Functional Regulation of ZnAl-LDHs and Mechanism of Photocatalytic Reduction of CO2: A DFT Study
Abstract
:1. Introduction
2. Results and Discussions
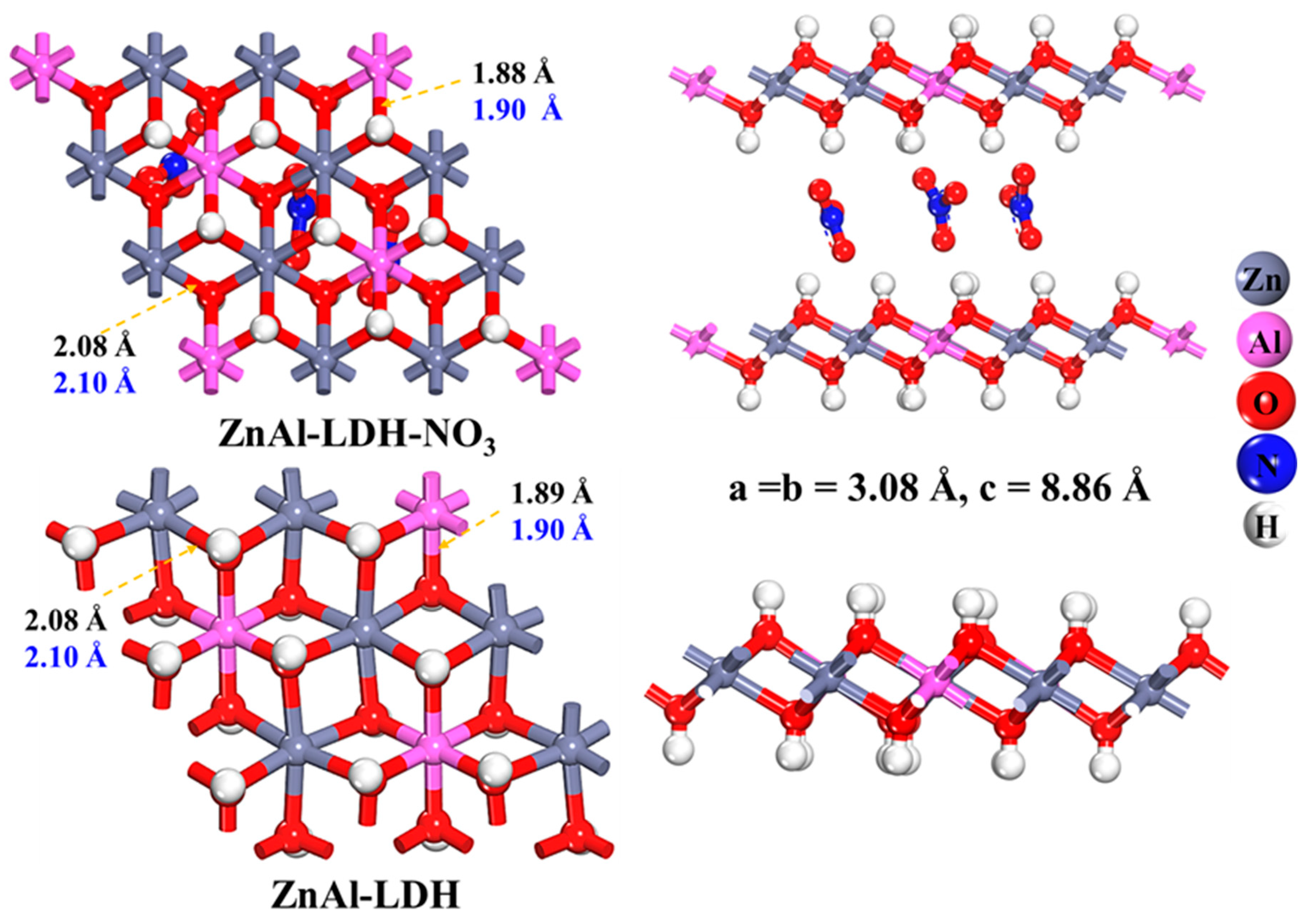
2.1. Structure Construction of the Functionalized ZnAl-LDHs
2.2. Charge Properties of Functionalized ZnAl-LDHs
2.3. Photocatalytic Activity of Functionalized ZnAl-LDHs
2.4. Mechanism of Functionalized ZnAl-LDHs Photocatalytic Reduction of CO2
3. Computational Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, L.; Wang, S.; Huang, H.; Zhang, Y.; Ma, T. Surface sites engineering on semiconductors to boost photocatalytic CO2 reduction. Nano Energy 2020, 75, 104959. [Google Scholar] [CrossRef]
- Zeng, G.; Qiu, J.; Li, Z.; Pavaskar, P.; Cronin, S.B. CO2 Reduction to Methanol on TiO2-Passivated GaP Photocatalysts. ACS Catal. 2014, 4, 3512–3516. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 Reduction: From the Electrochemical to Photochemical Approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef]
- Saravanan, A.; Vo, D.-V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.; Swetha, S.; Reshma, B. A comprehensive review on dofferent approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Shaya, J.; Srour, H.; Karamé, I. Introductory Chapter: An Outline of Carbon Dioxide Chemistry, Uses and Technology. In Carbon Dioxide Chemistry, Capture and Oil Recovery; IntechOpen: London, UK, 2018; pp. 1–12. [Google Scholar]
- Parida, K.; Mohapatra, L. Recent progress in the development of carbonate-intercalated Zn/Cr-LDH as a novel photocatalyst for hydrogen evolution aimed at the utilization of solar light. Dalton Trans. 2012, 41, 1173–1178. [Google Scholar] [CrossRef]
- Chen, Z.R.; Zhu, Y.Q.; Xu, S.M.; Zhao, Y.; Peng, Q.; Yan, H. Theoretical study on the anisotropic photo-induced carrier mobilities in layered double hydroxide-based photocatalysts. J. Mater. Chem. A 2021, 9, 20466–20482. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.Q.; Zulfiqar, S.; Shah, S.; Bahadur, I. An overview of semiconductors/layered double hydroxides composites: Properties, synthesis, photocatalytic and photoelectrochemical applications. J. Mol. Liq. 2019, 289, 111114. [Google Scholar] [CrossRef]
- Teramura, K.; Iguchi, S.; Mizuno, Y.; Shishido, T.; Tanaka, T. Photocatalytic conversion of CO2 in water over layered double hydroxides. Angew Chem. Int. Ed. Engl. 2012, 51, 8008–8011. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Ai, Z.; Wu, L.; Zhang, Q. Mechanochemical synthesis of CdS/MgAl-LDH-precursor as improved visible-light-driven photocatalyst for organic dye. Appl. Clay Sci. 2018, 163, 265–272. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Bian, T.; Yu, H.; Fan, H.; Zhou, C.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T. Ni3+ doped monolayer layered double hydroxide nanosheets as efficient electrodes for supercapacitors. Nanoscale 2015, 7, 7168–7173. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Shen, T.; Chang, W.; Zhao, Y.; Qi, B.; Song, Y.F. Defect engineering of NiCo-layered double hydroxide hollow nanocages for highly selective photoreduction of CO2 to CH4 with suppressing H2 evolution. Inorg. Chem. Front. 2021, 8, 996–1004. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T. Layered Double Hydroxide Nanostructured Photocatalysts for Renewable Energy Production. Adv. Energy Mater. 2016, 6, 1501974. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, J.; Wu, Z. Recent advances in catalytic conversion of carbon dioxide to propiolic acids over coinage-metal-based catalysts. J. Energy Chem. 2021, 59, 572–580. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhao, Y.; Shi, R.; Yin, W.; Zhao, Y.; Waterhouse, G.I.N.; Zhang, T. Selective photocatalytic CO2 reduction over Zn-based layered double hydroxides containing tri or tetravalent metals. Sci. Bull. 2020, 65, 987–994. [Google Scholar] [CrossRef]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered double hydroxide (LDH)-based materials: A mini-review on strategies to improve the performance for photocatalytic water splitting. J. Energy Chem. 2022, 64, 406–431. [Google Scholar] [CrossRef]
- Zhao, Y.; Waterhouse, G.I.N.; Chen, G.; Xiong, X.; Wu, L.Z.; Tung, C.H.; Zhang, T. Two-dimensional-related catalytic materials for solar-driven conversion of COx into valuable chemical feedstocks. Chem. Soc. Rev. 2019, 48, 1972–2010. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; He, F.; Dai, Y.; Xie, Y.; Ling, Y.; Liu, L.; Zhao, J.; Ye, H.; Hou, Y. A heterostructured ZnAl-LDH@ZIF-8 hybrid as a bifunctional photocatalyst/adsorbent for CO2 reduction under visible light irradiation. Chem. Eng. J. 2022, 446, 137003. [Google Scholar] [CrossRef]
- Li, W.; Hou, P.; Wang, Z.; Kang, P. Synergistic effect of N-doped layered double hydroxide derived NiZnAl oxides in CO2 electroreduction. Sustain. Energy Fuels 2019, 3, 1455–1460. [Google Scholar] [CrossRef]
- Fang, X.; Chen, C.; Jia, H.; Li, Y.; Liu, J.; Wang, Y.; Song, Y.; Du, T.; Liu, L. Progress in Adsorption-Enhanced Hydrogenation of CO2 on Layered Double Hydroxide (LDH) Derived Catalysts. J. Ind. Eng. Chem. 2021, 95, 16–27. [Google Scholar] [CrossRef]
- Tang, N.; He, T.; Liu, J.; Li, L.; Shi, H.; Cen, W.; Ye, Z. New Insights into CO2 Adsorption on Layered Double Hydroxide (LDH)-Based Nanomaterials. Nanoscale Res. Lett. 2018, 13, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, G.; Bian, T.; Zhou, C.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; Smith, L.J.; O’Hare, D.; Zhang, T. Defect-Rich Ultrathin ZnAl-Layered Double Hydroxide Nanosheets for Efficient Photoreduction of CO2 to CO with Water. Adv. Mater. 2015, 27, 7824–7831. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T. Efficient Photocatalytic Nitrogen Fixation over Cuδ+-Modified Defective ZnAl-Layered Double Hydroxide Nanosheets. Adv. Energy Mater. 2020, 10, 1901973. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Waterhouse, G.I.N.; Zheng, L.; Cao, X.; Teng, F.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T. Layered-Double-Hydroxide Nanosheets as Efficient Visible-Light-Driven Photocatalysts for Dinitrogen Fixation. Adv. Mater. 2017, 29, 1703828. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, J.; Shen, T.; An, S.; Qi, B.; Song, Y.F. In Situ Construction of MIL-100@NiMn-LDH Hierarchical Architectures for Highly Selective Photoreduction of CO2 to CH4. ACS Appl. Mater. Interfaces 2022, 14, 16369–16378. [Google Scholar] [CrossRef]
- Gu, K.; Zhu, X.; Wang, D.; Zhang, N.; Huang, G.; Li, W.; Long, P.; Tian, J.; Zou, Y.; Wang, Y.; et al. Ultrathin defective high-entropy layered double hydroxides for electrochemical water oxidation. J. Energy Chem. 2021, 60, 121–126. [Google Scholar] [CrossRef]
- Ma, Y.; Qiu, B.; Zhang, J.; Xing, M. Vacancy Engineering of Ultrathin 2D Materials for Photocatalytic CO2 Reduction. ChemNanoMat 2021, 7, 368–379. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Fast formation of single-unit-cell-thick and defect-rich layered double hydroxide nanosheets with highly enhanced oxygen evolution reaction for water splitting. Nano Res. 2018, 11, 1883–1894. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Mikhailau, A.; Maltanava, H.; Poznyak, S.K.; Salak, A.N.; Zheludkevich, M.L.; Yasakau, K.A.; Ferreira, M.G.S. One-step synthesis and growth mechanism of nitrate intercalated ZnAl-LDH conversion coatings on zinc. Chem. Commun. 2019, 55, 6878–6881. [Google Scholar] [CrossRef] [PubMed]
- Israëli, Y.; Taviot Guého, C.; Besse, J.P.; Morel, J.P.; Morel Desrosiers, N. Thermodynamics of anion exchange on a chloride-intercalated zinc-aluminum layered double hydroxide: A microcalorimetric study. J. Chem. Soc. Dalton Trans. 2000, 5, 791–796. [Google Scholar] [CrossRef]
- Aisawa, S.; Kudo, H.; Hoshi, T.; Takahashi, S.; Hirahara, H.; Umetsu, Y.; Narita, E. Intercalation behavior of amino acids into Zn-Al-layered double hydroxide by the calcination-rehydration reaction. J. Solid State Chem. 2004, 177, 3987–3994. [Google Scholar] [CrossRef]
- Guo, J.; Shen, H.; Wu, G.; Li, J.; Mu, M.; Fan, W.; Yin, X. Synergy of Various Defects in CoAl-Layered Double Hydroxides Photocatalyzed CO2 Reduction: A First-Principles Study. Catal. Lett. 2022, 219, 1–12. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, X.; Cong, B.; Hong, W.; Chen, G. Tailoring the d-Band Centers Endows (NixFe1–x)2P Nanosheets with Efficient Oxygen Evolution Catalysis. ACS Catal. 2020, 10, 9086–9097. [Google Scholar] [CrossRef]
- He, J.; Zhou, X.; Xu, P.; Sun, J. Regulating Electron Redistribution of Intermetallic Iridium Oxide by Incorporating Ru for Efficient Acidic Water Oxidation. Adv. Energy Mater. 2021, 11, 2102883. [Google Scholar] [CrossRef]
- Gao, M.; Tian, F.; Guo, Z.; Zhang, X.; Li, Z.; Zhou, J.; Zhou, X.; Yu, Y.; Yang, W. Mutual-modification effect in adjacent Pt nanoparticles and single atoms with sub-nanometer inter-site distances to boost photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 446, 137127. [Google Scholar] [CrossRef]
- Xu, S.M.; Pan, T.; Dou, Y.B.; Yan, H.; Zhang, S.T.; Ning, F.Y.; Shi, W.Y.; Wei, M. Theoretical and Experimental Study on MIIMIII-Layered Double Hydroxides as Efficient Photocatalysts toward Oxygen Evolution from Water. J. Phys. Chem. C 2015, 119, 18823–18834. [Google Scholar] [CrossRef]
- Costa, D.G.; Rocha, A.B.; Souza, W.F.; Chiaro, S.S.X.; Leitão, A.A. Comparative Structural, thermodynamic and electronic analyses of ZnAlAn- hydrotalcite-like compounds (An−, Cl−, F−, Br−, OH−, CO32− or NO3−): An ab initio study. Appl. Clay Sci. 2012, 56, 16–22. [Google Scholar] [CrossRef]
- Tang, Q.L.; Luo, Q.H. Adsorption of CO2 at ZnO: A Surface Structure Effect from DFT+U Calculations. J. Phys. Chem. C 2013, 117, 22954–22966. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Chen, C.; Huang, M.; Huang, Y.; Liu, S.; Li, B. Engineering Catalytic Interfaces in Cuδ+/CeO2-TiO2 Photocatalysts for Synergistically Boosting CO2 Reduction to Ethylene. ACS Nano 2022, 16, 2306–2318. [Google Scholar] [CrossRef] [PubMed]
- Orio, M.; Pantazis, D.A.; Neese, F. Density functional theory. Photosynth. Res. 2009, 102, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Kürti, J.; Rajczy, P.; Kertesz, M.; Hafner, J.; Kresse, G. Performance of the Vienna ab initio simulation package (VASP) in chemical applications. J. Mol. Struct. THEOCHEM 2003, 624, 37–45. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-Funct. Theory Beyond 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Yan, H.; Wei, M.; Ma, J.; Evans, D.G.; Duan, X. Plane-Wave Density Functional Theory Study on the Structural and Energetic Properties of Cation-Disordered Mg-Al Layered Double Hydroxides. J. Phys. Chem. A 2010, 114, 7369–7376. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.M.; Yan, H.; Wei, M. Band Structure Engineering of Transition-Metal-Based Layered Double Hydroxides toward Photocatalytic Oxygen Evolution from Water: A Theoretical-Experimental Combination Study. J. Phys. Chem. C 2017, 121, 2683–2695. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H.J.T.J.o.C.P. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Hostaš, J.; Řezáč, J. Accurate DFT-D3 Calculations in a Small Basis Set. J. Chem. Theory Comput. 2017, 13, 3575–3585. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Rohrbach, A.; Hafner, J.; Kresse, G. Electronic correlation effects in transition-metal sulfides. J. Phys. Condens. Matter 2003, 15, 979–996. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Xu, J.; Shao, Y.; Wu, J.; Xu, X.; Pan, Y.; Ju, H.; Zhu, J.; Xie, Y. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 2019, 4, 690–699. [Google Scholar] [CrossRef]
- Miao, P.; Zhao, J.; Shi, R.; Li, Z.; Zhao, Y.; Zhou, C.; Zhang, T. Layered Double Hydroxide Engineering for the Photocatalytic Conversion of Inactive Carbon and Nitrogen Molecules. ACS EST Eng. 2022, 2, 1088–1102. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Wei, J.J.; Zeng, G.M.; Zhang, H.Q.; Tan, X.F.; Ma, C.; Li, X.C.; Li, Z.H.; Zhang, C. A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2. Coord. Chem. Rev. 2019, 386, 154–182. [Google Scholar] [CrossRef]
- Zhao, X.J.; Xu, S.M.; Zhong, Y.; Chen, Z.R.; Yin, P.; Miao, Y.C.; Guo, J.Y.; Zhang, W.; Jie, Y.; Yan, H. Theoretical Study on Photocatalytic CO2 Reduction to CO and CH4 over M(II)2M(III/IV)-Layered Double Hydroxides. J. Phys. Chem. C 2022, 126, 1356–1365. [Google Scholar] [CrossRef]
- Hao, X.; Tan, L.; Xu, Y.; Wang, Z.; Wang, X.; Bai, S.; Ning, C.; Zhao, J.; Zhao, Y.; Song, Y.F. Engineering Active Ni Sites in Ternary Layered Double Hydroxide Nanosheets for a Highly Selective Photoreduction of CO2 to CH4 under Irradiation above 500 nm. Ind. Eng. Chem. Res. 2020, 59, 3008–3015. [Google Scholar] [CrossRef]
- Ouyang, T.; Fan, W.; Guo, J.; Zheng, Y.; Yin, X.; Shen, Y. DFT study on Ag loaded 2H-MoS2 for understanding the mechanism of improved photocatalytic reduction of CO2. Phys. Chem. Chem. Phys. 2020, 22, 10305–10313. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Yan, L.; Su, Z. DFT Study on Sulfur-Doped g-C3N4 Nanosheets as a Photocatalyst for CO2 Reduction Reaction. J. Phys. Chem. C 2018, 122, 7712–7719. [Google Scholar] [CrossRef]
- Jiao, X.; Li, X.; Jin, X.; Sun, Y.; Xu, J.; Liang, L.; Ju, H.; Zhu, J.; Pan, Y.; Yan, W.; et al. Partially Oxidized SnS2 Atomic Layers Achieving Efficient Visible-Light-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 18044–18051. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhou, X.; Zhou, Y.; Li, M. Theoretical insights into mechanisms of electrochemical reduction of CO2 to ethylene catalyzed by Pd3Au. Appl. Surf. Sci. 2022, 572, 151474. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Y.; Bao, W.; Wang, B.; Meng, Q.; Fan, L.; Zhang, Q. Two-dimensional stable transition metal carbides (MnC and NbC) with prediction and novel functionalizations. Phys. Chem. Chem. Phys. 2018, 20, 25437–25445. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Fu, G.; Li, Z.; Zhen, W.; Wang, H.; Liu, M.; Sun, J.; Zhang, J.; Yang, L. Functional Regulation of ZnAl-LDHs and Mechanism of Photocatalytic Reduction of CO2: A DFT Study. Molecules 2023, 28, 738. https://doi.org/10.3390/molecules28020738
Xu D, Fu G, Li Z, Zhen W, Wang H, Liu M, Sun J, Zhang J, Yang L. Functional Regulation of ZnAl-LDHs and Mechanism of Photocatalytic Reduction of CO2: A DFT Study. Molecules. 2023; 28(2):738. https://doi.org/10.3390/molecules28020738
Chicago/Turabian StyleXu, Dongcun, Gang Fu, Zhongming Li, Wenqing Zhen, Hongyi Wang, Meiling Liu, Jianmin Sun, Jiaxu Zhang, and Li Yang. 2023. "Functional Regulation of ZnAl-LDHs and Mechanism of Photocatalytic Reduction of CO2: A DFT Study" Molecules 28, no. 2: 738. https://doi.org/10.3390/molecules28020738





