Hepatoprotective Effects of Rosmarinic Acid on Ovalbumin-Induced Intestinal Food Allergy Mouse Model
Abstract
:1. Introduction
2. Results
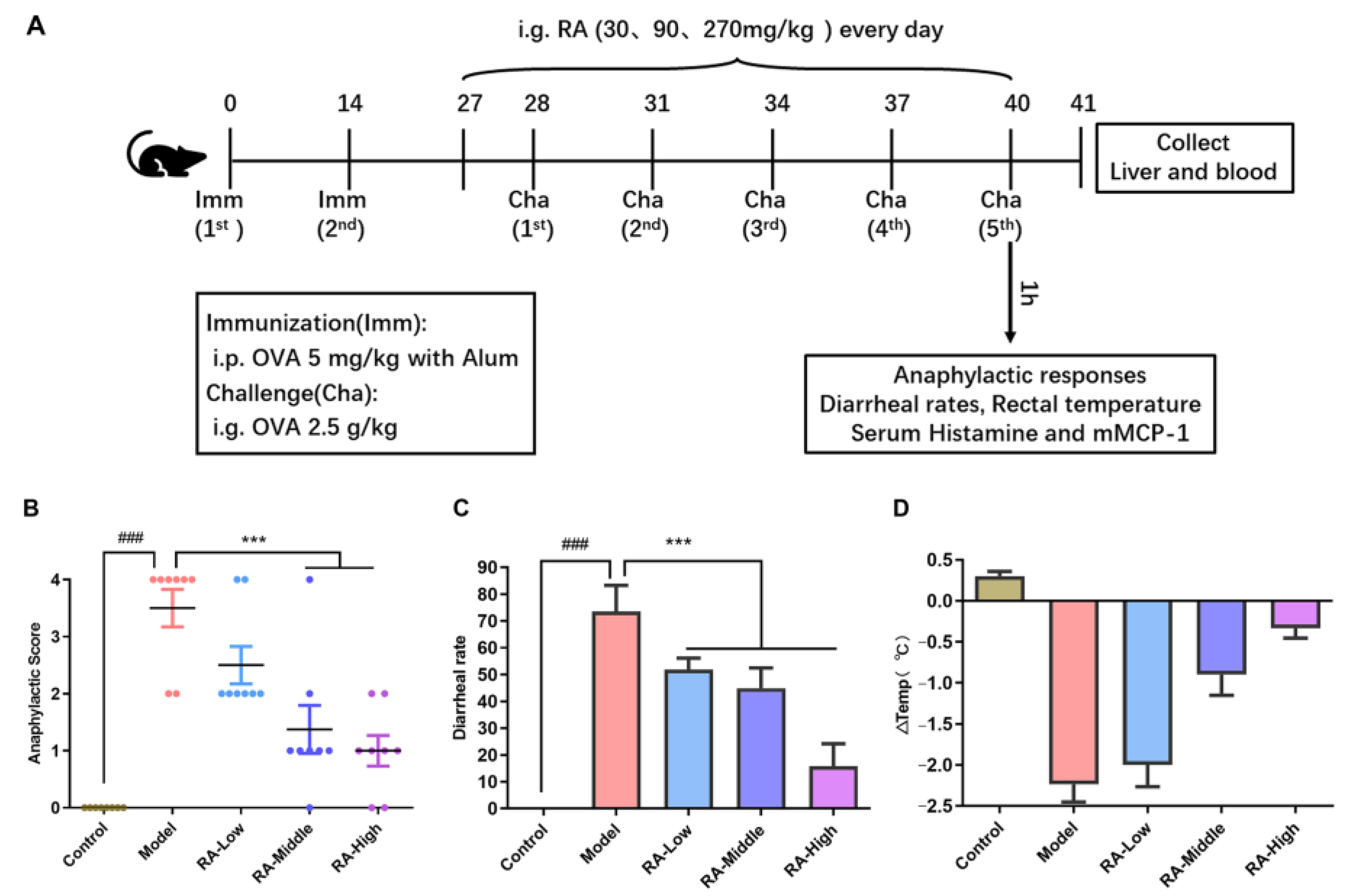
2.1. Attenuation of Anaphylaxis by Rosmarinic Acid on OVA-Induced Allergic Mice
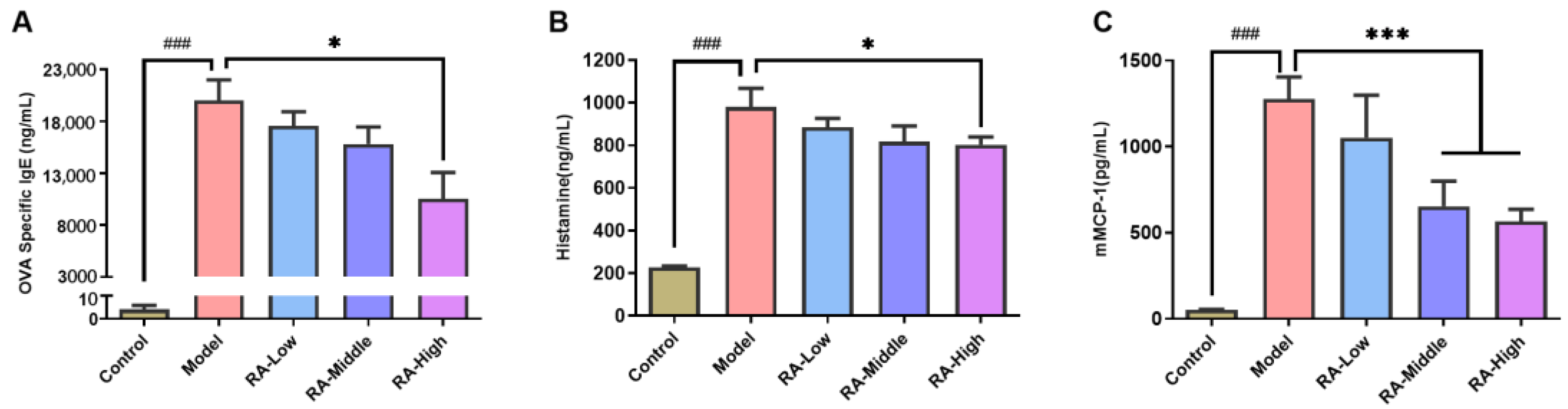
2.2. Regulating Effects of RA on Antibodies and Anaphylactic Mediators
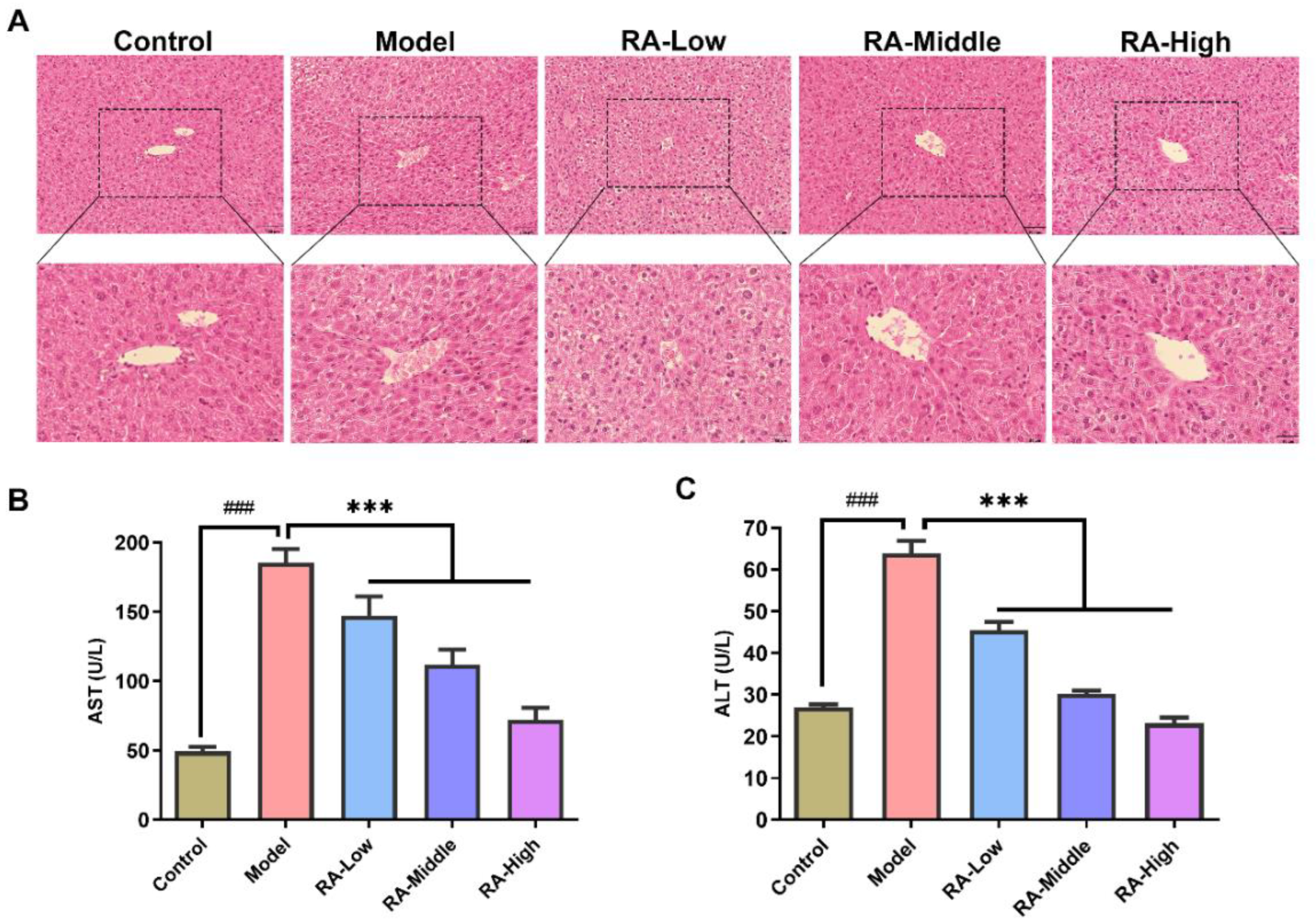
2.3. Effects of RA on Liver Tissue Morphology and Serum ALT and AST
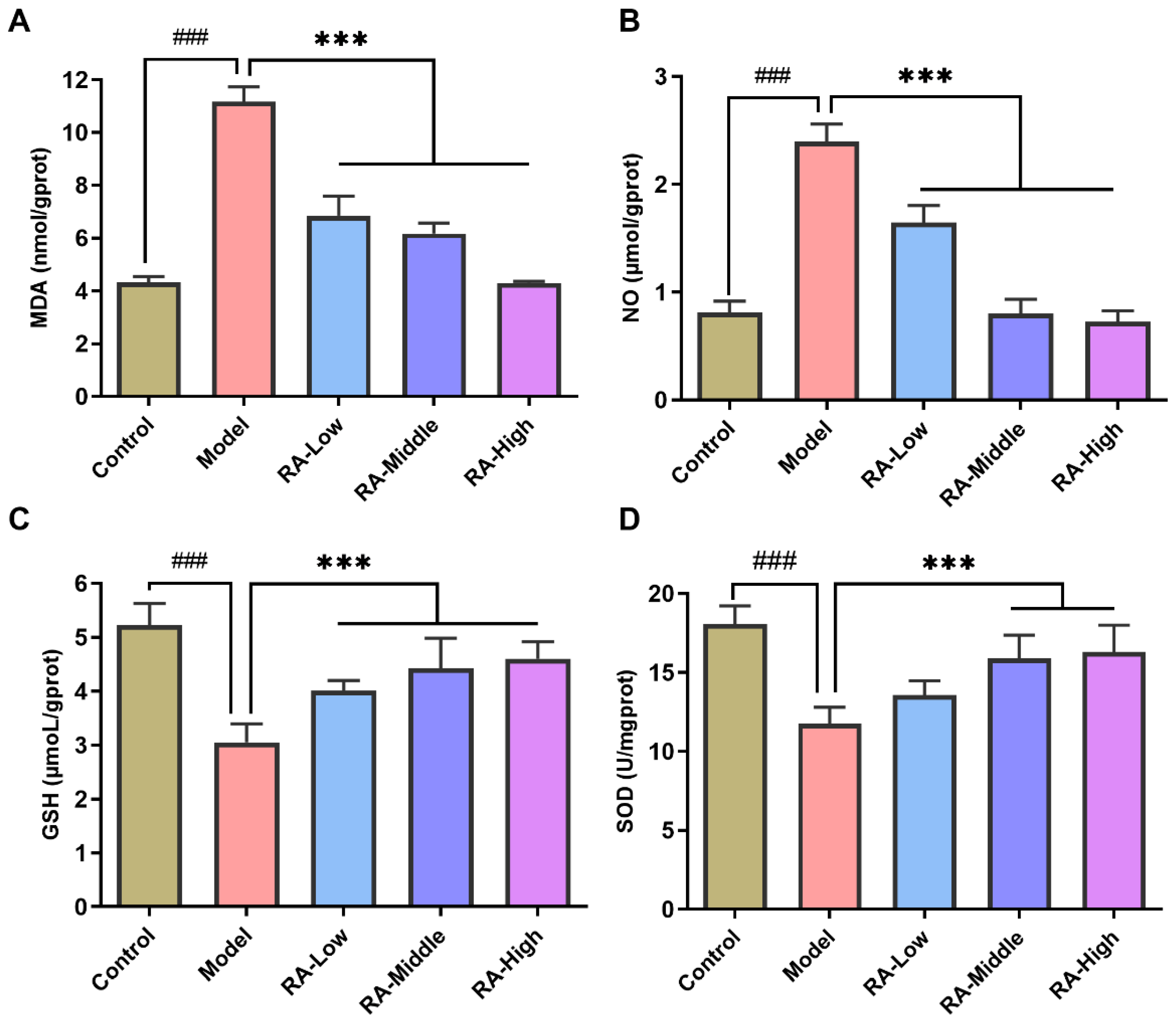
2.4. Effects of RA on OVA Challenge-Induced Liver Oxidative Stress
2.5. Effects of RA on Cytokine mRNA Expression in Liver
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animal
4.3. Animal Experimental Design
4.4. Measurements of Specific Antibodies and Allergic Mediators in Serum
4.5. Histopathologic Examination
4.6. Analysis of Serum and Liver Biochemical Parameters
4.7. RNA Preparation and Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| Gene | Primer Concentration (μM) | Tm |
|---|---|---|
| GAPDH | 0.2 | 56 |
| TNF-α | 0.4 | 60 |
| IL-6 | 0.2 | 58 |
| IL-4 | 0.2 | 60 |
| IL-1β | 0.4 | 56 |
| MCP-1 | 0.2 | 57 |
| TGF-β | 0.4 | 60 |
| iNOS | 0.2 | 60 |
| IL-10 | 0.2 | 60 |
| FOXP-3 | 0.2 | 60 |
References
- Fiocchi, A.; Risso, D.; DunnGalvin, A.; Díaz, S.N.G.; Monaci, L.; Fierro, V.; Ansotegui, I.J. Food labeling issues for severe food allergic patients. World Allergy Organ. J. 2021, 14, 100598. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food allergy across the globe. J. Allergy Clin. Immunol. 2021, 148, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Dona, D.W.; Suphioglu, C. Egg Allergy: Diagnosis and Immunotherapy. Int. J. Mol. Sci. 2020, 21, 5010. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chen, Y.; Chen, H.; Liu, C.; Zhou, W.; Wang, L.; Wu, Y. A Methodology of Epidemiologic Study in the General Population Focusing on Food Allergy—China, 2020. China CDC Wkly. 2022, 4, 749–755. [Google Scholar]
- Caubet, J.C.; Bencharitiwong, R.; Moshier, E.; Godbold, J.H.; Sampson, H.A.; Nowak-Węgrzyn, A. Significance of ovomucoid-and ovalbumin-specific IgE/IgG4 ratios in egg allergy. J. Allergy Clin. Immunol. 2012, 129, 739–747. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, Q.; Zhang, Y.; Yin, Q.; Zhu, X.; Zhang, P.; Wang, C.; Liu, J. Effects of fucoidans and alginates from Sargassum graminifolium on allergic symptoms and intestinal microbiota in mice with OVA-induced food allergy. Food Funct. 2022, 13, 6702–6715. [Google Scholar] [CrossRef]
- Saito, M.; Obi, M.; Kimura, M. Infantile hepatic dysfunction improved by elimination of cows’ milk formulas. Pediatr. Allergy Immunol. 2005, 16, 445–448. [Google Scholar] [CrossRef]
- Kaneko, R.; Ohishi, C.; Kim, M.; Shiina, M.; Kusayanagi, S.; Ogawa, M.; Munakata, K.; Mizuno, K.; Sato, Y. Two cases of food additive-induced severe liver damage associated with positive results on lymphocyte stimulation test and for antinuclear antibodies. Clin. J. Gastroenterol. 2012, 5, 268–274. [Google Scholar] [CrossRef]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Abbate, J.M.; Macrì, F.; Capparucci, F.; Iaria, C.; Briguglio, G.; Cicero, L.; Salvo, A.; Arfuso, F.; Ieni, A.; Piccione, G.; et al. Administration of Protein Hydrolysates from Anchovy (Engraulis Encrasicolus) Waste for Twelve Weeks Decreases Metabolic Dysfunction-Associated Fatty Liver Disease Severity in ApoE(-/-)Mice. Animals 2020, 10, 2303. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D and Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): An Update. Nutrients 2020, 12, 3302. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Liu, Q. Parthenolide plays a protective role in the liver of mice with metabolic dysfunction-associated fatty liver disease through the activation of the HIPPO pathway. Mol. Med. Rep. 2021, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid-From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharmacal Res. 2022, 45, 1–24. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Han, Y.; Yang, B.; Lin, H.; Li, Z. The natural substances with anti-allergic properties in food allergy. Trends Food Sci. Technol. 2022, 128, 53–67. [Google Scholar] [CrossRef]
- Yang, T.; Li, C.; Xue, W.; Huang, L.; Wang, Z. Natural immunomodulating substances used for alleviating food allergy. Crit. Rev. Food Sci. Nutr. 2021, 1–19. [Google Scholar] [CrossRef]
- do Nascimento, R.F.; de Oliveira Formiga, R.; Machado, F.D.F.; de Sales, I.R.P.; de Lima, G.M.; Alves Júnior, E.B.; Vieira, G.C.; Pereira, R.F.; de Araújo, A.A.; de Araújo Junior, R.F.; et al. Rosmarinic acid prevents gastric ulcers via sulfhydryl groups reinforcement, antioxidant and immunomodulatory effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2265–2278. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Nie, H.; Xu, Y.; Peng, J.; Zeng, Y.; Wei, Y.; Wen, X.; Qiu, J.; Zhong, W.; Deng, X.; et al. Therapeutic effects of rosmarinic acid on airway responses in a murine model of asthma. Int. Immunopharmacol. 2016, 41, 90–97. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.; Han, Z.; Wang, J.; Sun, N.; Zhang, R.; Dong, W.; Deng, C.; Zhuang, G. Effects of rosmarinic acid on the inflammatory response in allergic rhinitis rat models after PM2.5 exposure. J. Clin. Lab. Anal. 2022, 36, e24316. [Google Scholar] [CrossRef]
- Zhang, M.; Li, N.; Cai, R.; Gu, J.; Xie, F.; Wei, H.; Lu, C.; Wu, D. Rosmarinic acid protects mice from imiquimod induced psoriasis-like sk in lesions by inhibiting the IL-23/Th17 axis via regulating Jak2/Stat3 signaling pathway. Phytother. Res. 2021, 35, 4526–4537. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Chirumbolo, S. Dietary Assumption of Plant Polyphenols and Prevention of Allergy. Curr. Pharm. Des. 2014, 20, 811–839. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, Z. Liver-Mediated Adaptive Immune Tolerance. Front. Immunol. 2019, 10, 2525. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, Q.D.J.S.; Bachur, T.P.R.; Aragão, G.F. Whey protein supplementation and its potentially adverse effects on health: A systematic review. Appl. Physiol. Nutr. Metab. 2021, 46, 27–33. [Google Scholar] [CrossRef]
- Watanabe, T.; Katsukura, H.; Shirai, Y.; Yamori, M.; Chiba, T.; Kita, T.; Wakatsuki, Y. Helper CD4+ T cells for IgE response to a dietary antigen develop in the liver. J. Allergy Clin. Immunol. 2003, 111, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.M. Alanine aminotransferase levels: What’s normal? Ann. Intern. Med. 2002, 137, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Ztopuz, Ö.; Coşkun, Ö.; Büyük, B. The effect of Oleuropein on liver in experimental allergic rhinitis model. Ann. Clin. Anal. Med. 2020, 11, 61–66. [Google Scholar]
- Pourmehdi, A.; Sakhaei, Z.; Alirezaei, M.; Dezfoulian, O. Betaine effects against asthma-induced oxidative stress in the liver and kidney of mice. Mol. Biol. Rep. 2020, 47, 5729–5735. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.; Pohin, M.; Jégou, J.; Favot, L.; Venisse, N.; Mcheik, J.; Morel, F.; Lecron, J.; Silvain, C. Liver fibrosis is associated with cutaneous inflammation in the imiquimod-induced murine model of psoriasiform dermatitis. Br. J. Dermatol. 2018, 179, 101–109. [Google Scholar] [CrossRef]
- Alhusayan, R.M.; Aldahmash, B.A.; El-Nagar, D.M.; Rady, A.; Ibrahim, K.E.; Alkahtani, S. Butterbur (Petasites hybridus) Extract Ameliorates Hepatic Damage Induced by Ovalbumin in Mice. Oxidative Med. Cell. Longev. 2020, 2020, 3178214. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; El-Twab, A.; Sanaa, M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. 2020, 27, 7910–7921. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef]
- Kasdallah-Grissa, A.; Mornagui, B.; Aouani, E.; Hammami, M.; El May, M.; Gharbi, N.; Kamoun, A.; El-Fazaâ, S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007, 80, 1033–1039. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Mocci, G.; Marzo, M.; D’Aversa, F.; Rapaccini, G.L.; Guidi, L.; Armuzzi, A.; Gasbarrini, A.; Papa, A. Harmful Effects and Potential Benefits of Anti-Tumor Necrosis Factor (TNF)-α on the Liver. Int. J. Mol. Sci. 2018, 19, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muntane, J.; Rodriguez, F.; Segado, O.; Quintero, A.; Lozano, J.; Siendones, E.; Pedraza, C.; Delgado, M.; O’Valle, F.; Garcia, R. TNF-α dependent production of inducible nitric oxide is involved in PGE1 protection against acute liver injury. Gut 2000, 47, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njoku, D.B.; Li, Z.; Washington, N.D.; Mellerson, J.L.; Talor, M.V.; Sharma, R.; Rose, N.R. Suppressive and pro-inflammatory roles for IL-4 in the pathogenesis of experimental drug-induced liver injury. Eur. J. Immunol. 2010, 39, 1652–1663. [Google Scholar] [CrossRef] [Green Version]
- Klimenko, O.V. Regulation of immune responses, apoptosis, and tumorigenesis by separate FOXP-3-dependent genes: Connection with clinical manifestations. J. Microbiol. Immunol. Infect. 2011, 44, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Jia, B.; Liu, X.; Fang, J.; Zhao, L.; Xu, L.; Fang, M.; Gong, Z.; Sun, H. Molecular Cloning, Expression and Macrophage Activation of an Immunoregulatory Protein from Cordyceps militaris. Molecules 2021, 26, 7107. [Google Scholar] [CrossRef]

| Scores | Allergy Symptoms |
|---|---|
| 0 | Without any symptoms |
| 1 | Scratching and rubbing around the mouth and nose |
| 2 | Swelling around the mouth and eyes; diarrhea; reduced activity; increased respiratory |
| 3 | Wheezing; labored respiration; cyanosis around the mouth and the tail |
| 4 | No activity after stimulation; shivering; muscle contractions |
| 5 | Death by shock |
| Genes | Forward Primer | Reverse Primer | Product Size (bp) |
|---|---|---|---|
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA | 123 |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG | 61 |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC | 76 |
| IL-4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT | 102 |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT | 89 |
| MCP-1 | GAGGACAGATGTGGTGGGTTT | AGGAGTCAACTCAGCTTTCTCTT | 231 |
| TGF-β | CTCCCGTGGCTTCTAGTGC | GCCTTAGTTTGGACAGGATCTG | 133 |
| iNOS | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC | 127 |
| IL-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG | 105 |
| FOXP-3 | CCCATCCCCAGGAGTCTTG | ACCATGACTAGGGGCACTGTA | 183 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, B.; Shang, J.; Zeng, H.; Wang, X.; Fang, M.; Xu, L.; Liu, X.; Wu, K.; Gong, Z.; Yang, Q. Hepatoprotective Effects of Rosmarinic Acid on Ovalbumin-Induced Intestinal Food Allergy Mouse Model. Molecules 2023, 28, 788. https://doi.org/10.3390/molecules28020788
Jia B, Shang J, Zeng H, Wang X, Fang M, Xu L, Liu X, Wu K, Gong Z, Yang Q. Hepatoprotective Effects of Rosmarinic Acid on Ovalbumin-Induced Intestinal Food Allergy Mouse Model. Molecules. 2023; 28(2):788. https://doi.org/10.3390/molecules28020788
Chicago/Turabian StyleJia, Binmei, Jieli Shang, Haolong Zeng, Xuanpei Wang, Min Fang, Lin Xu, Xin Liu, Kejia Wu, Zhiyong Gong, and Qing Yang. 2023. "Hepatoprotective Effects of Rosmarinic Acid on Ovalbumin-Induced Intestinal Food Allergy Mouse Model" Molecules 28, no. 2: 788. https://doi.org/10.3390/molecules28020788
APA StyleJia, B., Shang, J., Zeng, H., Wang, X., Fang, M., Xu, L., Liu, X., Wu, K., Gong, Z., & Yang, Q. (2023). Hepatoprotective Effects of Rosmarinic Acid on Ovalbumin-Induced Intestinal Food Allergy Mouse Model. Molecules, 28(2), 788. https://doi.org/10.3390/molecules28020788






