Radiosynthesis and Early Evaluation of a Positron Emission Tomography Imaging Probe [18F]AGAL Targeting Alpha-Galactosidase A Enzyme for Fabry Disease
Abstract
:1. Introduction
2. Results and Discussion
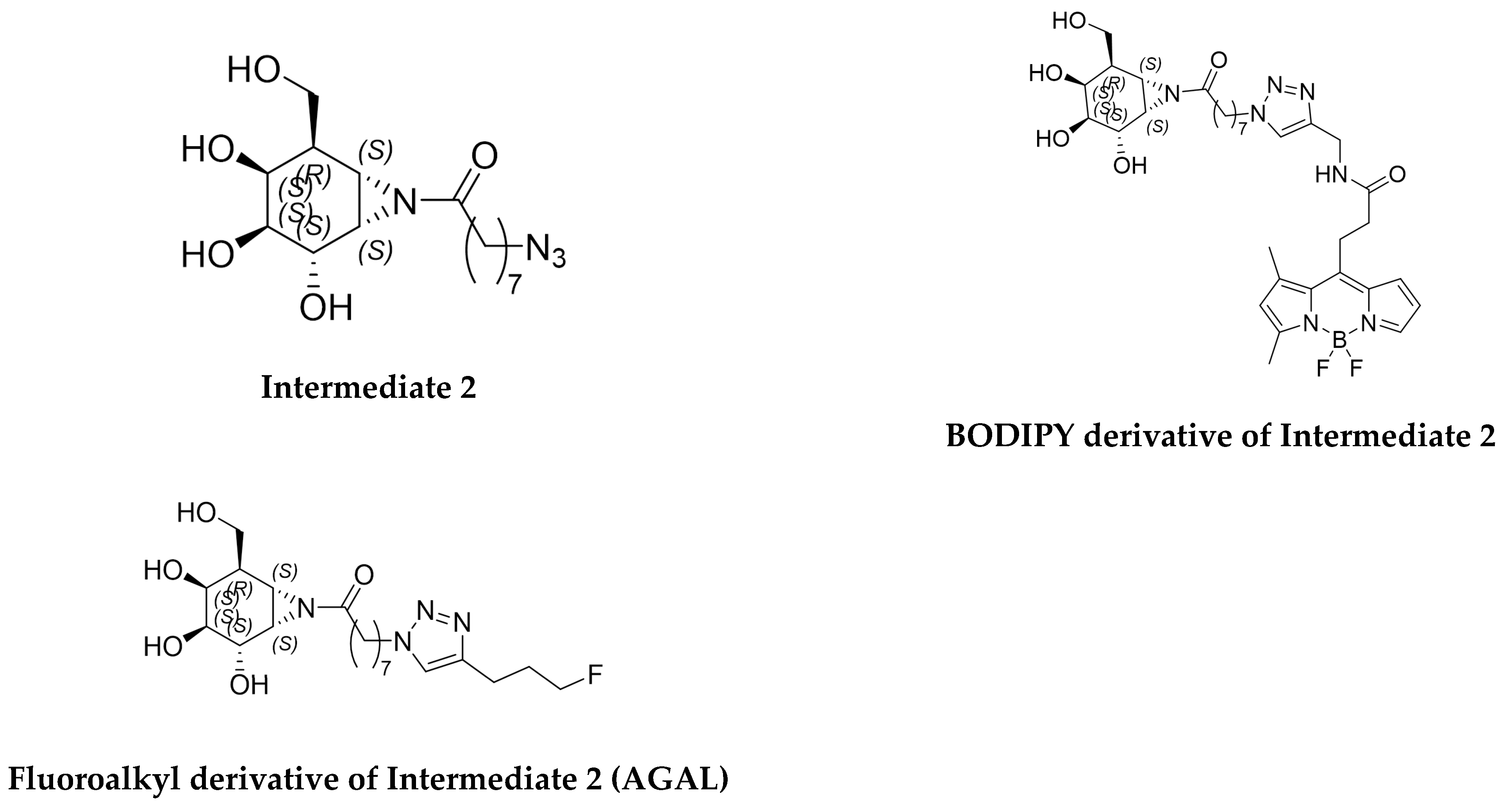
2.1. Synthesis of Radiolabeling Precursors and Reference Standards
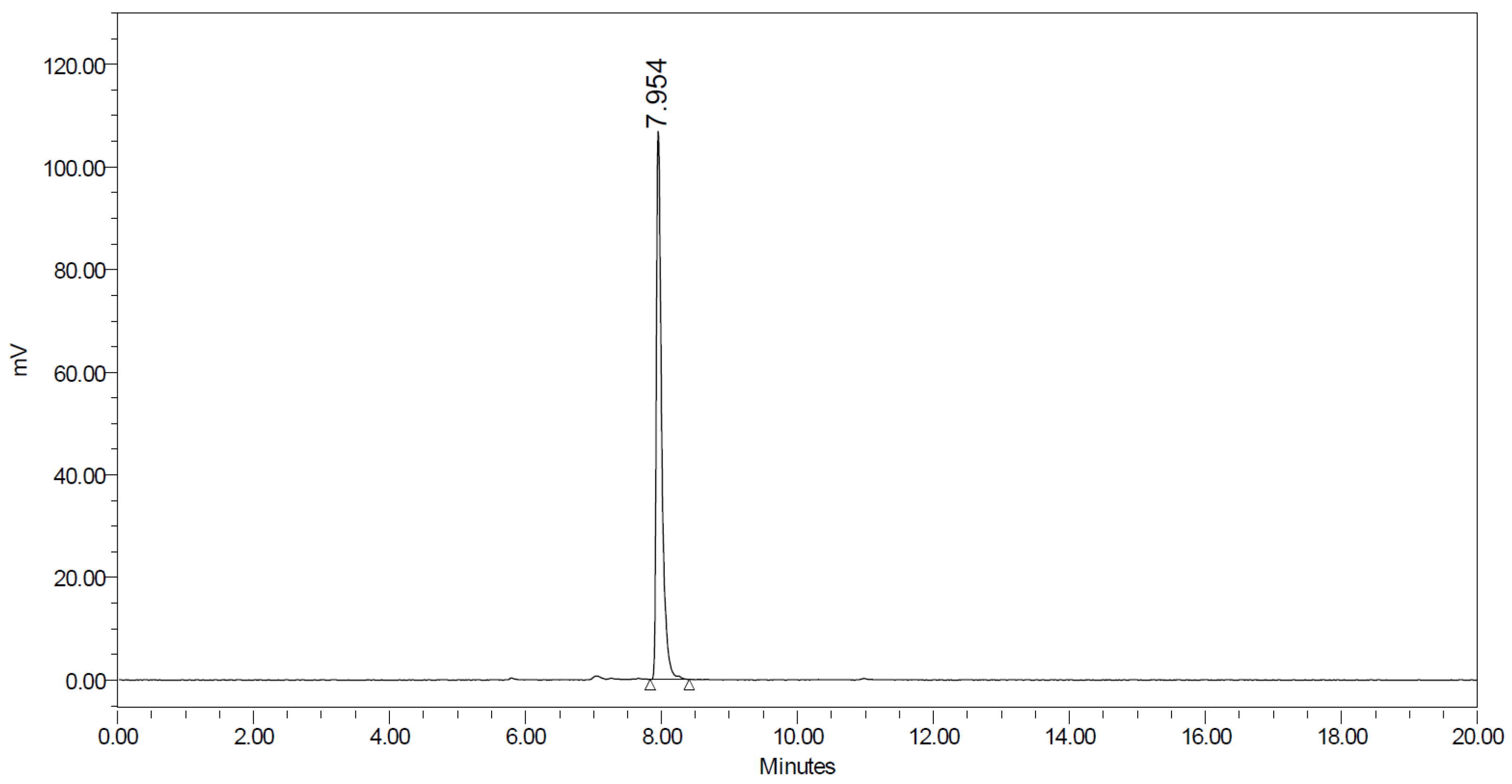
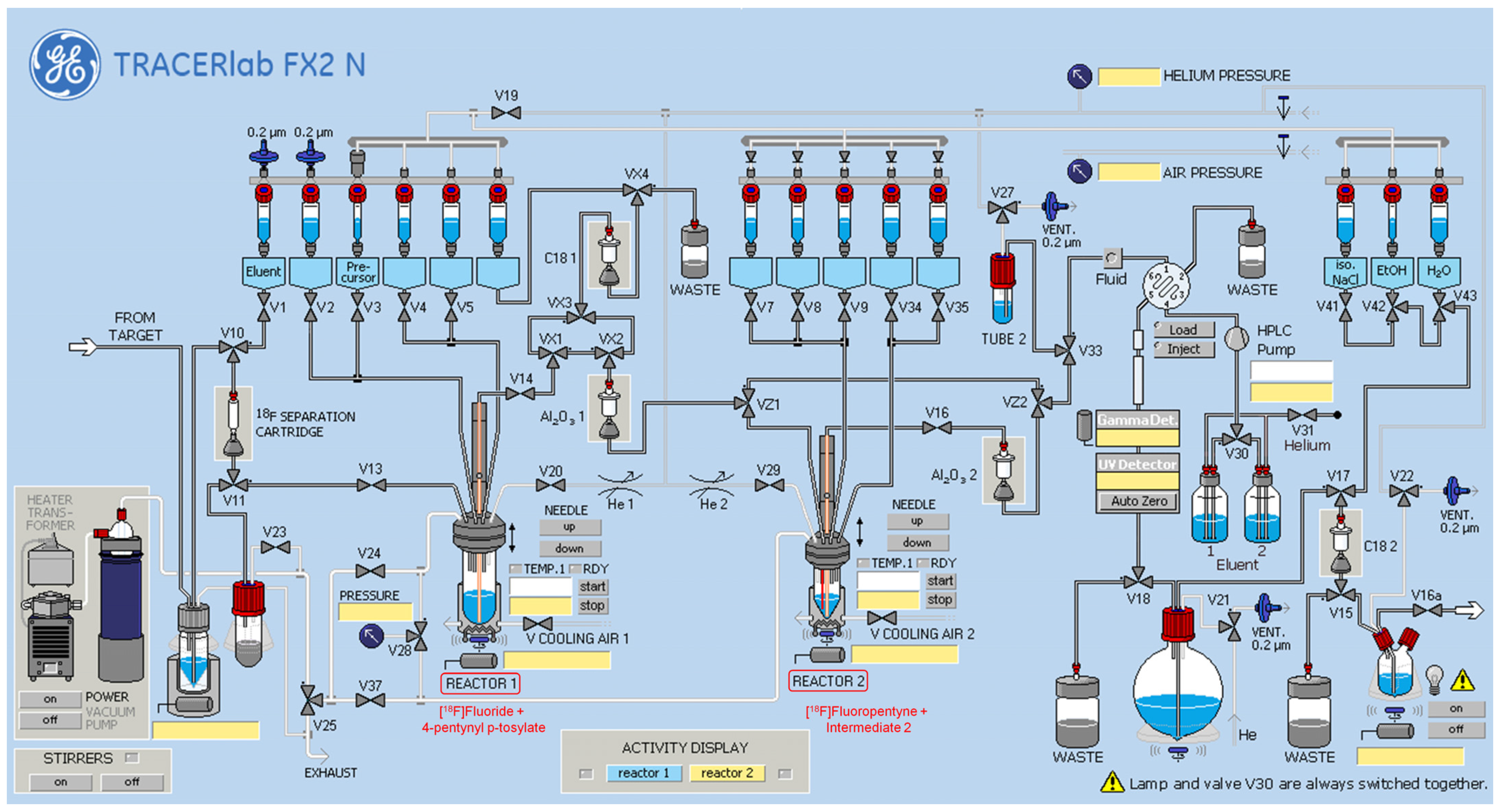
2.2. Synthesis of [18F]AGAL
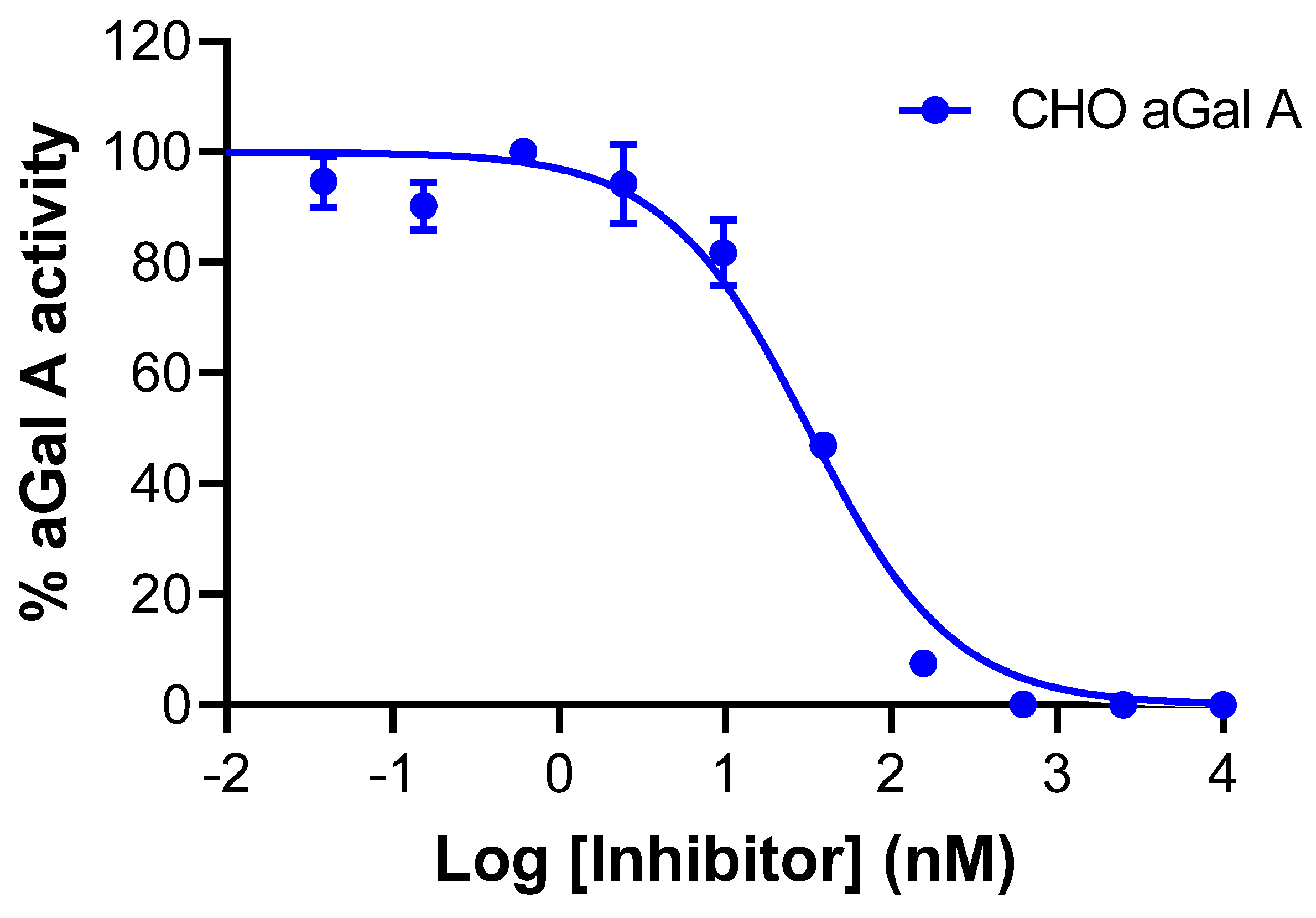
2.3. In Vitro Evaluation of AGAL
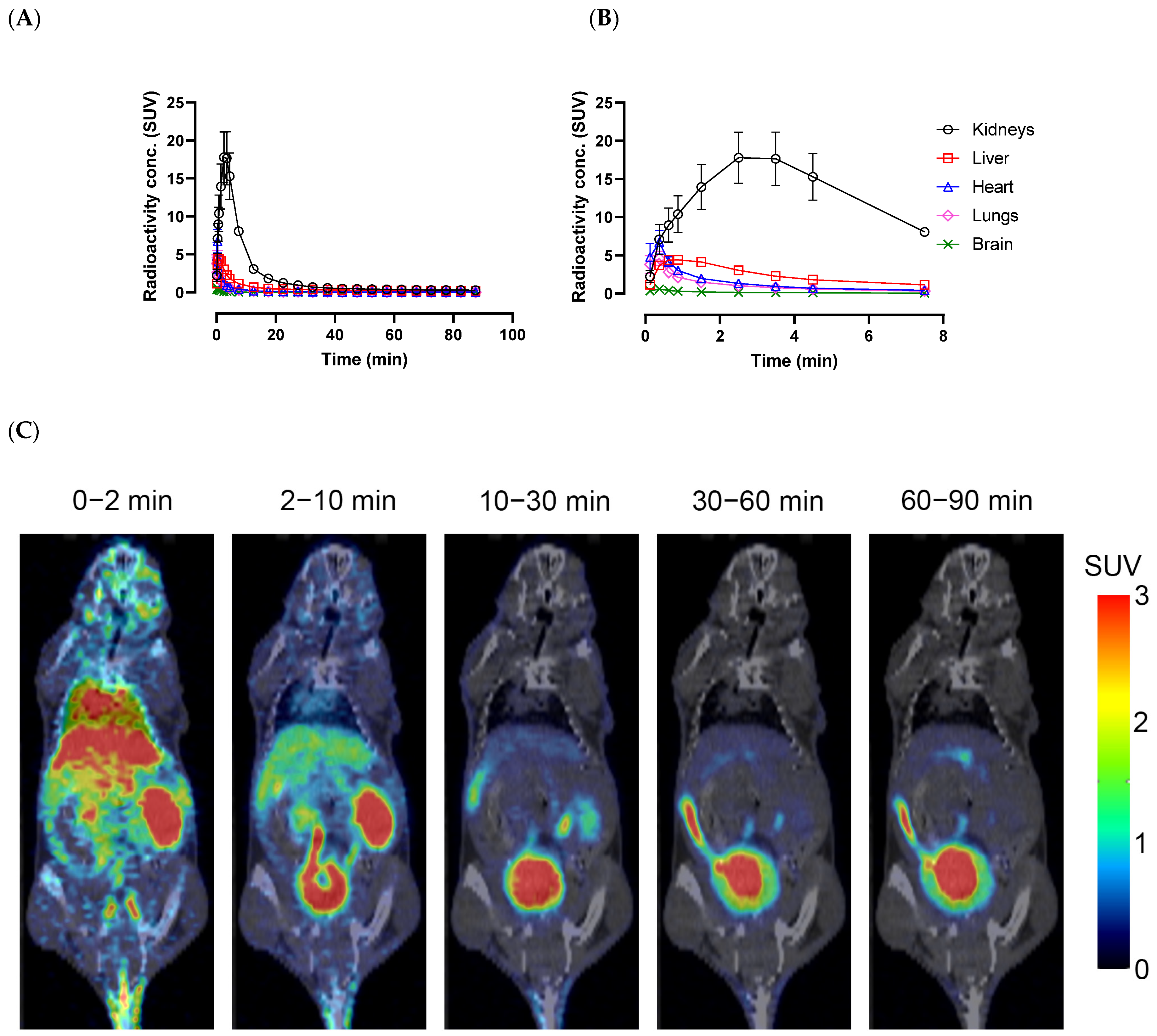
2.4. In Vivo PET/CT Imaging
3. Materials and Methods
3.1. Radiolabeling of AGAL
3.2. In Vitro Testing of AGAL for Selectivity and Affinity
3.3. LogD Determination of AGAL Molecule
3.4. In Vitro Liver Microsomal Metabolism Study
(X mg of microsomal protein/gram of liver) × (Y g of liver/kg of body weight)
- CLint = intrinsic clearance (calculated from microsomal incubations)
- X = 40 for human, 41.5 for rat and 45 for mouse
- Y = 21.4 for human, 40 for rat and 50 for mouse
- t1/2 = half-life
- CLh = hepatic clearance
- Q = liver blood flow rate = 1.24 L/h/kg for human, 4.8 L/h/kg for rat, and 5.4 L/h/kg for mouse
- Eh = hepatic extraction ratio
- CLh = hepatic clearance
3.5. Plasma Stability Study
3.6. Cytochrome 450 Enzyme Inhibition Assays
3.7. AGAL Cell Permeability Studies
3.7.1. Caco-2 Cell Culture
3.7.2. Bidirectional Permeability Studies
- dQ/dt = total amount of drug present in the receiver chamber per unit time (nmol/s)
- A = surface area (cm2, in the present studies A= 0.33 cm2)
- C0 = initial drug concentration in the donor chamber (nmol/mL)
- Papp= apparent permeability (cm/s × 10−6).
- Papp, A−to−B = Papp calculated where the apical side is the donor, and the basolateral side is the receiver
- Papp, B−to−A = Papp calculated where the basolateral side is the donor, and the apical side is the receiver.
3.8. In Vivo PET/CT IMAGING
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kok, K.; Zwiers, K.C.; Boot, R.G.; Overkleeft, H.S.; Aerts, J.; Artola, M. Fabry Disease: Molecular Basis, Pathophysiology, Diagnostics and Potential Therapeutic Directions. Biomolecules 2021, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Simonetta, I.; Riolo, R.; Todaro, F.; Di Chiara, T.; Miceli, S.; Pinto, A. Pathogenesis and Molecular Mechanisms of Anderson-Fabry Disease and Possible New Molecular Addressed Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 10088. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.I.; Beenakker, T.J.; Murray, B.; Scheij, S.; Kallemeijn, W.W.; Boot, R.G.; Verhoek, M.; Donker-Koopman, W.E.; Ferraz, M.J.; van Rijssel, E.R.; et al. Potent and selective activity-based probes for GH27 human retaining alpha-galactosidases. J. Am. Chem. Soc. 2014, 136, 11622–11625. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, A.; Trapero, A.; Perez, Y.; Llebaria, A. Galacto configured N-aminoaziridines: A new type of irreversible inhibitor of beta-galactosidases. Org. Biomol. Chem. 2015, 13, 5690–5697. [Google Scholar] [CrossRef]
- Hansen, F.G.; Bundgaard, E.; Madsen, R. A short synthesis of (+)-cyclophellitol. J. Org. Chem. 2005, 70, 10139–10142. [Google Scholar] [CrossRef]
- Harrak, Y.; Barra, C.M.; Delgado, A.; Castano, A.R.; Llebaria, A. Galacto-configured aminocyclitol phytoceramides are potent in vivo invariant natural killer T cell stimulators. J. Am. Chem. Soc. 2011, 133, 12079–12084. [Google Scholar] [CrossRef]
- Kim, D.H.; Choe, Y.S.; Jung, K.H.; Lee, K.H.; Choi, J.Y.; Choi, Y.; Kim, B.T. A (18)F-labeled glucose analog: Synthesis using a click labeling method and in vitro evaluation. Arch. Pharm. Res. 2008, 31, 587–593. [Google Scholar] [CrossRef]
- Su, H.; Chen, G.; Gangadharmath, U.; Gomez, L.F.; Liang, Q.; Mu, F.; Mocharla, V.P.; Szardenings, A.K.; Walsh, J.C.; Xia, C.F.; et al. Evaluation of [(18)F]-CP18 as a PET imaging tracer for apoptosis. Mol. Imaging Biol. 2013, 15, 739–747. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Lu, Q.; Xing, D.; Zhang, R. Exploring Carbohydrates for Therapeutics: A Review on Future Directions. Front. Pharmacol. 2021, 12, 756724. [Google Scholar] [CrossRef]
- Sodano, F.; Cristiano, C.; Rolando, B.; Marini, E.; Lazzarato, L.; Cuozzo, M.; Albrizio, S.; Russo, R.; Rimoli, M.G. Galactosylated Prodrugs: A Strategy to Improve the Profile of Nonsteroidal Anti-Inflammatory Drugs. Pharmaceuticals 2022, 15, 552. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Kalsoom, S.; Zamir, A.; Rehman, A.U.; Ashraf, W.; Imran, I.; Saeed, H.; Majeed, A.; Alqahtani, F.; Rasool, M.F. Clinical pharmacokinetics of nadolol: A systematic review. J. Clin. Pharm. Ther. 2022, 47, 1506–1516. [Google Scholar] [CrossRef]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G.; et al. Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov. 2010, 9, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Bolleddula, J.; Chen, H.; Cohen, L.; Zhou, X.; Pusalkar, S.; Berger, A.; Sedarati, F.; Venkatakrishnan, K.; Chowdhury, S.K. Metabolism and Disposition of [(14)C]Pevonedistat, a First-in-Class NEDD8-Activating Enzyme Inhibitor, after Intravenous Infusion to Patients with Advanced Solid Tumors. Drug Metab. Dispos. 2022, 50, 989–997. [Google Scholar] [CrossRef]
- Sandoval, P.; Chuang, B.C.; Cohen, L.; Yoneyama, T.; Pusalkar, S.; Yucha, R.W.; Chowdhury, S.K.; Chothe, P.P. Sinusoidal Uptake Determines the Hepatic Clearance of Pevonedistat (TAK-924) as Explained by Extended Clearance Model. Drug Metab. Dispos. 2022, 50, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Tanigawara, Y. Role of P-glycoprotein in drug disposition. Ther. Drug Monit. 2000, 22, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P-450 3A4: Regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 1–17. [Google Scholar] [CrossRef]
- Shimada, T.; Yamazaki, H.; Mimura, M.; Inui, Y.; Guengerich, F.P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994, 270, 414–423. [Google Scholar]
- Shi, P.; Liao, M.; Chuang, B.C.; Griffin, R.; Shi, J.; Hyer, M.; Fallon, J.K.; Smith, P.C.; Li, C.; Xia, C.Q. Efflux transporter breast cancer resistance protein dominantly expresses on the membrane of red blood cells, hinders partitioning of its substrates into the cells, and alters drug-drug interaction profiles. Xenobiotica 2018, 48, 1173–1183. [Google Scholar] [CrossRef]
- Yang, J.J.; Milton, M.N.; Yu, S.; Liao, M.; Liu, N.; Wu, J.T.; Gan, L.; Balani, S.K.; Lee, F.W.; Prakash, S.; et al. P-glycoprotein and breast cancer resistance protein affect disposition of tandutinib, a tyrosine kinase inhibitor. Drug Metab. Lett. 2010, 4, 201–212. [Google Scholar] [CrossRef] [PubMed]

| Sample | Human | Rat | Mouse | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CLint (L/h/Kg) | CLh (L/h/Kg) | Eh | CLint (L/h/Kg) | CLh (L/h/Kg) | Eh | CLint (L/h/Kg) | CLh (L/h/Kg) | Eh | |
| AGAL | <0.363 | <0.281 | <0.226 | 10.9 | 3.34 | 0.695 | 17.6 | 4.13 | 0.766 |
| Dextromethorphan | 2.59 | 0.838 | 0.676 | 38.5 | 4.27 | 0.889 | 33.1 | 4.64 | 0.860 |
| Sample | Plasma Half-Life, t1/2 (Min) | |
|---|---|---|
| Human | Mouse | |
| AGAL | 208 | 9.83 |
| Enalapril (Positive Control) | 283 | 30.6 |
| Propantheline (Positive Control) | 7.22 | 26 |
| Sample | Papp, A-to-B (×10−6 cm/s) | Papp, B-to-A (×10−6 cm/s) | Ratio (B/A) | ||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| Pevonedistat (positive control for P-gp) | 3.37 | 0.42 | 63.4 | 3.3 | 18.8 |
| Nadolol (low permeability control) | 0.36 | 0.02 | 1.45 | 0.03 | 4.1 |
| Propranolol (high permeability control) | 43.7 | 4.8 | 44.4 | 0.98 | 1 |
| Minoxidil (medium permeability control) | 7.81 | 0.64 | 9.78 | 0.48 | 1.3 |
| AGAL | 0.11 | 0.02 | 1.36 | 0.04 | 12 |
| AGAL + LY335979 (P-gp inhibitor) | 0.25 | 0.04 | 0.95 | 0.01 | 3.9 |
| AGAL + KO143 (BCRP inhibitor) | 0.15 | 0 | 1.31 | 0.01 | 8.7 |
| AGAL + Elacridar (GF918) (dual inhibitor of P-gp and BCRP) | 0.25 | 0.03 | 0.74 | 0.07 | 2.9 |
| CYP | Substrate | Final Incubation Concentrations (µM) |
|---|---|---|
| 1A2 | Phenacetin | 50 |
| 2B6 | Bupropion | 100 |
| 2C8 | Amodiaquine | 2 |
| 2C9 | Diclofenac | 5 |
| 2C19 | S-mephenytoin | 50 |
| 2D6 | Dextromethorphan | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohith, T.G.; Kaittanis, C.; Belanger, A.P.; Ahn, S.H.; Sandoval, P.; Cohen, L.; Rajarshi, G.; Ruangsiriluk, W.; Islam, R.; Winkelmann, C.T.; et al. Radiosynthesis and Early Evaluation of a Positron Emission Tomography Imaging Probe [18F]AGAL Targeting Alpha-Galactosidase A Enzyme for Fabry Disease. Molecules 2023, 28, 7144. https://doi.org/10.3390/molecules28207144
Lohith TG, Kaittanis C, Belanger AP, Ahn SH, Sandoval P, Cohen L, Rajarshi G, Ruangsiriluk W, Islam R, Winkelmann CT, et al. Radiosynthesis and Early Evaluation of a Positron Emission Tomography Imaging Probe [18F]AGAL Targeting Alpha-Galactosidase A Enzyme for Fabry Disease. Molecules. 2023; 28(20):7144. https://doi.org/10.3390/molecules28207144
Chicago/Turabian StyleLohith, Talakad G., Charalambos Kaittanis, Anthony P. Belanger, Shin Hye Ahn, Phil Sandoval, Lawrence Cohen, Girija Rajarshi, Wanida Ruangsiriluk, Rizwana Islam, Christopher T. Winkelmann, and et al. 2023. "Radiosynthesis and Early Evaluation of a Positron Emission Tomography Imaging Probe [18F]AGAL Targeting Alpha-Galactosidase A Enzyme for Fabry Disease" Molecules 28, no. 20: 7144. https://doi.org/10.3390/molecules28207144
APA StyleLohith, T. G., Kaittanis, C., Belanger, A. P., Ahn, S. H., Sandoval, P., Cohen, L., Rajarshi, G., Ruangsiriluk, W., Islam, R., Winkelmann, C. T., & McQuade, P. (2023). Radiosynthesis and Early Evaluation of a Positron Emission Tomography Imaging Probe [18F]AGAL Targeting Alpha-Galactosidase A Enzyme for Fabry Disease. Molecules, 28(20), 7144. https://doi.org/10.3390/molecules28207144






