Research Progress of Perovskite-Based Bifunctional Oxygen Electrocatalyst in Alkaline Conditions
Abstract
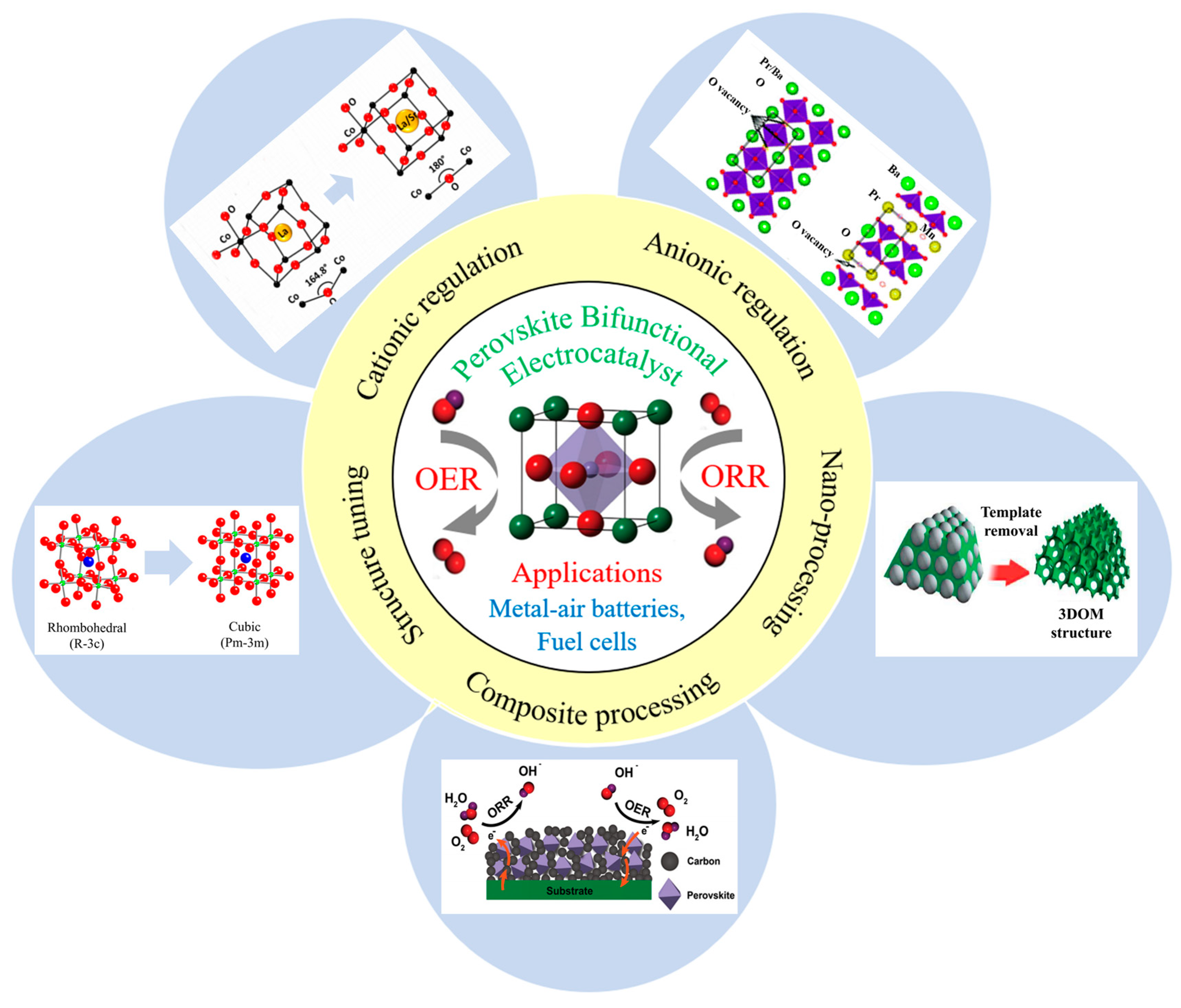
:1. Introduction
2. Crystallographic Structure Tuning
3. Cationic Regulation
3.1. A-Site Cation Regulation
3.2. B-Site Cation Regulation

3.3. A-Site and B-Site Cation Regulation
3.4. Cation Vacancies
4. Anionic Regulation
5. Nano-Processing
6. Composite Processing
6.1. Dual Components Integrated Electrocatalyst
6.2. Multiple Components Integrated Electrocatalyst
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Fang, B.; Wang, X.; Ignaszak, A.; Liu, Y.; Li, A.; Zhang, L.; Zhang, J. Recent advancements in the development of bifunctional electrocatalysts for oxygen electrodes in unitized regenerative fuel cells (URFCs). Prog. Mater. Sci. 2018, 98, 108–167. [Google Scholar] [CrossRef]
- Chao, S.; Zhang, Y.; Wang, K.; Bai, Z.; Yang, L. Flower–like Ni and N codoped hierarchical porous carbon microspheres with enhanced performance for fuel cell storage. Appl. Energy 2016, 175, 421–428. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part-A: Unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2016, 65, 961–977. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Lin, J.; Shen, Z. Recent advances in air electrodes for Zn–air batteries: Electrocatalysis and structural design. Mater. Horiz. 2017, 4, 945–976. [Google Scholar] [CrossRef]
- Velraj, S.; Zhu, J.H. Sm0.5Sr0.5CoO3−δ—A new bi-functional catalyst for rechargeable metal-air battery applications. J. Power Sources 2013, 227, 48–52. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Sharma, A.; Mathur, A.; Halder, A.; Kushwaha, H.S. Novel guar-gum electrolyte to aggrandize the performance of LaMnO3 perovskite-based zinc-air batteries. Electrochem. Sci. Adv. 2021, 2, e202100056. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, Q.; Xia, B.; Jiang, L.; Duan, J.; Chen, S. Perovskite Catalysts for Oxygen Evolution and Reduction Reactions in Zinc-Air Batteries. Catalysts 2022, 12, 1490. [Google Scholar] [CrossRef]
- Park, H.S.; Seo, E.; Yang, J.; Lee, Y.; Kim, B.S.; Song, H.K. Bifunctional hydrous RuO2 nanocluster electrocatalyst embedded in carbon matrix for efficient and durable operation of rechargeable zinc-air batteries. Sci. Rep. 2017, 7, 7150. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Mohamad, A.A.; Nguyen, M.T.; Yonezawa, T.; Qin, J.; Thamyongkit, P.; Somwangthanaroj, A.; Kheawhom, S. Recent advances in oxygen electrocatalysts based on tunable structural polymers. Mater. Today Chem. 2022, 23, 100632. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Masa, J.; Xia, W.; Sinev, I.; Zhao, A.; Sun, Z.; Grutzke, S.; Weide, P.; Muhler, M.; Schuhmann, W. MnxOy/NC and CoxOy/NC nanoparticles embedded in a nitrogen-doped carbon matrix for high-performance bifunctional oxygen electrodes. Angew. Chem. Int. Ed. 2014, 53, 8508–8512. [Google Scholar] [CrossRef] [PubMed]
- Elumeeva, K.; Masa, J.; Tietz, F.; Yang, F.; Xia, W.; Muhler, M.; Schuhmann, W. A Simple Approach towards High-Performance Perovskite-Based Bifunctional Oxygen Electrocatalysts. ChemElectroChem 2016, 3, 138–143. [Google Scholar] [CrossRef]
- May, K.J.; Carlton, C.E.; Stoerzinger, K.A.; Risch, M.; Suntivich, J.; Lee, Y.-L.; Grimaud, A.; Shao-Horn, Y. Influence of Oxygen Evolution during Water Oxidation on the Surface of Perovskite Oxide Catalysts. J. Phys. Chem. Lett. 2012, 3, 3264–3270. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Y.; Yu, J.; Xu, X.; Su, C.; Tadé, M.O.; Shao, Z. Boosting Oxygen Reduction Reaction Activity of Palladium by Stabilizing Its Unusual Oxidation States in Perovskite. Chem. Mater. 2015, 27, 3048–3054. [Google Scholar] [CrossRef]
- Liu, R.; Liang, F.; Zhou, W.; Yang, Y.; Zhu, Z. Calcium-doped lanthanum nickelate layered perovskite and nickel oxide nano-hybrid for highly efficient water oxidation. Nano Energy 2015, 12, 115–122. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Sharma, A.; Gupta, V.; Batoo, K.M.; Hussain, S.; Kushwaha, H.S. High energy storage capabilities of CaCu3Ti4O12 for paper-based zinc-air battery. Sci. Rep. 2022, 12, 3999. [Google Scholar] [CrossRef]
- Chen, T.W.; Kalimuthu, P.; Anushya, G.; Chen, S.M.; Ramachandran, R.; Mariyappan, V.; Muthumala, D.C. High-Efficiency of Bi-Functional-Based Perovskite Nanocomposite for Oxygen Evolution and Oxygen Reduction Reaction: An Overview. Materials 2021, 14, 2976. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Han, Z.; Yang, Y.; Li, J.; Deng, Q. Recent Advances in Oxygen Electrocatalysts Based on Perovskite Oxides. Nanomaterials 2019, 9, 1161. [Google Scholar] [CrossRef]
- Elumeeva, K.; Masa, J.; Sierau, J.; Tietz, F.; Muhler, M.; Schuhmann, W. Perovskite-based bifunctional electrocatalysts for oxygen evolution and oxygen reduction in alkaline electrolytes. Electrochim. Acta 2016, 208, 25–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Liu, T.; Feng, F.; Wang, C.; Hu, H.; Wu, M.; Ni, M.; Shao, Z. The Synergistic Effect Accelerates the Oxygen Reduction/Evolution Reaction in a Zn-Air Battery. Front. Chem. 2019, 7, 524. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Mai, L.; Han, C.; An, Q.; Xu, X.; Liu, X.; Zhang, Q. Hierarchical mesoporous perovskite La0.5Sr0.5CoO2.91 nanowires with ultrahigh capacity for Li-air batteries. Proc. Natl. Acad. Sci. USA 2012, 109, 19569–19574. [Google Scholar] [CrossRef] [PubMed]
- Grimaud, A.; Carlton, C.E.; Risch, M.; Hong, W.T.; May, K.J.; Shao-Horn, Y. Oxygen Evolution Activity and Stability of Ba6Mn5O16, Sr4Mn2CoO9, and Sr6Co5O15: The Influence of Transition Metal Coordination. J. Phys. Chem. C 2013, 117, 25926–25932. [Google Scholar] [CrossRef]
- Lu, F.; Sui, J.; Su, J.; Jin, C.; Shen, M.; Yang, R. Hollow spherical La0.8Sr0.2MnO3 perovskite oxide with enhanced catalytic activities for the oxygen reduction reaction. J. Power Sources 2014, 271, 55–59. [Google Scholar] [CrossRef]
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y.C. Rechargeable Zn-air batteries: Recent trends and future perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Christy, M.; Rajan, H.; Yang, H.; Kim, Y.-B. Optimizing the Surface Characteristics of La0.6Sr0.4CoO3−δ Perovskite Oxide by Rapid Flash Sintering Technology for Easy Fabrication and Fast Reaction Kinetics in Alkaline Medium. Energy Fuels 2020, 34, 16838–16846. [Google Scholar] [CrossRef]
- Gupta, S.; Kellogg, W.; Xu, H.; Liu, X.; Cho, J.; Wu, G. Bifunctional Perovskite Oxide Catalysts for Oxygen Reduction and Evolution in Alkaline Media. Chem. Asian J. 2016, 11, 10–21. [Google Scholar] [CrossRef]
- Lee, D.G.; Gwon, O.; Park, H.S.; Kim, S.H.; Yang, J.; Kwak, S.K.; Kim, G.; Song, H.K. Conductivity-Dependent Completion of Oxygen Reduction on Oxide Catalysts. Angew. Chem. 2015, 127, 15956–15959. [Google Scholar] [CrossRef]
- Kuai, L.; Kan, E.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.-P.; Huotari, S.; Wang, W.; Geng, B. Mesoporous LaMnO3+δ perovskite from spray−pyrolysis with superior performance for oxygen reduction reaction and Zn−air battery. Nano Energy 2018, 43, 81–90. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, W.; Niu, Y.; Zhu, Z.; Shao, Z. Phase Transition of a Cobalt-Free Perovskite as a High-Performance Cathode for Intermediate-Temperature Solid Oxide Fuel Cells. ChemSusChem 2012, 5, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Oh, S.; Hwang, J.-Y.; Choi, M.; Youn, C.; Kim, J.W.; Chang, S.H.; Woo, S.; Bae, J.-S.; Park, S.; et al. Enhanced electrocatalytic activity via phase transitions in strongly correlated SrRuO3 thin films. Energy Environ. Sci. 2017, 10, 924–930. [Google Scholar] [CrossRef]
- Retuerto, M.; Calle-Vallejo, F.; Pascual, L.; Lumbeeck, G.; Fernandez-Diaz, M.T.; Croft, M.; Gopalakrishnan, J.; Peña, M.A.; Hadermann, J.; Greenblatt, M.; et al. La1.5Sr0.5NiMn0.5Ru0.5O6 Double Perovskite with Enhanced ORR/OER Bifunctional Catalytic Activity. ACS Appl. Mater. Interfaces 2019, 11, 21454–21464. [Google Scholar] [CrossRef] [PubMed]
- Efimov, K.; Xu, Q.; Feldhoff, A. Transmission Electron Microscopy Study of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Perovskite Decomposition at Intermediate Temperatures. Chem. Mater. 2010, 22, 5866–5875. [Google Scholar] [CrossRef]
- Arnold, M.; Gesing, T.M.; Martynczuk, J.; Feldhoff, A. Correlation of the formation and the decomposition process of the BSCF perovskite at intermediate temperatures. Chem. Mater. 2008, 20, 5851–5858. [Google Scholar] [CrossRef]
- Jiang, M.; Li, J.; Zhao, Y.; Pan, L.; Cao, Q.; Wang, D.; Du, Y. Double Perovskites as Model Bifunctional Catalysts toward Rational Design: The Correlation between Electrocatalytic Activity and Complex Spin Configuration. ACS Appl. Mater. Interfaces 2018, 10, 19746–19754. [Google Scholar] [CrossRef]
- Zhou, W.; Sunarso, J. Enhancing Bi-functional Electrocatalytic Activity of Perovskite by Temperature Shock: A Case Study of LaNiO3−δ. J. Phys. Chem. Lett. 2013, 4, 2982–2988. [Google Scholar] [CrossRef]
- Jung, J.-I.; Park, S.; Kim, M.-G.; Cho, J. Tunable Internal and Surface Structures of the Bifunctional Oxygen Perovskite Catalysts. Adv. Energy Mater. 2015, 5, 1501560. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Iwasa, Y.; Kawasaki, M.; Keimer, B.; Nagaosa, N.; Tokura, Y. Emergent phenomena at oxide interfaces. Nat. Mater. 2012, 11, 103–113. [Google Scholar] [CrossRef]
- Rondinelli, J.M.; Spaldin, N.A. Structure and properties of functional oxide thin films: Insights from electronic-structure calculations. Adv. Mater. 2011, 23, 3363–3381. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, R.; Shukla, D.K.; Gautam, S.; Hwa Chae, K.; Kumar, R. Electronic structure and electrical transport properties of LaCo1−xNixO3 (0 ≤ x ≤ 0.5). J. Appl. Phys. 2013, 114, 073704. [Google Scholar] [CrossRef]
- Prabu, M.; Ramakrishnan, P.; Ganesan, P.; Manthiram, A.; Shanmugam, S. LaTi0.65Fe0.35O3−δ nanoparticle-decorated nitrogen-doped carbon nanorods as an advanced hierarchical air electrode for rechargeable metal-air batteries. Nano Energy 2015, 15, 92–103. [Google Scholar] [CrossRef]
- Bie, S.; Zhu, Y.; Su, J.; Jin, C.; Liu, S.; Yang, R.; Wu, J. One-pot fabrication of yolk–shell structured La0.9Sr0.1CoO3 perovskite microspheres with enhanced catalytic activities for oxygen reduction and evolution reactions. J. Mater. Chem. A 2015, 3, 22448–22453. [Google Scholar] [CrossRef]
- Yu, X.; Sui, C.; Ren, R.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Construction of Heterointerfaces with Enhanced Oxygen Reduction Kinetics for Intermediate-Temperature Solid Oxide Fuel Cells. ACS Appl. Energy Mater. 2019, 3, 447–455. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Richins, S.; Liaw, K.; Yan, L.; Zhou, M.; Luo, H. Polymer-assisted approach to LaCo1−xNixO3 network nanostructures as bifunctional oxygen electrocatalysts. Electrochim. Acta 2019, 296, 945–953. [Google Scholar] [CrossRef]
- Gonell, F.; Sánchez-Sánchez, C.M.; Vivier, V.; Méthivier, C.; Laberty-Robert, C.; Portehault, D. Structure–Activity Relationship in Manganese Perovskite Oxide Nanocrystals from Molten Salts for Efficient Oxygen Reduction Reaction Electrocatalysis. Chem. Mater. 2020, 32, 4241–4247. [Google Scholar] [CrossRef]
- Kim, N.I.; Sa, Y.J.; Yoo, T.S.; Choi, S.R.; Afzal, R.A.; Choi, T.; Seo, Y.-S. Oxygen-deficient triple perovskites as highly active and durable bifunctional electrocatalysts for oxygen electrode reactions. Sci. Adv. 2018, 4, eaap9360. [Google Scholar] [CrossRef]
- Sunarso, J.; Torriero, A.A.J.; Zhou, W.; Howlett, P.C.; Forsyth, M. Oxygen Reduction Reaction Activity of La-Based Perovskite Oxides in Alkaline Medium: A Thin-Film Rotating Ring-Disk Electrode Study. J. Phys. Chem. C 2012, 116, 5827–5834. [Google Scholar] [CrossRef]
- Bian, J.; Su, R.; Yao, Y.; Wang, J.; Zhou, J.; Li, F.; Wang, Z.L.; Sun, C. Mg Doped Perovskite LaNiO3 Nanofibers as an Efficient Bifunctional Catalyst for Rechargeable Zinc–Air Batteries. ACS Appl. Energy Mater. 2019, 2, 923–931. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Y.; Sun, S.; Li, S.; Miao, H.; Liu, Z. La0.8Sr0.2Co1−xMnxO3 perovskites as efficient bi-functional cathode catalysts for rechargeable zinc-air batteries. Electrochim. Acta 2017, 254, 14–24. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Zhou, W.; Zhu, Y.; Chen, Y.; Shao, Z. Co-doping Strategy for Developing Perovskite Oxides as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Adv. Sci. 2016, 3, 1500187. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Gwon, O.; Lim, C.; Kim, J.; Galindev, O.; Kim, G. Advanced Electrochemical Properties of PrBa0.5Sr0.5Co1.9Ni0.1O5+δ as a Bifunctional Catalyst for Rechargeable Zinc-Air Batteries. ChemElectroChem 2019, 6, 3154–3159. [Google Scholar] [CrossRef]
- Xia, W.; Liu, X.; Jin, F.; Jia, X.; Shen, Y.; Li, J. Evaluation of calcium codoping in double perovskite PrBaCo2O5+δ as cathode material for IT-SOFCs. Electrochim. Acta 2020, 364, 137274. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.-P.; Xia, T.; Huo, L.-H.; Zhao, H.; Rougier, A.; Grenier, J.-C. Effect of Sr doping on the electrochemical properties of bi-functional oxygen electrode PrBa1−xSrxCo2O5+δ. J. Power Sources 2016, 334, 86–93. [Google Scholar] [CrossRef]
- Gobaille-Shaw, G.P.A.; Celorrio, V.; Calvillo, L.; Morris, L.J.; Granozzi, G.; Fermin, D.J. Effect of Ba Content on the Activity of La1−xBaxMnO3 Towards the Oxygen Reduction Reaction. ChemElectroChem 2018, 5, 1922–1927. [Google Scholar] [CrossRef]
- Wang, G.; Bao, Y.; Tian, Y.; Xia, J.; Cao, D. Electrocatalytic activity of perovskite La1−xSrxMnO3 towards hydrogen peroxide reduction in alkaline medium. J. Power Sources 2010, 195, 6463–6467. [Google Scholar] [CrossRef]
- Ovenstone, J.; Ponton, C.B. Emulsion processing of SOFC materials Ca0.3La0.7CrO3, Sr0.16La0.84CrO3, and Sr0.2La0.8MnO3. J. Mater. Sci. 2000, 35, 4115–4119. [Google Scholar] [CrossRef]
- Malkhandi, S.; Yang, B.; Manohar, A.K.; Manivannan, A.; Prakash, G.K.; Narayanan, S.R. Electrocatalytic Properties of Nanocrystalline Calcium-Doped Lanthanum Cobalt Oxide for Bifunctional Oxygen Electrodes. J. Phys. Chem. Lett. 2012, 3, 967–972. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Shi, L.; Huang, H. Preparation of La1−xCaxMnO3 perovskite-graphene composites as oxygen reduction reaction electrocatalyst in alkaline medium. J. Power Sources 2014, 269, 144–151. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, M.; Pal, R.; Azad, U.P.; Singh, A.K.; Singh, D.P.; Ganesan, V.; Singh, A.K.; Prakash, R. Lanthanide based double perovskites: Bifunctional catalysts for oxygen evolution/reduction reactions. Int. J. Hydrogen Energy 2021, 46, 17163–17172. [Google Scholar] [CrossRef]
- Cheng, X.; Fabbri, E.; Nachtegaal, M.; Castelli, I.E.; El Kazzi, M.; Haumont, R.; Marzari, N.; Schmidt, T.J. Oxygen Evolution Reaction on La1–xSrxCoO3 Perovskites: A Combined Experimental and Theoretical Study of Their Structural, Electronic, and Electrochemical Properties. Chem. Mater. 2015, 27, 7662–7672. [Google Scholar] [CrossRef]
- Sun, N.; Liu, H.; Yu, Z.; Zheng, Z.; Shao, C. The electrochemical performance of La0.6Sr0.4Co1−xNixO3 perovskite catalysts for Li-O2 batteries. Ionics 2015, 22, 869–876. [Google Scholar] [CrossRef]
- Zhang, D.; Song, Y.; Du, Z.; Wang, L.; Li, Y.; Goodenough, J.B. Active LaNi1−xFexO3 bifunctional catalysts for air cathodes in alkaline media. J. Mater. Chem. A 2015, 3, 9421–9426. [Google Scholar] [CrossRef]
- Du, Z.; Yang, P.; Wang, L.; Lu, Y.; Goodenough, J.B.; Zhang, J.; Zhang, D. Electrocatalytic performances of LaNi1−xMgxO3 perovskite oxides as bi-functional catalysts for lithium air batteries. J. Power Sources 2014, 265, 91–96. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, J.-I.; Park, H.; Lee, S.W.; Kim, D.Y.; An, W.Y.; Kim, J.H.; Kim, J.; Cho, H.-S.; Park, J.-Y. Multiple perovskite layered lanthanum nickelate Ruddlesden-Popper systems as highly active bifunctional oxygen catalysts. Chem. Eng. J. 2021, 409, 128226. [Google Scholar] [CrossRef]
- Costa, A.; Jorge, M.E.M.; Carvalho, M.D.; Gomes, A.; da Silva Pereira, M.I. LaNi1−xCuxO3 (x = 0.05, 0.10, 0.30) coated electrodes for oxygen evolution in alkaline medium. J. Solid State Electrochem. 2013, 17, 2311–2318. [Google Scholar] [CrossRef]
- Shui, Z.; Tian, H.; Yu, S.; Xiao, H.; Zhao, W.; Chen, X. La0.75Sr0.25MnO3-based perovskite oxides as efficient and durable bifunctional oxygen electrocatalysts in rechargeable Zn-air batteries. Sci. China Mater. 2022, 66, 1002–1012. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Almomani, F.; Malik, S.S.; Suslov, S.; Tarlochan, F. Combustion synthesis of bifunctional LaMO3 (M = Cr, Mn, Fe, Co, Ni) perovskites for oxygen reduction and oxygen evolution reaction in alkaline media. J. Electroanal. Chem. 2018, 809, 22–30. [Google Scholar] [CrossRef]
- Vignesh, A.; Prabu, M.; Shanmugam, S. Porous LaCo1−xNixO3-δ Nanostructures as an Efficient Electrocatalyst for Water Oxidation and for a Zinc-Air Battery. ACS Appl. Mater. Interfaces 2016, 8, 6019–6031. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; You, Y.; Yuan, J.; Yin, Y.-X.; Li, Y.-T.; Xin, S.; Zhang, D. Nickel-Doped La0.8Sr0.2Mn1−xNixO3 Nanoparticles Containing Abundant Oxygen Vacancies as an Optimized Bifunctional Catalyst for Oxygen Cathode in Rechargeable Lithium–Air Batteries. ACS Appl. Mater. Interfaces 2016, 8, 6520–6528. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, T.; Lei, L.; Huang, K. Ta-Doped SrCoO3−δ as a promising bifunctional oxygen electrode for reversible solid oxide fuel cells: A focused study on stability. J. Mater. Chem. A 2017, 5, 8989–9002. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.; Xi, S.; Ren, X.; Zhou, Y.; Zhang, G.; Yang, H.; Du, Y.; Xu, Z.J. Tailoring the Co 3d-O 2p Covalency in LaCoO3 by Fe Substitution To Promote Oxygen Evolution Reaction. Chem. Mater. 2017, 29, 10534–10541. [Google Scholar] [CrossRef]
- Sun, J.; Du, L.; Sun, B.; Han, G.; Ma, Y.; Wang, J.; Huo, H.; Zuo, P.; Du, C.; Yin, G. A bifunctional perovskite oxide catalyst: The triggered oxygen reduction/evolution electrocatalysis by moderated Mn-Ni co-doping. J. Energy Chem. 2021, 54, 217–224. [Google Scholar] [CrossRef]
- Wang, J.; Saccoccio, M.; Chen, D.; Gao, Y.; Chen, C.; Ciucci, F. The effect of A-site and B-site substitution on BaFeO3−δ: An investigation as a cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2015, 297, 511–518. [Google Scholar] [CrossRef]
- Yuan, R.-H.; He, Y.; He, W.; Ni, M.; Leung, M.K.H. La0.8Sr0.2MnO3 based perovskite with A-site deficiencies as high performance bifunctional electrocatalyst for oxygen reduction and evolution reaction in alkaline. Energy Procedia 2019, 158, 5804–5810. [Google Scholar] [CrossRef]
- Xu, W.; Yan, L.; Teich, L.; Liaw, S.; Zhou, M.; Luo, H. Polymer-assisted chemical solution synthesis of La0.8Sr0.2MnO3-based perovskite with A-site deficiency and cobalt-doping for bifunctional oxygen catalyst in alkaline media. Electrochim. Acta 2018, 273, 80–87. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Du, Y.; Cai, X.; Hu, Q.; Zhang, J. Bi0.15Sr0.85Co0.8Fe0.2O3−δ perovskite: A novel bifunctional oxygen electrocatalyst with superior durability in alkaline media. J. Mater. Sci. Technol. 2022, 108, 158–163. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3−δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Yuan, R.-h.; Chen, B.; Zhang, Y.; Tan, F.; Liu, T. Boosting the bifunctional electrocatalytic activity of cobalt free perovskite oxide (La0.8Sr0.2)0.95MnO3 via iron doping for high-efficiency Zn–air batteries. Sep. Purif. Technol. 2022, 300, 121858. [Google Scholar] [CrossRef]
- Li, P.; Wei, B.; Lü, Z.; Wu, Y.; Zhang, Y.; Huang, X. La1.7Sr0.3Co0.5Ni0.5O4+δ layered perovskite as an efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Appl. Surf. Sci. 2019, 464, 494–501. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, H.; Sun, S.; Wang, Q.; Li, S.; Liu, Z. (La1−xSrx)0.98MnO3 perovskite with A-site deficiencies toward oxygen reduction reaction in aluminum-air batteries. J. Power Sources 2017, 342, 192–201. [Google Scholar] [CrossRef]
- Miao, H.; Wu, X.; Chen, B.; Wang, Q.; Wang, F.; Wang, J.; Zhang, C.; Zhang, H.; Yuan, J.; Zhang, Q. A-site deficient/excessive effects of LaMnO3 perovskite as bifunctional oxygen catalyst for zinc-air batteries. Electrochim. Acta 2020, 333, 135566. [Google Scholar] [CrossRef]
- Konysheva, E.Y.; Xu, X.; Irvine, J.T. On the existence of A-site deficiency in perovskites and its relation to the electrochemical performance. Adv. Mater. 2012, 24, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.; Huang, D.; Zhou, M.; Ding, D.; Luo, H. Cation Deficiency Tuning of LaCoO3 Perovskite as Bifunctional Oxygen Electrocatalyst. ChemCatChem 2020, 12, 2768–2775. [Google Scholar] [CrossRef]
- Yan, L.; Lin, Y.; Yu, X.; Xu, W.; Salas, T.; Smallidge, H.; Zhou, M.; Luo, H. La0.8Sr0.2MnO3-Based Perovskite Nanoparticles with the A-Site Deficiency as High Performance Bifunctional Oxygen Catalyst in Alkaline Solution. ACS Appl. Mater. Interfaces 2017, 9, 23820–23827. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Menard, H.; Irvine, J.T. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef]
- Cheng, X.; Fabbri, E.; Yamashita, Y.; Castelli, I.E.; Kim, B.; Uchida, M.; Haumont, R.; Puente-Orench, I.; Schmidt, T.J. Oxygen Evolution Reaction on Perovskites: A Multieffect Descriptor Study Combining Experimental and Theoretical Methods. ACS Catal. 2018, 8, 9567–9578. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Yu, J.; Chen, Y.; Liu, M.; Shao, Z. Enhancing Electrocatalytic Activity of Perovskite Oxides by Tuning Cation Deficiency for Oxygen Reduction and Evolution Reactions. Chem. Mater. 2016, 28, 1691–1697. [Google Scholar] [CrossRef]
- Yuan, R.-H.; He, Y.; He, W.; Ni, M.; Leung, M.K.H. Bifunctional electrocatalytic activity of La0.8Sr0.2MnO3-based perovskite with the A-site deficiency for oxygen reduction and evolution reactions in alkaline media. Appl. Energy 2019, 251, 113406. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Remarkable Oxygen-Evolution Activity of Ca2-xSrxFe2O6-δ. Angew. Chem. Int. Ed. 2019, 58, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Zheng, Y.; Guo, Z.; Zhu, Y.; Chen, H.; Li, F.; Liu, P.; Yu, B.; Wang, X.; et al. Uncovering the effect of lattice strain and oxygen deficiency on electrocatalytic activity of perovskite cobaltite thin films. Adv. Sci. 2019, 6, 1801898. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Jin, S.; Chen, H.; Lee, W.; Liu, M.; Chen, Y. Anionic defect engineering of transition metal oxides for oxygen reduction and evolution reactions. J. Mater. Chem. A 2019, 7, 5875–5897. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, Y.; Sunarso, J.; Guan, D.; Liu, B.; Liu, H.; Zhou, W.; Shao, Z. New Phosphorus-Doped Perovskite Oxide as an Oxygen Reduction Reaction Electrocatalyst in an Alkaline Solution. Chemistry 2018, 24, 6950–6957. [Google Scholar] [CrossRef]
- Devi, V.S.; Athika, M.; Elumalai, P. Vacancy-induced LaMnO3 Perovskite as Bifunctional Air-breathing Electrode for Rechargeable Lithium-Air Battery. ChemistrySelect 2022, 7, e202202554. [Google Scholar] [CrossRef]
- Chen, C.-F.; King, G.; Dickerson, R.M.; Papin, P.A.; Gupta, S.; Kellogg, W.R.; Wu, G. Oxygen-deficient BaTiO3-x perovskite as an efficient bifunctional oxygen electrocatalyst. Nano Energy 2015, 13, 423–432. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Boosting oxygen reduction/evolution reaction activities with layered perovskite catalysts. Chem. Commun. 2016, 52, 10739–10742. [Google Scholar] [CrossRef]
- Kim, J.; Yin, X.; Tsao, K.-C.; Fang, S.; Yang, H. Ca2Mn2O5 as Oxygen-Deficient Perovskite Electrocatalyst for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 14646–14649. [Google Scholar] [CrossRef]
- Du, J.; Zhang, T.; Cheng, F.; Chu, W.; Wu, Z.; Chen, J. Nonstoichiometric Perovskite CaMnO3−δ for Oxygen Electrocatalysis with High Activity. Inorg. Chem. 2014, 53, 9106–9114. [Google Scholar] [CrossRef]
- Bian, J.; Li, Z.; Li, N.; Sun, C. Oxygen Deficient LaMn0.75Co0.25O3−δ Nanofibers as an Efficient Electrocatalyst for Oxygen Evolution Reaction and Zinc-Air Batteries. Inorg. Chem. 2019, 58, 8208–8214. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Jeen, H.; Barron, S.C.; Meyer, T.L.; Lee, H.N. Enhancing Perovskite Electrocatalysis through Strain Tuning of the Oxygen Deficiency. J. Am. Chem. Soc. 2016, 138, 7252–7255. [Google Scholar] [CrossRef]
- Li, Z.; Lv, L.; Wang, J.; Ao, X.; Ruan, Y.; Zha, D.; Hong, G.; Wu, Q.; Lan, Y.; Wang, C.; et al. Engineering phosphorus-doped LaFeO3−δ perovskite oxide as robust bifunctional oxygen electrocatalysts in alkaline solutions. Nano Energy 2018, 47, 199–209. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Sunarso, J.; Zhong, Y.; Shao, Z. Phosphorus-Doped Perovskite Oxide as Highly Efficient Water Oxidation Electrocatalyst in Alkaline Solution. Adv. Funct. Mater. 2016, 26, 5862–5872. [Google Scholar] [CrossRef]
- Li, X.; Xia, T.; Dong, Z.; Wang, J.; Li, Q.; Sun, L.; Huo, L.; Zhao, H. Unveiling positive impacts of fluorine anion doping on extraordinary catalytic activity of bifunctional-layered double perovskite electrodes for solid oxide fuel cells and electrolysis cells. Mater. Today Chem. 2023, 29, 101469. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Lü, W.; Li, C.; Ariando; Venkatesan, T.; Shao-Horn, Y. Highly Active Epitaxial La(1–x)SrxMnO3 Surfaces for the Oxygen Reduction Reaction: Role of Charge Transfer. J. Phys. Chem. Lett. 2015, 6, 1435–1440. [Google Scholar] [CrossRef]
- Peng, S.; Han, X.; Li, L.; Chou, S.; Ji, D.; Huang, H.; Du, Y.; Liu, J.; Ramakrishna, S. Electronic and Defective Engineering of Electrospun CaMnO3 Nanotubes for Enhanced Oxygen Electrocatalysis in Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2018, 8, 1800612. [Google Scholar] [CrossRef]
- Ran, J.; Wang, T.; Zhang, J.; Liu, Y.; Xu, C.; Xi, S.; Gao, D. Modulation of Electronics of Oxide Perovskites by Sulfur Doping for Electrocatalysis in Rechargeable Zn–Air Batteries. Chem. Mater. 2020, 32, 3439–3446. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, J.; Chen, P.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Vibronic Superexchange in Double Perovskite Electrocatalyst for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2018, 140, 11165–11169. [Google Scholar] [CrossRef]
- Hardin, W.G.; Slanac, D.A.; Wang, X.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Highly Active, Nonprecious Metal Perovskite Electrocatalysts for Bifunctional Metal–Air Battery Electrodes. J. Phys. Chem. Lett. 2013, 4, 1254–1259. [Google Scholar] [CrossRef]
- Zhuang, S.; Huang, C.; Huang, K.; Hu, X.; Tu, F.; Huang, H. Preparation of homogeneous nanoporous La0.6Ca0.4CoO3 for bi-functional catalysis in an alkaline electrolyte. Electrochem. Commun. 2011, 13, 321–324. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Lu, F.; Wang, Y.; Jin, C.; Li, F.; Yang, R.; Chen, F. Microporous La0.8Sr0.2MnO3 perovskite nanorods as efficient electrocatalysts for lithium–air battery. J. Power Sources 2015, 293, 726–733. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Li, Y.; Chen, L.; Shu, Z.; Chen, H.; Shi, J. High surface area mesoporous LaFexCo1−xO3 oxides: Synthesis and electrocatalytic property for oxygen reduction. Dalton Trans. 2013, 42, 9448. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, P.; Wang, S.; Zhang, Z.; Dai, Z.; Tan, S.; Chen, D. A highly efficient electrocatalyst for oxygen reduction reaction: Three-dimensionally ordered macroporous perovskite LaMnO3. J. Power Sources 2019, 412, 701–709. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, L.; Zhen, D.; Yoo, S.; Ding, Y.; Chen, D.; Chen, Y.; Zhang, Q.; Doyle, B.; Xiong, X.; et al. A tailored double perovskite nanofiber catalyst enables ultrafast oxygen evolution. Nat. Commun. 2017, 8, 14586. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Zhao, X.; Liu, Z.; Chen, W. Porous Perovskite LaNiO3 Nanocubes as Cathode Catalysts for Li-O2 Batteries with Low Charge Potential. Sci. Rep. 2014, 4, 6005. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef]
- Xu, J.-J.; Xu, D.; Wang, Z.-L.; Wang, H.-G.; Zhang, L.-L.; Zhang, X.-B. Synthesis of Perovskite-Based Porous La0.75Sr0.25MnO3 Nanotubes as a Highly Efficient Electrocatalyst for Rechargeable Lithium-Oxygen Batteries. Angew. Chem. Int. Ed. 2013, 52, 3887–3890. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.; Xia, L.; Wang, S.; Ding, L.X.; Li, D.; Xiao, K.; Dai, S.; Wang, H. Hierarchical Mesoporous/Macroporous Perovskite La0.5Sr0.5CoO3-x Nanotubes: A Bifunctional Catalyst with Enhanced Activity and Cycle Stability for Rechargeable Lithium Oxygen Batteries. ACS Appl. Mater. Interfaces 2015, 7, 22478–22486. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Jiang, Y.; Zou, L.; Gong, Y.; Chi, B.; Pu, J.; Jian, L. Perovskite La0.6Sr0.4Co0.2Fe0.8O3 as an effective electrocatalyst for non-aqueous lithium air batteries. Electrochim. Acta 2016, 191, 106–115. [Google Scholar] [CrossRef]
- Bu, Y.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A Highly Efficient and Robust Cation Ordered Perovskite Oxide as a Bifunctional Catalyst for Rechargeable Zinc-Air Batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-I.; Risch, M.; Park, S.; Kim, M.G.; Nam, G.; Jeong, H.-Y.; Shao-Horn, Y.; Cho, J. Optimizing nanoparticle perovskite for bifunctional oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 176–183. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, K.; Gong, Y.; Wang, H.; Wang, R.; Zhao, L.; He, B. An integrated bifunctional catalyst of metal-sulfide/perovskite oxide for lithium-oxygen batteries. J. Power Sources 2019, 437, 226908. [Google Scholar] [CrossRef]
- Malkhandi, S.; Trinh, P.; Manohar, A.K.; Jayachandrababu, K.C.; Kindler, A.; Surya Prakash, G.K.; Narayanan, S.R. Electrocatalytic Activity of Transition Metal Oxide-Carbon Composites for Oxygen Reduction in Alkaline Batteries and Fuel Cells. J. Electrochem. Soc. 2013, 160, F943–F952. [Google Scholar] [CrossRef]
- Sakthivel, M.; Bhandari, S.; Drillet, J.F. On Activity and Stability of Rhombohedral LaNiO3 Catalyst towards ORR and OER in Alkaline Electrolyte. ECS Electrochem. Lett. 2015, 4, A56–A58. [Google Scholar] [CrossRef]
- Mattick, V.F.; Jin, X.; White, R.E.; Huang, K. Understanding the role of carbon in alkaline oxygen electrocatalysis: A case study on La0.6Sr0.4CoO3−δ/Vulcan carbon composite electrocatalyst. Int. J. Hydrogen Energy 2019, 44, 2760–2769. [Google Scholar] [CrossRef]
- Poux, T.; Napolskiy, F.S.; Dintzer, T.; Kéranguéven, G.; Istomin, S.Y.; Tsirlina, G.A.; Antipov, E.V.; Savinova, E.R. Dual role of carbon in the catalytic layers of perovskite/carbon composites for the electrocatalytic oxygen reduction reaction. Catal. Today 2012, 189, 83–92. [Google Scholar] [CrossRef]
- Miyazaki, K.; Kawakita, K.-i.; Abe, T.; Fukutsuka, T.; Kojima, K.; Ogumi, Z. Single-step synthesis of nano-sized perovskite-type oxide/carbon nanotube composites and their electrocatalytic oxygen-reduction activities. J. Mater. Chem. 2011, 21, 1913–1917. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Park, M.G.; Ismayilov, V.; Chen, Z. Synergistic bifunctional catalyst design based on perovskite oxide nanoparticles and intertwined carbon nanotubes for rechargeable zinc-air battery applications. ACS Appl. Mater. Interfaces 2015, 7, 902–910. [Google Scholar] [CrossRef]
- Nandikes, G.; Gouse Peera, S.; Singh, L. Perovskite-Based Nanocomposite Electrocatalysts: An Alternative to Platinum ORR Catalyst in Microbial Fuel Cell Cathodes. Energies 2021, 15, 272. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, D.U.; Zamani, P. Electrospun porous nanorod perovskite oxide/nitrogen-doped graphene composite as a bi-functional catalyst for metal air batteries. Nano Energy 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Wu, X.; Yu, J.; Yang, G.; Liu, H.; Zhou, W.; Shao, Z. Perovskite oxide/carbon nanotube hybrid bifunctional electrocatalysts for overall water splitting. Electrochim. Acta 2018, 286, 47–54. [Google Scholar] [CrossRef]
- Ge, X.; Goh, F.W.; Li, B.; Hor, T.S.; Zhang, J.; Xiao, P.; Wang, X.; Zong, Y.; Liu, Z. Efficient and durable oxygen reduction and evolution of a hydrothermally synthesized La(Co0.55Mn0.45)0.99O3−δ nanorod/graphene hybrid in alkaline media. Nanoscale 2015, 7, 9046–9054. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xue, L.; Shang, Z.; Zhang, Z.; Chen, D. An enhanced non-noble perovskite-based oxygen electrocatalyst for efficient oxygen reduction and evolution reactions. J. Solid State Chem. 2020, 282, 121119. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Zhang, Y.; Xin, S.; He, X.; Zhang, D.; Shui, J. Electrocatalytic performances of g-C3N4-LaNiO3 composite as bi-functional catalysts for lithium-oxygen batteries. Sci. Rep. 2016, 6, 24314. [Google Scholar] [CrossRef]
- Bu, Y.; Jang, H.; Gwon, O.; Kim, S.H.; Joo, S.H.; Nam, G.; Kim, S.; Qin, Y.; Zhong, Q.; Kwak, S.K.; et al. Synergistic interaction of perovskite oxides and N-doped graphene in versatile electrocatalyst. J. Mater. Chem. A 2019, 7, 2048–2054. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Shao, Z. Perovskite/carbon composites: Applications in oxygen electrocatalysis. Small 2017, 13, 1603793. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Cheng, X.; Schmidt, T.J. Superior Bifunctional Electrocatalytic Activity of Ba0.5Sr0.5Co0.8Fe0.2O3−δ/Carbon Composite Electrodes: Insight into the Local Electronic Structure. Adv. Energy Mater. 2015, 5, 1402033. [Google Scholar] [CrossRef]
- Liu, J.; Jin, X.; Song, W. Facile preparation of modified carbon black-LaMnO3 hybrids and the effect of covalent coupling on the catalytic activity for oxygen reduction reaction. Chin. J. Catal. 2014, 35, 1173–1188. [Google Scholar] [CrossRef]
- Hosseini-Benhangi, P.; Garcia-Contreras, M.A.; Alfantazi, A.; Gyenge, E.L. Method for Enhancing the Bifunctional Activity and Durability of Oxygen Electrodes with Mixed Oxide Electrocatalysts: Potential Driven Intercalation of Potassium. J. Electrochem. Soc. 2015, 162, F1356–F1366. [Google Scholar] [CrossRef]
- Yu, J.; Chen, D.; Saccoccio, M.; Lam, K.; Ciucci, F. Promotion of Oxygen Reduction with Both Amorphous and Crystalline MnOx through the Surface Engineering of La0.8Sr0.2MnO3−δ Perovskite. ChemElectroChem 2018, 5, 1105–1112. [Google Scholar] [CrossRef]
- Karuppiah, C.; Thirumalraj, B.; Alagar, S.; Piraman, S.; Li, Y.-J.J.; Yang, C.-C. Solid-State Ball-Milling of Co3O4 Nano/Microspheres and Carbon Black Endorsed LaMnO3 Perovskite Catalyst for Bifunctional Oxygen Electrocatalysis. Catalysts 2021, 11, 76. [Google Scholar] [CrossRef]
- Christy, M.; Rajan, H.; Lee, H.; Rabani, I.; Koo, S.M.; Yi, S.C. Surface engineering of perovskites for rechargeable zinc–air battery application. ACS Appl. Energy Mater. 2021, 4, 1876–1886. [Google Scholar] [CrossRef]
- Fang, F.; Feng, N.; Zhao, P.; Chen, C.; Li, X.; Meng, J.; Liu, G.; Chen, L.; Wan, H.; Guan, G. In situ exsolution of Co/CoOx core-shell nanoparticles on double perovskite porous nanotubular webs: A synergistically active catalyst for soot efficient oxidation. Chem. Eng. J. 2019, 372, 752–764. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Luo, J.-L. A facile surface chemistry approach to bifunctional excellence for perovskite electrocatalysis. Nano Energy 2018, 49, 117–125. [Google Scholar] [CrossRef]
- Benhangi, P.H.; Alfantazi, A.; Gyenge, E. Manganese dioxide-based bifunctional oxygen reduction/evolution electrocatalysts: Effect of perovskite doping and potassium ion insertion. Electrochim. Acta 2014, 123, 42–50. [Google Scholar] [CrossRef]
- Wang, H.; Yan, L.; Nakotte, T.; Xu, W.; Zhou, M.; Ding, D.; Luo, H. IrO2-incorporated La0.8Sr0.2MnO3 as a bifunctional oxygen electrocatalyst with enhanced activities. Inorg. Chem. Front. 2019, 6, 1029–1039. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Wu, P.-W.; Wu, C.-Y.; Hsieh, Y.-C. Synthesis of La0.6Ca0.4Co0.8Ir0.2O3 perovskite for bi-functional catalysis in an alkaline electrolyte. J. Power Sources 2009, 189, 1003–1007. [Google Scholar] [CrossRef]
- Li, M.; Hua, B.; Chen, J.; Zhong, Y.; Luo, J.-L. Charge transfer dynamics in RuO2/perovskite nanohybrid for enhanced electrocatalysis in solid oxide electrolyzers. Nano Energy 2019, 57, 186–194. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Lyu, Y.-Q.; Lam, K.; Ciucci, F. In situ growth of Pt3Ni nanoparticles on an A-site deficient perovskite with enhanced activity for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 6399–6404. [Google Scholar] [CrossRef]
- Han, X.; Cheng, F.; Zhang, T.; Yang, J.; Hu, Y.; Chen, J. Hydrogenated uniform Pt clusters supported on porous CaMnO3 as a bifunctional electrocatalyst for enhanced oxygen reduction and evolution. Adv. Mater. 2014, 26, 2047–2051. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sunarso, J.; Lu, Q.; Zhou, Z.; Dai, J.; Guan, D.; Zhou, W.; Shao, Z. High-Performance Platinum-Perovskite Composite Bifunctional Oxygen Electrocatalyst for Rechargeable Zn–Air Battery. Adv. Energy Mater. 2019, 10, 1903271. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, C.; Xu, X.; Zhou, W.; Ran, R.; Shao, Z. A universal and facile way for the development of superior bifunctional electrocatalysts for oxygen reduction and evolution reactions utilizing the synergistic effect. Chem. Eur. J. 2014, 20, 15533–15542. [Google Scholar] [CrossRef]
- Risch, M.; Stoerzinger, K.A.; Maruyama, S.; Hong, W.T.; Takeuchi, I.; Shao-Horn, Y. La0.8Sr0.2MnO3−δ Decorated with Ba0.5Sr0.5Co0.8Fe0.2O3−δ: A Bifunctional Surface for Oxygen Electrocatalysis with Enhanced Stability and Activity. J. Am. Chem. Soc. 2014, 136, 5229–5232. [Google Scholar] [CrossRef]
- Kubba, D.; Ahmed, I.; Kour, P.; Biswas, R.; Kaur, H.; Yadav, K.; Haldar, K.K. LaCoO3 Perovskite Nanoparticles Embedded in NiCo2O4 Nanoflowers as Electrocatalysts for Oxygen Evolution. ACS Appl. Nano Mater. 2022, 5, 16344–16353. [Google Scholar] [CrossRef]





| Catalyst | Modification Strategy | ORR Potential (V) @ −1 mA cm−2 | OER Potential (V) @ 10 mA cm−2 | ΔE (V) | Reference |
|---|---|---|---|---|---|
| LaNiO3−δ | Crystallographic Structure Tuning | −0.25 vs. Ag/AgCl | 0.73 vs. Ag/AgCl | 0.98 | [38] |
| La0.8Sr0.2Co0.4Mn0.6O3 | B-site regulation | 0.81 vs. RHE | 1.72 vs. RHE | 0.91 | [52] |
| La0.75Sr0.25Mn0.5Fe0.5O3 | nano-processing and B-site regulation | 0.74 vs. RHE | 1.66 vs. RHE | 0.92 | [69] |
| LaMnxNiyCozO3 (x:y:z = 1:2:3) | B-site regulation | 0.84 vs. RHE | 1.60 vs. RHE | 0.76 | [75] |
| (La0.8Sr0.2)0.95Mn0.5Fe0.5O3 | A-site deficiency and B-site regulation | 0.12 vs. Ag/AgCl | 0.89 vs. Ag/AgCl | 0.77 | [91] |
| Vacancy-induced LaMnO3 | anionic regulation | 0.94 vs. RHE | 1.84 vs. RHE | 0.90 | [96] |
| nsLaNiO3/NC | nano-processing | 0.74 vs. RHE | 1.62 vs. RHE | 0.88 | [110] |
| Ni3S2/PrBa0.5Sr0.5Co2O5+δ | composite processing | 0.81 vs. RHE | 1.63 vs. RHE | 0.82 | [124] |
| La(Co0.55Mn0.45)0.99O3−δ/NrGO | composite processing | 0.84 vs. RHE | 1.72 vs. RHE | 0.88 | [134] |
| 10%g-C3N4-LaNiO3 | composite processing | -0.32 vs. SCE | 0.76 vs. SCE | 1.08 | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Chen, W.; Jiang, F.; Chen, X.; Liu, J. Research Progress of Perovskite-Based Bifunctional Oxygen Electrocatalyst in Alkaline Conditions. Molecules 2023, 28, 7114. https://doi.org/10.3390/molecules28207114
Fu K, Chen W, Jiang F, Chen X, Liu J. Research Progress of Perovskite-Based Bifunctional Oxygen Electrocatalyst in Alkaline Conditions. Molecules. 2023; 28(20):7114. https://doi.org/10.3390/molecules28207114
Chicago/Turabian StyleFu, Kailin, Weijian Chen, Feng Jiang, Xia Chen, and Jianmin Liu. 2023. "Research Progress of Perovskite-Based Bifunctional Oxygen Electrocatalyst in Alkaline Conditions" Molecules 28, no. 20: 7114. https://doi.org/10.3390/molecules28207114
APA StyleFu, K., Chen, W., Jiang, F., Chen, X., & Liu, J. (2023). Research Progress of Perovskite-Based Bifunctional Oxygen Electrocatalyst in Alkaline Conditions. Molecules, 28(20), 7114. https://doi.org/10.3390/molecules28207114






