Cyclodextrin Metal-Organic Framework as a Broad-Spectrum Potential Delivery Vehicle for the Gasotransmitters
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Characterization
2.2. Synthesis of γ-CD-MOF
2.3. Synthesis of γ-CD-MOF-Pluronics
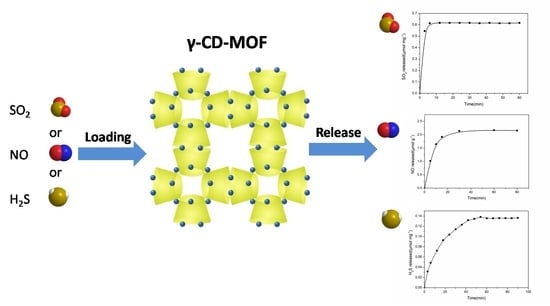
2.4. SO2, H2S, and NO Loading into γ-CD-MOF
2.5. Detection of SO2 Release from γ-CD-MOF and Pluronics Modified γ-CD-MOF
2.6. Detection of H2S Release
2.7. Detection of NO
2.8. DNA Cleavage Assay
2.9. Anti-Inflammation Study
3. Results and Discussion
3.1. Synthesis of Nano-γ-CD-MOF for Gasotransmitters
3.2. Sulfur Dioxide Adsorption and Release
3.3. Nitric Oxide Adsorption and Release Studies
3.4. H2S Adsorption and Release Studies
3.5. Regulation of Gasotransmitter Payloads and Release Rate
3.6. Bioactivity of Gasotransmitter-Loaded γ-CD-MOF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ignarro, L.J. Nitric Oxide: A unique endogenous signaling molecule in vascular biology. Biosci. Rep. 1999, 19, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, C.; Du, J.; Jin, H. Endogenous sulfur dioxide: A new member of gasotransmitter family in the cardiovascular system. Oxid. Med. Cell. Longev. 2016, 2016, 8961951–8961959. [Google Scholar] [CrossRef] [Green Version]
- Szabo, C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, J.M.; Maurya, R.C. A gentle introduction to gasotransmitters with special reference to nitric oxide: Biological and chemical implications. Rev. Inorg. Chem. 2018, 38, 193–220. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Mhatre, S.; Opere, C.A.; Singh, S. Unmet needs in glaucoma therapy: The potential role of hydrogen sulfide and its delivery strategies. J. Control. Release 2022, 347, 256–269. [Google Scholar] [CrossRef]
- Malwal, S.R.; Gudem, M.; Hazra, A.; Chakrapani, H. Benzosultines as sulfur dioxide (SO2) donors. Org. Lett. 2013, 15, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Foster, J.C.; Okyere, B.; Theus, M.H.; Matson, J.B. Therapeutic delivery of H2S via COS: Small molecule and polymeric donors with benign byproducts. J. Am. Chem. Soc. 2016, 138, 13477–13480. [Google Scholar] [CrossRef]
- Wang, W.; Ji, X.; Du, Z.; Wang, B. Sulfur dioxide prodrugs: Triggered release of SO2 via a click reaction. Chem. Commun. 2017, 53, 1370–1373. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.Y.; Zhong, Z.Y. External stimuli-responsive gasotransmitter prodrugs: Chemistry and spatiotemporal release. J. Control. Release 2022, 351, 81–101. [Google Scholar] [CrossRef]
- Fukushima, N.; Ieda, N.; Kawaguchi, M.; Sasakura, K.; Nagano, T.; Hanaoka, K.; Miyata, N.; Nakagawa, H. Development of photo-controllable hydrogen sulfide donor applicable in live cells. Bioorg. Med. Chem. Lett. 2015, 25, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstain, R.; Slanina, T.; Kand, D.; Klán, P. Visible-to-NIR-light activated release: From small molecules to nanomaterials. Chem. Rev. 2020, 120, 13135–13272. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Organ, C.L.; Park, C.M.; Yang, C.T.; Pacheco, A.; Wang, D.; Lefer, D.J.; Xian, M. pH-controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, K.A.; Malwal, S.R.; Banerjee, A.; Lahiri, S.; Rangarajan, R.; Chakrapani, H. Thiol activated prodrugs of sulfur dioxide (SO2) as MRSA inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 2694–2697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Xian, M. Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 2011, 133, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Pardeshi, K.A.; Ravikumar, G.; Chakrapani, H. Esterase sensitive self-immolative sulfur dioxide donors. Org. Lett. 2018, 20, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, B. Esterase-sensitive sulfur dioxide prodrugs inspired by modified Julia olefination. Chem. Commun. 2017, 53, 10124–10127. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yu, B.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-sensitive prodrugs with tunable release rates and direct generation of hydrogen sulfide. Angew. Chem.-Int. Ed. 2016, 55, 4514–4518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Henthorn, H.A.; Pluth, M.D. Kinetic insights into hydrogen sulfide delivery from caged-carbonyl sulfide isomeric donor platforms. J. Am. Chem. Soc. 2017, 139, 16365–16376. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; El-labbad, E.M.; Ji, K.; Lasheen, D.S.; Serya, R.A.T.; Abouzid, K.A.; Wang, B. Click and release: SO2 prodrugs with tunable release rates. Org. Lett. 2017, 19, 818–821. [Google Scholar] [CrossRef]
- Geng, J.; Zhang, Y.; Gao, Q.; Neumann, K.; Dong, H.; Porter, H.; Potter, M.; Ren, H.; Argyle, D.; Bradley, M. Switching on prodrugs using radiotherapy. Nat. Chem. 2021, 13, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Guo, Z.; Zheng, Y.; Wang, Z.; Fu, Q.; Liu, Z. Radiotherapy reduces N-oxides for prodrug activation in tumors. J. Am. Chem. Soc. 2022, 144, 9458–9464. [Google Scholar] [CrossRef]
- DeRosa, F.; Kibbe, M.R.; Najjar, S.F.; Citro, M.L.; Keefer, L.K.; Hrabie, J.A. Nitric oxide-releasing fabrics and other acrylonitrile-based diazeniumdiolates. J. Am. Chem. Soc. 2007, 129, 3786–3787. [Google Scholar] [CrossRef]
- Foster, J.C.; Radzinski, S.C.; Zou, X.; Finkielstein, C.V.; Matson, J.B. H2S-releasing polymer micelles for studying selective cell toxicity. Mol. Pharm. 2017, 14, 1300–1306. [Google Scholar] [CrossRef]
- Pinto, M.L.; Fernandes, A.C.; Rocha, J.; Ferreira, A.; Antunes, F.; Pires, J. Microporous titanosilicates Cu2+– and Co2+–ETS-4 for storage and slow release of therapeutic nitric oxide. J. Mater. Chem. B 2014, 2, 224–230. [Google Scholar] [CrossRef]
- Wheatley, P.S.; Butler, A.R.; Crane, M.S.; Fox, S.; Xiao, B.; Rossi, A.G.; Megson, I.L.; Morris, R.E. NO-releasing zeolites and their antithrombotic properties. J. Am. Chem. Soc. 2006, 128, 502–509. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Pinto, M.L.; Antunes, F.; Pires, J. Synthetic cobalt clays for the storage and slow release of therapeutic nitric oxide. RSC Adv. 2016, 6, 41195–41203. [Google Scholar] [CrossRef]
- Cattaneo, D.; Warrender, S.J.; Duncan, M.J.; Kelsall, C.J.; Doherty, M.K.; Whitfield, P.D.; Megson, I.L.; Morris, R.E. Tuning the nitric oxide release from CPO-27 MOFs. RSC Adv. 2016, 6, 14059–14067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinlay, A.C.; Xiao, B.; Wragg, D.S.; Wheatley, P.S.; Megson, I.L.; Morris, R.E. Exceptional behavior over the whole adsorption-storage-delivery cycle for NO in porous metal organic frameworks. J. Am. Chem. Soc. 2008, 130, 10440–10444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.V.; Antunes, F.; Pires, J.; Graça, V.; Brandão, P.; Pinto, M.L. Vitamin B3 metal-organic frameworks as potential delivery vehicles for therapeutic nitric oxide. Acta Biomater. 2017, 51, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.J.; Rojas, S.; Romão, C.C.; Navarro, J.A.R.; Barea, E.; Maldonado, C.R. One-pot preparation of a novel CO-releasing material based on a CO-releasing molecule@ metal–organic framework system. Chem. Commun. 2017, 53, 6581–6584. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metal–organic frameworks from edible natural products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef]
- Carson, R.J.; Seyffarth, G.M.R.; Maddock, H.; Wang, R. Gasotransmitters: Past, Present, and Future; Humana Press: Totowa, NJ, USA, 2005; pp. 33–35. [Google Scholar]
- Li, Y.; Zhao, M. Simple methods for rapid determination of sulfite in food products. Food Control. 2006, 17, 975–980. [Google Scholar] [CrossRef]
- Sharma, A.K.; Nair, M.; Chauhan, P.; Gupta, K.; Saini, D.K.; Chakrapani, H. Visible-light-triggered uncaging of carbonyl sulfide for hydrogen sulfide (H2S) release. Org. Lett. 2017, 19, 4822–4825. [Google Scholar] [CrossRef] [PubMed]
- Dinh, B.T.; Price, S.E.; Majul, A.; El-Hajj, M.; Morozov, V.; Hrabie, J.A.; Davies, K.M. Diazeniumdiolate reactivity in model membrane systems. Nitric Oxide-Biol. Chem. 2008, 18, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Furukawa, Y.; Ishiwata, T.; Sugikawa, K.; Kokado, K.; Sada, K. Nano- and microsized cubic gel particles from cyclodextrin metal–organic frameworks. Angew. Chem. Int. Ed. 2012, 51, 10566–10569. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef]
- Liu, B.; He, Y.; Han, L.; Singh, V.; Xu, X.; Guo, T.; Meng, F.; Xu, X.; York, P.; Liu, Z.; et al. Microwave-assisted rapid synthesis of γ-cyclodextrin metal–organic frameworks for size control and efficient drug loading. Cryst. Growth Des. 2017, 17, 1654–1660. [Google Scholar] [CrossRef]
- Gassensmith, J.J.; Furukawa, H.; Smaldone, R.A.; Forgan, R.S.; Botros, Y.Y.; Yaghi, O.M.; Stoddart, J.F. Strong and reversible binding of carbon dioxide in a green metal–organic framework. J. Am. Chem. Soc. 2011, 133, 15312–15315. [Google Scholar] [CrossRef]
- Wang, L.; Liang, X.Y.; Chang, Z.Y.; Ding, L.S.; Zhang, S.; Li, B.J. Effective formaldehyde capture by green cyclodextrin-based metal–organic framework. ACS Appl. Mater. Interfaces 2018, 10, 42–46. [Google Scholar] [CrossRef]
- Song, X.D.; Wang, S.; Hao, C.; Qiu, J.S. Investigation of SO2 gas adsorption in metal–organic frameworks by molecular simulation. Inorg. Chem. Commun. 2014, 46, 277–281. [Google Scholar] [CrossRef]
- Savage, M.; Cheng, Y.; Easun, T.L.; Eyley, J.E.; Argent, S.P.; Warren, M.R.; Lewis, W.; Murray, C.; Tang, C.C.; Frogley, M.D.; et al. Selective adsorption of sulfur dioxide in a robust metal–organic framework material. Adv. Mater. 2016, 28, 8705–8711. [Google Scholar] [CrossRef]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef]
- Singh, V.; Guo, T.; Xu, H.; Wu, L.; Gu, J.; Wu, C.; Gref, R.; Zhang, J. Moisture resistant and biofriendly CD-MOF nanoparticles obtained via cholesterol shielding. Chem. Commun. 2017, 53, 9246–9249. [Google Scholar] [CrossRef] [Green Version]
- McKinlay, A.C.; Eubank, J.F.; Wuttke, S.; Xiao, B.; Wheatley, P.S.; Bazin, P.; Lavalley, J.C.; Daturi, M.; Vimont, A.; De Weireld, G.; et al. Nitric Oxide Adsorption and Delivery in Flexible MIL-88(Fe) Metal–Organic Frameworks. Chem. Mater. 2013, 25, 1592–1599. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, X.; Liu, J.; Shen, J. Application of density functional theory to the nitric oxide heterogeneous reduction mechanism in the presence of hydroxyl and carbonyl groups. Energy Convers. Manag. 2014, 83, 167–176. [Google Scholar] [CrossRef]
- Xiao, B.; Wheatley, P.S.; Zhao, X.; Fletcher, A.J.; Fox, S.; Rossi, A.G.; Megson, I.L.; Bordiga, S.; Regli, L.; Thomas, K.M.; et al. High-capacity hydrogen and nitric oxide adsorption and storage in a metal-organic framework. J. Am. Chem. Soc. 2007, 129, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Noelia, F.; Natalia, C.A.; Jerome, S.; Lorenzo, L. Analysis of the Expression and Activity of Nitric Oxide Synthase from Marine Photosynthetic Microorganisms. Methods Mol. Biol. 2016, 1424, 149–162. [Google Scholar]
- Chavan, S.; Bonino, F.; Valenzano, L.; Civalleri, B.; Lamberti, C.; Acerbi, N.; Cavka, J.H.; Leistner, M.; Bordiga, S. Fundamental aspects of H2S adsorption on CPO-27-Ni. J. Phys. Chem. C 2013, 117, 15615–15622. [Google Scholar] [CrossRef]
- Fernandez, C.A.; Nune, S.K.; Annapureddy, H.V.; Dang, L.X.; McGrail, B.P.; Zheng, F.; Polikarpov, E.; King, D.L.; Freeman, C.; Brooks, K.P. Hydrophobic and moisture-stable metal–organic frameworks. Dalton Trans. 2015, 44, 13490–13497. [Google Scholar] [CrossRef] [PubMed]
- Burrows, C.J. Oxidative nucleobase modifications leading to strand scission. Chem. Rev. 1998, 98, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.K.; Zachary, J.B.; Yamil, J.C.; Paul, W.S.; Karl, A.S.; Randall, Q.S.; Joseph, T.H.; Omar, K.F. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar]







| Materials | SO2 Adsorption Capacity (μmol/mg) |
|---|---|
| γ-CD-MOF-K | 0.62 |
| γ-CD | 0.37 |
| UIO-66-(OH)2 | 0.53 |
| UIO-66 | 0.32 |
| γ-CD-MOF-Cs | 0.64 |
| Materials | H2S Adsorption Capacity (μmol/mg) |
|---|---|
| γ-CD-MOF-K | 0.14 |
| γ-CD | 0.05 |
| UIO-66-(OH)2 | 0.004 |
| UIO-66 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.-G.; Ke, D.; Li, G.-C.; Zhang, S.; Li, B.-J. Cyclodextrin Metal-Organic Framework as a Broad-Spectrum Potential Delivery Vehicle for the Gasotransmitters. Molecules 2023, 28, 852. https://doi.org/10.3390/molecules28020852
Liao L-G, Ke D, Li G-C, Zhang S, Li B-J. Cyclodextrin Metal-Organic Framework as a Broad-Spectrum Potential Delivery Vehicle for the Gasotransmitters. Molecules. 2023; 28(2):852. https://doi.org/10.3390/molecules28020852
Chicago/Turabian StyleLiao, Li-Guo, Duo Ke, Guo-Chen Li, Sheng Zhang, and Bang-Jing Li. 2023. "Cyclodextrin Metal-Organic Framework as a Broad-Spectrum Potential Delivery Vehicle for the Gasotransmitters" Molecules 28, no. 2: 852. https://doi.org/10.3390/molecules28020852