Effective Usage of Biochar and Microorganisms for the Removal of Heavy Metal Ions and Pesticides
Abstract
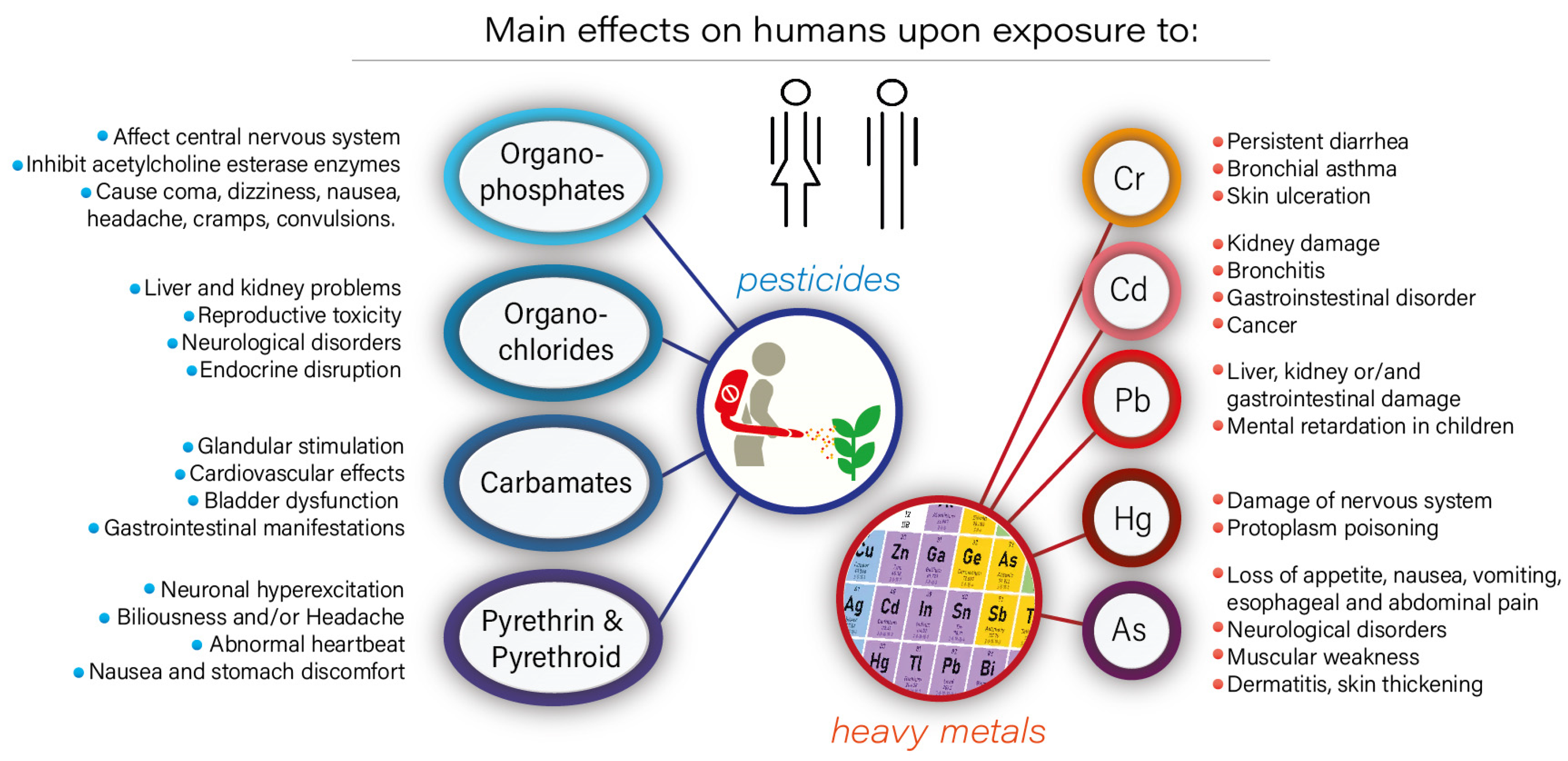
1. Introduction
2. Role of Biochar in the Removal of Metal Ions and Pesticides
2.1. Biochar Production, Properties, and Characterization
2.1.1. Biochar Production
2.1.2. Biochar: Physicochemical Properties and Characterization
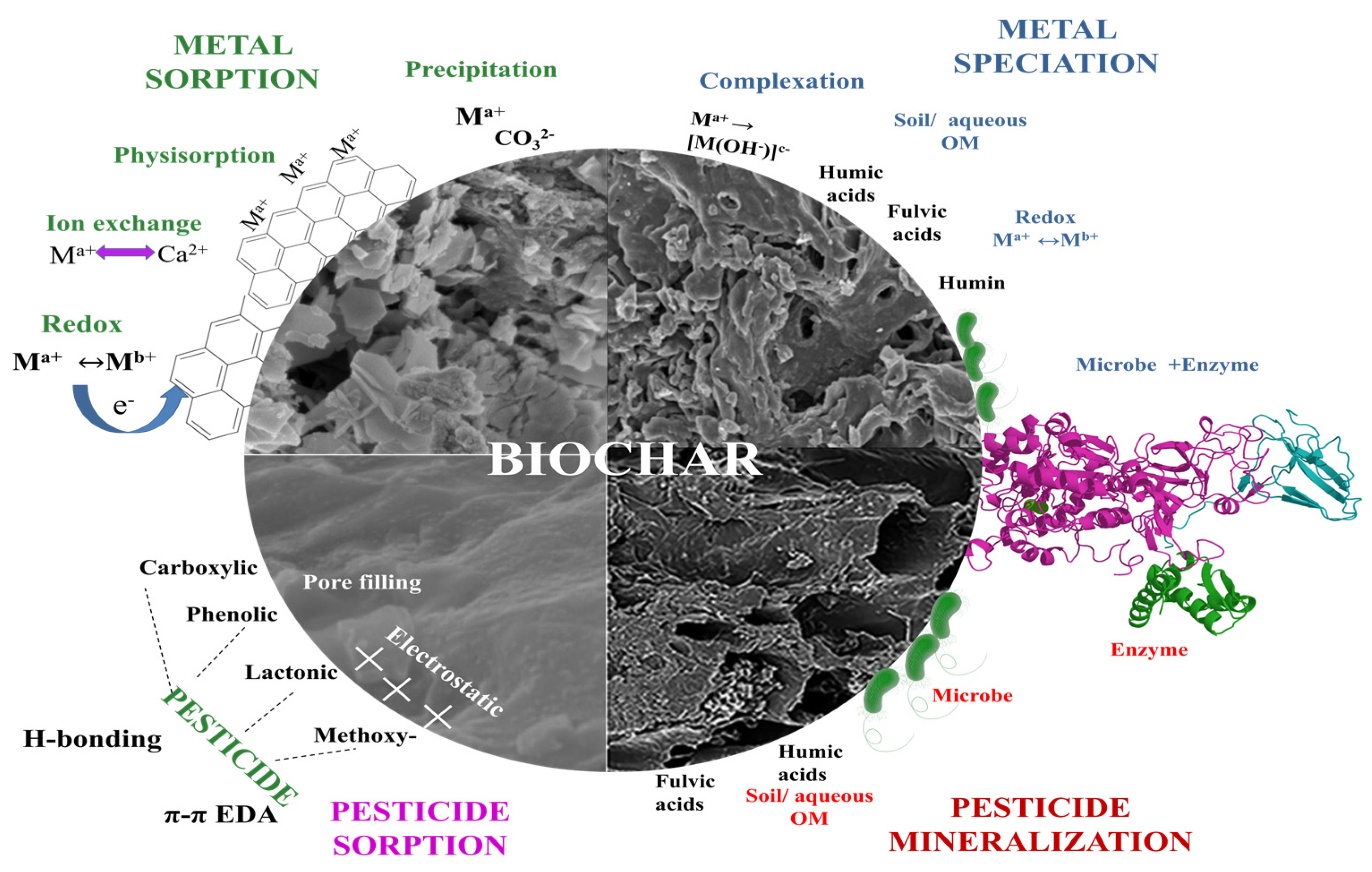
2.2. Biochar as Adsorbents
2.2.1. Removal of Metal Ions
2.2.2. Adsorption/Removal of Pesticides
2.3. Biochar as a Bioremediation Catalyst Support
3. Role of Microorganisms in the Removal of Metal Ions and Pesticides
- (i)
- Environmental factors
- (ii)
- Type of microorganism and degradation capacity
- (iii)
- Bioavailability of the contaminants
- (iv)
- Aerobic or anaerobic operating conditions
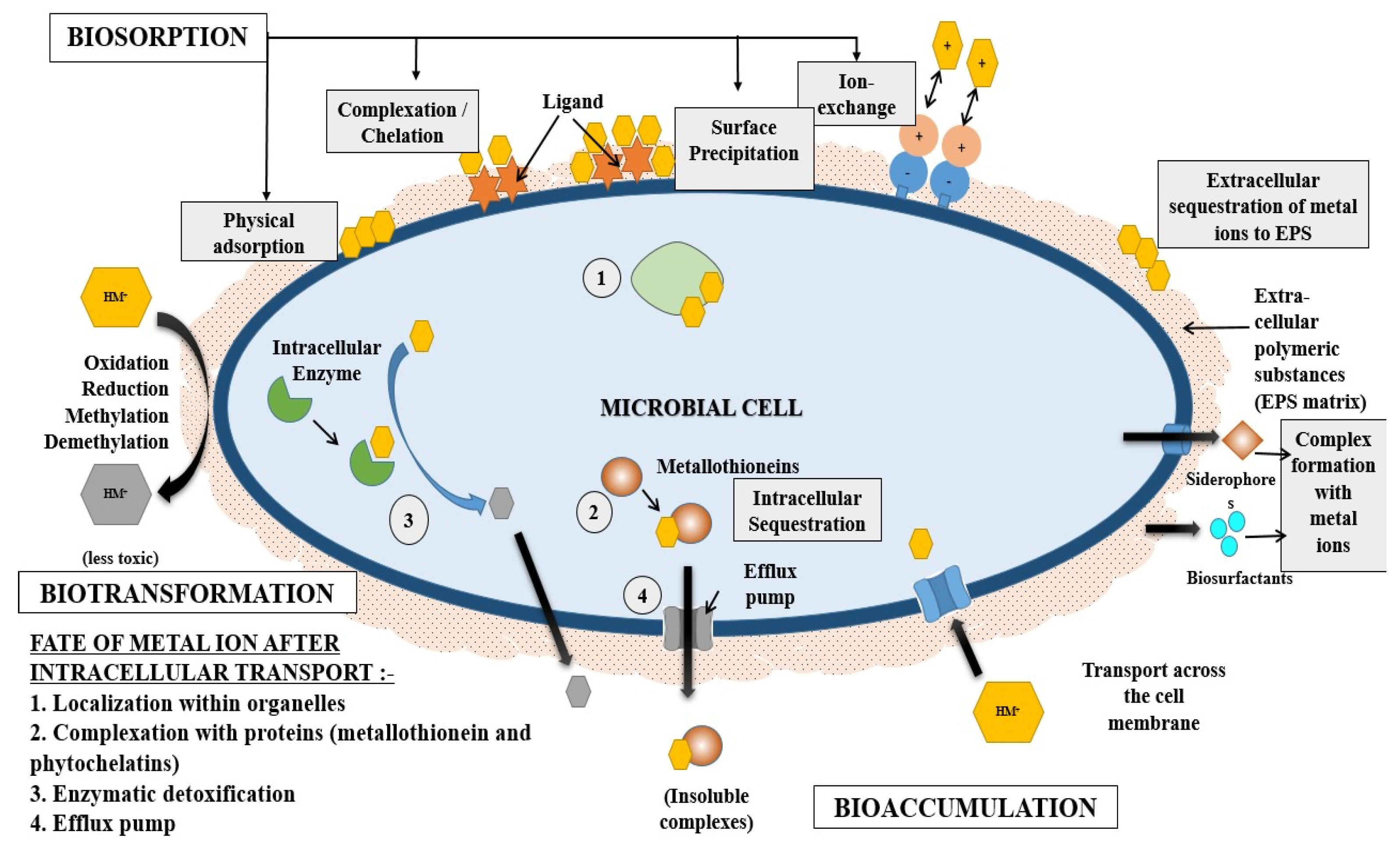
3.1. Removal of Heavy Metals Using Microorganisms
The Mechanism of Heavy Metal Removal by Microorganisms
3.2. Removal of Pesticides Using Microorganisms
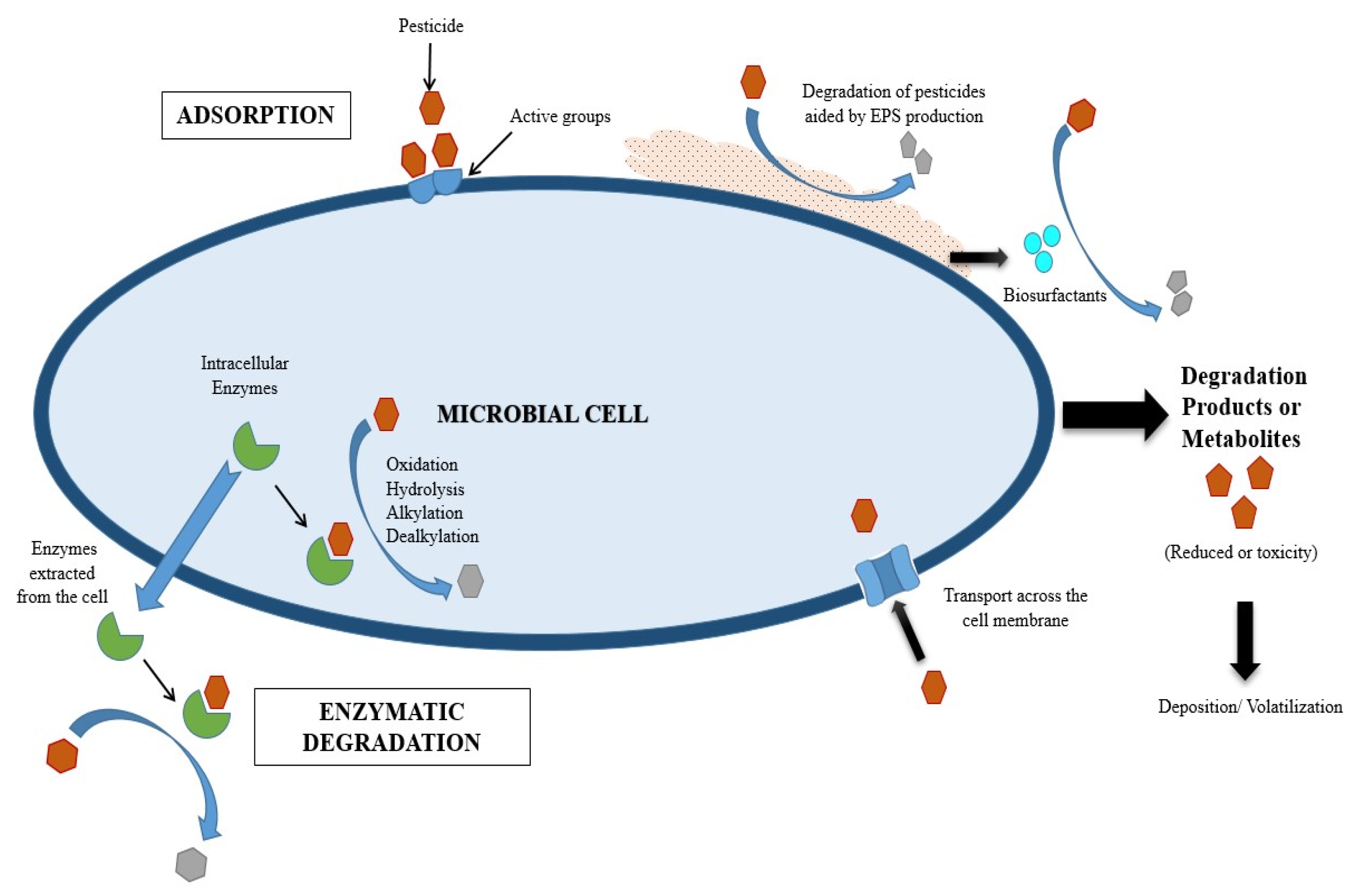
Mechanisms Involved in Pesticide Removal by Microorganisms
3.3. Challenges of Using Microorganisms as a Catalyst
4. Microbial Cell-Immobilized Biochar for the Removal of Metal Ions and Pesticides
4.1. Immobilization Methods
4.2. Factors that Influence Bioremediation Using Immobilized Microorganisms
4.3. Heavy Metal Ions and Pesticide Removal Using MCB
5. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priyadarshanee, M.; Das, S. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104686. [Google Scholar] [CrossRef]
- Amin, M.; Chetpattananondh, P. Biochar from extracted marine Chlorella sp. residue for high efficiency adsorption with ultrasonication to remove Cr(VI), Zn(II) and Ni(II). Bioresour. Technol. 2019, 289, 121578. [Google Scholar] [CrossRef]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide pollution in agricultural soils and sustainable remediation methods: A review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.M.; Qin, L.; et al. Degradation of naphthalene with magnetic biochar activate hydrogen peroxide: Synergism of bio-char and Fe–Mn binary oxides. Water Res. 2019, 160, 238–248. [Google Scholar] [CrossRef]
- Kung, C.C.; Mu, J.E. Prospect of China’s renewable energy development from pyrolysis and biochar applications under climate change. Renew. Sustain. Energy Rev. 2019, 114, 109343. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Kumar, S.; Loganathan, V.A.; Gupta, R.B.; Barnett, M.O. An Assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J. Environ. Manag. 2011, 92, 2504–2512. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Dai, M.; Ali, I.; Shen, X.; Hou, X.; Alhewairini, S.S.; Peng, C.; Naz, I. Application of microbial immobilization technology for remediation of Cr(VI) contamination: A review. Chemosphere 2022, 286, 131721. [Google Scholar] [CrossRef]
- Karimi, H.; Mahdavi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rajput, V.D.; Minkina, T.; Astatkie, T. Insights on the bioremediation technologies for pesticide-contaminated soils. Environ. Geochem. Health 2022, 44, 1329–1354. [Google Scholar] [CrossRef]
- Abu Talha, M.; Goswami, M.; Giri, B.S.; Sharma, A.; Rai, B.N.; Singh, R.S. Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Bioresour. Technol. 2018, 252, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Noronha, F.R.; Manikandan, S.K.; Nair, V. Role of coconut shell biochar and earthworm (Eudrilus euginea) in bioremediation and palak spinach (Spinacia oleracea L.) growth in cadmium-contaminated soil. J. Environ. Manag. 2022, 302, 114057. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Gyenge, E.; Ellis, N. A novel method to tailor the porous structure of KOH-activated biochar and its application in capacitive deionization and energy storage. Biomass Bioenergy 2016, 87, 107–121. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Zhou, X.; Zhang, C.; Chen, L.; Yan, H.; Qin, L.; Huang, D.; Ye, H.; Chen, W.; et al. Peroxydisulfate activation by sulfur-doped ordered mesoporous carbon: Insight into the intrinsic relationship between defects and 1O2 generation. Water Res. 2022, 221, 118797. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.-V.N.; Arun, J.; Gopinath, K.P. Production, characterization, activation and environmental applications of engineered biochar: A review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, Y.J.; Kang, J.K.; Lee, J.; Lee, C.G. Application of Fe-impregnated biochar from cattle manure for removing pentavalent antimony from aqueous solution. Appl. Sci. 2021, 11, 9257. [Google Scholar] [CrossRef]
- Kostas, E.T.; Durán-Jiménez, G.; Shepherd, B.J.; Meredith, W.; Stevens, L.A.; Williams, O.S.A.; Lye, G.J.; Robinson, J.P. Microwave pyrolysis of olive pomace for bio-oil and bio-char production. Chem. Eng. J. 2020, 387, 123404. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Wu, X.; Zhou, W.; Zhu, L. Impact of mineral components in cow manure biochars on the adsorption and competitive adsorption of oxytetracycline and carbaryl. RSC Adv. 2017, 7, 2127–2136. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Ju, N.; Ai, Y.; Liu, Y.; Liang, J.; Hu, Z.N.; Guo, R.; Xu, W.; Zhang, W.; et al. Ultimate resourcization of waste: Crab shell-derived biochar for antimony removal and sequential utilization as an anode for a Li-Ion Battery. ACS Sustain. Chem. Eng. 2021, 9, 8813–8823. [Google Scholar] [CrossRef]
- Ding, T.; Huang, T.; Wu, Z.; Li, W.; Guo, K.; Li, J. Adsorption-desorption behavior of carbendazim by sewage sludge-derived biochar and its possible mechanism. RSC Adv. 2019, 9, 35209–35216. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N.; et al. Advances and future directions of biochar characterization methods and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.P.; Lima, J.Z.; Marques, J.P.; Massaro Sousa, L.; Rodrigues, V.G.S.; Espíndola, E.L.G. A review of pesticides sorption in biochar from maize, rice, and wheat residues: Current status and challenges for soil application. J. Environ. Manag. 2021, 300, 113753. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod. 2020, 265, 121762. [Google Scholar] [CrossRef]
- Xu, Z.; He, M.; Xu, X.; Cao, X.; Tsang, D.C.W. Impacts of different activation processes on the carbon stability of biochar for oxidation resistance. Bioresour. Technol. 2021, 338, 125555. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xu, Y.; Wang, L.; Liang, X.; Sun, Y.; Jia, H. Effect of different pyrolysis temperatures on physico-chemical characteristics and lead(II) removal of biochar derived from chicken manure. RSC Adv. 2020, 10, 3667–3674. [Google Scholar]
- Pandey, D.; Daverey, A.; Arunachalam, K. Biochar: Production, properties and emerging role as a support for enzyme immobilization. J. Clean. Prod. 2020, 255, 120267. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. Recent advancements in biochar preparation, feedstocks, modification, characterization and future applications. Environ. Technol. Rev. 2019, 8, 47–64. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Watcharenwong, A.; Chokejaroenrat, C.; Rittirat, A. Leonardite-derived biochar suitability for effective sorption of herbicides. Water Air Soil Pollut. 2021, 232, 36. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lamb, D.; Rahman, M.M.; Bahar, M.M.; Sanderson, P.; Abbasi, S.; Bari, A.S.M.F.; Naidu, R. Removal of arsenate from contaminated waters by novel zirconium and zirconium-iron modified biochar. J. Hazard. Mater. 2021, 409, 124488. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Wang, J.J.; Kim, S.H.; Kang, S.W.; Jeong, C.Y.; Jeon, J.R.; Park, K.H.; Cho, J.S.; Delaune, R.D.; Seo, D.C. Cadmium adsorption characteristics of biochars derived using various pine tree residues and pyrolysis temperatures. J. Colloid Interface Sci. 2019, 553, 298–307. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Imran, M.; Iqbal, M.M.; Iqbal, J.; Shah, N.S.; Khan, Z.U.H.; Murtaza, B.; Amjad, M.; Ali, S.; Rizwan, M. Synthesis, characterization and application of novel MnO and CuO impregnated biochar composites to sequester arsenic (As) from water: Modeling, thermodynamics and reusability. J. Hazard. Mater. 2021, 401, 123338. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, J.; Jin, Q.; Zhang, X.; Yang, H.; Chen, Y.; Zhang, S.; Chen, H. Effect of deashing on activation process and lead adsorption capacities of sludge-based biochar. Sci. Total Environ. 2020, 716, 137016. [Google Scholar] [CrossRef]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef]
- Ma, J.; Huang, W.; Zhang, X.; Li, Y.; Wang, N. The utilization of lobster shell to prepare low-cost biochar for high-efficient removal of copper and cadmium from aqueous: Sorption properties and mechanisms. J. Environ. Chem. Eng. 2021, 9, 104703. [Google Scholar] [CrossRef]
- Khan, Z.H.; Gao, M.; Qiu, W.; Song, Z. Properties and adsorption mechanism of magnetic biochar modified with molybdenum disulfide for cadmium in aqueous solution. Chemosphere 2020, 255, 126995. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Qiu, L.; Li, Y.; Sun, J.; Gao, B.; He, F.; Wan, W. Accelerated antimony and copper removal by manganese oxide embedded in biochar with enlarged pore structure. Chem. Eng. J. 2020, 402, 126021. [Google Scholar] [CrossRef]
- He, R.; Peng, Z.; Lyu, H.; Huang, H.; Nan, Q.; Tang, J. Synthesis and characterization of an iron-impregnated biochar for aqueous arsenic removal. Sci. Total Environ. 2018, 612, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Peng, Y.; Guo, L.; Wang, Q.; Guan, C.Y.; Yang, F.; Chen, Q. Arsenic adsorption on layered double hydroxides biochars and their amended red and calcareous soils. J. Environ. Manag. 2020, 271, 111045. [Google Scholar] [CrossRef]
- Kwak, J.H.; Islam, M.S.; Wang, S.; Messele, S.A.; Naeth, M.A.; El-Din, M.G.; Chang, S.X. Biochar properties and lead(II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation. Chemosphere 2019, 231, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yan, C.; Luo, Z.; Fang, H.; Hu, S.; Cao, Y. Synthesis of a novel ternary HA/Fe-Mn oxides-loaded biochar composite and its application in cadmium(II) and arsenic(V) adsorption. J. Environ. Sci. 2019, 85, 168–176. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Gui, C.; Li, P.; Zhang, J.; Zhong, H.; Wei, Y. Chemical forms and risk assessment of heavy metals in sludge-biochar produced by microwave-induced low temperature pyrolysis. RSC Adv. 2016, 6, 101960–101967. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, S.; Liu, S.; Xu, S.; Li, J.; Zhang, Y. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Shao, Y.; Huang, W.; Cheng, X. Different adsorption behaviors and mechanisms of a novel amino-functionalized hydrothermal biochar for hexavalent chromium and pentavalent antimony. Bioresour. Technol. 2020, 310, 123438. [Google Scholar] [CrossRef]
- Binh, Q.A.; Nguyen, H.H. Investigation the isotherm and kinetics of adsorption mechanism of herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) on corn cob biochar. Bioresour. Technol. Rep. 2020, 11, 100520. [Google Scholar] [CrossRef]
- Binh, Q.A.; Tungtakanpoung, D.; Kajitvichyanukul, P. Similarities and differences in adsorption mechanism of dichlorvos and pymetrozine insecticides with coconut fiber biowaste sorbent. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2020, 55, 103–114. [Google Scholar] [CrossRef]
- Liu, L.; Fang, W.; Yuan, M.; Li, X.; Wang, X.; Dai, Y. Metolachlor-adsorption on the walnut shell biochar modified by the fulvic acid and citric acid in water. J. Environ. Chem. Eng. 2021, 9, 106238. [Google Scholar] [CrossRef]
- Vimal, V.; Patel, M.; Mohan, D. Aqueous carbofuran removal using slow pyrolyzed sugarcane bagasse biochar: Equilibrium and fixed-bed studies. RSC Adv. 2019, 9, 26338–26350. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U.; Mohan, D.; Mlsna, T. Adsorption of metribuzin from aqueous solution using magnetic and nonmagnetic sustainable low-cost biochar adsorbents. Environ. Sci. Pollut. Res. 2017, 24, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, J.; Chang, J.; Guo, T.; Yang, Q.; Jia, W.; Shen, S. Characterization of an Hg(II)-volatilizing Pseudomonas sp. strain, DC-B1, and its potential for soil remediation when combined with biochar amendment. Ecotoxicol. Environ. Saf. 2018, 163, 172–179. [Google Scholar] [CrossRef]
- Qiao, J.; Li, X.; Li, F. Roles of different active metal-reducing bacteria in arsenic release from arsenic-contaminated paddy soil amended with biochar. J. Hazard. Mater. 2018, 344, 958–967. [Google Scholar] [CrossRef]
- Qiao, J.T.; Li, X.M.; Hu, M.; Li, F.B.; Young, L.Y.; Sun, W.M.; Huang, W.; Cui, J.H. Transcriptional activity of arsenic-reducing bacteria and genes regulated by lactate and biochar during arsenic transformation in flooded paddy soil. Environ. Sci. Technol. 2018, 52, 61–70. [Google Scholar] [CrossRef]
- An, X.; Chen, Y.; Ao, M.; Jin, Y.; Zhan, L.; Yu, B.; Wu, Z.; Jiang, P. Sequential photocatalytic degradation of organophosphorus pesticides and recovery of orthophosphate by biochar/α-Fe2O3/MgO composite: A new enhanced strategy for reducing the impacts of organophosphorus from wastewater. Chem. Eng. J. 2022, 435, 135087. [Google Scholar] [CrossRef]
- Huang, W.H.; Wu, R.M.; Chang, J.S.; Juang, S.Y.; Lee, D.J. Pristine and manganese ferrite modified biochars for copper ion adsorption: Type-wide comparison. Bioresour. Technol. 2022, 360, 127529. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, M.; Qin, H.; Zhou, J.; Shen, Q.; Wang, K.; Chen, W.; Liu, M.; Li, N. Synergy effect between adsorption and heterogeneous photo-Fenton-like catalysis on LaFeO3/lignin-biochar composites for high efficiency degradation of ofloxacin under visible light. Sep. Purif. Technol. 2022, 280, 119751. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, D.; Wu, J.; Jiang, S.; Yu, H.; Cheng, Y.; Zhang, S.; Zhang, X. Study on adsorption-degradation of 2,4-dichlorophenol by modified biochar immobilized laccase. Int. J. Environ. Sci. Technol. 2022, 19, 1393–1406. [Google Scholar] [CrossRef]
- Castro, C.; Urbieta, M.S.; Plaza Cazón, J.; Donati, E.R. Metal biorecovery and bioremediation: Whether or not thermophilic are better than mesophilic microorganisms. Bioresour. Technol. 2019, 279, 317–326. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Tariq, M.; Waseem, M.; Rasool, M.H.; Zahoor, M.A.; Hussain, I. Isolation and molecular characterization of the indigenous Staphylococcus aureus strain K1 with the ability to reduce hexavalent chromium for its application in bioremediation of metal-contaminated sites. PeerJ 2019, 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Shukla, P. Lead bioaccumulation mediated by Bacillus cereus BPS-9 from an industrial waste contaminated site encoding heavy metal resistant genes and their transporters. J. Hazard. Mater. 2021, 401, 123285. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Arabelo, A.; Banda, K.; Nyambe, I.; Chirwa, M.; Ito, M.; Sato, T.; Igarashi, T.; et al. Biosorption of Pb (II) and Zn (II) from aqueous solution by Oceanobacillus profundus isolated from an abandoned mine. Sci. Rep. 2020, 10, 21189. [Google Scholar] [CrossRef]
- Li, Y.; Xin, M.; Xie, D.; Fan, S.; Ma, J.; Liu, K.; Yu, F. Variation in extracellular polymeric substances from Enterobacter sp. and their Pb2+ adsorption behaviors. ACS Omega 2021, 6, 9617–9628. [Google Scholar] [CrossRef]
- Liu, J.; Xue, J.; Wei, X.; Su, H.; Xu, R. Optimization of Cr6+ removal by Bacillus subtilis strain SZMC 6179J from chromium-containing soil. Indian J. Microbiol. 2020, 60, 430–435. [Google Scholar] [CrossRef]
- Oyewole, O.A.; Zobeashia, S.S.L.T.; Oladoja, E.O.; Raji, R.O.; Odiniya, E.E.; Musa, A.M. Biosorption of heavy metal polluted soil using bacteria and fungi isolated from soil. SN Appl. Sci. 2019, 1, 857. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Murugan, K.; Benelli, G.; Higuchi, A.; Rajasekar, A. Bioreduction of hexavalent chromium by Pseudomonas stutzeri L1 and Acinetobacter baumannii L2. Ann. Microbiol. 2017, 67, 91–98. [Google Scholar] [CrossRef]
- Kumaresan Sarankumar, R.; Arulprakash, A.; Devanesan, S.; Selvi, A.; AlSalhi, M.S.; Rajasekar, A.; Ahamed, A. Bioreduction of hexavalent chromium by chromium resistant alkalophilic bacteria isolated from tannery effluent. J. King Saud Univ.-Sci. 2020, 32, 1969–1977. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Wang, T. Cadmium biosorption by lactic acid bacteria Weissella viridescens ZY-6. Food Control 2021, 123, 107747. [Google Scholar] [CrossRef]
- Dey, U.; Chatterjee, S.; Mondal, N.K. Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol. Rep. 2016, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yin, C.; Abbas, N.; Mao, Z.; Zhang, Y. Multiple heavy metal tolerance and removal by an earthworm gut fungus Trichoderma brevicompactum QYCD-6. Sci. Rep. 2020, 10, 6940. [Google Scholar] [CrossRef] [PubMed]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Perera, I.A.; Megharaj, M. Acid-tolerant microalgae can withstand higher concentrations of invasive cadmium and produce sustainable biomass and biodiesel at pH 3.5. Bioresour. Technol. 2019, 281, 469–473. [Google Scholar] [CrossRef]
- Cheng, J.; Yin, W.; Chang, Z.; Lundholm, N.; Jiang, Z. Biosorption capacity and kinetics of cadmium(II) on live and dead Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 211–221. [Google Scholar] [CrossRef]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Vega, M.; Bolado, S. Comparative uptake study of arsenic, boron, copper, manganese and zinc from water by different green microalgae. Bioresour. Technol. 2018, 263, 49–57. [Google Scholar] [CrossRef]
- Rugnini, L.; Costa, G.; Congestri, R.; Bruno, L. Testing of two different strains of green microalgae for Cu and Ni removal from aqueous media. Sci. Total Environ. 2017, 601–602, 959–967. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Mycoremediation of heavy metals: Processes, mechanisms, and affecting factors. Environ. Sci. Pollut. Res. 2021, 28, 10375–10412. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Srivastava, R.R.; Ilyas, N. Biosorption of strontium from aqueous solutions. Handb. Environ. Chem. 2020, 88, 65–83. [Google Scholar] [CrossRef]
- Escudero, L.B.; Quintas, P.Y.; Wuilloud, R.G.; Dotto, G.L. Recent advances on elemental biosorption. Environ. Chem. Lett. 2019, 17, 409–427. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V.; Anshumali. A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 170. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Shen, L.; Li, Z.; Wang, J.; Liu, A.; Li, Z.; Yu, R.; Wu, X.; Liu, Y.; Li, J.; Zeng, W. Characterization of extracellular polysaccharide/protein contents during the adsorption of Cd(II) by Synechocystis sp. PCC6803. Environ. Sci. Pollut. Res. 2018, 25, 20713–20722. [Google Scholar] [CrossRef]
- Xie, Y.; He, N.; Wei, M.; Wen, T.; Wang, X.; Liu, H.; Zhong, S.; Xu, H. Cadmium biosorption and mechanism investigation using a novel Bacillus subtilis KC6 isolated from pyrite mine. J. Clean. Prod. 2021, 312, 127749. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, Y.; Bhatt, P.; Chen, S. Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils. J. Hazard. Mater. 2021, 418, 126253. [Google Scholar] [CrossRef]
- Lopes, C.S.C.; Teixeira, D.B.; Braz, B.F.; Santelli, R.E.; de Castilho, L.V.A.; Gomez, J.G.C.; Castro, R.P.V.; Seldin, L.; Freire, D.M.G. Application of rhamnolipid surfactant for remediation of toxic metals of long- and short-term contamination sites. Int. J. Environ. Sci. Technol. 2021, 18, 575–588. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. Genomic analysis of Bacillus cereus NWUAB01 and its heavy metal removal from polluted soil. Sci. Rep. 2020, 10, 19660. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Nokman, W.; Benluvankar, V.; Maria Packiam, S.; Vincent, S. Screening and molecular identification of heavy metal resistant Pseudomonas putida S4 in tannery effluent wastewater. Biocatal. Agric. Biotechnol. 2019, 18, 101052. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.V.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Dash, D.M.; Osborne, W.J. Rapid biodegradation and biofilm-mediated bioremoval of organophosphorus pesticides using an indigenous Kosakonia oryzae strain -VITPSCQ3 in a Vertical-flow Packed Bed Biofilm Bioreactor. Ecotoxicol. Environ. Saf. 2020, 192, 110290. [Google Scholar] [CrossRef]
- Xu, B.; Sun, Q.J.; Lan, J.C.W.; Chen, W.M.; Hsueh, C.C.; Chen, B.Y. Exploring the glyphosate-degrading characteristics of a newly isolated, highly adapted indigenous bacterial strain, Providencia rettgeri GDB 1. J. Biosci. Bioeng. 2019, 128, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kolekar, P.D.; Patil, S.M.; Suryavanshi, M.V.; Suryawanshi, S.S.; Khandare, R.V.; Govindwar, S.P.; Jadhav, J.P. Microcosm study of atrazine bioremediation by indigenous microorganisms and cytotoxicity of biodegraded metabolites. J. Hazard. Mater. 2019, 374, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Zhou, X.; Huang, Y.; Zhang, W.; Chen, S. Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazard. Mater. 2021, 411, 125026. [Google Scholar] [CrossRef]
- Aswathi, A.; Pandey, A.; Sukumaran, R.K. Rapid degradation of the organophosphate pesticide—Chlorpyrifos by a novel strain of Pseudomonas nitroreducens AR-3. Bioresour. Technol. 2019, 292, 122025. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Xu, D.; Zhao, X.H.; Song, Y.; Liu, Y.L.; Li, H.N. Biodegradation of two organophosphorus pesticides in whole corn silage as affected by the cultured Lactobacillus plantarum. 3 Biotech 2016, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, S.; Khan, M.A.; Qader, S.A.U.; Iqbal, S.; Sattar, H.; Ansari, A. A comparative study on degradation of complex malathion organophosphate using of Escherichia coli IES-02 and a novel carboxylesterase. Int. J. Biol. Macromol. 2020, 145, 445–455. [Google Scholar] [CrossRef]
- Carles, L.; Joly, M.; Bonnemoy, F.; Leremboure, M.; Donnadieu, F.; Batisson, I.; Besse-Hoggan, P. Biodegradation and toxicity of a maize herbicide mixture: Mesotrione, nicosulfuron and S-metolachlor. J. Hazard. Mater. 2018, 354, 42–53. [Google Scholar] [CrossRef]
- Ekram, M.A.E.; Sarker, I.; Rahi, M.S.; Rahman, M.A.; Saha, A.K.; Reza, M.A. Efficacy of soil-borne Enterobacter sp. for carbofuran degradation: HPLC quantitation of degradation rate. J. Basic Microbiol. 2020, 60, 390–399. [Google Scholar] [CrossRef]
- Soares, P.R.S.; Birolli, W.G.; Ferreira, I.M.; Porto, A.L.M. Biodegradation pathway of the organophosphate pesticides chlorpyrifos, methyl parathion and profenofos by the marine-derived fungus Aspergillus sydowii CBMAI 935 and its potential for methylation reactions of phenolic compounds. Mar. Pollut. Bull. 2021, 166, 112185. [Google Scholar] [CrossRef]
- Kaur, P.; Balomajumder, C. Effective mycoremediation coupled with bioaugmentation studies: An advanced study on newly isolated Aspergillus sp. in Type-II pyrethroid-contaminated soil. Environ. Pollut. 2020, 261, 114073. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, T.J.; DiRusso, C.C.; Wilson, M.; Black, P.N. Reactive Oxygen Species (ROS) mediated degradation of organophosphate pesticides by the green microalgae Coccomyxa subellipsoidea. Bioresour. Technol. Rep. 2020, 11, 100461. [Google Scholar] [CrossRef]
- Hu, N.; Xu, Y.; Sun, C.; Zhu, L.; Sun, S.; Zhao, Y.; Hu, C. Removal of atrazine in catalytic degradation solutions by microalgae Chlorella sp. and evaluation of toxicity of degradation products via algal growth and photosynthetic activity. Ecotoxicol. Environ. Saf. 2021, 207, 111546. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Fan, Z.; Liu, F.; Liu, H.; Wang, L.; Wu, H. Soil bacterial community dynamics following bioaugmentation with Paenarthrobacter sp. W11 in atrazine-contaminated soil. Chemosphere 2021, 282, 130976. [Google Scholar] [CrossRef]
- Papadopoulou, E.S.; Genitsaris, S.; Omirou, M.; Perruchon, C.; Stamatopoulou, A.; Ioannides, I.; Karpouzas, D.G. Bioaugmentation of thiabendazole-contaminated soils from a wastewater disposal site: Factors driving the efficacy of this strategy and the diversity of the indigenous soil bacterial community. Environ. Pollut. 2018, 233, 16–25. [Google Scholar] [CrossRef]
- Aldas-Vargas, A.; van der Vooren, T.; Rijnaarts, H.H.M.; Sutton, N.B. Biostimulation is a valuable tool to assess pesticide biodegradation capacity of groundwater microorganisms. Chemosphere 2021, 280, 130793. [Google Scholar] [CrossRef]
- Raimondo, E.E.; Aparicio, J.D.; Bigliardo, A.L.; Fuentes, M.S.; Benimeli, C.S. Enhanced bioremediation of lindane-contaminated soils through microbial bioaugmentation assisted by biostimulation with sugarcane filter cake. Ecotoxicol. Environ. Saf. 2020, 190, 110143. [Google Scholar] [CrossRef]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Rathour, R.; Singh, R.; Thakur, I.S. Production and characterization of extracellular polymeric substances (EPS) generated by a carbofuran degrading strain Cupriavidus sp. ISTL7. Bioresour. Technol. 2019, 282, 417–424. [Google Scholar] [CrossRef]
- Satapute, P.; Jogaiah, S. A biogenic microbial biosurfactin that degrades difenoconazole fungicide with potential antimicrobial and oil displacement properties. Chemosphere 2022, 286, 131694. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.N.; Saxena, R.; Paul, D.; Tomar, R.S. Biodiversity of pesticides degrading microbial communities and their environmental impact. Biocatal. Agric. Biotechnol. 2021, 31, 101883. [Google Scholar] [CrossRef]
- Dash, D.M.; Osborne, J.W. Biodegradation of monocrotophos by a plant growth promoting Bacillus aryabhattai (VITNNDJ5) strain in artificially contaminated soil. Int. J. Environ. Sci. Technol. 2020, 17, 1475–1490. [Google Scholar] [CrossRef]
- Marican, A.; Durán-Lara, E.F. A review on pesticide removal through different processes. Environ. Sci. Pollut. Res. 2018, 25, 2051–2064. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; López-chuken, U.J.; Villarreal-chiu, J.F. Microbial Degradation of Organophosphate Pesticides: A Review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Gangola, S.; Sharma, A.; Bhatt, P.; Khati, P.; Chaudhary, P. Presence of esterase and laccase in Bacillus subtilis facilitates biodegradation and detoxification of cypermethrin. Sci. Rep. 2018, 8, 12755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Wang, X.; Hu, B.; Zhang, C.; Jin, W.; Zhu, S.; Hu, G.; Hong, Q. Identification of the key amino acid sites of the carbendazim hydrolase (MheI) from a novel carbendazim-degrading strain Mycobacterium sp. SD-4. J. Hazard. Mater. 2017, 331, 55–62. [Google Scholar] [CrossRef]
- Bigley, A.N.; Desormeaux, E.; Xiang, D.F.; Bae, S.Y.; Harvey, S.P.; Raushel, F.M. Overcoming the challenges of enzyme evolution to adapt phosphotriesterase for V-Agent decontamination. Biochemistry 2019, 58, 2039–2053. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Jha, B. Pilot scale production of extracellular thermo-alkali stable laccase from Pseudomonas sp. S2 using agro waste and its application in organophosphorous pesticides degradation. J. Chem. Technol. Biotechnol. 2018, 93, 1022–1030. [Google Scholar] [CrossRef]
- Sarker, A.; Lee, S.H.; Kwak, S.Y.; Nandi, R.; Kim, J.E. Comparative catalytic degradation of a metabolite 3,5-dichloroaniline derived from dicarboximide fungicide by laccase and MnO2 mediators. Ecotoxicol. Environ. Saf. 2020, 196, 110561. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Qin, X.; Xia, L. Degradation of the herbicide isoproturon by laccase-mediator systems. Biochem. Eng. J. 2017, 119, 92–100. [Google Scholar] [CrossRef]
- Sun, T.; Miao, J.; Saleem, M.; Zhang, H.; Yang, Y.; Zhang, Q. Bacterial compatibility and immobilization with biochar improved tebuconazole degradation, soil microbiome composition and functioning. J. Hazard. Mater. 2020, 398, 122941. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, L.; Ge, S.; Liu, X.; Zhang, X.; Chen, X. Remediation potential of immobilized bacterial consortium with biochar as carrier in pyrene-Cr(VI) co-contaminated soil. Environ. Technol. 2019, 40, 2345–2353. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J. Removal of chlortetracycline from water by Bacillus cereus immobilized on Chinese medicine residues biochar. Environ. Technol. Innov. 2021, 24, 101930. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Toan, N.C.; Kajitvichyanukul, P. Enhanced paraquat removal from contaminated water using cell-immobilized biochar. Clean Technol. Environ. Policy 2022, 24, 1073–1085. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Giannakoudakis, D.A.; Prekodravac, J.R.; Carlos, J. Role of catalyst supports in biocatalysis. J. Chem. Technol. Biotechnol. 2022, 98, 7–21. [Google Scholar] [CrossRef]
- Guo, Q.; Bandala, E.R.; Goonetilleke, A.; Hong, N.; Li, Y.; Liu, A. Application of Chlorella pyrenoidosa embedded biochar beads for water treatment. J. Water Process Eng. 2021, 40, 1073–1085. [Google Scholar] [CrossRef]
- Huang, S.W.; Chen, X.; Wang, D.D.; Jia, H.L.; Wu, L. Bio-reduction and synchronous removal of hexavalent chromium from aqueous solutions using novel microbial cell/algal-derived biochar particles: Turning an environmental problem into an opportunity. Bioresour. Technol. 2020, 309, 123304. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K. Biosorption and bioaccumulation—The prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Nair, V. Pseudomonas stutzeri immobilized sawdust biochar for nickel ion removal. Catalysts 2022, 12, 1495. [Google Scholar] [CrossRef]
- Qi, X.; Gou, J.; Chen, X.; Xiao, S.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.; Han, M.; Luo, X. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Luo, X.; Liu, S.; Wan, W.; Huang, Q.; Chen, W. A novel eco-friendly recycling of food waste for preparing biofilm-attached biochar to remove Cd and Pb in wastewater. J. Clean. Prod. 2021, 311, 127514. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.I.A.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef]
- Chuaphasuk, C.; Prapagdee, B. Effects of biochar-immobilized bacteria on phytoremediation of cadmium-polluted soil. Environ. Sci. Pollut. Res. 2019, 26, 23679–23688. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Li, H. Enhanced bio-immobilization of Pb contaminated soil by immobilized bacteria with biochar as carrier. Pol. J. Environ. Stud. 2017, 26, 413–418. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Ma, H.; Wei, M.; Wang, Z.; Hou, S.; Li, X.; Xu, H. Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J. Hazard. Mater. 2020, 388, 122065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tie, B.; Peng, O.; Luo, H.; Li, D.; Liu, S.; Lei, M.; Wei, X.; Liu, X.; Du, H. Inoculation of Cd-contaminated paddy soil with biochar-supported microbial cell composite: A novel approach to reducing cadmium accumulation in rice grains. Chemosphere 2020, 247, 125850. [Google Scholar] [CrossRef]
- Shen, Y.; Li, H.; Zhu, W.; Ho, S.H.; Yuan, W.; Chen, J.; Xie, Y. Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour. Technol. 2017, 244, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Shao, W.; Zhang, K.; Yu, F.; Huo, Y.; Li, M. Enhanced passivation of lead with immobilized phosphate solubilizing bacteria beads loaded with biochar/ nanoscale zero valent iron composite. J. Hazard. Mater. 2020, 384, 121505. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Ren, X.; Li, B.; Mao, H. Adsorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis loaded on biochars derived from different stock materials. Ecotoxicol. Environ. Saf. 2018, 148, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Youngwilai, A.; Kidkhunthod, P.; Jearanaikoon, N.; Chaiprapa, J.; Supanchaiyamat, N.; Hunt, A.J.; Ngernyen, Y.; Ratpukdi, T.; Khan, E.; Siripattanakul-Ratpukdi, S. Simultaneous manganese adsorption and biotransformation by Streptomyces violarus strain SBP1 cell-immobilized biochar. Sci. Total Environ. 2020, 713, 136708. [Google Scholar] [CrossRef] [PubMed]
- Wahla, A.Q.; Anwar, S.; Mueller, J.A.; Arslan, M.; Iqbal, S. Immobilization of metribuzin degrading bacterial consortium MB3R on biochar enhances bioremediation of potato vegetated soil and restores bacterial community structure. J. Hazard. Mater. 2020, 390, 121493. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Luo, T.; Ma, Y.; Wang, B.; Huang, Q. Remediation potential of immobilized bacterial strain with biochar as carrier in petroleum hydrocarbon and Ni co-contaminated soil. Environ. Technol. 2022, 43, 1068–1081. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, M.; Qian, L. Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J. Soils Sediments 2012, 12, 1350–1359. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.; Li, Y.; Yang, J.; Li, N.; An, N. Effects of biochar and straw application on the physicochemical and biological properties of paddy soils in Northeast China. Sci. Rep. 2019, 9, 16531. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, Y.; Ma, L.; Gao, G.; Wang, Y. Combination of biochar and immobilized bacteria in cypermethrin-contaminated soil remediation. Int. Biodeterior. Biodegrad. 2017, 120, 15–20. [Google Scholar] [CrossRef]

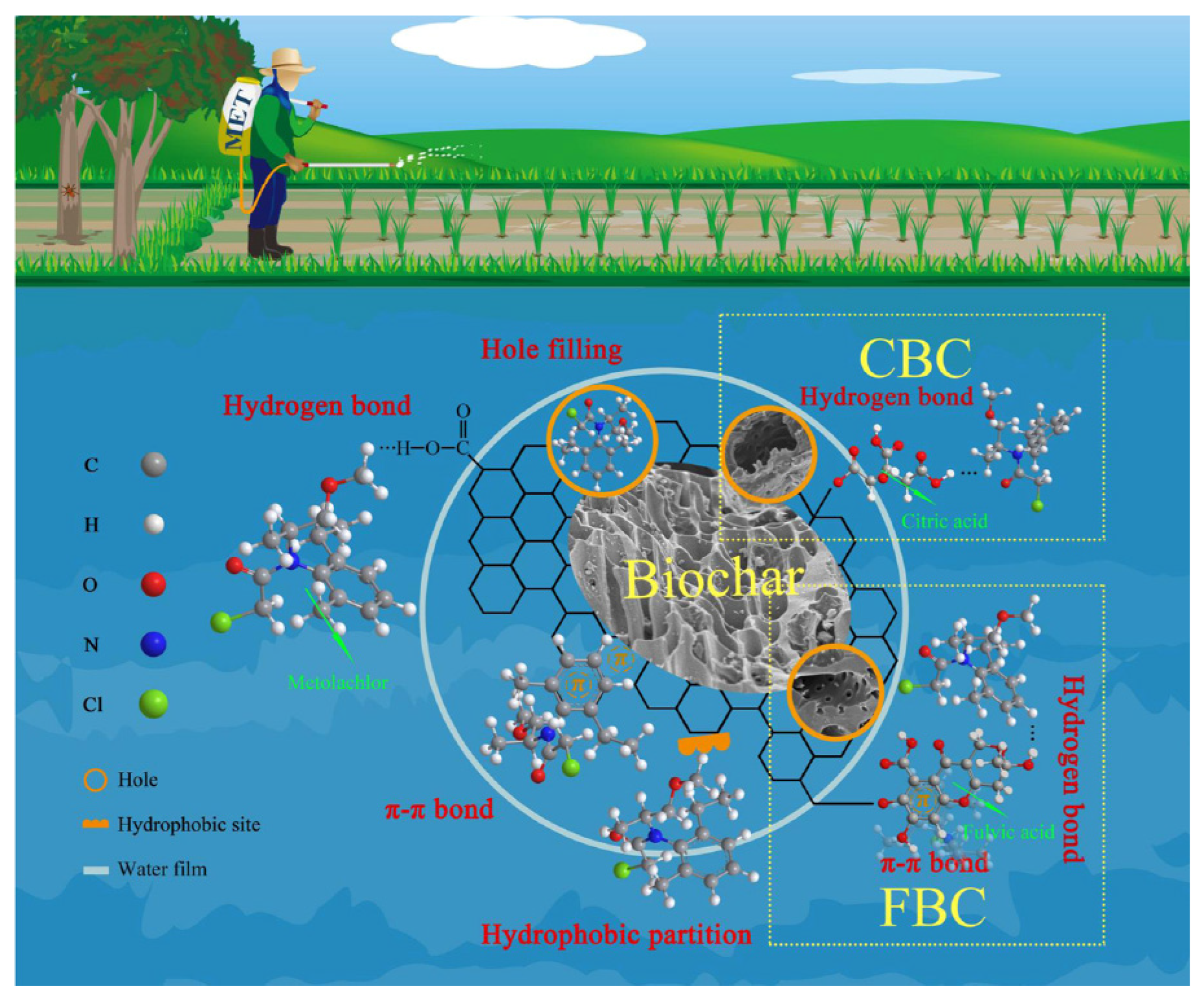

| Biomass Type | Pyrolysis Temperature (°C) | Modification | Metal Ion | System | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|---|
| Crab shell | 350 | Fe-La doped | Sb3+ | Water | 498 | [25] |
| Crab shell | 350 | Fe-La doped | SbO67− | Water | 337 | [25] |
| Cattle manure | 500 | Fe-impregnated | Sb5+ | Water | 58.3 | [37] |
| Wood chip | 600 | Sulfurized | Hg2+ | Water | 107.5 | [37] |
| Sesbania bispinosa | 450 | MnO | AsO43− | Water | 7.35 | [39] |
| Sesbania bispinosa | 450 | CuO | AsO43− | Water | 12.47 | [39] |
| Rice straw | 500 | Thiol-modified | Cd2+ | Soil | 45.1 | [41] |
| Rice straw | 500 | Thiol-modified | Pb2+ | Soil | 61.4 | [41] |
| Lobster shell | 600 | HCl treatment | Cu2+ | Water | 71.4 | [42] |
| Lobster shell | 600 | HCl treatment | Cd2+ | Water | 126 | [42] |
| Peanut shell | 600 | MnO-embedded | Sb3+ | Water | 248 | [44] |
| Corn straw | 600 | Fe-impregnated | HAsO42− | Water | 6.80 | [45] |
| Cornstalk | 550 | Mg-Al-LDH | As5+ | Soil | 0.820 | [46] |
| Cornstalk | 550 | Zn–Al-LDH | As5+ | Soil | 0.916 | [46] |
| Cornstalk | 550 | Cu–Al-LDH | As5+ | Soil | 0.787 | [46] |
| Canola straw | 700 | Steam activation | Pb2+ | Water | 195 | [47] |
| Rice husk | 500 | HA/Fe-Mn oxide-loaded | Cd2+ | Water | 67.11 | [48] |
| Rice husk | 500 | HA/Fe-Mn oxide-loaded | As5+ | Water | 35.59 | [48] |
| Rice husk | 1 kW (microwave) | Fe3O4-magnetic | Cr6+ | Water | 8.35 | [49] |
| Pomelo peel | 300 | K2FeO4-promoted | Cr6+ | Water | 209.64 | [50] |
| Sawdust | 180 | Amino-functionalized (HNO3, nicotinamide) | Sb5+ | Water | 241.92 | [51] |
| Sawdust | 180 | Amino-functionalized (HNO3, nicotinamide) | Cr6+ | Water | 132.74 | [51] |
| Biomass Type | Pyrolysis Temperature (°C) | Modification | Pesticide | System | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|---|
| Cow manure | 600 | HCl/HF | Carbaryl | Water | ~55 | [24] |
| Dewatered sludge | 700 | - | Carbendazim | Soil | 0.144 | [26] |
| Leonardite | 550 | - | Alachlor | Water | 3.802 | [35] |
| Corn cob | 600 | HF | 2,4-dichloro-phenoxyacetic acid | Water | [52] | |
| Coconut fiber | 600 | HCl | Dichlorvos | Water | 90.9 | [53] |
| Walnut shell powder | 700 | Fulvic acid | Metolachlor | Water | 99.01 | [54] |
| Walnut shell powder | 700 | Citric acid | Metolachlor | Water | 74.07 | [54] |
| Bagasse | 500 | - | Carbofuran | Water | 18.9 | [55] |
| Switchgrass | 425 | Fe3+/Fe2+ magnetic | Metribuzin | Water | 205 | [56] |
| Switch grass | 425 | - | Metribuzin | Water | 223 | [56] |
| Heavy Metal | Microorganism | Initial Heavy Metal Concentration | Incubation Time | Degradation Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| Pb | Bacillus cereus BPS-9 | - | 48 h | 77.57 | [67] |
| Oceanobacillus profundus KBZ 3-2 | 50 mg/L | 24 h | 97 | [68] | |
| Enterobacter sp. FM-1 | 100 mg/L | 24 h | 93.85 | [69] | |
| Cr | Bacillus subtilis SZMC 6179J | 55 mg/L | 24 h | 93.50 | [70] |
| Pseudomonas aeruginosa | 20 ppm | 21 days | 89.67 | [71] | |
| Pseudomonas stutzeri L1 | 100 mg/L | 24 h | 97 | [72] | |
| Bacillus cohnii | 100 mg/L | 25 h | 94 | [73] | |
| Bacillus licheniformis | 100 mg/L | 25 h | 95 | [73] | |
| Cd | Weissella viridescens ZY-6 | NM | 2 h | 69.45–79.91 | [74] |
| Zn | Oceanobacillus profundus KBZ 3-2 | 2 mg/L | 24 h | 54 | [68] |
| Cu | Pseudomonas aeruginosa | 15 ppm | 14 days | 90.89 | [71] |
| As | Bacillus sp. | 100 ppm | 72 h | 53.29 | [75] |
| Aneurinibacillus aneurinilyticus | 100 ppm | 72 h | 50.37 | [75] | |
| Fungi | |||||
| Pb | Trichoderma brevicompactum QYCD-6 | 50 mg/L | 5 days | 97.5 | [76] |
| Cr | Trichoderma brevicompactum QYCD-6 | 100 mg/L | 5 days | 31.83 | [76] |
| Cd | Penicillium notatum | 10 ppm | 14 days | 77.67 | [71] |
| Trichoderma brevicompactum QYCD-6 | 30 mg/L | 5 days | 20.13 | [76] | |
| Cu | Trichoderma brevicompactum QYCD-6 | 50 mg/L | 5 days | 64.46 | [76] |
| Ni | Aspergillus niger | 20 ppm | 28 days | 81.07 | [71] |
| Microalgae | |||||
| Cd | Desmodesmus sp. MAS1 | 5 mg/L | 7 days | >58% | [77] |
| Heterochlorella sp. MAS3 | 5 mg/L | 7 days | >58% | [77] | |
| Chlorella vulgaris | 100 mg/L | 5–15 min | Live cells—95.2 Dead cells—96.8 | [78] | |
| Zn | Chlorophyceae spp. | 3 mg/L | 3 h | 91.9 | [79] |
| Cu | Chlorella vulgaris | 1.9–11.9 mg/L | 12 days | 39 | [80] |
| Chlorophyceae spp. | 3 mg/L | 10 min | 88 | [79] | |
| As | Scenedesmus almeriensis | 12 mg/L | 3 h | 40.7 | [79] |
| Ni | Chlorella vulgaris | 1.9–11.9 mg/L | 12 days | 32 | [80] |
| Mn | Scenedesmus almeriensis | 3 mg/L | 3 h | 99.4 | [79] |
| Pesticide | Microorganism | Initial Pesticide Concentration | Incubation Time | Degradation Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| Chlorpyrifos | Pseudomonas nitroreducens AR-3 | 100 mg/L | 8 h | 97 | [98] |
| Chlorpyrifos | Lactobacillus plantarum | 0.20–0.80 mg/kg | - | 24.9–34.4 | [99] |
| Malathion | Escherichia coli IES-02 | 50 ppm | 4 h | 99 | [100] |
| Mesotrione | Bacillus megaterium Mes11 | 1 mM | 5 h | 99 | [101] |
| Carbofuran | Enterobacter sp. | 4 µg/ml | 7 days | 80 | [102] |
| Fungi | |||||
| Chlorpyrifos | Aspergillus sydowii CBMAI 935 | 50 mg/L | 30 days | 32 | [103] |
| Methyl parathion | 80 | ||||
| Profenos | 52 | ||||
| Pyrethroid mixture (cypermethrin, cyfluthrin, cyhalothrin) | Aspergillus sp. | 500 mg/L | 15 days | ≈100 | [104] |
| Microalgae | |||||
| Paraoxon, Malathion and Diazinon | Coccomyxa subellipsoidea | 0.1 mg/ml | 10 days | - | [105] |
| Atrazine | Chlorella sp. | 40 µg/L | 8 days | 83.0 | [106] |
| 80 µg/L | 64.3 | ||||
| Microorganism | Catalyst Support | Pollutant Type | Mechanism | System Water/Soil | Quantification of Heavy Metal Removal | Reference |
|---|---|---|---|---|---|---|
| Bacillus sp.TZ5 | Coconut shell | Cd2+ | Adsorption | Soil | 48.49% | [141] |
| Delftia sp B9 | Cornstalk | Cd2+ | Adsorption | soil | 0.33 mg/kg reduced to 0.06–0.13 mg/kg | [142] |
| Chlorella sp. | Water hyacinth | Cd2+ | Adsorption | water | 92.5% | [143] |
| Leclercia adecarboxylata | Rice hull | Pb2+ | Entrapment | water | 93% | [144] |
| Bacillus subtilis | Pig manure | Hg2+, Pb2+ co-contamination | Adsorption | water | 69 mg/g Hg 112.3 mg/g Pb | [145] |
| Bacillus subtilis | Corn straw | Hg2+, Pb2+ co-contamination | Adsorption | water | 53.7 mg/g Hg; 83.0 mg/g Pb | [145] |
| Enterobacter sp. | Rice husk BC | Pb2+ | Adsorption | - | 24.1% | [146] |
| Enterobacter sp. | Sludge BC | Pb2+ | Adsorption | - | 60.9% | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manikandan, S.K.; Pallavi, P.; Shetty, K.; Bhattacharjee, D.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Nair, V. Effective Usage of Biochar and Microorganisms for the Removal of Heavy Metal Ions and Pesticides. Molecules 2023, 28, 719. https://doi.org/10.3390/molecules28020719
Manikandan SK, Pallavi P, Shetty K, Bhattacharjee D, Giannakoudakis DA, Katsoyiannis IA, Nair V. Effective Usage of Biochar and Microorganisms for the Removal of Heavy Metal Ions and Pesticides. Molecules. 2023; 28(2):719. https://doi.org/10.3390/molecules28020719
Chicago/Turabian StyleManikandan, Soumya K., Pratyasha Pallavi, Krishan Shetty, Debalina Bhattacharjee, Dimitrios A. Giannakoudakis, Ioannis A. Katsoyiannis, and Vaishakh Nair. 2023. "Effective Usage of Biochar and Microorganisms for the Removal of Heavy Metal Ions and Pesticides" Molecules 28, no. 2: 719. https://doi.org/10.3390/molecules28020719
APA StyleManikandan, S. K., Pallavi, P., Shetty, K., Bhattacharjee, D., Giannakoudakis, D. A., Katsoyiannis, I. A., & Nair, V. (2023). Effective Usage of Biochar and Microorganisms for the Removal of Heavy Metal Ions and Pesticides. Molecules, 28(2), 719. https://doi.org/10.3390/molecules28020719









