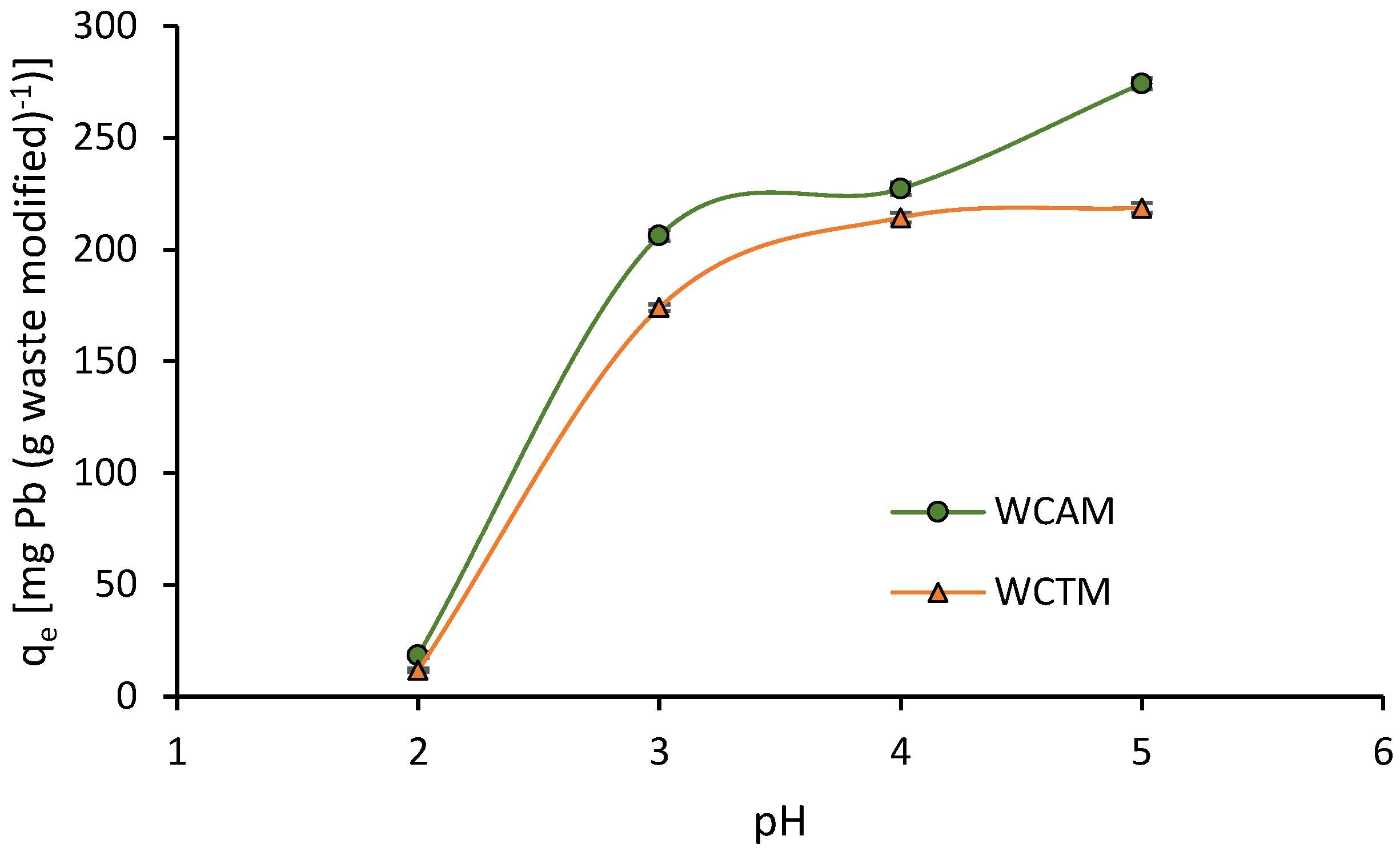
2.3.1. Influence of pH Solution
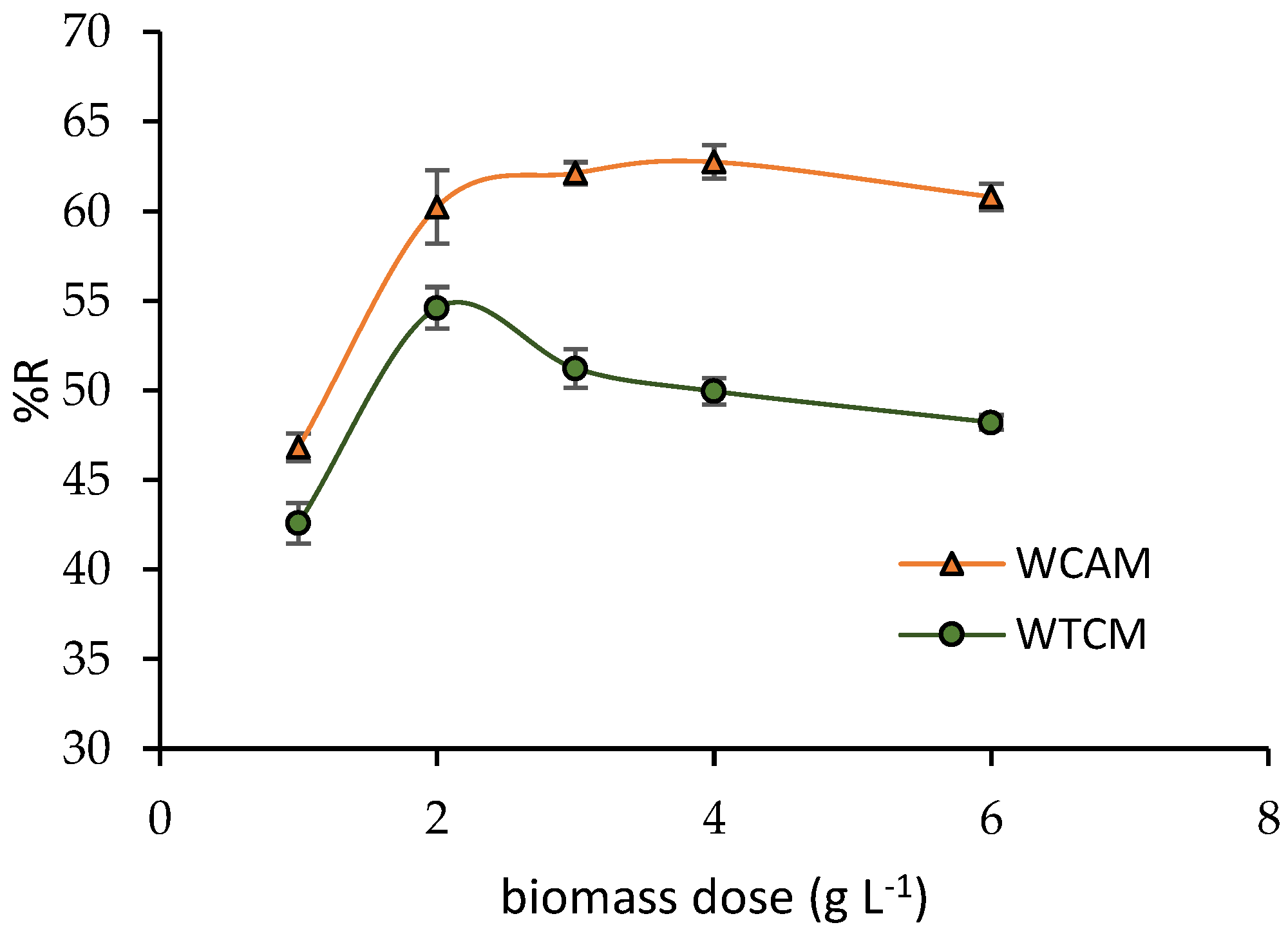
2.3.2. Influence of Biomass Dosage
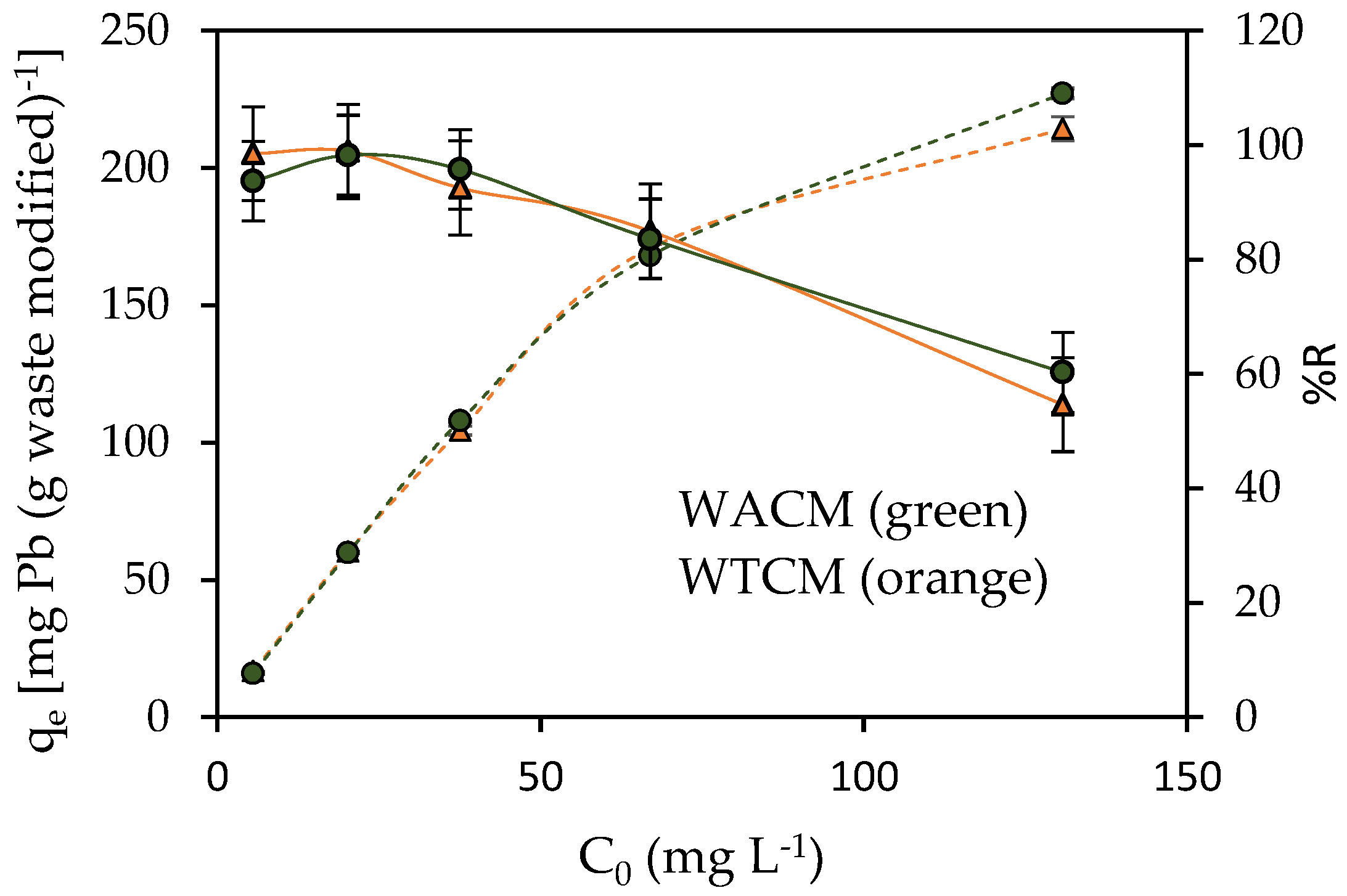
2.3.3. Influence of Initial Pb(II) ion Concentration, C0
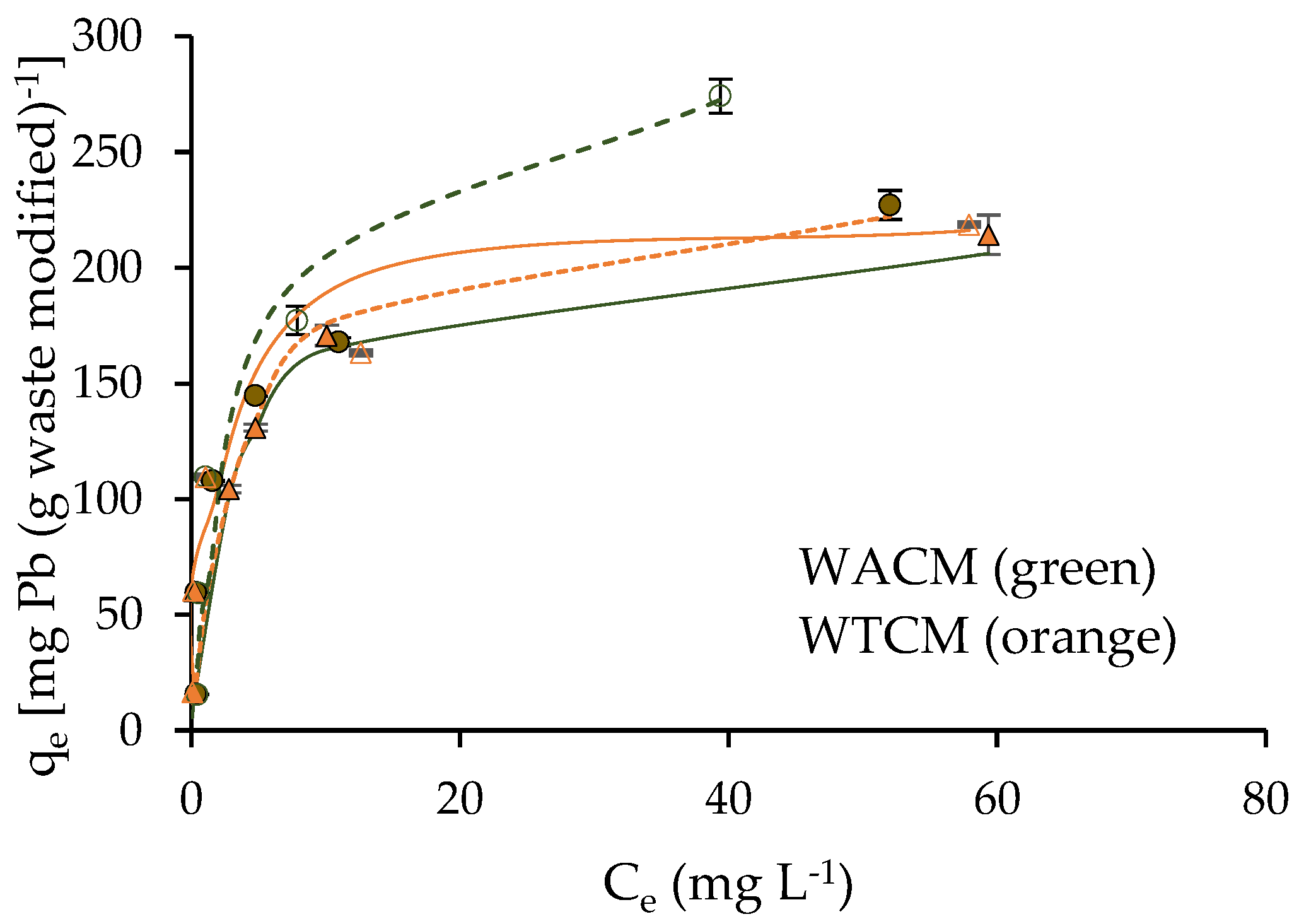
2.4. Adsorption Isotherms
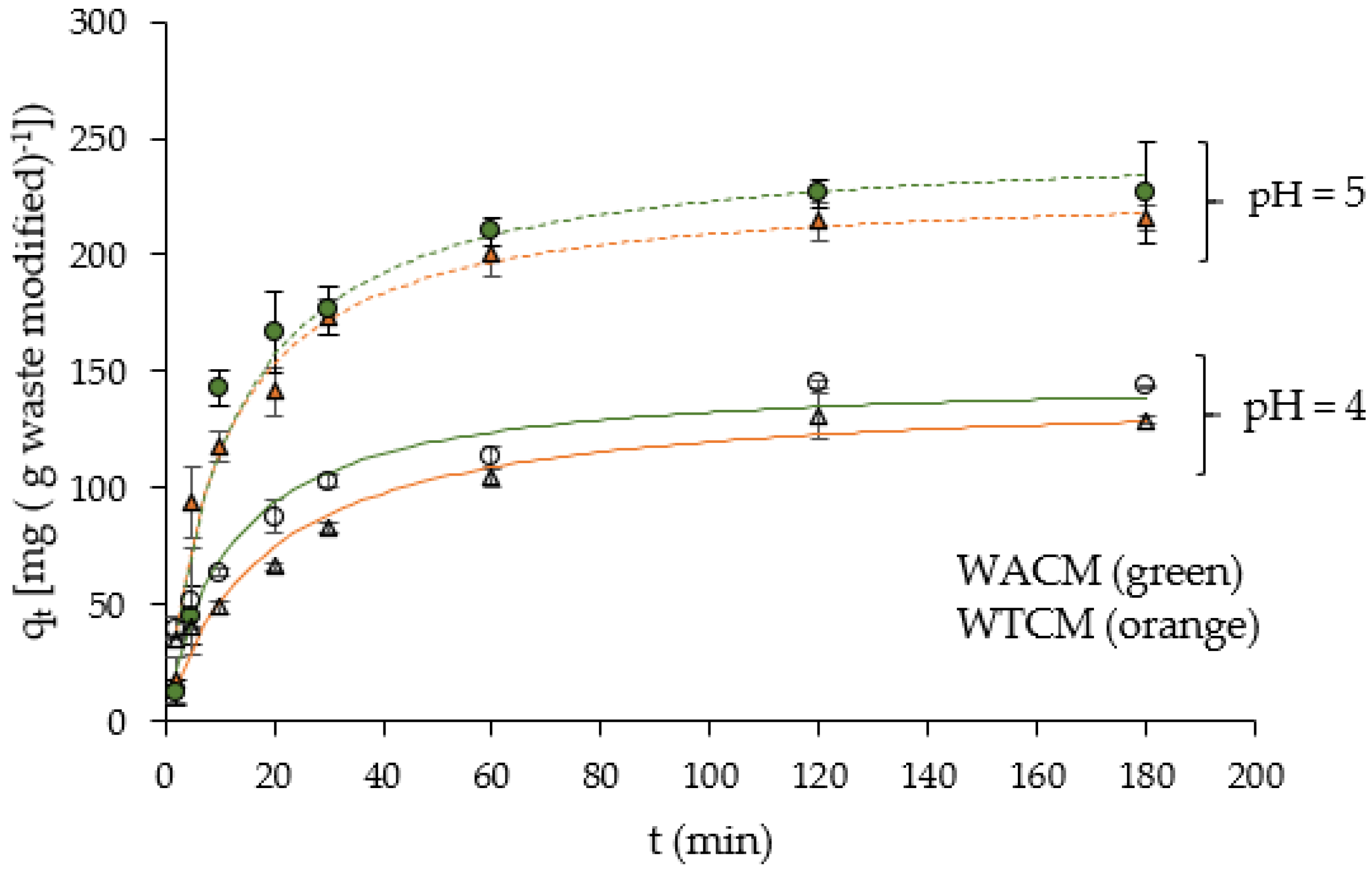
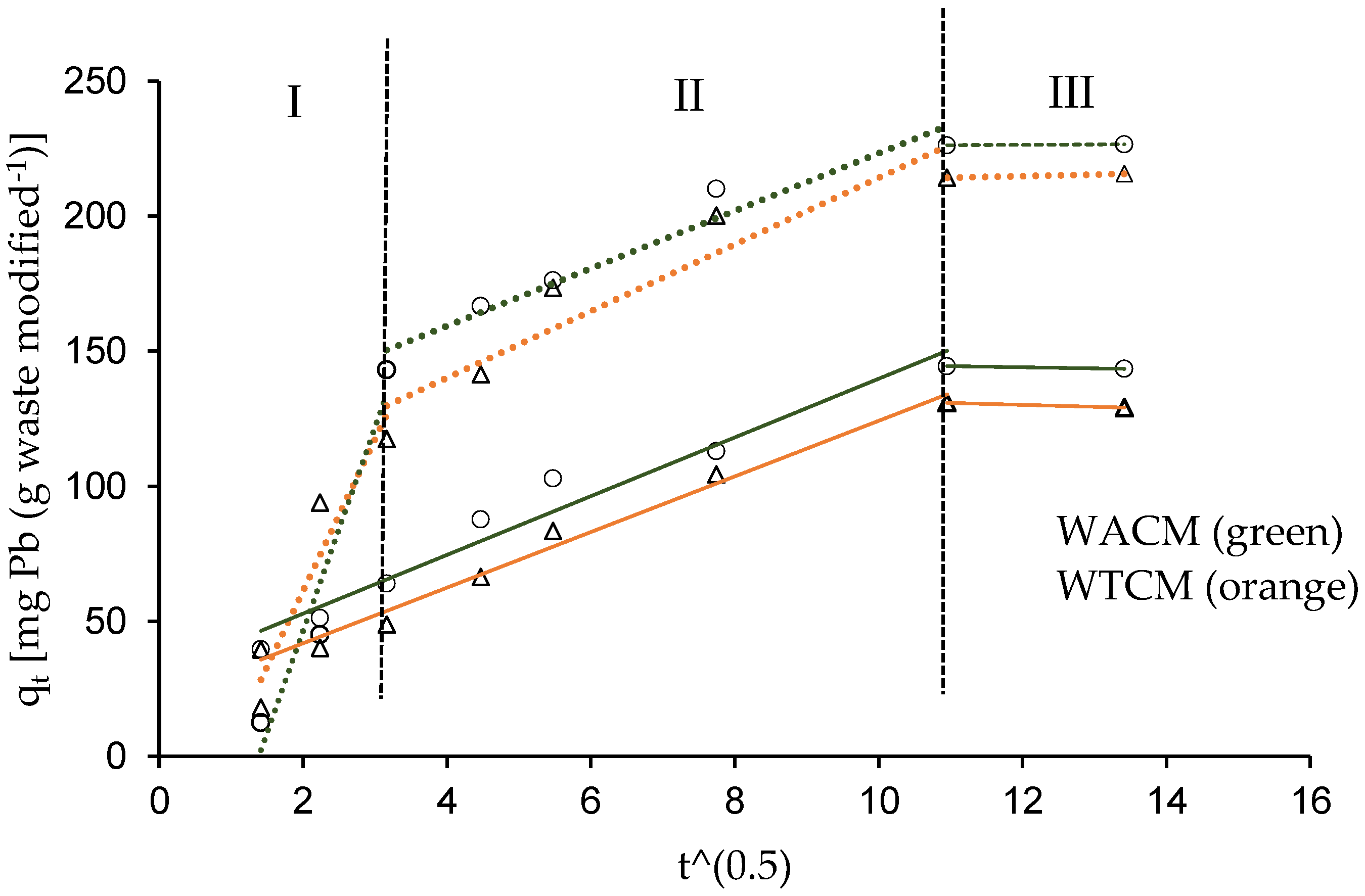
2.5. Kinetic of Biosorption
2.6. Biosorption Thermodynamics
3. Materials and Methods
3.1. Preparation of Biosorbents
3.2. Biosorbent Characterization
- The point of zero charge (pHPZC) study was evaluated according to the methodology reported by do-Nascimento et al. [45]. A mixture of 0.05 g of biomass with 50 mL of an aqueous solution under different initial pHs (pH0) ranging from 1 to 12 was prepared. The acid dilutions were prepared from a 1 M HCl solution, while basic dilutions were from 1 M NaOH. After 24 h of equilibrium, the final pHs (pHf) were measured.
- The concentrations of the acid and basic groups (or acid/basic titrable sites) on the surface of WACM and WTCM were determined using the Boehm method reported by Aygun et al. [46]. For acid titrable sites (between brackets for basic sites), mixtures of 0.25 g (0.5 g) of the biosorbent with 50 mL of a standardized 0.05 M NaOH (0.1 M HCl) solution were prepared. All the mixtures were shaken, at room temperature, for 24 h at 100 rpm, and then, for each mixture, 20 mL (10 mL) of the supernatant liquid was pipetted and excess acid (base) was adequately titrated using bromocresol blue or phenolphtalien) as an indicator.
- Fourier transform infrared (FTIR, SHIMADZU IR Affinity) spectroscopy, over a spectral range of 4000 to 500 cm−1 was used to characterize the functional groups present on the surface of WACM and WTCM before and after Pb(II) biosorption.
- Morphological and elemental analysis on the surface of biosorbents were performed by Scanning Electron Microscopy (SEM) coupled with EDX (Energy Dispersive X-rays spectroscopy) (LEO 440 model).
3.3. Adsorption Experiments
3.4. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, X.; Bing, J.; Zhang, J.; Guo, L.; Deng, Z.; Wang, D.; Liu, L. Ecological risk assessment and sources identification of heavy metals in surface sediments of a river–reservoir system. Sci. Total Environ. 2022, 842, 156683. [Google Scholar] [CrossRef] [PubMed]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Ali, M.N.V.S.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Lanphear, B.P. The effects of iniquitous lead exposure on health. Nat. Sustain. 2020, 3, 77–79. [Google Scholar] [CrossRef]
- Taguchi, R.; Seki, H.; Maruyama, H. Biosorption of Pb and Cd onto Polygonum sachalinense. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 650, 129210. [Google Scholar] [CrossRef]
- Jaihan, W.; Mohdee, V.; Sanongraj, S.; Pancharoen, U.; Nootong, K. Biosorption of lead (II) from aqueous solution using Cellulose-based Bio-adsorbents prepared from unripe papaya (Carica papaya) peel waste: Removal Efficiency, Thermodynamics, kinetics and isotherm analysis. Arab. J. Chem. 2022, 15, 103883. [Google Scholar] [CrossRef]
- Mangwandi, C.; Kurniawan, T.A.; Albadarin, A.B. Comparative biosorption of chromium (VI) using chemically modified date pits (CM-DP) and olive stone (CM-OS): Kinetics, isotherms and influence of co-existing ions. Chem. Eng. Res. Des. 2020, 156, 251–262. [Google Scholar] [CrossRef]
- Brazesh, B.; Mousavi, S.M.; Zarei, M.; Ghaedi, M.; Bahrani, S.; Hashemi, S.A. Biosorption. Interface Sci. Technol. 2021, 33, 587–628. [Google Scholar]
- Cheng, S.Y.; Show, P.L.; Lau, B.F.; Chang, J.S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Basu, A.; Ali, S.S.; Hossain, S.K.S.; Asif, M. A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process. Processes 2022, 10, 1154. [Google Scholar] [CrossRef]
- Syeda, H.I.; Sultan, I.; Razavi, K.S.; Yap, P.S. Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes: A review. J. Water Process Eng. 2022, 46, 102446. [Google Scholar] [CrossRef]
- Ren, B.; Jin, Y.; Zhao, L.; Cui, C.; Song, X. Enhanced Cr(VI) adsorption using chemically modified dormant Aspergillus niger spores: Process and mechanisms. J. Environ. Chem. Eng. 2020, 10, 106955. [Google Scholar] [CrossRef]
- Calero, M.; Pérez, A.; Blázquez, G.; Ronda, A.; Martín-lara, M.A. Characterization of chemically modified biosorbents from olive tree pruning for the biosorption of lead. Ecol. Eng. 2013, 58, 344–354. [Google Scholar] [CrossRef]
- Moyo, M.; Pakade, V.E.; Modise, S.J. Biosorption of lead(II) by chemically modified Mangifera indica seed shells: Adsorbent preparation, characterization and performance assessment. Process Saf. Environ. Prot. 2017, 111, 40–51. [Google Scholar] [CrossRef]
- Petrović, J.T.; Stojanović, M.D.; Milojković, J.V.; Petrović, M.S.; Šoštarić, T.D.; Laušević, M.D.; Mihajlović, M.L. Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb2+ from aqueous solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Yu, Z. Adsorption of Pb(II) onto Modified Rice Bran. Nat. Resour. 2010, 01, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Informe Mensual del “Valor Bruto de la Producción Agropecuaria” Diciembre 2021. Available online: https://cdn.www.gob.pe/uploads/document/file/3282594/Informe%20Mensual%20del%20%22Valor%20Bruto%20de%20la%20Producci%C3%B3n%20Agropecuaria%22%20Diciembre%202021.pdf?v=1655825207 (accessed on 15 December 2022).
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.; Saari, J.; Melo, Y.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Evaluation of thermochemical routes for the valorization of solid coffee residues to produce biofuels: A Brazilian case. Renew. Sustain. Energy Rev. 2021, 137, 110585. [Google Scholar] [CrossRef]
- Park, S.J.; Yang, H.K. Ultra-fast synthesis of carbon dots using the wasted coffee residues for environmental remediation. Curr. Appl. Phys. 2022, 36, 9–15. [Google Scholar] [CrossRef]
- Lavado-Meza, C.; De la Cruz-Cerrón, L.; Cisneros-Santos, G.; De la Cruz, A.H.; Angeles-Suazo, J.; Dávalos-Prado, J.Z. Arabica-coffee and teobroma-cocoa agro-industrial waste biosorbents, for Pb(II) removal in aqueous solutions. Environ. Sci. Pollut. Res. 2022. [CrossRef]
- Santos, S.C.R.; Ungureanu, G.; Volf, I.; Boaventura, R.A.R.; Botelho, C.M.S. Macroalgae Biomass as Sorbent for Metal Ions. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; pp. 69–112. [Google Scholar]
- Bulgariu, L.; Bulgariu, D. Enhancing Biosorption Characteristics of Marine Green Algae (Ulva lactuca) for Heavy Metals Removal by Alkaline Treatment. J. Bioprocess. Biotech. 2014, 04, 1–8. [Google Scholar] [CrossRef]
- Blázquez, G.; Calero, M.; Ronda, A.; Tenorio, G.; Martín-Lara, M.A. Study of kinetics in the biosorption of lead onto native and chemically treated olive stone. J. Ind. Eng. Chem. 2014, 20, 2754–2760. [Google Scholar] [CrossRef]
- Gupta, N.K.; Gupta, A.; Ramteke, P.; Sahoo, H.; Sengupta, A. Biosorption-a green method for the preconcentration of rare earth elements (REEs) from waste solutions: A review. J. Mol. Liq. 2019, 274, 148–164. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Lead on Cucumber Peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Song, T.; Yu, S.; Wang, X.; Teng, C.; Bai, X.; Liang, J.; Dong, L.; Ouyang, F.; Qu, J.; Jin, Y. Biosorption of Lead(II) from Aqueous Solution by Sodium Hydroxide Modified Auricularia auricular Spent Substrate: Isotherms, Kinetics, and Mechanisms. Water. Air. Soil Pollut. 2017, 228, 1–17. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead(II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Mahyoob, W.; Alakayleh, Z.; Abu Hajar, H.A.; Al-Mawla, L.; Altwaiq, A.M.; Al-Remawi, M.; Al-Akayleh, F. A novel co-processed olive tree leaves biomass for lead adsorption from contaminated water. J. Contam. Hydrol. 2022, 248, 104025. [Google Scholar] [CrossRef]
- Fawzy, M.; Nasr, M.; Abdel-Rahman, A.M.; Hosny, G.; Odhafa, B.R. Techno-economic and environmental approaches of Cd2+ adsorption by olive leaves (Olea europaea L.) waste. Int. J. Phytoremediat. 2019, 21, 1205–1214. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Paduraru, C.; Ibanescu, D.; Tofan, L. Biosorption of lead ions from aqueous effluents by rapeseed biomass. N. Biotechnol. 2017, 39, 110–124. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Removal of Copper and Lead using Banana Biochar in Batch Adsorption Systems: Isotherms and Kinetic Studies. Arab. J. Sci. Eng. 2018, 43, 5711–5722. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; El Makhfouk, M.; Qourzal, S. Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J. Environ. Chem. Eng. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Lavado-Meza, C.; Asencios, O.Y.J.; Cisneros-Santos, G.; Unchupaico-Payano, I. Revista Mexicana de Ingeniería Química. Rev. Mex. Ing. Química 2021, 2, 941–954. [Google Scholar] [CrossRef]
- Šoštarić, T.D.; Petrović, M.S.; Pastor, F.T.; Lončarević, D.R.; Petrović, J.T.; Milojković, J.V.; Stojanović, M.D. Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment. J. Mol. Liq. 2018, 259, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.H.K.; Harinath, Y.; Seshaiah, K.; Reddy, A.V.R. Biosorption of Pb(II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem. Eng. J. 2010, 162, 626–634. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Lee, S.M. Optimization of Cd(II), Cu(II) and Ni(II) biosorption by chemically modified Moringa oleifera leaves powder. Carbohydr. Polym. 2012, 88, 1077–1086. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Schwantes, D.; Campagnolo, M.A.; Dragunski, D.C.; Tarley, C.R.T.; Dos Santos Silva, A.K. Removal of toxic metals using endocarp of açaí berry as biosorbent. Water Sci. Technol. 2018, 77, 1547–1557. [Google Scholar] [CrossRef] [Green Version]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Lavado-Meza, C.; Sun-Kou, M.R.; Castro-Arroyo, T.K.; Bonilla-Mancilla, H.D. Biosorción de plomo (II) en solución acuosa con biomasa de los cladodios de la tuna (opuntia ficus indica). Rev. Colomb. Quim. 2020, 49, 36–46. [Google Scholar] [CrossRef]
- Oliveira, M.R.F.; Abreu, K.V.; Romão, A.L.E.; Davi, D.M.V.; Magalhães, C.E.C.; Carrilho, E.N.V.; Alves, C.R. Carnauba (Copernicia prunifera) palm tree biomass as adsorbent for Pb(II) and Cd(II) from water medium. Environ. Sci. Pollut. Res. 2021, 28, 18941–18952. [Google Scholar] [CrossRef]
- Tavana, M.; Pahlavanzadeh, H.; Zarei, M.J. The novel usage of dead biomass of green algae of Schizomeris leibleinii for biosorption of copper(II) from aqueous solutions: Equilibrium, kinetics and thermodynamics. J. Environ. Chem. Eng. 2020, 8, 104272. [Google Scholar] [CrossRef]
- Milojković, J.V.; Mihajlović, M.L.; Stojanović, M.D.; Lopičić, Z.R.; Petrović, M.S.; Šoštarić, T.D.; Ristić, M.D. Pb(II) removal from aqueous solution by Myriophyllum spicatum and its compost: Equilibrium, kinetic and thermodynamic study. J. Chem. Technol. Biotechnol. 2014, 89, 662–670. [Google Scholar] [CrossRef]
- do Nascimento, J.M.; de Oliveira, J.D.; Leite, S.G.F. Chemical characterization of biomass flour of the babassu coconut mesocarp (Orbignya speciosa) during biosorption process of copper ions. Environ. Technol. Innov. 2019, 16, 100440. [Google Scholar] [CrossRef]
- Aygun, A.; Yenisoy-Karakaş, S.; Duman, I. Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M.; Bhila, B.; Moloto, H.; Naidoo, E.B. Lead ions and methylene blue dye removal from aqueous solution by mibuna beans (velvet beans) adsorbents. J. Environ. Chem. Eng. 2020, 8, 103557. [Google Scholar] [CrossRef]
- Kushwaha, S.; Suhas; Chaudhary, M.; Tyagi, I.; Bhutiani, R.; Goscianska, J.; Ahmed, J.; Manila; Chaudhary, S. Utilization of Phyllanthus emblica fruit stone as a Potential Biomaterial for Sustainable Remediation of Lead and Cadmium Ions from Aqueous Solutions. Molecules 2022, 27, 3355. [Google Scholar] [CrossRef] [PubMed]
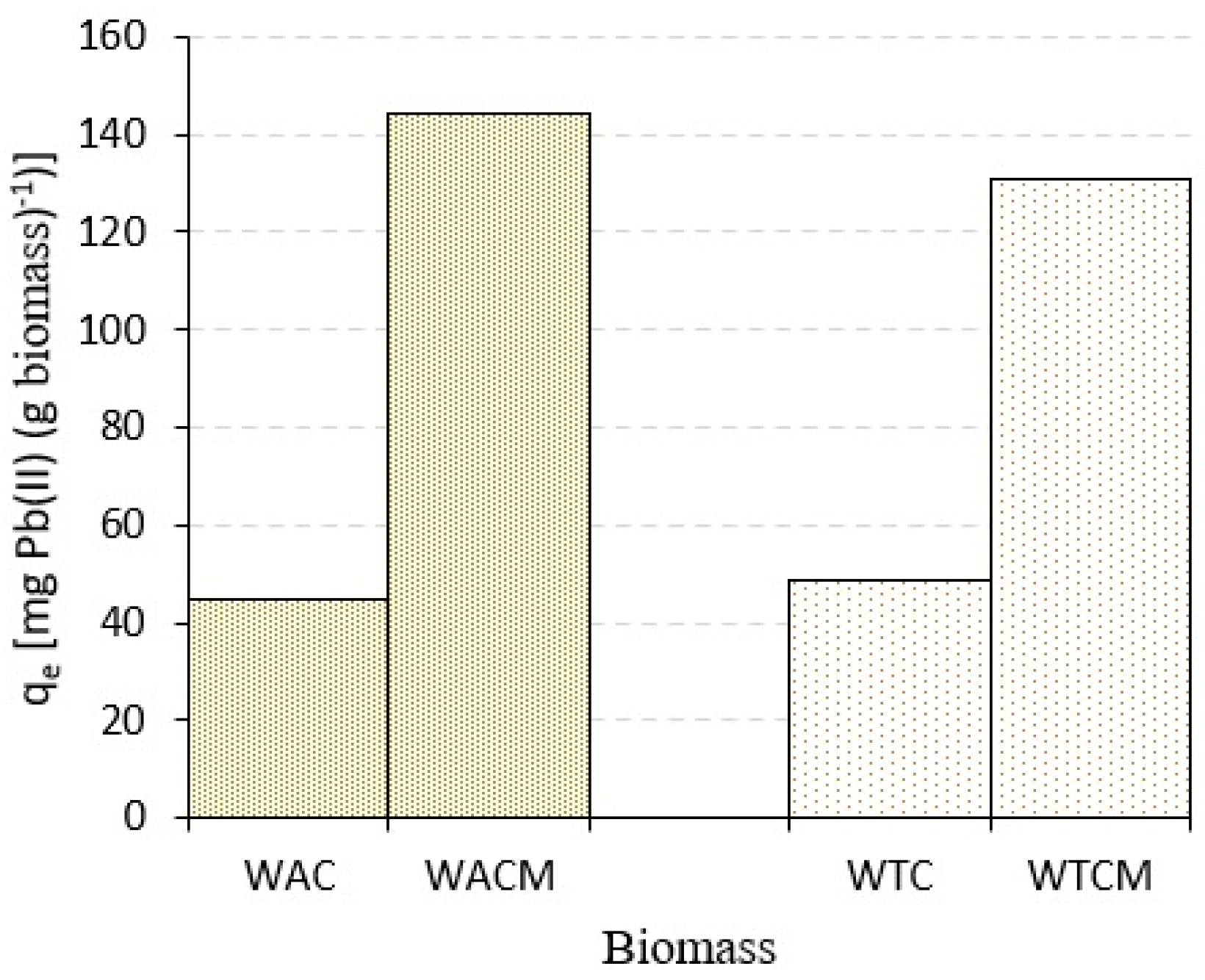
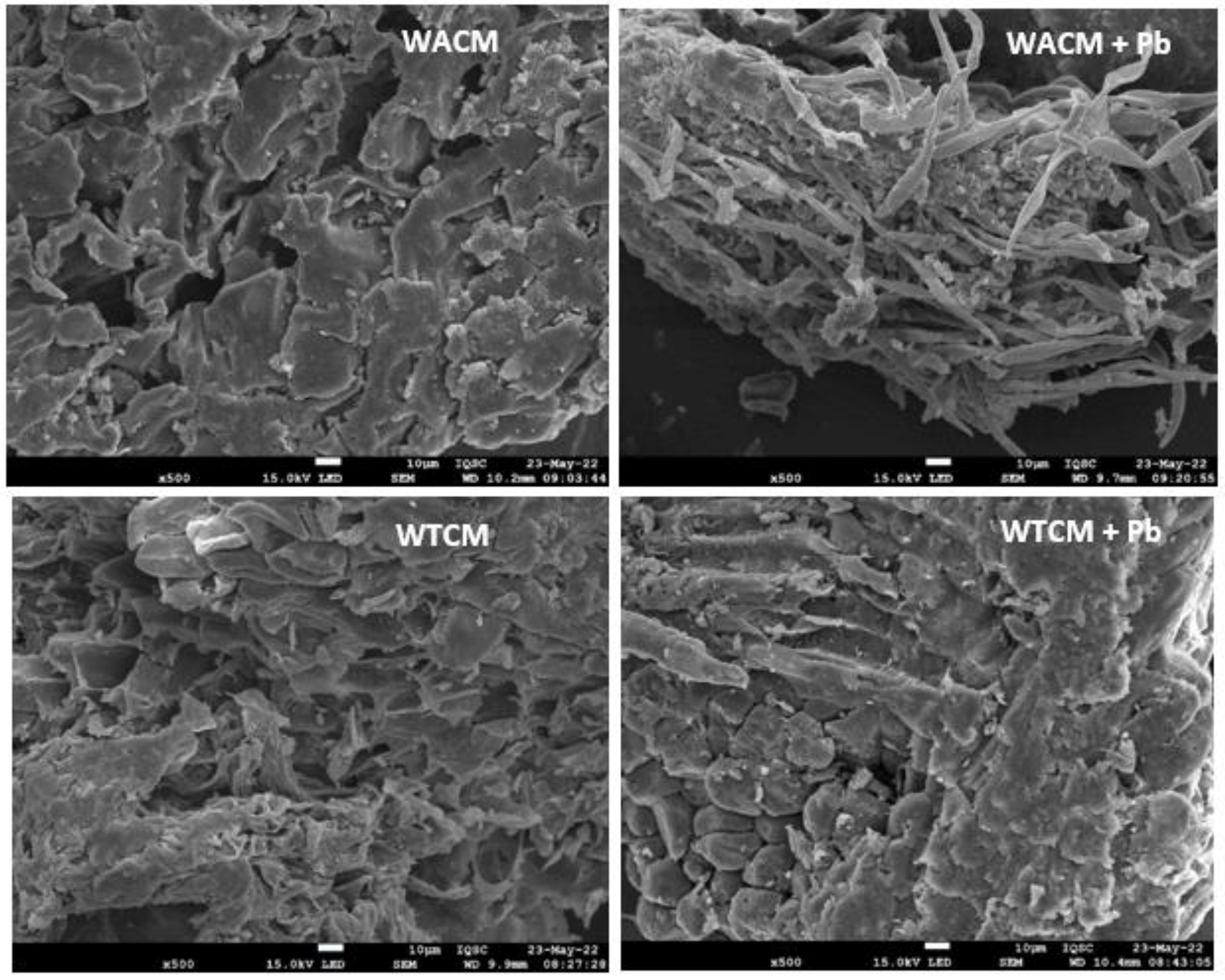
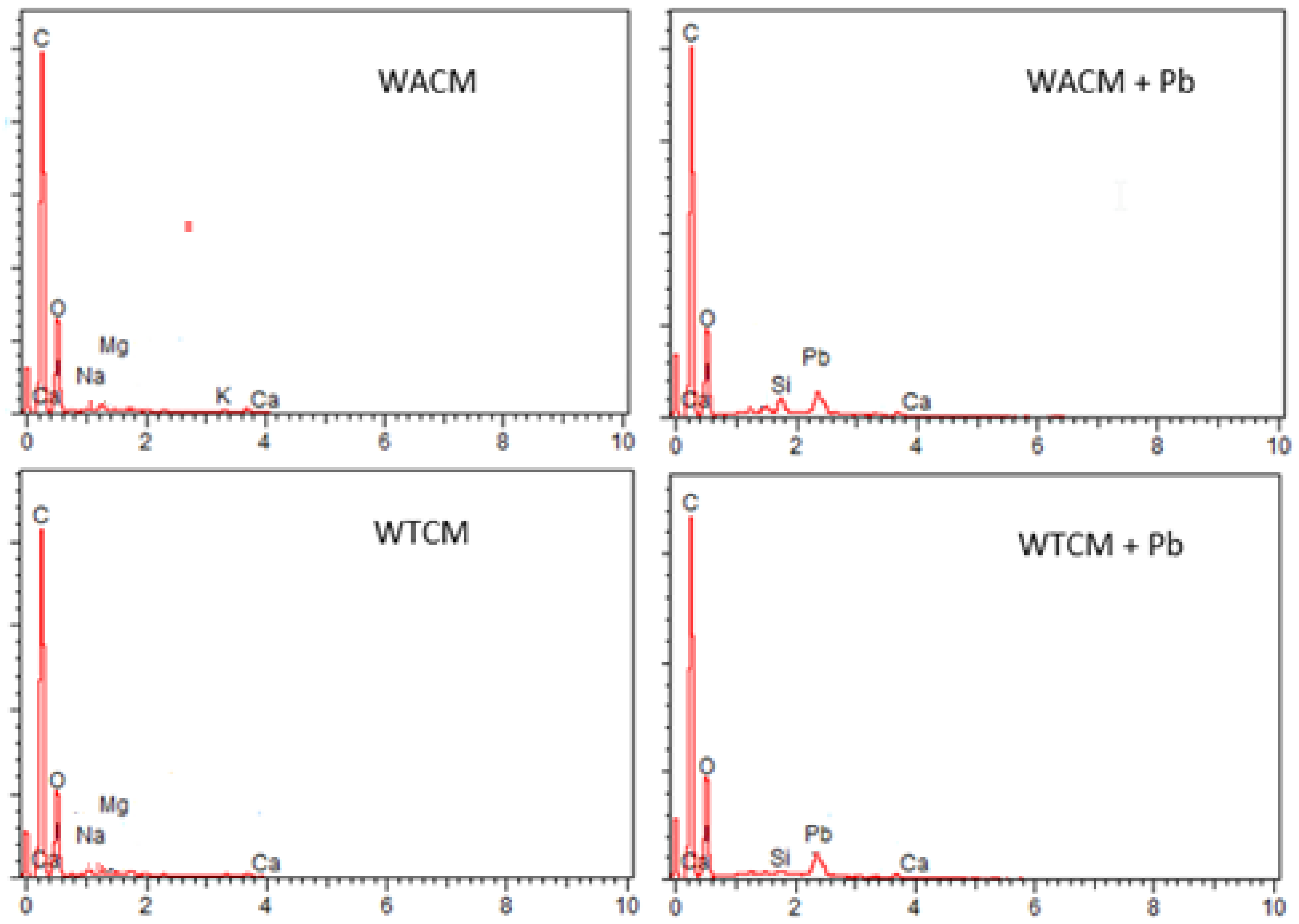
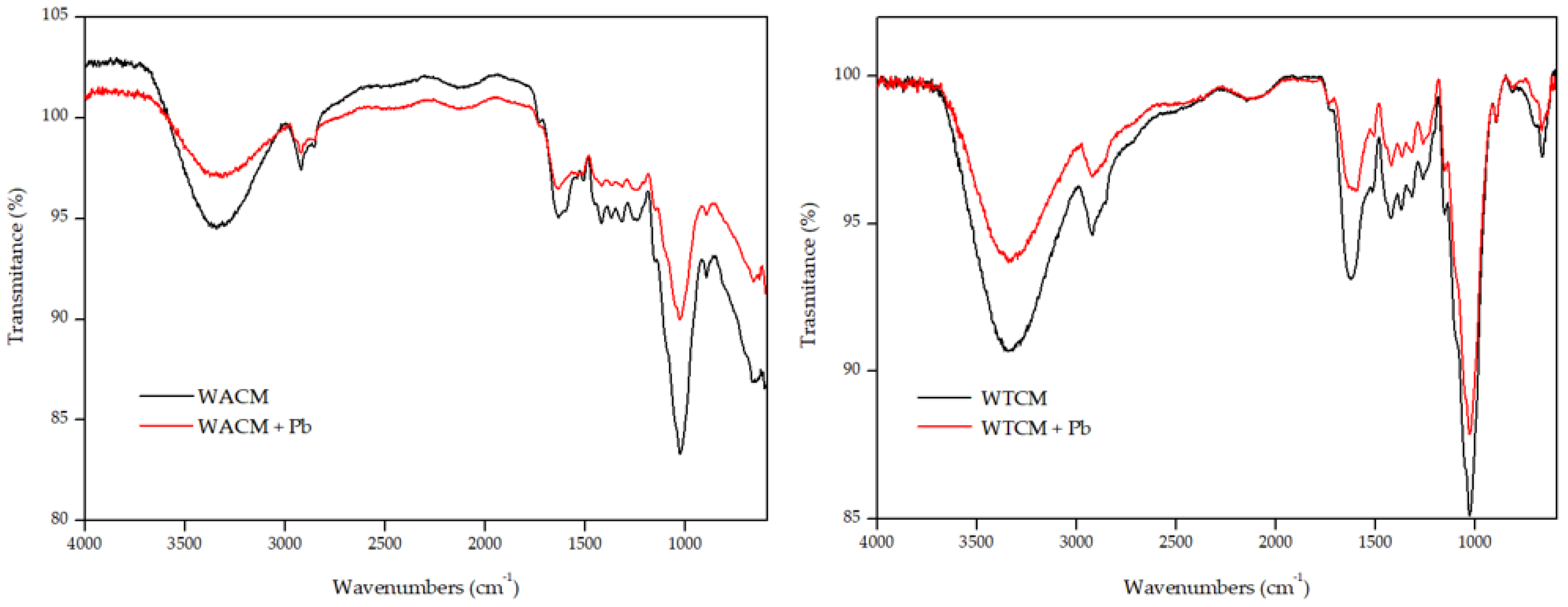

| WAC Coffe Waste | WTC Cocoa Waste | WACM Coffe Waste | WTCM Cocoa Waste | |
|---|---|---|---|---|
| Untreated | Alkaline Treated a | |||
| Point of Zero Charge, pHPZC | 4.8 | 6 | 6 | 6.8 |
| Acid titrable sites (mmol g−1) | 2.8 × 10−2 | 1.97 × 10−2 | 0 | 0 |
| Basic titrable sites (mmol g−1) | 2.12 × 10−2 | 1.84 × 10−2 | 2.97 × 10−2 | 2.63 × 10−2 |
| % Biomass loss due to treatment | 40.2 | 38.4 | ||
| WACM | WTCM | ||||
|---|---|---|---|---|---|
| Parameters | pH = 4 | pH = 5 | pH = 4 | pH = 5 | |
| Langmuir model | KL (L·mg−1) qmax (mg·g−1) R2 | 0.32 238.1 ~1 | 0.22 303.0 0.98 | 0.45 222.2 ~1 | 0.61 223.1 ~1 |
| Freundlich model | KF (mg·g−1 L(1/n)·mg−(1/n)) nF R2 | 58.47 2.39 0.73 | 56.78 2.04 0.72 | 66.08 2.80 0.87 | 71.69 3.11 0.79 |
| Biosorbent Wastes | qmax (mg g−1) | Reference |
|---|---|---|
| Apricot shells Mangifera indica seed shells Olive tree pruning Grape pomace Moringa oleifera tree leaves Theobroma cacao; WTCM Arabica coffee; WACM | 37.37 59.25 121.60 137 209.54 223.1 303.0 | [35] [13] [12] [14] [36] This work This work |
| Model | Parameters | WACM | WTCM | ||
|---|---|---|---|---|---|
| C0 = 130.8 mg L−1 | C0 = 48.42 mg L−1 | C0 = 130.8 mg L−1 | C0 = 48.42 mg L−1 | ||
| Pseudo 1st order | k1(min−1) qe,cal (mg g−1) a R2 | 0.075 206.62 0.94 | 0.063 132.93 0.84 | 0.075 206.62 0.94 | 0.043 124.92 0.87 |
| Pseudo 2nd order | k2 (g mg−1 min−1) qe,cal (mg g−1) a h R2 | 0.0003 249.53 20.99 0.94 | 0.0006 147.98 13.14 0.93 | 0.0004 229.99 21.16 0.97 | 0.0004 141.23 7.98 0.92 |
| Weber and Morris model | kd,I (mg g−1 min−1/2) b R2 kd,II (mg g−1 min−1/2) b R2 kd,III (mg g−1 min−1/2) b R2 | 75.4 0.94 10.7 0.95 0.12 1 | 10.9 0.96 0.4 1 - - | 56.2 0.90 12.4 0.89 0.58 1 | 10.3 0.99 0.7 1 - - |
| ∆H0 (kJ mol−1) | ∆S0 (J mol−1 K−1) | ∆G0 (kJ mol−1) | |||
|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | |||
| WACM | 22.5 | 88.9 | −35.90 | −44.42 | −53.70 |
| WTCM | 25.4 | 97.2 | −31.26 | −41.32 | −50.70 |
| Model | Equation | Parameters |
|---|---|---|
| Langmuir | qe (mg g−1): adsorption capacity Ce (mg L−1): adsorbate concentration in equilibrium qmax (mg g−1): maximum sorption capacity kL (L mg−1): Langmuir constant related to the affinity between sorbent and sorbate | |
| Freundlich | kF (mg g−1 L(1/n)·mg−(1/n)): equilibrium constant n: constant related to the affinity between sorbent and sorbate |
| Model | Equation | Parameters |
|---|---|---|
| Pseudo-first order | qe (mg g−1): adsorption capacity qt (mg g−1): amount of Pb(II) retained per unit biomass at time t. k1 (min−1): first-order kinetic constant k2 (g (mg min)−1): rate constant adsorption h (mg(g min)−1): initial adsorption rate | |
| Pseudo-second order | ||
| Weber and Morris | kd (mg g−1 min−1/2): intraparticle diffusion rate constant B (mg g−1): constant related to the thickness of the adsorbent boundary layer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavado-Meza, C.; De la Cruz-Cerrón, L.; Asencios, Y.J.O.; Marcos, F.C.F.; Dávalos-Prado, J.Z. Alkaline Modification of Arabica-Coffee and Theobroma-Cocoa Agroindustrial Waste for Effective Removal of Pb(II) from Aqueous Solutions. Molecules 2023, 28, 683. https://doi.org/10.3390/molecules28020683
Lavado-Meza C, De la Cruz-Cerrón L, Asencios YJO, Marcos FCF, Dávalos-Prado JZ. Alkaline Modification of Arabica-Coffee and Theobroma-Cocoa Agroindustrial Waste for Effective Removal of Pb(II) from Aqueous Solutions. Molecules. 2023; 28(2):683. https://doi.org/10.3390/molecules28020683
Chicago/Turabian StyleLavado-Meza, Carmencita, Leonel De la Cruz-Cerrón, Yvan J.O. Asencios, Francielle Candian Firmino Marcos, and Juan Z. Dávalos-Prado. 2023. "Alkaline Modification of Arabica-Coffee and Theobroma-Cocoa Agroindustrial Waste for Effective Removal of Pb(II) from Aqueous Solutions" Molecules 28, no. 2: 683. https://doi.org/10.3390/molecules28020683
APA StyleLavado-Meza, C., De la Cruz-Cerrón, L., Asencios, Y. J. O., Marcos, F. C. F., & Dávalos-Prado, J. Z. (2023). Alkaline Modification of Arabica-Coffee and Theobroma-Cocoa Agroindustrial Waste for Effective Removal of Pb(II) from Aqueous Solutions. Molecules, 28(2), 683. https://doi.org/10.3390/molecules28020683







