Comparing the Effects of Encapsulated and Non-Encapsulated Propolis Extracts on Model Lipid Membranes and Lactic Bacteria, with Emphasis on the Synergistic Effects of Its Various Compounds
Abstract
:1. Introduction
2. Results and Discussion
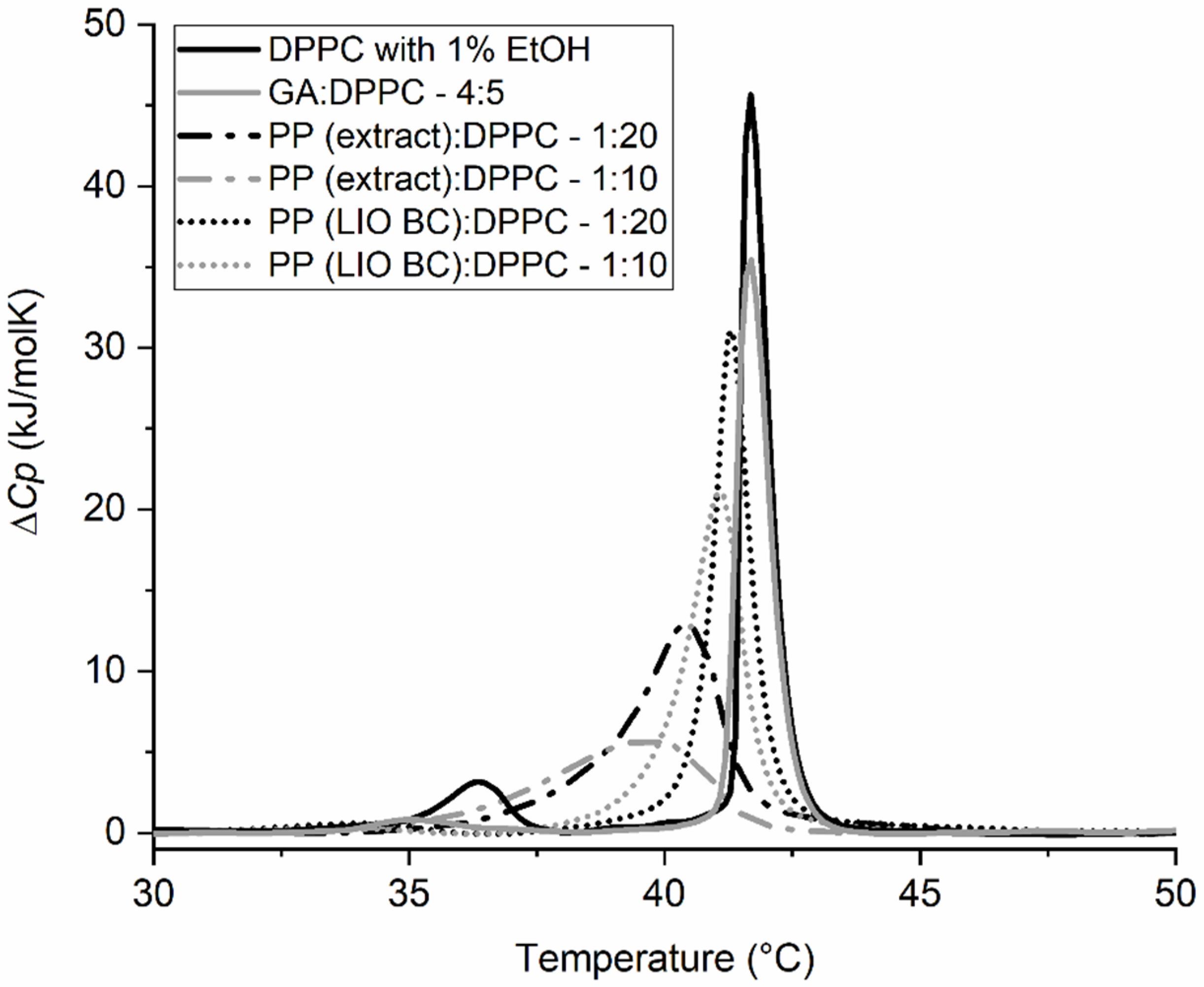
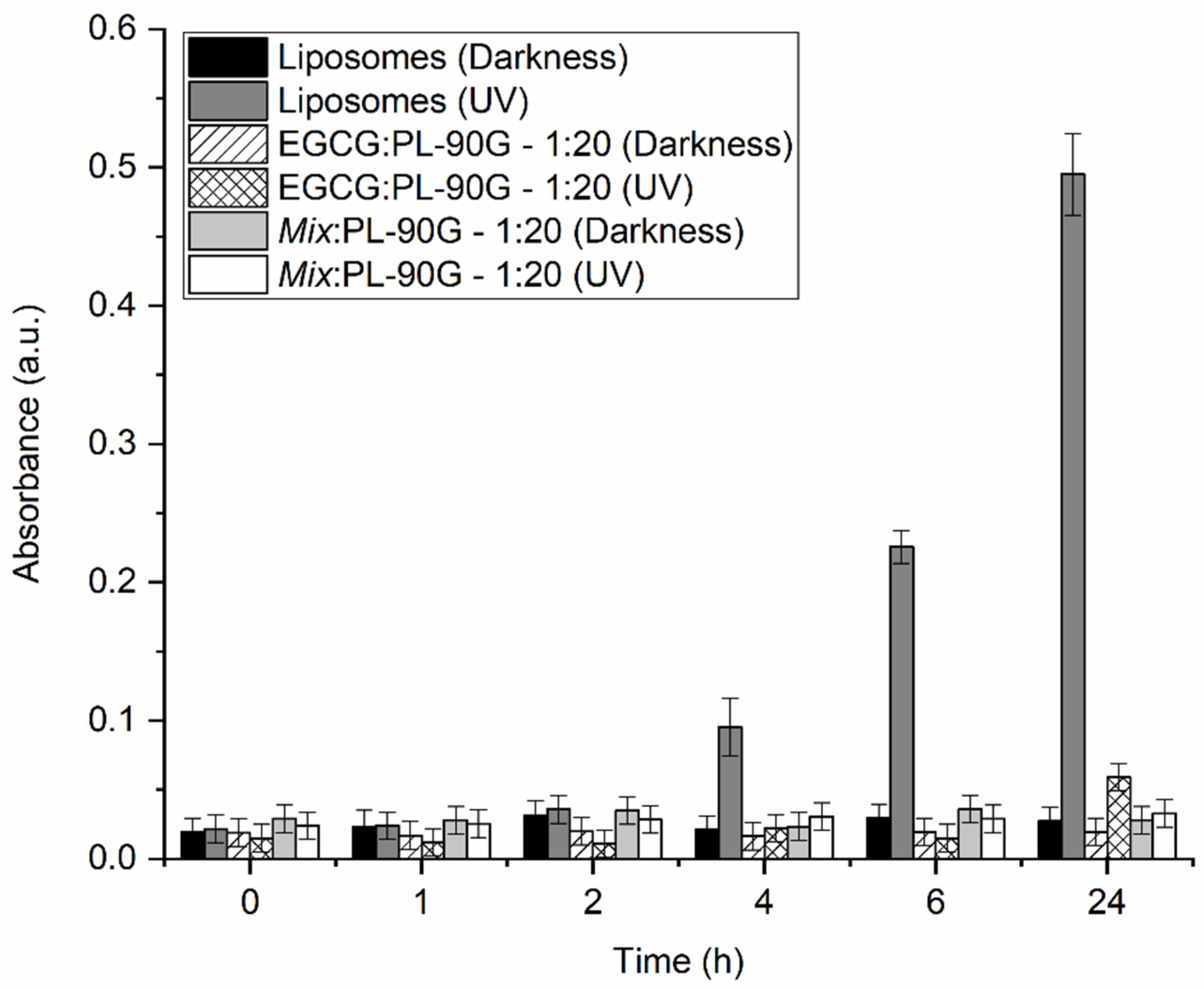
2.1. Effects of Encapsulated and Non-Encapsulated Propolis on Lipid Membrane Integrity and Lactic Bacteria
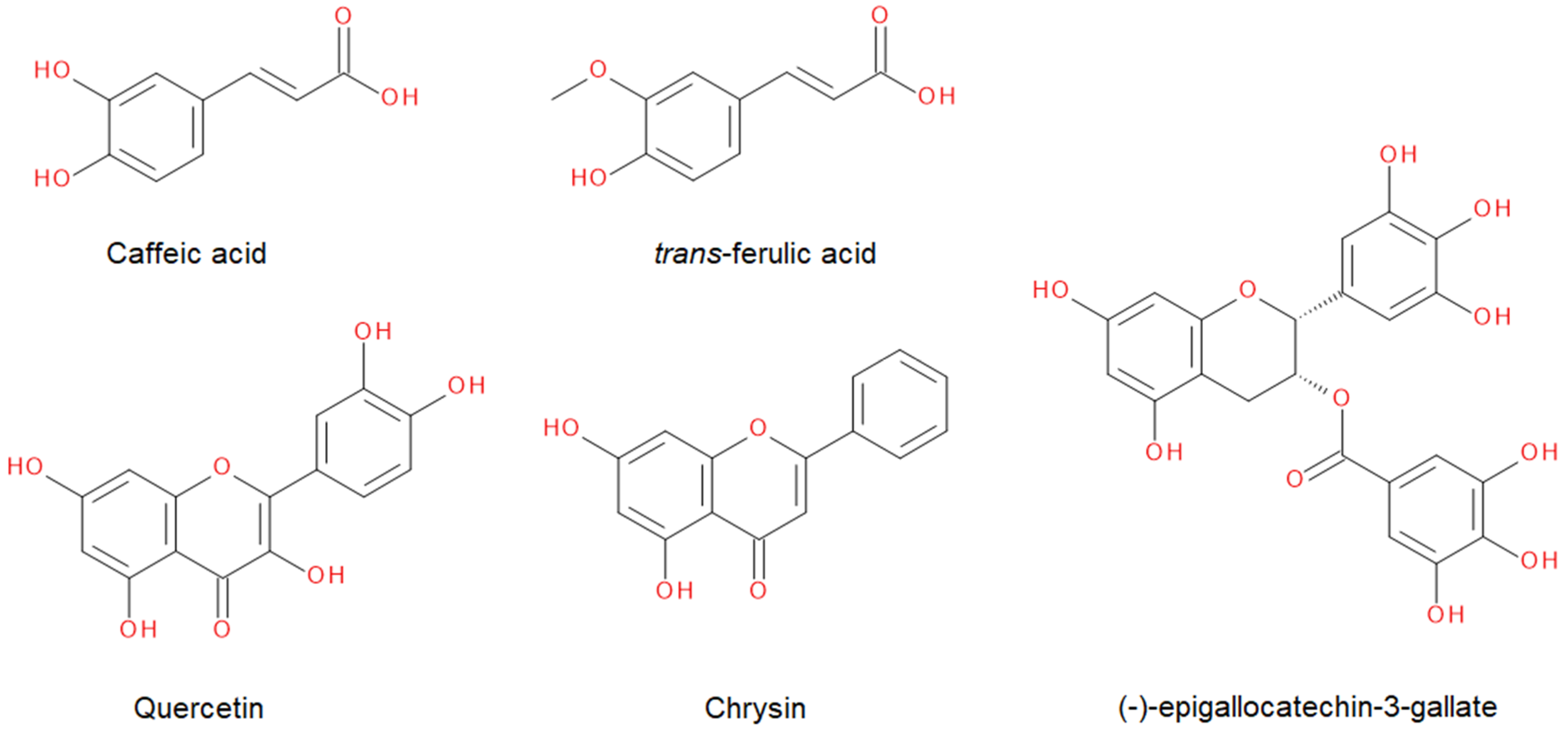
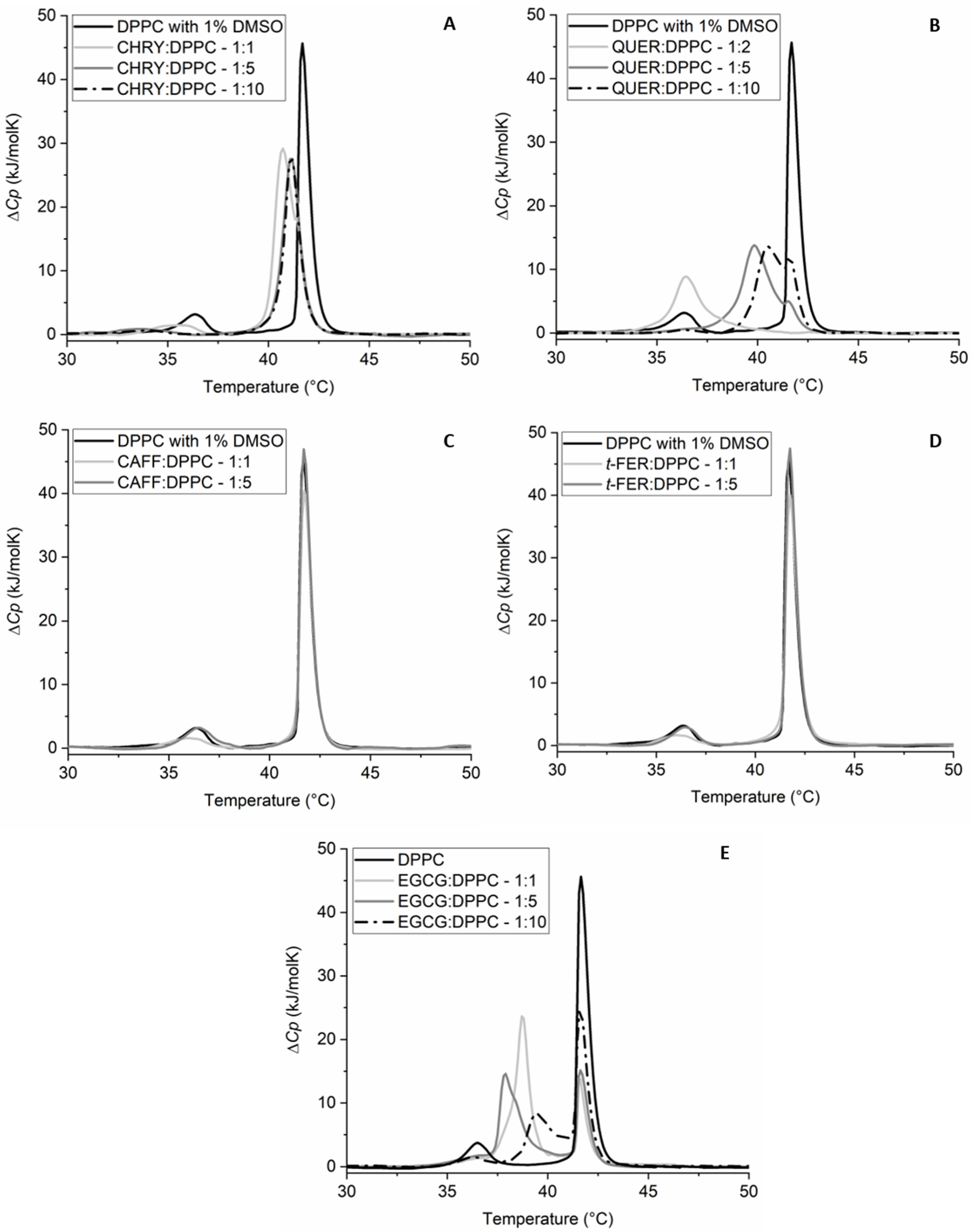
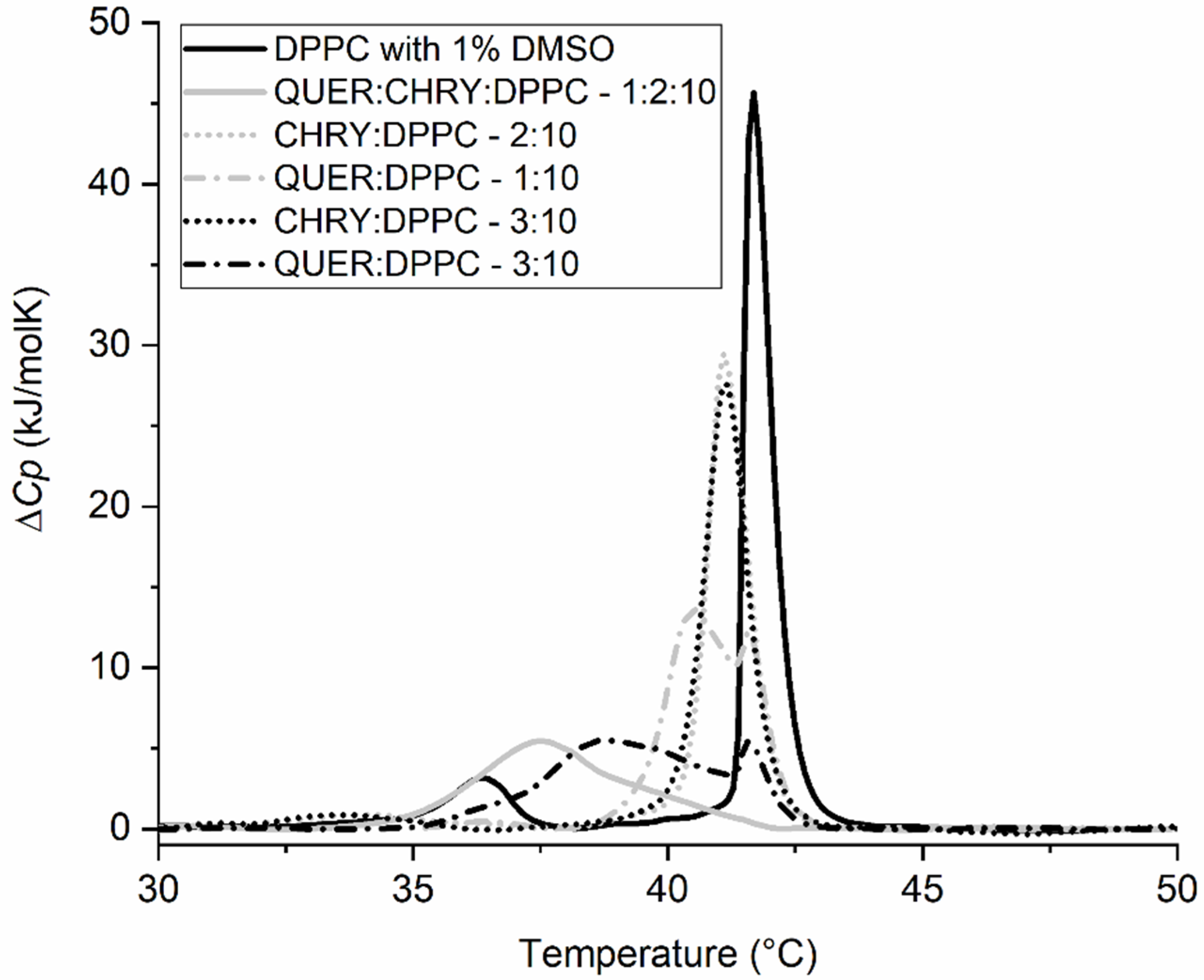
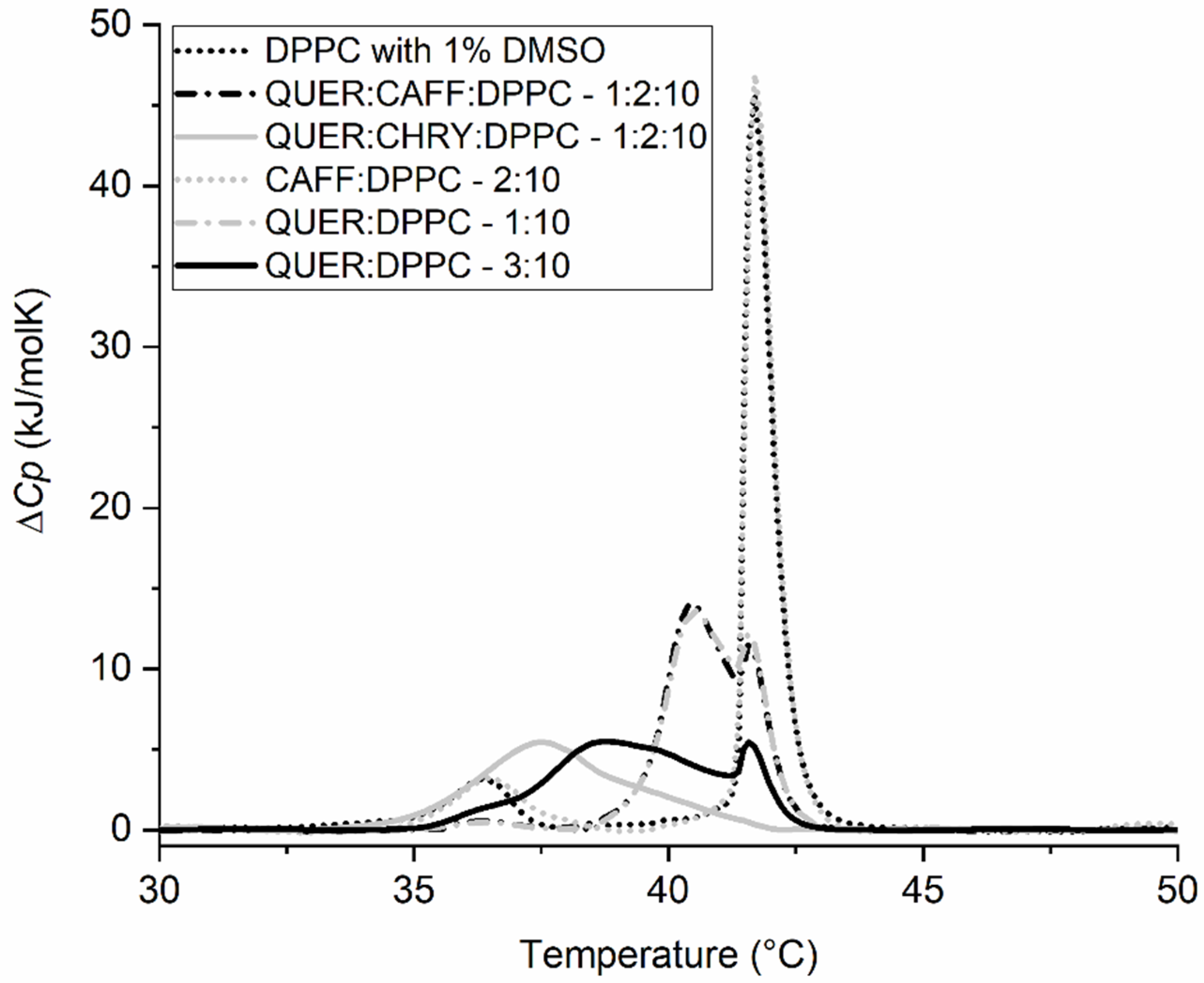
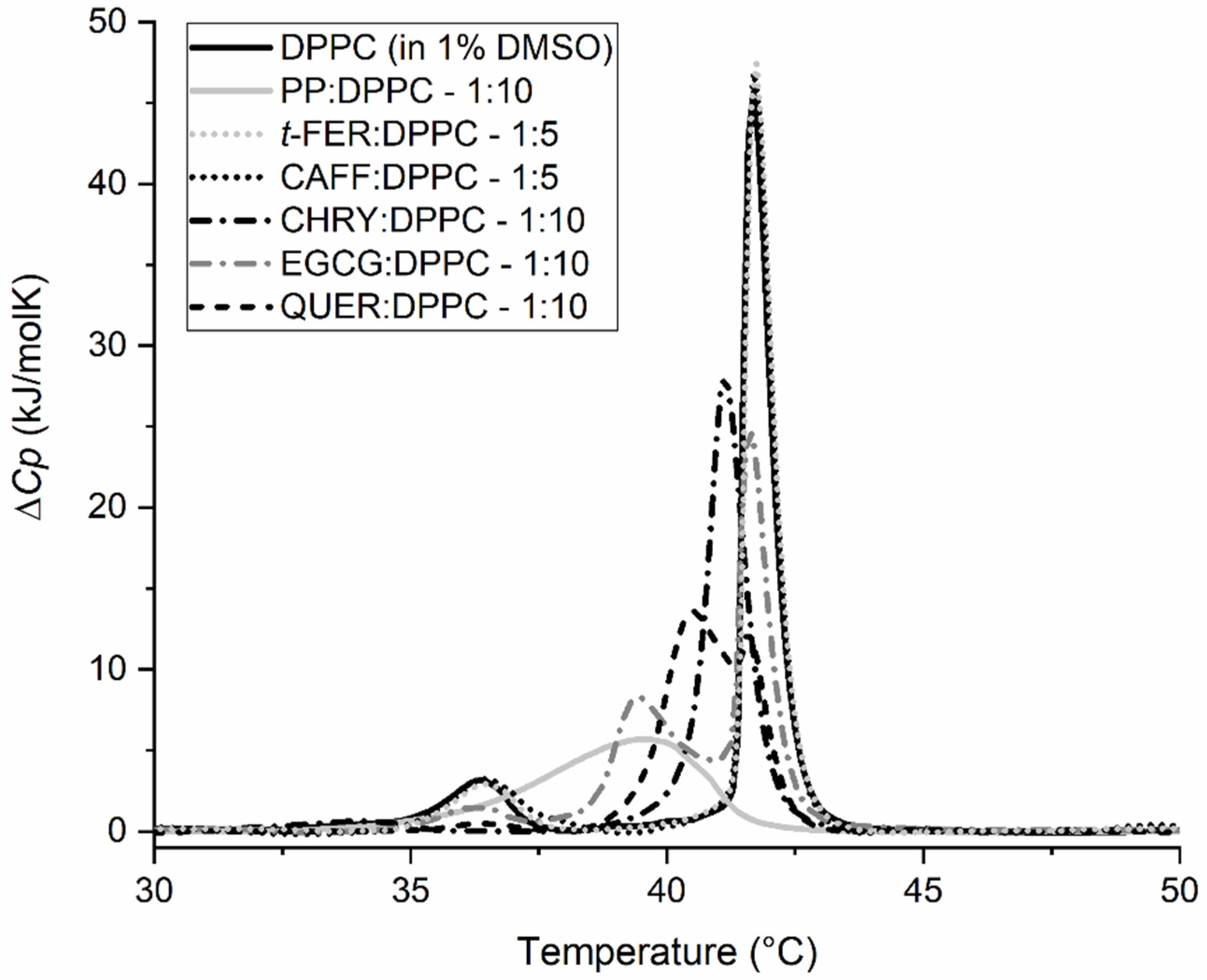
2.2. Synergistic Effects of Compounds from Propolis on the Gel-to-Liquid Crystalline Phase Transition of DPPC Lipids
3. Materials and Methods
3.1. Materials
3.2. Bacterial Strains and Culture Preparations
3.3. Preparation of Liposomes
3.4. Differential Scanning Calorimetry
3.5. Antimicrobial Activity of Propolis Extract/Powder Based on Agar Disc-Diffusion Assay
3.6. Thiobarbituric Acid Reacting Substances (TBARS) Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, X.; Wang, K.; Cao, X.; Zhang, C.; Zheng, H.; Hu, F. Antioxidant activities and molecular mechanisms of the ethanol extracts of Baccharis propolis and Eucalyptus propolis in RAW64.7 cells. Pharm. Biol. 2016, 54, 2220–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šturm, L.; Poklar Ulrih, N. Advances in the Propolis Chemical Composition between 2013 and 2018: A Review. eFood 2019, 1, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Mello, B.C.B.S.; Petrus, J.C.C.; Hubinger, M.D. Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nanofiltration. J. Food Eng. 2010, 96, 533–539. [Google Scholar] [CrossRef]
- Busch, V.M.; Pereyra-Gonzalez, A.; Šegatin, N.; Santagapita, P.R.; Poklar Ulrih, N.; Buera, M.P. Propolis encapsulation by spray drying: Characterization and stability. LWT 2017, 75, 227–235. [Google Scholar] [CrossRef]
- da Silva, F.C.; da Fonseca, C.R.; de Alencar, S.M.; Thomazini, M.; Balieiro, J.C.d.C.; Pittia, P.; Favaro-Trindade, C.S. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using gum Arabic and OSA starch-based carrier systems. Food Bioprod. Process. 2013, 91, 28–36. [Google Scholar] [CrossRef]
- Elbaz, N.M.; Khalil, I.A.; Abd-Rabou, A.A.; El-Sherbiny, I.M. Chitosan-based nano-in-microparticle carriers for enhanced oral delivery and anticancer activity of propolis. Int. J. Biol. Macromol. 2016, 92, 254–269. [Google Scholar] [CrossRef]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of non-dewaxed propolis by freeze-drying and spray-drying using gum Arabic, maltodextrin and inulin as coating materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Mascheroni, E.; Figoli, A.; Musatti, A.; Limbo, S.; Drioli, E.; Suevo, R.; Talarico, S.; Rollini, M. An alternative encapsulation approach for production of active chitosan propolis beads. Int. J. Food Sci. Technol. 2014, 49, 1401–1407. [Google Scholar] [CrossRef]
- Mavri, A.; Abramovič, H.; Polak, T.; Bertoncelj, J.; Jamnik, P.; Smole Možina, S.; Jeršek, B. Chemical Properties and Antioxidant and Antimicrobial Activities of Slovenian Propolis. Chem. Biodivers. 2012, 9, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.R.; Mendonça, R.Z.; Vilar, K.d.S.; Figueiredo, C.A.; Badari, J.C.; Taniwaki, N.; Namiyama, G.; Oliveira, M.I.d.; Curti, S.P.; Evelyn Silva, P.; et al. Antiviral Action of Hydromethanolic Extract of Geopropolis from Scaptotrigona postica against Antiherpes Simplex Virus (HSV-1). Evid. -Based Complement. Altern. Med. 2015, 2015, 296086. [Google Scholar] [CrossRef]
- Agüero, M.B.; Svetaz, L.; Sánchez, M.; Luna, L.; Lima, B.; López, M.L.; Zacchino, S.; Palermo, J.; Wunderlin, D.; Feresin, G.E.; et al. Argentinean Andean propolis associated with the medicinal plant Larrea nitida Cav. (Zygophyllaceae). HPLC–MS and GC–MS characterization and antifungal activity. Food Chem. Toxicol. 2011, 49, 1970–1978. [Google Scholar] [CrossRef]
- Iadnut, A.; Mamoon, K.; Thammasit, P.; Pawichai, S.; Tima, S.; Preechasuth, K.; Kaewkod, T.; Tragoolpua, Y.; Tragoolpua, K. In Vitro Antifungal and Antivirulence Activities of Biologically Synthesized Ethanolic Extract of Propolis-Loaded PLGA Nanoparticles against Candida albicans. Evid. -Based Complement. Altern. Med. 2019, 2019, 3715481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta-Rubio, O.; Campo Fernández, M.; Márquez Hernández, I.; Jaramillo, C.G.J.; González, V.H.; Montes De Oca Porto, R.; Marrero Delange, D.; Monzote Fidalgo, L.; Piccinelli, A.L.; Campone, L.; et al. Chemical profile and anti-leishmanial activity of three Ecuadorian propolis samples from Quito, Guayaquil and Cotacachi regions. Fitoterapia 2017, 120, 177–183. [Google Scholar] [CrossRef]
- Mitsui, T.; Hotta, S.; Tazawa, S.; Arai, Y.; Kato, K.; Ichihara, K. Chemical constituents of Brazilian Propolis from the state of Bahia and their growth inhibitory activities against cancer cells. Biosci. Biotechnol. Biochem. 2018, 82, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Nori, M.P.; Favaro-Trindade, C.S.; Matias de Alencar, S.; Thomazini, M.; de Camargo Balieiro, J.C.; Contreras Castillo, C.J. Microencapsulation of propolis extract by complex coacervation. LWT-Food Sci. Technol. 2011, 44, 429–435. [Google Scholar] [CrossRef]
- Aytekin, A.A.; Tuncay Tanrıverdi, S.; Aydın Köse, F.; Kart, D.; Eroğlu, İ.; Özer, Ö. Propolis loaded liposomes: Evaluation of antimicrobial and antioxidant activities. J. Liposome Res. 2020, 30, 107–116. [Google Scholar] [CrossRef]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Siddalingam, R.P.; Yuen, K.H.; Ambu, S.P.; Davamani, F. Chitosan-propolis nanoparticle formulation demonstrates anti-bacterial activity against Enterococcus faecalis biofilms. PLoS ONE 2017, 12, e0174888. [Google Scholar] [CrossRef] [Green Version]
- Abram, V.; Berlec, B.; Ota, A.; Šentjurc, M.; Blatnik, P.; Ulrih, N.P. Effect of flavonoid structure on the fluidity of model lipid membranes. Food Chem. 2013, 139, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Abramovič, H.; Abram, V.; Poklar Ulrih, N. Interactions of p-coumaric, caffeic and ferulic acids and their styrenes with model lipid membranes. Food Chem. 2011, 125, 1256–1261. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Misiak, L.E.; Zarzyka, B.; Paduch, R.; Gawron, A.; Gruszecki, W.I. FTIR, 1H NMR and EPR spectroscopy studies on the interaction of flavone apigenin with dipalmitoylphosphatidylcholine liposomes. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, A.; D’Errico, G.; Lauro, M.R.; Sansone, F.; Di Marino, S.; D’Ursi, A.M.; Aquino, R.P. Effect of flavonoids on the Aβ(25-35)-phospholipid bilayers interaction. Eur. J. Med. Chem. 2010, 45, 3998–4003. [Google Scholar] [CrossRef]
- Tsuchiya, H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. [Google Scholar] [CrossRef]
- Ulrih, N.P.; Maričić, M.; Ota, A.; Šentjurc, M.; Abram, V. Kaempferol and quercetin interactions with model lipid membranes. Food Res. Int. 2015, 71, 146–154. [Google Scholar] [CrossRef]
- Mendanha, S.A.; Alonso, A. Effects of terpenes on fluidity and lipid extraction in phospholipid membranes. Biophys. Chem. 2015, 198, 45–54. [Google Scholar] [CrossRef]
- Mendanha, S.A.; Moura, S.S.; Anjos, J.L.V.; Valadares, M.C.; Alonso, A. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol. In Vitro 2013, 27, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Witzke, S.; Duelund, L.; Kongsted, J.; Petersen, M.; Mouritsen, O.G.; Khandelia, H. Inclusion of terpenoid plant extracts in lipid bilayers investigated by molecular dynamics simulations. J. Phys. Chem. B 2010, 114, 15825–15831. [Google Scholar] [CrossRef]
- Zalba, S.; ten Hagen, T.L.M. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef] [Green Version]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid Based Complement Alternat Med 2013, 2013, 964149. [Google Scholar] [CrossRef] [Green Version]
- Amoros, M.; Simões, C.M.; Girre, L.; Sauvager, F.; Cormier, M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992, 55, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Ashida, H.; Danno, G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Berić, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crops Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postepy Higieny i Medycyny Doswiadczalnej 2014, 68, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793. [Google Scholar] [CrossRef] [Green Version]
- Ilhan-Ayisigi, E.; Ulucan, F.; Saygili, E.; Saglam-Metiner, P.; Gulce-Iz, S.; Yesil-Celiktas, O. Nano-vesicular formulation of propolis and cytotoxic effects in a 3D spheroid model of lung cancer. J. Sci. Food Agric. 2020, 100, 3525–3535. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Luo, Y.; Liu, Y.; Wang, X.-T.; Liu, F.; Guo, M.-Z.; Wang, Z.; Liu, A.-J.; Zhang, Y.-M. Inclusion of chrysin in β-cyclodextrin and its biological activities. J. Drug Deliv. Sci. Technol. 2016, 31, 176–186. [Google Scholar] [CrossRef]
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C.; Gualtar, C.D. How do bees prevent hive infections? The antimicrobial properties of propolis. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 481–493. [Google Scholar]
- Kalogeropoulos, N.; Konteles, S.J.; Troullidou, E.; Mourtzinos, I.; Karathanos, V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009, 116, 452–461. [Google Scholar] [CrossRef]
- Quinto, E.J.; Jiménez, P.; Caro, I.; Tejero, J.; Mateo, J.; Girbés, T. Probiotic Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2014, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, T.; Maeno, S.; Kishi, S.; Fujii, T.; Tanno, H.; Hirano, K.; Tochio, T.; Tanizawa, Y.; Arita, M.; Yokota, S.-i.; et al. Oligosaccharide Metabolism and Lipoteichoic Acid Production in Lactobacillus gasseri and Lactobacillus paragasseri. Microorganisms 2021, 9, 1590. [Google Scholar] [CrossRef]
- Osojnik Črnivec, I.G.; Neresyan, T.; Gatina, Y.; Kolmanič Bučar, V.; Skrt, M.; Dogša, I.; Bogovič Matijašić, B.; Kulikova, I.; Lodygin, A.; Poklar Ulrih, N. Polysaccharide Hydrogels for the Protection of Dairy-Related Microorganisms in Adverse Environmental Conditions. Molecules 2021, 26, 7484. [Google Scholar] [CrossRef]
- Petruzzi, L.; Rosaria Corbo, M.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. [Google Scholar] [CrossRef]
- da Cruz Almeida, E.T.; da Silva, M.C.D.; dos Santos Oliveira, J.M.; Kamiya, R.U.; dos Santos Arruda, R.E.; Vieira, D.A.; da Costa Silva, V.; Escodro, P.B.; Basílio-Júnior, I.D.; do Nascimento, T.G. Chemical and microbiological characterization of tinctures and microcapsules loaded with Brazilian red propolis extract. J. Pharm. Anal. 2017, 7, 280–287. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Maia, D.S.V.; Krumreich, F.D.; Crizel-Cardoso, M.M.; Fioravante, J.B.; da Silva, W.P.; Borges, C.D.; Zambiazi, R.C. Propolis microparticles produced with pea protein: Characterization and evaluation of antioxidant and antimicrobial activities. Food Hydrocoll. 2019, 87, 703–711. [Google Scholar] [CrossRef]
- Barbosa, V.T.; Souza, J.K.C.; Alvino, V.; Meneghetti, M.R.; Florez-Rodriguez, P.P.; Moreira, R.E.; Paulino, G.V.B.; Landell, M.F.; Basílio-Júnior, I.D.; do Nascimento, T.G.; et al. Biogenic synthesis of silver nanoparticles using Brazilian propolis. Biotechnol. Prog. 2019, 35, e2888. [Google Scholar] [CrossRef]
- Hegazi, A.G.; El-Houssiny, A.S.; Fouad, E.A. Egyptian propolis 14: Potential antibacterial activity of propolis-encapsulated alginate nanoparticles against different pathogenic bacteria strains. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045019. [Google Scholar] [CrossRef]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: A patent review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Stompor-Gorący, M.; Machaczka, M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. Int. J. Mol. Sci. 2021, 22, 12889. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- dos Santos, L.; Hochheim, S.; Boeder, A.M.; Kroger, A.; Tomazzoli, M.M.; Dal Pai Neto, R.; Maraschin, M.; Guedes, A.; de Cordova, C.M.M. Chemical characterization, antioxidant, cytotoxic and antibacterial activity of propolis extracts and isolated compounds from the Brazilian stingless bees Melipona quadrifasciata and Tetragonisca angustula. J. Apic. Res. 2017, 56, 543–558. [Google Scholar] [CrossRef]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of Flavonoids toward Cultured Normal Human Cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Wätjen, W.; Michels, G.; Steffan, B.r.; Niering, P.; Chovolou, Y.; Kampkötter, A.; Tran-Thi, Q.-H.; Proksch, P.; Kahl, R. Low Concentrations of Flavonoids Are Protective in Rat H4IIE Cells Whereas High Concentrations Cause DNA Damage and Apoptosis. J. Nutr. 2005, 135, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Šturm, L.; Prislan, I.; González-Ortega, R.; Mrak, P.; Snoj, T.; Anderluh, G.; Poklar Ulrih, N. Interactions of (−)-epigallocatechin-3-gallate with model lipid membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2022, 1864, 183999. [Google Scholar] [CrossRef]
- Nakayama, T.; Ono, K.; Hashimoto, K. Affinity of Antioxidative Polyphenols for Lipid Bilayers Evaluated with a Liposome System. Biosci. Biotechnol. Biochem. 1998, 62, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free. Radic. Biol. Med. 1995, 19, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Gadhwal, M.K.; Joshi, U.J.; Srivastava, S.; Govil, G.; Kundanani, K.M.; Parade, C. Interaction of quercetin with DPPC model membrane: Molecular dynamic simulation, DSC and multinuclear NMR studies. J. Indian Chem. Soc. 2011, 88, 1203. [Google Scholar]
- Sinha, R.; Joshi, A.; Joshi, U.J.; Srivastava, S.; Govil, G. Localization and interaction of hydroxyflavones with lipid bilayer model membranes: A study using DSC and multinuclear NMR. Eur. J. Med. Chem. 2014, 80, 285–294. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-type Propolis: Chemical Composition, Botanical Origin and Biological Activity. Nat. Prod. Commun. 2015, 10, 1934578X1501001117. [Google Scholar] [CrossRef] [Green Version]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance Liquid Chromatography and Mass Spectrometry (UHPLC–LTQ/Orbitrap/MS/MS) Study of Phenolic Profile of Serbian Poplar Type Propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef]
- Volpi, N. Separation of flavonoids and phenolic acids from propolis by capillary zone electrophoresis. Electrophoresis 2004, 25, 1872–1878. [Google Scholar] [CrossRef]
- Rodríguez Vaquero, M.J.; Aredes Fernández, P.A.; Manca de Nadra, M.C.; Strasser de Saad, A.M. Phenolic Compound Combinations on Escherichia coli Viability in a Meat System. J. Agric. Food Chem. 2010, 58, 6048–6052. [Google Scholar] [CrossRef]
- González-Búrquez, M.d.J.; González-Díaz, F.R.; García-Tovar, C.G.; Carrillo-Miranda, L.; Soto-Zárate, C.I.; Canales-Martínez, M.M.; Penieres-Carrillo, J.G.; Crúz-Sánchez, T.A.; Fonseca-Coronado, S. Comparison between In Vitro Antiviral Effect of Mexican Propolis and Three Commercial Flavonoids against Canine Distemper Virus. Evid. -Based Complement. Altern. Med. 2018, 2018, 7092416. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Amau, M.; Okamoto, Y.; Suga, K.; Umakoshi, H. Investigation of Quercetin interaction behaviors with lipid bilayers: Toward understanding its antioxidative effect within biomembrane. J. Biosci. Bioeng. 2021, 132, 49–55. [Google Scholar] [CrossRef]
- Keith, C.T.; Borisy, A.A.; Stockwell, B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ryall, K.A.; Tan, A.C. Systems biology approaches for advancing the discovery of effective drug combinations. J. Cheminformatics 2015, 7, 7. [Google Scholar] [CrossRef]
- Savka, M.A.; Dailey, L.; Popova, M.; Mihaylova, R.; Merritt, B.; Masek, M.; Le, P.; Nor, S.R.M.; Ahmad, M.; Hudson, A.O.; et al. Chemical Composition and Disruption of Quorum Sensing Signaling in Geographically Diverse United States Propolis. Evid.-Based Complement. Altern. Med. 2015, 2015, 472593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Isenberg, H.D.; Garcia, L.S. Clinical Microbiology Procedures Handbook, 2nd ed.; ASM Press: Washington, DC, USA, 2007; Volume 1, p. 2274. [Google Scholar]
- Lasch, J.; Weissig, V.; Brandl, M. Preparation of liposomes. In Liposomes—A practical approach, 2nd ed.; Torchilin, V.P., Weissig, V., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 3–30. [Google Scholar]
- Perrett, S.; Golding, M.; Williams, W.P. A simple method for the preparation of liposomes for pharmaceutical applications: Characterization of the liposomes. J. Pharm. Pharmacol. 1991, 43, 154–161. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Balanč, B.D.; Ota, A.; Djordjević, V.B.; Šentjurc, M.; Nedović, V.A.; Bugarski, B.M.; Ulrih, N.P. Resveratrol-loaded liposomes: Interaction of resveratrol with phospholipids. Eur. J. Lipid Sci. Technol. 2015, 117, 1615–1626. [Google Scholar] [CrossRef]




| Suspensions (mg of Propolis/mL) | E. coli | L. bulgaricus | L. paragasseri | L. plantarum | L. rhamnosus |
|---|---|---|---|---|---|
| 100% EtOH | / | 11.3 ± 3.0 | 13.8 ± 0.8 | 12.8 ± 1.3 | 14.3 ± 0.4 |
| 50% EtOH | / | / | / | / | 7.5 ± 0.5 * |
| Milli-Q water | / | / | / | / | / |
| 200 mg/mL (PP) (100% EtOH) | / | 18.5 ± 2.5 | 16.0 ± 0.5 | 13.5 ± 0.5 | 17.0 ± 0.5 |
| 10 mg/mL (PP) (50% EtOH) | / | 8.5 ± 0.5 | 7.8 ± 0.8 | 6.5 ± 0.5 * | 9.5 ± 0.5 |
| 2 mg/mL (PP) (50% EtOH) | / | / | / | / | 8.0 ± 1.0 |
| 0.2 mg/mL (PP) (50% EtOH) | / | / | / | / | 8.0 ± 0.5 * |
| 0.02 mg/mL (PP) (50% EtOH) | / | / | / | / | 7.3 ± 0.8 * |
| Compound | Ratio (n:n) | Tpre (°C) | T1 (°C) | T2 (°C) | ΔHpre (kJ/mol) | ΔH1+2 (kJ/mol) |
|---|---|---|---|---|---|---|
| CHRY:DPPC | 1:1 | 35.6 ± 0.2 | 40.7 ± 0.1 | / | 3.6 ± 0.5 | 40.1 ± 1.0 |
| CHRY:DPPC | 1:5 | 33.7 ± 0.2 | 41.2 ± 0.1 | / | 2.8 ± 0.5 | 32.6 ± 1.0 |
| CHRY:DPPC | 1:10 | 34.0 ± 0.2 | 41.2 ± 0.1 | / | 1.8 | 30.1 ± 1.0 |
| QUER:DPPC | 1:2 | / | 36.4 ± 0.1 | / | / | 19.5 ± 1.0 |
| QUER:DPPC | 1:5 | / | 39.8 ± 0.1 | 41.5 ± 0.1 | / | 30.6 ± 1.0 |
| QUER:DPPC | 1:10 | 36.4 ± 0.2 | 40.5 ± 0.1 | 41.6 ± 0.1 | 0.7 ± 0.1 | 28.8 ± 1.0 |
| CAFF:DPPC | 1:1 | 35.9 ± 0.2 | 41.7 ± 0.1 | / | 3.4 ± 0.5 | 31.9 ± 1.0 |
| CAFF:DPPC | 1:5 | 36.5 ± 0.2 | 41.7 ± 0.1 | / | 5.7 ± 0.5 | 33.8 ± 1.0 |
| t-FER:DPPC | 1:1 | 36.0 ± 0.2 | 41.7 ± 0.1 | / | 3.5 ± 0.5 | 34.0 ± 1.0 |
| t-FER:DPPC | 1:5 | 36.4 ± 0.2 | 41.7 ± 0.1 | / | 4.8 ± 0.5 | 34.8 ± 1.0 |
| EGCG:DPPC | 1:1 | / | 38.7 ± 0.1 | 41.6 ± 0.1 | / | 38.4 ± 1.0 |
| EGCG:DPPC | 1:5 | / | 37.9 ± 0.1 | 41.6 ± 0.1 | / | 35.9 ± 1.0 |
| EGCG:DPPC | 1:10 | 36.2 ± 0.2 | 39.4 ± 0.1 | 41.7 ± 0.1 | 2.7 ± 0.5 | 34.1 ± 1.0 |
| Mix:DPPC | 9:10 | / | 36.8 ± 0.1 | / | / | 20.7 ± 1.0 |
| DPPC (1% DMSO) | / | 36.4 ± 0.2 | 41.7 ± 0.1 | / | 5.4 ± 0.5 | 33.8 ± 1.0 |
| DPPC | / | 36.5 ± 0.2 | 41.6 ± 0.1 | / | 5.6 ± 1.0 | 34.2 ± 1.0 |
| Compound | Ratio (n:n) | Tpre (°C) | T1 (°C) | T2 (°C) | ΔHpre (kJ/mol) | ΔH1+2 (kJ/mol) |
|---|---|---|---|---|---|---|
| QUER:CAFF:DPPC | 1:2:10 | 36.4 ± 0.2 | 40.4 ± 0.1 | 41.6 ± 0.1 | 0.8 ± 0.1 | 28.9 ± 1.0 |
| QUER:CHRY:DPPC | 1:2:10 | / | 37.5 ± 0.1 | / | / | 19.8 ± 1.0 |
| QUER:DPPC | 1:10 | 36.4 ± 0.2 | 40.5 ± 0.1 | 41.6 ± 0.1 | 0.7 ± 0.1 | 28.8 ± 1.0 |
| QUER:DPPC | 3:10 | / | 38.8 ± 0.1 | 41.6 ± 0.1 | / | 24.3 ± 1.0 |
| CAFF:DPPC | 2:10 | 36.5 ± 0.2 | 41.7 ± 0.1 | / | 5.7 ± 0.5 | 33.8 ± 1.0 |
| CHRY:DPPC | 2:10 | 33.7 ± 0.2 | 41.2 ± 0.1 | / | 2.8 ± 0.5 | 32.6 ± 1.0 |
| CHRY:DPPC | 3:10 | 34.1 ± 0.2 | 41.1 ± 0.1 | / | 3.1 ± 0.5 | 31.5 ± 1.0 |
| DPPC (1% DMSO) | / | 36.4 ± 0.2 | 41.7 ± 0.1 | / | 5.4 ± 0.5 | 33.8 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šturm, L.; Osojnik Črnivec, I.G.; Prislan, I.; Ulrih, N.P. Comparing the Effects of Encapsulated and Non-Encapsulated Propolis Extracts on Model Lipid Membranes and Lactic Bacteria, with Emphasis on the Synergistic Effects of Its Various Compounds. Molecules 2023, 28, 712. https://doi.org/10.3390/molecules28020712
Šturm L, Osojnik Črnivec IG, Prislan I, Ulrih NP. Comparing the Effects of Encapsulated and Non-Encapsulated Propolis Extracts on Model Lipid Membranes and Lactic Bacteria, with Emphasis on the Synergistic Effects of Its Various Compounds. Molecules. 2023; 28(2):712. https://doi.org/10.3390/molecules28020712
Chicago/Turabian StyleŠturm, Luka, Ilja Gasan Osojnik Črnivec, Iztok Prislan, and Nataša Poklar Ulrih. 2023. "Comparing the Effects of Encapsulated and Non-Encapsulated Propolis Extracts on Model Lipid Membranes and Lactic Bacteria, with Emphasis on the Synergistic Effects of Its Various Compounds" Molecules 28, no. 2: 712. https://doi.org/10.3390/molecules28020712






