Development of Different Kinds of Electrocatalyst for the Electrochemical Reduction of Carbon Dioxide Reactions: An Overview
Abstract
:1. Introduction
2. Carbon-Based Electrode Materials
3. Graphene-Based Electrode Materials
4. Metal-Oxide-Based Electrode Materials
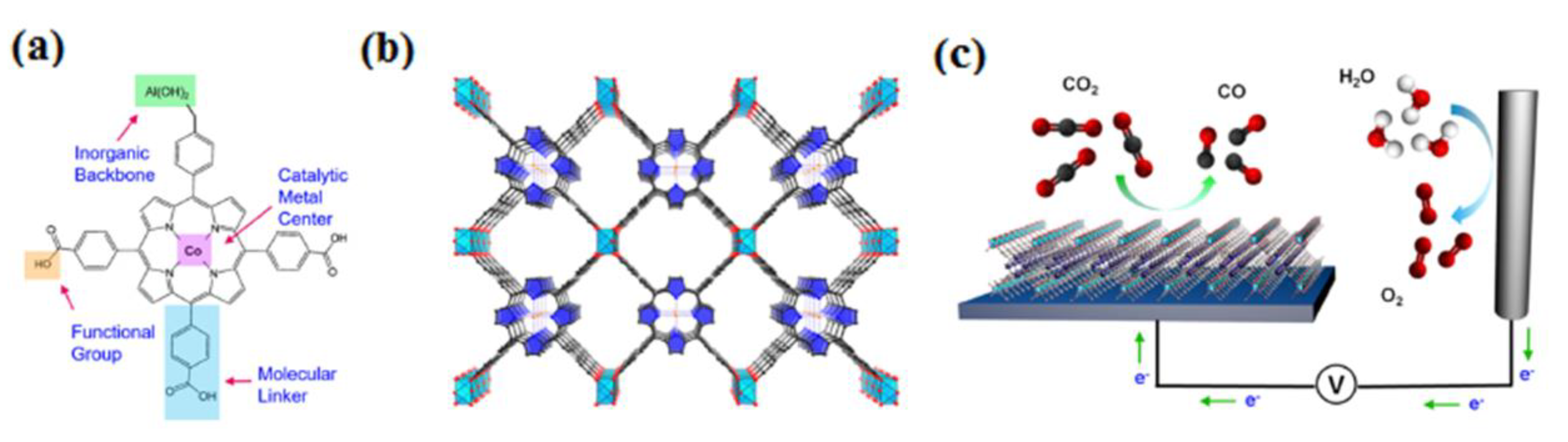
5. MOF-Based Electrode Materials
6. Metal-Based Electrocatalysts
7. Transition-Metal-Based Electrocatalyst
8. Conducting Polymers
9. MXenen-Based Electrodes
10. Free-Standing Electrode Materials
11. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRR | Carbon dioxide reduction reaction |
| eCO2R | Electrochemical carbon dioxide reduction |
| CNT | Carbon nanotube |
| MWCNT | Multi-walled carbon nanotube |
| MOFs | Metal-organic frameworks |
| CCS | Carbon capture and storage |
| CCU | Carbon capture and utilization |
| TMD | Transition metal dichalcogenides |
| PANI | Polyaniline |
References
- Dey, R.S.; Raj, C.R. Development of an amperometric cholesterol biosensorbasedon graphene-Ptnanoparticlehybrid material. J. Phys. Chem. C 2010, 114, 21427–21433. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.G.; Quéré, C.L.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Conway, T.J.; Gillett, N.P.; Houghton, R.A.; Marland, G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc Natl. Acad Sci. USA 2007, 104, 18866–18870. [Google Scholar] [CrossRef]
- Huan, T.N.; Corte, D.A.; Lamaison, S.; Karapinar, D.; Lutz, L.; Menguy, N.; Foldyna, M.; Turren-Cruz, S.-H.; Hagfeldt, A.; Federico Bella, F.; et al. Low-cost high-efficiency system for solar-driven conversion of CO to hydrocarbons. Proc. Natl. Acad. Sci. USA 2019, 116, 9735–9740. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.; Speranza, R.; Savino, U.; Zeng, J.; Farkhondehfal, M.A.; Lamberti, A.; Chiodoni, A.; Pirri, C.F. An integrated device for the solar-driven electrochemical conversion of CO2 to CO. ACS Sustain. Chem. Eng. 2020, 8, 7563–7568. [Google Scholar] [CrossRef]
- Yin, Z.; Palmore, G.T.R.; Sun, S. Electrochemical reduction of CO2 catalyzed by metal nanocatalysts. Trends Chem. 2019, 1, 739–750. [Google Scholar] [CrossRef]
- Gurudayal, G.; Bullock, J.; Srankó, D.F.; Towle, C.M.; Lum, Y.; Hettick, M.; Scott, M.C.; Javey, A.; Ager, J. Efficient solar-driven electrochemical CO2 reduction to hydrocarbons and oxygenates. Energy Environ. Sci. 2017, 10, 2222–2230. [Google Scholar] [CrossRef]
- Poon, K.C.; Wan, W.Y.; Su, H.; Sato, H. A review on recent advances in the electrochemical reduction of CO2 to CO with nano-electrocatalysts. RSC Adv. 2022, 12, 22703–22721. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- Psarras, P.C.; Comello, S.; Bains, P.; Charoensawadpong, P.; Reichelstein, S.; Wilcox, J. Carbon capture and utilization in the industrial sector. Environ. Sci. Technol. 2017, 51, 11440–11449. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, P.; Li, A.; Wei, B.; Si, K.; Wei, Y.; Wang, X.; Zhu, G.; Chen, Q.; Gu, X.; et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nature Commun. 2022, 13, 1877. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, C.; Zhang, H.; Chang, X.; Chen, J.G.; Goddard, W.A., III; Cheng, M.; Xu, B.; Lu, Q. Oxygen induced promotion of electrochemical reduction of CO2 via co-electrolysis. Nature Commun. 2020, 11, 3844. [Google Scholar] [CrossRef]
- Li, Y.; He, Z.; Wu, F.; Wang, S.; Cheng, Y.; Jian, S. Defect engineering of high-loading single-atom catalysts for electrochemical carbon dioxide reduction. Mater. Rep. Energy 2023, 3, 100197. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Dong, J.; Jia, K.; Sun, T.; Xu, L. Recent advances in designing efficient electrocatalysts for electrochemical carbon dioxide reduction to formic acid/formate. J. Electroanal. Chem. 2023, 928, 117018. [Google Scholar] [CrossRef]
- Yan, L.; Wu, Z.; Li, C.; Wang, J. Sb-doped SnS2 nanosheets enhance electrochemical reduction of carbon dioxide to formate. J. Ind. Eng. Chem. 2023, 123, 33–40. [Google Scholar] [CrossRef]
- Hongrutai, N.; Watmanee, S.; Pinthong, P.; Panpranot, J. Electrochemical reduction of carbon dioxide on the oxide-containing electrocatalysts. J. CO2 Util. 2022, 64, 102194. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, X.; Gao, T.; Wang, X.B. Metal-free carbon-based nanomaterials for electrochemical nitrogen and carbon dioxide reductions. Mater. Res. Bull. 2021, 140, 111294. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chiu, K.Y.; Fan, C.H.; Chen, H.L. Electrocatalytic carbon dioxide reduction on graphene-supported Ni cluster and its hydride: Insight from first-principles calculations. Appl. Surf. Sci. 2023, 629, 157418. [Google Scholar] [CrossRef]
- Kayan, D.B.; Köleli, F. Simultaneous electrocatalytic reduction of dinitrogen and carbon dioxide on conducting polymer electrodes. Appl. Catal. B Environ. 2016, 181, 88–93. [Google Scholar] [CrossRef]
- Wijaya, D.T.; Haryanto, A.; Lim, H.W.; Jin, K.; Lee, C.W. Sub-2nm mixed metal oxide for electrochemical reduction of carbon dioxide to carbon monoxide. J. Energy Chem. 2023, 84, 303–310. [Google Scholar] [CrossRef]
- Liang, X.; Ji, S.; Chen, Y.; Wang, D. Synthetic strategies for MOF-based single-atom catalysts for photo- and electro-catalytic CO2 reduction. iScience 2022, 25, 104177. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Mignosa, M.; Monti, N.B.D.; Sacco, A.; Pirri, C.F. Engineering copper nanoparticle electrodes for tunable electrochemical reduction of carbon dioxide. Electrochim. Acta 2023, 464, 142862. [Google Scholar] [CrossRef]
- Franco, F.; Rettenmaier, C.; Jeon, H.S.; Cuenya, B.R. Transition metal-based catalysts for the electrochemical CO2 reduction: From atoms and molecules to nanostructured material. Chem. Soc. Rev. 2020, 49, 6884–6946. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.J.; Handoko, A.D.; Seh, Z.W. A realistic take on MXenes for electrochemical reduction of carbon dioxide. Diam. Relat. Mater. 2022, 130, 109461. [Google Scholar] [CrossRef]
- Yang, H.; Lin, Q.; Wu, Y.; Li, G.; Hu, Q.; Chai, X.; Ren, X.; Zhang, Q.; Liu, J.; He, C. Highly efficient utilization of single atoms via constructing 3D and free-standing electrodes for CO2reduction with ultrahigh current density. Nano Energy 2020, 70, 104454. [Google Scholar] [CrossRef]
- Yao, P.; Qiu, Y.; Zhang, T.; Su, P.; Li, X.; Zhang, H. N-doped nanoporous carbon from biomass as a highly efficient electrocatalyst for the CO2 reduction reaction. ACS Sustain. Chem. Eng. 2019, 7, 5249–5255. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, P.; Wang, Y.; Xiang, X.; Kang, P. Acidic electrochemical reduction of CO2 using nickel nitride on multi-walled carbon nanotube as selective catalyst. ACS Sustain. Chem. Eng. 2019, 7, 6106–6112. [Google Scholar] [CrossRef]
- Zhu, H.L.; Zheng, Y.Q.; Shui, M. Synergistic interaction of nitrogen-doped carbon nanorod array anchored with cobalt phthalocyanine for electrochemical reduction of CO2. ACS Appl. Energy Mater. 2020, 3, 3893–3901. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Ding, J.; Xu, N.; Bernards, M.T.; Yi He, Y.; Shi, Y. Three-dimensional nitrogen-doped graphene aerogel-supported MnO nanoparticles as efficient electrocatalysts for CO2 reduction to CO. ACS Sustain. Chem. Eng. 2020, 8, 4983–4994. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Yue, G.; Hu, Q.; Wang, L. Boosting CH3OH production in electrocatalytic CO2 reduction over partially oxidized 5 nm cobalt nanoparticles dispersed on single layer nitrogen-doped graphene. ACS Appl. Mater. Interfaces 2018, 10, 44403–44414. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Swain, G.M.; Jiang, X. Nanocarbon electrochemistry and electroanalysis: Current status and future perspectives. Electroanalysis 2015, 28, 27–34. [Google Scholar] [CrossRef]
- Zhao, K.; Quan, X. Carbon-based materials for electrochemical reduction of CO2 to C2+ oxygenates: Recent progress and remaining challenges. ACS Catal. 2021, 11, 2076–2097. [Google Scholar] [CrossRef]
- Tian, H.; Wang, T.; Zhang, F.; Zhao, S.; Wan, S.; He, F.; Wang, G. Tunable porous carbon spheres for high-performance rechargeable batteries. J. Mater. Chem. A 2018, 6, 12816–12841. [Google Scholar] [CrossRef]
- Shi, H.; Shen, Y.; He, F.; Li, Y.; Liu, A.; Liu, S.; Zhang, Y. Recent advances of doped carbon as non-precious catalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 15704–15716. [Google Scholar] [CrossRef]
- Deng, L.; Fang, H.; Zhang, P.; Abdelkader, A.; Ren, X.; Li, Y.; Xie, N. Nitrogen and sulfur dual-doped carbon microtubes with enhanced performances for oxygen reduction reaction. J. Electrochem. Soc. 2016, 163, H343–H349. [Google Scholar] [CrossRef]
- Lum, Y.; Kwon, Y.; Lobaccaro, P.; Chen, L.; Clark, E.L.; Bell, A.T.; Ager, J.W. Trace levels of copper in carbon materials show significant electrochemical CO2 reduction activity. ACS Catal. 2016, 6, 202–209. [Google Scholar] [CrossRef]
- Hu, X.; Joo, P.H.; Matios, E.; Wang, C.; Luo, J.; Yang, K.; Li, W. Designing an All-solid-state sodium-carbon dioxide battery enabled by nitrogen-doped nanocarbon. Nano Lett. 2020, 20, 3620–3626. [Google Scholar] [CrossRef]
- Berger, C. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Lira, P.; Hu, G.; Luo, J.; Sun, Z.; Davis, R.; Huang, Y.; Toan, S. Graphene-based electrodes and catalysts for electroreduction of CO2 to low-carbon alcohols. Mater. Rep. Energy 2023, 3, 100192. [Google Scholar]
- Vasileff, A.; Zheng, Y.; Qiao, S.Z. Carbon solving carbon’s problems: Recent progress of nanostructured carbon-based catalysts for the electrochemical reduction of CO2. Adv. Energy Mater. 2017, 7, 1700759. [Google Scholar] [CrossRef]
- Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B.A.; Haasch, R.; Abiade, J.; Yarin, A.L.; Salehi-Khojin, A. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 2013, 4, 3819. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Z.; Zhang, C.; Zhou, J.; Liu, S.; Cao, Q. Single-atom catalysts supported on the graphene/graphdiyne heterostructure for effective CO2 electroreduction. Inorg. Chem. 2022, 61, 12012–12022. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.N.; Wen, J.; Chen, A. Unique copper and reduced graphene oxide nanocomposite toward the efficient electrochemical reduction of carbon dioxide. Sci. Rep. 2017, 7, 3184. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Javed, M.S.; Najam, T.; Molochas, C.; Khan, N.A.; Nazir, M.A.; Xu, M.; Tsiakaras, P.; Bao, S.J. Metal oxides for the electrocatalytic reduction of carbon dioxide: Mechanism of active sites, composites, interface and defect engineering strategies. Coord. Chem. Rev. 2022, 471, 214716. [Google Scholar] [CrossRef]
- Zhang, L.; Garcia, I.M.; Albo, J.; Sánchez, C.M.S. Electrochemical CO2 reduction reaction on cost-effective oxide-derived copper and transition metal-nitrogen-carbon catalysts. Curr. Opin. Electrochem. 2020, 23, 65–73. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Rutkowska, I.A. Mixed-metal-oxide based hybrid electrocatalytic materials for efficient reduction of carbon dioxide, The Electrochemical Society. In Electrochemical Society Meeting Abstracts 237; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2020; p. 2599. [Google Scholar] [CrossRef]
- Hao, L.; Sun, Z. Metal oxide-based materials for electrochemical CO2 reduction. Acta Phys.-Chim. 2020, 37, 2009033. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition metal oxide electrode materials for supercapacitors: A review of recent developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Wan, J.H.; Yeo, B.S. Electrochemical reduction of carbon dioxide to ethane using nanostructured Cu2O-derived copper catalyst and Palladium(II)chloride. J. Phys. Chem. C 2015, 119, 6875–26882. [Google Scholar] [CrossRef]
- Jiwantia, P.K.; Ichzanb, A.M.; Dewandarub, R.K.P.; Atriardib, S.R.; Einagac, Y.; Ivandini, T.A. Improving the CO2 electrochemical reduction to formic acid using iridium oxide-modified boron-doped diamond electrodes. Diam. Relat. Mater. 2020, 106, 107874. [Google Scholar] [CrossRef]
- Dongare, S.; Singh, N.; Bhunia, H. Oxide-derived Cu-Zn nanoparticles supported on N-doped graphene for electrochemical reduction of CO2 to ethanol. Appl. Surf. Sci. 2021, 556, 149790. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, H.; Cai, W.B.; Wang, H. Li Electrochemical tuning of metal oxide for highly selective CO2 reduction. ACS Nano 2017, 11, 6451–6458. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on Copper(I) oxide catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Rowaili, F.N.A.; Jamal, A.; Mohammad, S.; Shammakh, M.S.B.; Rana, A. A review on recent advances for electrochemical reduction of carbon dioxide to methanol using metal-organic framework (MOF) and non-MOF catalysts: Challenges and future prospects. ACS Sustain. Chem. Eng. 2018, 6, 15895–15914. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Ma, Y.; Yin, J.; Wang, Y.; Fan, Z. Electrocatalytic reduction of carbon dioxide to high-value multi-carbon products with metal-organic frameworks and their derived materials. ACS Materials Lett. 2022, 4, 2058–2079. [Google Scholar] [CrossRef]
- Wang, G.D.; Krishna, R.; Li, Y.Z.; Ma, Y.Y.; Hou, L.; Wang, Y.Y.; Zhu, Z. Rational Construction of Ultrahigh Thermal Stable MOF for Efficient Separation of MTO Products and Natural Gas. ACS Mater. Lett. 2023, 5, 1091–1099. [Google Scholar] [CrossRef]
- Kang, S.J.; Won, J.H.; Choi, H.; Sim, W.; Kim, M.K.; Sultan, S.; Kwon, Y.; Jeong, H.M. Compensating the impurities on the Cu surface by MOFs for enhanced hydrocarbon production in the electrochemical reduction of carbon dioxide. J. Energy Chem. 2022, 66, 68–73. [Google Scholar] [CrossRef]
- Vaitsis, C.; Kanellou, E.; Pandis, P.K.; Papamichael, I.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Sonochemical synthesis of zinc adipate Metal-Organic Framework(MOF) for the electrochemical reduction of CO: MOF and circulareconomy potential. Sustain. Chem. Pharm. 2022, 29, 100786. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Liang, Z.L.; Hou, S.L.; Xie, Y.; Ma, Y.; Zhang, Y.; Zhao, B. Efficient CO electroreduction coupled with semi-dehydrogenation of tetrahydroisoquinoline by MOFs modified electrodes. J. Energy Chem. 2021, 63, 328–335. [Google Scholar] [CrossRef]
- Xu, B.; Hasan, I.M.U.; Peng, L.; Liu, J.; Xu, N.; Fan, M.; Niazi, N.K.; Qiao, J. Anion-regulation engineering toward Cu/In/MOF bimetallic electrocatalysts for selective electrochemical reduction of CO2 to CO/formate. Mater. Rep. Energy 2022, 2, 100139. [Google Scholar] [CrossRef]
- Meng, Z.; Luo, J.; Li, W.; Mirica, K.A. Hierarchical Tuning of the Performance of Electrochemical Carbon Dioxide Reduction Using Conductive Two-Dimensional Metallo phthalocyanine Based Metal-Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 21656–21669. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Song Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal-Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef]
- Rayer, A.V.; Reid, E.; Kataria, A.; Luz, I.; Thompson, S.J.; Lail, M.; Zhou, J.; Soukri, M. Electrochemical carbon dioxide reduction to isopropanol using novel carbonized copper metal organic framework derived electrodes. J. CO2 Util. 2020, 39, 101159. [Google Scholar] [CrossRef]
- Gruber, N.; Clement, D.; Carter, B.R.; Feely, R.A. The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science 2019, 363, 1193–1199. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, Z.; Gao, N.; Huang, L.; Lin, Z.; Liu, Y.; Meng, F.; Deng, J.; Jin, S.; Zhang, Q.; et al. Coupled vacancy pairs in Ni-doped CoSe for improved electrocatalytic hydrogen production through topochemical deintercalation. Angew. Chem. Int. Ed. 2020, 59, 22743–22748. [Google Scholar] [CrossRef]
- De Sousa, L.; Benes, N.E.; Mul, G. Evaluating the Effects of Membranes, Cell Designs, and Flow Configurations on the Performance of Cu-GDEs in Converting CO to CO. ACS EST Eng. 2022, 2, 2034–2042. [Google Scholar] [CrossRef]
- Nielsen, I.M.; Leung, K. Cobalt−Porphyrin Catalyzed Electrochemical Reduction of Carbon Dioxide in Water. 1. A Density Functional Study of Intermediates. J. Phys. Chem. A 2010, 114, 10166–10173. [Google Scholar] [CrossRef] [PubMed]
- Can, M.; Armstrong, F.A.; Ragsdale, S.W. Structure, Function, and Mechanism of the Nickel Metallo enzymes, CO Dehydrogenase, and Acetyl-CoA Synthase. Chem. Rev. 2014, 114, 4149–4174. [Google Scholar] [CrossRef] [PubMed]
- Corbin, N.; Zeng, J.; Williams, K.; Manthiram, K. Heterogeneous Molecular Catalysts for Electrocatalytic CO2 Reduction. Nano Res. 2019, 12, 2093–2125. [Google Scholar] [CrossRef]
- Smith, P.T.; Nichols, E.M.; Cao, Z.; Chang, C.J. Hybrid Catalysts for Artificial Photosynthesis: Merging Approaches from Molecular, Materials, and Biological Catalysis. Acc. Chem. Res. 2020, 53, 575–587. [Google Scholar] [CrossRef]
- Derrick, J.S.; Loipersberger, M.; Chatterjee, R.; Iovan, D.A.; Smith, P.T.; Chakarawet, K.; Yano, J.; Long, J.R.; Head-Gordon, M.; Chang, C.J. Metal−Ligand Cooperativity via Exchange Coupling Promotes Iron-Catalyzed Electrochemical CO2 Reduction at Low Overpotentials, Journal of the American Chemical Society. J. Am. Chem. Soc. 2020, 142, 20489–20501. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; Zhong, H.X.; Zhang, T.T.; Xu, W.B.; Su, P.P.; Li, X.F.; Zhang, H.M. Selective Electrochemical Reduction of Carbon Dioxide Using Cu Based Metal Organic Framework for CO2 Capture. ACS Appl. Mater. Interfaces 2018, 10, 2480–2489. [Google Scholar] [CrossRef]
- Néstor, E. Mendieta-Reyes, Ana Korina Díaz-García, and Roberto Gómez Simultaneous electrocatalytic CO2 reduction and enhanced electrochromic effect at WO3 nanostructured electrodes in acetonitrile. ACS Catal. 2018, 8, 1903–1912. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.S.; Hwang, Y.J.; Min, B.K. Surface-Morphology-Dependent Electrolyte Effects on Gold-Catalyzed Electrochemical CO2 Reduction. J. Phys. Chem. C 2017, 121, 22637–22643. [Google Scholar] [CrossRef]
- Tomisaki, M.; Kasahara, S.; Natsui, K.; Ikemiya, N.; Einaga, Y. Switchable Product Selectivity in the Electrochemical Reduction of Carbon Dioxide Using Boron-Doped Diamond Electrodes. J. Am. Chem. Soc. 2019, 141, 7414–7420. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-Based Catalysts on Various Carbon Supports and Conducting Polymers for Direct Methanol Fuel Cell Applications: A Review. Nanoscale Res. Lett. 2018, 13, 410. [Google Scholar] [CrossRef]
- Tan, D.; Wulan, B.; Cao, X.; Zhang, J. Strong interactions of metal-support for efficient reduction of carbon dioxide into ethylene. Nano Energy 2021, 89, 106460. [Google Scholar] [CrossRef]
- Li, M.; Garg, S.; Chang, X.; Ge, L.; Li, L.; Konarova, M.; Rufford, T.E.; Rudolph, V.; Wang, G. Toward Excellence of Transition Metal-Based Catalysts for CO2 Electrochemical Reduction: An Overview of Strategies and Rationales. Small Methods 2020, 4, 2000033. [Google Scholar] [CrossRef]
- Kang, S.; Ju, S.; Han, S.; Kang, Y. Computational Identification of Transition-Metal Dichalcogenides for Electrochemical CO2 Reduction to Highly Reduced Species Beyond CO and HCOOH. J. Phys. Chem. C 2020, 124, 25812–25820. [Google Scholar] [CrossRef]
- Nazir, R.; Khalfani, A.; Abdelfattah, O.; Kumar, A.; Saleh Saad, M.A.; Ali, S. Nanosheet Synthesis of Mixed Co3O4/CuO via Combustion Method for Methanol Oxidation and Carbon Dioxide Reduction. Langmuir 2020, 36, 12760–12771. [Google Scholar] [CrossRef]
- Halilu, A.; Hadj-Kali, M.K.; Hashim, M.A.; Ali, E.M.; Bhargava, S.K. Electroreduction of CO and Quantifi cation in New Transition-metal-Based Deep Eutectic Solvents Using Single-Atom AgElectrocatalyst. ACS Omega 2022, 7, 14102–14112. [Google Scholar] [CrossRef]
- Landers, A.T.; Fields, M.; Torelli, D.A.; Xiao, J.; Hellstern, T.R.; Francis, S.A.; Tsai, C.; Kibsgaard, J.; Lewis, N.S.; Chan, K.; et al. The Predominance of Hydrogen Evolution on Transition Metal Sulfides and Phosphides under CO2 Reduction Conditions: An Experimental and Theoretical Study. ACS Energy Lett. 2018, 3, 1450–1457. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, Y.; Li, K.; Tang, H.; Wang, Y.; Wu, Z. Transition metal doped C3N monolayer as efficient electrocatalyst for carbon dioxide electroreduction: A computational study. Appl. Surf. Sci. 2021, 542, 148568. [Google Scholar] [CrossRef]
- Grice, K.A. Carbon dioxide reduction with homogenous early transition metal complexes: Opportunities and challenges for developing CO2 catalysis. Coord. Chem. Rev. 2017, 336, 78–95. [Google Scholar] [CrossRef]
- Watkins, J.D.; Bocarsly, A.B. Direct Reduction of Carbon Dioxide to Formate in High-Gas-Capacity Ionic Liquids at Post-Transition-Metal Electrodes. ChemSusChem 2014, 7, 284–290. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Yu, J.; Hu, B.; Zhao, H.; Tsiakaras, P.; Song, S. Copper oxide derived nanostructured self-supporting Cu electrodes for electrochemical reduction of carbon dioxide. Electrochim. Acta 2019, 328, 135083. [Google Scholar] [CrossRef]
- Esmaeilirad, M.; Baskin, A.; Kondori, A.; Matias, A.S.; Qian, J.; Song, B.; Saray, M.T.; Kucuk, K.; Belmonte, A.R.; Delgado, P.N.M.; et al. Gold-like activity copper-like selectivity of heteroatomic transition metal carbides for electrocatalytic carbon dioxide reduction reaction. Nature Commun. 2021, 12, 5067. [Google Scholar] [CrossRef] [PubMed]
- Sassone, D.; Zeng, J.; Fontana, M.; Sacco, A.; Farkhondafal, M.A.; Periolatto, M.; Pirri, C.F.; Bocchini, S. Polymer-metal complexes as emerging catalysts for electrochemical reduction of carbon dioxide. J. Appl. Electrochem. 2021, 51, 1301–1311. [Google Scholar] [CrossRef]
- Diao, Y.; Jung, S.; Kouhnavard, M.; Woon, R.; Yang, H.; Biswas, P.; Arcy, J.M.D. Single PEDOT Catalyst Boosts CO2Photoreduction Efficiency. ACS Cent. Sci. 2021, 7, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Ponnurangam, S.; Cheryshova, I.V.; Somasundaran, P. Nitrogen-containing polymers as a platform for CO2 electroreduction. Adv. Colloid. Inter. Sci. 2017, 244, 184–198. [Google Scholar] [CrossRef]
- Lui, S.R.; Luna, P.D. How increasing proton and electron conduction benefits electrocatalytic CO2 reduction. Cell Matter 2021, 4, 1555–1577. [Google Scholar]
- Karim, K.M.R.; Tarek, M.; Sarkar, S.M.; Mouras, R.; Ong, H.; Abdullah, C.K.; Cheng, M.M.; Khan, R. Photoelectrocatalytic reduction of CO2 to methanol over CuFe2O4@pani Photocathode. Int. J. Hydrogen Energy 2021, 46, 24709–24720. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Chen, B.; Wang, L.; Yang, J.; Wang, B. Nitrogen-doped hierarchically constrcted interconnected porous carbon nanofiners derived from PANI for highly selective CO2 capture and effective methanol adsorption. J. Environ. Chem. Eng. 2022, 10, 108847. [Google Scholar] [CrossRef]
- Khalilim, S.; Afhami, A.; Medrakian, T. Electrochemical simultaneous treatment approach: Electro-reduction of CO2 at Pt/PANI@ZnO paired with waste water electro oxidation over PbO2. Appl. Catal. B Environ. 2023, 326, 122545. [Google Scholar] [CrossRef]
- Bharath, G.; Hai, A.; Rambabu, K.; Haija, M.A.; Banat, F. Sustainable electrochemical process for the recovery of metal ions in syntheric mining wastewater and their utilization in photocathodic CO2 reduction into formic acid, Resources. Conserv. Recycl. 2023, 10, 106778. [Google Scholar] [CrossRef]
- Fontmorin, J.M.; Lzadi, P.; Lim, S.S.; Faroo, S.; Bilal, S.S.; Cheng, S.; Yu, E.H. Gas diffusion electrodes modified with binary doped polyaniline for enhanced CO2 conversion during microbial electrosynthesis. Electrochim. Acta 2021, 372, 137853. [Google Scholar] [CrossRef]
- Li, S.H.; Hu, S.; Liu, H.; Liu, J.; Kang, X.; Ge, S.; Zhang, Z.; Yu, Q.; Liu, B. Two-Dimensional Metal Coordination polymerDerived Indium Nanosheet for Efficient carbon dioxide reduction to Formate. ACS Nano 2023, 17, 9338–9346. [Google Scholar] [CrossRef] [PubMed]
- Amrillah, T.; Supandi, A.R.; Puspasari, V.; Hemawan, A.; Seh, Z.W. MXene-based Photocatalysts and electrocatalysts for CO2 Conversion to chemicals. Trans. Tlanjin Univ. 2022, 28, 307–322. [Google Scholar] [CrossRef]
- Handoko, A.D.; Chen, H.; Lum, Y.; Zhang, B.; Anasori, Z.; Seh, W. Two-dimensional Titanim and molybdenum carbide Mxenes as electrocatalysts for CO2 Reduction. iScience 2023, 23, 101181. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Jung, J. Mo2CS2-MXene supported single –atom catalysts for efficient and selective CO2 electrochemical reduction. Appl. Surf. Sci. 2022, 592, 15333. [Google Scholar] [CrossRef]
- Kadja, G.T.M.; Ilmi, M.M.; Azhari, N.J.; Febrianti, A.; Siregar, J.J.M.; Nurdini, N.; Pratomo, U.; Khalil, M. MXene-based nanocomposites or electrocatalystic reduction of CO2: Experimental and theoretical results. Flat. Chem. 2023, 38, 100481. [Google Scholar] [CrossRef]
- Attanyake, N.H.; Banjade, H.R.; Thenwara, A.C.; Anasori, B.; Yan, O.; Strongin, D.R. Electrocatalytic CO2 reduction on earth abundant 2D Mo2C and Ti3C2Mxenes. Chem. Comm. 2021, 57, 1675–1678. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, Y.; Yu, D.; Liu, Q.; Zhou, L.; Zhang, K.; Li, P.; Hu, F.; Li, L.; Chou, S.; et al. Hierarchical Ti3C2Tx MXene/Carbon Nanotubes for Low Overpotential and Long-Life Li-CO2 Batteries. ACS Nano 2021, 15, 8407–8417. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhou, K.; Liu, C.; Hu, Q.; Fang, H.; Yang, H.; He, C. Superbase and Hydrophobic Ionic Liquid Confined within NiFoams as a Free-Standing Catalyst for CO Electroreduction. ACS Appl. Mater. Interfaces 2022, 14, 38717–38726. [Google Scholar] [CrossRef]
- Chatterjee, S.; Griego, C.; Hart, J.L.; Li, Y.; Taheri, M.L.; Keith, J.; Snyder, J.D. Free Standing Nanoporous Palladium Alloys as CO Poisoning Tolerant Electrocatalysts for the Electrochemical Reduction of CO2 to Formate. ACS Catal. 2019, 9, 5290–5301. [Google Scholar] [CrossRef]














| S.No | Electrode | Product | Electrolyte (M) | Current Density (mA cm−2) | Faradaic Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Cu/Cu2O | Ethylene | 1 KCl | 200 | 84.5 | [11] |
| 2 | Sb-SnS2nanosheets | Formate | 0.1 KHCO3 | 17.17 | 90.86 | [15] |
| 3 | Ni-rich (Pd20-Ni80/ZC) | Carbon monoxide | 1 KOH | 200 | 95.3 | [20] |
| 4 | Cu NPs | Ethylene | 1 KOH | 300 | 35.0 | [22] |
| 5 | 3D net-like CoSA/HCNFs nanofibers | Carbon monoxide | 0.1 KHCO3 | 67 | 91.0 | [25] |
| 6 | N-Doped Nanoporous Carbon | Carbon monoxide | 0.5 NaHCO3 | −0.40 | 90.0 | [26] |
| 7 | Ni3N/MCNT | Carbon monoxide | 0.5 NaHCO3 | 6.5 | 89.0 | [27] |
| 8 | N-C-CoPc NR | Carbon monoxide | 0.1 KHCO3 | 30 | 85.3 | [28] |
| 9 | MnO/NGA-1 | Carbon monoxide | 0.5 KHCO3 | - | 86.0 | [29] |
| 10 | PO-5 nm Co/SL-NG | Methanol | 0.1 NaHCO3 | 10 | 71.4 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-W.; Chen, S.-M.; Anushya, G.; Kannan, R.; G. Al-Sehemi, A.; Alargarsamy, S.; Gajendran, P.; Ramachandran, R. Development of Different Kinds of Electrocatalyst for the Electrochemical Reduction of Carbon Dioxide Reactions: An Overview. Molecules 2023, 28, 7016. https://doi.org/10.3390/molecules28207016
Chen T-W, Chen S-M, Anushya G, Kannan R, G. Al-Sehemi A, Alargarsamy S, Gajendran P, Ramachandran R. Development of Different Kinds of Electrocatalyst for the Electrochemical Reduction of Carbon Dioxide Reactions: An Overview. Molecules. 2023; 28(20):7016. https://doi.org/10.3390/molecules28207016
Chicago/Turabian StyleChen, Tse-Wei, Shen-Ming Chen, Ganesan Anushya, Ramanujam Kannan, Abdullah G. Al-Sehemi, Saranvignesh Alargarsamy, Pandi Gajendran, and Rasu Ramachandran. 2023. "Development of Different Kinds of Electrocatalyst for the Electrochemical Reduction of Carbon Dioxide Reactions: An Overview" Molecules 28, no. 20: 7016. https://doi.org/10.3390/molecules28207016







