Dark-Chocolate-Coated BRS Clara Raisins: Phenolic Composition and Sensory Attributes
Abstract
:1. Introduction
2. Results and Discussion
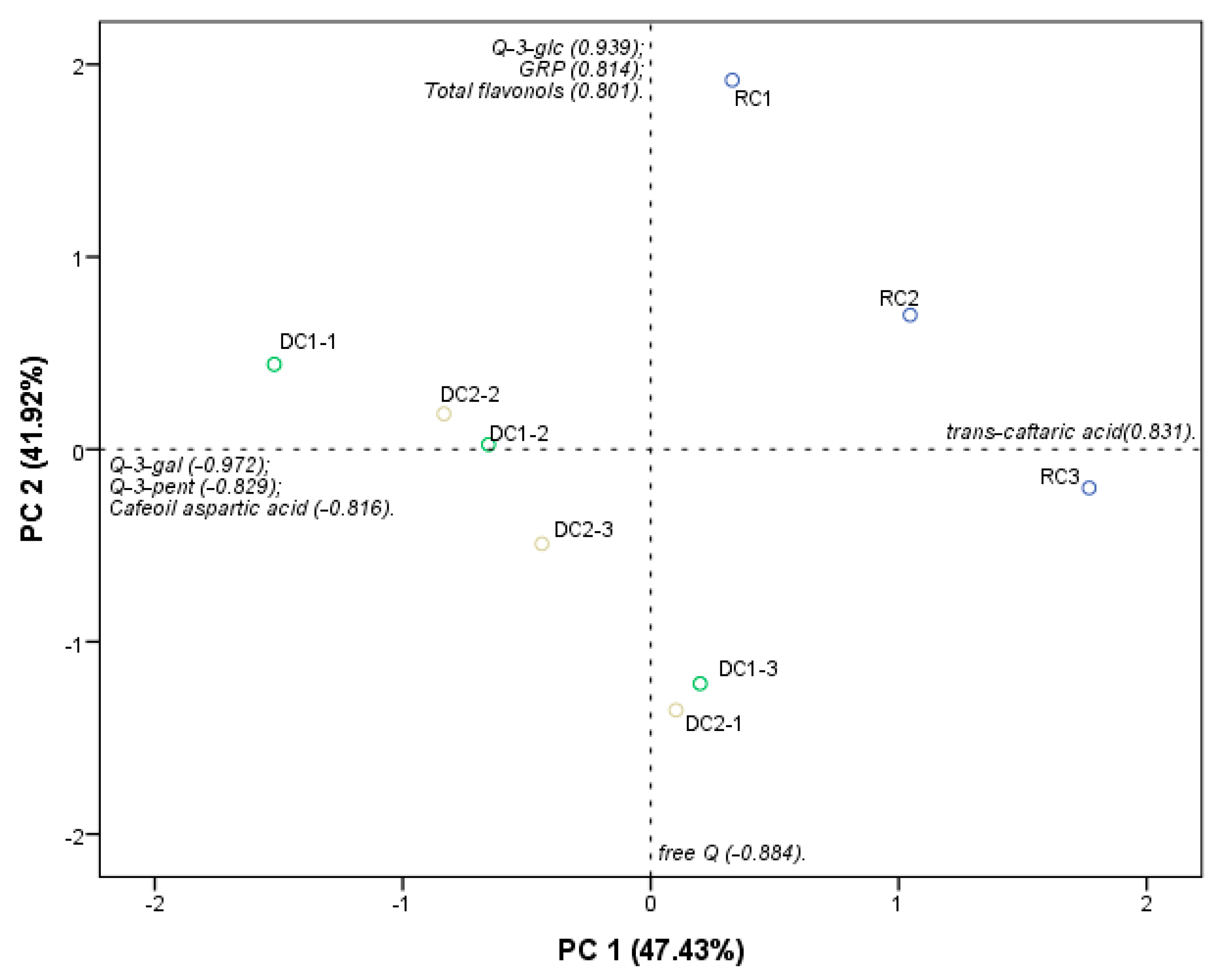
2.1. Flavonols
2.2. HCADs
2.3. Stilbenes
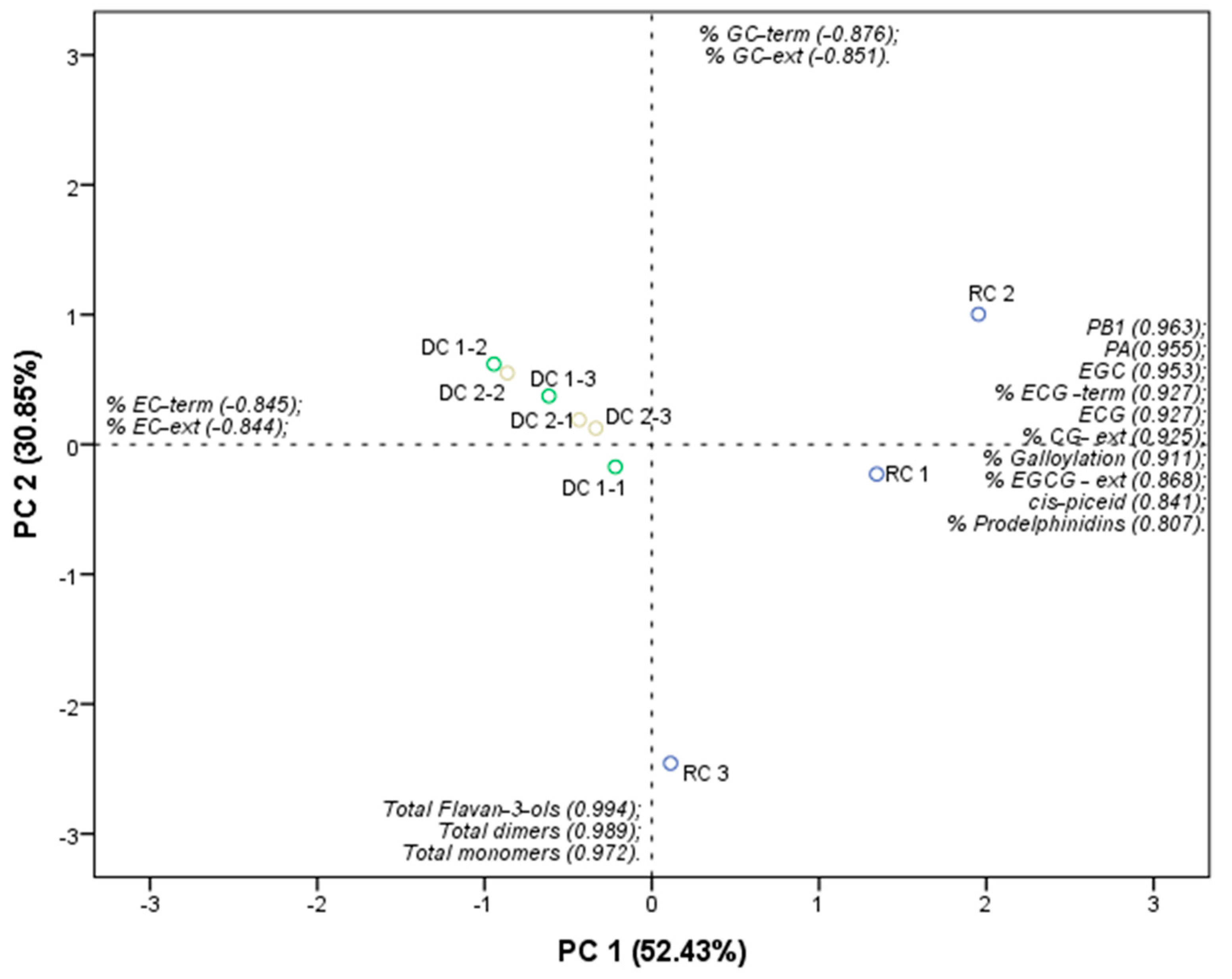
2.4. Flavan-3-ol Monomers, Dimers, and PA
2.5. Sensory Analysis
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Ingredients for the Dark-Chocolate-Panned Confections
4.3. Production of Raisin Centers
4.4. Chocolate Dragée Production
4.5. Identification and Quantification of PCs of RC and DC Using HPLC-DAD-ESI-MS/MS
4.6. Sensory Analysis
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Komes, D.; Belščak-Cvitanović, A.; Škrabal, S.; Vojvodić, A.; Bušić, A. The Influence of Dried Fruits Enrichment on Sensory Properties of Bitter and Milk Chocolates and Bioactive Content of Their Extracts Affected by Different Solvents. LWT 2013, 53, 360–369. [Google Scholar] [CrossRef]
- Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Rosa, M.D.; Rosa, M.D.; Mozzon, M. Design of Healthy Snack Based on Kiwifruit. Molecules 2020, 25, 3309. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Oracz, J.; Bilicka, M.; Kulbat-Warycha, K.; Klewicka, E. Influence of Freeze-Dried Phenolic-Rich Plant Powders on the Bioactive Compounds Profile, Antioxidant Activity and Aroma of Different Types of Chocolates. Molecules 2021, 26, 7058. [Google Scholar] [CrossRef] [PubMed]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape Bioactive Molecules, and the Potential Health Benefits in Reducing the Risk of Heart Diseases. Food Chem. X 2020, 12, 100149. [Google Scholar] [CrossRef] [PubMed]
- California Raisins Application Research Review. Using Raisins Ingredients as a Binder and Protein Bar Base. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://californiaraisins.de/wp-content/uploads/2022/10/CRMB460_003_BinderBarBase_WhPaper_R4.pdf (accessed on 12 November 2022).
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of Dried Fruits: Phytochemicals, Antioxidant Efficacies, and Health Benefits. J. Funct. Foods 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Deus, V.L.; Resende, L.M.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. FTIR and PLS-Regression in the Evaluation of Bioactive Amines, Total Phenolic Compounds and Antioxidant Potential of Dark Chocolates. Food Chem. 2021, 357, 129754. [Google Scholar] [CrossRef]
- Özbal, B.; Çelekli, A.; Gün, D.; Bozkurt, H. Effect of Arthrospira Platensis Incorporation on Nutritional and Sensory Attributes of White Chocolate. Int. J. Gastron. Food Sci. 2022, 28, 100544. [Google Scholar] [CrossRef]
- Yeganehzad, S.; Kiumarsi, M.; Nadali, N.; Rabie Ashkezary, M. Formulation, Development and Characterization of a Novel Functional Fruit Snack Based on Fig (Ficus Carica L.) Coated with Sugar-Free Chocolate. Heliyon 2020, 6, e04350. [Google Scholar] [CrossRef]
- Mudenuti, N.V.d.R.; de Camargo, A.C.; Shahidi, F.; Madeira, T.B.; Hirooka, E.Y.; Grossmann, M.V.E. Soluble and Insoluble-Bound Fractions of Phenolics and Alkaloids and Their Antioxidant Activities in Raw and Traditional Chocolate: A Comparative Study. J. Funct. Foods 2018, 50, 164–171. [Google Scholar] [CrossRef]
- Kita, A.; Lachowicz, S.; Filutowska, P. Effects of Package Type on the Quality of Fruits and Nuts Panned in Chocolate during Long-Time Storage. LWT 2020, 125, 109212. [Google Scholar] [CrossRef]
- Loss, C.R.; Zellner, D.; Migoya, F. Innovation Influences Liking for Chocolates among Neophilic Consumers. Int. J. Gastron. Food Sci. 2017, 10, 7–10. [Google Scholar] [CrossRef]
- Freitas, D.d.G.C.; Machado, J.A.; Mattos, C.T.G.B.; Nogueira, R.I.; Cornejo, F.E.P.; De Souza, R.T.; Maia, J.D.G.; Ritschel, P.S. Aceitação de Uvas Passas Brasileiras e Suas Características Sensoriais Segundo a Percepção Do Consumidor. Comun. Técnico 2013, 1, 1–5. [Google Scholar]
- Lago-Vanzela, E.S.; Da-Silva, R.; Gomes, E.; García-Romero, E.; Hermosín-Gutiérrez, I. Phenolic Composition of the Brazilian Seedless Table Grape Varieties BRS Clara and BRS Morena. J. Agric. Food Chem. 2011, 59, 8314–8323. [Google Scholar] [CrossRef] [PubMed]
- Olivati, C.; Nishiyama, Y.P.O.; da Silva, R.; Gómez-Alonso, S.; Lago-Vanzela, E.S. BRS Clara Raisins Production: Effect of the Pre-Treatment and the Drying Process on the Phenolic Composition. J. Food Compos. Anal. 2022, 114, 104771. [Google Scholar] [CrossRef]
- Acan, B.G.; Kilicli, M.; Bursa, K.; Toker, O.S.; Palabiyik, I.; Gulcu, M.; Yaman, M.; Gunes, R.; Konar, N. Effect of Grape Pomace Usage in Chocolate Spread Formulation on Textural, Rheological and Digestibility Properties. LWT 2021, 138, 110451. [Google Scholar] [CrossRef]
- Olivati, C. Composição Fenólica de Uvas Passas e Drageados de Uva Passa Com Chocolate; UNESP: São José do Rio Preto, Brazil, 2020. [Google Scholar]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Changes in the Flavan-3-Ols, Anthocyanins, and Flavanols Composition of Cocoa Beans of Different Theobroma Cacao L. Groups Affected by Roasting Conditions. Eur. Food Res. Technol. 2015, 241, 663–681. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Monagas, M.; Khan, N.; Izquterdo-Pulido, M.; Urpi-Sarda, M.; Permanyer, J.; Lamuela-Raventós, R.M. Flavanol and Flavonol Contents of Cocoa Powder Products: Influence of the Manufacturing Process. J. Agric. Food Chem. 2008, 56, 3111–3117. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comprehensive Evaluation of Phenolic Profile in Dark Chocolate and Dark Chocolate Enriched with Sakura Green Tea Leaves or Turmeric Powder. Food Res. Int. 2018, 112, 1–16. [Google Scholar] [CrossRef]
- Efraim, P.; Barreto Alves, A.; Calil Pereira Jardim, D. Revisão: Polifenóis Em Cacau e Derivados: Teores, Fatores de Variação e Efeitos Na Saúde. Braz. J. Food Technol. 2011, 14, 181–201. [Google Scholar] [CrossRef]
- Tavares, I.M.C.; de Castilhos, M.B.M.; Mauro, M.A.; Ramos, A.M.; de Souza, R.T.; Gómez-Alonso, S.; Gomes, E.; Da-Silva, R.; Hermosín-Gutiérrez, I.; Lago-Vanzela, E.S. BRS Violeta (BRS Rúbea × IAC 1398-21) grape juice powder produced by foam mat drying. Part I: Effect of drying temperature on phenolic compounds and antioxidant activity. Food Chem. 2019, 298, 124971. [Google Scholar] [CrossRef]
- Counet, C.; Callemien, D.; Collin, S. Food Chemistry Chocolate and Cocoa: New Sources of Trans -Resveratrol and Trans -Piceid. Food Chem. 2006, 98, 649–657. [Google Scholar] [CrossRef]
- Hurst, W.J.; Glinski, J.A.; Miller, K.B.; Apgar, J.; Davey, M.H.; Stuart, D.A. Survey of the Trans-Resveratrol and Trans-Piceid Content of Cocoa-Containing and Chocolate Products. J. Agric. Food Chem. 2008, 56, 8374–8378. [Google Scholar] [CrossRef] [PubMed]
- Wollgast, J.; Anklam, E. Review on Polyphenols in Theobroma Cacao: Changes in Composition during the Manufacture of Chocolate and Methodology for Identification and Quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Komes, D.; Dujmović, M.; Karlović, S.; Biškić, M.; Brnčić, M.; Ježek, D. Physical, Bioactive and Sensory Quality Parameters of Reduced Sugar Chocolates Formulated with Natural Sweeteners as Sucrose Alternatives. Food Chem. 2015, 167, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Belščak, A.; Komes, D.; Horžić, D.; Ganić, K.K.; Karlović, D. Comparative Study of Commercially Available Cocoa Products in Terms of Their Bioactive Composition. Food Res. Int. 2009, 42, 707–716. [Google Scholar] [CrossRef]
- Todorovic, V.; Redovnikovic, I.R.; Todorovic, Z.; Jankovic, G.; Dodevska, M.; Sobajic, S. Polyphenols, Methylxanthines, and Antioxidant Capacity of Chocolates Produced in Serbia. J. Food Compos. Anal. 2015, 41, 137–143. [Google Scholar] [CrossRef]
- Cambrai, A.; Marchioni, E.; Julien-David, D.; Marcic, C. Discrimination of Cocoa Bean Origin by Chocolate Polyphenol Chromatographic Analysis and Chemometrics. Food Anal. Methods 2017, 10, 1991–2000. [Google Scholar] [CrossRef]
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; McHale, N.L.; Flanagan, J.A.; Ou, B.; Hurst, W.J. Antioxidant Activity and Polyphenol and Procyanidin Contents of Selected Commercially Available Cocoa-Containing and Chocolate Products in the United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef]
- Samaniego, I.; Espín, S.; Quiroz, J.; Ortiz, B.; Carrillo, W.; García-Viguera, C.; Mena, P. Effect of the Growing Area on the Methylxanthines and Flavan-3-Ols Content in Cocoa Beans from Ecuador. J. Food Compos. Anal. 2020, 88, 103448. [Google Scholar] [CrossRef]
- Hurst, W.J.; Krake, S.H.; Bergmeier, S.C.; Payne, M.J.; Miller, K.B.; Stuart, D.A. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem. Cent. J. 2011, 5, 53. [Google Scholar] [CrossRef]
- Miller, K.B.; Hurst, W.J.; Flannigan, N.; Ou, B.; Lee, C.Y.; Smith, N.; Stuart, D.A. Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J. Agric. Food Chem. 2009, 57, 9169–9180. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, S.R.; Ares, G. RATA Questions Are Not Likely to Bias Hedonic Scores. Food Qual. Prefer. 2015, 44, 157–161. [Google Scholar] [CrossRef]
- Tassi, A.L.W.; Bento, J.A.C.; Ferreira, K.C.; Caliari, M.; da Silva, V.S.N.; Pacheco, M.T.B.; Ida, E.I.; Soares Júnior, M.S. Roasting soybeans in a microwave for manufacturing chocolate dragée. Food Technol. Cienc. Rural. 2019, 49, e20180743. [Google Scholar] [CrossRef]
- Olivati, C.; de Oliveira Nishiyama, Y.P.; de Souza, R.T.; Janzantti, N.S.; Mauro, M.A.; Gomes, E.; Hermosín-Gutiérrez, I.; da Silva, R.; Lago-Vanzela, E.S. Effect of the Pre-Treatment and the Drying Process on the Phenolic Composition of Raisins Produced with a Seedless Brazilian Grape Cultivar. Food Res. Int. 2019, 116, 190–199. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005; ISBN 0935584757. [Google Scholar]
- Rebello, L.P.G.; Lago-Vanzela, E.S.; Barcia, M.T.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Gómez-Alonso, S.; Hermosín-gutiérrez, I. Phenolic Composition of the Berry Parts of Hybrid Grape Cultivar BRS Violeta (BRS Rubea×IAC 1398-21) Using HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 54, 354–366. [Google Scholar] [CrossRef]
- Bordiga, M.; Coïsson, J.D.; Locatelli, M.; Arlorio, M.; Travaglia, F. Pyrogallol: An Alternative Trapping Agent in Proanthocyanidins Analysis. Food Anal. Methods 2013, 6, 148–156. [Google Scholar] [CrossRef]
- Ares, G.; Bruzzone, F.; Vidal, L.; Cadena, R.S.; Giménez, A.; Pineau, B.; Hunter, D.C.; Paisley, A.G.; Jaeger, S.R. Evaluation of a Rating-Based Variant of Check-All-That-Apply Questions: Rate-All-That-Apply (RATA). Food Qual. Prefer. 2014, 36, 87–95. [Google Scholar] [CrossRef]
- Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 5th ed.; Taylor & Francis: Abingdon, UK, 2015; ISBN 9781482216905. [Google Scholar]
- Ares, G.; Jaeger, S.R. Check-All-That-Apply Questions: Influence of Attribute Order on Sensory Product Characterization. Food Qual. Prefer. 2013, 28, 141–153. [Google Scholar] [CrossRef]
| Parameter | DC-1 | DC-2 |
|---|---|---|
| Length (cm) | 2.25 ± 0.13 | 2.13 ± 0.04 |
| Width (cm) | 1.38 ± 0.17 | 1.58 ± 0.17 |
| Mass (g) 1 | 2.66 ± 0.17 | 2.91 ± 0.55 |
| pH | 4.45 ± 0.11 | 4.53 ± 0.07 |
| Acidity 2 | 1.23 ± 0.15 | 1.19 ± 0.04 |
| Moisture % (m/m) | 9.16 ± 0.00 | 9.14 ± 0.00 |
| Flavonols | Deprotonated Molecule and Product Ions (m/z) | Molar Ratios (%) 1 | ||
| RC | DC-1 | DC-2 | ||
| Q-3-gal | 463; 301 | 3.39 b ± 0.90 | 5.70 a ± 0.75 | 5.23 a ± 0.44 |
| Q-3-glcU | 477; 301 | 48.97 a ± 3.13 | 24.05 b ± 0.97 | 24.08 b ± 1.53 |
| Q-3-glc | 463; 301 | 36.15 a ± 4.30 | 30.52 a ± 1.49 | 29.44 a ± 2.13 |
| Q-3-rut | 609; 301 | 4.17 a ± 0.04 | 2.07 b ± 0.19 | 1.81 b ± 0.31 |
| Q-3-pent | 433; 301 | ND | 23.00 a ± 1.35 | 21.64 a ± 1.53 |
| K-3-rut | 593; 285 | ND | 2.19 a ± 0.35 | 1.95 a ± 0.22 |
| free Q | 301 | 7.32 a ± 2.03 | 12.47 a ± 4.50 | 15.85 a ± 5.67 |
| Total (mg·kg−1, DW) | 60.96 a ± 28.35 | 32.92 a ± 10.73 | 34.65 a ± 7.16 | |
| Total (mg·kg−1, FW) | 54.84 ± 8.89 | 28.33 ± 9.18 | 29.32 ± 5.76 | |
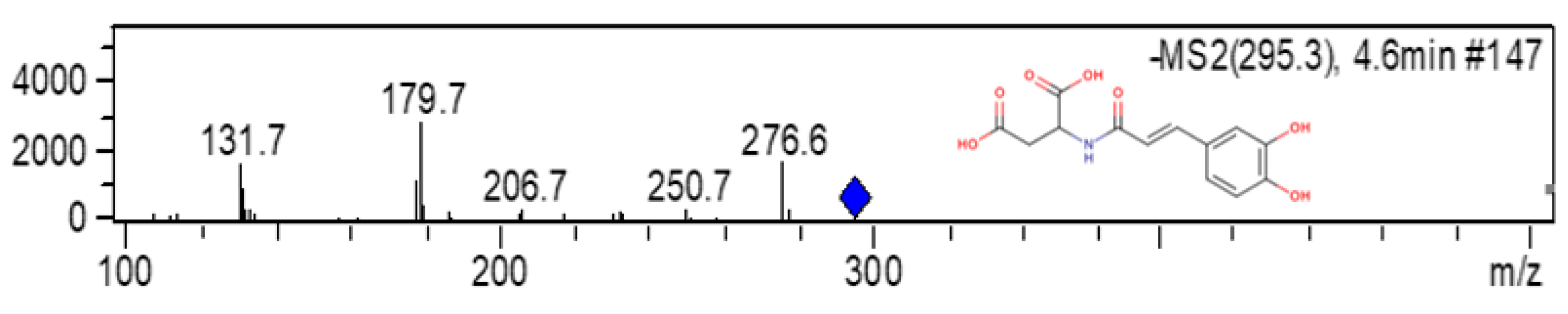
| HCAD | Deprotonated molecule and product ions (m/z) | RC | DC-1 | DC-2 |
| GRP | 618; 543; 489; 264 | 8.82 a ± 1.57 | 5.13 b ± 1.18 | 5.10 b ± 1.16 |
| trans-caftaric acid | 311; 179; 149; 135 | 69.40 a ± 4.14 | 28.29 b ± 4.72 | 29.59 b ± 2.58 |
| cafeoil aspartic acid | 294; 179; 131 | ND | 26.32 a ± 1.00 | 24.58 a ± 2.94 |
| trans-coutaric acid | 295; 163; 149; 119 | 7.62 a ± 0.86 | 3.41 b ± 0.36 | 3.61 b ± 0.25 |
| cis-coutaric acid | 295; 163; 149; 120 | 5.41 a ± 0.76 | 2.51 b ± 0.19 | 2.61 b ± 0.10 |
| trans-fertaric acid | 325; 193; 149 | 5.51 b ± 0.93 | 14.17 a ± 2.53 | 13.06 a ± 1.40 |
| cis-fertaric acid | 325; 193; 149 | 3.23 a ± 0.33 | 2.60 a ± 1.30 | 2.27 a ± 0.30 |
| p-coumaric acid | 325; 163, 145, 119, 117 | ND | 17.56 a ± 3.98 | 19.17 a ± 5.27 |
| Total DAHC (mg·kg−1, DW) | 285.67 a ± 28.02 | 128.04 b ± 73.57 | 107.84 b ± 51.15 | |
| Total DAHC (mg·kg−1, FW) | 129.36 ± 71.31 | 73.12 ± 44.40 | 62.16 ± 30.10 | |
| Stilbenes | m/z Pairs | mg·kg−1 of Product—DW (mg·kg−1 of Product—FW) | ||
|---|---|---|---|---|
| RC | DC-1 | DC-2 | ||
| trans-piceid | 389/227; 389/185 | 0.59 a ± 0.03 (0.51 ± 0.02) | 0.40 a ± 0.13 (0.36 ± 0.12) | 0.39 a ± 0.02 (0.35 ± 0.02) |
| cis-piceid | 389/227; 389/185 | 0.67 a ± 0.01 (0.59 ± 0.01) | 0.17 b ± 0.02 (0.16 ± 0.01) | 0.18 b ± 0.01 (0.16 ± 0.00) |
| Sum of cis- and trans-piceid (mg eq. of trans-piceid·kg−1 of product) | 1.26 a ± 0.04 (1.10 ± 0.04) | 0.57 b ± 0.15 (0.52 ± 0.14) | 0.57 b ± 0.01 (0.52 ± 0.01) | |
| Flavan-3-ols Monomers | m/z Pairs | mg·kg−1 of Product—DW (mg·kg−1 of Product—FW) | ||
| RC | DC-1 | DC-2 | ||
| (+)-catechin | 289/137; 289/164 | 10.12 b ± 5.02 (8.77 ± 4.34) | 60.23 a ± 8.60 (54.71 ± 7.82) | 57.42 a ± 1,90 (52.17 ± 1.73) |
| (−)-epicatechin | 289/137; 289/164 | 3.46 b ± 1.89 (3.00 ± 1.64) | 117.86 a ± 13.80 (107.06 ± 12.53) | 117.66 a ± 4.55 (106.90 ± 4.13) |
| (−)-galocatechin | 305/109; 305/137 | 0.40 a ± 0.10 (0.35 ± 0.09) | 0.38 a ± 0.08 (0.35 ± 0.07) | 0.30 a ± 0.09 (0.27 ± 0.08) |
| (−)-epigalocatechin | 305/109; 305/137 | 0.16 b ± 0.10 (0.14 ± 0.09) | 0.30 b ± 0.13 (0.27 ± 0.12) | 0.52 a ± 0.09 (0.47 ± 0.08) |
| (−)-epicatechin-3-galate | 441/245; 441/289 | 0.87 a ± 0.18 (0.76 ± 0.15) | 0.14 b ± 0.05 (0.13 ±0.05) | 0.17 b ± 0.03 (0.16 ± 0.03) |
| Total monomers | 21.19 b ± 11.70 (18.37 ± 10.12) | 253.24 a ± 29.70 (230.05 ± 26.98) | 251.13 a ± 5.10 (228.17 ± 4.64) | |
| Flavan-3-ols dimers | m/z pairs | mg·kg−1 of product—DW (mg·kg−1 of product—FW) | ||
| RC | DC-1 | DC-2 | ||
| Procianidin B1 | 577/425; 577/407 | 19.06 a ± 7.04 (16.53 ± 6.08) | 10.16 a ± 2.61 (9.23 ± 2.38) | 10.37 a ± 1.16 (9.43 ± 1.06) |
| Procianidin B2 | 577/425; 577/407 | 10.06 b ± 6.19 (8.72 ± 5.36) | 208.76 a ± 30.31 (189.65 ± 27.53) | 209.74 a ± 12.31 (190.57 ± 11.18) |
| Procianidin B4 | 577/425; 577/407 | 11.94 b ± 7.30 (10.35 ± 6.36) | 247.76 a ± 35.97 (225.07 ± 32.68) | 248.92 a ± 14.61 (226.16 ± 13.27) |
| Total dimers | 26.80 b ± 12.72 (23.23 ± 10.99) | 263.50 a ± 39.90 (239.37 ± 36.25) | 263.83 a ± 15.37 (239.71 ± 13.97) | |
| Sum of monomers and dimers (mg eq. of catechin·kg−1 of product) | 47.99 b ± 24.36 (41.60 ± 21.07) | 516.73 a ± 68.70 (469.41 ± 62.41) | 514.97 a ± 20.34 (467.88 ± 18.49) | |
| PA 1 | RC | DC-1 | DC-2 |
|---|---|---|---|
| mDP | 4.15 a ± 0.31 | 2.70 b ± 0.12 | 2.71 b ± 0.02 |
| % galoylation | 5.90 a ± 0.94 | 0.51 b ± 0.16 | 0.42 b ± 0.01 |
| % prodelphinidin | 6.76 a ± 0.47 | 2.24 b ± 0.73 | 2.08 b ± 0.21 |
| % C-terminal | 49.03 a ± 3.54 | 23.75 b ± 0.99 | 22.86 b ± 0.29 |
| % EC-terminal | 16.21 b ± 1.64 | 46.55 a ± 0.67 | 46.84 a ± 1.01 |
| % GC-terminal | 2.60 a ± 2.35 | 0.14 a ± 0.03 | 0.11 a ± 0.03 |
| % EGC-terminal | 0.69 a ± 0.15 | 0.12 b ± 0.06 | 0.20 b ± 0.03 |
| % ECG-terminal | 3.14 a ± 1.46 | 0.03 b ± 0.01 | 0.04 b ± 0.01 |
| % C-extension | 3.15 b ± 0.51 | 4.86 a ± 0.76 | 4.72 a ± 0.25 |
| % EC-extension | 84.63 b ± 1.53 | 91.58 a ± 1.95 | 92.22 a ± 0.47 |
| % EGC-extension | 7.18 a ± 0.39 | 2.99 b ± 1.03 | 2.60 b ± 0.31 |
| % CG-extension | 4.54 a ± 0.94 | 0.51 b ± 0.15 | 0.41 b ± 0.01 |
| % GC-extension | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.00 |
| % ECG-extension | trace | trace | trace |
| % GCG-extension | ND | 0.01 a ± 0.00 | 0.01 a ± 0.00 |
| % EGCG-extension | 0.49 a ± 0.04 | 0.04 b ± 0.02 | 0.03 b ± 0.01 |
| Total PA 2 mg·kg−1 of product —DW (mg·kg−1 of product —FW) | 421.65 a ± 137.60 (365.62 ± 118.61) | 768.32 a ± 208.22 (697.96 ± 189.15) | 783.14 a ± 57.57 (711.54 ± 52.30) |
| Sensory Analysis | Mean ± Standard Deviation | |
|---|---|---|
| Sensory descriptors 1 | ||
| Appearance | Brown color | 4.57 ± 0.87 |
| Opacity | 3.71 ± 1.35 | |
| Icing roughness | 1.65 ± 1.51 | |
| Odor | Dark chocolate | 3.33 ± 1.08 |
| Milk chocolate | 2.47 ± 1.71 | |
| Fat/Cocoa butter | 2.65 ± 1.73 | |
| Taste | Sweet | 3.86 ± 1.08 |
| Sour | 2,75 ± 1.81 | |
| Bitter | 1.88 ± 1.54 | |
| Flavor | Dark chocolate | 2.71 ± 1.65 |
| Milk chocolate | 2.90 ± 1.61 | |
| Raisins | 3.88 ± 1.45 | |
| Fat/Cocoa butter/Butter | 2.12 ± 1.70 | |
| Texture | Soft | 4.20 ± 0.96 |
| Adhesive | 2.02 ± 1.70 | |
| Hedonic Terms | Tasty | 4.37 ± 0.88 |
| Pleasant | 4.18 ± 1.15 | |
| Natural | 2.98 ± 1.73 | |
| Interesting | 4.14 ± 1.32 | |
| Delightful | 4.31 ± 1.08 | |
| Acceptance 2 | 8.20 ± 0.89 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivati, C.; Nishiyama-Hortense, Y.P.; Soares Janzantti, N.; da Silva, R.; Lago Vanzela, E.S.; Gómez-Alonso, S. Dark-Chocolate-Coated BRS Clara Raisins: Phenolic Composition and Sensory Attributes. Molecules 2023, 28, 7006. https://doi.org/10.3390/molecules28207006
Olivati C, Nishiyama-Hortense YP, Soares Janzantti N, da Silva R, Lago Vanzela ES, Gómez-Alonso S. Dark-Chocolate-Coated BRS Clara Raisins: Phenolic Composition and Sensory Attributes. Molecules. 2023; 28(20):7006. https://doi.org/10.3390/molecules28207006
Chicago/Turabian StyleOlivati, Carolina, Yara Paula Nishiyama-Hortense, Natália Soares Janzantti, Roberto da Silva, Ellen Silva Lago Vanzela, and Sergio Gómez-Alonso. 2023. "Dark-Chocolate-Coated BRS Clara Raisins: Phenolic Composition and Sensory Attributes" Molecules 28, no. 20: 7006. https://doi.org/10.3390/molecules28207006
APA StyleOlivati, C., Nishiyama-Hortense, Y. P., Soares Janzantti, N., da Silva, R., Lago Vanzela, E. S., & Gómez-Alonso, S. (2023). Dark-Chocolate-Coated BRS Clara Raisins: Phenolic Composition and Sensory Attributes. Molecules, 28(20), 7006. https://doi.org/10.3390/molecules28207006