Design and Performance of a New Zn0.5Mg0.5FeMnO4 Porous Spinel as Anode Material for Li-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
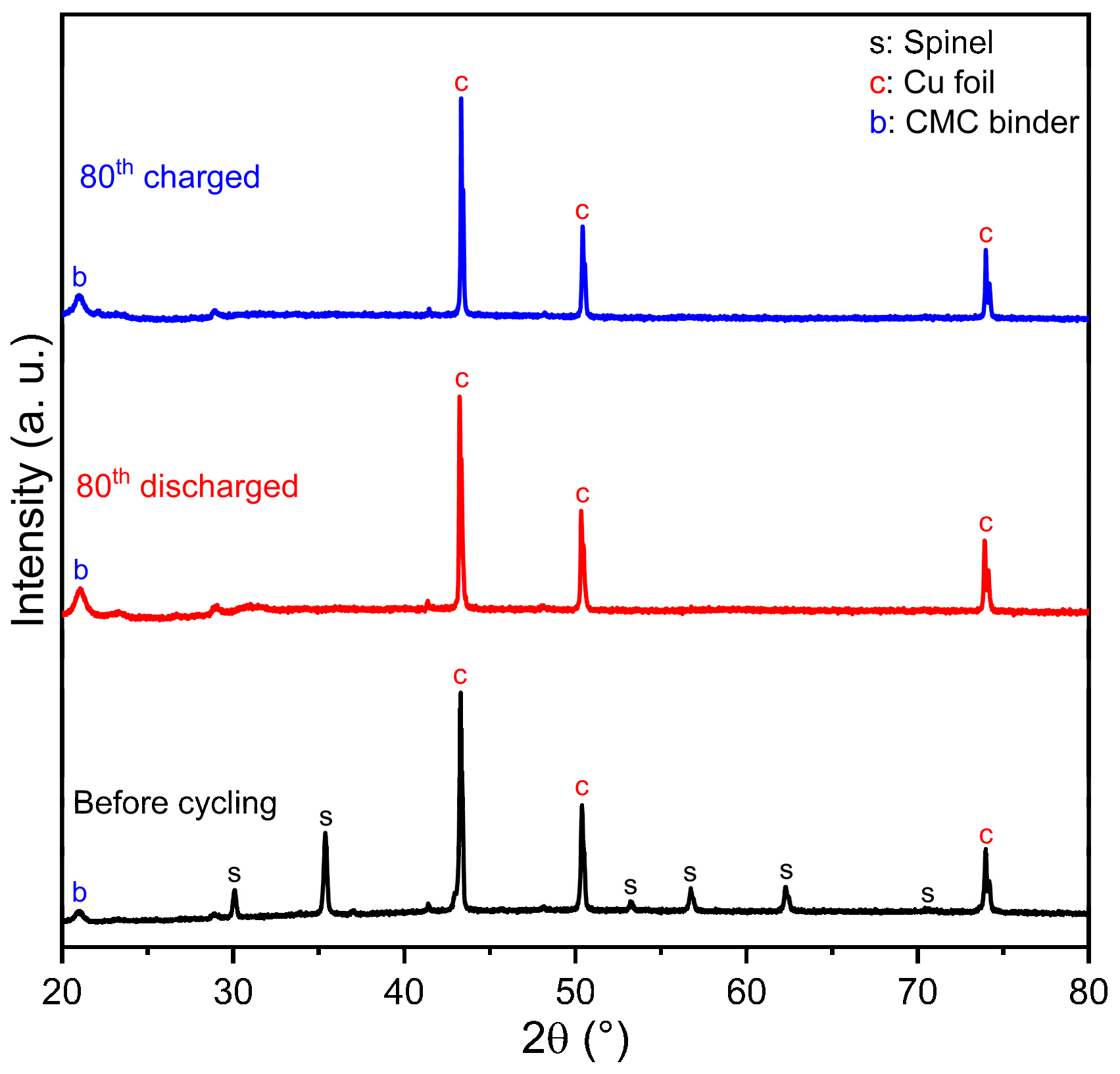
2.1. X-ray Diffraction, Rietveld Refinement, and Crystal Structure
2.2. Thermal Analysis
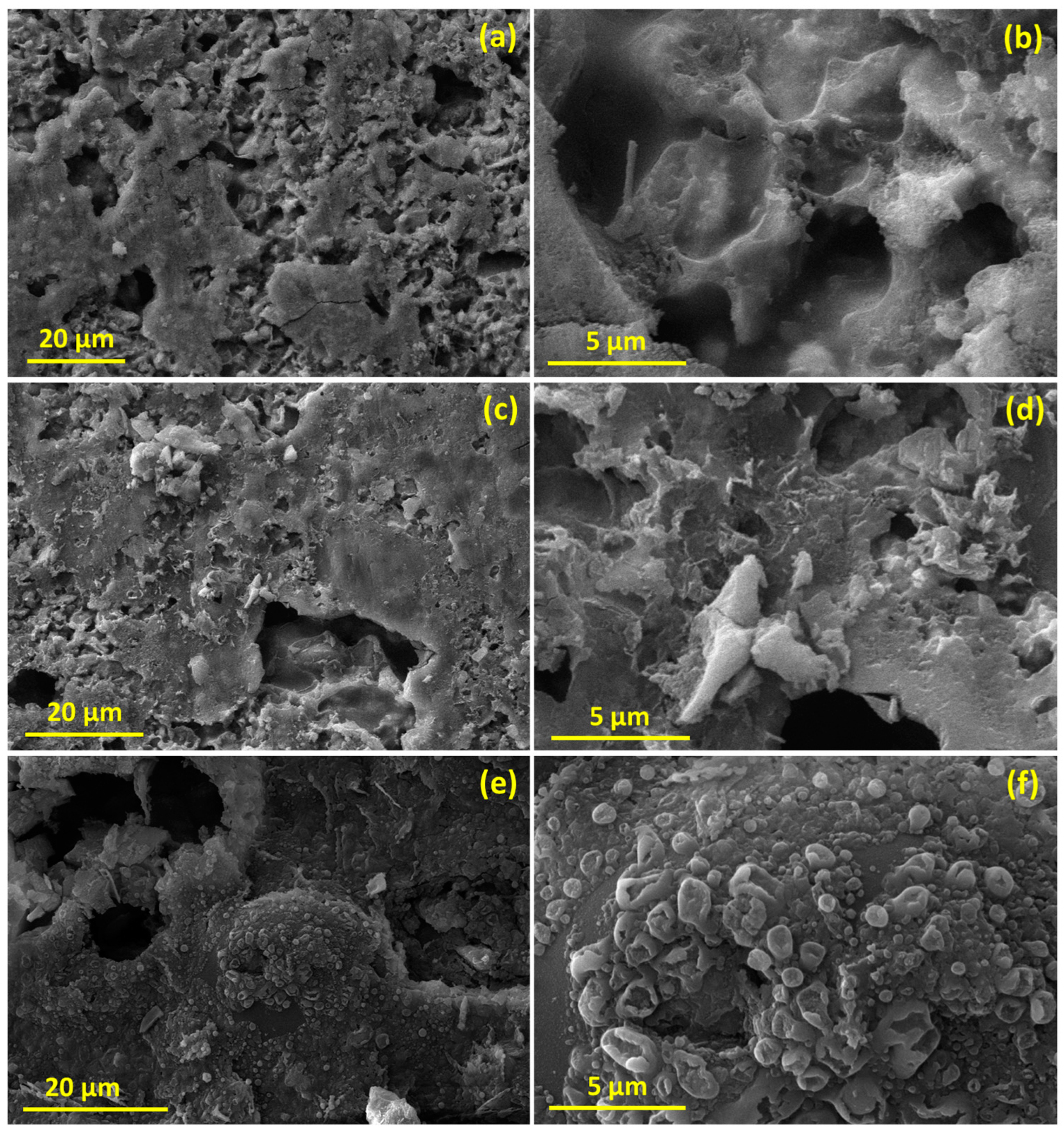
2.3. Morphology, Purity, and Specific Surface
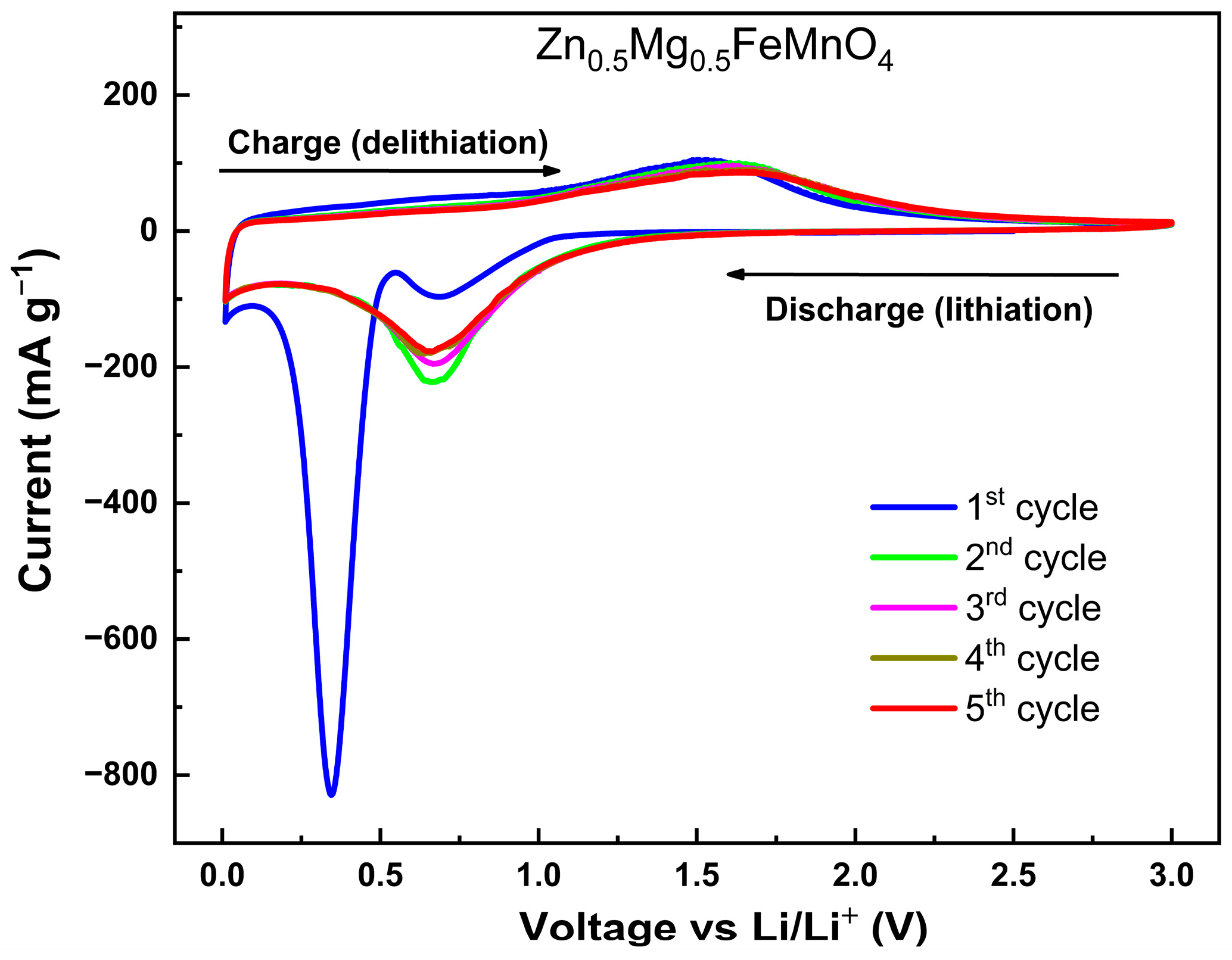
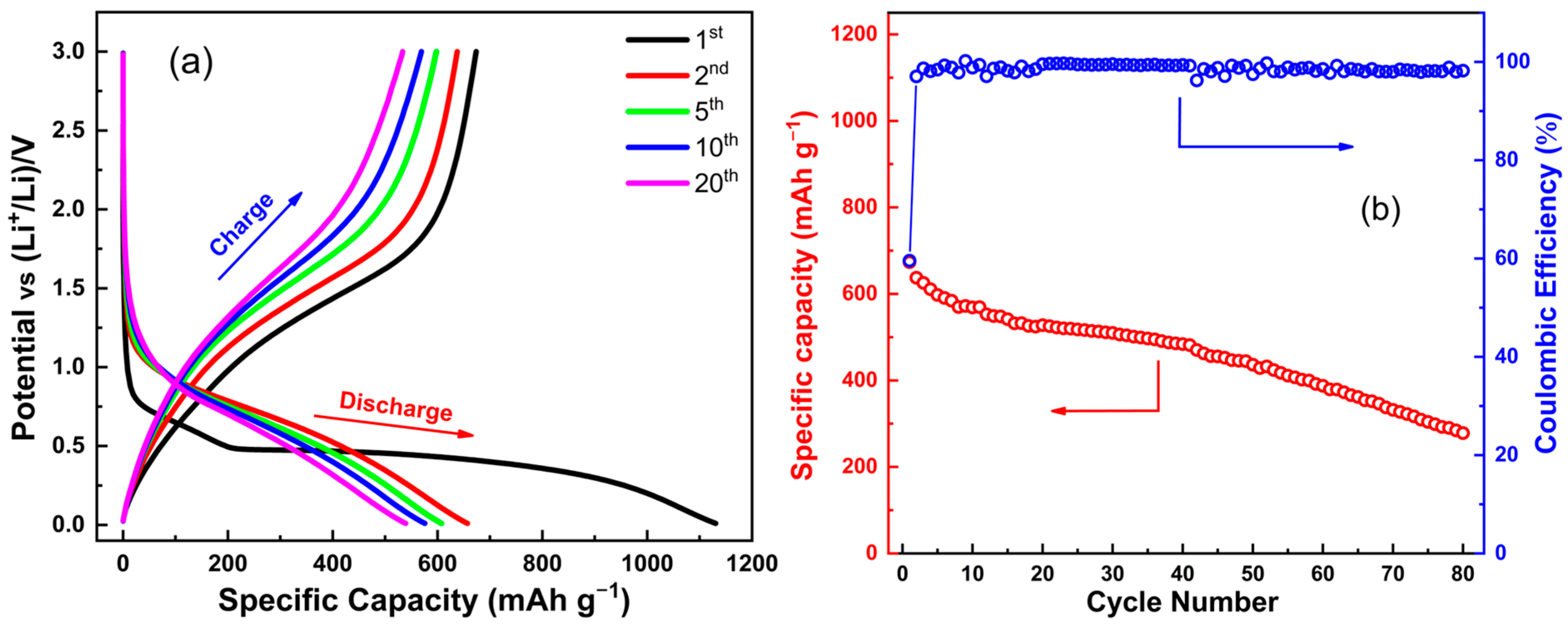
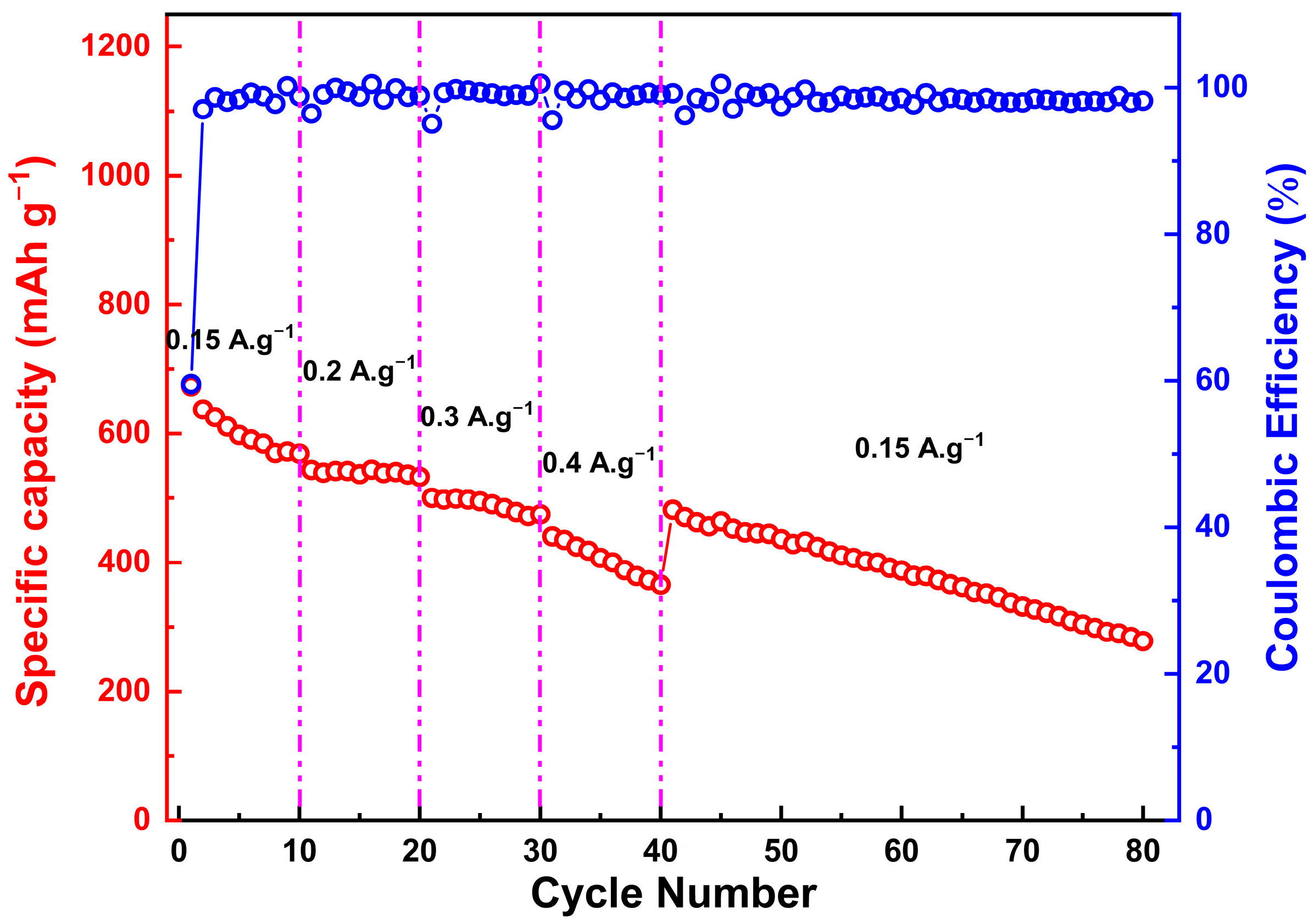
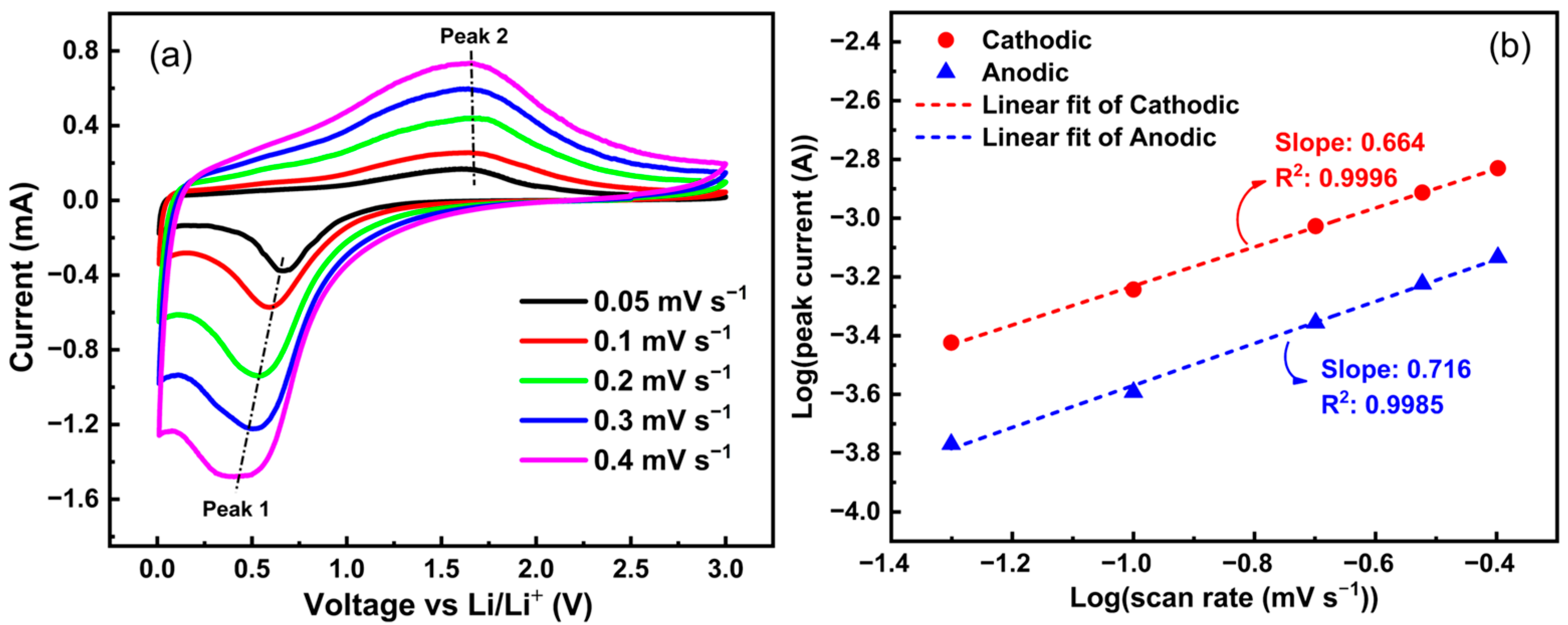
2.4. Electrochemical Results
- First cycle:
- Subsequent cycles:
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Porous Zn0.5Mg0.5FeMnO4 Spinel
Zn0.5Mg0.5FeMnO4 + 0.5 Cl2↑ + 6 NO2↑ + 16 H2O↑
3.3. Characterization Techniques
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, L.; Song, J.; Lu, Q.; Tan, X. Synthesis of Nano-Crystalline Sm0.5Sr0.5Co(Fe)O3−δ Perovskite Oxides by a Microwave-Assisted Sol–Gel Combustion Process. Ceram. Int. 2014, 40, 1189–1194. [Google Scholar] [CrossRef]
- Shen, D.; Jia, M.; Li, M.; Fu, X.; Liu, Y.; Dong, W.; Yang, S. High Coulomb Efficiency Sn–Co Alloy/RGO Composite Anode Material for Li–Ion Battery with Long Cycle–Life. Molecules 2023, 28, 3923. [Google Scholar] [CrossRef]
- Liu, G.; Jin, B.; Zhang, R.; Bao, K.; Xie, H.; Guo, J.; Wei, M.; Jiang, Q. Synthesis of Ti2Nb10O29/C Composite as an Anode Material for Lithium-Ion Batteries. Int. J. Hydrogen Energy 2016, 41, 14807–14812. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Q.; Tang, S.; Zhang, L.; Deng, S.; Shi, Z.; Chen, G. One-Pot Synthesis of ZnFe2O4/C Hollow Spheres as Superior Anode Materials for Lithium Ion Batteries. Chem. Commun. 2011, 47, 6828. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-Sacrifice Template Fabrication of Hierarchical Mesoporous Bi-Component-Active ZnO/ZnFe2O4 Sub-Microcubes as Superior Anode Towards High-Performance Lithium-Ion Battery. Adv. Funct. Mater. 2015, 25, 238–246. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, M.; Li, C.; Wu, B.; Chen, J.; Xiang, W.; Wen, X.; Zhang, D.; Cao, G.; Li, W. Surface Spinel and Interface Oxygen Vacancies Enhanced Lithium-Rich Layered Oxides with Excellent Electrochemical Performances. Chem. Eng. J. 2022, 443, 136434. [Google Scholar] [CrossRef]
- Meister, P.; Jia, H.; Li, J.; Kloepsch, R.; Winter, M.; Placke, T. Best Practice: Performance and Cost Evaluation of Lithium Ion Battery Active Materials with Special Emphasis on Energy Efficiency. Chem. Mater. 2016, 28, 7203–7217. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, R.; Fan, C.; Xie, D.; Li, B.; Zhang, J.; Wu, X. Engineering All-Purpose Amorphous Carbon Nanotubes with High N/O-Co-Doping Content to Bridge the Alkali-Ion Batteries and Li Metal Batteries. Small 2021, 17, 2006566. [Google Scholar] [CrossRef]
- Zha, G.; Hu, W.; Agarwal, S.; Ouyang, C.; Hu, N.; Hou, H. High Performance Layered LiNi0.8Co0.07Fe0.03Mn0.1O2 Cathode Materials for Li-Ion Battery. Chem. Eng. J. 2021, 409, 128343. [Google Scholar] [CrossRef]
- Lim, Y.E.; Choi, W.S.; Kim, J.H.; Ahn, Y.N.; Kim, I.T. The Sn–Red P–Fe–Based Alloy Materials for Efficient Li–Ion Battery Anodes. J. Ind. Eng. Chem. 2023, 121, 299–311. [Google Scholar] [CrossRef]
- Bae, J.-H.; Nyamaa, O.; Lee, J.-S.; Yun, S.; Woo, S.-M.; Yang, J.-H.; Kim, M.-S.; Noh, J.-P. Electrochemical Properties of the Si Thin-Film Anode Deposited on Ti-Nb-Zr Shape Memory Alloy in Li-Ion Batteries. Electrochem. Commun. 2022, 140, 107315. [Google Scholar] [CrossRef]
- Shang, C.; Li, X.; Wei, R.; Liu, X.; Xu, S.; Zhang, J. Research Progress of Metal Oxide Glass Anode Materials for Lithium-Ion Batteries: A Review. J. Non. Cryst. Solids 2023, 618, 122547. [Google Scholar] [CrossRef]
- Lamhani, M.; Chchiyai, Z.; Elomrani, A.; Manoun, B.; Hasnaoui, A. The Effect of Sr Substitution on the Structural and Physical Properties of Manganite Perovskites Ca1−xSrxMnO3−δ (0 ≤ x ≤ 1). Phys. Chem. Chem. Phys. 2022, 24, 19414–19431. [Google Scholar] [CrossRef]
- Kouchi, K.; Tayoury, M.; Chari, A.; Chchiyai, Z.; Hdidou, L.; Tamraoui, Y.; Alami, J.; Manoun, B.; Dahbi, M. Sol Gel Synthesis of Nanoparticles Ni0.5Mg0.5Fe1.7Mn0.3O4 as Anode Material for Lithium-Ion Batteries. In Proceedings of the 2021 9th International Renewable and Sustainable Energy Conference (IRSEC), Tetouan, Morocco, 23–27 November 2021; IEEE: Piscataway, NJ, USA; pp. 1–5. [Google Scholar]
- Huang, S.; Li, H.; Xu, G.; Liu, X.; Zhang, Q.; Yang, L.; Cao, J.; Wei, X. Porous N-Doped Carbon Sheets Wrapped MnO in 3D Carbon Networks as High-Performance Anode for Li-Ion Batteries. Electrochim. Acta 2020, 342, 136115. [Google Scholar] [CrossRef]
- Chu, K.; Li, Z.; Xu, S.; Yao, G.; Xu, Y.; Niu, P.; Zheng, F. NiO Nanocrystals Encapsulated into a Nitrogen-Doped Porous Carbon Matrix as Highly Stable Li-Ion Battery Anodes. J. Alloys Compd. 2021, 854, 157264. [Google Scholar] [CrossRef]
- Su, Y.; Liu, T.; Zhang, P.; Zheng, P. CuO Nanowire Arrays Synthesized at Room Temperature as a High-Performance Anode Material for Li/Na-Ion Batteries. Thin Solid Films 2019, 690, 137522. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Lu, X.; Zhao, H.; Cui, J.; Zhang, Y.; He, W. Hydrothermal Synthesis of Carbon-Coated Mixed Crystalline Phase TiO2 Nanoparticle Carbon Microsphere Composites as High Performance Anode Materials for Li-Ion Batteries. Diam. Relat. Mater. 2023, 136, 109913. [Google Scholar] [CrossRef]
- Liu, D.; Choi, W.M. Hierarchical Hollow Urchin-like Structured MnO2 Microsphere/Carbon Nanofiber Composites as Anode Materials for Li-Ion Batteries. Curr. Appl. Phys. 2019, 19, 768–774. [Google Scholar] [CrossRef]
- Buga, M.-R.; Spinu-Zaulet, A.A.; Ungureanu, C.G.; Mitran, R.-A.; Vasile, E.; Florea, M.; Neatu, F. Carbon-Coated SiO2 Composites as Promising Anode Material for Li-Ion Batteries. Molecules 2021, 26, 4531. [Google Scholar] [CrossRef]
- Golmohammad, M.; Sazvar, A.; Maleki Shahraki, M.; Golestanifard, F. Synthesis and Characterization of Bar-like Maghemite (γ-Fe2O3) as an Anode for Li-Ion Batteries. Ceram. Int. 2022, 48, 27148–27153. [Google Scholar] [CrossRef]
- Wen, Z.; Rong, Z.; Yin, Y.; Ren, H.; Woo Joo, S.; Huang, J. N-Doped Carbon Coated SnO2 Nanospheres as Li-Ion Battery Anode with High Capacity and Good Cycling Stability. J. Electroanal. Chem. 2021, 899, 115694. [Google Scholar] [CrossRef]
- Allen, J.L.; Crear, B.A.; Choudhury, R.; Wang, M.J.; Tran, D.T.; Ma, L.; Piccoli, P.M.; Sakamoto, J.; Wolfenstine, J. Fast Li-Ion Conduction in Spinel-Structured Solids. Molecules 2021, 26, 2625. [Google Scholar] [CrossRef] [PubMed]
- Drozhzhin, O.A.; Shevchenko, V.A.; Bobyleva, Z.V.; Alekseeva, A.M.; Antipov, E.V. Rational Screening of High-Voltage Electrolytes and Additives for Use in LiNi0.5Mn1.5O4-Based Li-Ion Batteries. Molecules 2022, 27, 3596. [Google Scholar] [CrossRef] [PubMed]
- Sanad, M.M.S.; Azab, A.A.; Taha, T.A. Introduced Oxygen Vacancies in Cadmium Ferrite Anode Materials via Zn2+ Incorporation for High Performance Lithium-Ion Batteries. Mater. Sci. Semicond. Process. 2022, 143, 106567. [Google Scholar] [CrossRef]
- Mazur, Ł.; Koszelow, D.; Zajusz, M.; Łapiński, M.; Bik, M.; Zając, P.; Adamczyk, A.; Rutkowski, P.; Molin, S.; Brylewski, T. Comparison of Cu1.3Mn1.7O4 Spinels Doped with Ni or Fe and Synthesized via Wet Chemistry and Solid-State Reaction Methods, Designed as Potential Coating Materials for Metallic Interconnects. J. Eur. Ceram. Soc. 2023, 43, 5557–5574. [Google Scholar] [CrossRef]
- Nyamaa, O.; Kang, G.-H.; Huh, S.-C.; Yang, J.-H.; Nam, T.-H.; Noh, J.-P. Unraveling the Mechanism and Practical Implications of the Sol-Gel Synthesis of Spinel LiMn2O4 as a Cathode Material for Li-Ion Batteries: Critical Effects of Cation Distribution at the Matrix Level. Molecules 2023, 28, 3489. [Google Scholar] [CrossRef]
- Afzia, M.; Khan, R.A.; Ismail, B.; Zaki, M.E.A.; Althagafi, T.M.; Alanazi, A.A.; Khan, A.U. Correlation between Magnetic and Dielectric Response of CoFe2O4:Li1+/Zn2+ Nanopowders Having Improved Structural and Morphological Properties. Molecules 2023, 28, 2824. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Tsai, C.-C.; Patra, J.; Clemens, O.; Chang, J.-K.; Ting, J.-M. Co-Free High Entropy Spinel Oxide Anode with Controlled Morphology and Crystallinity for Outstanding Charge/Discharge Performance in Lithium-Ion Batteries. Chem. Eng. J. 2022, 430, 132658. [Google Scholar] [CrossRef]
- Saroha, R.; Seon, Y.H.; Jin, B.; Kang, Y.C.; Kang, D.-W.; Jeong, S.M.; Cho, J.S. Self-Supported Hierarchically Porous 3D Carbon Nanofiber Network Comprising Ni/Co/NiCo2O4 Nanocrystals and Hollow N-Doped C Nanocages as Sulfur Host for Highly Reversible Li–S Batteries. Chem. Eng. J. 2022, 446, 137141. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Gu, A.; Wei, W.; Lv, H.; Lou, Z.; Zhou, Q. Ethylene Glycol Combustion Strategy towards 3D Mesoporous ZnCo2O4 as Anodes for Li-Ion Batteries. Solid State Ionics 2020, 356, 115461. [Google Scholar] [CrossRef]
- Chen, R.; Wen, B.; Li, H.; Xiang, M.; Su, C.; Guo, J.; Bai, W.; Sa, Z. Facile Preparation of High-Performance Spinel LiMn2−XCuxO4 Cathodes by Microwave-Induced Solution Flameless Combustion. Vacuum 2021, 187, 110077. [Google Scholar] [CrossRef]
- Wu, S.; Su, H.-J. Electrochemical Characteristics of Partially Cobalt-Substituted LiMn2−yCoyO4 Spinels Synthesized by Pechini Process. Mater. Chem. Phys. 2003, 78, 189–195. [Google Scholar] [CrossRef]
- Qi, C.; Zhao, M.; Fang, T.; Zhu, Y.; Wang, P.; Xie, A.; Shen, Y. Multifunctional Hollow Porous Fe3O4@N-C Nanocomposites as Anodes of Lithium-Ion Battery, Adsorbents and Surface-Enhanced Raman Scattering Substrates. Molecules 2023, 28, 5183. [Google Scholar] [CrossRef] [PubMed]
- Sankar, A.; Paramasivaganesh, K.; Parthibavarman, M.; Meganathan, K.L. ZIF-8 Derived CuFe2O4 Nanoparticles: Evolution of Composition and Microstructures, and Their Electrochemical Performances as Anode for Lithium-Ion Batteries. Inorg. Chem. Commun. 2022, 140, 109424. [Google Scholar] [CrossRef]
- Karunakaran, G.; Kundu, M.; Maduraiveeran, G.; Kolesnikov, E.; Gorshenkov, M.V.; Balasingam, S.K.; Kumari, S.; Sasidharan, M.; Kuznetsov, D. Hollow Mesoporous Heterostructures Negative Electrode Comprised of CoFe2O4@Fe3O4 for next Generation Lithium Ion Batteries. Microporous Mesoporous Mater. 2018, 272, 1–7. [Google Scholar] [CrossRef]
- Mujahid, M.; Ullah Khan, R.; Mumtaz, M.; Mubasher; Soomro, S.A.; Ullah, S. NiFe2O4 Nanoparticles/MWCNTs Nanohybrid as Anode Material for Lithium-Ion Battery. Ceram. Int. 2019, 45, 8486–8493. [Google Scholar] [CrossRef]
- Du, F.-H.; Li, S.-Q.; Yan, Y.; Lu, X.-M.; Guo, C.; Ji, Z.; Hu, P.; Shen, X. Facile Fabrication of Fe0.8Mn1.2O3 with Various Nanostructures for High-Performance Lithium-Ion Batteries. Chem. Eng. J. 2022, 427, 131697. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Wei, X.; Yuan, C.; Yin, J.; Cao, D.; Wang, G. MgFe2O4 Nanoparticles as Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2013, 109, 89–94. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Tan, Q.; Zhong, Z.; Su, F. Facile Solvothermal Synthesis of Mesoporous Manganese Ferrite (MnFe2O4) Microspheres as Anode Materials for Lithium-Ion Batteries. J. Colloid Interface Sci. 2013, 398, 185–192. [Google Scholar] [CrossRef]
- Guo, X.; Lu, X.; Fang, X.; Mao, Y.; Wang, Z.; Chen, L.; Xu, X.; Yang, H.; Liu, Y. Lithium Storage in Hollow Spherical ZnFe2O4 as Anode Materials for Lithium Ion Batteries. Electrochem. Commun. 2010, 12, 847–850. [Google Scholar] [CrossRef]
- Wu, W.; Wei, Y.; Chen, H.; Wei, K.; Li, Z.; He, J.; Deng, L.; Yao, L.; Yang, H. In-Situ Encapsulation of α-Fe2O3 Nanoparticles into ZnFe2O4 Micro-Sized Capsules as High-Performance Lithium-Ion Battery Anodes. J. Mater. Sci. Technol. 2021, 75, 110–117. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, Y.; Meng, Y.; Huang, X.; Chen, S.; Chang, L.; Shen, J. ZnFe2O4 Nanoparticles Embedded Dispersedly inside 3D Porous Carbon Framework as Advanced Anode Materials of Li-Ion Batteries. J. Alloys Compd. 2022, 913, 165279. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, D.; Wang, X.; Qiu, H.; Zhang, T.; Zhang, M.; Tian, G.; Yue, H.; Feng, S.; Chen, G. Porous ZnFe2O4 Nanospheres as Anode Materials for Li-Ion Battery with High Performance. J. Alloys Compd. 2017, 721, 697–704. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Wang, L.; Yan, W. Fabrication of the ZnFe2O4 Fiber-in-Tube and Tubular Mesoporous Nanostructures via Single-Spinneret Electrospinning: Characterization, Mechanism and Performance as Anodes for Li-Ion Batteries. Electrochim. Acta 2016, 222, 1176–1185. [Google Scholar] [CrossRef]
- Chen, W.; Du, R.; Liang, H.; Zhou, Z.; Shao, L.; Shu, J.; Wang, Z. Lithium Storage Behavior of Manganese Based Complex Spinel Titanate as Anode Material for Li-Ion Batteries. J. Power Sources 2014, 272, 622–628. [Google Scholar] [CrossRef]
- Liang, Z.; Tu, H.; Kong, Z.; Yao, X.; Xu, D.; Liu, S.; Shao, Y.; Wu, Y.; Hao, X. Urchin like Inverse Spinel Manganese Doped NiCo2O4 Microspheres as High Performances Anode for Lithium-Ion Batteries. J. Colloid Interface Sci. 2022, 616, 509–519. [Google Scholar] [CrossRef]
- Cai, K.; Luo, S.H.; Cong, J.; Li, K.; Yan, S.X.; Hou, P.Q.; Wang, Q.; Zhang, Y.; Liu, X.; Lei, X.; et al. Facile Microwave-Assisted Hydrothermal Synthesis and Improved Electrochemical Performance of Micro Rhombus ZnMn2O4 Anodes for Li-Ion Batteries. J. Electroanal. Chem. 2022, 912, 116237. [Google Scholar] [CrossRef]
- Wang, Z.; Ai, L.; Ding, J.; Zhu, P.; Zhuang, J.; Zhao, J.; Li, B.; Yu, F.; Duan, X. MgMn2O4 Anode Materials for Lithium-Ion Batteries: Synthesis and Cationic Distribution Study. Vacuum 2022, 201, 111075. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Z.; Li, Y.; Jin, C.; Dong, F.; Zhao, W.; Qin, C.; Wang, Z. Spinel-Structured, Multi-Component Transition Metal Oxide (Ni,Co,Mn)Fe2O4−x as Long-Life Lithium-Ion Battery Anode Material. Batteries 2023, 9, 54. [Google Scholar] [CrossRef]
- Tian, K.-H.; Duan, C.-Q.; Ma, Q.; Li, X.-L.; Wang, Z.-Y.; Sun, H.-Y.; Luo, S.-H.; Wang, D.; Liu, Y.-G. High-Entropy Chemistry Stabilizing Spinel Oxide (CoNiZnXMnLi)3O4 (X = Fe, Cr) for High-Performance Anode of Li-Ion Batteries. Rare Met. 2022, 41, 1265–1275. [Google Scholar] [CrossRef]
- Liu, Y.; Russo, P.A.; Montoro, L.A.; Pinna, N. Recent Developments in Nb-based Oxides with Crystallographic Shear Structures as Anode Materials for High-rate Lithium-ion Energy Storage. Batter. Energy 2023, 2, 20220037. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Li, Q.; Zhang, J.; Sun, M.; Qi, W.; Li, J. Core-Shell MgFe2O4@C Nano-Composites Derived via Thermal Decomposition-Reduction Dual Strategy for Superior Lithium Storage. J. Alloys Compd. 2020, 834, 155207. [Google Scholar] [CrossRef]
- Chchiyai, Z.; El Bachraoui, F.; Tamraoui, Y.; Bih, L.; Faik, A.; Alami, J.; Manoun, B. Design and Characterization of Novel Manganite Perovskites Ba1−XBixTi1−XMnxO3 (0 ≤ x ≤ 0.2). Ceram. Int. 2020, 46, 26911–26922. [Google Scholar] [CrossRef]
- Chchiyai, Z.; El Bachraoui, F.; Tamraoui, Y.; Haily, E.M.; Bih, L.; Lahmar, A.; Alami, J.; Manoun, B. Design, Structural Evolution, Optical, Electrical and Dielectric Properties of Perovskite Ceramics Ba1−XBixTi1−XFexO3 (0 ≤ x ≤ 0.8). Mater. Chem. Phys. 2021, 273, 125096. [Google Scholar] [CrossRef]
- Chchiyai, Z.; El Bachraoui, F.; Tamraoui, Y.; Bih, L.; Lahmar, A.; Faik, A.; Alami, J.; Manoun, B. Synthesis, Structural Refinement and Physical Properties of Novel Perovskite Ceramics Ba1−XBixTi1−XMnxO3 (x = 0.3 and 0.4). Mater. Chem. Phys. 2021, 262, 124302. [Google Scholar] [CrossRef]
- Chchiyai, Z.; El Bachraoui, F.; Tamraoui, Y.; Mehdi Haily, E.; Bih, L.; Lahmar, A.; El Marssi, M.; Alami, J.; Manoun, B. Effect of Cobalt Doping on the Crystal Structure, Magnetic, Dielectric, Electrical and Optical Properties of PbTi1−xCoxO3−δ Perovskite Materials. J. Alloys Compd. 2022, 927, 166979. [Google Scholar] [CrossRef]
- El Bachraoui, F.; Chchiyai, Z.; Tamraoui, Y.; Louihi, S.; Alami, J.; Manoun, B. Effect of the Composition and Structure on the Optical Properties of Ba1−XLaxTi1−XFexO3 (0 ≤ x ≤ 1) Solid Solution: Correlation Study Using Rietveld Refinement. Mater. Charact. 2021, 175, 111058. [Google Scholar] [CrossRef]
- El Bachraoui, F.; Chchiyai, Z.; Tamraoui, Y.; Louihi, S.; Alami, J.; Manoun, B.; El Moussaoui, H.; Alami, J.; Manoun, B. Optical and Magnetic Properties of Perovskite Materials: Ba0.3La0.7Ti0.3Fe0.7O3 and Ba0.1La0.9Ti0.1Fe0.9O3. J. Rare Earths 2022, 40, 652–659. [Google Scholar] [CrossRef]
- Placke, T.; Siozios, V.; Schmitz, R.; Lux, S.F.; Bieker, P.; Colle, C.; Meyer, H.-W.; Passerini, S.; Winter, M. Influence of Graphite Surface Modifications on the Ratio of Basal Plane to “Non-Basal Plane” Surface Area and on the Anode Performance in Lithium Ion Batteries. J. Power Sources 2012, 200, 83–91. [Google Scholar] [CrossRef]
- Chchiyai, Z.; Hdidou, L.; Tayoury, M.; Chari, A.; Tamraoui, Y.; Alami, J.; Dahbi, M.; Manoun, B. Synthesis and Electrochemical Properties of Mn-Doped Porous Mg0.9Zn0.1Fe2−xMnxO4 (0 ≤ x ≤ 1.25) Spinel Oxides as Anode Materials for Lithium-Ion Batteries. J. Alloys Compd. 2023, 935, 167997. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Deng, X.; He, C.; Liu, E.; He, F.; Shi, C.; Zhao, N. Ultrathin-Nanosheet-Induced Synthesis of 3D Transition Metal Oxides Networks for Lithium Ion Battery Anodes. Adv. Funct. Mater. 2017, 27, 1605017. [Google Scholar] [CrossRef]
- Tian, L.; Zou, H.; Fu, J.; Yang, X.; Wang, Y.; Guo, H.; Fu, X.; Liang, C.; Wu, M.; Shen, P.K.; et al. Topotactic Conversion Route to Mesoporous Quasi-Single-Crystalline Co3O4 Nanobelts with Optimizable Electrochemical Performance. Adv. Funct. Mater. 2010, 20, 617–623. [Google Scholar] [CrossRef]
- Wang, Z.; Fei, P.; Xiong, H.; Qin, C.; Zhao, W.; Liu, X. CoFe2O4 Nanoplates Synthesized by Dealloying Method as High Performance Li-Ion Battery Anodes. Electrochim. Acta 2017, 252, 295–305. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Wang, Y.; Gu, S.; Yang, C.; Gao, T.; Cao, P.; Zhou, G. Preparation of Yolk–Double Shell Mn0.5Zn0.5Co2O4/C Nanomaterials as Anodes for High–Performance Lithium–Ion Batteries. Appl. Mater. Today 2022, 27, 101452. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, G.; Wang, T.; Wei, Z.; Sui, Y.; Shen, B.; Qi, J.; Wei, F.; Zheng, J. High-Entropy Oxides as Advanced Anode Materials for Long-Life Lithium-Ion Batteries. Nano Energy 2022, 95, 106962. [Google Scholar] [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent Progress of Surface Coating on Cathode Materials for High-Performance Lithium-Ion Batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, W.; Jin, B.; Li, H.; Li, S.; Zhu, G.; Jiang, Q. ZnFe2O4/MoS2/RGO Composite as an Anode for Rechargeable Lithium-Ion Batteries. J. Electroanal. Chem. 2018, 823, 407–415. [Google Scholar] [CrossRef]
- Liu, W.; Gao, R.; Yin, Y.; Cui, Y.; Yue, H.; Dong, H.; Yang, S. 3D Hierarchical Porous N-Doped Carbon Nanosheets/MgFe2O4 Composite as Anode Material with Excellent Cycling Stability and Rate Performance. Scr. Mater. 2020, 189, 36–41. [Google Scholar] [CrossRef]
- Yin, Y.; Huo, N.; Liu, W.; Shi, Z.; Wang, Q.; Ding, Y.; Zhang, J.; Yang, S. Hollow Spheres of MgFe2O4 as Anode Material for Lithium-Ion Batteries. Scr. Mater. 2016, 110, 92–95. [Google Scholar] [CrossRef]
- Rai, A.K.; Thi, T.V.; Gim, J.; Kim, J. Combustion Synthesis of MgFe2O4/Graphene Nanocomposite as a High-Performance Negative Electrode for Lithium Ion Batteries. Mater. Charact. 2014, 95, 259–265. [Google Scholar] [CrossRef]
- Jin, R.; Liu, J.; Qiu, H.; Xu, C.; Weng, L.; Liu, C.; Zeng, Y. Synthesis of Porous Nanosheet-Assembled ZnFe2O4@polypyrrole Yolk-Shell Microspheres as Anode Materials for High-Rate Lithium-Ion Batteries. J. Electroanal. Chem. 2020, 863, 114038. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Meng, Y.; Chang, L.; Huang, X.; Zheng, Y.; Shen, J.; Zhao, T. CNTs Boosting Superior Cycling Stability of ZnFe2O4/C Nanoparticles as High-Capacity Anode Materials of Li-Ion Batteries. J. Alloys Compd. 2022, 912, 165135. [Google Scholar] [CrossRef]
- Cai, K.; Luo, S.; Cong, J.; Li, K.; Yan, S.; Hou, P.; Song, Y.; Wang, Q.; Zhang, Y.; Liu, X.; et al. Sol-Gel Synthesis of Nano Block-like ZnMn2O4 Using Citric Acid Complexing Agent and Electrochemical Performance as Anode for Lithium-Ion Batteries. J. Alloys Compd. 2022, 909, 164882. [Google Scholar] [CrossRef]
- Dun, C.; Ji, X.; Xi, Y.; Yao, L.; Zhang, X.; Wang, Q.; Wu, H. Effect of N-Doped Carbon Layer on the Electrochemical Performance of CoFe2O4 Anode Materials in Lithium-Ion Batteries Synthesized from Spent LiCoO2 Batteries. J. Alloys Compd. 2022, 908, 164661. [Google Scholar] [CrossRef]
- Min, X.; Zhang, Y.; Yu, M.; Wang, Y.; Yuan, A.; Xu, J. A Hierarchical Dual-Carbon Supported ZnMn2O4/C Composite as an Anode Material for Li-Ion Batteries. J. Alloys Compd. 2021, 877, 160242. [Google Scholar] [CrossRef]
- Zou, L.; Kang, F.; Li, X.; Zheng, Y.-P.; Shen, W.; Zhang, J. Investigations on the Modified Natural Graphite as Anode Materials in Lithium Ion Battery. J. Phys. Chem. Solids 2008, 69, 1265–1271. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Yao, T.; Li, Y.; Zhu, L.; Li, F.; Han, X.; Cheng, Y.; Wang, H. Integrating Amorphous Vanadium Oxide into Carbon Nanofibers via Electrospinning as High-Performance Anodes for Alkaline Ion (Li+/Na+/K+) Batteries. Electrochim. Acta 2021, 369, 137711. [Google Scholar] [CrossRef]
- Wei, J.-L.; Wang, Z.-Y.; Sun, Y.-H.; Zhang, G.-L.; Guan, D.-C.; Nan, J.-M. The Kinetics Investigation of Nitrogen/Sulfur Co-Doped Reduced Graphene Oxide@spinel SnFe2O4/Sn0.205Fe1.727O3 as High Performance Anode for Lithium-Ion Batteries and Its Application in Full Cells. Electrochim. Acta 2021, 375, 138026. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Y.; Yan, J.; Bin, H.; Li, Y.; Xiao, S. Nanoparticles-Constructed Spinel ZnFe2O4 Anode Material with Superior Lithium Storage Performance Boosted by Pseudocapacitance. Mater. Res. Bull. 2018, 104, 188–193. [Google Scholar] [CrossRef]
- Mei, C.; Hou, S.; Liu, M.; Guo, Y.; Liu, T.; Li, J.; Fu, W.; Wang, L.; Zhao, L. MOF Derived ZnFe2O4 Nanoparticles Scattered in Hollow Octahedra Carbon Skeleton for Advanced Lithium-Ion Batteries. Appl. Surf. Sci. 2021, 541, 148475. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, I.Y.; Adpakpang, K.; Hwang, S.-J. The Beneficial Effect of Nanocrystalline and Amorphous Nature on the Anode Performance of Manganese Oxide for Lithium Ion Batteries. Electrochim. Acta 2015, 174, 391–399. [Google Scholar] [CrossRef]
- Duan, C.; Tian, K.; Li, X.; Wang, D.; Sun, H.; Zheng, R.; Wang, Z.; Liu, Y. New Spinel High-Entropy Oxides (FeCoNiCrMnXLi)3O4 (X = Cu, Mg, Zn) as the Anode Material for Lithium-Ion Batteries. Ceram. Int. 2021, 47, 32025–32032. [Google Scholar] [CrossRef]
- Lamhani, M.; Chchiyai, Z.; Elomrani, A.; Manoun, B.; Hasnaoui, A. Enhanced Photocatalytic Water Splitting of SrTiO3 Perovskite through Cobalt Doping: Experimental and Theoretical DFT Understanding. Inorg. Chem. 2023, 62, 13405–13418. [Google Scholar] [CrossRef] [PubMed]
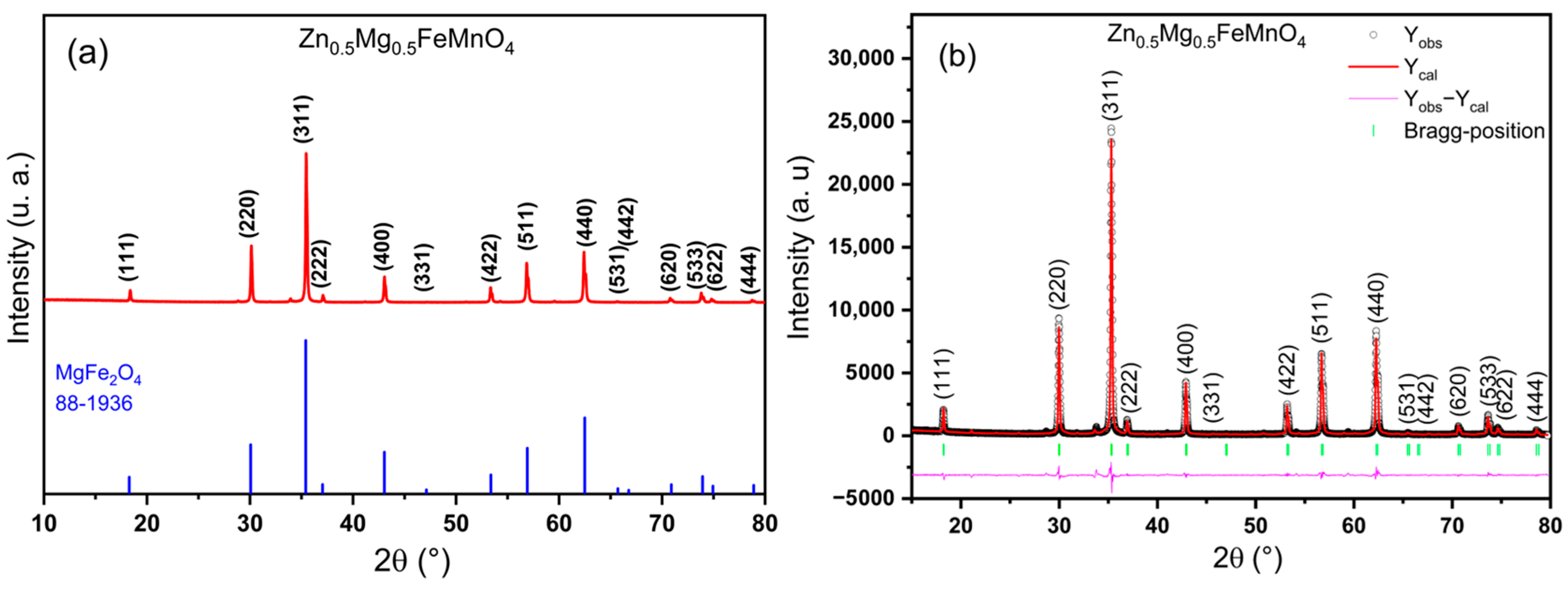

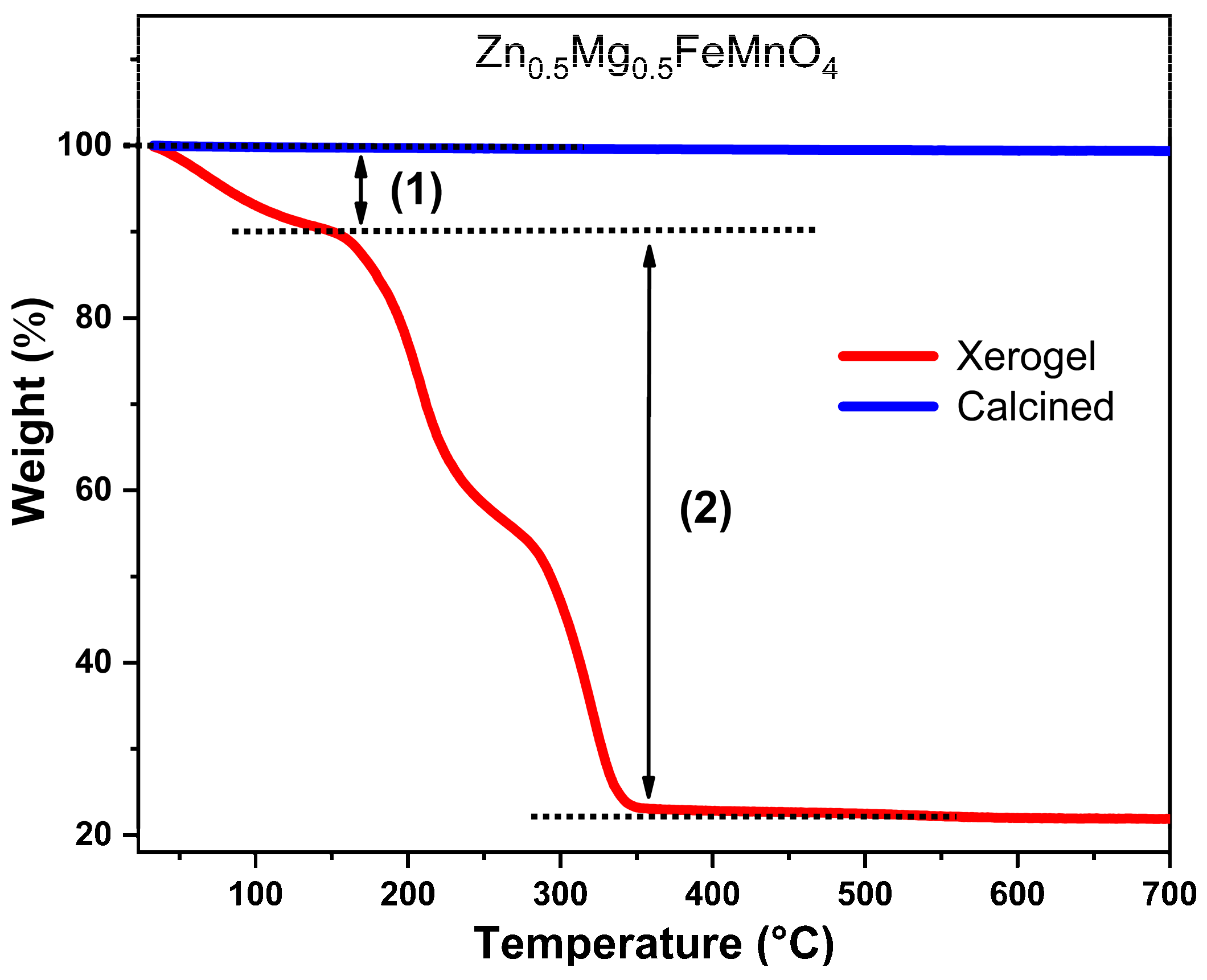
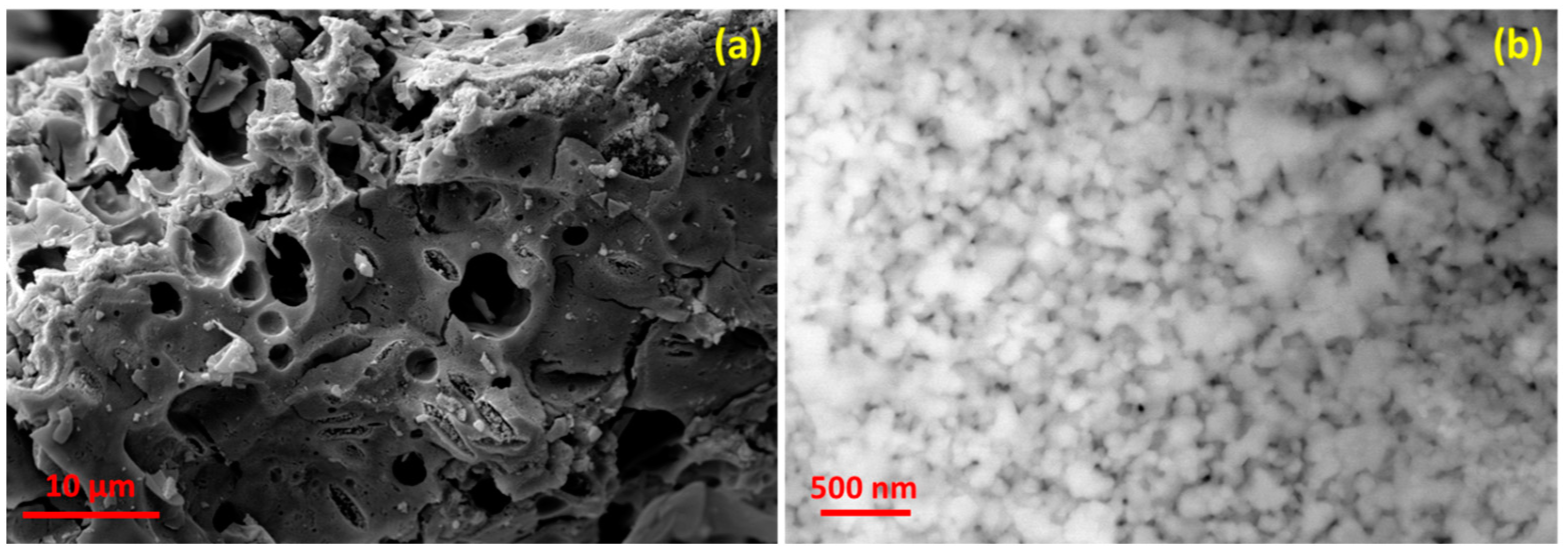
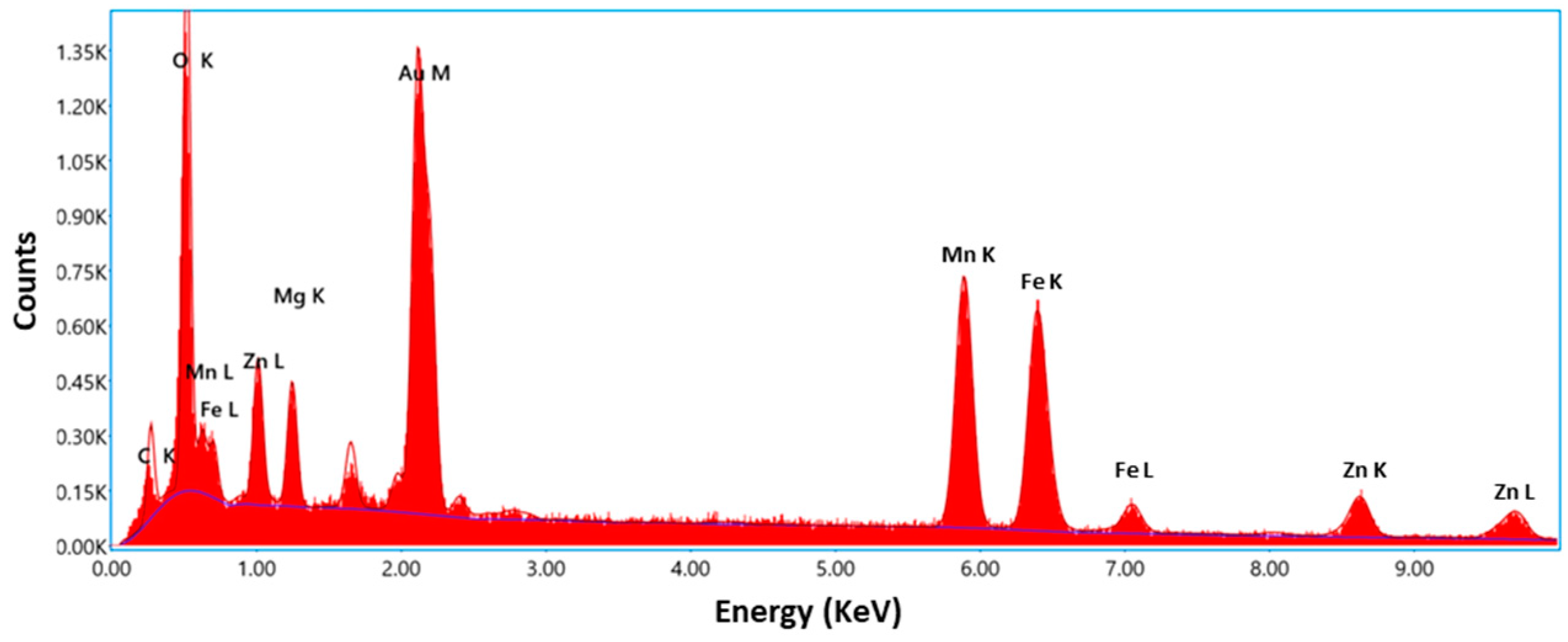
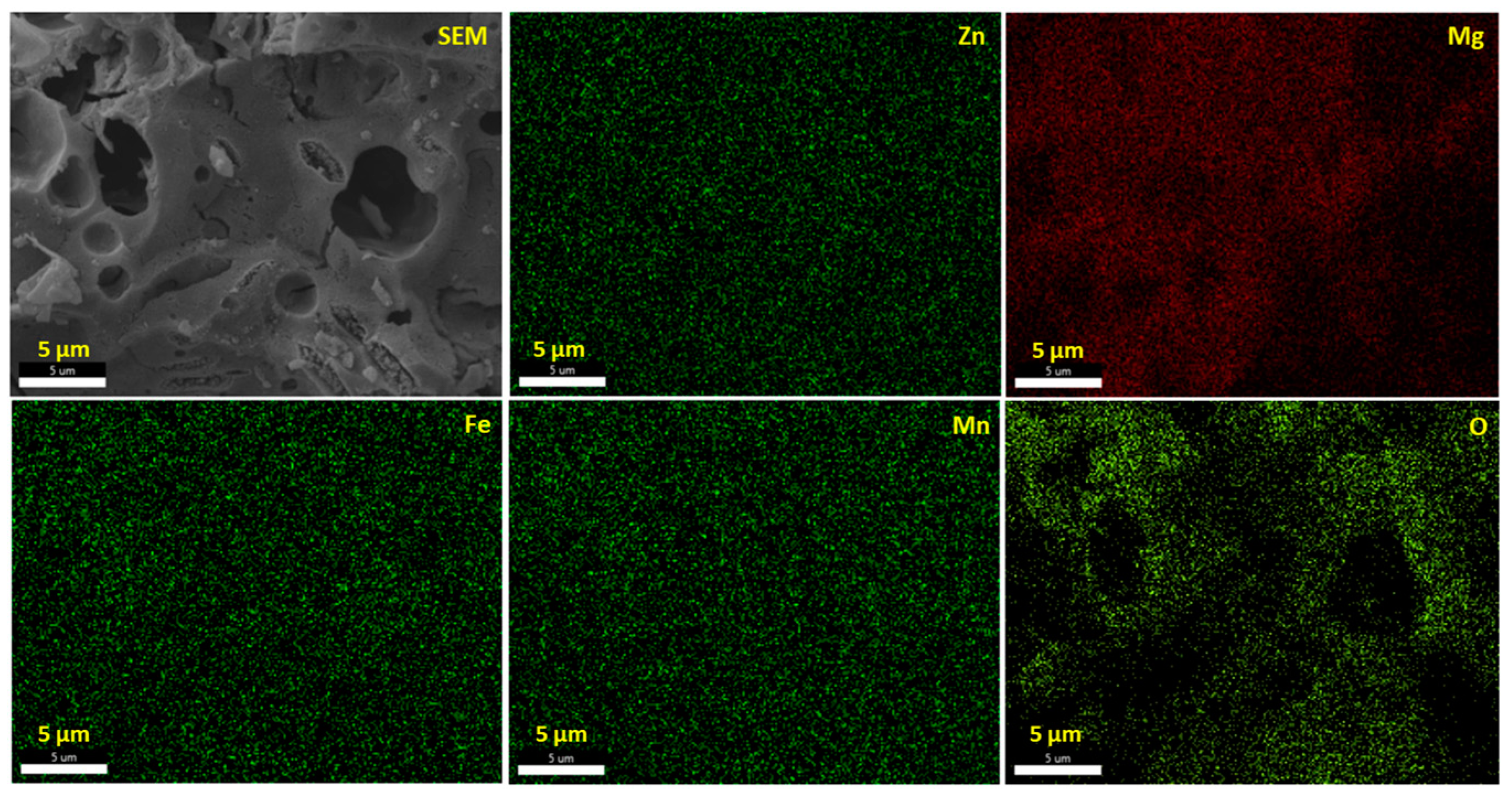
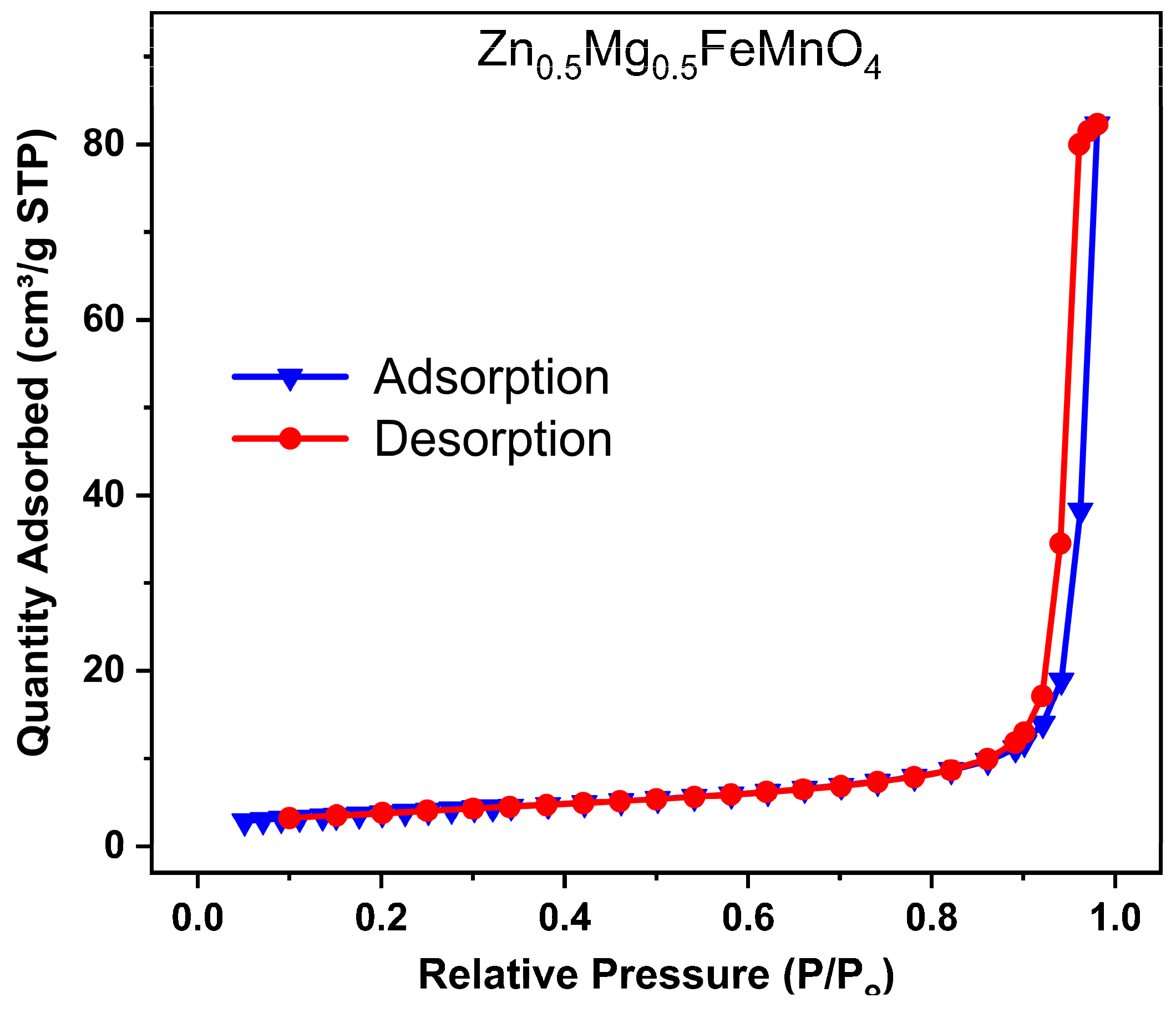
| Wavelengths (Å) | Lattice Parameters | Crystal Structure | Mixing Factor | Caglioti Parameters | R-Factors |
|---|---|---|---|---|---|
| λkα1 = 1.54056 λkα2 = 1.54439 | a = 8.4265 (2) Å V = 598.33 (1) Å3 | Cubic Fdm | η = 635 (7) | U = 0.029 (6) V = −0.011 (6) W = 0.0154 (2) | RF = 2.41, RB= 2.83 RP = 7.27, Rexp = 11.5 Rwp = 12.3 |
| Anode | Applied Current Density (mA g−1) | Cycle Number | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| Graphite | 76 | 50 | 265 | [78] |
| MgFe2O4 nanoparticles | 90 | 50 | 474 | [40] |
| MnFe2O4 mesoporous microspheres | 744 | 50 | 552 | [41] |
| ZnFe2O4 porous nanospheres | 200 | 80 | 869 | [45] |
| ZnMn2O4 microsheets | 90 | 40 | 337 | [49] |
| MgMn2O4 nanoparticles | 50 | 100 | 484 | [50] |
| Porous Zn0.5Mg0.5FeMnO4 | 150 | 80 | 300 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chchiyai, Z.; El Ghali, O.; Lahmar, A.; Alami, J.; Manoun, B. Design and Performance of a New Zn0.5Mg0.5FeMnO4 Porous Spinel as Anode Material for Li-Ion Batteries. Molecules 2023, 28, 7010. https://doi.org/10.3390/molecules28207010
Chchiyai Z, El Ghali O, Lahmar A, Alami J, Manoun B. Design and Performance of a New Zn0.5Mg0.5FeMnO4 Porous Spinel as Anode Material for Li-Ion Batteries. Molecules. 2023; 28(20):7010. https://doi.org/10.3390/molecules28207010
Chicago/Turabian StyleChchiyai, Zakaria, Oumayema El Ghali, Abdelilah Lahmar, Jones Alami, and Bouchaib Manoun. 2023. "Design and Performance of a New Zn0.5Mg0.5FeMnO4 Porous Spinel as Anode Material for Li-Ion Batteries" Molecules 28, no. 20: 7010. https://doi.org/10.3390/molecules28207010






