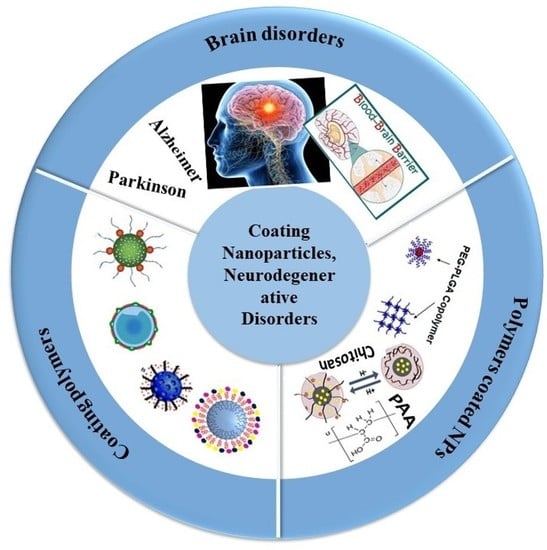
Management of Brain Cancer and Neurodegenerative Disorders with Polymer-Based Nanoparticles as a Biocompatible Platform
Abstract
:1. Introduction
2. Brain Disorders
2.1. Brain Cancer
2.2. Neurodegenerative Diseases
2.2.1. Alzheimer’s Disease (AD)
2.2.2. Parkinson’s Disease (PD)
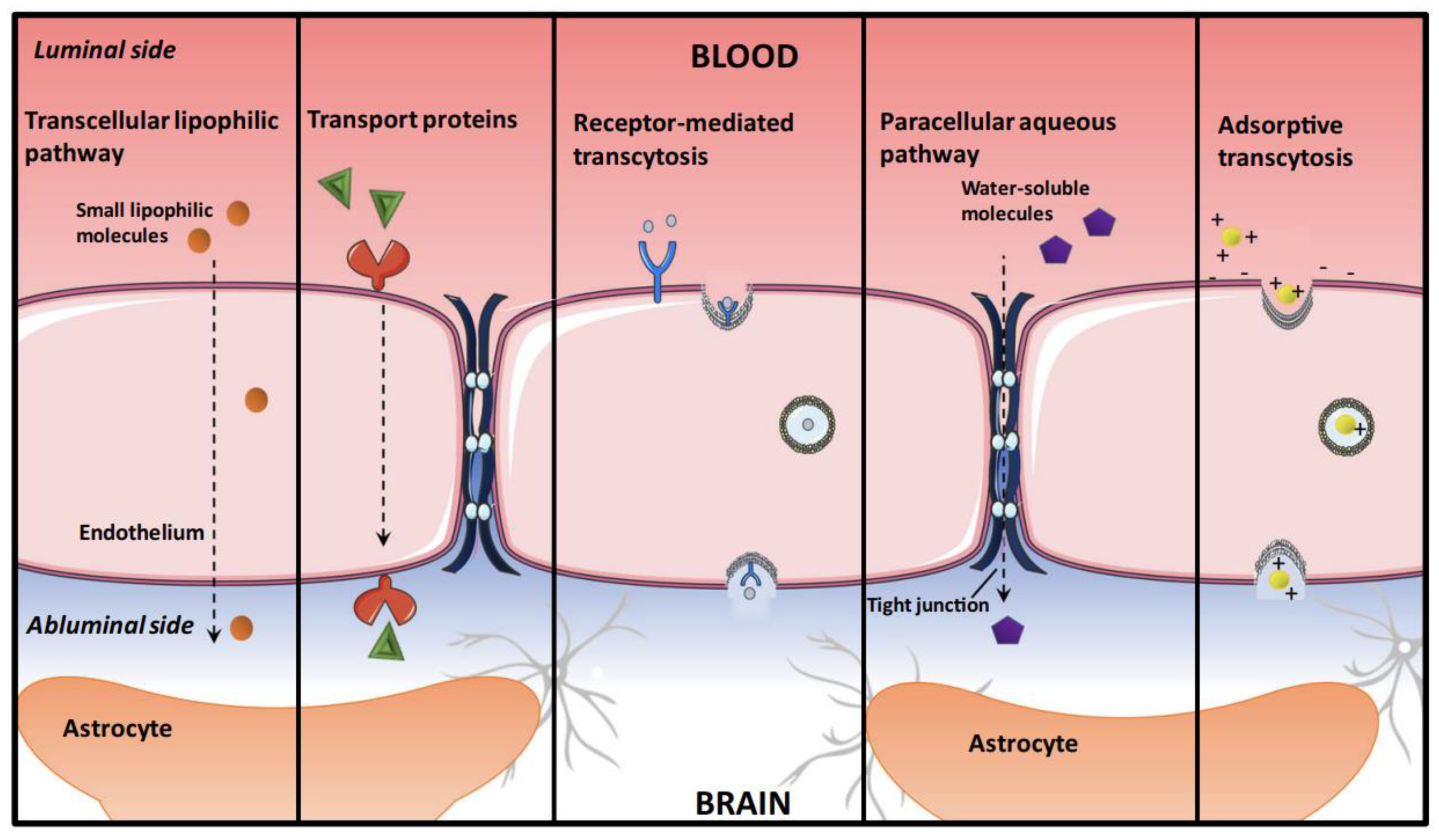
3. The Challenge of the BBB
4. Coating Polymers
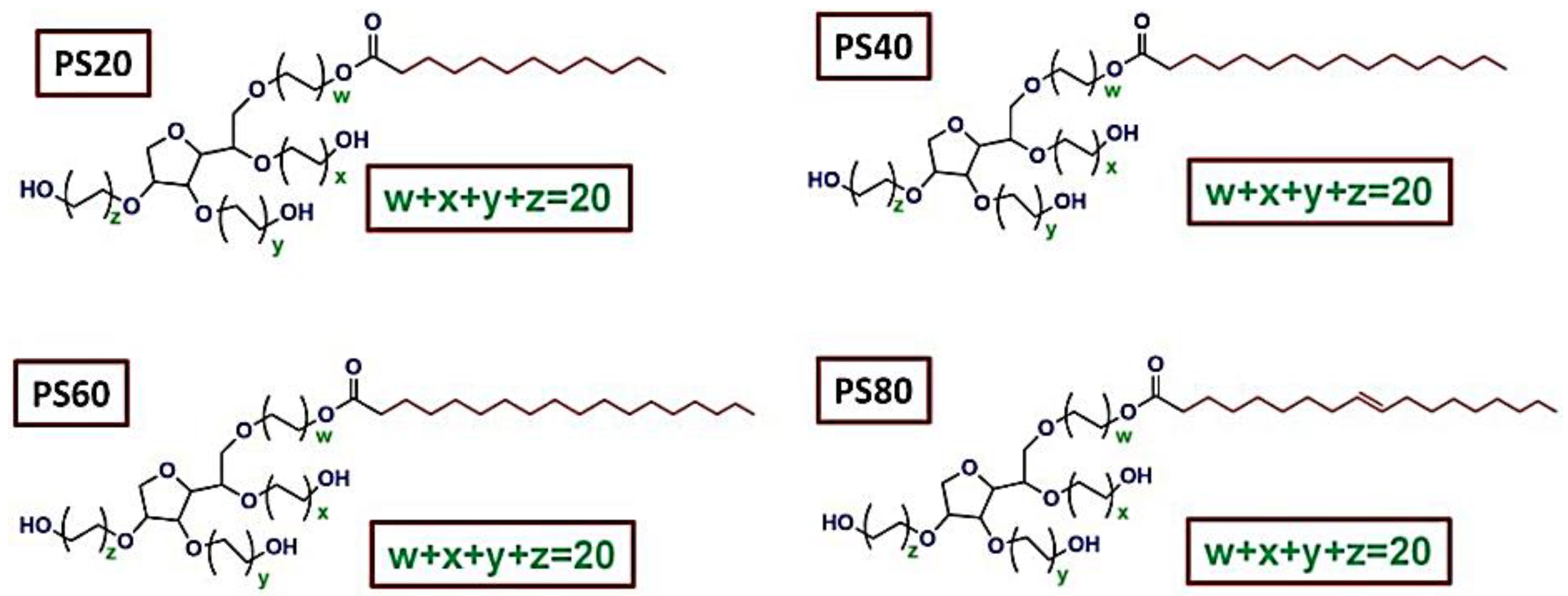
4.1. Polysorbate (PS)
4.2. Polyethylene Glycol (PEG)
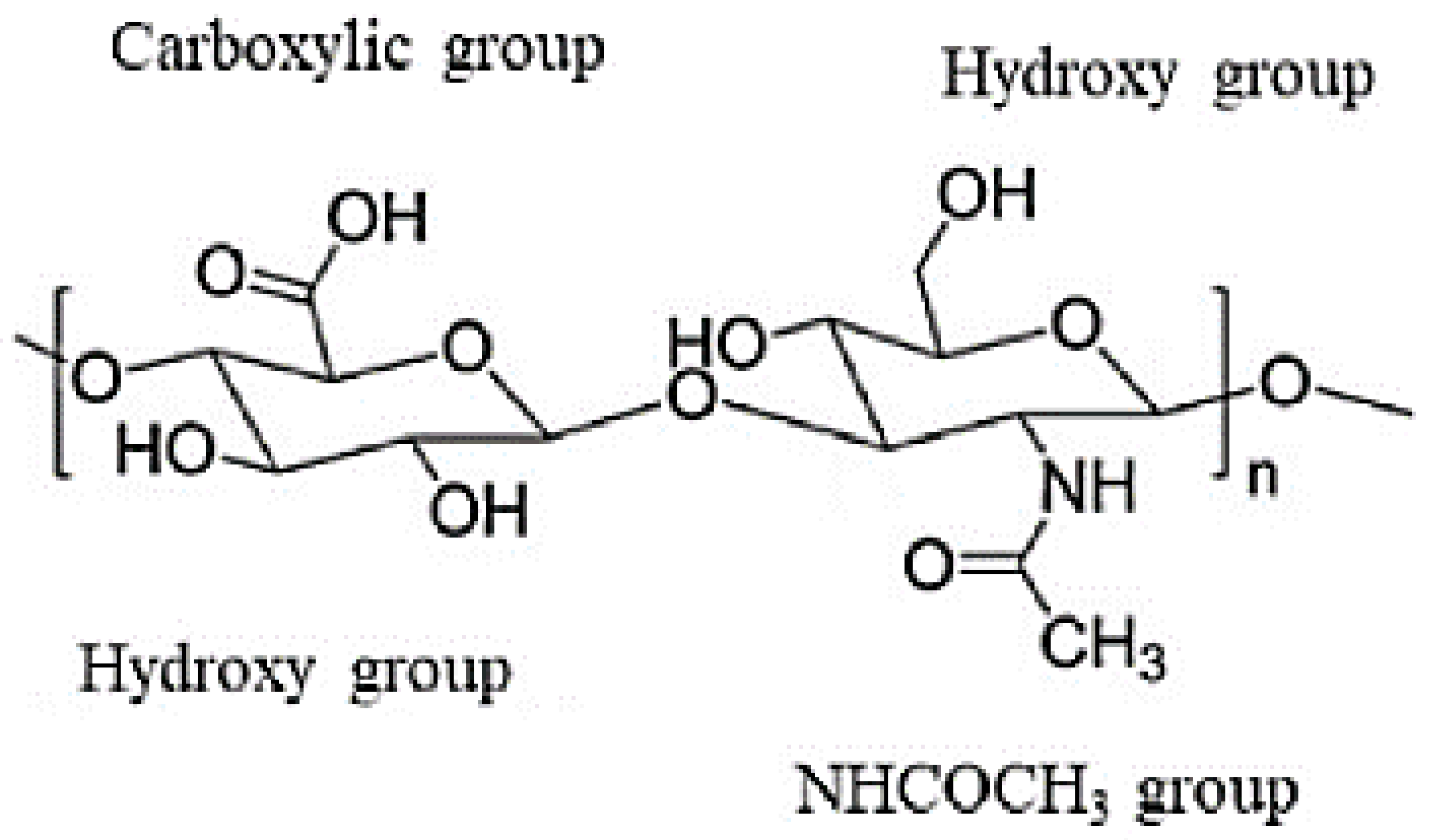
4.3. Chitosan
4.4. Poly-Ɛ-Caprolactone (PCL)
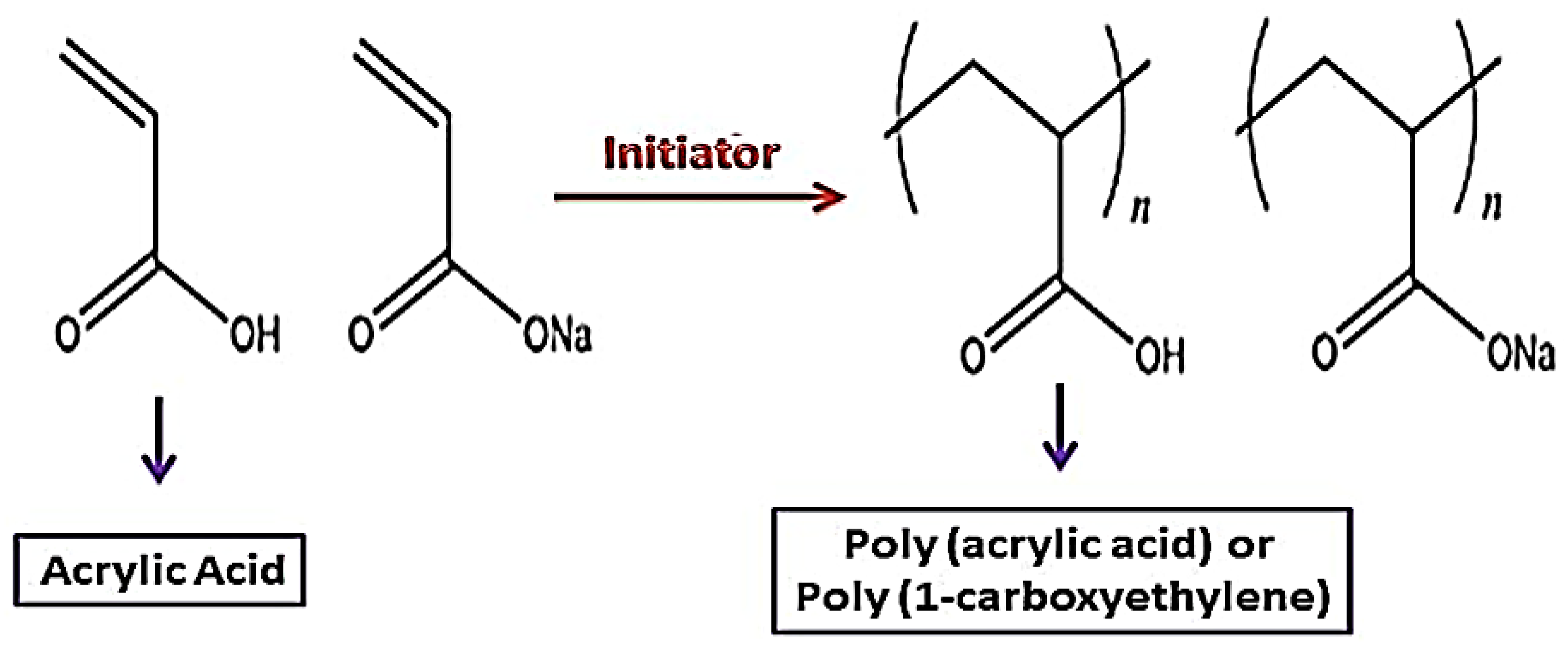
4.5. Polyacrylic Acid (PAA)
4.6. Poly (Lactic-co-Glycolic Acid) (PLGA)
4.7. Hyaluronic Acid (HA)
4.8. Cyclodextrins (CDs)
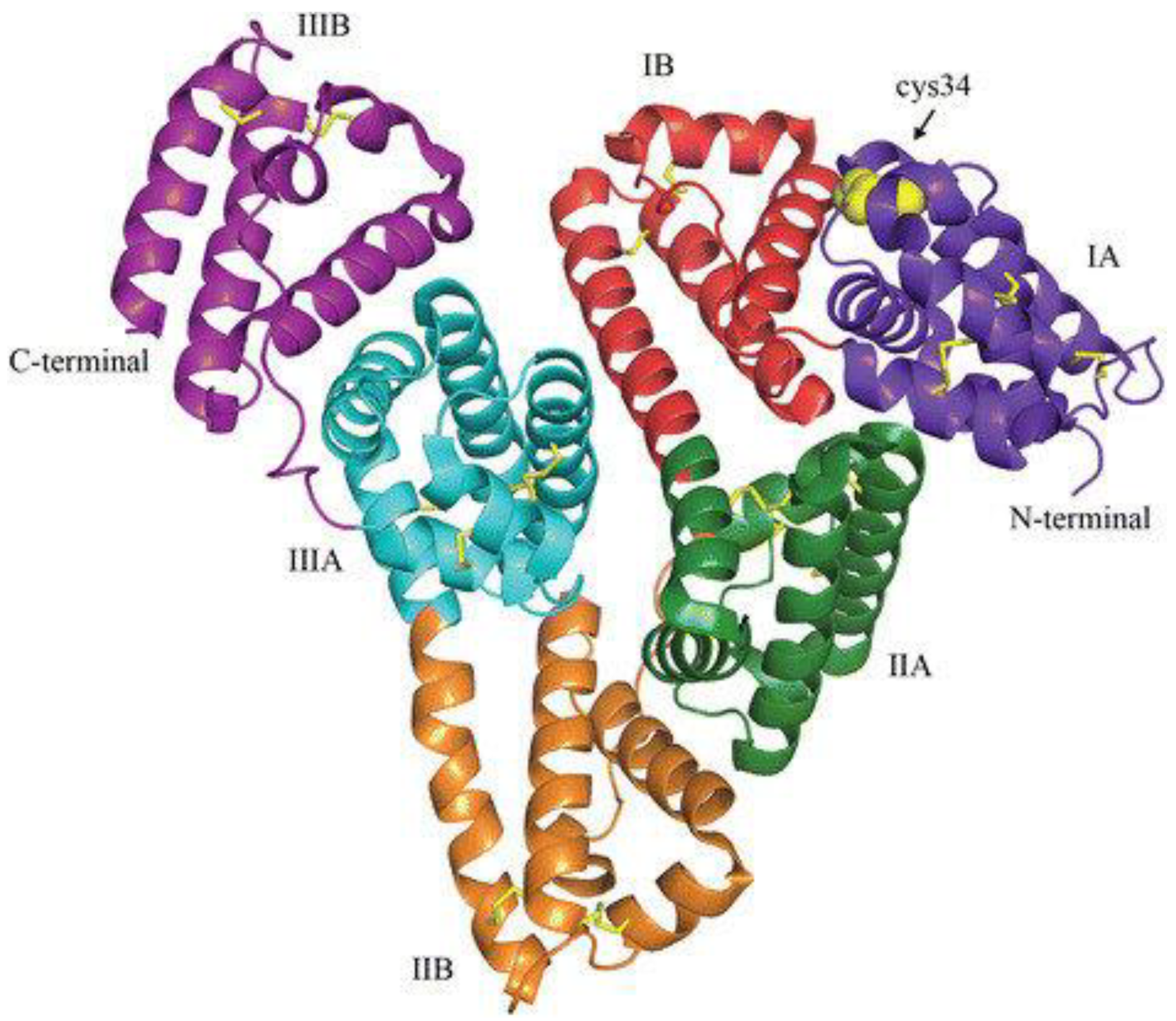
4.9. Human Serum Albumin (HSA)
5. Polymer-Coated Nanoparticles
6. Challenges
7. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sikdar, S.; Guha, S. Advancements of healthcare technologies: Paradigm towards smart healthcare systems. In Recent Trends in Image and Signal Processing in Computer Vision; Springer: Berlin/Heidelberg, Germany, 2020; pp. 113–132. [Google Scholar]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Santo Signorelli, S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Sabir, F.; Ain, Q.U.; Rahdar, A.; Yang, Z.; Barani, M.; Bilal, M.; Bhalla, N. Correction to: Functionalized Nanoparticles in Drug Delivery: Strategies to Enhance Direct Nose-to-Brain Drug Delivery via Integrated Nerve Pathways. In Synthesis and Applications of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2022; p. C1. [Google Scholar]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Md, S.; Bhattmisra, S.K.; Zeeshan, F.; Shahzad, N.; Mujtaba, M.A.; Meka, V.S.; Radhakrishnan, A.; Kesharwani, P.; Baboota, S.; Ali, J. Nano-carrier enabled drug delivery systems for nose to brain targeting for the treatment of neurodegenerative disorders. J. Drug Deliv. Sci. Technol. 2018, 43, 295–310. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Salarpour, S.; Barani, M.; Pardakhty, A.; Khatami, M.; Chauhan, N.P.S. The application of exosomes and exosome-nanoparticle in treating brain disorders. J. Mol. Liq. 2022, 350, 118549. [Google Scholar] [CrossRef]
- Arkaban, H.; Shervedani, R.K.; Yaghoobi, F.; Kefayat, A.; Ghahremani, F. Imaging and therapeutic capabilities of the AuNPs@ MnCO3/Mn3O4, coated with PAA and integrated with folic acid, doxorubicin and propidium iodide for murine breast cancer. J. Drug Deliv. Sci. Technol. 2022, 67, 102818. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Li, L.; He, D.; Chi, J.; Li, Q.; Wu, Y.; Zhao, Y.; Zhang, S.; Wang, L. Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J. Control. Release 2022, 349, 679–698. [Google Scholar] [CrossRef]
- Roostaee, M.; Sheikhshoaie, I. Fabrication of a sensitive sensor for determination of xanthine in the presence of uric acid and ascorbic acid by modifying a carbon paste sensor with Fe3O4@ Au core–shell and an ionic liquid. J. Food Meas. Charact. 2022, 16, 731–739. [Google Scholar] [CrossRef]
- Sonbol, H.; AlYahya, S.; Ameen, F.; Alsamhary, K.; Alwakeel, S.; Al-Otaibi, S.; Korany, S. Bioinspired synthesize of CuO nanoparticles using Cylindrospermum stagnale for antibacterial, anticancer and larvicidal applications. Appl. Nanosci. 2021, in press. [Google Scholar] [CrossRef]
- Zeng, Q.; Bie, B.; Guo, Q.; Yuan, Y.; Han, Q.; Han, X.; Chen, M.; Zhang, X.; Yang, Y.; Liu, M. Hyperpolarized Xe NMR signal advancement by metal-organic framework entrapment in aqueous solution. Proc. Natl. Acad. Sci. USA 2020, 117, 17558–17563. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Yang, L.; Wang, Z.; Wang, Z.; Feng, J.; Wen, X.; Cheng, L.; Zhu, R. MgFe-LDH nanoparticles: A promising leukemia inhibitory factor replacement for self-renewal and pluripotency maintenance in cultured mouse embryonic stem cells. Adv. Sci. 2021, 8, 2003535. [Google Scholar] [CrossRef]
- Rabiee, N.; Ahmadi, S.; Afshari, R.; Khalaji, S.; Rabiee, M.; Bagherzadeh, M.; Fatahi, Y.; Dinarvand, R.; Tahriri, M.; Tayebi, L. Polymeric nanoparticles for nasal drug delivery to the brain: Relevance to Alzheimer’s disease. Adv. Ther. 2021, 4, 2000076. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of polymeric nanoparticles for blood–brain barrier transfer—Strategies and challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Bhaskar, S.; Panda, A.K. Inorganic nanoparticles for natural product delivery: A review. Environ. Chem. Lett. 2020, 18, 2107–2118. [Google Scholar] [CrossRef]
- Akbarizadeh, M.R.; Sarani, M.; Darijani, S. Study of antibacterial performance of biosynthesized pure and Ag-doped ZnO nanoparticles. Rend. Lincei. Sci. Fis. E Nat. 2022, 33, 613–621. [Google Scholar] [CrossRef]
- Akbarizadeh, M.R.; Naderifar, M.; Mousazadeh, F.; Zafarnia, N.; Sarani, M. Cytotoxic activity and Magnetic Behavior of green synthesized iron oxide nanoparticles on brain glioblastoma cells. Nanomed. Res. J. 2022, 7, 99–106. [Google Scholar]
- Mortezagholi, B.; Movahed, E.; Fathi, A.; Soleimani, M.; Forutan Mirhosseini, A.; Zeini, N.; Khatami, M.; Naderifar, M.; Abedi Kiasari, B.; Zareanshahraki, M. Plant-mediated synthesis of silver-doped zinc oxide nanoparticles and evaluation of their antimicrobial activity against bacteria cause tooth decay. Microsc. Res. Tech. 2022, 85, 3553–3564. [Google Scholar] [CrossRef]
- Satarzadeh, N.; Shakibaie, M.; Adeli-Sardou, M.; Jabari-Morouei, F.; Forootanfar, H.; Sadeghi-Dousari, A. Facile microwave-assisted biosynthesis of arsenic nanoparticles and evaluation their antioxidant properties and cytotoxic effects: A preliminary in vitro study. J. Clust. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Syed, A.; Ameen, F.; Bastia, A.K.; Mohanta, T.K. Bio-inspired synthesis of silver nanoparticles from leaf extracts of Cleistanthus collinus (Roxb.): Its potential antibacterial and anticancer activities. IET Nanobiotechnol. 2018, 12, 343–348. [Google Scholar] [CrossRef]
- Sonbol, H.; Ameen, F.; AlYahya, S.; Almansob, A.; Alwakeel, S. Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef]
- Kumar, B.; Pandey, M.; Pottoo, F.H.; Fayaz, F.; Sharma, A.; Sahoo, P. Liposomes: Novel drug delivery approach for targeting Parkinson’s disease. Curr. Pharm. Des. 2020, 26, 4721–4737. [Google Scholar] [CrossRef]
- Ozkizilcik, A.; Williams, R.; Tian, Z.R.; Muresanu, D.F.; Sharma, A.; Sharma, H.S. Synthesis of biocompatible titanate nanofibers for effective delivery of neuroprotective agents. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2018; pp. 433–442. [Google Scholar]
- Lai, W.-F.; Wong, W.-T. Property-tuneable microgels fabricated by using flow-focusing microfluidic geometry for bioactive agent delivery. Pharmaceutics 2021, 13, 787. [Google Scholar] [CrossRef]
- Lai, W.-F. Development of hydrogels with self-healing properties for delivery of bioactive agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.-F. Multi-component hydrogel beads incorporated with reduced graphene oxide for ph-responsive and controlled co-delivery of multiple agents. Pharmaceutics 2021, 13, 313. [Google Scholar] [CrossRef]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Nematollahi, M.H. Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: A Nano-herbal treatment for Cancer. DARU J. Pharm. Sci. 2018, 26, 11–17. [Google Scholar] [CrossRef]
- Hajizadeh, M.R.; Maleki, H.; Barani, M.; Fahmidehkar, M.A.; Mahmoodi, M.; Torkzadeh-Mahani, M. In vitro cytotoxicity assay of D-limonene niosomes: An efficient nano-carrier for enhancing solubility of plant-extracted agents. Res. Pharm. Sci. 2019, 14, 448. [Google Scholar]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Adeli-Sardou, M. Evaluation of carum-loaded niosomes on breast cancer cells: Physicochemical properties, in vitro cytotoxicity, flow cytometric, DNA fragmentation and cell migration assay. Sci. Rep. 2019, 9, 7139. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Wang, J.; Yang, M.; Du, R.; Pontrelli, G.; McGinty, S.; Wang, G.; Yin, T.; Wang, Y. Penetration of the blood–brain barrier and the anti-tumour effect of a novel PLGA-lysoGM1/DOX micelle drug delivery system. Nanoscale 2020, 12, 2946–2960. [Google Scholar] [CrossRef]
- Rahdar, A.; Taboada, P.; Hajinezhad, M.R.; Barani, M.; Beyzaei, H. Effect of tocopherol on the properties of Pluronic F127 microemulsions: Physico-chemical characterization and in vivo toxicity. J. Mol. Liq. 2019, 277, 624–630. [Google Scholar] [CrossRef]
- Muntoni, E.; Martina, K.; Marini, E.; Giorgis, M.; Lazzarato, L.; Salaroglio, I.C.; Riganti, C.; Lanotte, M.; Battaglia, L. Methotrexate-loaded solid lipid nanoparticles: Protein functionalization to improve brain biodistribution. Pharmaceutics 2019, 11, 65. [Google Scholar] [CrossRef]
- Kheirkhah, P.; Denyer, S.; Bhimani, A.D.; Arnone, G.D.; Esfahani, D.R.; Aguilar, T.; Zakrzewski, J.; Venugopal, I.; Habib, N.; Gallia, G.L. Magnetic drug targeting: A novel treatment for intramedullary spinal cord tumors. Sci. Rep. 2018, 8, 11417. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, M.; Wang, G.; Liu, X.; Liu, D.; Peng, H.; Wang, Q. Heart-targeted nanoscale drug delivery systems. J. Biomed. Nanotechnol. 2014, 10, 2038–2062. [Google Scholar] [CrossRef]
- Peng, H.; Liu, X.; Wang, R.; Jia, F.; Dong, L.; Wang, Q. Emerging nanostructured materials for musculoskeletal tissue engineering. J. Mater. Chem. B 2014, 2, 6435–6461. [Google Scholar] [CrossRef]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood–brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Poovaiah, N.; Davoudi, Z.; Peng, H.; Schlichtmann, B.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Treatment of neurodegenerative disorders through the blood–brain barrier using nanocarriers. Nanoscale 2018, 10, 16962–16983. [Google Scholar] [CrossRef] [Green Version]
- Barani, M.; Mukhtar, M.; Rahdar, A.; Sargazi, G.; Thysiadou, A.; Kyzas, G.Z. Progress in the Application of Nanoparticles and Graphene as Drug Carriers and on the Diagnosis of Brain Infections. Molecules 2021, 26, 186. [Google Scholar] [CrossRef]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for diagnosis and treatment of brain cancer: Recent updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Jena, L.; McErlean, E.; McCarthy, H. Delivery across the blood-brain barrier: Nanomedicine for glioblastoma multiforme. Drug Deliv. Transl. Res. 2020, 10, 304–318. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Lai, W.-F. Non-conjugated polymers with intrinsic luminescence for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101916. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Tandel, G.S.; Biswas, M.; Kakde, O.G.; Tiwari, A.; Suri, H.S.; Turk, M.; Laird, J.R.; Asare, C.K.; Ankrah, A.A.; Khanna, N. A review on a deep learning perspective in brain cancer classification. Cancers 2019, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.A.; Chung, I.-M.; Rajakumar, G.; Alzohairy, M.A.; Alomary, M.N.; Thiruvengadam, M.; Pottoo, F.H.; Ahmad, N. Current nanoparticle approaches in nose to brain drug delivery and anticancer therapy-a review. Curr. Pharm. Des. 2020, 26, 1128–1137. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Parodi, A.; Rudzińska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A. Established and emerging strategies for drug delivery across the blood-brain barrier in brain cancer. Pharmaceutics 2019, 11, 245. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
- Gregory, J.V.; Kadiyala, P.; Doherty, R.; Cadena, M.; Habeel, S.; Ruoslahti, E.; Lowenstein, P.R.; Castro, M.G.; Lahann, J. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020, 11, 5687. [Google Scholar] [CrossRef]
- Fayazi, N.; Sheykhhasan, M.; Soleimani Asl, S.; Najafi, R. Stem cell-derived exosomes: A new strategy of neurodegenerative disease treatment. Mol. Neurobiol. 2021, 58, 3494–3514. [Google Scholar] [CrossRef]
- Peplow, P.V.; Martinez, B.; Gennarelli, T.A. Prevalence, Needs, Strategies, and Risk Factors for Neurodegenerative Diseases. In Neurodegenerative Diseases Biomarkers; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–8. [Google Scholar]
- Ross, C.; Taylor, M.; Fullwood, N.; Allsop, D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 8507. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Q.; Meng, Y.; Zhou, W.; Tong, J.; Cheng, Z.; Zhu, Q. New advances in brain-targeting nano-drug delivery systems for Alzheimer’s disease. J. Drug Target. 2022, 30, 61–81. [Google Scholar] [CrossRef]
- Karthivashan, G.; Ganesan, P.; Park, S.-Y.; Kim, J.-S.; Choi, D.-K. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Deliv. 2018, 25, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, A.C.; Bharadwaj, S.; Kumar, S.; Wei, D.-Q. Nano-particle mediated inhibition of Parkinson’s disease using computational biology approach. Sci. Rep. 2018, 8, 9169. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Wei, C.-X.; Lyu, Y.-Q.; Chen, H.-Z.; Jiang, G.; Gao, X.-L. Biomimetic drug-delivery systems for the management of brain diseases. Biomater. Sci. 2020, 8, 1073–1088. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: A comprehensive review. Int. J. Nanomed. 2020, 15, 2563. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Bera, A.; De, P. Current status, challenges and future directions in the treatment of neurodegenerative diseases by polymeric materials. J. Indian Chem. Soc. 2021, 98, 100011. [Google Scholar] [CrossRef]
- Tao, X.; Li, Y.; Hu, Q.; Zhu, L.; Huang, Z.; Yi, J.; Yang, X.; Hu, J.; Feng, X. Preparation and drug release study of novel nanopharmaceuticals with polysorbate 80 surface adsorption. J. Nanomater. 2018, 2018, 4718045. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. Performance of the biocompatible surfactant Tween 80, for the formation of microemulsions suitable for new pharmaceutical processing. J. Appl. Chem. 2013, 2013, 930356. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.K.; Mishra, J.; Mishra, A.K. Introducing Tween-curcumin niosomes: Preparation, characterization and microenvironment study. Soft Matter 2020, 16, 1779–1791. [Google Scholar] [CrossRef]
- Deng, L.-L.; Taxipalati, M.; Que, F.; Zhang, H. Physical characterization and antioxidant activity of thymol solubilized Tween 80 micelles. Sci. Rep. 2016, 6, 38160. [Google Scholar] [CrossRef]
- Hekmat, A.; Attar, H.; Seyf Kordi, A.A.; Iman, M.; Jaafari, M.R. New oral formulation and in vitro evaluation of docetaxel-loaded nanomicelles. Molecules 2016, 21, 1265. [Google Scholar] [CrossRef] [Green Version]
- Garidel, P.; Hoffmann, C.; Blume, A. A thermodynamic analysis of the binding interaction between polysorbate 20 and 80 with human serum albumins and immunoglobulins: A contribution to understand colloidal protein stabilisation. Biophys. Chem. 2009, 143, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef]
- El-Setouhy, D.A.; Basalious, E.B.; Abdelmalak, N.S. Bioenhanced sublingual tablet of drug with limited permeability using novel surfactant binder and microencapsulated polysorbate: In vitro/in vivo evaluation. Eur. J. Pharm. Biopharm. 2015, 94, 386–392. [Google Scholar] [CrossRef]
- Su, R.; Yang, L.; Wang, Y.; Yu, S.; Guo, Y.; Deng, J.; Zhao, Q.; Jin, X. Formulation, development, and optimization of a novel octyldodecanol-based nanoemulsion for transdermal delivery of ceramide IIIB. Int. J. Nanomed. 2017, 12, 5203. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, V.; Lee, M.; Nguyen Cao, T.G.; Shim, M.S. Polysorbate-based drug formulations for brain-targeted drug delivery and anticancer therapy. Appl. Sci. 2021, 11, 9336. [Google Scholar] [CrossRef]
- Torosantucci, R.; Furtmann, B.; Elshorst, B.; Pfeiffer-Marek, S.; Hartleb, T.; Andres, N.; Bussemer, T. Protein-excipient interactions evaluated via nuclear magnetic resonance studies in polysorbate-based multidose protein formulations: Influence on antimicrobial efficacy and potential study approach. J. Pharm. Sci. 2018, 107, 2531–2537. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Miller, D.W. Salinomycin-loaded iron oxide nanoparticles for glioblastoma therapy. Nanomaterials 2020, 10, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Tröster, S.; Müller, U.; Kreuter, J. Modification of the body distribution of poly (methyl methacrylate) nanoparticles in rats by coating with surfactants. Int. J. Pharm. 1990, 61, 85–100. [Google Scholar] [CrossRef]
- Ray, S.; Sinha, P.; Laha, B.; Maiti, S.; Bhattacharyya, U.K.; Nayak, A.K. Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting. J. Drug Deliv. Sci. Technol. 2018, 48, 21–29. [Google Scholar] [CrossRef]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond poly (ethylene glycol): Linear polyglycerol as a multifunctional polyether for biomedical and pharmaceutical applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Lohrasbi-Nejad, A.; Nematollahi, M.H. A new formulation of hydrophobin-coated niosome as a drug carrier to cancer cells. Mater. Sci. Eng. C 2020, 113, 110975. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, F.; Khalili Yazdi, A.; Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M. Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. DARU J. Pharm. Sci. 2018, 26, 57–64. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly (ethylene glycol)-and poly (phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.D.; Jay, M.; Dziubla, T.D.; Lu, X. PEGylation of nanocarrier drug delivery systems: State of the art. J. Biomed. Nanotechnol. 2008, 4, 133–148. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.D.; Nguyen, T.K.O.; Vo, T.K. Advances in developing therapeutic strategies for Alzheimer’s disease. Biomed. Pharmacother. 2021, 139, 111623. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef]
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosic, L.; Calabró, D.; Avallone, B.; Balsamo, G. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994, 15, 1215–1220. [Google Scholar] [CrossRef]
- Majedi, F.S.; Hasani-Sadrabadi, M.M.; VanDersarl, J.J.; Mokarram, N.; Hojjati-Emami, S.; Dashtimoghadam, E.; Bonakdar, S.; Shokrgozar, M.A.; Bertsch, A.; Renaud, P. On-chip fabrication of paclitaxel-loaded chitosan nanoparticles for cancer therapeutics. Adv. Funct. Mater. 2014, 24, 432–441. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652–9673. [Google Scholar]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Monsalve, Y.; Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F.; Lopez, B.L.; Sierra, L. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine 2015, 10, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, C.; Sousa, F.; Sarmento, B. Chitosan-based nanomedicine for brain delivery: Where are we heading? React. Funct. Polym. 2020, 146, 104430. [Google Scholar] [CrossRef]
- Wilson, B.; Alobaid, B.N.M.; Geetha, K.M.; Jenita, J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102176. [Google Scholar] [CrossRef]
- Manek, E.; Darvas, F.; Petroianu, G.A. Use of biodegradable, chitosan-based nanoparticles in the treatment of Alzheimer’s disease. Molecules 2020, 25, 4866. [Google Scholar] [CrossRef]
- Sinha, V.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Woodruff, M.; Hutmacher, D. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Woodward, S.C.; Brewer, P.; Moatamed, F.; Schindler, A.; Pitt, C. The intracellular degradation of poly (ε-caprolactone). J. Biomed. Mater. Res. 1985, 19, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Nakaya, A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002, 30, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Varan, C.; Bilensoy, E. Cationic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017, 8, 1446–1456. [Google Scholar] [CrossRef]
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-conjugated poly (ethylene glycol)-co-poly (ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32, 4293–4305. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.-H.; Li, S.; Vert, M. Synthesis and degradation of PLA–PCL–PLA triblock copolymer prepared by successive polymerization of ε-caprolactone and dl-lactide. Polymer 2004, 45, 8675–8681. [Google Scholar] [CrossRef]
- Mohamadpour, H.; Azadi, A.; Rostamizadeh, K.; Andalib, S.; Saghatchi Zanjani, M.R.; Hamidi, M. Preparation, Optimization, and Evaluation of Methoxy Poly (ethylene glycol)-co-poly (ε-caprolactone) Nanoparticles Loaded by Rivastigmine for Brain Delivery. ACS Chem. Neurosci. 2020, 11, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly (ϵ-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef]
- Pavlinec, J.; Novák, I.; Rychlý, J.; Kleinová, A.; Nógellová, Z.; Preťo, J.; Vanko, V.; Žigo, O.; Chodák, I. Hot melt adhesives prepared by grafting of acrylic and crotonic acids onto metallocene ethylene–octene copolymers. J. Plast. Film Sheet. 2019, 35, 239–259. [Google Scholar] [CrossRef]
- Dashtizadeh, A.; Abdouss, M.; Khorassani, M.; Mahdavi, H. Preparation of silica-filled water-based acrylic nanocomposite paints with improved scratch resistance. J. Plast. Film Sheet. 2012, 28, 120–135. [Google Scholar] [CrossRef]
- Foroushani, M.S.; Zahmatkeshan, A.; Arkaban, H.; Shervedani, R.K.; Kefayat, A. A drug delivery system based on nanocomposites constructed from metal-organic frameworks and Mn3O4 nanoparticles: Preparation and physicochemical characterization for BT-474 and MCF-7 cancer cells. Colloids Surf. B Biointerfaces 2021, 202, 111712. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Müller, A.H.; Klee, J.E. Intelligent colloidal hybrids via reversible pH-induced complexation of polyelectrolyte and silica nanoparticles. J. Am. Chem. Soc. 2003, 125, 3712–3713. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1444. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: Poly (acrylic acid)-functionalization is superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Poly (acrylic acid) nanocomposites: Design of advanced materials. J. Plast. Film Sheet. 2021, 37, 409–428. [Google Scholar] [CrossRef]
- Jing, X.; Feng, P.; Chen, Z.; Xie, Z.; Li, H.; Peng, X.-F.; Mi, H.-Y.; Liu, Y. Highly stretchable, self-healable, freezing-tolerant, and transparent polyacrylic acid/nanochitin composite hydrogel for self-powered multifunctional sensors. ACS Sustain. Chem. Eng. 2021, 9, 9209–9220. [Google Scholar] [CrossRef]
- Ebrahimi, A.K.; Sheikhshoaie, I.; Salimi, S.; Arkaban, H. In-situ facile synthesis of superparamagnetic porous core-shell structure for dye adsorption. J. Mol. Struct. 2021, 1228, 129797. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Wang, X.; Tang, L.; Ma, J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 323–336. [Google Scholar] [CrossRef]
- Borkar, N.; Mu, H.; Holm, R. Challenges and trends in apomorphine drug delivery systems for the treatment of Parkinson’s disease. Asian J. Pharm. Sci. 2018, 13, 507–517. [Google Scholar] [CrossRef]
- Durazo, S.A.; Kompella, U.B. Functionalized nanosystems for targeted mitochondrial delivery. Mitochondrion 2012, 12, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic acid nanoplatforms: Antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Hines, D.J.; Kaplan, D.L. Poly (lactic-co-glycolic) acid-controlled-release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, S.; Sowmya, S.; Cinu, T.; Aleykutty, N.; Thomas, S.; Souto, E. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014, 63, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Mirzaei, M.; Behzadi, M. Magnetic solid-phase extraction of lawsone using polyphenol-coated magnetic nanoparticles: Synthesis, characterization and examination. Chromatographia 2021, 84, 455–462. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Stayshich, R.M.; Weiss, R.M.; Li, J.; Meyer, T.Y. Periodic incorporation of pendant hydroxyl groups in repeating sequence PLGA copolymers. Macromol. Rapid Commun. 2011, 32, 220–225. [Google Scholar] [CrossRef]
- Choi, S.H.; Park, T.G. Synthesis and characterization of elastic PLGA/PCL/PLGA tri-block copolymers. J. Biomater. Sci. Polym. Ed. 2002, 13, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.S.; Hasan, W.; Rawal, S.; Walsh, M.D.; Enlow, E.M.; Luft, J.C.; Bridges, A.S.; Kuijer, J.L.; Napier, M.E.; Zamboni, W.C. Plasma, tumor and tissue pharmacokinetics of Docetaxel delivered via nanoparticles of different sizes and shapes in mice bearing SKOV-3 human ovarian carcinoma xenograft. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G. PLGA-PEG-ANG-2 Nanoparticles for Blood–Brain Barrier Crossing: Proof-of-Concept Study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambaryan, P.; Kondrasheva, I.; Severin, E.; Guseva, A.; Kamensky, A. Increasing the Efficiency of Parkinson’s Disease Treatment Using a poly (lactic-co-glycolic acid) (PLGA) Based L-DOPA Delivery System. Exp. Neurobiol. 2014, 23, 246. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, C. Tuning the size of poly (lactic-co-glycolic acid) (PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol. J. 2018, 13, 1700203. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Gul, M.; Nguyen, T.-T.-L.; Maeng, H.-J. Controlled release and targeted drug delivery with poly (lactic-co-glycolic acid) nanoparticles: Reviewing two decades of research. J. Pharm. Investig. 2022, 52, 683–724. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The role of mTOR signaling as a therapeutic target in cancer. Int. J. Mol. Sci. 2021, 22, 1743. [Google Scholar] [CrossRef]
- Su, M.; Soomro, S.H.; Jie, J.; Fu, H. Effects of the extracellular matrix on myelin development and regeneration in the central nervous system. Tissue Cell 2021, 69, 101444. [Google Scholar] [CrossRef]
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent advances in the treatment of bone metastases and primary bone tumors: An up-to-date review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef]
- Voci, S.; Fresta, M.; Cosco, D. Gliadins as versatile biomaterials for drug delivery applications. J. Control. Release 2021, 329, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.; Hossainy, S.; Healy, K. Hyaluronic Acid macromer molecular weight dictates the biophysical properties and in vitro cellular response to semisynthetic hydrogels. ACS Biomater. Sci. Eng. 2019, 6, 1135–1143. [Google Scholar] [CrossRef]
- Zakusilo, F.T.; O’Banion, M.K.; Gelbard, H.A.; Seluanov, A.; Gorbunova, V. Matters of size: Roles of hyaluronan in CNS aging and disease. Ageing Res. Rev. 2021, 72, 101485. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Ursini, O.; Palamà, I.E.; Gigli, G.; Moroni, L.; Cortese, B. HYDRHA: Hydrogels of hyaluronic acid. New biomedical approaches in cancer, neurodegenerative diseases, and tissue engineering. Mater. Today Bio 2022, 17, 100453. [Google Scholar] [CrossRef] [PubMed]
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as emerging therapeutic tools in the treatment of cholesterol-associated vascular and neurodegenerative diseases. Molecules 2016, 21, 1748. [Google Scholar] [CrossRef]
- Loftus, S.K.; Morris, J.A.; Carstea, E.D.; Gu, J.Z.; Cummings, C.; Brown, A.; Ellison, J.; Ohno, K.; Rosenfeld, M.A.; Tagle, D.A. Murine model of Niemann-Pick C disease: Mutation in a cholesterol homeostasis gene. Science 1997, 277, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Carstea, E.D.; Morris, J.A.; Coleman, K.G.; Loftus, S.K.; Zhang, D.; Cummings, C.; Gu, J.; Rosenfeld, M.A.; Pavan, W.J.; Krizman, D.B. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 1997, 277, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Sebak, S.; Mirzaei, M.; Malhotra, M.; Kulamarva, A.; Prakash, S. Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: Preparation and in vitro analysis. Int. J. Nanomed. 2010, 5, 525. [Google Scholar]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Shabani-Nooshabadi, M.; Roostaee, M. Coupling of NiO nanoparticles and room temperature ionic liquid for fabrication of highly sensitive voltammetric sensor in tryptophan analysis. Anal. Bioanal. Electrochem. 2016, 8, 578–588. [Google Scholar]
- Vidhya, M.S.; Ameen, F.; Dawoud, T.; Yuvakkumar, R.; Ravi, G.; Kumar, P.; Velauthapillai, D. Anti-cancer applications of Zr, Co, Ni-doped ZnO thin nanoplates. Mater. Lett. 2021, 283, 128760. [Google Scholar] [CrossRef]
- Swathi, S.; Ameen, F.; Ravi, G.; Yuvakkumar, R.; Hong, S.; Velauthapillai, D.; AlKahtani, M.D.; Thambidurai, M.; Dang, C. Cancer targeting potential of bioinspired chain like magnetite (Fe3O4) nanostructures. Curr. Appl. Phys. 2020, 20, 982–987. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Maary, K.S.; Almansob, A.; AlNadhari, S. Antioxidant, antibacterial and anticancer efficacy of Alternaria chlamydospora-mediated gold nanoparticles. Appl. Nanosci. 2022, in press. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Tomaro-Duchesneau, C.; Prakash, S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials 2013, 34, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Fei, W.; Tang, H.; Li, C.; Mu, C.; Zheng, H.; Li, F.; Zhu, Z. Angiopep-2-conjugated “core–shell” hybrid nanovehicles for targeted and pH-triggered delivery of arsenic trioxide into glioma. Mol. Pharm. 2019, 16, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Gouritin, B.; Villarroya, H.; Eclancher, F.; Giannavola, C.; Klein, C.; Andreux, J.P.; Couvreur, P. Quantification and localization of PEGylated polycyanoacrylate nanoparticles in brain and spinal cord during experimental allergic encephalomyelitis in the rat. Eur. J. Neurosci. 2002, 15, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Ebrahimi, A.K.; Yarahmadi, A.; Zarrintaj, P.; Barani, M. Development of a multifunctional system based on CoFe2O4@ polyacrylic acid NPs conjugated to folic acid and loaded with doxorubicin for cancer theranostics. Nanotechnology 2021, 32, 305101. [Google Scholar] [CrossRef]
- Arkaban, H.; Shervedani, R.K.; Yaghoobi, F.; Kefayat, A. A nanocomposite theranostic system, consisting of AuNPs@ MnCO3/Mn3O4 coated with PAA and integrated with folic acid, doxorubicin, and propidium iodide: Synthesis, characterization and examination for capturing of cancer cells. Inorg. Chem. Commun. 2021, 128, 108566. [Google Scholar] [CrossRef]
- Rahim, M.; Iram, S.; Syed, A.; Ameen, F.; Hodhod, M.S.; Khan, M.S. Nutratherapeutics approach against cancer: Tomato-mediated synthesised gold nanoparticles. IET Nanobiotechnol. 2018, 12, 1–5. [Google Scholar] [CrossRef]
- Kolev, S.K.; Aleksandrov, H.A.; Atanasov, V.A.; Popov, V.N.; Milenov, T.I. Surface chemistry of reduced graphene oxide: H-atom transfer reactions. Appl. Surf. Sci. 2021, 567, 150815. [Google Scholar] [CrossRef]
- Ginestra, P. Manufacturing of polycaprolactone-Graphene fibers for nerve tissue engineering. J. Mech. Behav. Biomed. Mater. 2019, 100, 103387. [Google Scholar] [CrossRef] [Green Version]
- Rauti, R.; Medelin, M.; Newman, L.; Vranic, S.; Reina, G.; Bianco, A.; Prato, M.; Kostarelos, K.; Ballerini, L. Graphene oxide flakes tune excitatory neurotransmission in vivo by targeting hippocampal synapses. Nano Lett. 2019, 19, 2858–2870. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Agarwal, S.; Kaur, R.; Pancham, P.; Kaur, H.; Bhardwaj, S.; Singh, M. In-silico validation and development of chlorogenic acid (CGA) loaded polymeric nanoparticle for targeting neurodegenerative disorders. J. Biomater. Nanobiotechnol. 2020, 11, 279. [Google Scholar] [CrossRef]
- Pongrac, I.M.; Ahmed, L.B.; Mlinarić, H.; Jurašin, D.D.; Pavičić, I.; Čermak, A.M.M.; Milić, M.; Gajović, S.; Vrček, I.V. Surface coating affects uptake of silver nanoparticles in neural stem cells. J. Trace Elem. Med. Biol. 2018, 50, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Men, P.; Kudo, W.; Perry, G.; Smith, M.A. Nanoparticle–chelator conjugates as inhibitors of amyloid-β aggregation and neurotoxicity: A novel therapeutic approach for Alzheimer disease. Neurosci. Lett. 2009, 455, 187–190. [Google Scholar] [CrossRef]
- Trapani, A.; De Giglio, E.; Cafagna, D.; Denora, N.; Agrimi, G.; Cassano, T.; Gaetani, S.; Cuomo, V.; Trapani, G. Characterization and evaluation of chitosan nanoparticles for dopamine brain delivery. Int. J. Pharm. 2011, 419, 296–307. [Google Scholar] [CrossRef]
- García-Pardo, J.; Novio, F.; Nador, F.; Cavaliere, I.; Suárez-García, S.; Lope-Piedrafita, S.; Candiota, A.P.; Romero-Gimenez, J.; Rodríguez-Galván, B.; Bové, J. Bioinspired theranostic coordination polymer nanoparticles for intranasal dopamine replacement in parkinson’s disease. ACS Nano 2021, 15, 8592–8609. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Liu, R.; Shen, Q.; Li, Y.; Shi, Z.; Shen, J.; Ji, W.; Zhang, X. Switchable nanoparticle for programmed gene-chem delivery with enhanced neuronal recovery and CT imaging for neurodegenerative disease treatment. Mater. Horiz. 2019, 6, 1923–1929. [Google Scholar] [CrossRef]
- Bittner, A.; Ducray, A.D.; Widmer, H.R.; Stoffel, M.H.; Mevissen, M. Effects of gold and PCL-or PLLA-coated silica nanoparticles on brain endothelial cells and the blood–brain barrier. Beilstein J. Nanotechnol. 2019, 10, 941–954. [Google Scholar] [CrossRef]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood–brain barrier. J. Nanobiotechnol. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandić, L.; Sadžak, A.; Strasser, V.; Baranović, G.; Domazet Jurašin, D.; Dutour Sikirić, M.; Šegota, S. Enhanced protection of biological membranes during lipid peroxidation: Study of the interactions between flavonoid loaded mesoporous silica nanoparticles and model cell membranes. Int. J. Mol. Sci. 2019, 20, 2709. [Google Scholar] [CrossRef] [Green Version]
- Baldim, V.; Yadav, N.; Bia, N.; Graillot, A.; Loubat, C.; Singh, S.; Karakoti, A.S.; Berret, J.-F. Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Appl. Mater. Interfaces 2020, 12, 42056–42066. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Wang, L.; Liu, Y.; Zhang, W.; Fan, B.; Ma, X.; Yuan, Q.; Ma, G.; Su, Z. Enhanced humoral and cell-mediated immune responses generated by cationic polymer-coated PLA microspheres with adsorbed HBsAg. Mol. Pharm. 2014, 11, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Feiner-Gracia, N.; Calderó, G.; García-Celma, M.; Solans, C. Galantamine-loaded PLGA nanoparticles, from nano-emulsion templating, as novel advanced drug delivery systems to treat neurodegenerative diseases. Nanoscale 2015, 7, 12076–12084. [Google Scholar] [CrossRef]
- Sun, W.; Xie, C.; Wang, H.; Hu, Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials 2004, 25, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qiu, J.; Li, X.-L.; Aday, S.; Zhang, J.; Conley, G.; Xu, J.; Joseph, J.; Lan, H.; Langer, R. BBB pathophysiology–independent delivery of siRNA in traumatic brain injury. Sci. Adv. 2021, 7, eabd6889. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Xu, N.; Cao, C.; Yuan, W.; Yu, X.; Chen, J.; Ren, J. Preparation and therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. J. Biomater. Sci. Polym. Ed. 2009, 20, 1369–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.S.; Ramasamy, M.; Suresh, B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 144–152. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Zhao, N.; Hao, B.; Wang, X.; Kong, P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ. Toxicol. Pharmacol. 2012, 34, 272–279. [Google Scholar] [CrossRef]
- Ahlschwede, K.M.; Curran, G.L.; Rosenberg, J.T.; Grant, S.C.; Sarkar, G.; Jenkins, R.B.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Cationic carrier peptide enhances cerebrovascular targeting of nanoparticles in Alzheimer’s disease brain. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 258–266. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.-H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Calpena, A.C.; Folch, J.; Barenys, M.; Sánchez-López, E.; Camins, A.; García, M.L. Epigallocatechin-3-gallate loaded PEGylated-PLGA nanoparticles: A new anti-seizure strategy for temporal lobe epilepsy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Manhas, P.; Swami, A.; Bhandari, R.; Sharma, K.K.; Jain, R.; Kumar, R.; Pandey, S.K.; Kuhad, A.; Sharma, R.K. Bioengineered PLGA-chitosan nanoparticles for brain targeted intranasal delivery of antiepileptic TRH analogues. Chem. Eng. J. 2018, 346, 630–639. [Google Scholar] [CrossRef]
- Mukherjee, S.; Madamsetty, V.S.; Bhattacharya, D.; Roy Chowdhury, S.; Paul, M.K.; Mukherjee, A. Recent advancements of nanomedicine in neurodegenerative disorders theranostics. Adv. Funct. Mater. 2020, 30, 2003054. [Google Scholar] [CrossRef]
- Lang, A.E. Clinical trials of disease-modifying therapies for neurodegenerative diseases: The challenges and the future. Nat. Med. 2010, 16, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of nanomaterials: An up-to-date overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Venkataramani, V.; Doeppner, T.R.; Willkommen, D.; Cahill, C.M.; Xin, Y.; Ye, G.; Liu, Y.; Southon, A.; Aron, A.; Au-Yeung, H.Y. Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J. Neurochem. 2018, 147, 831–848. [Google Scholar] [CrossRef] [PubMed]


| Components | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Cerium oxide |
|
| [158] |
| Chitosan |
|
| [159] [160] |
| Gold |
|
| [78,158] |
| Silica |
|
| [78] |
| Component | Synthesis Method | Structure | Ref. |
|---|---|---|---|
| GO/PCL | electrospinning | porous framework | [166] |
| GO | hummer | nanosheets | [167] |
| SiO2@PSA | ionic gelation method | core-shell | [168] |
| Ag NPs | precipitation | core-shell | [169] |
| Polystyrene | --- | nanosphere–chelator conjugate | [170] |
| Chitosan | ionic gelation | nanospheres | [171] |
| Dopamine | reversible coordination complexation | coordination polymer nanoparticles | [172] |
| SiO2@ PLLA | Stober | core-shell | [174] |
| Polymer-coated cerium oxide | precipitation−redispersion | stair-like | [177] |
| Cationic polymer-coated PLA | emulsification and emulsion solvent evaporation | microspheres | [178] |
| Drug Carriers | Therapeutic Agents | Diseases | Surface Coating | Study Model | Reference |
|---|---|---|---|---|---|
| PLA | FITC-dextran | BBB | PS 80 | Kunming mice | [180] |
| PLGA | siRNA | Traumatic brain injury | PS 80 | C57BL/6J mice | [181] |
| PLA-b-PEG | Amphotericin B | Cryptococcal Meningitis | PS 80 | BALB/c mice | [182] |
| Chitosan | Tacrine | Alzheimer’s Disease | PS 80 | in vitro release | [183] |
| liposomes | Galantamine | Alzheimer’s disease and vascular dementia | PEG | rat brains | [184] |
| Silica-encapsulated liposomes | Arsenic trioxide | Glioma | PAA | rat brains | [160] |
| MSNs | Resveratrol | Parkinson’s disease | PLA | rat brains | [175] |
| PLGA | Curcumin | Alzheimer’s disease | Chitosan | Tg2576 mice | [185] |
| PLGA | Epigallocatechin gallate | Alzheimer’s disease | PEG | APP/PS1 mice | [186] |
| PLGA | Epigallocatechin gallate | Epilepsy | PEG | KA–C57BL/6 mice | [187] |
| PLGA | TRH analogs | Epilepsy | Chitosan | rat brains | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazi Alahri, M.; Jibril Ibrahim, A.; Barani, M.; Arkaban, H.; Shadman, S.M.; Salarpour, S.; Zarrintaj, P.; Jaberi, J.; Turki Jalil, A. Management of Brain Cancer and Neurodegenerative Disorders with Polymer-Based Nanoparticles as a Biocompatible Platform. Molecules 2023, 28, 841. https://doi.org/10.3390/molecules28020841
Bazi Alahri M, Jibril Ibrahim A, Barani M, Arkaban H, Shadman SM, Salarpour S, Zarrintaj P, Jaberi J, Turki Jalil A. Management of Brain Cancer and Neurodegenerative Disorders with Polymer-Based Nanoparticles as a Biocompatible Platform. Molecules. 2023; 28(2):841. https://doi.org/10.3390/molecules28020841
Chicago/Turabian StyleBazi Alahri, Mehdi, Alhawarin Jibril Ibrahim, Mahmood Barani, Hassan Arkaban, Seyedeh Malahat Shadman, Soodeh Salarpour, Payam Zarrintaj, Javad Jaberi, and Abduladheem Turki Jalil. 2023. "Management of Brain Cancer and Neurodegenerative Disorders with Polymer-Based Nanoparticles as a Biocompatible Platform" Molecules 28, no. 2: 841. https://doi.org/10.3390/molecules28020841
APA StyleBazi Alahri, M., Jibril Ibrahim, A., Barani, M., Arkaban, H., Shadman, S. M., Salarpour, S., Zarrintaj, P., Jaberi, J., & Turki Jalil, A. (2023). Management of Brain Cancer and Neurodegenerative Disorders with Polymer-Based Nanoparticles as a Biocompatible Platform. Molecules, 28(2), 841. https://doi.org/10.3390/molecules28020841