Electroreductively Induced Radicals for Organic Synthesis
Abstract
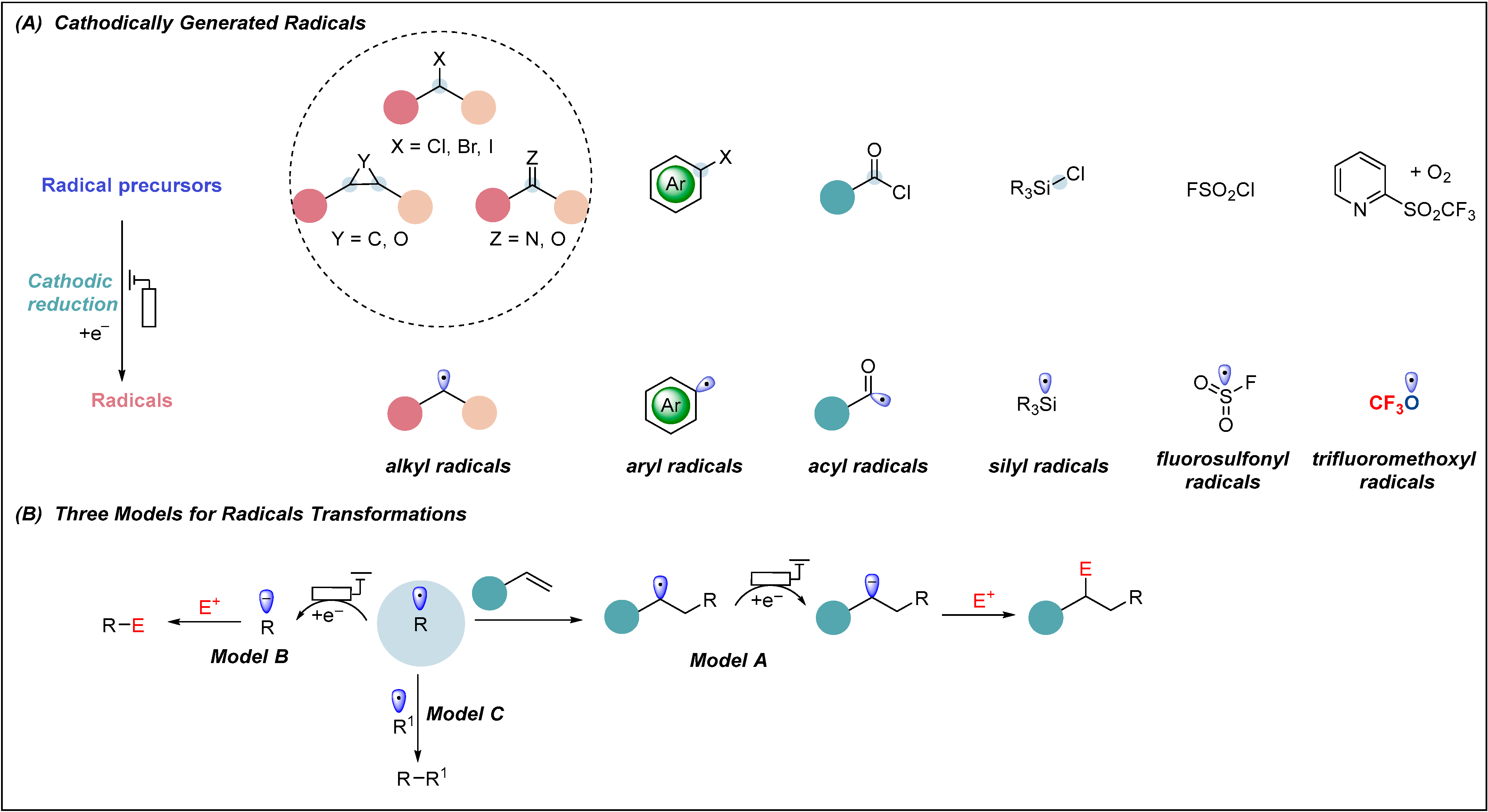
1. Introduction
2. Electrochemical Reduction
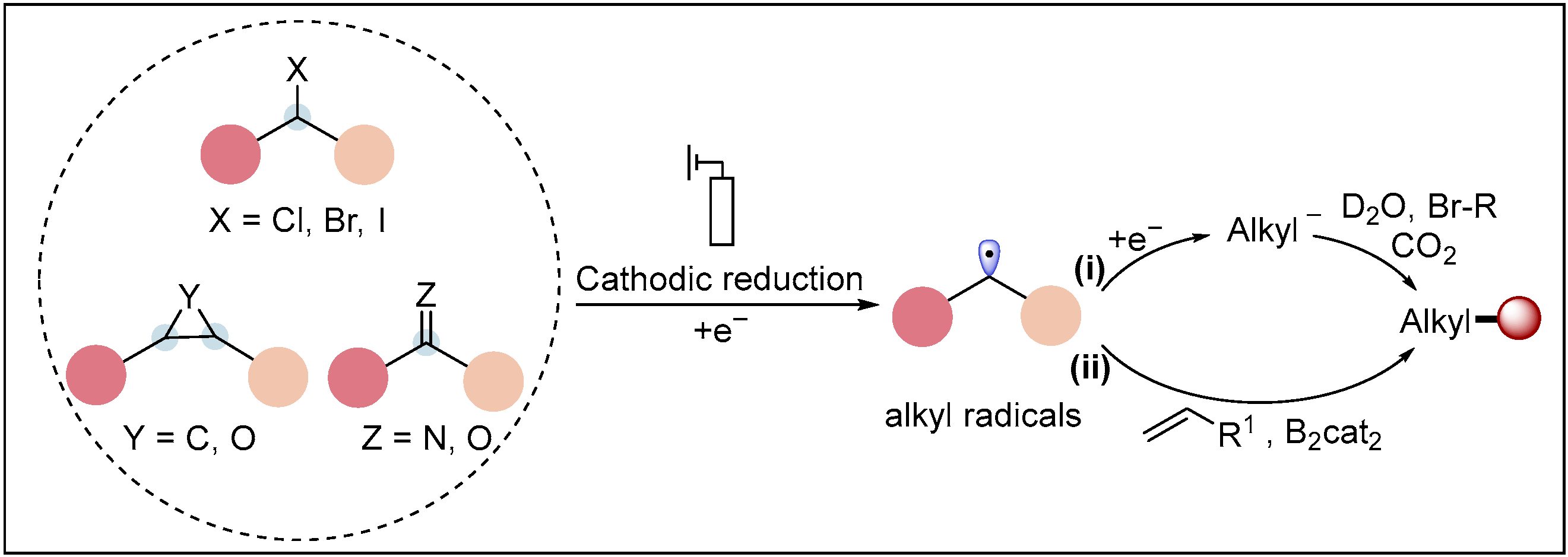
2.1. Electroreductive Reaction of Alkyl Radicals
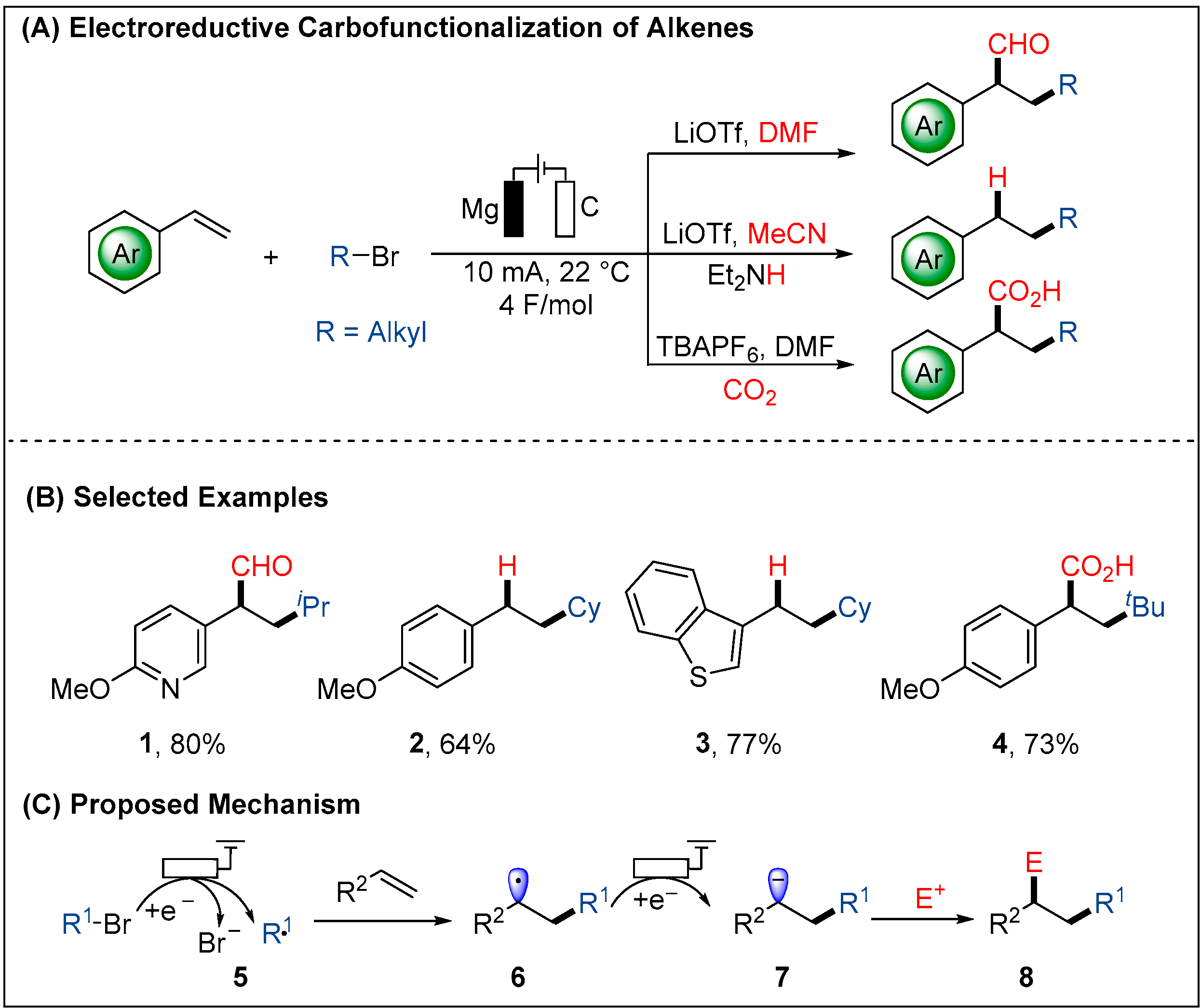
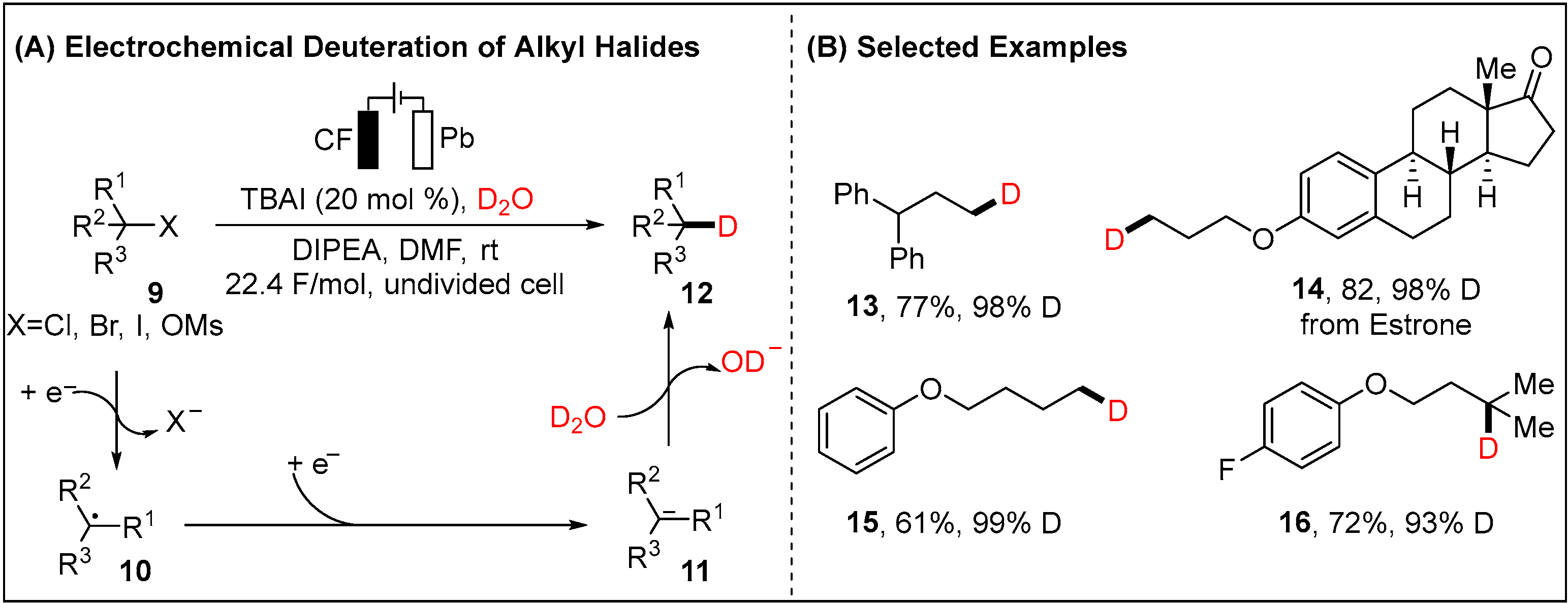
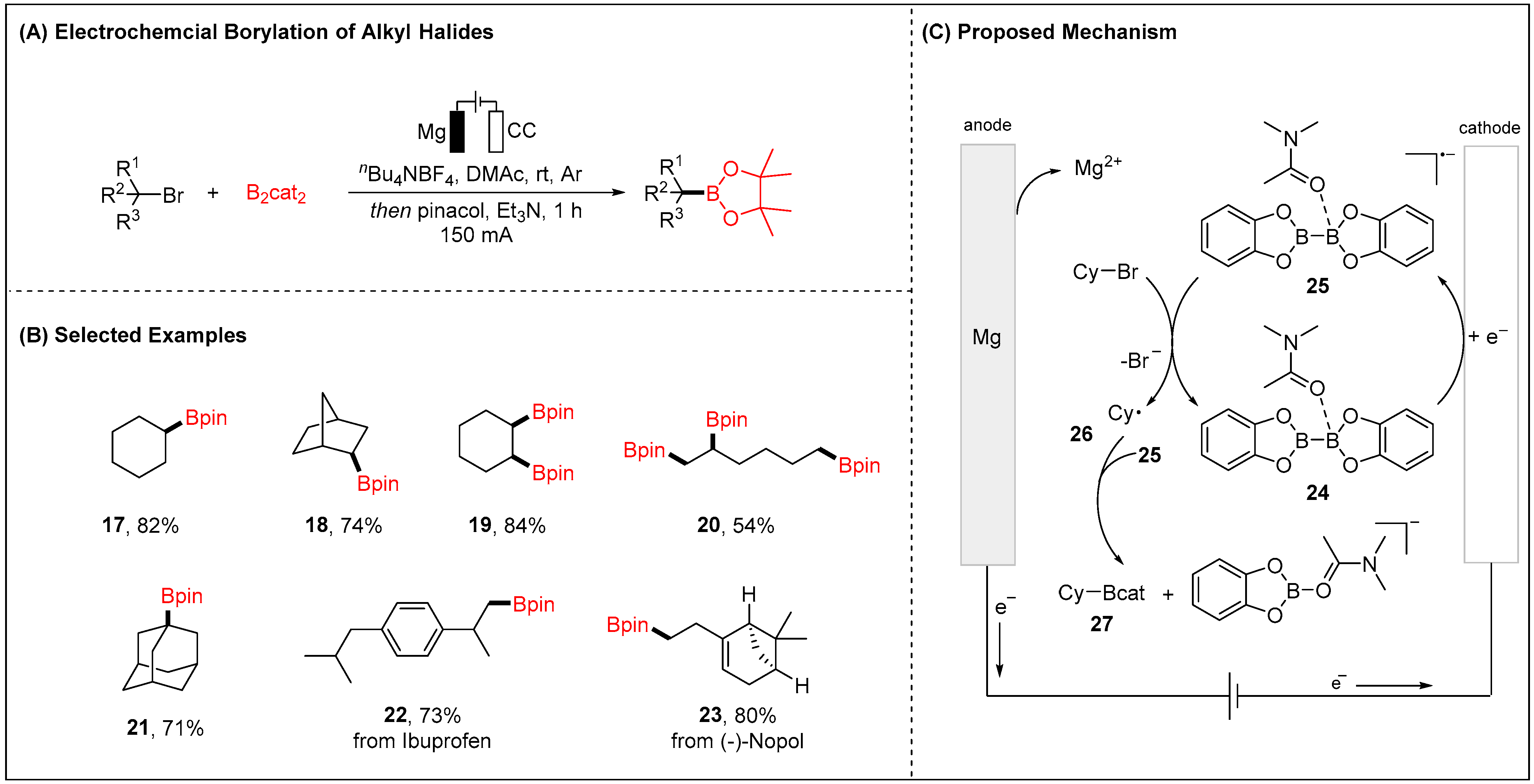
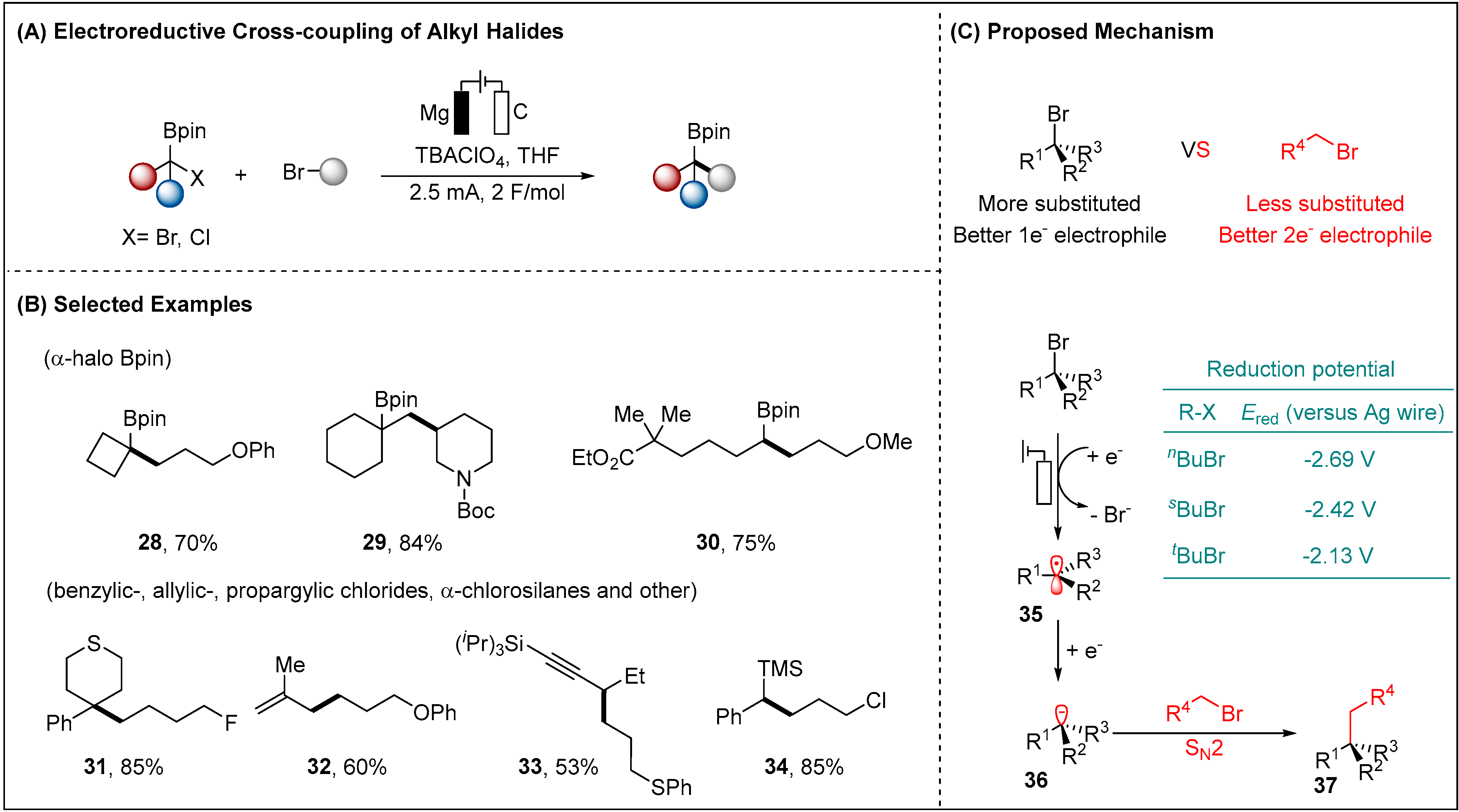
2.1.1. Electroreductive Reaction of Alkyl Halides
2.1.2. Electroreductive Reaction of Epoxides and Cyclopropanes
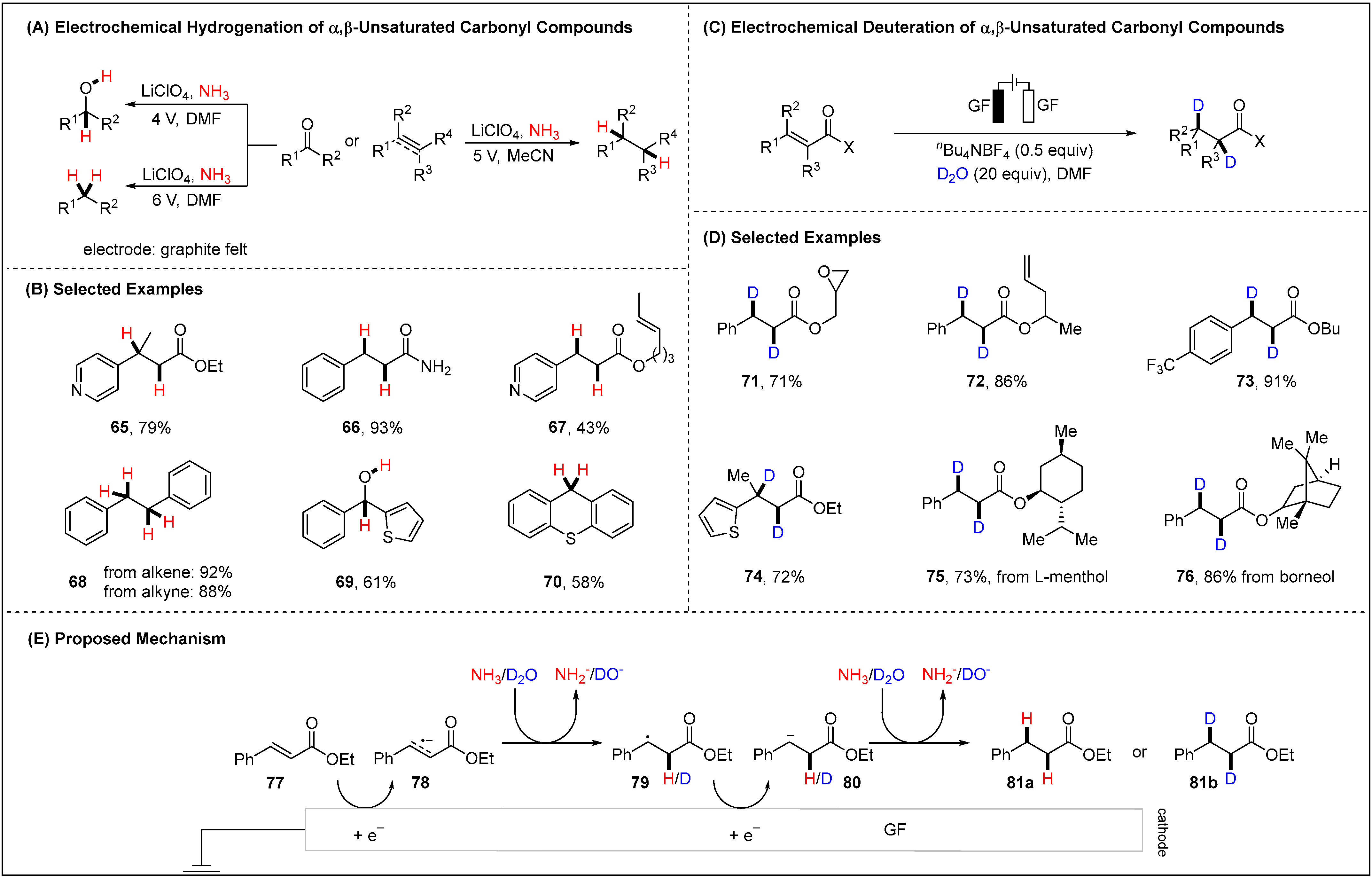
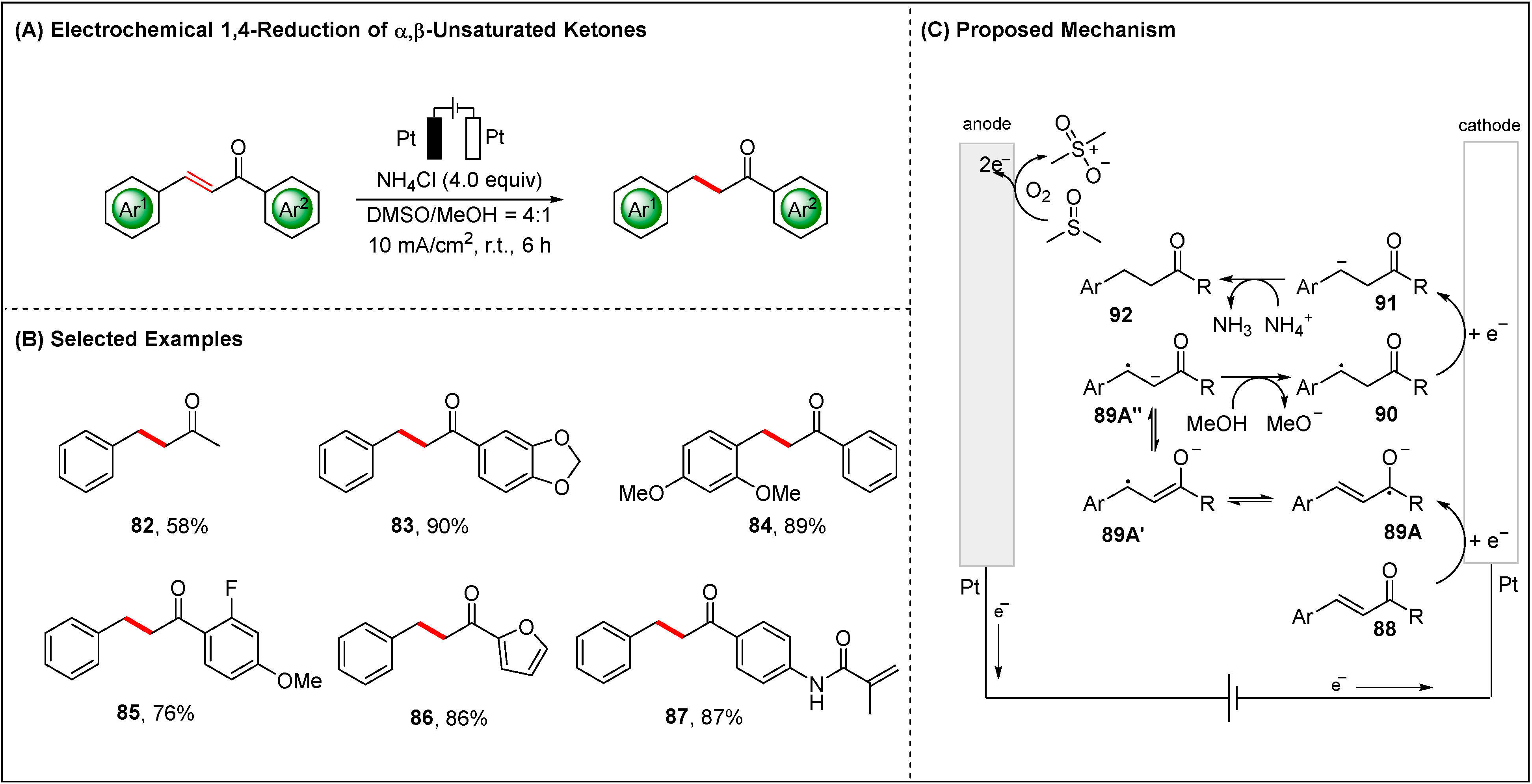
2.1.3. Electroreductive Reaction of Ketones, Alkenes and Alkynes
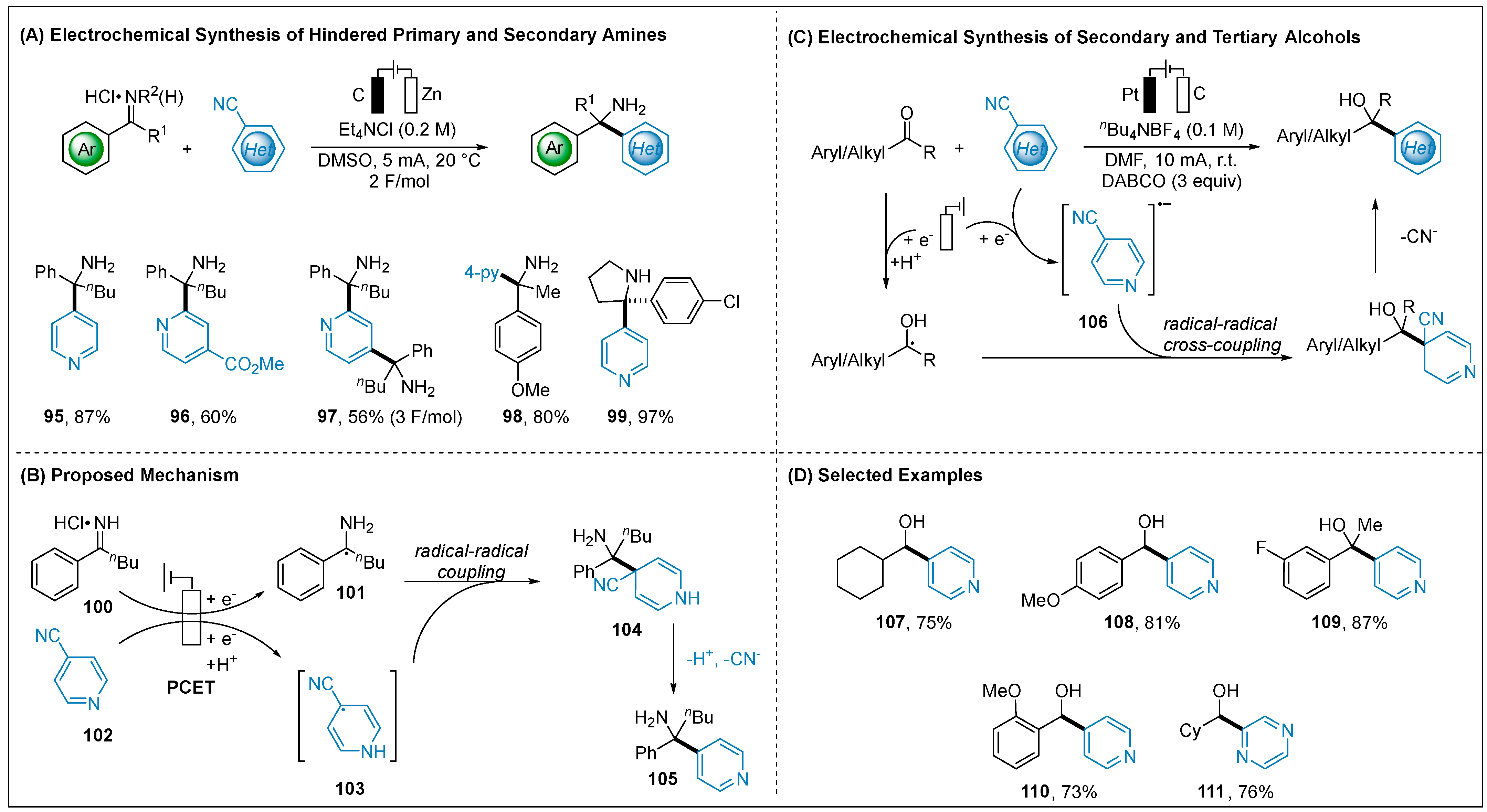
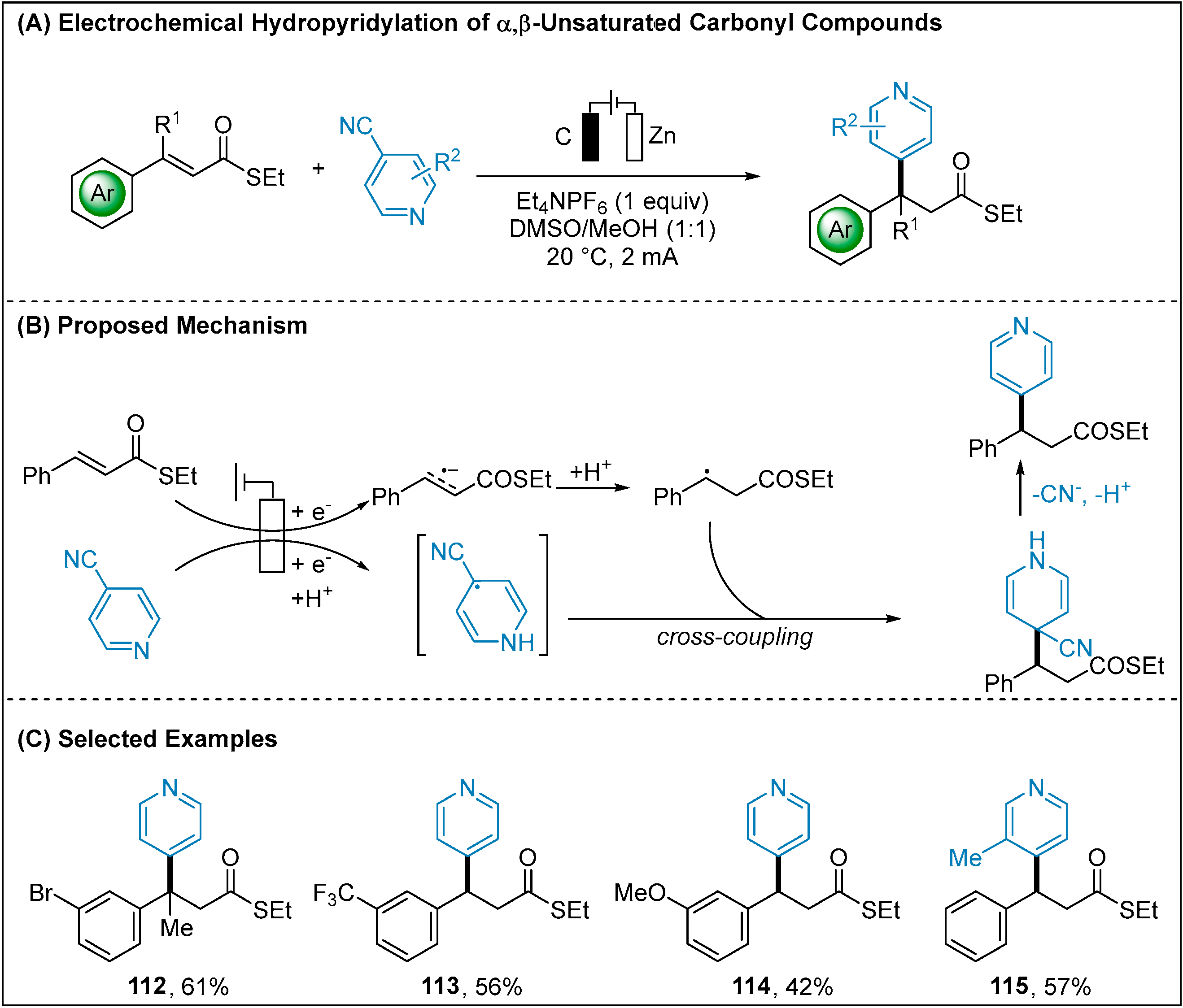
2.1.4. Electroreductive Reaction of Cyanoheteroarenes
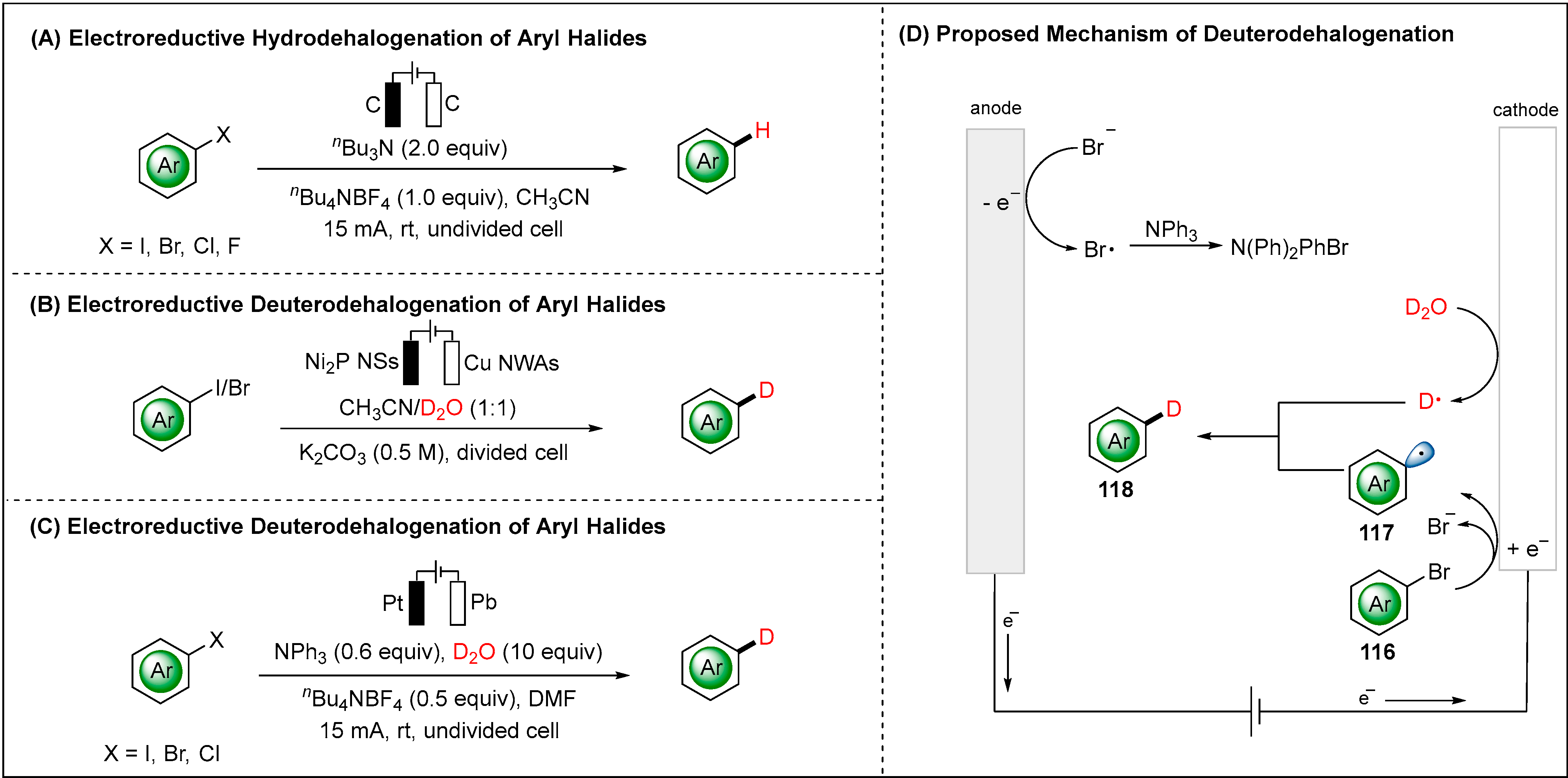
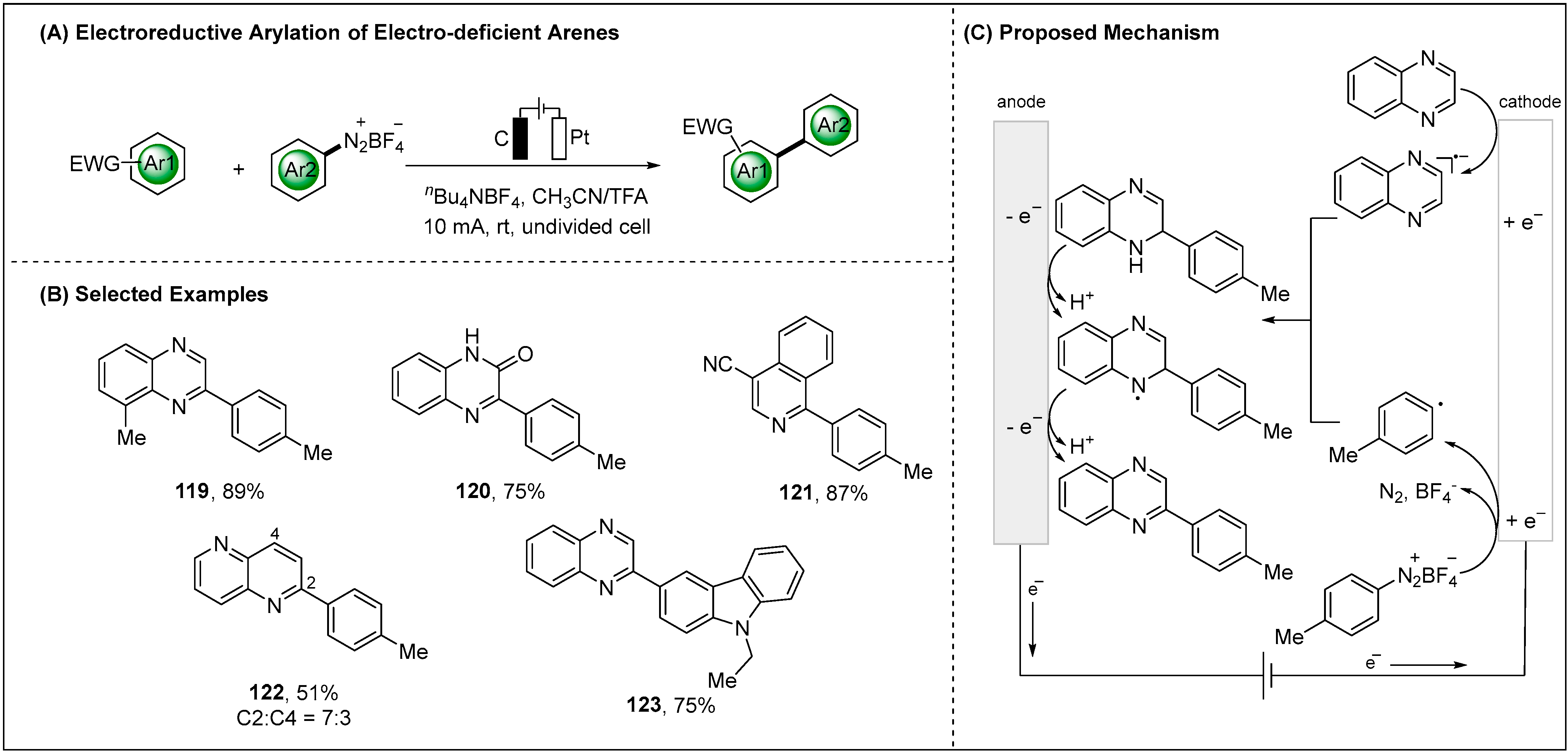
2.2. Electroreductive Reaction of Aryl Radicals
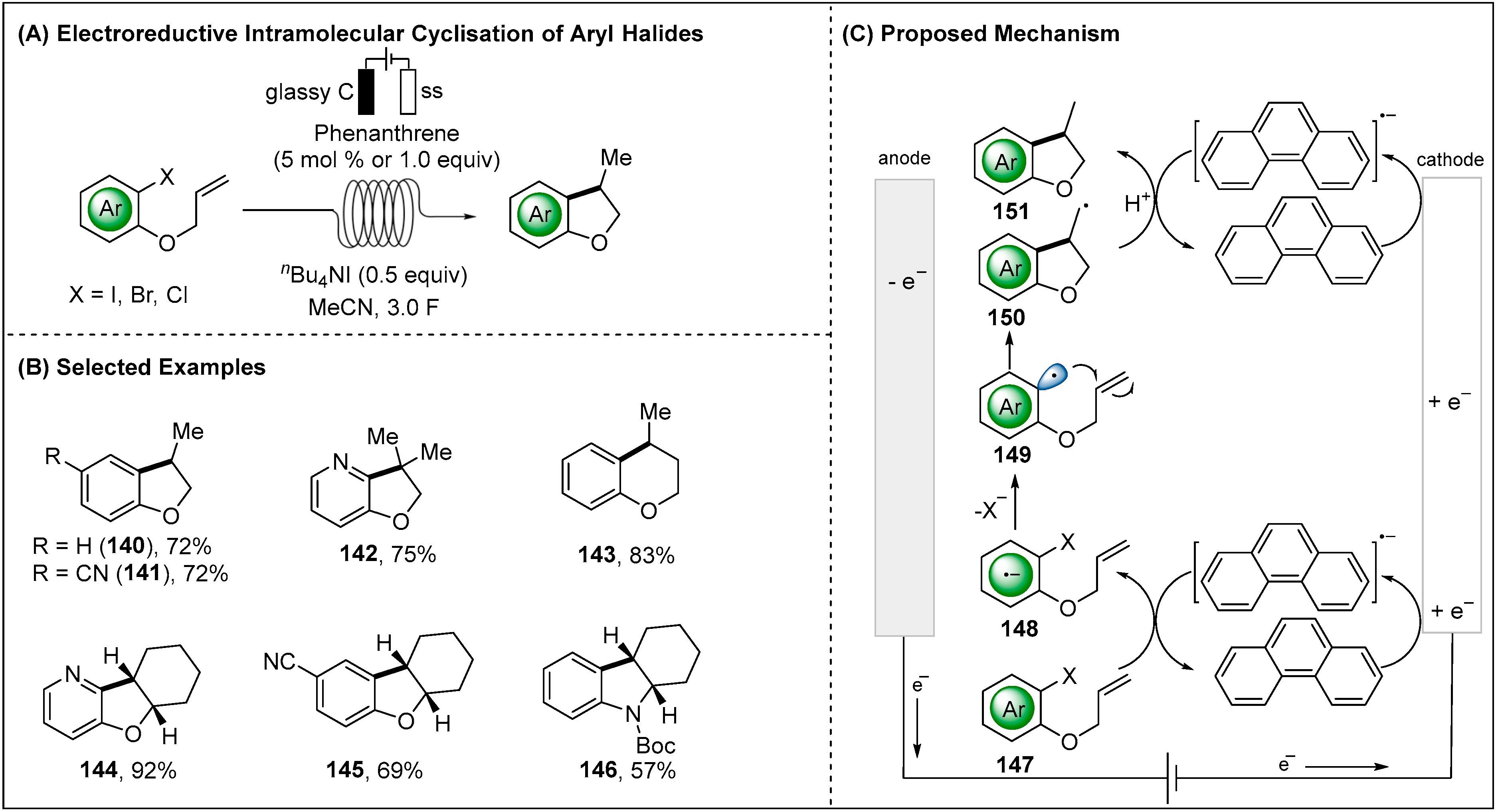
2.2.1. Direct Electroreductive Reaction of Aryl Radicals
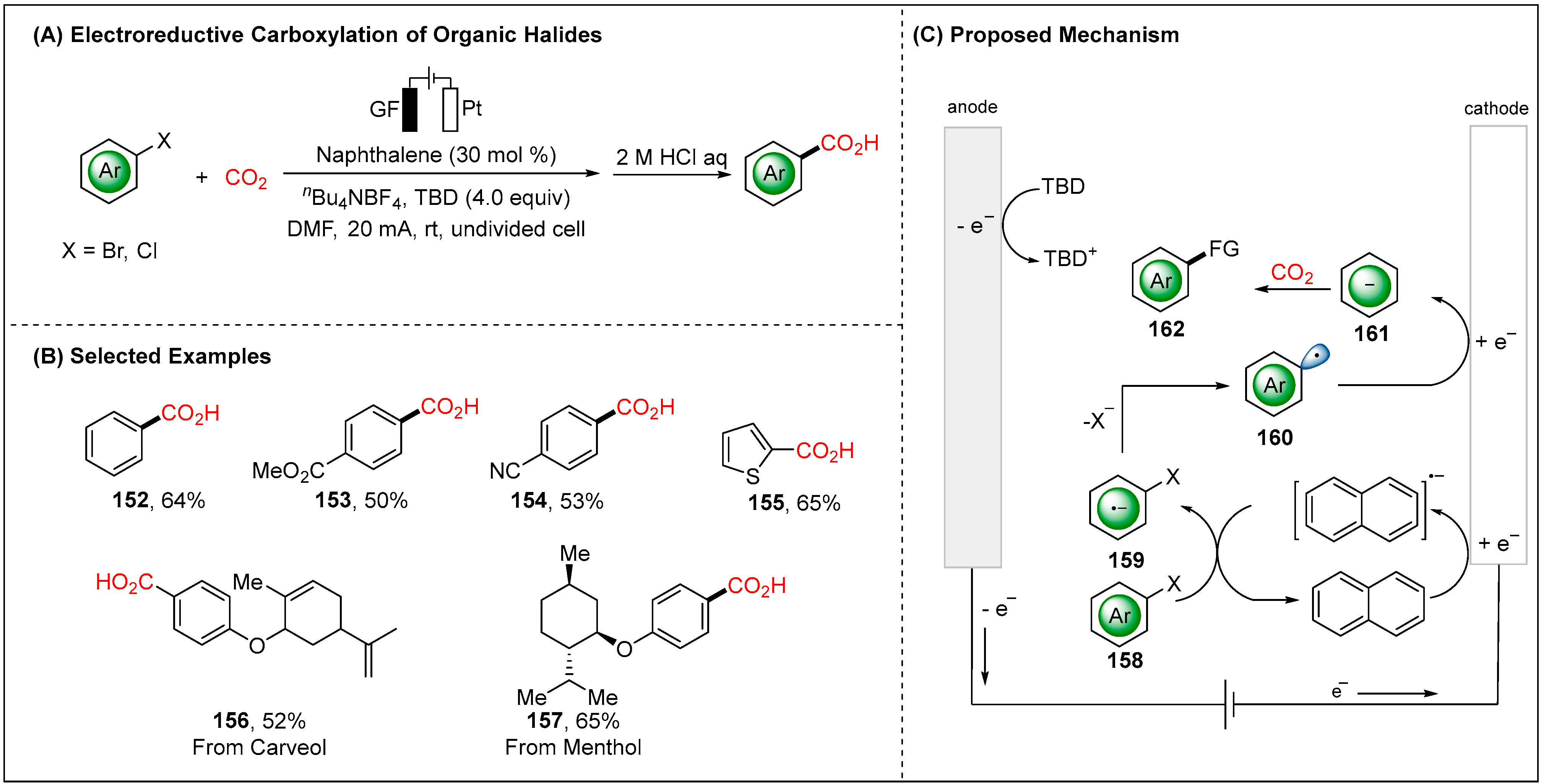
2.2.2. Indirect Electroreductive Reaction of Aryl Radicals
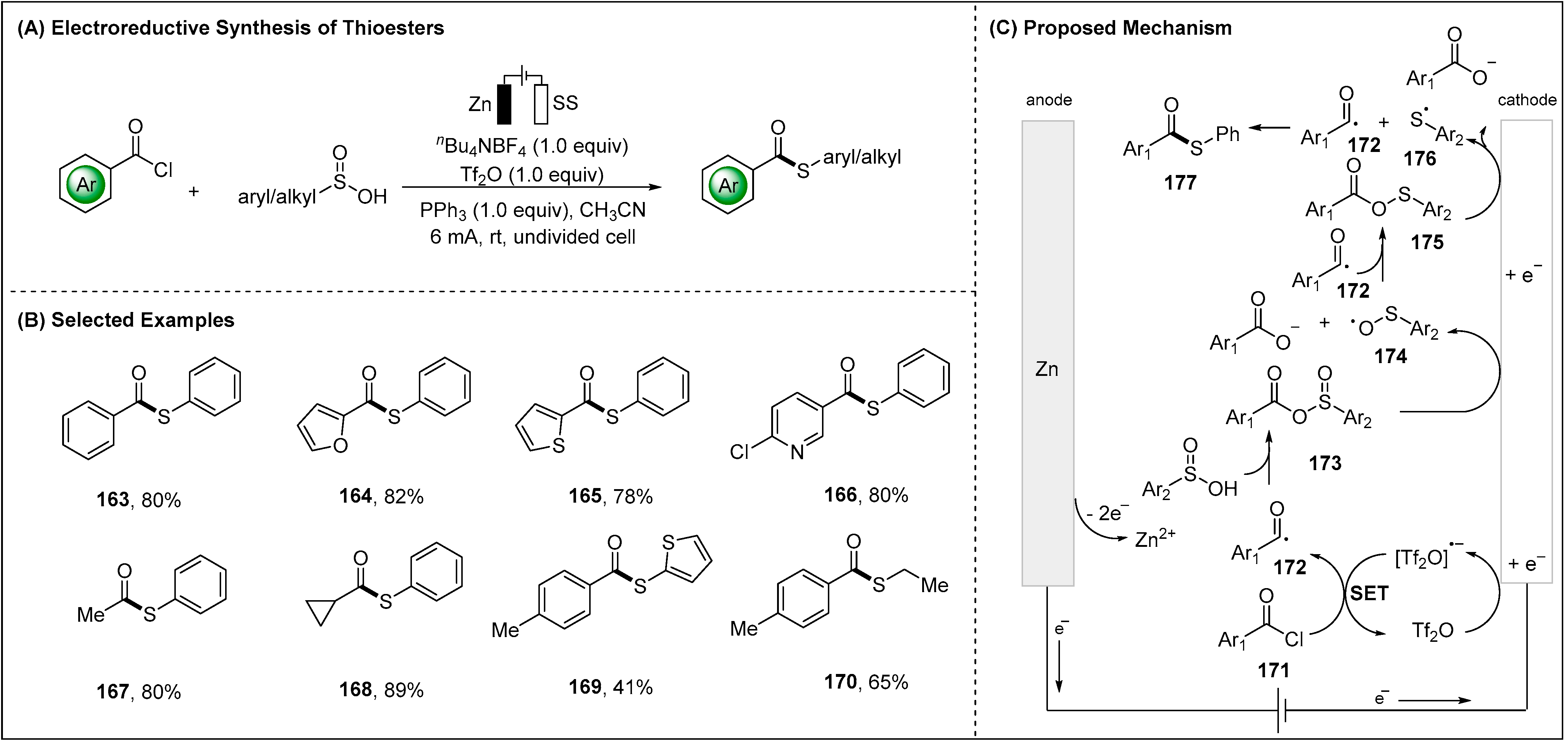
2.3. Electroreductive Reaction of Acyl Radicals
2.4. Electroreductive Reaction of Silyl Radicals
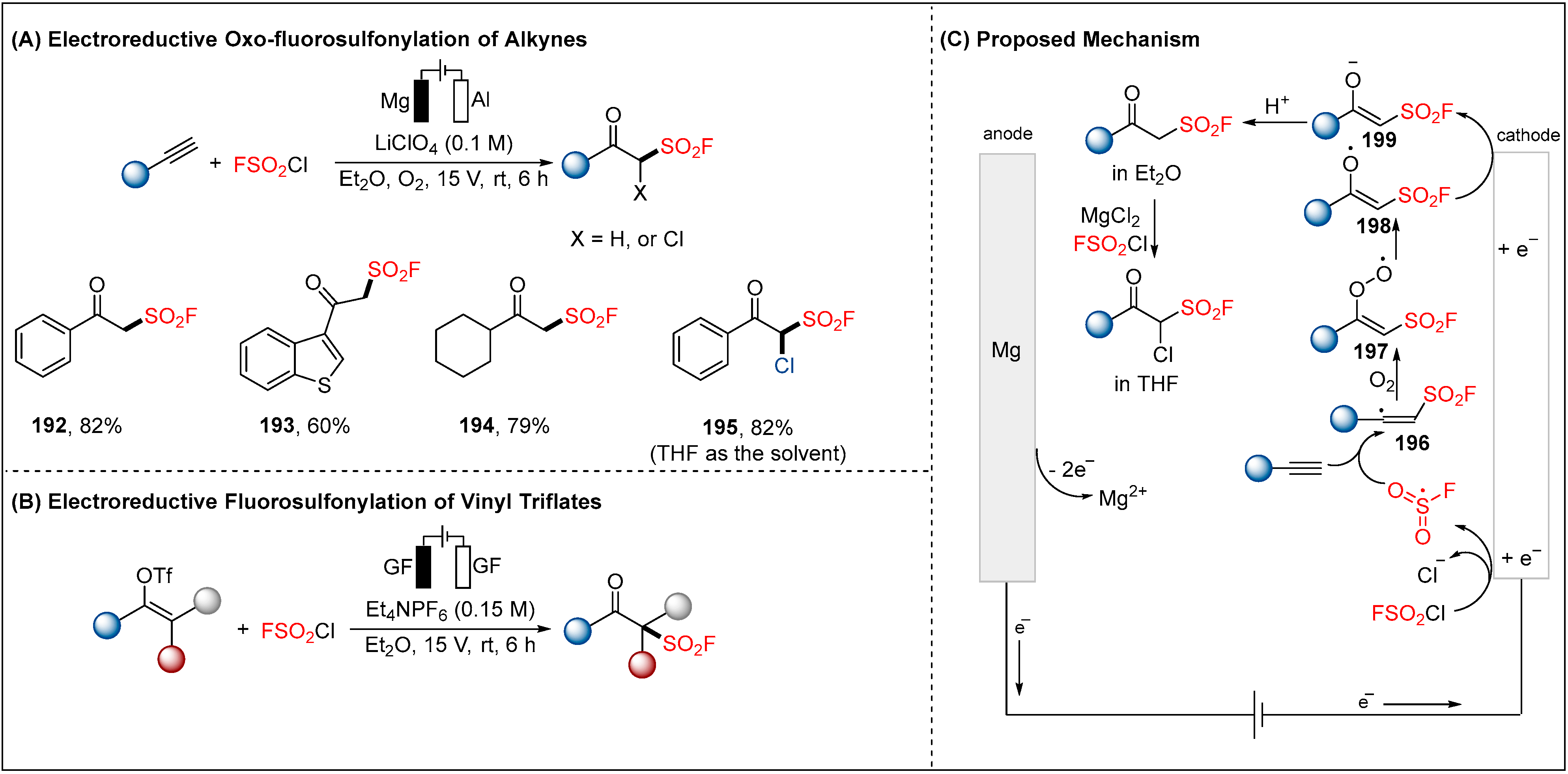
2.5. Electroreductive Reaction of Fluorosulfonyl Radical
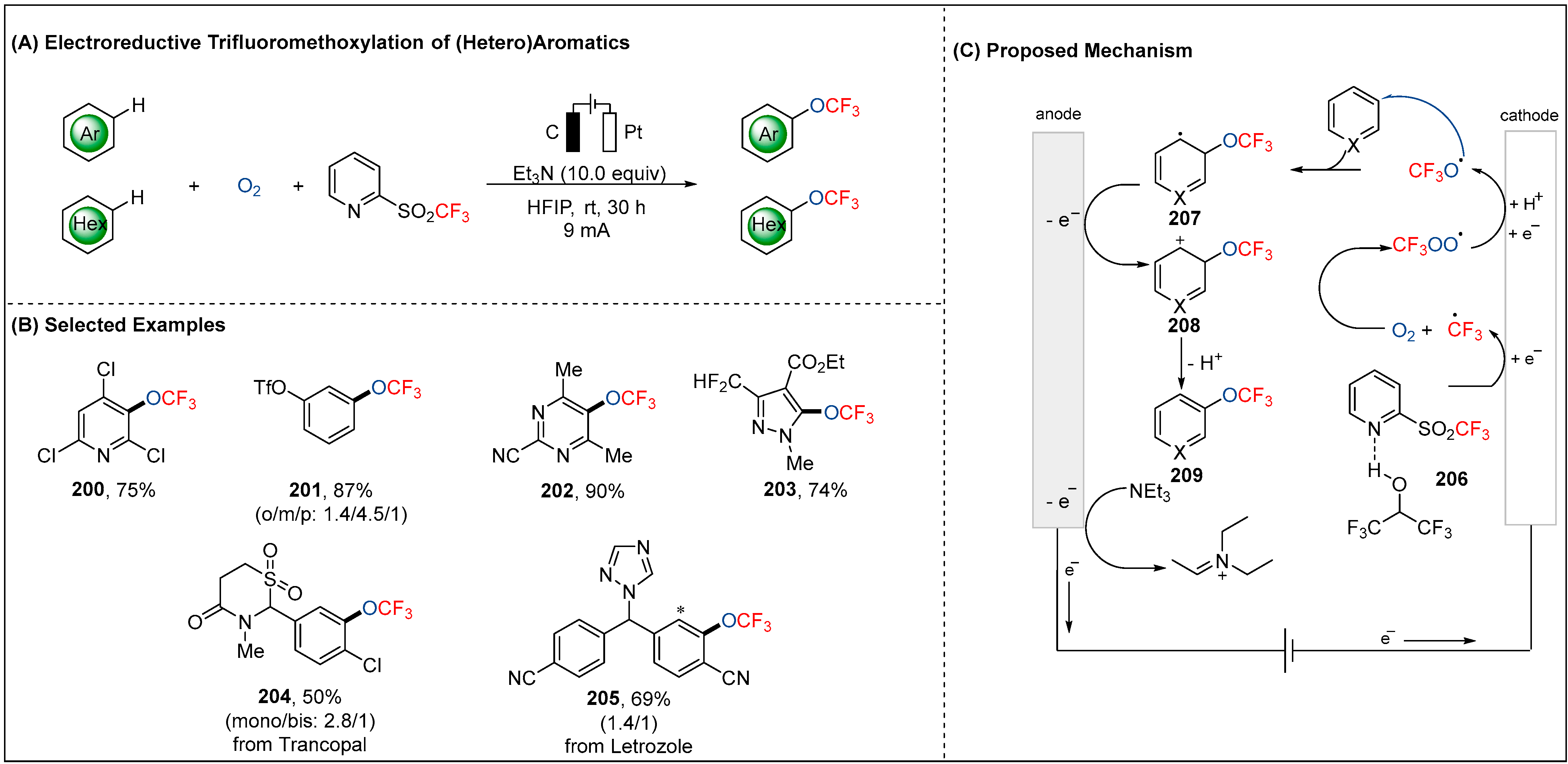
2.6. Electroreductive Reaction of OCF3 Radicals (•OCF3)
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jasperse, C.P.; Curran, D.P.; Fevig, T.L. Radical Reactions in Natural Product Synthesis. Chem. Rev. 1991, 91, 1237–1286. [Google Scholar] [CrossRef]
- Curran, D.P. The Design and Application of Free Radical Chain Reactions in Organic Synthesis. Part 1. Synthesis 1988, 417–439. [Google Scholar] [CrossRef]
- Curran, D.P. The Design and Application of Free Radical Chain Reactions in Organic Synthesis. Part 2. Synthesis 1988, 489–513. [Google Scholar] [CrossRef]
- Yan, M.; Lo, J.C.; Edwards, J.T.; Baran, P.S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Harwood, S.J.; Baran, P.S. Radical Retrosynthesis. Acc. Chem. Res. 2018, 51, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Parsaee, F.; Senarathna, M.C.; Kannangara, P.B.; Alexander, S.N.; Arche, P.D.E.; Welin, E.R. Radical Philicity and Its Role in Selective Organic Transformations. Nat. Rev. Chem. 2021, 5, 486–499. [Google Scholar] [CrossRef]
- Goddard, J.-P.; Ollivier, C.; Fensterbank, L. Photoredox Catalysis for the Generation of Carbon Centered Radicals. Acc. Chem. Res. 2016, 49, 1924–1936. [Google Scholar] [CrossRef]
- Chan, A.Y.; Perry, I.B.; Bissonnette, N.B.; Buksh, B.F.; Edwards, G.A.; Frye, L.I.; Garry, O.L.; Lavagnino, M.N.; Li, B.X.; Liang, Y.; et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Visible Light-Driven Radical-Mediated C–C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar Synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef]
- Gandeepan, P.; Finger, L.H.; Meyer, T.H.; Ackermann, L. 3d Metallaelectrocatalysis for Resource Economical Syntheses. Chem. Soc. Rev. 2020, 49, 4254–4272. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.H.; Choi, I.; Tian, C.; Ackermann, L. Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis? Chem 2020, 6, 2484–2496. [Google Scholar] [CrossRef]
- Zhu, C.; Ang, N.W.J.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Organic Electrochemistry: Molecular Syntheses with Potential. ACS Cent. Sci. 2021, 7, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S.R. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619. [Google Scholar] [CrossRef]
- Siu, J.C.; Fu, N.; Lin, S. Catalyzing Electrosynthesis: A Homogeneous Electrocatalytic Approach to Reaction Discovery. Acc. Chem. Res. 2020, 53, 547–560. [Google Scholar] [CrossRef]
- Liu, J.; Lu, L.; Wood, D.; Lin, S. New Redox Strategies in Organic Synthesis by Means of Electrochemistry and Photochemistry. ACS Cent. Sci. 2020, 6, 1317–1340. [Google Scholar] [CrossRef] [PubMed]
- He, J.-Y.; Qian, W.-F.; Wang, Y.-Z.; Yao, C.; Wang, N.; Liu, H.; Zhong, B.; Zhu, C.; Xu, H. Sustainable Electrochemical Dehydrogenative C(sp3)–H Mono/di-alkylations. Green Chem. 2022, 24, 2483–2491. [Google Scholar] [CrossRef]
- Pham, P.H.; Petersen, H.A.; Katsirubas, J.L.; Luca, O.R. Recent Synthetic Methods Involving Carbon Radicals Generated by Electrochemical Catalysis. Org. Biomol. Chem. 2022, 20, 5907–5932. [Google Scholar] [CrossRef]
- Chen, N.; Xu, H.-C. Electrochemical Generation of Nitrogen-Centered Radicals for Organic Synthesis. Green Synth. Catal. 2021, 2, 165–178. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.; Lei, A. Recent Advances in Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution involving Radicals. Chem. Soc. Rev. 2021, 50, 10058–10086. [Google Scholar] [CrossRef]
- Sauer, G.S.; Lin, S. An Electrocatalytic Approach to the Radical Difunctionalization of Alkenes. ACS Catal. 2018, 8, 5175–5187. [Google Scholar] [CrossRef]
- Xiong, P.; Xu, H.-C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 2019, 52, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, H. Zersetzung der Valeriansäure durch den elektrischen Strom. Justus Liebigs Ann. Chem. 1848, 64, 339–341. [Google Scholar]
- Baizer, M.M. Electrolytic Reductive Coupling: I. Acrylonitrile. J. Electrochem. 1964, 111, 215. [Google Scholar] [CrossRef]
- Ackermann, L. Metalla-Electrocatalyzed C–H Activation by Earth-Abundant 3d Metals and Beyond. Acc. Chem. Res. 2020, 53, 84–104. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Y.; Lei, A. Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution: A Green and Sustainable Way for Bond Formation. Chem 2018, 4, 27–45. [Google Scholar] [CrossRef]
- Kingston, C.; Palkowitz, M.D.; Takahira, Y.; Vantourout, J.C.; Peters, B.K.; Kawamata, Y.; Baran, P.S. A Survival Guide for the “Electro-curious”. Acc. Chem. Res. 2020, 53, 72–83. [Google Scholar] [CrossRef]
- Rosen, B.R.; Werner, E.W.; O’Brien, A.G.; Baran, P.S. Total Synthesis of Dixiamycin B by Electrochemical Oxidation. J. Am. Chem. Soc. 2014, 136, 5571–5574. [Google Scholar] [CrossRef]
- Shi, S.-H.; Liang, Y.; Jiao, N. Electrochemical Oxidation Induced Selective C–C Bond Cleavage. Chem. Rev. 2021, 121, 485–505. [Google Scholar] [CrossRef]
- Park, S.H.; Ju, M.; Ressler, A.J.; Shim, J.; Kim, H.; Lin, S. Reductive Electrosynthesis: A New Dawn. Aldrichimica Acta 2021, 54, 17–27. [Google Scholar]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.B.; Wright, D.L. The Application of Cathodic Reductions and Anodic Oxidations in the Synthesis of Complex Molecules. Chem. Soc. Rev. 2006, 35, 605–621. [Google Scholar] [CrossRef]
- Wirtanen, T.; Prenzel, T.; Tessonnier, J.-P.; Waldvogel, S.R. Cathodic Corrosion of Metal Electrodes—How to Prevent It in Electroorganic Synthesis. Chem. Rev. 2021, 121, 10241–10270. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Sun, Z.; Sun, G. Recent Progress in Cathodic Reduction-Enabled Organic Electrosynthesis: Trends, Challenges, and Opportunities. eScience 2022, 2, 243–277. [Google Scholar] [CrossRef]
- Zhang, S.-K.; Samanta, R.C.; Del Vecchio, A.; Ackermann, L. Evolution of High-Valent Nickela-Electrocatalyzed C−H Activation: From Cross(-Electrophile)-Couplings to Electrooxidative C−H Transformations. Chem. Eur. J. 2020, 26, 10936–10947. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, S.; Lei, A. Tuning Radical Reactivity for Selective Radical/radical Cross-Coupling. Sci. Bull. 2018, 63, 1006–1009. [Google Scholar] [CrossRef]
- Andrieux, C.P.; Gallardo, I.; Saveant, J.M. Outer-Sphere Electron-Transfer Reduction of Alkyl Halides. A Source of Alkyl Radicals or of Carbanions? Reduction of Alkyl Radicals. J. Am. Chem. Soc. 1989, 111, 1620–1626. [Google Scholar] [CrossRef]
- Benderskii, V.A.; Krivenko, A.G. The Electrochemistry of Short-Lived Intermediate Species. Russ. Chem. Rev. 1990, 59, 1–22. [Google Scholar] [CrossRef]
- Schäfer, H.J. Electrochemical Generation of Radicals. In Radicals in Organic Synthesis; Renaud, P., Sibi, M.P., Eds.; Wiley: New York, NY, USA, 2001; pp. 250–297. [Google Scholar]
- Roth, H.G.; Romero, N.A.; Nicewicz, D.A. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714–723. [Google Scholar]
- Zhang, W.; Lin, S. Electroreductive Carbofunctionalization of Alkenes with Alkyl Bromides via a Radical-Polar Crossover Mechanism. J. Am. Chem. Soc. 2020, 142, 20661–20670. [Google Scholar] [CrossRef]
- Wiberg, K.B. The Deuterium Isotope Effect. Chem. Rev. 1955, 55, 713–743. [Google Scholar] [CrossRef]
- Kushner, D.J.; Baker, A.; Dunstall, T.G. Pharmacological Uses and Perspectives of Heavy Water and Deuterated Compounds. Can. J. Physiol. Pharmacol. 1999, 77, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, M.; Sierra, M.A. Kinetic Isotope Effects in the Study of Organometallic Reaction Mechanisms. Chem. Rev. 2011, 111, 4857–4963. [Google Scholar] [CrossRef] [PubMed]
- Pirali, T.; Serafini, M.; Cargnin, S.; Genazzani, A.A. Applications of Deuterium in Medicinal Chemistry. J. Med. Chem. 2019, 62, 5276–5297. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Bourriquen, F.; Li, W.; Neumann, H.; Junge, K.; Beller, M. Recent Developments for the Deuterium and Tritium Labeling of Organic Molecules. Chem. Rev. 2022, 122, 6634–6718. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Rabeah, J.; Bourriquen, F.; Yang, D.; Kreyenschulte, C.; Rockstroh, N.; Lund, H.; Bartling, S.; Surkus, A.-E.; Junge, K.; et al. Scalable and Selective Deuteration of (Hetero)arenes. Nat. Chem. 2022, 14, 334–341. [Google Scholar] [CrossRef]
- Norcott, P.L. Current Electrochemical Approaches to Selective Deuteration. Chem. Comm. 2022, 58, 2944–2953. [Google Scholar] [CrossRef]
- Li, P.; Guo, C.; Wang, S.; Ma, D.; Feng, T.; Wang, Y.; Qiu, Y. Facile and General Electrochemical Deuteration of Unactivated Alkyl Halides. Nat. Commun. 2022, 13, 3774. [Google Scholar] [CrossRef]
- Lu, L.; Li, H.; Zheng, Y.; Bu, F.; Lei, A. Facile and Economical Electrochemical Dehalogenative Deuteration of (Hetero)Aryl Halides. CCS Chem. 2021, 3, 2669–2675. [Google Scholar] [CrossRef]
- Mitsudo, K.; Okada, T.; Shimohara, S.; Mandai, H.; Suga, S. Electro-reductive Halogen-Deuterium Exchange and Methylation of Aryl Halides in Acetonitrile. Electrochemistry 2013, 81, 362–364. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Lambert, T.H.; Lin, S. Reductive Electrophotocatalysis: Merging Electricity and Light To Achieve Extreme Reduction Potentials. J. Am. Chem. Soc. 2020, 142, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Liu, Q.; Li, F.; Bai, G.; Liu, G.; Li, M.; Nayal, O.S.; Fu, X.; Mo, F. Electrochemical Radical Borylation of Aryl Iodides. Chin. J. Chem. 2019, 37, 347–351. [Google Scholar] [CrossRef]
- Wang, B.; Peng, P.; Ma, W.; Liu, Z.; Huang, C.; Cao, Y.; Hu, P.; Qi, X.; Lu, Q. Electrochemical Borylation of Alkyl Halides: Fast, Scalable Access to Alkyl Boronic Esters. J. Am. Chem. Soc. 2021, 143, 12985–12991. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Fu, G.C. Transition Metal-Ctalyzed Alkyl-Alkyl Bond Formation: Another Dimension in Cross-Coupling Chemistry. Science 2017, 356, eaaf7230. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Gong, H. Nickel-Catalyzed Reductive Couplings. Top. Curr. Chem. 2016, 374, 43. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.L.; Jarvo, E.R. Stereospecific and Stereoconvergent Cross-Couplings Between Alkyl electrophiles. Nat. Rev. Chem. 2017, 1, 0065. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in Transition Metal (Pd, Ni, Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef]
- Everson, D.A.; Weix, D.J. Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem. 2014, 79, 4793–4798. [Google Scholar] [CrossRef]
- Weix, D.J. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res. 2015, 48, 1767–1775. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, L.; Zhang, W.; Wang, Y.; Ware, S.D.; Mondragon, J.; Rein, J.; Strotman, N.; Lehnherr, D.; See, K.A.; et al. Electrochemically Driven Cross-Electrophile Coupling of Alkyl Halides. Nature 2022, 604, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ma, W.; Zheng, X.; Xu, M.; Qi, X.; Lu, Q. Epoxide Electroreduction. J. Am. Chem. Soc. 2022, 144, 1389–1395. [Google Scholar] [CrossRef]
- Liao, L.-L.; Wang, Z.-H.; Cao, K.-G.; Sun, G.-Q.; Zhang, W.; Ran, C.-K.; Li, Y.; Chen, L.; Cao, G.-M.; Yu, D.-G. Electrochemical Ring-Opening Dicarboxylation of Strained Carbon–Carbon Single Bonds with CO2: Facile Synthesis of Diacids and Derivatization into Polyesters. J. Am. Chem. Soc. 2022, 144, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.; Petzold, M.; Brandt, F.; Jones, P.G.; Jacob, C.R.; Werz, D.B. Electrocatalytic Activation of Donor–Acceptor Cyclopropanes and Cyclobutanes: An Alternative C(sp3)−C(sp3) Cleavage Mode. Angew. Chem., Int. Ed. 2021, 60, 15928–15934. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Yan, X.; Zhang, K.; Liu, Z.; Zeng, L.; Chen, Y.; Zhang, H.; Lei, A. Electrochemical C−C Bond Cleavage of Cyclopropanes Towards the Synthesis of 1,3-Difunctionalized Molecules. Nat. Commun. 2021, 12, 3075. [Google Scholar] [CrossRef]
- Kolb, S.; Ahlburg, N.L.; Werz, D.B. Friedel–Crafts-Type Reactions with Electrochemically Generated Electrophiles from Donor–Acceptor Cyclopropanes and -Butanes. Org. Lett. 2021, 23, 5549–5553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, S.; Yang, G.; Wang, S.; Ma, D.; Qiu, Y. Electrocarboxylation of Aryl Epoxides with CO2 for the Facile and Selective Synthesis of beta-Hydroxy Acids. Angew. Chem. Int. Ed. 2022, 61, e202207746. [Google Scholar]
- Zhang, K.; Ren, B.-H.; Liu, X.-F.; Wang, L.-L.; Zhang, M.; Ren, W.-M.; Lu, X.-B.; Zhang, W.-Z. Direct and Selective Electrocarboxylation of Styrene Oxides with CO2 for Accessing β-Hydroxy Acids. Angew. Chem., Int. Ed. 2022, 61, e202207660. [Google Scholar]
- Hu, P.; Peters, B.K.; Malapit, C.A.; Vantourout, J.C.; Wang, P.; Li, J.; Mele, L.; Echeverria, P.-G.; Minteer, S.D.; Baran, P.S. Electroreductive Olefin–Ketone Coupling. J. Am. Chem. Soc. 2020, 142, 20979–20986. [Google Scholar] [CrossRef]
- Mori, A.; Miyakawa, Y.; Ohashi, E.; Haga, T.; Maegawa, T.; Sajiki, H. Pd/C-Catalyzed Chemoselective Hydrogenation in the Presence of Diphenylsulfide. Org. Lett. 2006, 8, 3279–3281. [Google Scholar] [CrossRef]
- Mendes-Burak, J.; Ghaffari, B.; Copéret, C. Selective Hydrogenation of α,β-Unsaturated Carbonyl Compounds on Silica-Supported Copper Nanoparticles. Chem. Comm. 2019, 55, 179–181. [Google Scholar] [CrossRef]
- Mori, A.; Fujita, A.; Nishihara, Y.; Hiyama, T. Copper(I) Salt Mediated 1,4-Reduction of α,β-Unsaturated Ketones Using Hydrosilanes. Chem. Comm. 1997, 2159–2160. [Google Scholar] [CrossRef]
- Sugiura, M.; Sato, N.; Kotani, S.; Nakajima, M. Lewis Base-Catalyzed Conjugate Reduction and Reductive Aldol Reaction of α,β-Unsaturated Ketones Using Trichlorosilane. Chem. Comm. 2008, 4309–4311. [Google Scholar] [CrossRef] [PubMed]
- Broggi, J.; Jurčík, V.; Songis, O.; Poater, A.; Cavallo, L.; Slawin, A.M.Z.; Cazin, C.S.J. The Isolation of [Pd{OC(O)H}(H)(NHC)(PR3)] (NHC = N-Heterocyclic Carbene) and Its Role in Alkene and Alkyne Reductions Using Formic Acid. J. Am. Chem. Soc. 2013, 135, 4588–4591. [Google Scholar]
- Baán, Z.; Finta, Z.; Keglevich, G.; Hermecz, I. Unexpected Chemoselectivity in the Rhodium-Catalyzed Transfer Hydrogenation of α,β-Unsaturated Ketones in Ionic Liquids. Green Chem. 2009, 11, 1937–1940. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Leng, X.; Zhu, H.; Liu, G.; Huang, Z. Transfer Hydrogenation of Alkenes Using Ethanol Catalyzed by a NCP Pincer Iridium Complex: Scope and Mechanism. J. Am. Chem. Soc. 2018, 140, 4417–4429. [Google Scholar] [PubMed]
- Li, J.; He, L.; Liu, X.; Cheng, X.; Li, G. Electrochemical Hydrogenation with Gaseous Ammonia. Angew. Chem. Int. Ed. 2019, 58, 1759–1763. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Qiu, J.; Cheng, X.; Li, G. Chemical-Reductant-Free Electrochemical Deuteration Reaction using Deuterium Oxide. Angew. Chem. Int. Ed. 2020, 59, 13962–13967. [Google Scholar] [CrossRef]
- Huang, B.; Li, Y.; Yang, C.; Xia, W. Electrochemical 1,4-Reduction of α,β-Unsaturated Ketones with Methanol and Ammonium Chloride as Hydrogen Sources. Chem. Comm. 2019, 55, 6731–6734. [Google Scholar] [CrossRef]
- Vittimberga, B.M.; Minisci, F.; Morrocchi, S. Photoinitiated Electron Transfer-Substitution Reaction of Diphenyl Ketyl with Protonated 4-Cyanopyridine. J. Am. Chem. Soc. 1975, 97, 4397–4398. [Google Scholar] [CrossRef]
- McNally, A.; Prier, C.K.; MacMillan, D.W.C. Discovery of an α-Amino C–H Arylation Reaction Using the Strategy of Accelerated Serendipity. Science 2011, 334, 1114–1117. [Google Scholar] [CrossRef]
- Pirnot, M.T.; Rankic, D.A.; Martin, D.B.C.; MacMillan, D.W.C. Photoredox Activation for the Direct β-Arylation of Ketones and Aldehydes. Science 2013, 339, 1593–1596. [Google Scholar] [CrossRef]
- Lehnherr, D.; Lam, Y.-h.; Nicastri, M.C.; Liu, J.; Newman, J.A.; Regalado, E.L.; DiRocco, D.A.; Rovis, T. Electrochemical Synthesis of Hindered Primary and Secondary Amines via Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2020, 142, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, C.; Gao, H.; Wang, L.; Guo, L.; Xia, W. Reductive Arylation of Aliphatic and Aromatic Aldehydes with Cyanoarenes by Electrolysis for the Synthesis of Alcohols. Org. Lett. 2021, 23, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, J.; Nie, F.; Zhao, X.; Jiang, Z. Metal-Free Hydropyridylation of Thioester-Activated Alkenes via Electroreductive Radical Coupling. J. Org. Chem. 2021, 86, 16204–16212. [Google Scholar] [CrossRef]
- Ding, W.; Li, M.; Fan, J.; Cheng, X. Palladium-Catalyzed Asymmetric Allylic 4-Pyridinylation via Electroreductive Substitution Reaction. Nat. Commun. 2022, 13, 5642. [Google Scholar] [CrossRef]
- Ke, J.; Wang, H.; Zhou, L.; Mou, C.; Zhang, J.; Pan, L.; Chi, Y.R. Hydrodehalogenation of Aryl Halides through Direct Electrolysis. Chem. Eur. J. 2019, 25, 6911–6914. [Google Scholar] [CrossRef]
- Liu, C.; Han, S.; Li, M.; Chong, X.; Zhang, B. Electrocatalytic Deuteration of Halides with D2O as the Deuterium Source over a Copper Nanowire Arrays Cathode. Angew. Chem. Int. Ed. 2020, 59, 18527–18531. [Google Scholar] [CrossRef]
- Huang, B.; Guo, L.; Xia, W. A Facile and Versatile Electro-Reductive System for Hydrodefunctionalization Under Ambient Conditions. Green Chem. 2021, 23, 2095–2103. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Z.; Wang, Z.; Xu, C.; Huang, L.; Wang, S.; Zhang, H.; Lei, A. Electrochemical Arylation of Electron-Deficient Arenes through Reductive Activation. Angew. Chem. Int. Ed. 2019, 58, 15747–15751. [Google Scholar] [CrossRef]
- Francke, R.; Little, R.D. Redox Catalysis in Organic Electrosynthesis: Basic Principles and Recent Developments. Chem. Soc. Rev. 2014, 43, 2492–2521. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Guan, X.; Liu, J.; Luo, J.; Xia, Y. Redox Mediators as Charge Agents for Changing Electrochemical Reactions. Chem. Soc. Rev. 2020, 49, 7454–7478. [Google Scholar] [CrossRef]
- Sun, G.; Ren, S.; Zhu, X.; Huang, M.; Wan, Y. Direct Arylation of Pyrroles via Indirect Electroreductive C–H Functionalization Using Perylene Bisimide as an Electron-Transfer Mediator. Org. Lett. 2016, 18, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Cowper, N.G.W.; Chernowsky, C.P.; Williams, O.P.; Wickens, Z.K. Potent Reductants via Electron-Primed Photoredox Catalysis: Unlocking Aryl Chlorides for Radical Coupling. J. Am. Chem. Soc. 2020, 142, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Folgueiras-Amador, A.A.; Teuten, A.E.; Salam-Perez, M.; Pearce, J.E.; Denuault, G.; Pletcher, D.; Parsons, P.J.; Harrowven, D.C.; Brown, R.C.D. Cathodic Radical Cyclisation of Aryl Halides Using a Strongly-Reducing Catalytic Mediator in Flow. Angew. Chem. Int. Ed. 2022, 61, e202203694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Pan, D.; Wang, S.; Jia, K.; Ma, D.; Yang, G.; Xue, X.S.; Qiu, Y. Metal-Free Electrochemical Carboxylation of Organic Halides in the Presence of Catalytic Amounts of an Organomediator. Angew. Chem. Int. Ed. 2022, 134, e202210201. [Google Scholar]
- Xu, J.; Lu, F.L.; Sun, L.H.; Huang, M.N.; Jiang, J.W.; Wang, K.; Ouyang, D.D.; Lu, L.J.; Lei, A. Electrochemical Reductive Cross-coupling of Acyl Chlorides and Sulfinic Acids Towards the Synthesis of Thioesters. Green Chem. 2022, 24, 7350–7354. [Google Scholar] [CrossRef]
- Bogonda, G.; Patil, D.V.; Kim, H.Y.; Oh, K. Visible-Light-Promoted Thiyl Radical Generation from Sodium Sulfinates: A Radical–Radical Coupling to Thioesters. Org. Lett. 2019, 21, 3774–3779. [Google Scholar] [CrossRef]
- Lu, L.; Siu, J.C.; Lai, Y.; Lin, S. An Electroreductive Approach to Radical Silylation via the Activation of Strong Si–Cl Bond. J. Am. Chem. Soc. 2020, 142, 21272–21278. [Google Scholar] [CrossRef]
- Xu, R.; Xu, T.; Yang, M.; Cao, T.; Liao, S. A Rapid Access to Aliphatic Sulfonyl Fluorides. Nat. Commun. 2019, 10, 3752. [Google Scholar] [CrossRef]
- Nie, X.; Xu, T.; Song, J.; Devaraj, A.; Zhang, B.; Chen, Y.; Liao, S. Radical Fluorosulfonylation: Accessing Alkenyl Sulfonyl Fluorides from Alkenes. Angew. Chem. Int. Ed. 2021, 60, 3956–3960. [Google Scholar] [CrossRef]
- Chen, D.; Nie, X.; Feng, Q.; Zhang, Y.; Wang, Y.; Wang, Q.; Huang, L.; Huang, S.; Liao, S. Electrochemical Oxo-Fluorosulfonylation of Alkynes under Air: Facile Access to β-Keto Sulfonyl Fluorides. Angew. Chem. Int. Ed. 2021, 60, 27271–27276. [Google Scholar] [CrossRef]
- Feng, Q.; Fu, Y.; Zheng, Y.; Liao, S.; Huang, S. Electrochemical Synthesis of β-Keto Sulfonyl Fluorides via Radical Fluorosulfonylation of Vinyl Triflates. Org. Lett. 2022, 24, 3702–3706. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Wang, P.; Li, Y.; Lei, A. Electrochemical Oxidation with Lewis-Acid Catalysis Leads to Trifluoromethylative Difunctionalization of Alkenes Using CF3SO2Na. Org. Lett. 2018, 20, 7396–7399. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Electrochemical Trifluoromethoxylation of (Hetero)aromatics with a Trifluoromethyl Source and Oxygen. Angew. Chem. Int. Ed. 2022, 61, e202114048. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.-R.; Wang, Z.-H.; Ma, C.; Herbert, S.; Schirok, H.; Mei, T.-S. Paired Electrolysis-Enabled Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Between α-Chloroesters and Aryl Bromides. Nat. Commun. 2022, 13, 7318. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, W.; Wang, X.; Li, L.; Zhang, Y.; Li, C. Electrochemically Enabled, Nickel-Catalyzed Dehydroxylative Cross-Coupling of Alcohols with Aryl Halides. J. Am. Chem. Soc. 2021, 143, 3536–3543. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Luo, S. Tailoring Radicals by Asymmetric Electrochemical Catalysis. Org. Chem. Front. 2020, 7, 2997–3000. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; He, J.; Qian, W.; Qiu, M.; Xu, H.; Duan, W.; Ouyang, Y.; Wang, Y.; Zhu, C. Electroreductively Induced Radicals for Organic Synthesis. Molecules 2023, 28, 857. https://doi.org/10.3390/molecules28020857
Xiang H, He J, Qian W, Qiu M, Xu H, Duan W, Ouyang Y, Wang Y, Zhu C. Electroreductively Induced Radicals for Organic Synthesis. Molecules. 2023; 28(2):857. https://doi.org/10.3390/molecules28020857
Chicago/Turabian StyleXiang, Huaming, Jinyu He, Weifeng Qian, Mingqiang Qiu, Hao Xu, Wenxi Duan, Yanyan Ouyang, Yanzhao Wang, and Cuiju Zhu. 2023. "Electroreductively Induced Radicals for Organic Synthesis" Molecules 28, no. 2: 857. https://doi.org/10.3390/molecules28020857
APA StyleXiang, H., He, J., Qian, W., Qiu, M., Xu, H., Duan, W., Ouyang, Y., Wang, Y., & Zhu, C. (2023). Electroreductively Induced Radicals for Organic Synthesis. Molecules, 28(2), 857. https://doi.org/10.3390/molecules28020857




