A Multi-Method Approach for Impact Assessment of Some Heavy Metals on Lactuca sativa L.
Abstract
:1. Introduction
2. Results
2.1. Analysis of Plant Tissues
2.1.1. Assimilating Pigments Evaluation
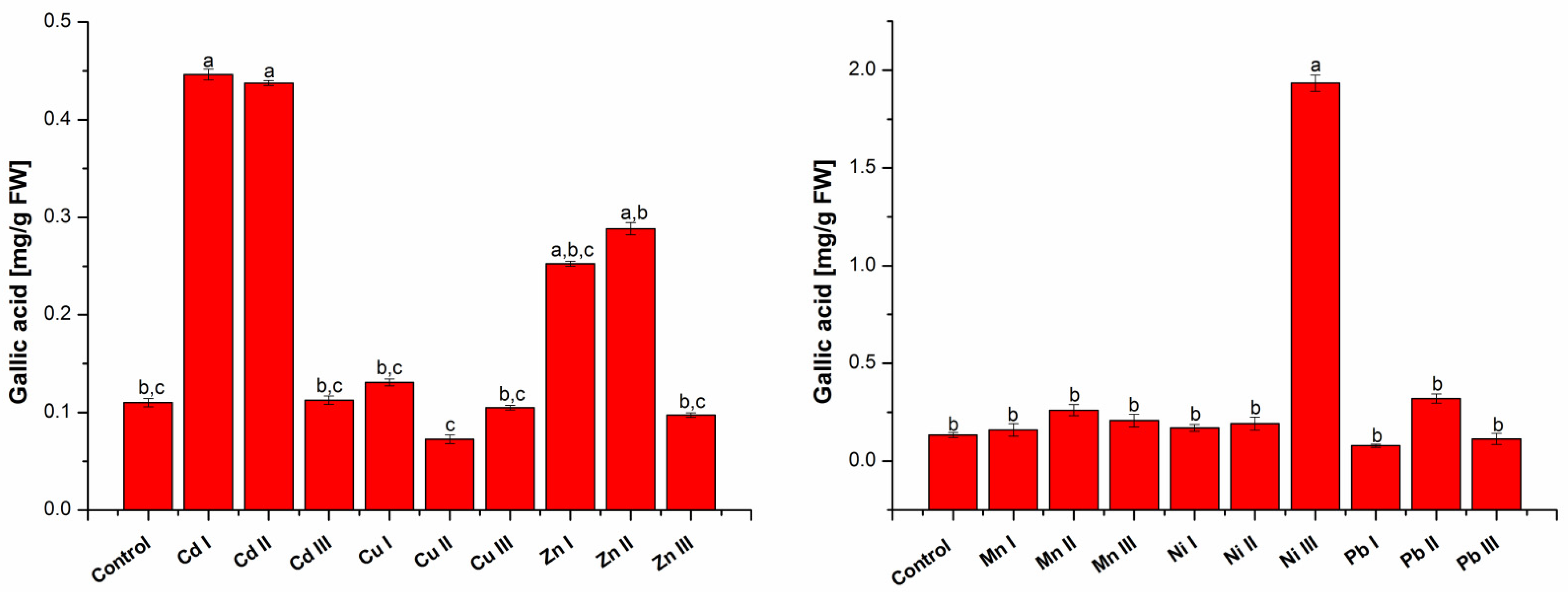
2.1.2. Determination of Total Phenolic Content
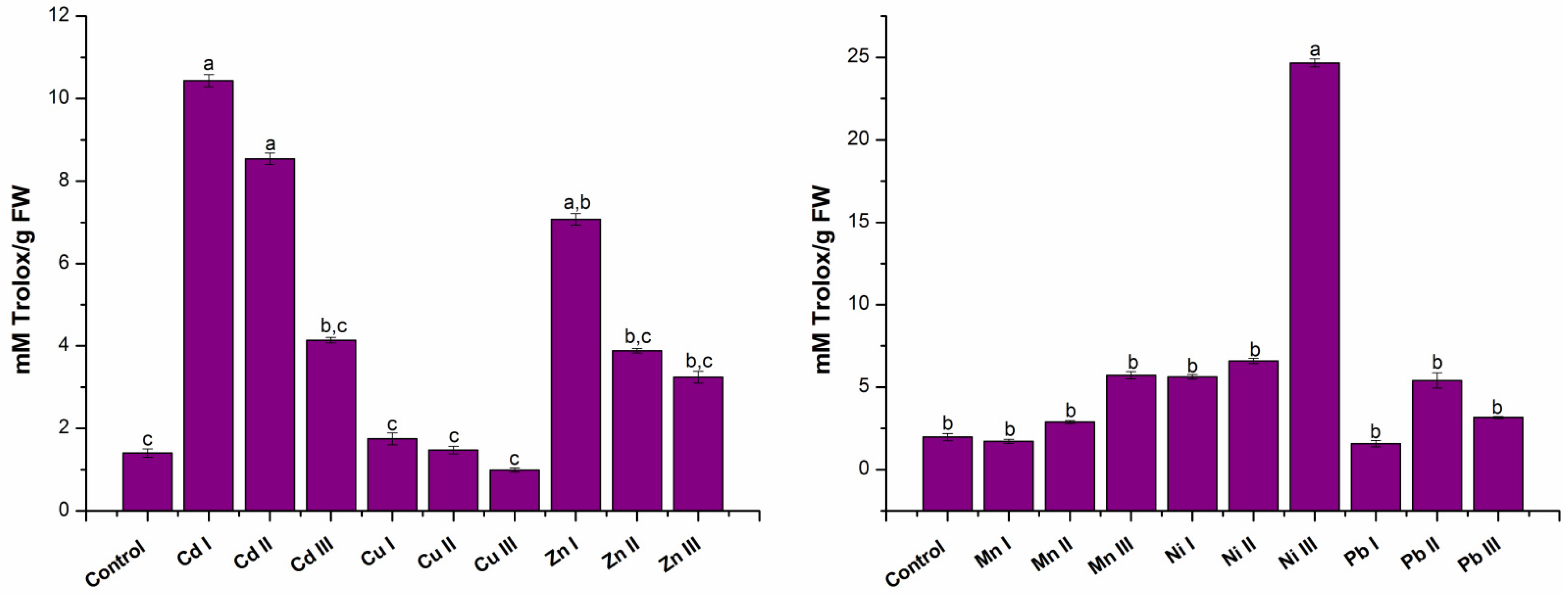
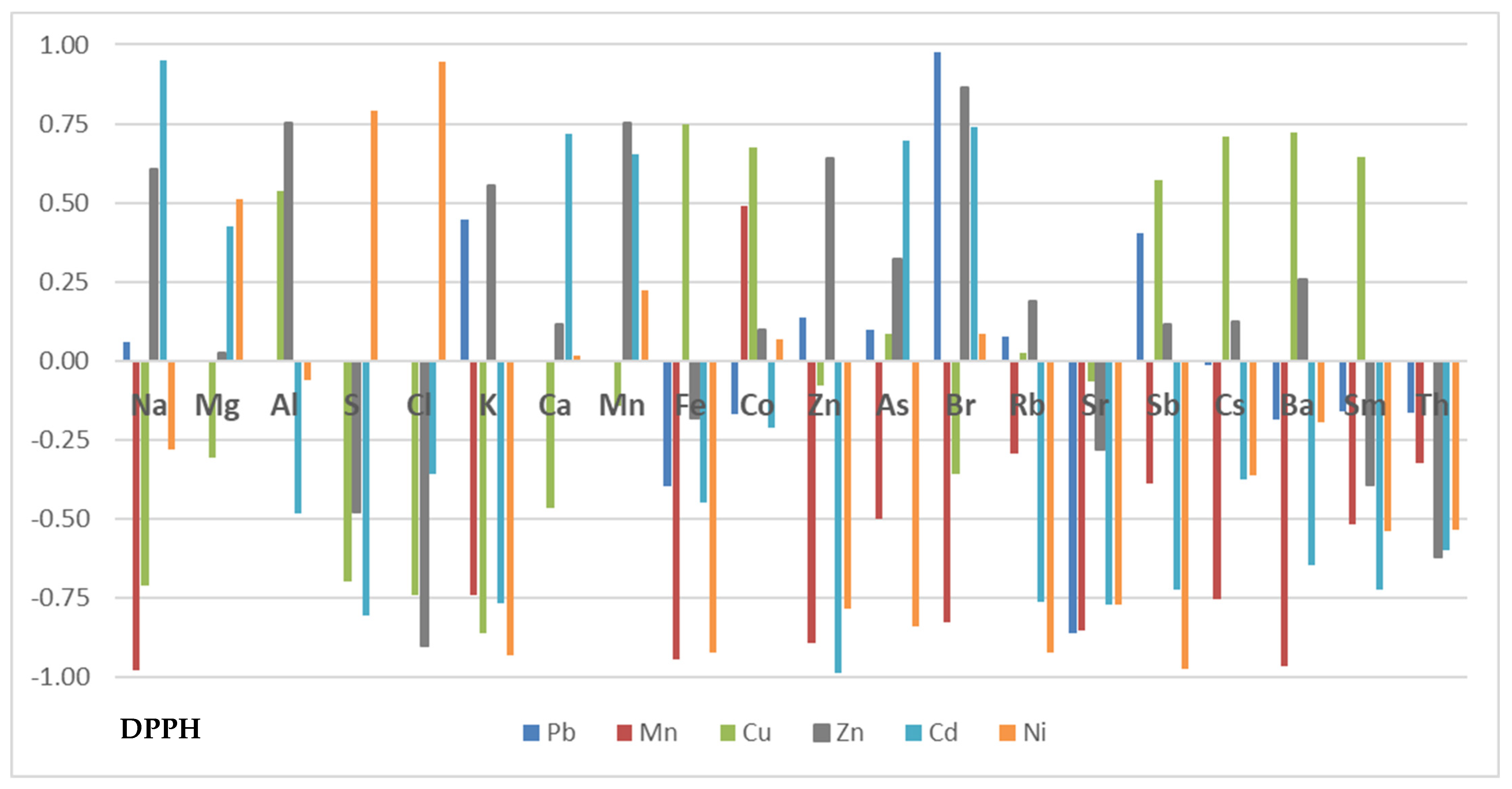
2.1.3. Establishing Antioxidant Capacity
2.1.4. Elemental Content Determination
2.1.5. Variation of the Elemental Content in Plant Compared to Control
2.1.6. Elemental Content in Plant Parts versus Content of Applied HMs
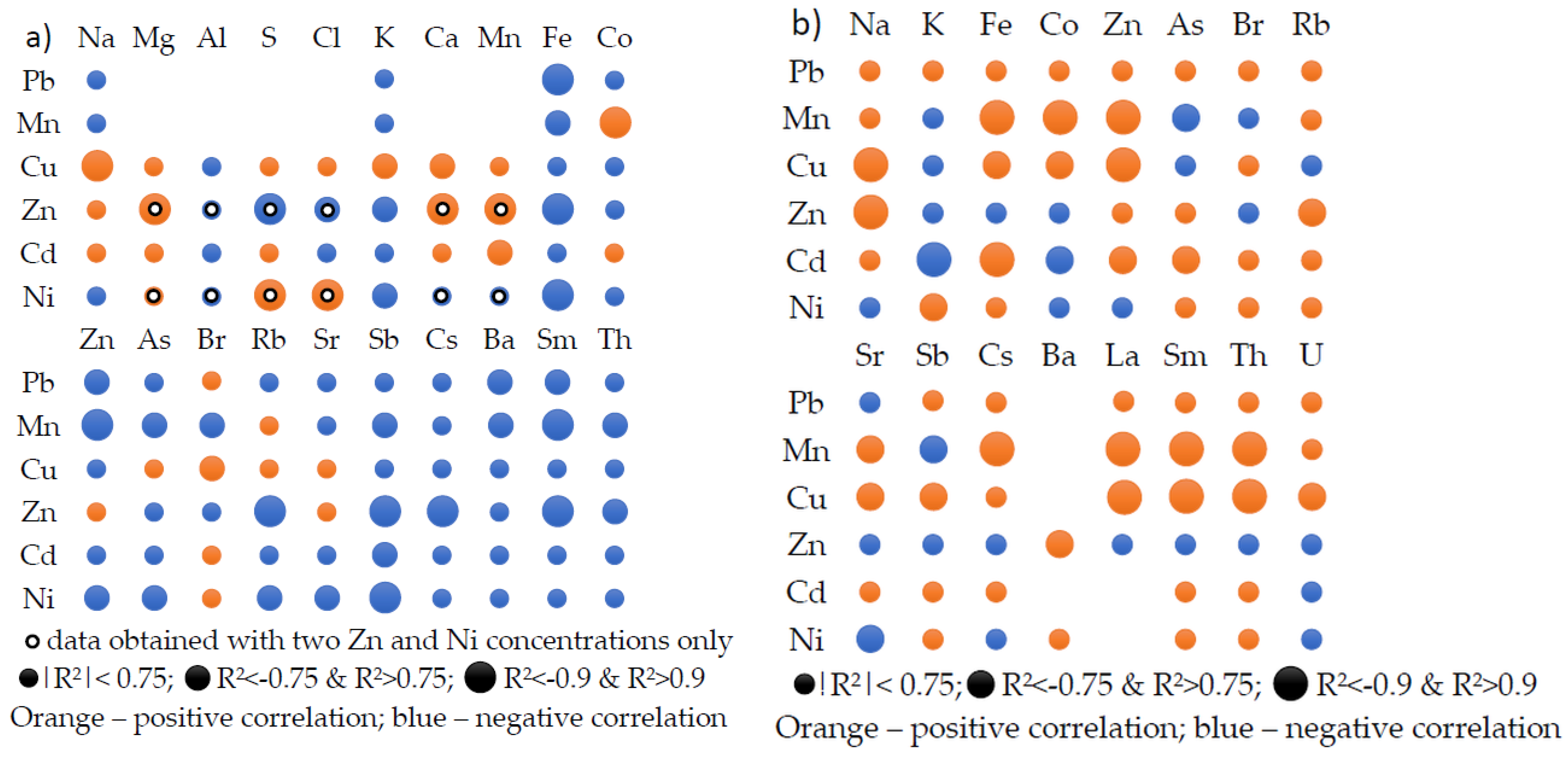
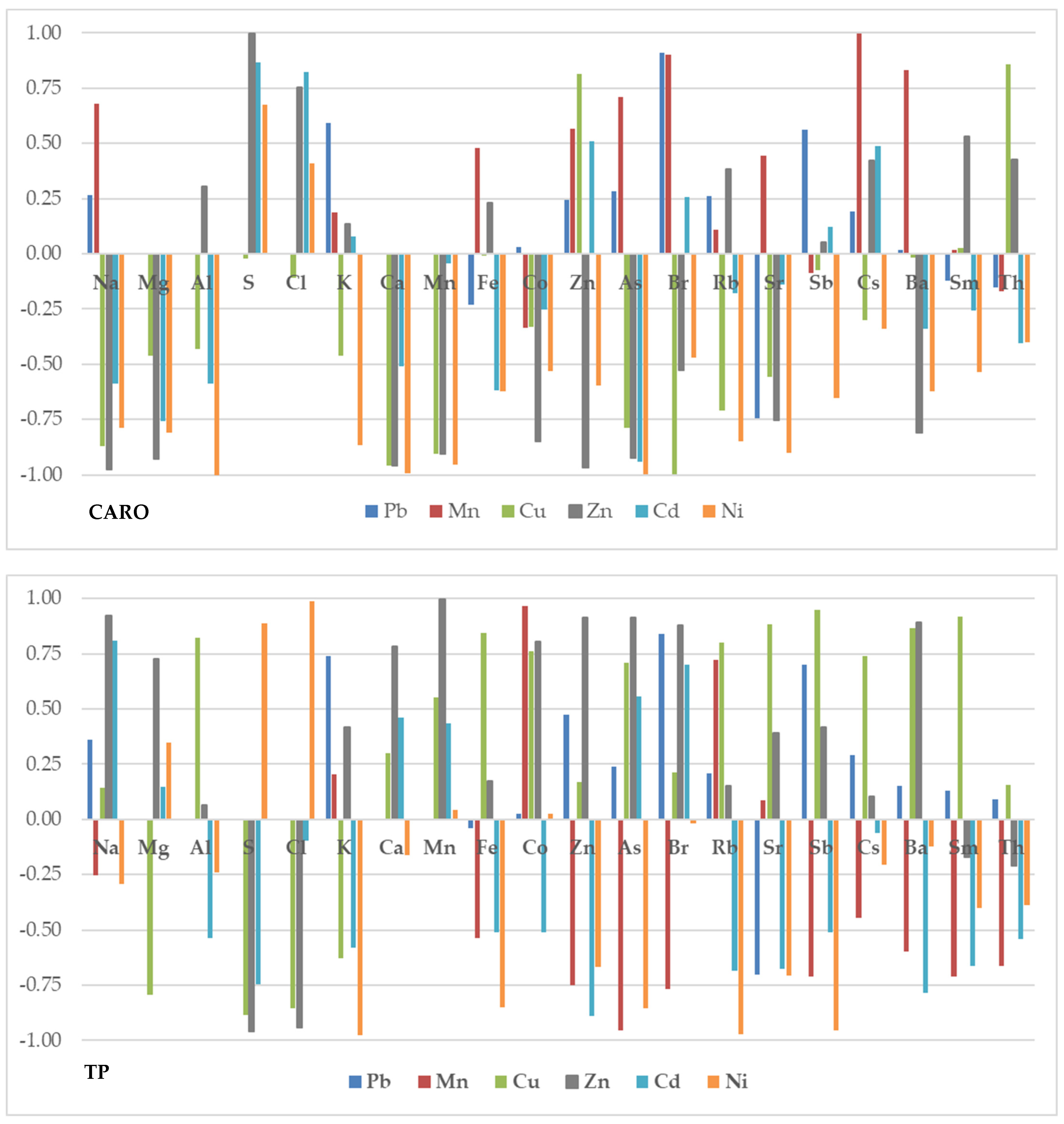
2.2. Correlation between Elemental Content and Bioactive Compounds
2.3. Translocation Factor
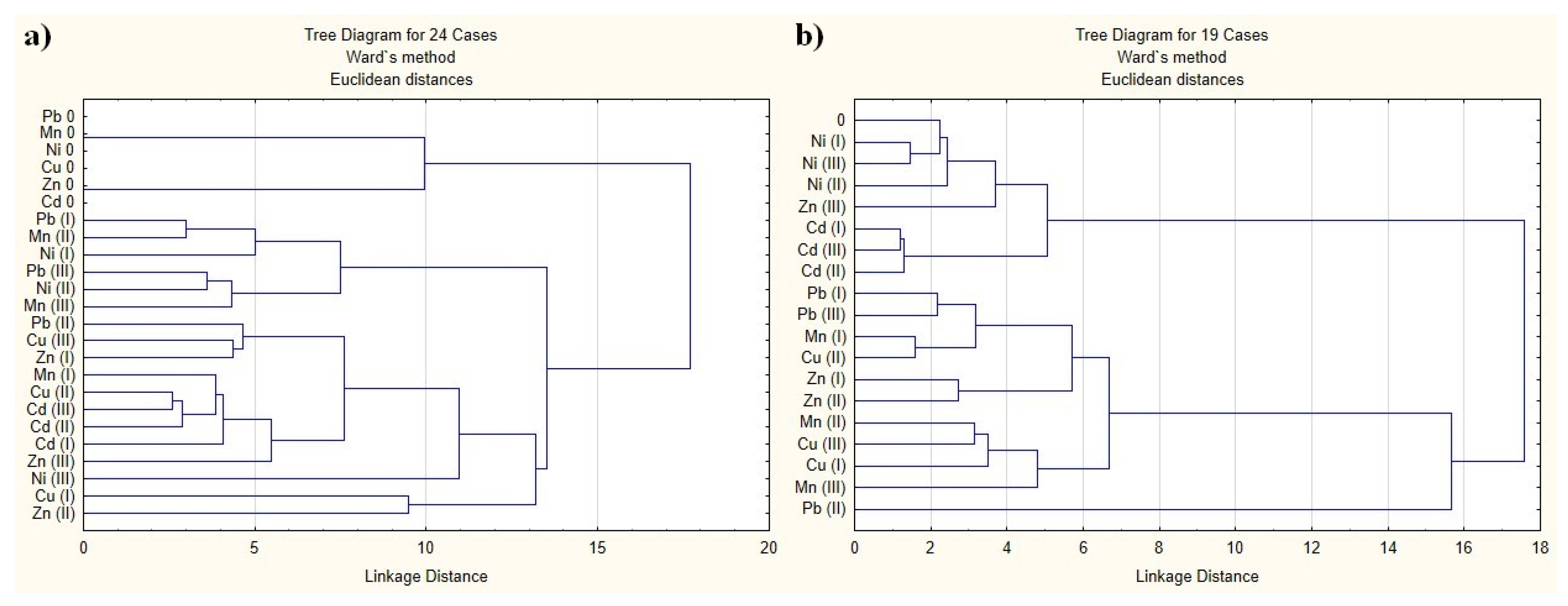
2.4. Cluster Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Plant Growth Conditions
3.3. Plants and Soil Analysis after Harvesting
3.3.1. Determination of Chlorophylls and Total Carotenoids Content
3.3.2. Total Polyphenols Evaluation
3.3.3. Antioxidant Capacity Determination by DPPH Method
3.4. Multielemental Investigation of Lettuce and Soil Substrate by NAA
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xie, N.; Kang, C.; Ren, D.; Zhang, L. Assessment of the variation of heavy metal pollutants in soil and crop plants through field and laboratory tests. Sci. Total Environ. 2022, 811, 152343. [Google Scholar] [CrossRef] [PubMed]
- Sanjosé, I.; Navarro-Roldán, F.; Infante-Izquierdo, M.D.; Martínez-Sagarra, G.; Devesa, J.A.; Polo, A.; Ramírez-Acosta, S.; Sánchez-Gullón, E.; Jiménez-Nieva, F.J.; Muñoz-Rodríguez, A.F. Accumulation and effect of heavy metals on the germination and growth of Salsola vermiculata L. seedlings. Diversity 2021, 13, 539. [Google Scholar] [CrossRef]
- Klink, A. A comparison of trace metal bioaccumulation and distribution in Typha latifolia and Phragmites australis: Implication for phytoremediation. Environ. Sci. Pollut. Res. 2017, 24, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.B.; Singh, A.; Sharma, R.K.; Agrawal, M. Bioaccumulation of heavy metals in vegetables: A threat to human health. Terr. Aquat. Environ. Toxicol. 2007, 1, 13–23. [Google Scholar]
- Gope, M.; Masto, R.E.; George, J.; Hoque, R.R.; Balachandran, S. Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol, India. Ecotoxicol. Environ. Saf. 2017, 138, 231–241. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Ali, W.; Mao, K.; Zhang, H.; Junaid, M.; Xu, N.; Rasool, A.; Feng, X.; Yang, Z. Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J. Hazard. Mater. 2020, 397, 122720. [Google Scholar] [CrossRef]
- Sihlahla, M.; Mouri, H.; Nomngongo, P.N. Uptake of trace elements by vegetable plants grown on agricultural soils. J. Afr. Earth Sci. 2019, 160, 103635. [Google Scholar] [CrossRef]
- Cabral-Pinto, M.M.; Inácio, M.; Neves, O.; Almeida, A.A.; Pinto, E.; Oliveiros, B.; Ferreira da Silva, E.A. Human health risk assessment due to agricultural activities and crop consumption in the surroundings of an industrial area. Expos. Health 2020, 12, 629–640. [Google Scholar] [CrossRef]
- Tong, S.; Yang, L.; Gong, H.; Wang, L.; Li, H.; Yu, J.; Li, Y.; Deji, Y.; Nima, C.; Zhao, S.; et al. Bioaccumulation characteristics, transfer model of heavy metals in soil-crop system and health assessment in plateau region, China. Ecotoxicol. Environ. Saf. 2022, 241, 113733. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Bolan, N.S.; Müller, K.; Laurenson, S.; Kim, W.I. The influence of wastewater irrigation on the transformation and bioavailability of heavy metal(loid)s in soil. Adv. Agron. 2012, 115, 215–297. [Google Scholar]
- Muhammad, M.S. Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere 2013, 93, 2230–2238. [Google Scholar]
- Xi, B.D.; Yu, H.; Li, Y.P.; Dang, Q.L.; Tan, W.B.; Wang, Y.; Cui, D.Y. Insights into the effects of heavy metal pressure driven by long-term treated wastewater irrigation on bacterial communities and nitrogen-transforming genes along vertical soil profiles. J. Hazard. Mater. 2020, 403, 123853. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Xue, P.Y.; Wei, L.; Liu, C.C. Characteristics of Cd, As, and Pb in soil and wheat grains and health risk assessment of grain-Cd/As/Pb on the field scale. Environ. Sci. 2020, 41, 367–375. [Google Scholar]
- Nodelkoska, T.V.; Doran, P.M. Interactive effects of temperature and metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Pollut. 2000, 107, 315–320. [Google Scholar]
- Diaconu, M.; Pavel, L.V.; Hlihor, R.M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of heavy metal toxicity in some plants and microorganisms–A preliminary approach for environmental bioremediation. Nat. Biotechnol. 2020, 56, 130–139. [Google Scholar] [CrossRef]
- Rehman, A.U.; Nazir, S.; Irshad, R.; Tahir, K.; ur Rehman, K.; Islam, R.U.; Wahad, Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J. Mol. Liq. 2020, 321, 114455. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, N.; Madawala, H.M.S.P.; Ok, Y.S.; Vithanage, M. Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health 2019, 41, 1813–1831. [Google Scholar] [CrossRef]
- Jin, C.; Fan, J.; Liu, R.; Sun, R. Single and joint toxicity of sulfamonomethoxine and Cadmium on three agricultural crops. Soil Sediment Contam. 2015, 24, 454–470. [Google Scholar] [CrossRef]
- Mirshekali, H.; Hadi, H.; Amirnia, R.; Khodaverdiloo, H. Effect of zinc toxicity on plant productivity, chlorophyll and Zn contents of Sorghum (Sorghum bicolor) and common Lambsquarter (Chenopodium album). Int. J. Agric. Res. 2012, 2, 247–254. [Google Scholar]
- Tauqeer, H.M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Iftikhar, U.; Ahmad, R.; Farid, M.; Abbasi, G.H. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicol. Environ. Saf. 2016, 126, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Andrade Júnior, W.V.; Oliveira Neto, C.F.; Santos Filho, B.G.; Amarante, C.B.; Cruz, E.D.; Okumura, R.S.; Barbosa, A.V.C.; Sousa, D.J.P.; Teixeira, J.S.S.; Botelho, A.S. Effect of cadmium on young plants of Virola surinamensis. AoB Plants 2019, 11, plz022. [Google Scholar] [CrossRef]
- Grajek, H.; Rydzyński, D.; Piotrowicz-Cieślak, A.; Herman, A.; Maciejczyk, M.; Wieczorek, Z. Cadmium ion-chlorophyll interaction–Examination of spectral properties and structure of the cadmium-chlorophyll complex and their relevance to photosynthesis inhibition. Chemosphere 2020, 261, 127434. [Google Scholar] [CrossRef]
- Mwamba, T.M.; Ali, S.; Ali, B.; Lwalaba, J.L.; Liu, H.; Farooq, M.A.; Shou, J.; Zhou, W. Interactive effects of cadmium and copper on metal accumulation, oxidative stress, and mineral composition in Brassica napus. Int. J. Environ. Sci. Technol. 2016, 13, 2163–2174. [Google Scholar] [CrossRef]
- Laporte, D.; Rodríguez, F.; González, A.; Zúñiga, A.; Castro-Nallar, E.; Sáez, C.A.; Moenne, A. Copper-induced concomitant increases in photosynthesis, respiration, and C, N and S assimilation revealed by transcriptomic analyses in Ulva compressa (Chlorophyta). BMC Plant Biol. 2020, 20, 25. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, J.; Li, E.; Zhang, L.; Wang, X.; Song, L. Combined toxic effects and mechanisms of microsystin-LR and copper on Vallisneria Natans (Lour.) Hara seedlings. J. Hazard. Mater. 2017, 328, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Yagura, R.; Imanishi, J.; Shibata, S. Effects of copper ions on the growth and photosynthetic activity of Scopelophila cataractae. Lindbergia 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Dey, S.; Mazumder, P.B.; Paul, S.B. Effect of copper on growth and chlorophyll content in tea plants (Camellia sinensis (L.) O. Kuntze). Int. J. Res. Appl. Nat. Soc. Sci. 2014, 2, 223–230. [Google Scholar]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, T.A.; Prajapati, D.H.; Burow, M. Differential partitioning of thiols and glucosinolates between shoot and root in Chinese cabbage upon excess zinc exposure. J. Plant Physiol. 2020, 244, 153088. [Google Scholar] [CrossRef] [PubMed]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, F.; Yasmeen, T.; Riaz, M.; Arif, M.S.; Ali, S.; Raza, S.H. Proteus mirabilis alleviates zinc toxicity by preventing oxidative stress in maize (Zea mays) plants. Ecotoxicol. Environ. Saf. 2014, 110, 143–152. [Google Scholar] [CrossRef]
- Betancourt, O.; Tapia, M.; Méndez, I. Decline of general intelligence in children exposed to manganese from mining contamination in Puyango river basin, Southern Ecuador. Ecohealth 2015, 12, 453–460. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Sieprawska, A.; Filek, M.; Tobiasz, A.; Walas, S.; Dudek-Adamska, D.; Grygo-Szymanko, E. Trace elements’ uptake and antioxidant response to excess of manganese in in vitro cells of sensitive and tolerant wheat. Acta Physiol. Plant. 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Yang, S.X.; Deng, H.; Li, M.S. Manganese uptake and accumulation in a woody hyperaccumulator, Schima superba. Plant Soil Environ. 2008, 54, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Gill, R.A.; Kanwar, M.K.; Rodrigues dos Reis, A.; Ali, B. Editorial: Heavy metal toxicity in plants: Recent insights on physiological and molecular aspects. Front. Plant Sci. 2022, 12, 830682. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Jatav, P.K.; Verma, R.; Kothari, S.L.; Kachhwaha, S. Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environ. Sci. Pollut. Res. Int. 2017, 24, 23915–23925. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Agnihotri, R.K.; Reshma, R.S.; Ahmad, M. Effect of lead and nickel toxicity on chlorophyll and proline content of Urd (Vigna mungo L.) seedlings. Int. J. Plant Physiol. Biochem. 2012, 4, 136–141. [Google Scholar]
- Cenkci, S.; Cigerci, I.H.; Yıldız, M.; Ozay, C.; Bozdag, A.; Terzi, H. Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environ. Exp. Bot. 2010, 67, 467–473. [Google Scholar] [CrossRef]
- Afaj, A.H.; Jassim, A.J.; Noori, M.M.; Schuth, C. Effects of lead toxicity on the total chlorophyll content and growth changes of the aquatic plant Ceratophyllum demersum L. Int. J. Environ. Stud. 2016, 74, 119–128. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Tajmir–Riahi, H.A. Interaction of toxic metal ions Cd2+, Hg2+ and Pb2+ with light–harvesting proteins of chloroplast thylakoid membranes. An FTIR spectroscopic study. J. Inorg. Biochem. 1993, 50, 235–243. [Google Scholar] [CrossRef]
- Vodnik, D.; Jentschke, G.; Fritz, E.; Gogala, N.; Godbold, D.L. Root–applied cytokinin reduces lead uptake and affects its distribution in Norway spruce seedlings. Physiol. Plant 1999, 106, 75–81. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of phytoremediation potential of Nerium indicum with inorganic modifier calcium carbonate and organic modifier mushroom residue to lead-zinc tailings. Int. J. Environ. Res. Public Health 2022, 19, 10353. [Google Scholar] [CrossRef]
- Yu, C.; Yan, C.; Liu, Y.; Liu, Y.; Jia, Y.; Lavelle, D.; An, G.; Zhang, W.; Zhang, L.; Han, R.; et al. Upregulation of a KN1 homolog by transposon insertion promotes leafy head development in lettuce. Proc. Natl. Acad. Sci. USA 2020, 117, 33668–33678. [Google Scholar] [CrossRef]
- Lopez, A.; Javier, G.A.; Fenoll, J.; Hellín, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- Nicolle, C.; Carnat, A.; Fraisse, D.; Lamaison, J.L.; Rock, E.; Michel, H.; Amouroux, P.; Remesy, C. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J. Sci. Food Agric. 2004, 84, 2061–2069. [Google Scholar] [CrossRef]
- Chaney, R.L. How does contamination of rice soils with Cd and Zn cause high incidence of human Cd disease in subsistence rice farmers. Curr. Pollut. Rep. 2015, 1, 13–22. [Google Scholar] [CrossRef]
- Achakzai, A.K.K.; Bazai, Z.A.; Kayani, S.A. Accumulation of heavy metals by lettuce (Lactuca sativa L.) irrigated with different levels of wastewater of Quetta city. Pak. J. Bot. 2011, 43, 2953–2960. [Google Scholar]
- Ferri, R.; Donna, F.; Smith, D.R.; Guazzetti, S.; Zacco, A.; Rizzo, L.; Bontempi, E.; Zimmerman, N.J.; Lucchini, R.G. Heavy metals in soil and salad in the proximity of historical ferroalloy emission. J. Environ. Prot. 2012, 3, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Al-Salama, Y.J. The use of neutron activation analysis technique to estimate some heavy metals in soil and plants irrigated with wastewater. R.J. Aleppo Univ. Basic Sci. Ser. 2013, 105, 1–14. [Google Scholar]
- Armelin, M.J.A.; Trevizam, A.R.; Muraoka, T.; Silva, M.L.S.; Saiki, M.; Maihara, V.A. Instrumental neutron activation analysis applied to multielement determination in a variety of lettuce grown in a contaminated soil and treated with phosphate. 3rd-INCC 2011, 58. Available online: http://repositorio.ipen.br/bitstream/handle/123456789/14193/17308.pdf?sequence=1&isAllowed=y (accessed on 26 August 2022).
- Pacheco, A.M.G.; Freitas, M.C.; Ventura, M.G.; Dionısio, I.; Ermakova, E. Chemical elements in common vegetable components of Portuguese diets, determined by k0-INAA. Nucl. Instrum. Methods Phys. Res. A 2006, 564, 721–728. [Google Scholar] [CrossRef]
- Ittipongse, A.; Fungklin, R. Determine of heavy metal contents in fresh vegetable by using nuclear activation analysis technique. SNRU J. Sci. Technol. 2016, 8, 187–191. [Google Scholar]
- Freitas, M.C.; Pacheco, A.M.G.; Bacchi, M.A.; Dionísio, I.; Landsberger, S.; Braisted, J.; Fernandes, E.A.N. Compton suppression instrumental neutron activation analysis performance in determining trace- and minor-element contents in foodstuff. J. Radioanal. Nucl. Chem. 2008, 276, 149–156. [Google Scholar] [CrossRef]
- Alsayed, E.M.; Elqusy, N.O. Heavy metals uptake and translocation by lettuce and spinach grown on a metal-contaminated soil. J. Soil Sci. Plant Nutr. 2018, 18, 1097–1107. [Google Scholar]
- Adu, A.A.; Aderinola, O.J.; Kusemiju, V. Heavy metals concentration in garden lettuce (Lactuca sativa L.) grown along Badagry expressway, Lagos, Nigeria. Transnatl. J. Sci. Technol. 2012, 2, 115–130. [Google Scholar]
- Boamponsem, G.A.; Kumi, M.; Debrah, I. Heavy metals accumulation in cabbage, lettuce and carrot irrigated with wastewater from Nagodi mining site in Ghana. Int. J. Sci. Technol. Res. 2012, 1, 124–129. [Google Scholar]
- Seka Yapoga, J.; Yapo Ossey, B.; Yapi Dopé, A.C. Heavy metals contamination in Lactuca sativa L. (lettuce) from two agricultural sites of Abidjan. Int. J. Pure Appl. Sci. Technol. 2015, 27, 59–64. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Current Protocols in Food Analytical Chemistry (Units: F4.3.1–F4.3.8); John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]

| Pb | Mn | Cu | Zn | Cd | Ni | Control | |
|---|---|---|---|---|---|---|---|
| Na * | 3.2–4.5 | 3.2–4.4 | 3.8–6.0 | 4.4–7.1 | 4.0–4.6 | 3.5–4.9 | 3.8–4.3 |
| Mg * | 2.7–3.1 a | - | 2.7–3.9 | 2.6–3.9 a | 2.5–3.7 | 2.7–4.3 a | 2.5–3.0 |
| Al ** | 70.4–79.6 a | - | 133–660 | 177–220 a | 96–181 | 132–293 a | 183–203 |
| S a ** | 7.2–11.8 a | - | 4.8–15.6 | 5.3–10.1 a | 5.0–12.6 | 7.7–15.2 a | 7.2–12.7 |
| Cl * | 30.6–35.0 a | - | 22.7–42.9 | 22.1–26.8 a | 25.3–36.1 | 32.7–40.4 a | 30.3–34.7 |
| K * | 93.0–129 | 91.2–130 | 98.3–136 | 87.6–136 | 96.5–119 | 82.8–132 | 101–125 |
| Ca * | 9.0–11.7 a | - | 10.5–17.7 | 11.6–25.2 a | 11.4–17.2 | 8.0–17.7 a | 9.7–12.7 |
| Mn ** | 92–106 a | - | 146–409 | 1256–1648 a | 229–292 | 84–389 a | 112–130 |
| Fe ** | 144–308 | 155–295 | 181–527 | 141–279 | 139–304 | 174–279 | 278–334 |
| Co *** | 58–248 | 17–306 | 93–360 | 74–242 | 95–146 | 94–201 | 102–134 |
| Zn ** | 111–286 | 197–302 | 102–137 | 729–1757 | 86–242 | 100–209 | 289–329 |
| As ** | 0.49–0.81 | 0.48–0.61 | 0.54–0.96 | 0.50–0.97 | 0.54–0.82 | 0.47–0.63 | 0.54–0.59 |
| Br ** | 17.6–49.9 | 13.7–28.9 | 33.0–61.8 | 17.6–49.9 | 34.8–53.1 | 28.0–53.1 | 21.2–25 |
| Rb ** | 40.1–65.2 | 40.0–65.2 | 40.1–61.7 | 35.9–57.0 | 38.5–54.7 | 32.6–60.5 | 41.0–57.0 |
| Sr ** | 20.9–42.0 | 22.2–38.6 | 24.6–40.7 | 24.8–47.3 | 24.9–32.4 | 21.0–35.3 | 28.7–35.3 |
| Sb *** | 11.5–68.9 | 25.7–35.7 | 21.5–76.5 | 12–53.2 | 20–33.8 | 26.7–54.2 | 39.4–56.6 |
| Cs *** | 46.4–82.6 | 58.5–83.5 | 61.1–133 | 48.3–76.8 | 50.7–74.0 | 53.2–71.0 | 62.6–75.4 |
| Ba ** | 7.3–15.7 | 5.3–16.4 | 6.8–22.0 | 7.8–21.4 | 7.5–14.9 | 6.4–16.8 | 10.2–15.0 |
| Sm *** | 8.1–24.7 | 6.4–16.1 | 14.3–70.1 | 6.4–26.1 | 12.0–31.1 | 11–33.5 | 28.9–47.1 |
| Th *** | 15.9–36.9 | 15.0–28.5 | 15.0–25.0 | 7.5–37.5 | 7.5–41.6 | 24.1–43.4 | 58–68 |
| [45] | [47] | [48] | [49] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CLL | LL | ILL (Evora) | OLL (Evora) | ILL (Coimbra) | OLL (Coimbra) | LL | OLL | OLL | ILL | ILL | |
| Na * | 4.2 | 6–10.3 | 0.14 ± 0.03 | 0.34 ± 0.07 | 0.17 ± 0.14 | 0.31 ± 0.11 | 2.9 ± 7 | 1.93 ± 0.01 | 2.4 ± 0.8 | 0.74 ± 0.04 | 1.3 ± 1.1 |
| Mg * | 5.2 | 3.6–6.8 | 3.6 ± 0.7 | 3.2 ± 0.014 | 2.04 ± 0.01 | ||||||
| Al ** | 3524 ± 318 | ||||||||||
| S * | |||||||||||
| Cl * | 22.8 | 8.5–15.1 | 17.9 ± 0.4 | ||||||||
| K * | 96.1 | 52.7–74.9 | 6.3 ± 1.4 | 8.3 ± 1.9 | 7.2 ± 0.8 | 10.7 ± 1.7 | 84.8 ± 1.7 | 82 ± 13 | 54.3 ± 0.1 | 55.1 ± 6 | |
| Ca * | 30.3 | 14.9–22.9 | 0.51 ± 0.08 | 1.17 ± 0.18 | 0.54 ± 0.05 | 1.29 ± 0.06 | 13.3 ± 0.6 | 12.9 ± 0.15 | 9.92 ± 0.45 | 5.0 ± 0.28 | 4.18 ± 0.04 |
| Mn ** | 0.042 | 0.013–0.038 | 68.81 ± 0.22 | 157.6 ± 3.1 | 38.2 ± 1.2 | ||||||
| Fe ** | 0.42 | 0.19–0.97 | 16.1 ± 3.0 | 44 ± 13 | 16.9 ± 4.6 | 53.2 ± 3.6 | 1506 ± 54.3 NAA 530.4 AAS | 343 ± 24 | 409 ± 28 | 102 ± 10 | 130 ± 35 |
| Co *** | 302 | 76–1026 | 7.9 ± 1.7 | 19.3 ± 5.6 | 8.4 ± 1.7 | 30 ± 10 | 1010 ± 160 NAA 7200 AAS | 0.13 ± 0.05 | 0.23 ± 0.08 | 0.067 ± 0.02 | |
| Zn ** | 0.84 | 0.35–1.2 | 4.99 ± 0.58 | 3.55 ± 0.6 | 7.24 ± 0.5 | 7.14 ± 0.33 | 45.7 ± 1 | 54.9 ± 2.5 | 56.2 ± 3.9 | 55.7 ± 3.8 | |
| As *** | 13 ± 6.7 | 43 ± 16 | 6.5 ± 2.1 | 24.3 ± 2.1 | |||||||
| Br ** | 87.5 | 62–80 | 1.27 ± 0.23 | 2.74 ± 0.45 | 3.41 ± 0.45 | 8.3 ± 1.8 | |||||
| Rb ** | 52.3 | 59.3–81 | 1.19 ± 0.1 | 1.56 ± 0.11 | 1.2 ± 0.12 | 1.72 ± 0.18 | 11.6 ± 0.94 | 13.2 ± 1.4 | 9.22 ± 0.34 | 9.23 ± 0.92 | |
| Sr ** | 2.45 ± 0.39 | 5.5 ± 0.54 | 2.00 ± 0.23 | 6.85 ± 0.59 | |||||||
| Sb *** | 85 | 30.3–79 | <8 | 3.94 ± 0.86 | 4.5 ± 1.5 | 11.5 ± 1.8 | 46.8 ± 7.1 | 88 ± 14 | 34.9 ± 7.3 | 35 ± 12 | |
| Cs *** | 50 | 170–2360 | |||||||||
| Ba | |||||||||||
| Sm | |||||||||||
| Th *** | 25 | 24–143 | |||||||||
| Pb | Mn | Cu | Zn | Cd | Ni | Control | |
|---|---|---|---|---|---|---|---|
| Na * | 5.2–10.9 | 4.4–6.7 | 4.7–7.2 | 5.0–6.9 | 5.4–7.5 | 5.1–7.6 | 4.9–5.6 |
| K * | 114–243 | 92.7–150 | 78.3–134 | 112–162 | 135–173 | 140–182 | 142–172 |
| Fe ** | 1811–7194 | 1398–3960 | 1033–2554 | 394–2494 | 285–475 | 317–474 | 259–351 |
| Co ** | 1.75–10.0 | 2.5–6.3 | 1.03–3.4 | 0.66–5.7 | 0.61–1.12 | 0.69–2.4 | 1.17–1.53 |
| Zn ** | 116–485 | 140–286 | 121–234 | 1896–3347 | 96.6–152 | 103–312 | 82.9–97.1 |
| As ** | 4.0–6.7 | 2.9–4.0 | 5.3–7.2 | 4.0–7.3 | 11.7–17.0 | 5.1–9.0 | 6.1–6.9 |
| Br ** | 40.3–158 | 33.9–57.5 | 51.4–79.1 | 43.1–80.1 | 66.9–95.3 | 75.2–103 | 49.5–58.5 |
| Rb ** | 51.8–129 | 42.6–87.4 | 39.4–63.6 | 51.0–77.7 | 55.2–79.2 | 56.8–82.6 | 52.6–73.4 |
| Sr ** | 27.4–125 | 30.4–155 | 32.3–77.0 | 36.4–75.1 | 36.7–61.1 | 36.5–56.4 | 40.0–54.1 |
| Sb *** | 87.7–4510 | 78.9–2251 | 67.2–1839 | 82.7–1819 | 69.6–157 | 77.1–209 | 120–159 |
| Cs *** | 216–975 | 167–686 | 209–819 | 133–422 | 124–201 | 100–485 | 98.6–147 |
| Ba ** | - | - | - | 38.9–67.3 | - | 23.9–45.6 | 14.3–23.8 |
| La ** | 1.23–4.4 | 0.83–2.3 | 0.57–2.8 | 0.54–2.2 | - | - | 0.62–0.84 |
| Sm *** | 234–699 | 128–279 | 105–410 | 24.6–26.9 | 17.8–46.9 | 12.5–49.1 | 5.6–10.4 |
| Th *** | 225–1262 | 203–602 | 234–766 | 45.0–423 | 52.5–123 | 67.5–152 | 45.0–75.0 |
| U *** | 157–304 | 94.4–278 | 85.3–230 | 67.8–230 | 67.3–133 | 75.4–187 | 121–177 |
| Pb | Mn | Cu | Zn | Cd | Ni | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | I | II | III | I | II | III | I | II | III | I | II | III | I | II | III | I | II | III |
| Na | - | - | * | - | - | * | - | - | *** | *** | *** | ** | * | - | - | * | - | - |
| Mg | - | - | *** | ** | - | ** | ** | - | * | *** | - | |||||||
| Al | *** | *** | *** | *** | - | - | * | *** | *** | *** | *** | |||||||
| S | - | - | - | - | - | ** | - | - | - | - | - | |||||||
| Cl | - | *** | *** | ** | *** | *** | ** | - | - | - | * | |||||||
| K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | * |
| Ca | - | - | - | ** | - | *** | ** | - | * | ** | - | |||||||
| Mn | ** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |||||||
| Fe | - | ** | *** | - | * | *** | *** | *** | *** | * | ** | *** | - | *** | * | * | * | *** |
| Co | *** | ** | *** | *** | *** | *** | *** | - | - | - | *** | * | - | - | - | *** | - | - |
| Zn | *** | * | *** | - | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| As | *** | *** | - | - | - | - | *** | - | *** | *** | *** | - | *** | - | - | - | - | ** |
| Br | * | *** | *** | - | *** | *** | *** | *** | *** | *** | *** | * | *** | *** | *** | *** | *** | *** |
| Rb | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sr | - | ** | * | - | - | ** | - | - | - | - | * | - | - | - | - | - | * | ** |
| Sb | - | * | *** | ** | ** | ** | ** | ** | - | - | - | *** | ** | ** | *** | - | - | * |
| Cs | - | - | ** | - | - | - | *** | - | - | - | - | - | - | - | * | - | - | - |
| Ba | - | - | * | - | - | ** | ** | * | - | - | * | - | - | - | - | - | * | - |
| Sm | *** | ** | *** | *** | *** | *** | - | ** | - | ** | ** | *** | - | *** | * | - | *** | ** |
| Th | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Pb | Mn | Cu | Zn | Cd | Ni | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | I | II | III | I | II | III | I | II | III | I | II | III | I | II | III | I | II | III |
| Na | ** | *** | - | - | - | - | - | - | ** | - | - | * | ** | - | - | ** | - | - |
| K | * | *** | - | ** | *** | - | *** | ** | *** | * | * | - | - | - | - | - | - | - |
| Fe | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | - | - | ** | ** | - | - |
| Co | *** | *** | ** | *** | *** | *** | *** | - | *** | *** | *** | *** | * | *** | *** | *** | *** | - |
| Zn | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | * | *** | *** | ** | *** |
| As | *** | - | - | *** | *** | *** | - | ** | - | *** | *** | - | *** | *** | *** | ** | ** | *** |
| Br | ** | *** | *** | - | *** | - | - | *** | - | *** | - | - | *** | *** | *** | *** | *** | *** |
| Rb | - | ** | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sr | - | ** | * | - | ** | ** | - | - | * | - | * | - | - | - | - | - | - | - |
| Sb | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | * | - | - | ** | - | ** | - |
| Cs | *** | *** | *** | ** | *** | *** | *** | *** | *** | *** | *** | - | - | ** | - | - | *** | - |
| Ba | *** | *** | *** | * | ** | ** | ||||||||||||
| La | *** | *** | *** | * | *** | *** | *** | - | *** | *** | * | - | - | - | - | - | - | - |
| Sm | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | *** |
| Th | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | - | *** | - | - | *** | - | *** |
| U | - | ** | - | - | - | * | - | - | ** | - | * | ** | ** | - | * | - | * | - |
| mg kg−1 | Na | K | Fe | Co | Zn | As | Br | Rb | Sr | Sb | Cs | Ba | Sm | Th | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 0 | 0.74 | 0.72 | 1.00 | 0.09 | 3.43 | 0.09 | 0.43 | 0.78 | 0.68 | 0.33 | 0.56 | 0.66 | 4.75 | 1.05 |
| Pb | I | 0.64 | 0.86 | 0.11 | 0.09 | 1.45 | 0.18 | 0.44 | 0.88 | 0.75 | 0.04 | 0.19 | 0.08 | 0.07 | |
| II | 0.42 | 0.53 | 0.04 | 0.02 | 0.60 | 0.11 | 0.32 | 0.50 | 0.24 | 0.02 | 0.09 | 0.03 | 0.03 | ||
| III | 0.61 | 0.76 | 0.08 | 0.04 | 0.66 | 0.09 | 0.44 | 0.76 | 0.78 | 0.01 | 0.20 | 0.07 | 0.08 | ||
| Mn | I | 0.85 | 0.93 | 0.17 | 0.07 | 1.87 | 0.19 | 0.50 | 0.95 | 0.87 | 0.04 | 0.37 | 0.09 | 0.08 | |
| II | 0.83 | 1.15 | 0.07 | 0.06 | 1.26 | 0.16 | 0.45 | 1.08 | 0.43 | 0.02 | 0.12 | 0.05 | 0.05 | ||
| III | 0.56 | 0.76 | 0.06 | 0.04 | 0.79 | 0.14 | 0.29 | 0.66 | 0.21 | 0.02 | 0.11 | 0.03 | 0.05 | ||
| Cu | I | 0.87 | 1.20 | 0.25 | 0.14 | 0.85 | 0.13 | 0.84 | 1.10 | 0.97 | 0.08 | 0.17 | 0.34 | 0.06 | |
| II | 0.78 | 0.98 | 0.18 | 0.09 | 0.71 | 0.10 | 0.49 | 0.89 | 0.59 | 0.04 | 0.29 | 0.16 | 0.08 | ||
| III | 0.85 | 1.40 | 0.09 | 0.04 | 0.58 | 0.14 | 0.92 | 0.96 | 0.55 | 0.02 | 0.15 | 0.07 | 0.03 | ||
| Zn | I | 1.04 | 0.98 | 0.11 | 0.02 | 0.61 | 0.17 | 0.62 | 0.79 | 0.54 | 0.03 | 0.18 | 0.26 | 0.08 | 0.05 |
| II | 1.18 | 0.82 | 0.20 | 0.10 | 0.53 | 0.19 | 0.58 | 0.68 | 0.64 | 0.04 | 0.21 | 0.37 | 0.15 | 0.12 | |
| III | 0.75 | 0.66 | 0.34 | 0.11 | 0.38 | 0.08 | 0.41 | 0.65 | 0.77 | 0.16 | 0.35 | 0.18 | 0.29 | 0.17 | |
| Cd | I | 0.68 | 0.68 | 0.76 | 0.12 | 0.71 | 0.05 | 0.51 | 0.69 | 0.67 | 0.20 | 0.41 | 0.61 | 0.35 | |
| II | 0.72 | 0.70 | 0.44 | 0.16 | 1.56 | 0.05 | 0.56 | 0.69 | 0.66 | 0.20 | 0.37 | 0.67 | 0.14 | ||
| III | 0.73 | 0.72 | 0.59 | 0.18 | 1.60 | 0.04 | 0.52 | 0.71 | 0.53 | 0.30 | 0.36 | 0.92 | 0.34 | ||
| Ni | I | 0.66 | 0.75 | 0.61 | 0.08 | 0.67 | 0.08 | 0.52 | 0.73 | 0.64 | 0.23 | 0.45 | 0.47 | 0.84 | 0.29 |
| II | 0.64 | 0.75 | 0.65 | 0.14 | 1.37 | 0.10 | 0.37 | 0.75 | 0.61 | 0.51 | 0.13 | 0.22 | 0.83 | 0.31 | |
| III | 0.70 | 0.55 | 0.50 | 0.12 | 0.86 | 0.06 | 0.38 | 0.56 | 0.50 | 0.23 | 0.53 | 0.35 | 0.51 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soran, M.-L.; Sîrb, A.N.; Lung, I.; Opriş, O.; Culicov, O.; Stegarescu, A.; Nekhoroshkov, P.; Gligor, D.-M. A Multi-Method Approach for Impact Assessment of Some Heavy Metals on Lactuca sativa L. Molecules 2023, 28, 759. https://doi.org/10.3390/molecules28020759
Soran M-L, Sîrb AN, Lung I, Opriş O, Culicov O, Stegarescu A, Nekhoroshkov P, Gligor D-M. A Multi-Method Approach for Impact Assessment of Some Heavy Metals on Lactuca sativa L. Molecules. 2023; 28(2):759. https://doi.org/10.3390/molecules28020759
Chicago/Turabian StyleSoran, Maria-Loredana, Aura Nicoleta Sîrb, Ildiko Lung, Ocsana Opriş, Otilia Culicov, Adina Stegarescu, Pavel Nekhoroshkov, and Delia-Maria Gligor. 2023. "A Multi-Method Approach for Impact Assessment of Some Heavy Metals on Lactuca sativa L." Molecules 28, no. 2: 759. https://doi.org/10.3390/molecules28020759






