Optical and Electrochemical Properties of a Photosensitive Pyromellitic Diimide Derivative of Cymantrene
Abstract
:1. Introduction
2. Results and Discussion
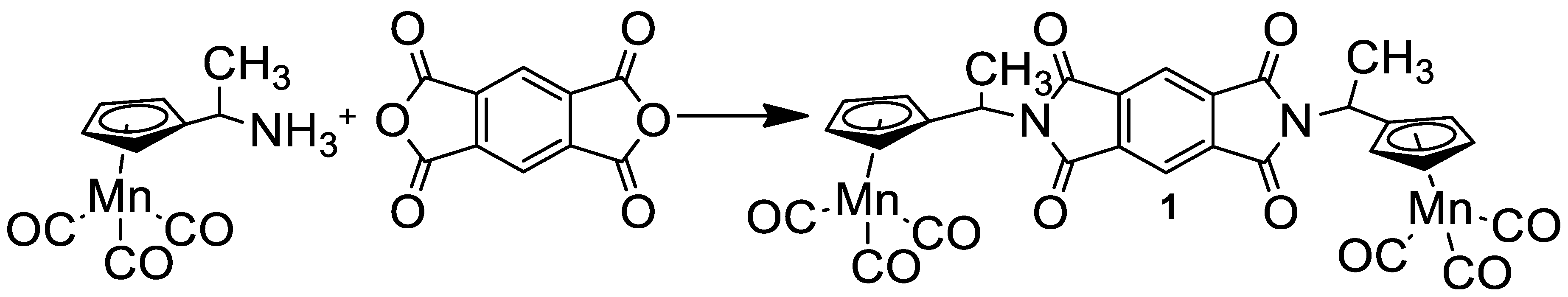
2.1. Synthesis of N,N′-Di(1-Cymantrenylethyl)Pyromellitic Diimide
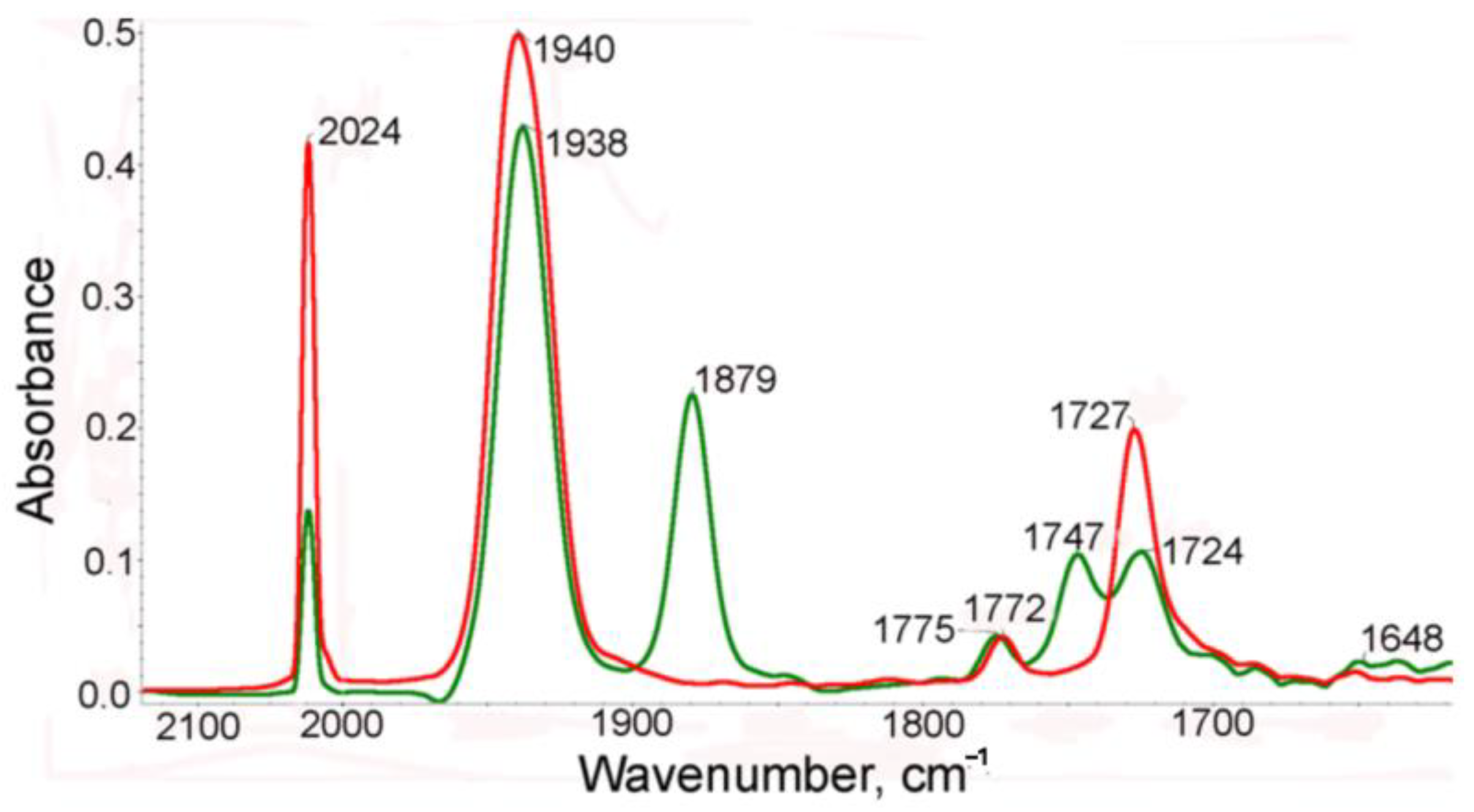
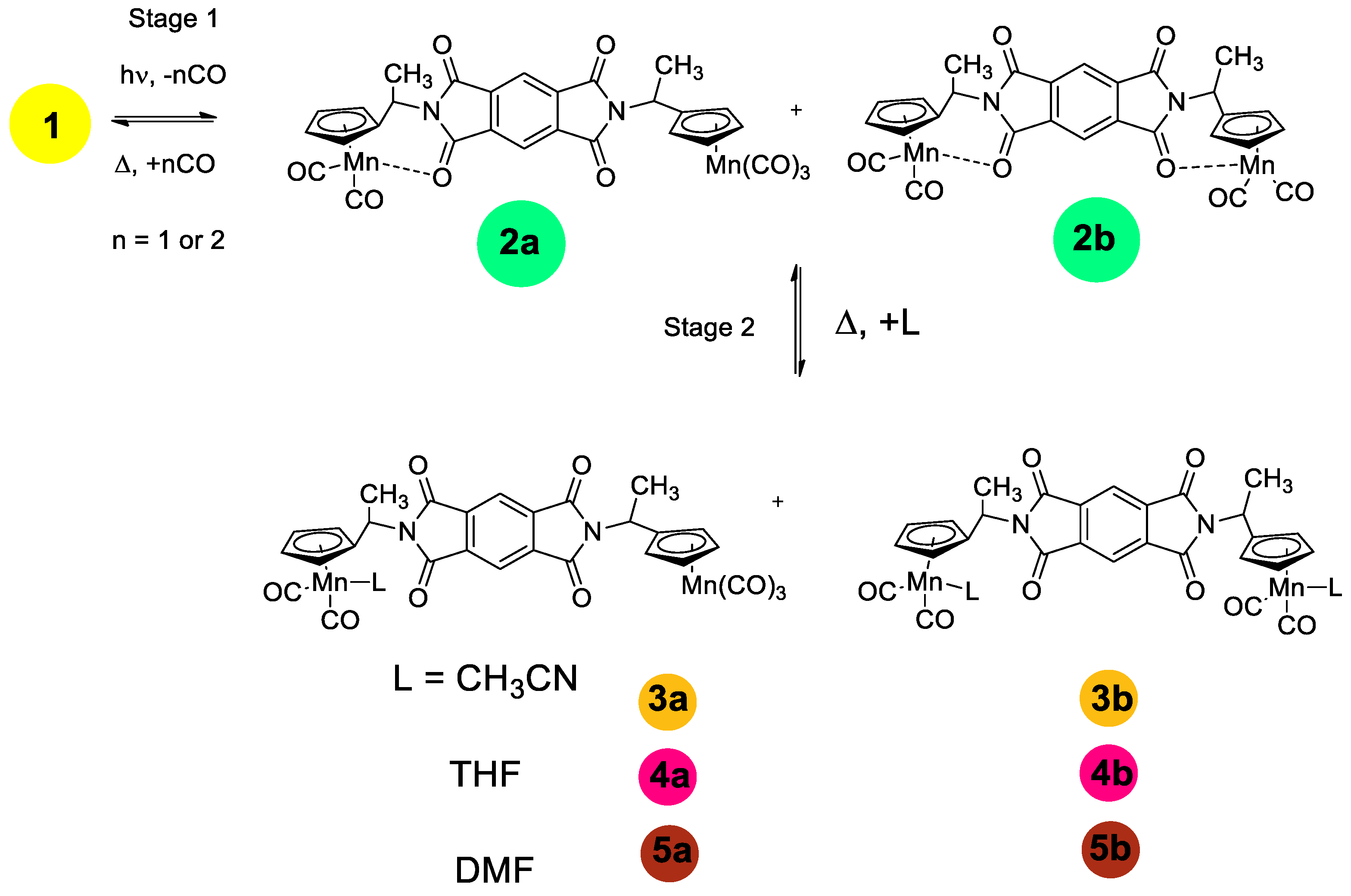
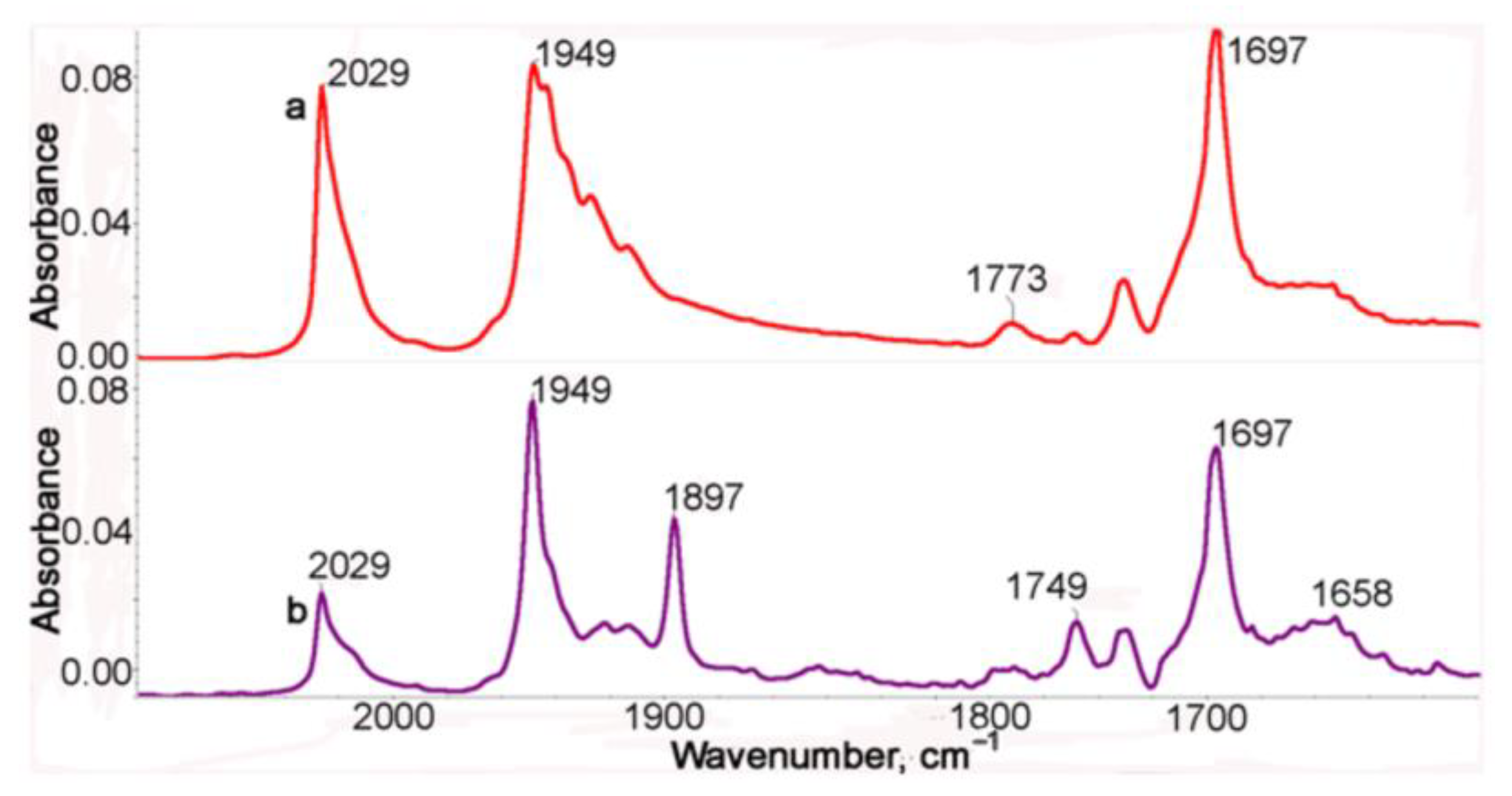
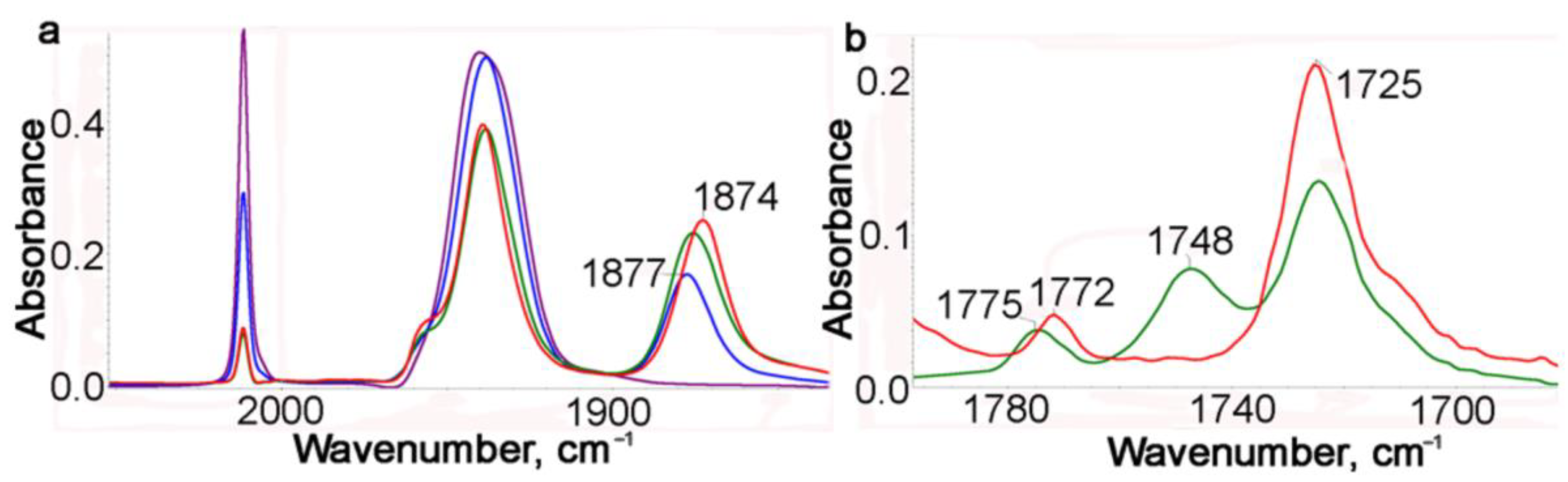
2.2. IR Spectroscopic Study of Photochemical and Hemilabile Properties of N,N′-Di(1-Cymantrenylethyl)Pyromellitic Diimide
2.3. DFT Calculation
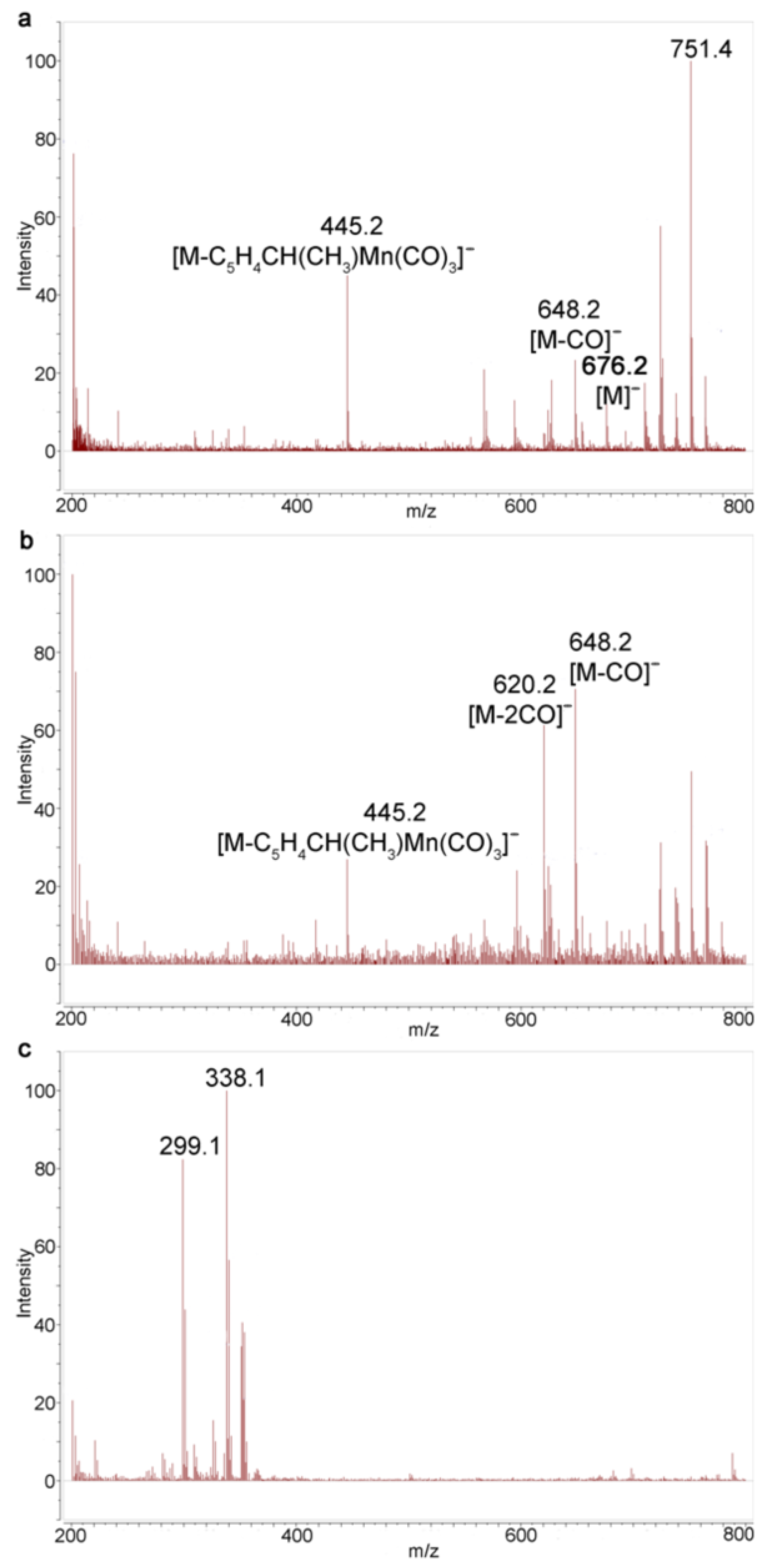
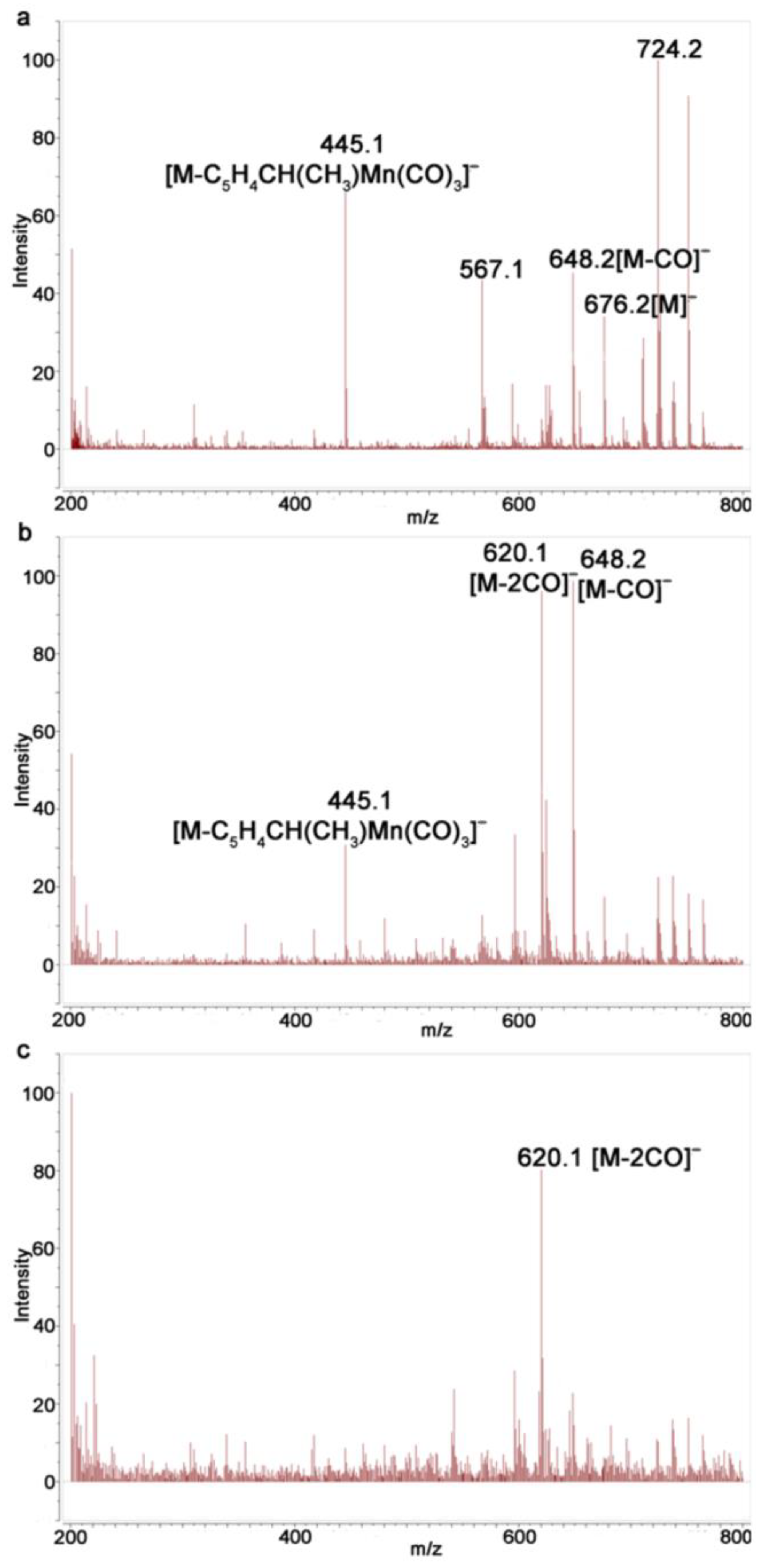
2.4. Study of Photochemical Properties of N,N′-Di(1-Cymantrenylethyl)Pyromellitic Diimide with 1H NMR Spectroscopy and Mass Spectrometry
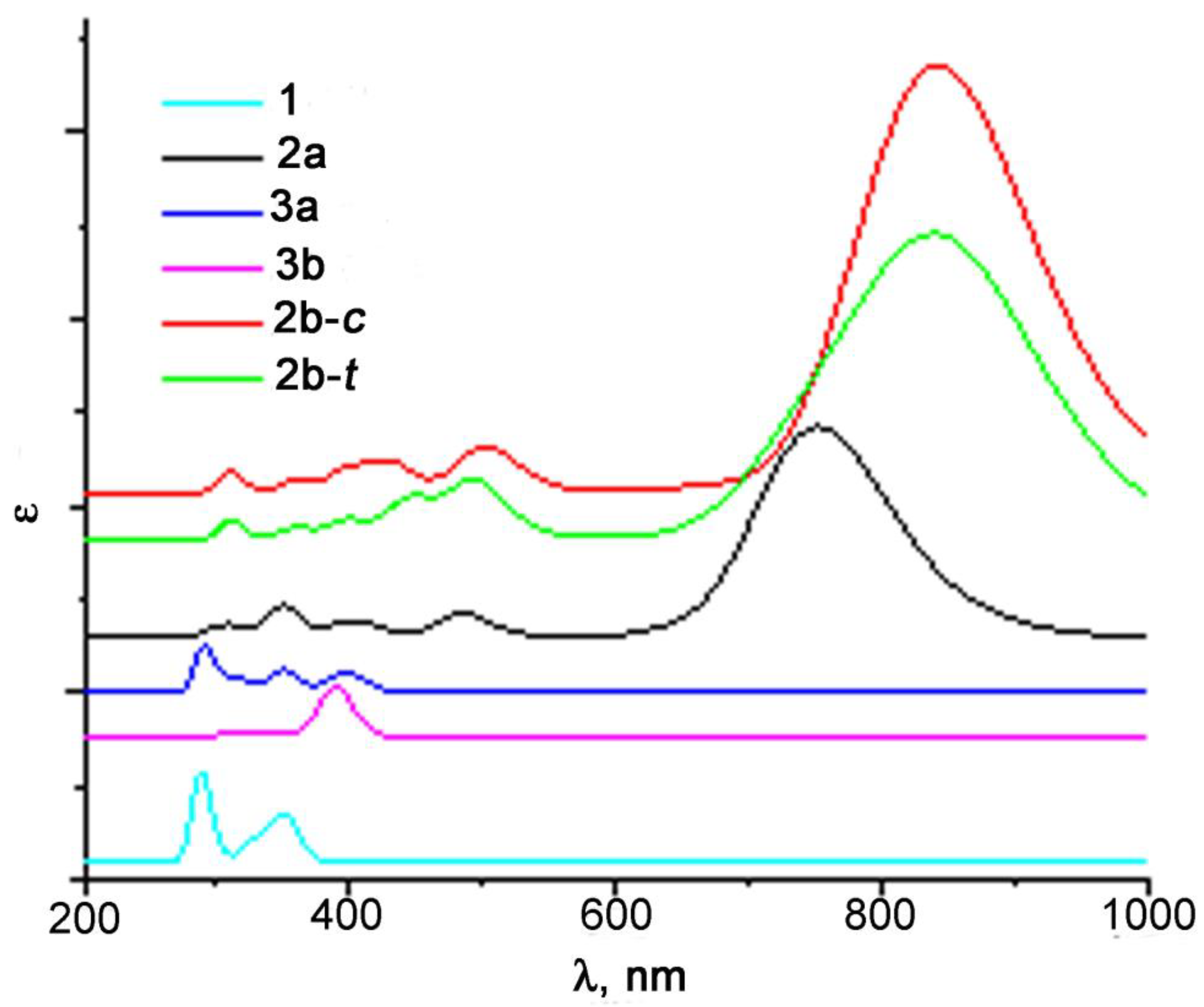
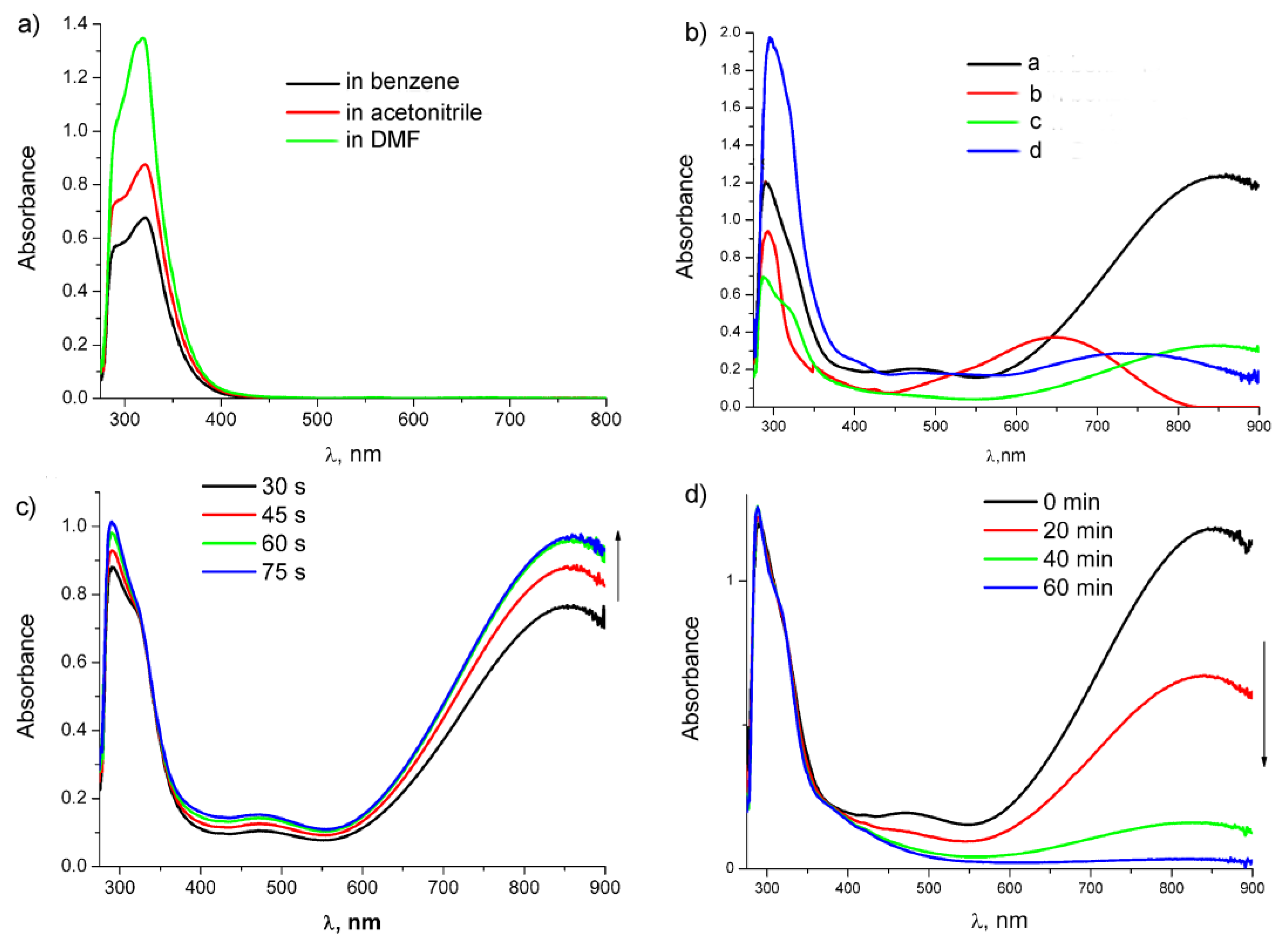
2.5. Study of Photochemical and Hemilabile Properties of N,N′-Di(1-Cymantrenylethyl)Pyromellitic Diimide with UV-Vis Spectroscopy
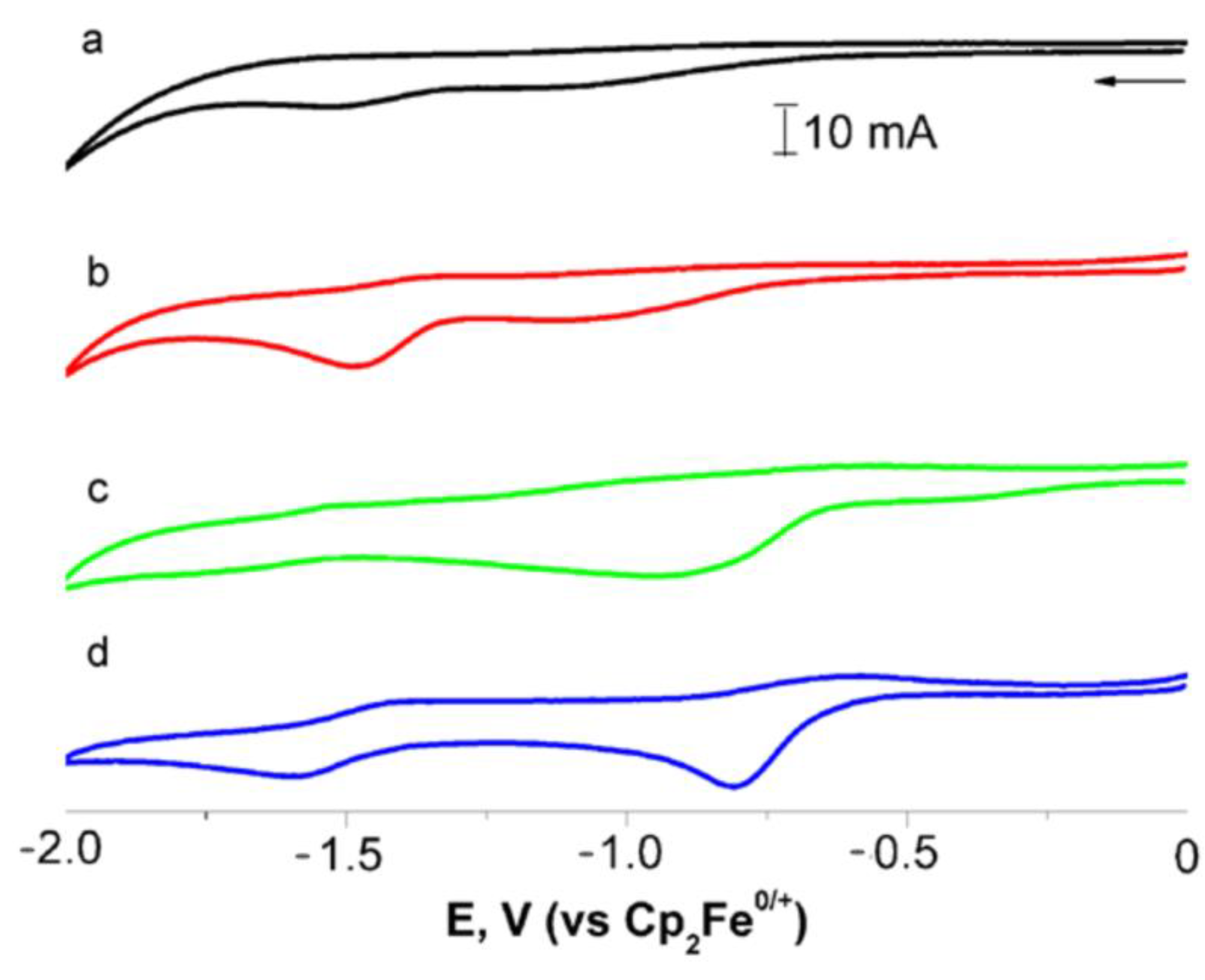
2.6. Cyclic Voltammetry Study of N,N′-Di(1-Cymantrenylethyl)Pyromellitic Diimide
3. Materials and Methods
3.1. N,N′-Di(1-Cymantrenylethyl)Pyromellitic Diimide 1
3.2. General Technique of Spectral Studies of Photochemical Reactions of Tricarbonyl Complexes and Their Dark Reaction
3.3. Mass Spectroscopic Study
3.4. Electrochemical Study
3.5. Computation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bredas, J.-L.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-Transfer and Energy-Transfer Processes in π-Conjugated Oligomersand Polymers: A Molecular Picture. Chem. Rev. 2004, 104, 4971–5003. [Google Scholar] [CrossRef]
- Jia, J.; Wang, J.; Li, M.; Gong, C.; Liang, G.; Song, Y.; She, Y. Phenothiazine metal-organic framework materials with excellent third-order nonlinear properties. Dyes Pigm. 2022, 205, 110398. [Google Scholar] [CrossRef]
- Yeab, J.-T.; Qiu, Y.-Q. The inspiration and challenge for through-space charge transfer architecture: From thermally activated delayed fluorescence to non-linear optical properties. Phys. Chem. Chem. Phys. 2021, 23, 15881–15898. [Google Scholar]
- Wang, L.; Liu, Y.-L.; Li, Q.-J.; Chen, S.-H.; He, D.; Wang, M.-S. Assembling of Perylene, Naphthalene, and Pyromellitic Diimide-Based Materials and Their Third-Order Nonlinear Optical Properties. J. Phys. Chem. A 2022, 126, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, G.; Qu, H.; Todarwal, Y.; Wang, Y.; Norman, P.; Linares, M.; Surin, M.; Zhang, H.-J.; Lin, J.; et al. Naphthodithiophene Diimide Based Chiral p-Conjugated Nanopillar Molecules. Angew. Chem. Int. Ed. 2021, 60, 24543–24548. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Che, Y.; Moore, J.S. One-Dimensional Self-Assembly of Planar πConjugated Molecules: Adaptable Building Blocks for Organic Nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and Related Diimides for Organic Electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef]
- Chen, W.-G.; Chen, Z.-J.; Zhang, L.; Wang, B.; Lin, Z.-Z.; Cao, R.; Wang, W.-R.; Chen, Y.; Wang, Y. Reversible, photoresponsive, dynamic wide-range emission color from polymer-matrixed naphthalene diimide single-luminogen. Chem. Eng. J. 2022, 432, 134411. [Google Scholar] [CrossRef]
- Hao, P.; Xu, Y.; Shen, J.; Fu, Y. Effect of positional isomerism on electron-transfer photochromism and photoluminescence of two pyromellitic diimide-based organic molecules. Dyes Pigm. 2021, 186, 108941. [Google Scholar] [CrossRef]
- Goncalves Dal-Bo, A.; de Costa Duarte, R.; Cercena, R.; Peterson, M.; Rafique, J.; Saba, S.; Zapp, E.; Sangiogo Gil, E.; Goncalves, P.F.B.; Rodembusch, F.S.; et al. New long-chain donor-acceptor-donor pyromellitic diimide (PMDI) derivatives. A combined theoretical and experimental study. Dyes Pigm. 2018, 157, 143–150. [Google Scholar] [CrossRef]
- Komissarova, E.A.; Zhulanov, V.E.; Mokrushin, I.G.; Vasyanin, A.N.; Shklyaeva, E.V.; Abasheva, G.G. Synthesis and study of N,N′-disubstituted derivatives of pyromellitic diimide. Russ. Chem. Bull. 2020, 69, 1944–1948. [Google Scholar] [CrossRef]
- Khatua, R.; Debata, S.; Sahu, S. Computational characterization of N-type characteristics and optoelectronic properties in air-stable pyromellitic diimide derivatives. New J. Chem. 2020, 44, 8412–8421. [Google Scholar] [CrossRef]
- Frizon, T.E.A.; Salla, C.A.M.; Grillo, F.; Rodembusch, F.S.; Camara, V.S.; Silva, H.C., Jr.; Zapp, E.; Junca, E.; Galetto, F.Z.; de Costa, A.M.; et al. ESIPT-based benzazole-pyromellitic diimide derivatives. A thermal, electrochemical, and photochemical investigation. Spectrochim. Acta Part A 2023, 288, 122050. [Google Scholar] [CrossRef]
- Krishnan, S.B.; Krishnan, R.; Gopidas, K.R. Effect of N-Alkyl Substituents on the Hierarchical Self-Assembly of β-Cyclodextrin-Linked Pyrene-Pyromellitic Diimide Charge-Transfer Complexes. Chem. Eur. J. 2018, 24, 11451–11460. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Fu, T.; Zhao, C.; Yu, H.; Liu, Z.; He, Q.; Zhang, D.; Meng, H.; Huang, W. Wide band gap pyromellitic diimides for photo stable n-channel thin film transistors. J. Mater. Chem. C 2020, 8, 7344–7349. [Google Scholar] [CrossRef]
- Yeh, M.-L.; Wang, S.-Y.; Hardigree, J.F.M.; Podzorov, V.; Katz, H.E. Effect of side chain length on film structure and electron mobility of core-unsubstituted pyromellitic diimides and enhanced mobility of the dibrominated core using the optimized side chain. J. Mater. Chem. C 2015, 3, 3029–3037. [Google Scholar] [CrossRef]
- Nambafu, G.S.; Delmo, E.P.; Shahid, U.B.; Zhang, C.; Chen, Q.; Zhao, T.; Gao, P.; Amine, K.; Shao, M. Pyromellitic diimide based bipolar molecule for total organic symmetric redox flow battery. Nano Energy 2022, 94, 106963. [Google Scholar] [CrossRef]
- Veerababu, M.; Nirmalendu, K.; Kothandaraman, R. Reversible Sodium Storage Behavior of Aromatic Diimide Disodium Carboxylates. J. Electrochem. Soc. 2017, 164, A6147–A6153. [Google Scholar]
- Veerababu, M.; Varadaraju, U.V.; Kothandaraman, R. Reversible lithium storage behaviour of aromatic diimide dilithium carboxylates. Electrochim. Acta 2016, 193, 80–87. [Google Scholar] [CrossRef]
- Kelbysheva, E.S.; Strelkova, T.V.; Ezernitskaya, M.G.; Alekseev, V.G.; Telegina, L.N. Synthesis, Spectral and Electrochemical Properties, and Computational Modeling of N-Cymantrenylmethylphthalimide. ChemistrySelect 2023, 8, e202204162. [Google Scholar] [CrossRef]
- Kelbysheva, E.S.; Telegina, L.N.; Godovikov, I.A.; Strelkova, T.V.; Borisov, Y.A.; Ezernitskaya, M.G.; Lokshin, B.V.; Loim, N.M. Dicarbonyl chelates from 1-cymantrenylalkylamides: Formation, properties, and kinetics of the dark reaction with carbon monoxide. Russ. Chem. Bull. 2015, 64, 914–922. [Google Scholar] [CrossRef]
- Gies, A.P.; Nonidez, W.K.; Anthamatten, M.; Cook, R.C.; Mays, J.W. Characterization of an insoluble polyimide oligomer by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2002, 16, 1903–1910. [Google Scholar] [CrossRef]
- Yang, P.F.; Yang, G.K. Haloalkanes as ligands. Spectroscopic and energetic studies of CpMn(CO)2XR. J. Am. Chem. Soc. 1992, 114, 6937–6938. [Google Scholar] [CrossRef]
- Kelbysheva, E.S.; Gordey, Y.A.; Ezernitskaya, M.G.; Smol’yakov, A.F.; Telegina, L.N. Synthesis of Sulfur-Containing Cymantrene Derivatives Having Potential Photo- and Electrochemical Properties. Russ. J. Coord. Chem. 2020, 46, 28–36. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, Z.; Ding, Y.; Zhang, L.; Zhou, Y.; Guo, X.; Zhang, X.; Zhao, Y.; Yum, G. Highly Concentrated Phthalimide-Based Anolytes for Organic Redox Flow Batteries with Enhanced Reversibility. Chem 2018, 4, 2814–2825. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; McAfee, S.M.; Sun, J.-P.; McCormick, T.M.; Hill, I.G.; Welch, G.C. Phthalimide-based π-conjugated small molecules with tailored electronic energy levels for use as acceptors in organic solar cells. J. Mater. Chem. C 2015, 3, 8904–8915. [Google Scholar] [CrossRef]
- Greenlee, A.J.; Ofosu, C.K.; Xiao, Q.; Modan, M.M.; Janzen, D.E.; Cao, D.D. Pyridinium-Functionalized Pyromellitic Diimides with Stabilized Radical Anion States. ACS Omega 2018, 3, 240–245. [Google Scholar] [CrossRef]
- Kelbysheva, E.S.; Ezernitskaya, M.G.; Strelkova, T.V.; Borisov, Y.A.; Telegina, L.N. Hemilabile Properties of Sulfur-Containing Cymantrene Derivatives. ChemistrySelect 2021, 6, 9861–9866. [Google Scholar] [CrossRef]
- Wu, K.; Park, J.Y.; Al-Saadon, R.; Nam, H.; Lee, Y.; Top, S.; Jaouen, G.; Baik, M.-H.; Geiger, W.E. Oxidation of Cymantrene-Tagged Tamoxifen Analogues: Effect of Diphenyl Functionalization on the Redox Mechanism. Organometallics 2020, 39, 679–687. [Google Scholar] [CrossRef]
- Guo, X.; Watson, M.D. Pyromellitic Diimide-Based Donor–Acceptor Poly(phenylene ethynylene)s. Macromolecules 2011, 44, 6711–6716. [Google Scholar] [CrossRef]
- Kostyuchenko, A.S.; Kurowska, A.; Zassowski, P.; Zheleznova, T.Y.; Ulyankin, E.B.; Domagala, W.; Pron, A.; Fisyuk, A.S. Synthesis of Bis([2,2′-bithiophen]-5-yl)-Substituted Oligothiadiazoles: Effect of the Number of Acceptor Units on Electrochemical and Spectroscopic Properties. J. Org. Chem. 2019, 84, 10040–10049. [Google Scholar] [CrossRef]
- Loim, N.M.; Parnes, Z.N.; Andrianov, V.G.; Struchkov, Y.T.; Kursanov, D.N. Synthesis and absolute configuration of the enantiomers α-aminoethylcymantrene and some of its derivatives. J. Organomet. Chem. 1980, 201, 301–310. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V.J. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree-Fock and Hybrid DFT Calculations. A “Chain-of-Spheres” Algorithm for the Hartree-Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Dutta, A.K.; Neese, F.; Izsak, R. Speeding up equation of motion coupled cluster theory with the chain of spheres approximation. J. Chem. Phys. 2016, 144, 034102. [Google Scholar] [CrossRef] [PubMed]

| Compound | r2-SCAN-3c/Def2-TZVP | B3LYP/Def2-TZVP | ||||
|---|---|---|---|---|---|---|
| ΔEtot | ΔHo298 | ΔGo298 | ΔEtot | ΔHo298 | ΔGo298 | |
| 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2 | 34.4 | 34.7 | 24.8 | 27.8 | 28.1 | 18.3 |
| 2b-t 1 | 69.0 | 69.6 | 49.7 | 55.5 | 56.1 | 36.5 |
| 2b-c 1 | 69.1 | 69.7 | 49.9 | 55.6 | 56.3 | 36.6 |
| 3a | 22.6 | 22.9 | 24.1 | 17.9 | 18.2 | 19.5 |
| 3b | 45.4 | 46.6 | 47.3 | 35.9 | 37.1 | 37.2 |
| Compound | Benzene λmax, nm (ε (l× ∗ mol−1 ∗ cm−1)) | Acetonitrile λmax, nm (ε (l× ∗ mol−1 ∗ cm−1)) | DMF λmax, nm (ε (l× ∗ mol−1 ∗ cm−1)) |
|---|---|---|---|
| 1 | 291 (2800), 321 (3300) | 291 (3100), 321 (3700) | 291 (2900), 316 (3800) |
| 2 | 290 (7100), 475 (700), 859 (6900) | 290 (8600), 849 (6300) | 294 (5400), 405 (700), 740 (800) |
| 3 | - | 290 (9000), 366 (700) | - |
| 5 | - | - | 294 (6400),488 (500), 555 (500) |
| 7 | 293 (1500), 327 (800) | 293 (1700), 326 (900) | 296 (2100), 324 (1200) |
| 8 | 358 (500), 651 (1000) | 605 (100) | 581 (600) |
| 9 | - | 371 (400), 435 (200) | - |
| 10 | - | - | 398 (3800), 528 (600) |
| Compound | EpRed1, V | ia/ic | EpRed2, V | ia/ic | EpOx1, V | ia/ic | EpOx2, V | ia/ic | IP 1, eV | EA 1, eV | Eg 1, eV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | - | −0.94 | 0.55 | 1.45 | 0.18 | - | - | 6.25 | 4.96 | 1.29 |
| 2 | −0.81 | 0.26 | −1.59 | 0.96 | 0.17 | - | 1.29 | 0.1 | 5.59 | 4.52 | 0.49 |
| 5 | −0.81 | 0.26 | −1.59 | 0.96 | 0.91 | - | 1.29 | 0.1 | 5.01 | 4.52 | 1.07 |
| 7 | −1.10 | - | −1.52 | - | 1.08 | 0.47 | - | - | 6.06 | 4.45 | 1.61 |
| 10 | −1.06 | - | −1.49 | 0.37 | 0.93 | - | - | - | 5.90 | 4.48 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelbysheva, E.S.; Ezernitskaya, M.G.; Aysin, R.R.; Strelkova, T.V.; Rodionov, A.N.; Telegina, L.N. Optical and Electrochemical Properties of a Photosensitive Pyromellitic Diimide Derivative of Cymantrene. Molecules 2023, 28, 7098. https://doi.org/10.3390/molecules28207098
Kelbysheva ES, Ezernitskaya MG, Aysin RR, Strelkova TV, Rodionov AN, Telegina LN. Optical and Electrochemical Properties of a Photosensitive Pyromellitic Diimide Derivative of Cymantrene. Molecules. 2023; 28(20):7098. https://doi.org/10.3390/molecules28207098
Chicago/Turabian StyleKelbysheva, Elena S., Mariam G. Ezernitskaya, Rinat R. Aysin, Tatyana V. Strelkova, Alexey N. Rodionov, and Lyudmila N. Telegina. 2023. "Optical and Electrochemical Properties of a Photosensitive Pyromellitic Diimide Derivative of Cymantrene" Molecules 28, no. 20: 7098. https://doi.org/10.3390/molecules28207098





