Cytotoxic Indole Diterpenoids from a Sphagneticola trilobata-Derived Fungus Aspergillus sp. PQJ-1
Abstract
:1. Introduction
2. Results and Discussion
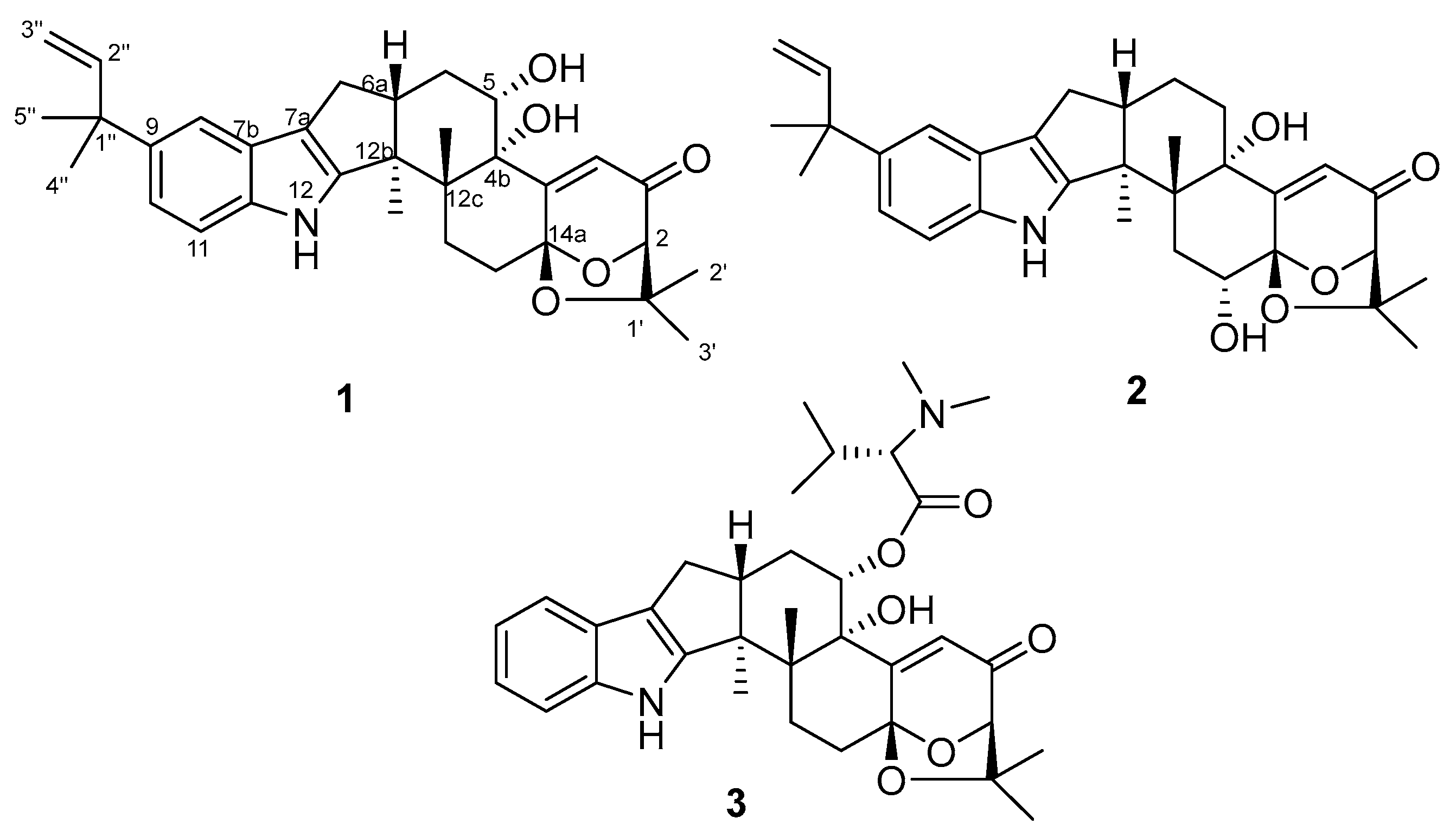
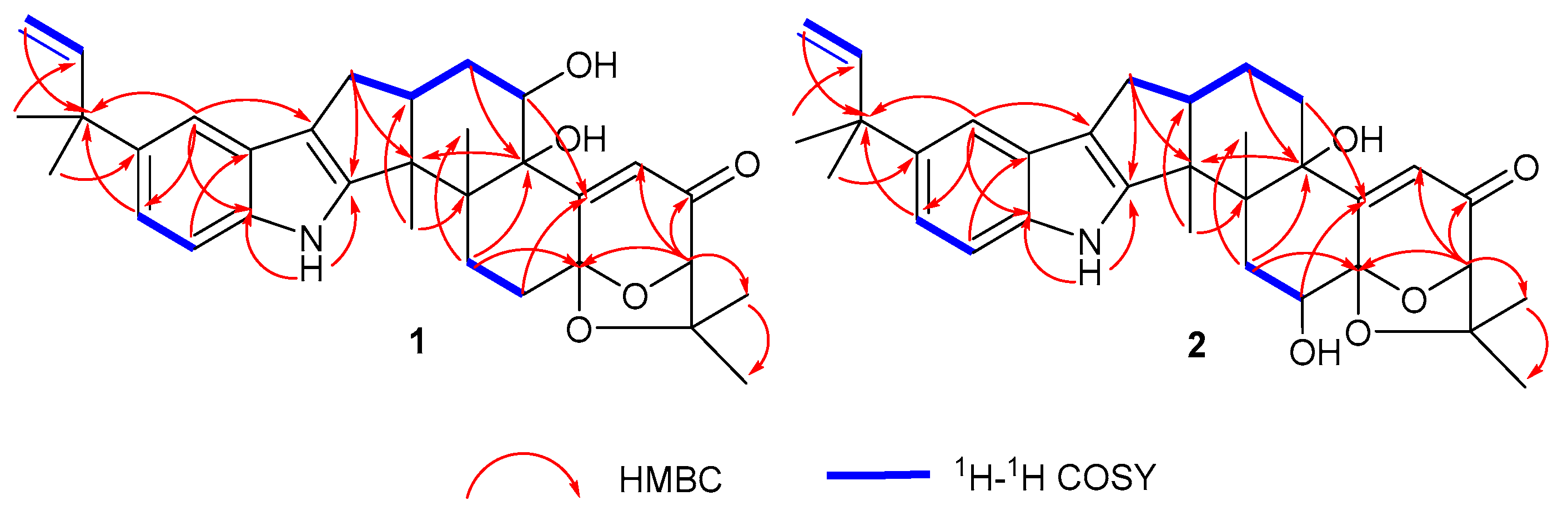
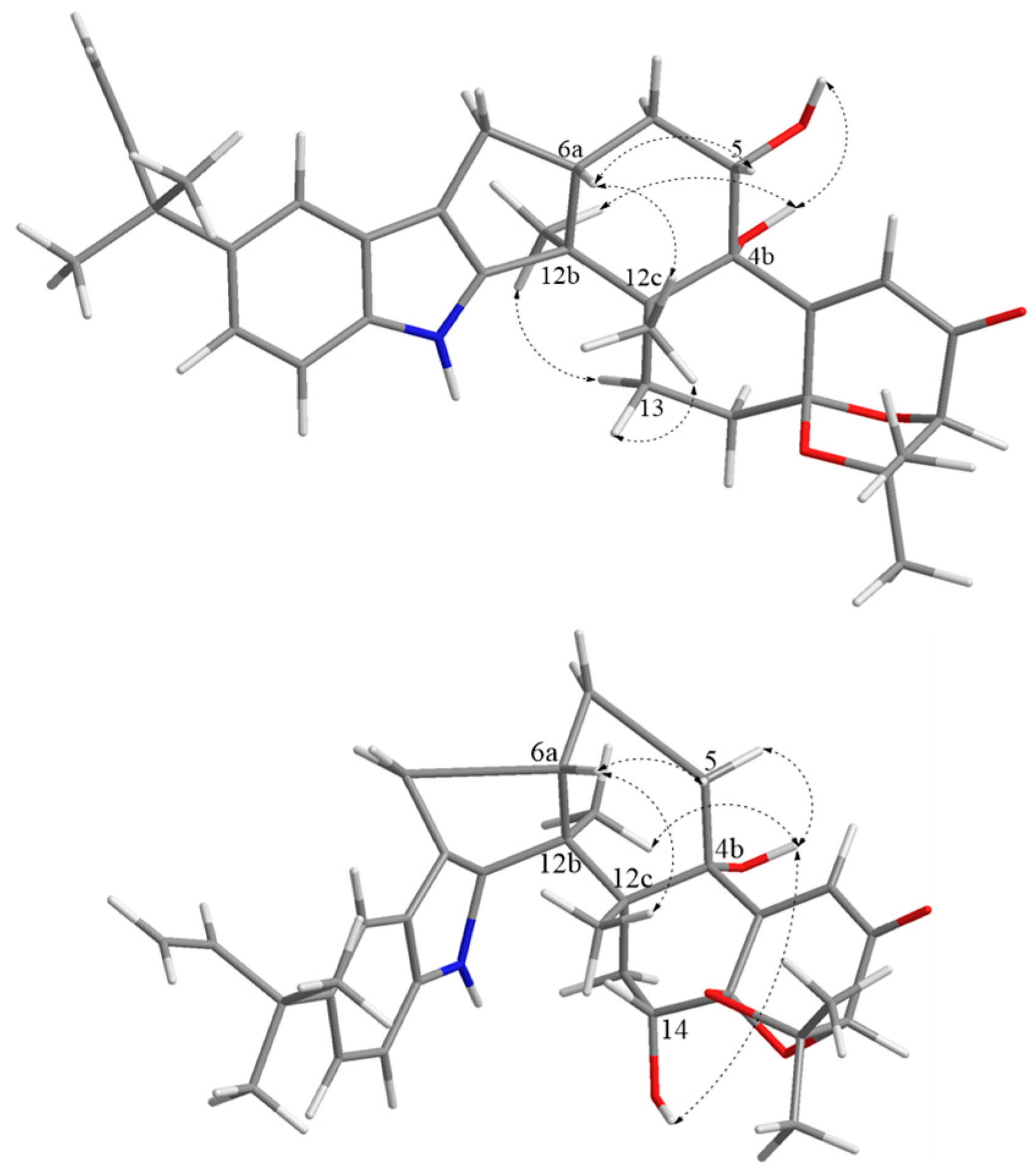
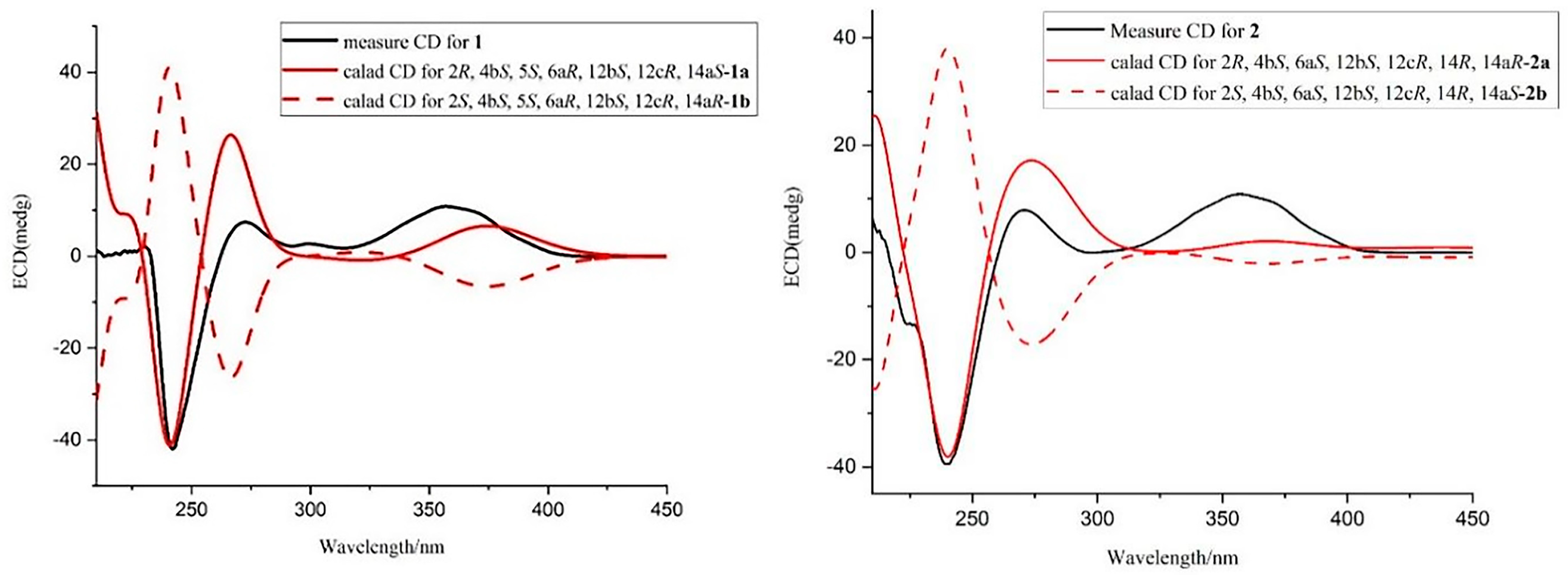
2.1. Structural Identification of Compounds 1–2
2.2. Cytotoxic Activity of Compounds 1–3
2.3. Compound 1 Could Cause Apoptosis of Hela Cell
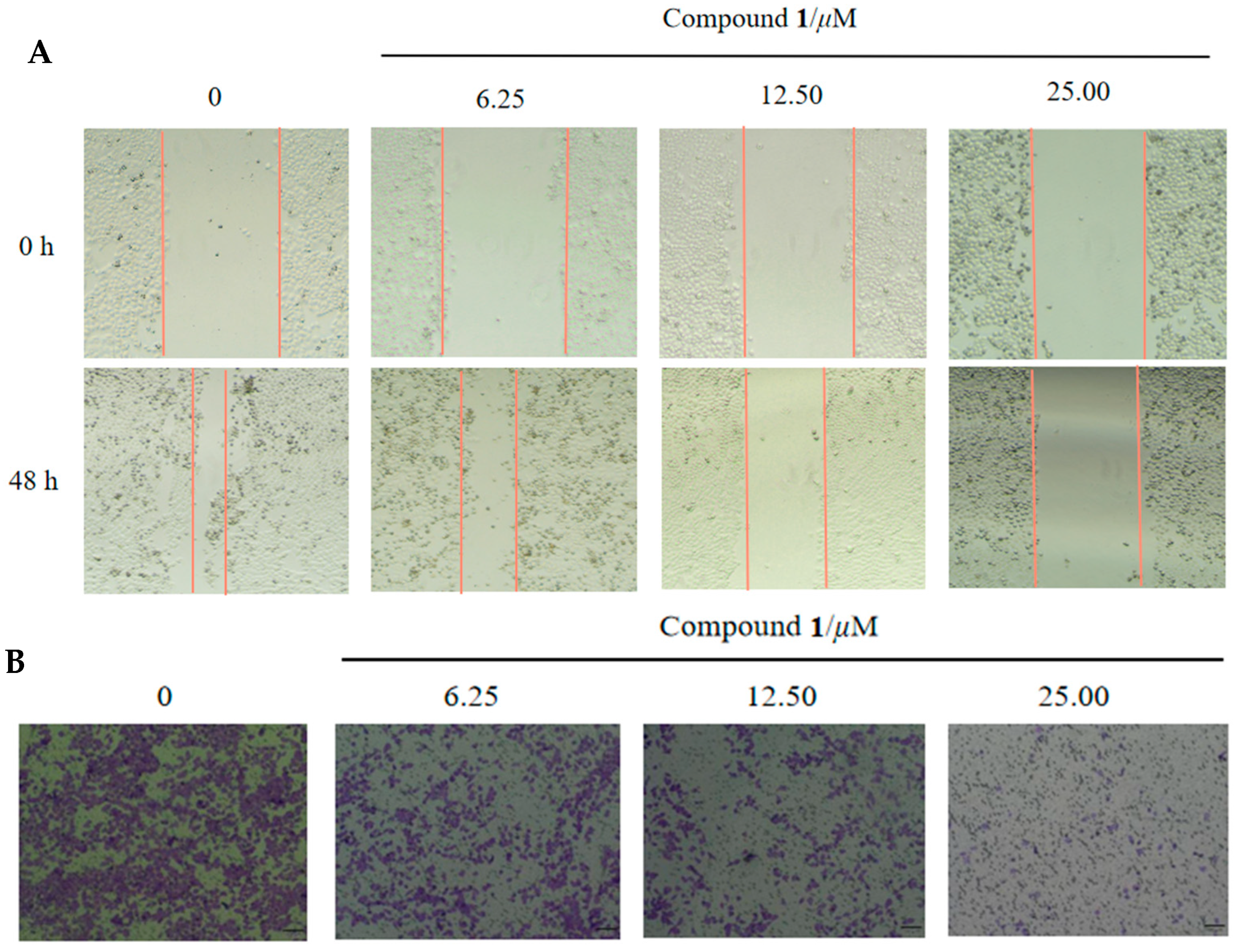
2.4. Wound Healing and Transwell Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Materials
3.3. Fermentation, Extraction, and Isolation
3.4. Cytotoxicity Assays
3.4.1. Cell Line and Cell Culture
3.4.2. Wound-Healing Migration Assay
3.4.3. Transwell Migration Assay
3.4.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
3.4.5. Mitochondrial Membrane Assay
3.4.6. Observation of Morphological Changes in Hela Cells by Hoechst 33258 Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, W.; Gavia, D.J.; Tang, Y. Biosynthesis of fungal indole alkaloids. Nat. Prod. Rep. 2014, 31, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, J.P.; Liu, S.F.; Yin, C.Y.; Tang, D.Y.; Li, Y.H.; Zhang, L.X. 7-Methoxy-13-dehydroxypaxilline: New indole diterpenoid from an endophytic fungus Penicillium sp. Nb 19. Nat. Prod. Res. 2022, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Corsello, M.A.; Kim, J.; Garg, N.K. Indole diterpenoid natural products as the inspiration for new synthetic methods and strategies. Chem. Sci. 2017, 8, 5836–5844. [Google Scholar] [CrossRef]
- Zheng, C.J.; Bai, M.; Zhou, X.M.; Huang, G.L.; Shao, T.M.; Luo, Y.P.; Niu, Z.G.; Niu, Y.Y.; Chen, G.Y.; Han, C.R. Penicilindoles A-C, cytotoxic indole diterpenes from the mangrove-derived fungus Eupenicillium sp. HJ002. J. Nat. Prod. 2018, 81, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, C.; Hou, X.; Che, Q.; Zhang, G.; Gu, Q.; Liu, C.; Zhu, T.; Li, D. Ascandinines A-D, indole diterpenoids, from the sponge-derived fungus Aspergillus candidus HDN15-152. J. Org. Chem. 2021, 86, 2431–2436. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Hai, P.; Zhang, S.B.; Xiao, J.F.; Gao, Y.; Ma, B.J.; Fu, H.Y.; Chen, Y.M.; Yang, X.L. Prenylated indole diterpene alkaloids from a mine-soil-derived Tolypocladium sp. J. Nat. Prod. 2019, 82, 221–231. [Google Scholar] [CrossRef]
- Li, C.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Thiersinines A and B: Novel antiinsectan indole diterpenoids from a new fungicolous Penicillium species (NRRL 28147). Org. Lett. 2002, 4, 3095–3098. [Google Scholar] [CrossRef]
- Gatenby, W.A.; Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O. Terpendole M, a novel indole-diterpenoid isolated from Lolium perenne infected with the endophytic fungus Neotyphodium lolii. J. Agric. Food Chem. 1999, 47, 1092–1097. [Google Scholar] [CrossRef]
- Li, C.; Gloer, J.B.; Wicklow, D.T. Thiersindoles A-C: New indole diterpenoids from Penicillium thiersii. J. Nat. Prod. 2003, 66, 1232–1235. [Google Scholar] [CrossRef]
- Laakso, J.A.; Gloer, J.B. Radarins A–D: New antiinsectan and cytotoxic indole diterpenoids from the sclerotia of Aspergillus sulphureus. J. Org. Chem. 1992, 57, 138–141. [Google Scholar] [CrossRef]
- Calvo, A.M.; Cary, J.W. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 2015, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Dowd, P.F.; Cole, R.J.; Vesonder, R.F. Toxicity of selected tremorgenic mycotoxins and related compounds to Spodoptera frugiperda and Heliothis zea. J. Antibiot. 1988, 41, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Huo, X.K.; Cheng, Z.B.; Sun, C.P.; Zhao, J.C.; Kang, X.H.; Zhang, T.Y.; Chen, Z.J.; Yang, T.M.; Wu, Y.Y.; et al. An indole diterpenoid isolated from the fungus Drechmeria sp. and its antimicrobial activity. Nat. Prod. Res. 2019, 33, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Guo, Z.G.; Wang, L.; Guo, Q.F.; Cao, F. Anti-IAV indole-diterpenoids from the marine-derived fungus Penicillium citrinum. Nat. Prod. Res. 2023, 37, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.R.; Welch, K.D.; Lee, S.T.; Cook, D.; Riet-Correa, F. Tremorgenic indole diterpenes from ipomoea asarifolia and ipomoea muelleri and the identification of 6,7-dehydro-11-hydroxy-12,13-epoxyterpendole A. J. Nat. Prod. 2018, 81, 1682–1686. [Google Scholar] [CrossRef]
- Uhlig, S.; Rangel-Huerta, O.D.; Divon, H.H.; Rolén, E.; Pauchon, K.; Sumarah, M.W.; Vrålstad, T.; Renaud, J.B. Unraveling the ergot alkaloid and indole diterpenoid metabolome in the claviceps purpurea species complex using LC-HRMS/MS diagnostic fragmentationfiltering. J. Agric. Food. Chem. 2021, 69, 7137–7148. [Google Scholar] [CrossRef]
- Kong, F.D.; Fan, P.; Zhou, L.M.; Ma, Q.Y.; Xie, Q.Y.; Zheng, H.Z.; Zheng, Z.H.; Zhang, R.S.; Yuan, J.Z.; Dai, H.F.; et al. Penerpenes A-D, four indole terpenoids with potent protein tyrosine phosphatase inhibitory activity from the marine-derived fungus Penicillium sp. KFD28. Org. Lett. 2019, 21, 4864–4867. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Sun, Z.; Ma, G.; Chen, D.; Wu, H.; Yang, J.; Li, Y.; Xu, X. The biosynthesis related enzyme, structure diversity and bioactivity abundance of indole-diterpenes: A review. Molecules 2022, 27, 6870. [Google Scholar] [CrossRef]
- Haiyosang, B.; Kanokmedhakul, K.; Yodsing, N.; Boonlue, S.; Yang, J.X.; Wang, Y.A.; Andersen, R.J.; Yahuafai, J.; Kanokmedhakul, S. Three new indole diterpenoids from Aspergillus aculeatus KKU-CT2. Nat. Prod. Res. 2022, 36, 4973–4981. [Google Scholar] [CrossRef]
- Li, W.X.; Zhou, X.Q.; Ji, S.D.; Wang, Y.N.; Sun, Z.F.; Huang, Z.Y.; Zhou, Z.M.; Hui, Y.; Chen, W.H. Two new lactam derivatives from a Sphagneticola trilobata derived fungus Penicillium rubens PQJ-2. Nat. Prod. Res. 2022, 35, 1–7. [Google Scholar] [CrossRef]
- Yang, J.N.; Hui, Y.; Chen, Z.X.; Chen, G.Y.; Song, X.P.; Sun, Z.F.; Han, C.R.; Chen, W.H. Four undescribed pyranones from the Scutellaria formosana-derived endophytic fungi Ascomycota sp. FAE17. Molecules 2023, 28, 5388. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Y.; Liu, P. Indole-diterpenoids with anti-H1N1 activity from the aciduric fungus Penicillium camemberti OUCMDZ-1492. J. Nat. Prod. 2013, 76, 1328–1336. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, L.; Li, Y.Q. Oxalierpenes A and B, unusual indole diterpenoid derivatives with antiviral activity from a marine-D. J. Nat. Prod. 2022, 85, 1880–1885. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, Y.; Guo, L. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs 2014, 12, 3970–3981. [Google Scholar] [CrossRef] [PubMed]
- Karoń, K.; Łapkowski, M.; Dobrowolski, J.C. ECD spectroelectrochemistry: A review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119349. [Google Scholar] [CrossRef]
- Li, W.S.; Lei, X.P.; Yan, X.T.; Qin, Y.Y.; Chen, G.Y.; Li, S.; Jiang, Z.P. Hainanxylogranolides A-F: New limonoids isolated from the seeds of Hainan mangrove plant Xylocarpus granatum. Fitoterapia 2023, 165, 105407. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Ma, Y.H.; Ma, W.T.; Zhou, Z.K.; Huang, X.; Jiang, X.R.; Du, K.J.; Sun, M.Z.; Zhang, H.; Fang, H.; Zhao, Y.; et al. Synthesis of 8-fluoroneocryptolepine and evaluation for cytotoxic activity against AGS cancer cells. J. Nat. Prod. 2022, 85, 963–971. [Google Scholar] [CrossRef]
- Meng, Y.; Qiu, L.; Zeng, X.; Hu, X.; Zhang, Y.; Wan, X.; Mao, X.; Wu, J.; Xu, Y.; Xiong, Q.; et al. Targeting CRL4 suppresses chemoresistant ovarian cancer growth by inducing mitophagy. Signal. Transduct. Target. Ther. 2022, 7, 388. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Sun, N.; Zhang, X.; Shah, M.; Zhang, G.; Che, Q.; Zhu, T.; Li, J.; Li, D. Cytotoxic nitrobenzoyl sesquiterpenoids from an sntarctica sponge-derived Aspergillus insulicola. J. Nat. Prod. 2022, 85, 987–996. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Buranaamnuay, K. The MTT assay application to measure the viability of spermatozoa: A variety of the assay protocols. Open Vet. J. 2021, 11, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 87.1 | 4.39, s | 87.1 | 4.39, s |
| 3 | 197.2 | 197.3 | ||
| 4 | 119.8 | 6.24, br s | 119.8 | 6.24, s |
| 4a | 167.7 | 167.8 | ||
| 4b | 78.8 | 77.6 | ||
| 5 | 69.8 | 4.10, m | 28.2 | 1.83, m 2.75, m |
| 6 | 31.3 | 1.84, m | 26.8 | 1.92, m 2.46, m |
| 6a | 44.8 | 2.67, m | 45.4 | 2.73, m |
| 7 | 26.6 | 1.95, m 2.49, m | 26.6 | 2.32, m 2.56, m |
| 7a | 114.6 | 115.3 | ||
| 7b | 122.9 | 124.3 | ||
| 8 | 115.6 | 6.85, d (1.2) | 114.4 | 7.18, d (1.8) |
| 9 | 138.8 | 138.4 | ||
| 10 | 118.9 | 6.87, d (7.2) | 118.2 | 6.94, dd (1.8, 9.0) |
| 11 | 110.8 | 7.18, d (7.8) | 111.4 | 7.19, d (8.6) |
| 11a | 140.8 | 138.2 | ||
| 12 | 10.64, s | 10.48, s | ||
| 12a | 151.4 | 152.4 | ||
| 12b | 49.9 | 50.6 | ||
| 12c | 39.6 | 39.6 | ||
| 13 | 33.1 | 2.44, m 2.72, m | 31.5 | 1.86, m |
| 14 | 28.2 | 1.84, m 2.74, m | 70.0 | 4.10, t (6.4) |
| 14a | 104.4 | 104.4 | ||
| 12b-Me | 16.0 | 1.27, s | 16.2 | 1.28, s |
| 12c-Me | 22.4 | 1.13, s | 22.9 | 1.08, s |
| 1′ | 77.6 | 78.7 | ||
| 2′ | 28.4 | 1.37, s | 28.4 | 1.36, s |
| 3′ | 22.9 | 1.08, s | 22.4 | 1.13, s |
| 1″ | 41.0 | 40.5 | ||
| 2″ | 149.2 | 6.19, t (7.2) | 149.0 | 6.03, q (6.4) |
| 3″ | 111.4 | 4.81, dd (1.2, 10.8) 4.96, dd (1.2, 17.4) | 109.8 | 5.00, t (10.4) |
| 4″ | 29.2 | 1.43, s | 28.6 | 1.37, s |
| 5″ | 29.4 | 1.44, s | 28.6 | 1.37, s |
| 4b-OH | 4.52, s | 4.54, s | ||
| 5-OH | 5.24, s | |||
| 14-OH | 5.29, s | |||
| IC50 (µM) | ||||
|---|---|---|---|---|
| Compounds | A549 | Hela | Hep G2 | MCF-7 |
| 1 | >50 | 12.54 | 15.06 | 26.56 |
| 2 | >50 | 15.61 | 20.03 | 29.47 |
| 3 | >50 | >50 | >50 | >50 |
| Adriamycin | 5.52 | 1.50 | 5.73 | 5.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Yi, G.; Lin, K.; Chen, G.; Hui, Y.; Chen, W. Cytotoxic Indole Diterpenoids from a Sphagneticola trilobata-Derived Fungus Aspergillus sp. PQJ-1. Molecules 2023, 28, 7003. https://doi.org/10.3390/molecules28207003
Li W, Yi G, Lin K, Chen G, Hui Y, Chen W. Cytotoxic Indole Diterpenoids from a Sphagneticola trilobata-Derived Fungus Aspergillus sp. PQJ-1. Molecules. 2023; 28(20):7003. https://doi.org/10.3390/molecules28207003
Chicago/Turabian StyleLi, Wenxing, Guohui Yi, Kaiwen Lin, Guangying Chen, Yang Hui, and Wenhao Chen. 2023. "Cytotoxic Indole Diterpenoids from a Sphagneticola trilobata-Derived Fungus Aspergillus sp. PQJ-1" Molecules 28, no. 20: 7003. https://doi.org/10.3390/molecules28207003
APA StyleLi, W., Yi, G., Lin, K., Chen, G., Hui, Y., & Chen, W. (2023). Cytotoxic Indole Diterpenoids from a Sphagneticola trilobata-Derived Fungus Aspergillus sp. PQJ-1. Molecules, 28(20), 7003. https://doi.org/10.3390/molecules28207003






