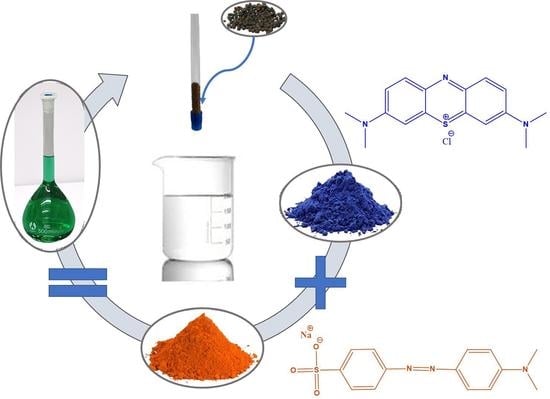
Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media
Abstract
:1. Introduction
2. Results
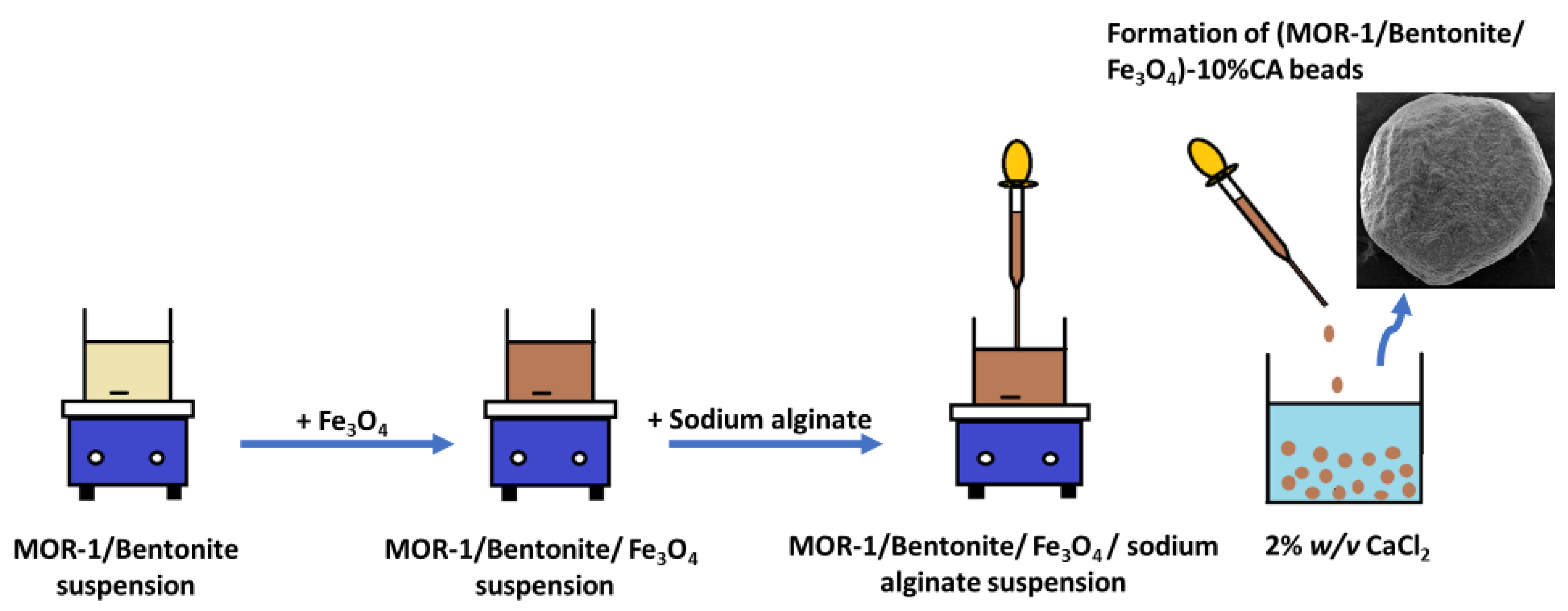
2.1. Synthesis of the Composite Materials
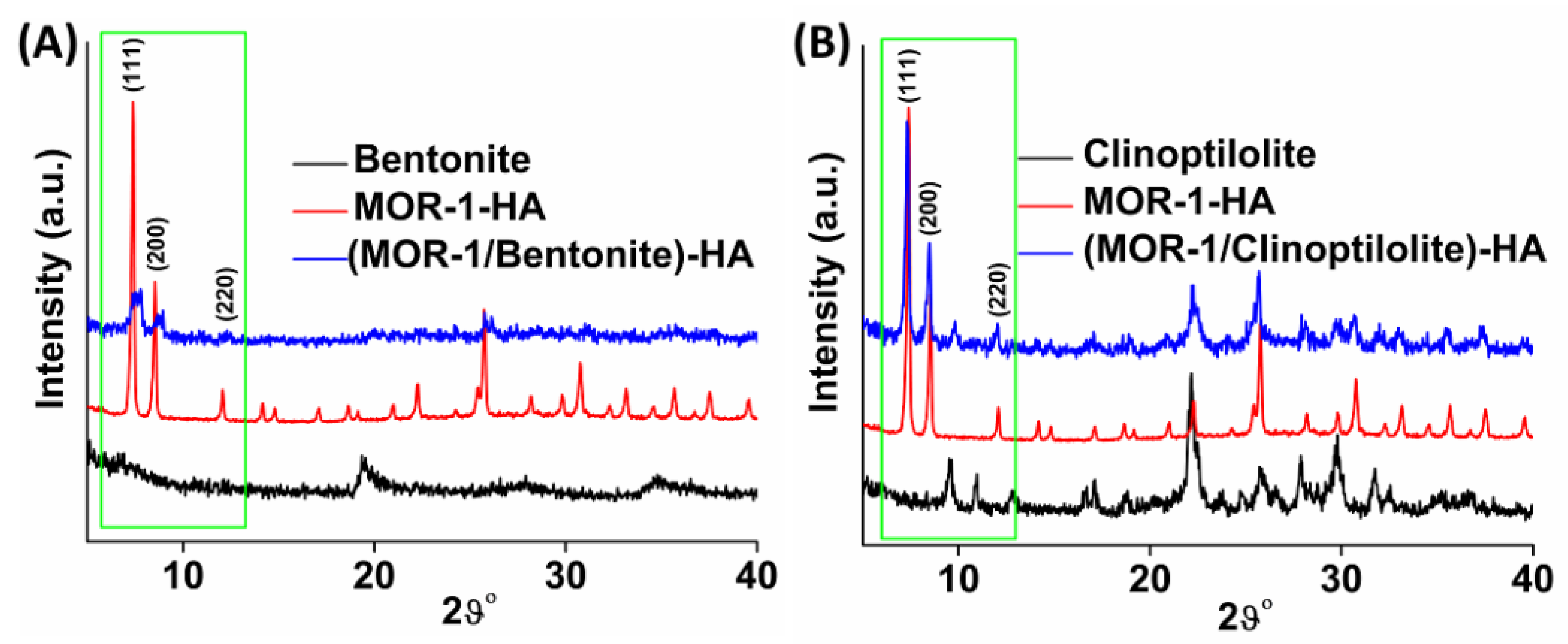
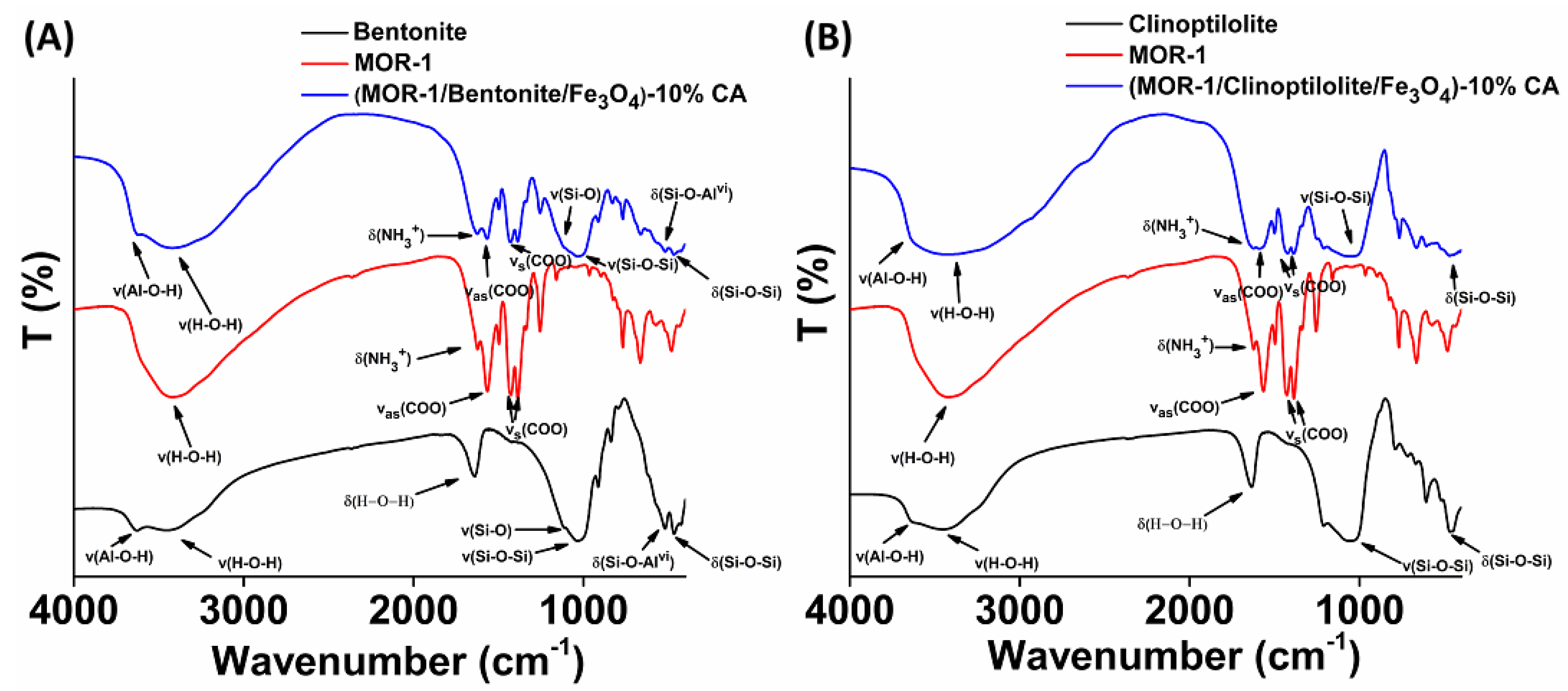
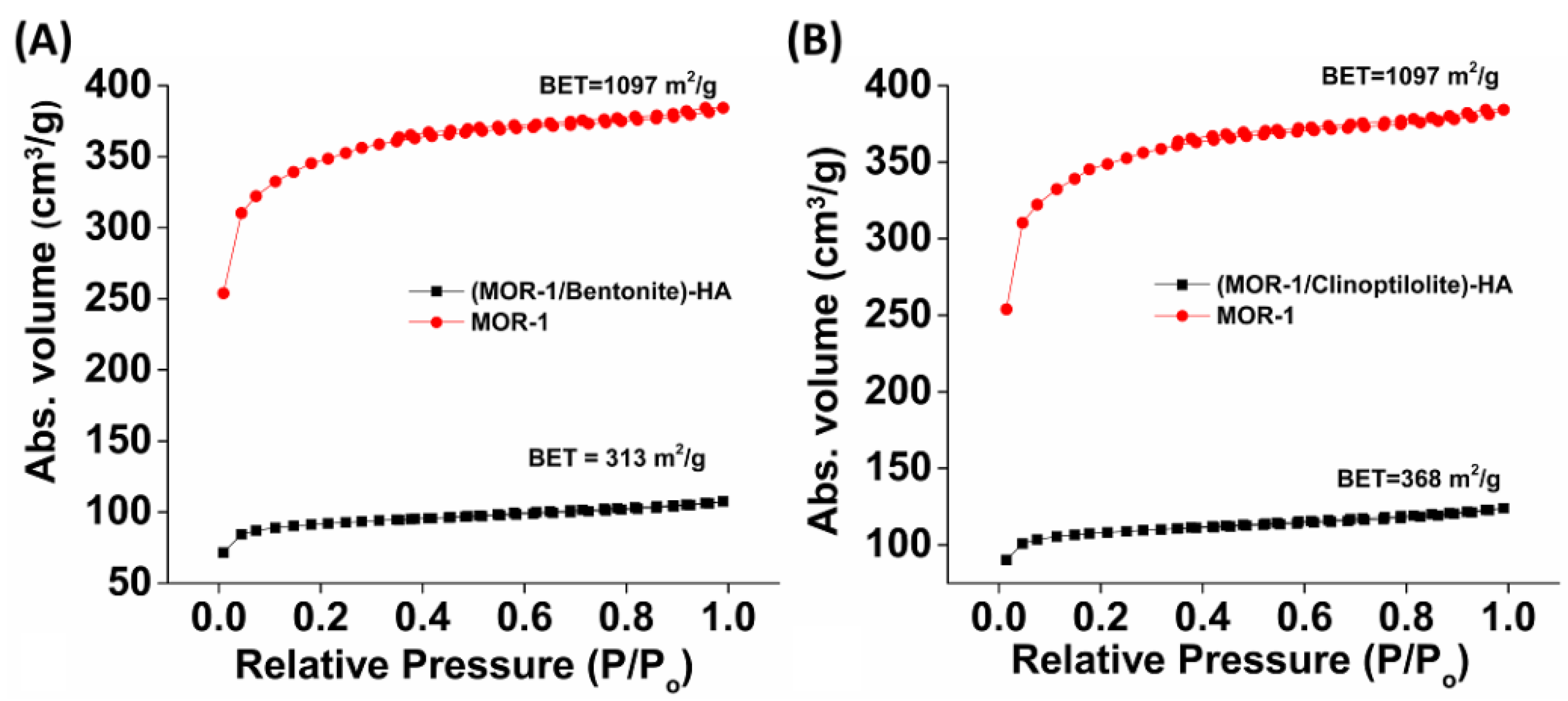
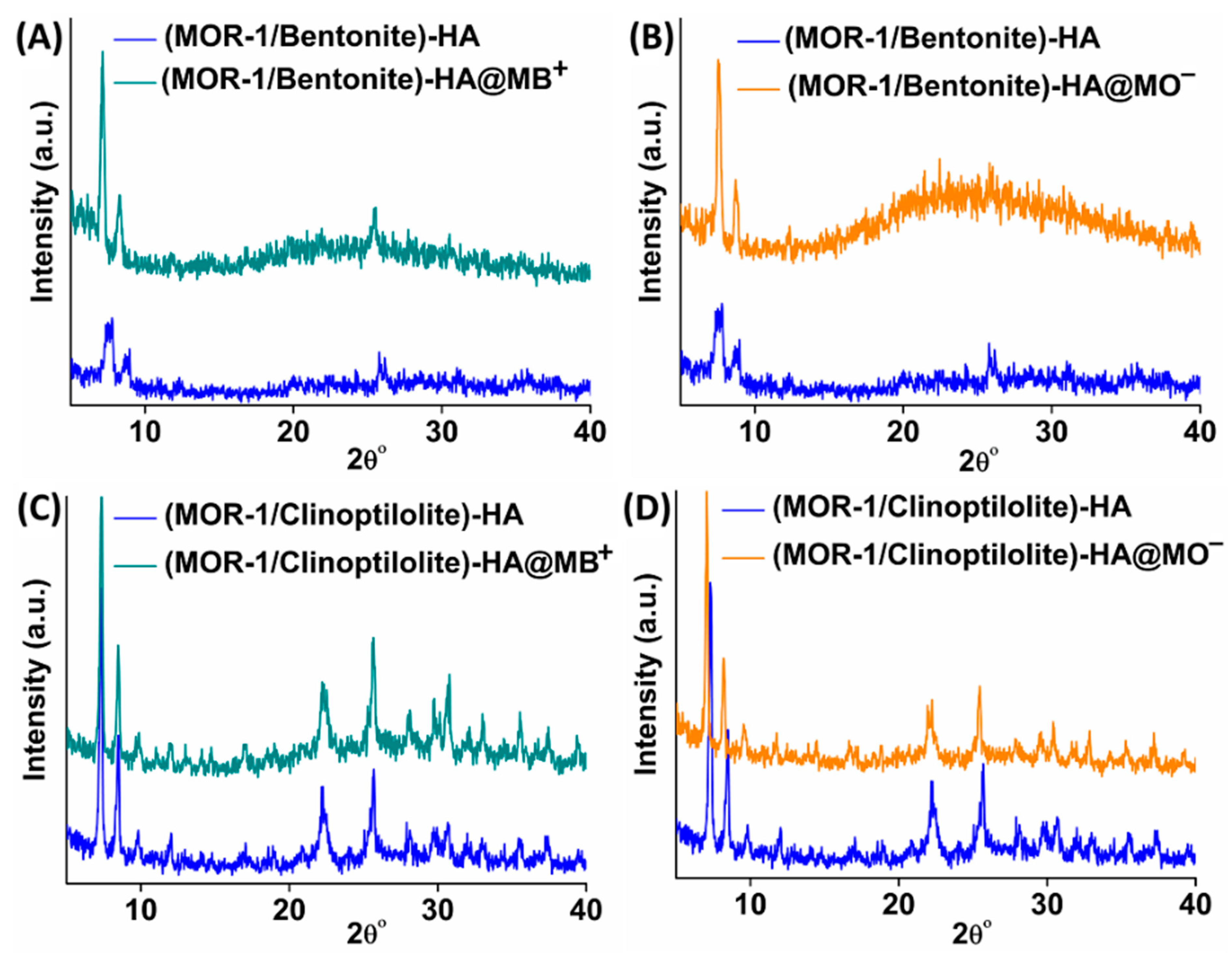
2.2. Characterization of the Composite Materials
2.3. Batch Sorption Studies
2.3.1. Sorption Kinetics
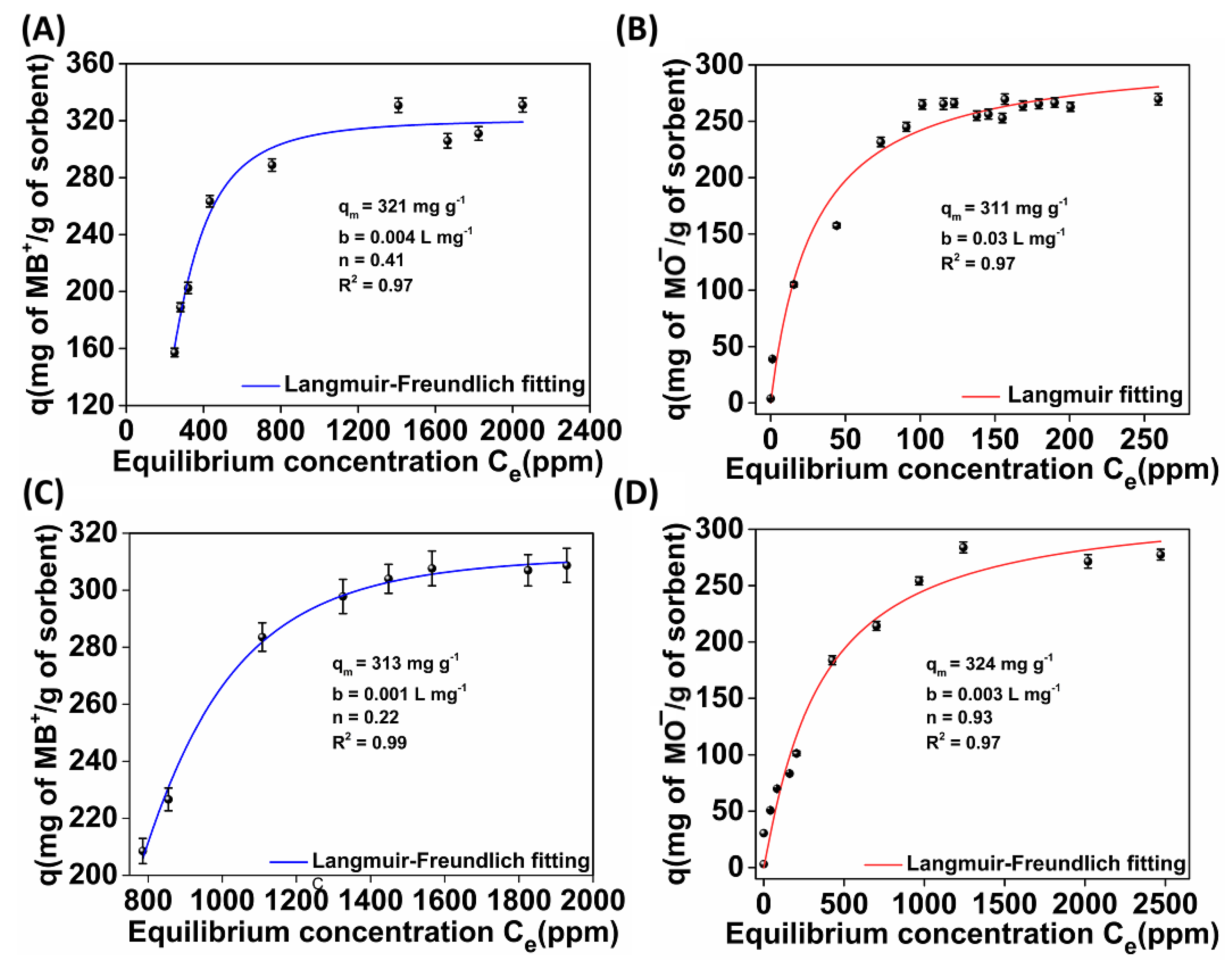
2.3.2. Sorption Isotherms
- (a)
- Langmuir
- (b)
- Freundlich
- (c)
- Langmuir–Freundlich
2.3.3. Variable pH Studies
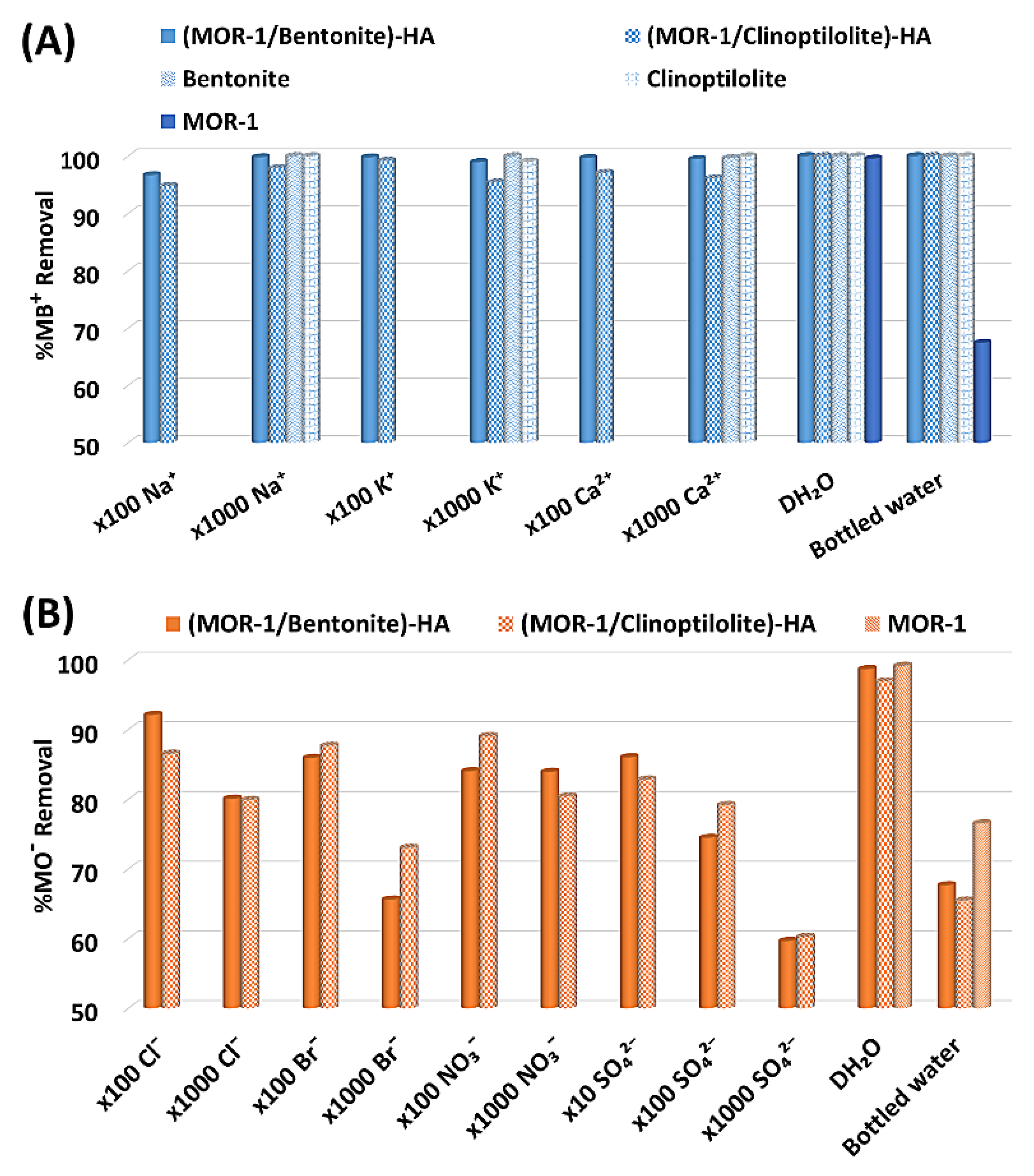
2.3.4. Selectivity Studies
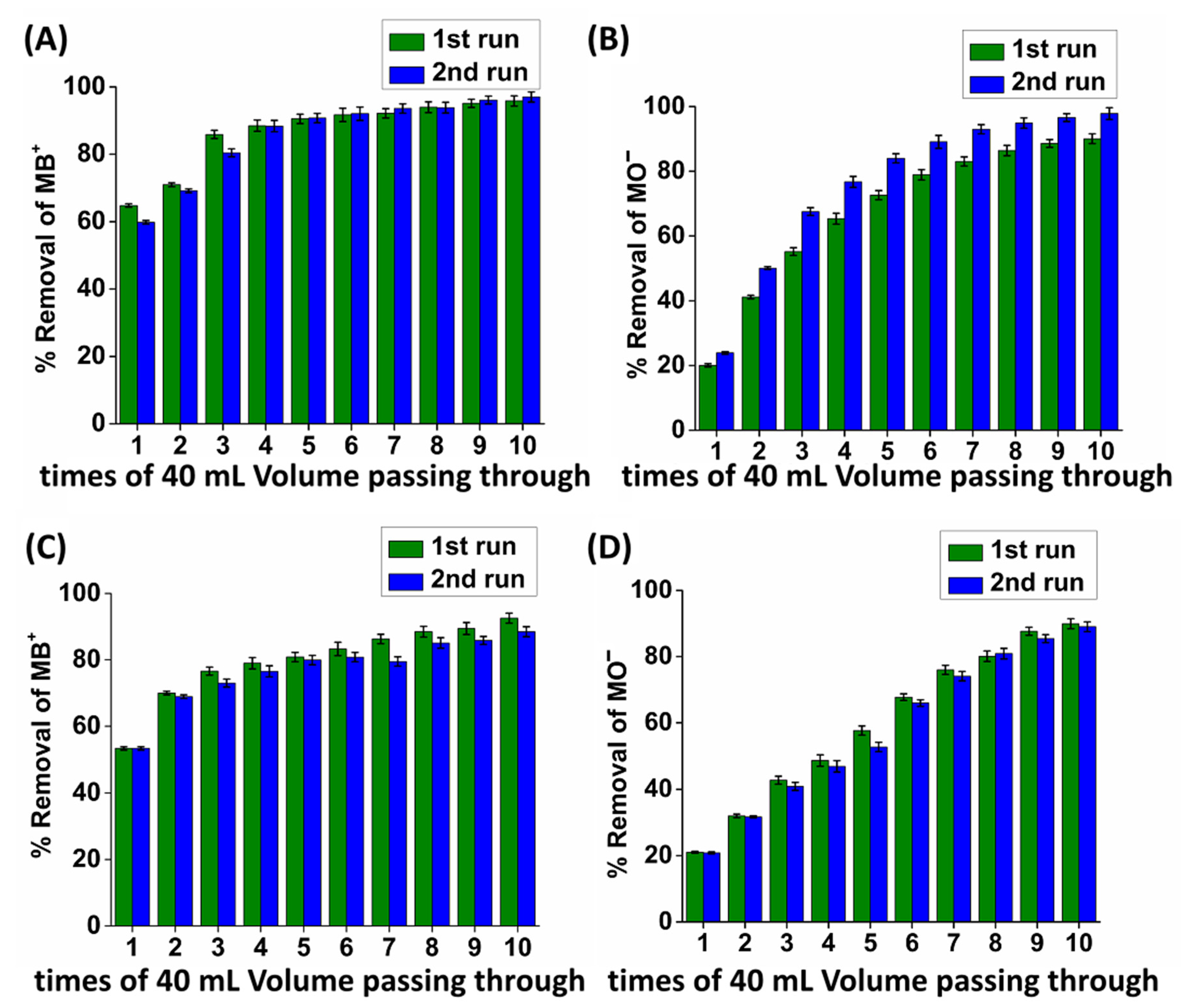
2.4. Column Sorption Study
2.5. Isolation and Characterization of the Composite Materials and Dye-Loaded Composite Materials
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, L.; Shi, J.; Long, C.; Li, A. Advanced treatment of textile dyeing secondary effluent using magnetic anion exchange resin and its effect on organic fouling in subsequent RO membrane. J. Hazard. Mater. 2015, 284, 50–57. [Google Scholar] [CrossRef]
- Rauf, M.A.; Salman Ashraf, S. Survey of recent trends in biochemically assisted degradation of dyes. Chem. Eng. J. 2012, 209, 520–530. [Google Scholar] [CrossRef]
- Golka, K.; Kopps, S.; Myslak, Z.W. Carcinogenicity of azo colorants: Influence of solubility and bioavailability. Toxicol. Lett. 2004, 151, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Lo, V.; Minett, A.I.; Harris, A.T.; Church, T.L. Dichotomous adsorption behaviour of dyes on an amino-functionalised metal-organic framework, amino-MIL-101(Al). J. Mater. Chem. A 2014, 2, 193–203. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manage. 2012, 102, 148–164. [Google Scholar] [CrossRef]
- Sarro, M.; Gule, N.P.; Laurenti, E.; Gamberini, R.; Paganini, M.C.; Mallon, P.E.; Calza, P. ZnO-based materials and enzymes hybrid systems as highly efficient catalysts for recalcitrant pollutants abatement. Chem. Eng. J. 2018, 334, 2530–2538. [Google Scholar] [CrossRef]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Pournara, A.D.; Rapti, S.; Skliri, E.; Armatas, G.S.; Tsipis, A.C.; Manos, M.J. Highly Efficient Sorption of Methyl Orange by a Metal–Organic Resin–Alginic Acid Composite. Chempluschem 2017, 82, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pournara, A.; Kim, K.H.; Bansal, V.; Rapti, S.; Manos, M.J. Metal-organic frameworks: Challenges and opportunities for ion-exchange/sorption applications. Prog. Mater. Sci. 2017, 86, 25–74. [Google Scholar] [CrossRef]
- Kang, K.; Liu, S.; Zhang, M.; Li, L.; Liu, C.; Lei, L.; Dai, X.; Xu, C.; Xiao, C. Fast Room-Temperature Synthesis of an Extremely Alkaline-Resistant Cationic Metal–Organic Framework for Sequestering TcO4− with Exceptional Selectivity. Adv. Funct. Mater. 2022, 32, 2208148. [Google Scholar] [CrossRef]
- Kang, K.; Shen, N.; Wang, Y.; Li, L.; Zhang, M.; Zhang, X.; Lei, L.; Miao, X.; Wang, S.; Xiao, C. Efficient sequestration of radioactive 99TcO4- by a rare 3-fold interlocking cationic metal-organic framework: A combined batch experiments, pair distribution function, and crystallographic investigation. Chem. Eng. J. 2022, 427, 130942. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.I.H.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Zagorodni, A.A. Ion Exchange Materials: Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2007; p. 280. [Google Scholar]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Tsipis, A.C.; Lazarides, T.; Kanatzidis, M.G.; Manos, M.J. Selective capture of hexavalent chromium from an anion-exchange column of metal organic resin-alginic acid composite. Chem. Sci. 2016, 7, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Hassan, Y.S.; Alkordi, M.H.; Kanatzidis, M.G.; Manos, M.J. Rapid, green and inexpensive synthesis of high quality UiO-66 amino-functionalized materials with exceptional capability for removal of hexavalent chromium from industrial waste. Inorg. Chem. Front. 2016, 3, 635–644. [Google Scholar] [CrossRef]
- Rapti, S.; Diamantis, S.A.; Dafnomili, A.; Pournara, A.; Skliri, E.; Armatas, G.S.; Tsipis, A.C.; Spanopoulos, I.; Malliakas, C.D.; Kanatzidis, M.G.; et al. Exceptional TcO4− sorption capacity and highly efficient ReO4− luminescence sensing by Zr4+ MOFs. J. Mater. Chem. A 2018, 6, 20813–20821. [Google Scholar] [CrossRef]
- Pournara, A.D.; Rapti, S.; Valmas, A.; Margiolaki, I.; Andreou, E.; Armatas, G.S.; Tsipis, A.C.; Plakatouras, J.C.; Giokas, D.L.; Manos, M.J. Alkylamino-terephthalate ligands stabilize 8-connected Zr4+ MOFs with highly efficient sorption for toxic Se species. J. Mater. Chem. A 2021, 9, 3379–3387. [Google Scholar] [CrossRef]
- Pournara, A.D.; Evangelou, D.A.; Roukounaki, C.; Andreou, E.K.; Armatas, G.S.; Lazarides, T.; Manos, M.J. Highly efficient sorption and luminescence sensing of oxoanionic species by 8-connected alkyl-amino functionalized Zr4+ MOFs. Dalton Trans. 2022, 51, 17301–17309. [Google Scholar] [CrossRef] [PubMed]
- Pournara, A.D.; Rizogianni, S.; Evangelou, D.A.; Andreou, E.K.; Armatas, G.S.; Manos, M.J. Zr4+ -terephthalate MOFs with 6-connected structures, highly efficient As (III/V) sorption and superhydrophobic properties. Chem. Commun. 2022, 58, 8862–8865. [Google Scholar] [CrossRef]
- Diamantis, S.A.; Pournara, A.D.; Koutsouroubi, E.D.; Moularas, C.; Deligiannakis, Y.; Armatas, G.S.; Hatzidimitriou, A.G.; Manos, M.J.; Lazarides, T. Detection and Sorption of Heavy Metal Ions in Aqueous Media by a Fluorescent Zr(IV) Metal–Organic Framework Functionalized with 2-Picolylamine Receptor Groups. Inorg. Chem. 2022, 61, 7847–7858. [Google Scholar] [CrossRef]
- Kubilay, Ş.; Gürkan, R.; Savran, A.; Şahan, T. Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption 2007, 13, 41–51. [Google Scholar] [CrossRef]
- Tahir, S.S.; Rauf, N. Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 2006, 63, 1842–1848. [Google Scholar] [CrossRef]
- Qiu, M.; Qian, C.; Xu, J.; Wu, J.; Wang, G. Studies on the adsorption of dyes into clinoptilolite. Desalination 2009, 243, 286–292. [Google Scholar] [CrossRef]
- Smičiklas, I.; Dimović, S.; Plećaš, I. Removal of Cs1+, Sr2+ and Co2+ from aqueous solutions by adsorption on natural clinoptilolite. Appl. Clay Sci. 2007, 35, 139–144. [Google Scholar] [CrossRef]
- Cadar, O.; Senila, M.; Hoaghia, M.A.; Scurtu, D.; Miu, I.; Levei, E.A. Effects of thermal treatment on natural clinoptilolite-rich zeolite behavior in simulated biological fluids. Molecules 2020, 25, 2570. [Google Scholar] [CrossRef]
- Eren, E.; Afsin, B. An investigation of Cu(II) adsorption by raw and acid-activated bentonite: A combined potentiometric, thermodynamic, XRD, IR, DTA study. J. Hazard. Mater. 2008, 151, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.R.; Prelot, B. Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Manos, M.J.; Kanatzidis, M.G. Sequestration of heavy metals from water with layered metal sulfides. Chem.—A Eur. J. 2009, 15, 4779–4784. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Samarghandi, M.R.; McKay, G. Simplified fixed bed design models for the adsorption of acid dyes on novel pine cone derived activated carbon. Water Air Soil Pollut. 2011, 218, 197–212. [Google Scholar] [CrossRef]
- Karickhoff, S.W.; Bailey, G.W. Optical absorption spectra of clay minerals. Clays Clay Miner. 1973, 21, 51–57. [Google Scholar] [CrossRef]
- Garbowski, E.D.; Mirodatos, C. Investigation of structural charge transfer in zeolites by ultraviolet spectroscopy. J. Phys. Chem. 1982, 86, 97–102. [Google Scholar] [CrossRef]
- Garcia-Basabe, Y.; Rodriguez-Iznaga, I.; De Menorval, L.C.; Llewellyn, P.; Maurin, G.; Lewis, D.W.; Binions, R.; Autie, M.; Ruiz-Salvador, A.R. Step-wise dealumination of natural clinoptilolite: Structural and physicochemical characterization. Microporous Mesoporous Mater. 2010, 135, 187–196. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Razavi, A. Identification and estimation of extra-framework aluminium in acidic mazzite by diffuse reflectance spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 119–127. [Google Scholar] [CrossRef]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and reactivity of framework and extraframework iron in Fe-silicalite as investigated by spectroscopic and physicochemical methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Kumar, M.S.; Brückner, A. Reduction of N2O with CO over FeMFI zeolites: Influence of the preparation method on the iron species and catalytic behavior. J. Catal. 2004, 223, 13–27. [Google Scholar] [CrossRef]
- Benhammou, A.; Yaacoubi, A.; Nibou, L.; Tanouti, B. Adsorption of metal ions onto Moroccan stevensite: Kinetic and isotherm studies. J. Colloid Interface Sci. 2005, 282, 320–326. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgianos, P.; Pournara, A.D.; Andreou, E.K.; Armatas, G.S.; Manos, M.J. Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media. Molecules 2023, 28, 815. https://doi.org/10.3390/molecules28020815
Georgianos P, Pournara AD, Andreou EK, Armatas GS, Manos MJ. Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media. Molecules. 2023; 28(2):815. https://doi.org/10.3390/molecules28020815
Chicago/Turabian StyleGeorgianos, Petros, Anastasia D. Pournara, Evangelos K. Andreou, Gerasimos S. Armatas, and Manolis J. Manos. 2023. "Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media" Molecules 28, no. 2: 815. https://doi.org/10.3390/molecules28020815
APA StyleGeorgianos, P., Pournara, A. D., Andreou, E. K., Armatas, G. S., & Manos, M. J. (2023). Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media. Molecules, 28(2), 815. https://doi.org/10.3390/molecules28020815