Review of Phytochemical Potency as a Natural Anti-Helicobacter pylori and Neuroprotective Agent
Abstract
:1. Introduction
2. H. pylori
2.1. VacA
2.2. CagA
2.3. Urease
2.4. Pathophysiology of H. pylori Infection
2.5. Diagnosis and Treatment
3. NDs
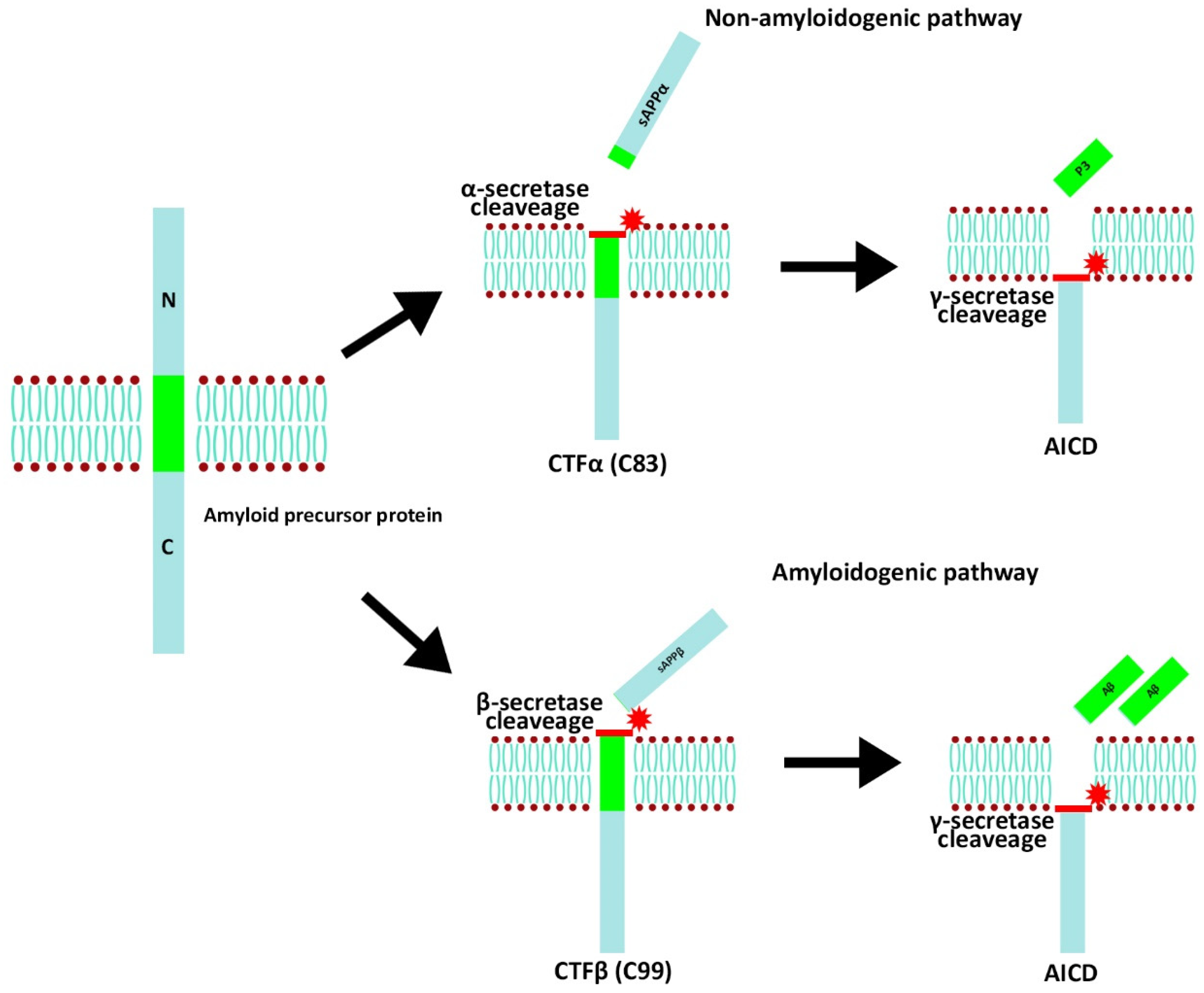
4. AD
5. PD
6. HD
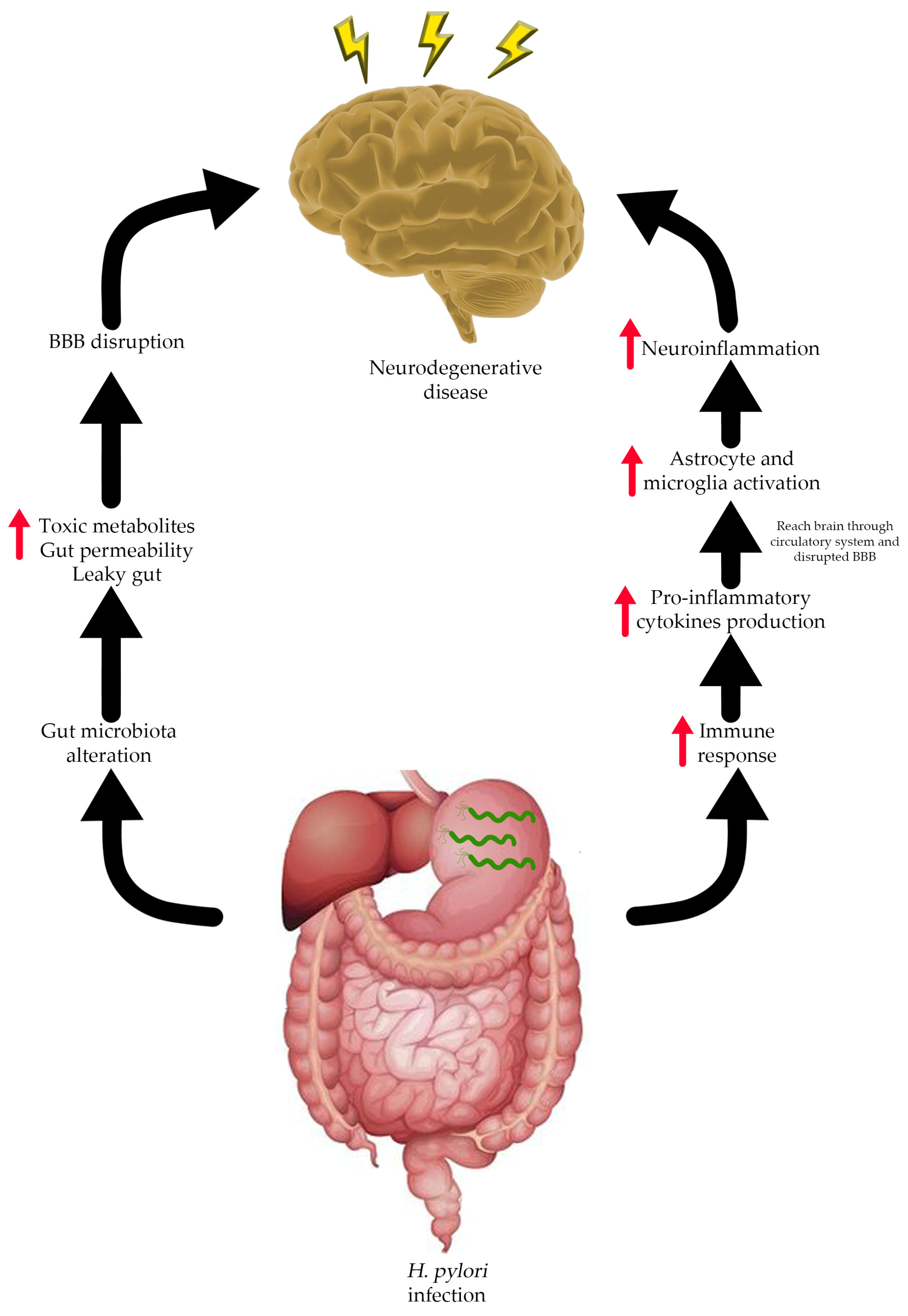
7. Connection between H. pylori Infection and Neurodegenerative Diseases
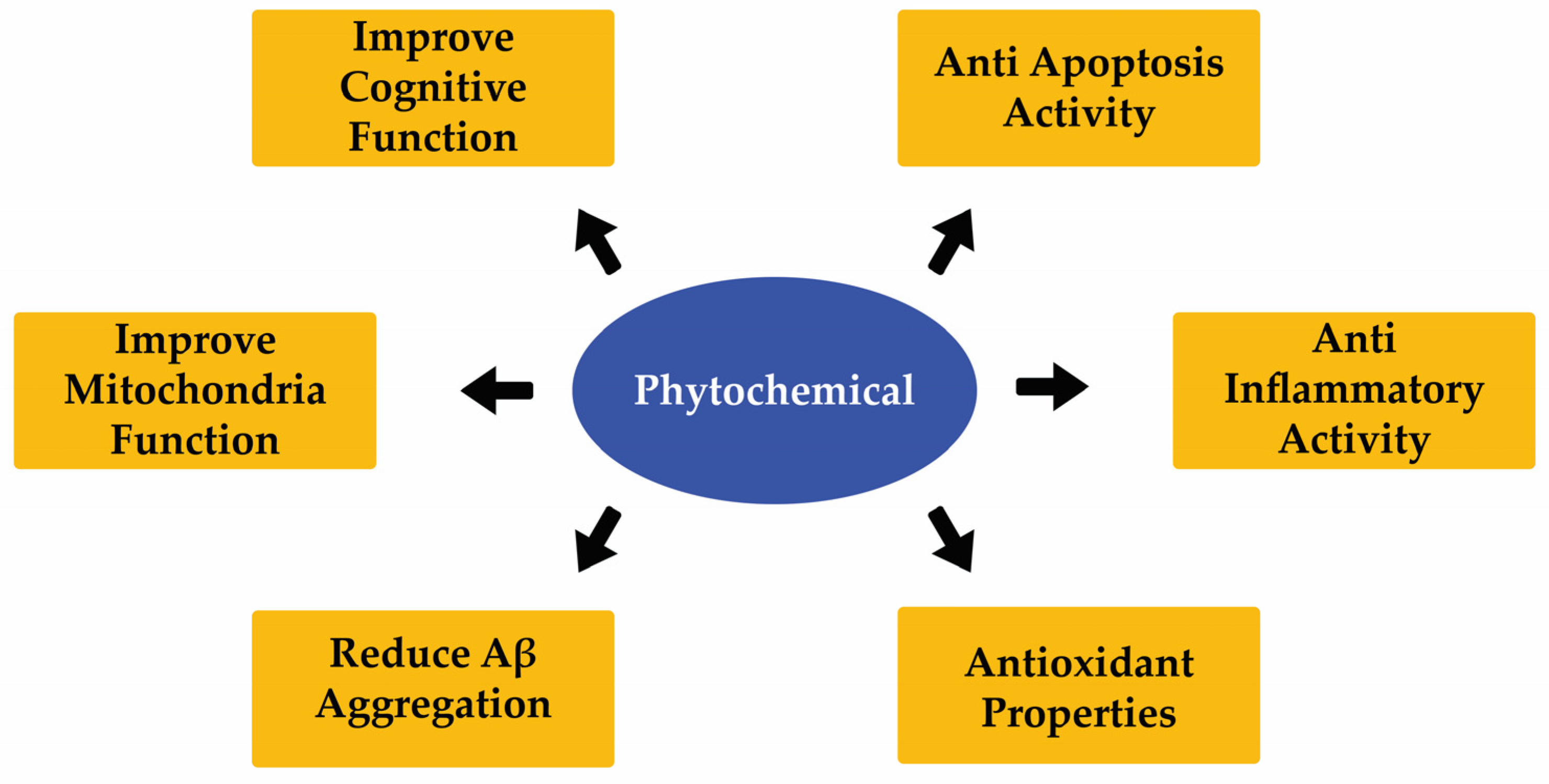
8. Phytochemicals
8.1. Phenolics
8.2. Carotenoids
8.3. Alkaloids
8.4. Saponins
9. Effect of Different Phytochemicals on H. pylori Infection
10. Effect of Different Phytochemicals on ND Development
11. H. pylori Eradication Improved Cognitive Function in an ND Subject
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kusters, J.G.; van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Dunn, B.E.; Cohen, H.; Blaser, M.J. Helicobacter pylori . Clin. Microbiol. Rev. 1997, 10, 720–741. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-Y.; Sheu, B.-S.; Wu, J.-J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Selgrad, M.; Malfertheiner, P. Treatment of Helicobacter pylori. Curr. Opin. Gastroenterol. 2011, 27, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Bytzer, P.; Dahlerup, J.F.; Eriksen, J.R.; Jarbøl, D.E.; Rosenstock, S.; Wildt, S.; Danish Society for Gastroenterology. Diagnosis and treatment of Helicobacter pylori infection. Dan. Med. Bull. 2011, 58, C4271. [Google Scholar] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef]
- Chi, H.; Chang, H.-Y.; Sang, T.-K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2005, 827, 65–75. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Elbejjani, M.; Dore, G.A.; Zonderman, A.B. Helicobacter pylori seropositivity and its association with incident all-cause and Alzheimer’s disease dementia in large national surveys. Alzheimer’s Dement. 2018, 14, 1148–1158. [Google Scholar] [CrossRef]
- Palacios, E.; Lobos-González, L.; Guerrero, S.; Kogan, M.J.; Shao, B.; Heinecke, J.W.; Quest, A.F.G.; Leyton, L.; Valenzuela-Valderrama, M. Helicobacter pylori outer membrane vesicles induce astrocyte reactivity through nuclear factor-κappa b activation and cause neuronal damage in vivo in a murine model. J. Neuroinflamm. 2023, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Mridula, K.R.; Borgohain, R.; Chandrasekhar Reddy, V.; Srinivasarao Bandaru, V.C.; Suryaprabha, T. Association of Helicobacter pylori with Parkinson’s disease. J. Clin. Neurol. 2017, 13, 181. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Overy, A.J.; Büsselberg, D. Phytochemicals and gastrointestinal cancer: Cellular mechanisms and effects to change cancer progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Cheung, K.-L.; Khor, T.O.; Chen, C.; Kong, A.-N. Phytochemicals: Cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010, 29, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.B. Historical review of medicinal plants′ usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective herbs for the management of Alzheimer’s disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Önem, E.; Sarısu, H.C.; Özaydın, A.G.; Muhammed, M.T.; Ak, A. Phytochemical profile, antimicrobial, and anti-quorum sensing properties of fruit stalks of Prunus Avium L. Lett. Appl. Microbiol. 2021, 73, 426–437. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Guo, Y.; Liu, W.; Li, M.; Zhang, L.; Zhang, Y.; Zhou, T.; Zhang, J.; Gao, H.; et al. Suppression of Helicobacter pylori infection by daily cranberry intake: A double-blind, randomized, placebo-controlled trial. J. Gastroenterol. Hepatol. 2020, 36, 927–935. [Google Scholar] [CrossRef]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.; et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The cocoa, cognition, and aging (cocoa) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2015, 56, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.J.; Armstrong, J.A.; McGechie, D.B.; Clancy, R.J. Attempt to fulfil Koch’s postulates for pyloric Campylobacter. Med. J. Aust. 1985, 142, 436–439. [Google Scholar] [CrossRef]
- Gotteland, M.; Andrews, M.; Toledo, M.; Muñoz, L.; Caceres, P.; Anziani, A.; Wittig, E.; Speisky, H.; Salazar, G. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition 2008, 24, 421–426. [Google Scholar] [CrossRef]
- Abbas, M.; Sharif, F.A.; Osman, S.M.; Osman, A.M.; El Sanousi, S.M.; Magzoub, M.; Ibrahim, M.E. Prevalence and associated symptoms of Helicobacter pylori infection among schoolchildren in Kassala state, east of Sudan. Interdiscip. Perspect. Infect. Dis. 2018, 2018, 4325752. [Google Scholar] [CrossRef] [PubMed]
- Palframan, S.L.; Kwok, T.; Gabriel, K. Vacuolating cytotoxin a (VacA), a key toxin for Helicobacter pylori pathogenesis. Front. Cell. Infect. Microbiol. 2012, 2, 92. [Google Scholar] [CrossRef]
- Abdullah, M.; Greenfield, L.K.; Bronte-Tinkew, D.; Capurro, M.I.; Rizzuti, D.; Jones, N.L. VacA promotes CagA accumulation in gastric epithelial cells during Helicobacter pylori Infection. Sci. Rep. 2019, 9, 38. [Google Scholar] [CrossRef]
- Willhite, D.C.; Cover, T.L.; Blanke, S.R. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J. Biol. Chem. 2003, 278, 48204–48209. [Google Scholar] [CrossRef]
- Rao, R.V.; Peel, A.; Logvinova, A.; del Rio, G.; Hermel, E.; Yokota, T.; Goldsmith, P.C.; Ellerby, L.M.; Ellerby, H.M.; Bredesen, D.E. Coupling endoplasmic reticulum stress to the cell death program: Role of the er chaperone grp78. FEBS Lett. 2002, 514, 122–128. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef]
- Rassow, J. Helicobacter pylori vacuolating toxin A and apoptosis. Cell Commun. Signal. 2011, 9, 26. [Google Scholar] [CrossRef]
- Foegeding, N.J.; Caston, R.R.; McClain, M.S.; Ohi, M.D.; Cover, T.L. An overview of Helicobacter pylori VacA toxin biology. Toxins 2016, 8, 173. [Google Scholar] [CrossRef]
- Jiménez-Soto, L.F.; Haas, R. The CagA toxin of Helicobacter pylori: Abundant production but relatively low amount translocated. Sci. Rep. 2016, 6, 23227. [Google Scholar] [CrossRef]
- Hatakeyama, M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef]
- Jones, K.R.; Whitmire, J.M.; Merrell, D.S. A Tale of two toxins: Helicobacter pylori CagA and VacA modulate host pathways that impact disease. Front. Microbiol. 2010, 1, 23227. [Google Scholar] [CrossRef]
- Suzuki, M.; Mimuro, H.; Kiga, K.; Fukumatsu, M.; Ishijima, N.; Morikawa, H.; Nagai, S.; Koyasu, S.; Gilman, R.H.; Kersulyte, D.; et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe 2009, 5, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.; The role of Helicobacter pylori urease in the pathogenesis of gastritis and peptic ulceration. Aliment. Pharmacol. Ther. 1996, 10 (Suppl. S1), 57–64. [Google Scholar]
- Olivera-Severo, D.; Uberti, A.F.; Marques, M.S.; Pinto, M.T.; Gomez-Lazaro, M.; Figueiredo, C.; Leite, M.; Carlini, C.R. A New Role for Helicobacter pylori urease: Contributions to angiogenesis. Front. Microbiol. 2017, 8, 1883. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, L.; Xu, C. Relationship between Helicobacter pylori infection and gastrointestinal microecology. Front. Cell. Infect. Microbiol. 2022, 12, 938608. [Google Scholar] [CrossRef] [PubMed]
- Mišak, Z.; Hojsak, I.; Homan, M. Review: Helicobacter pylori in pediatrics. Helicobacter 2019, 24, e12639. [Google Scholar] [CrossRef] [PubMed]
- Araújo, G.R.L.; Marques, H.S.; Santos, M.L.C.; da Silva, F.A.F.; de Brito, B.B.; Santos, G.L.C.; de Melo, F.F. Helicobacter pylori infection: How does age influence the inflammatory pattern? World J. Gastroenterol. 2022, 28, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Meliț, L.E.; Mărginean, C.O.; Mărginean, C.D.; Mărginean, M.O. The relationship between toll-like receptors and Helicobacter pylori-related gastropathies: Still a controversial topic. J. Immunol. Res. 2019, 2019, 8197048. [Google Scholar] [CrossRef]
- Kalali, B.; Mejías-Luque, R.; Javaheri, A.; Gerhard, M. H. pylori Virulence factors: Influence on immune system and pathology. Mediators Inflamm. 2014, 2014, 426309. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.P. Urea Breath Tests in the Management of Helicobacter pylori infection. Gut 1998, 43 (Suppl. S1), S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, B. Diagnosis of Helicobacter pylori infection and recent advances. Diagnostics 2021, 11, 1305. [Google Scholar] [CrossRef]
- Graham, D.Y.; Miftahussurur, M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: A mini review. J. Adv. Res. 2018, 13, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 2018, 145, 301–307. [Google Scholar] [CrossRef]
- Rachakonda, V.; Pan, T.H.; Le, W.D. Biomarkers of neurodegenerative disorders: How good are they? Cell Res. 2004, 14, 349–358. [Google Scholar] [CrossRef]
- Duncan, G.W. The aging brain and neurodegenerative diseases. Clin. Geriatr. Med. 2011, 27, 629–644. [Google Scholar] [CrossRef]
- Pierre, G. Neurodegenerative disorders and metabolic disease. Arch. Dis. Child. 2013, 98, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, H.; Manfredi, G. Introduction to neurodegenerative diseases and related techniques. Methods Mol. Biol. 2011, 793, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/29763097/ (accessed on 22 September 2022).
- Budelier, M.M.; Bateman, R.J. Biomarkers of Alzheimer disease. J. Appl. Lab. Med. 2019, 5, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef]
- Kametani, F.; Hasegawa, M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef]
- Rukmangadachar, L.A.; Bollu, P.C. Amyloid beta peptide. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/29083757/ (accessed on 12 October 2022).
- Zhang, Y.; Thompson, R.; Zhang, H.; Xu, H. App processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef]
- Cras, P.; Kawai, M.; Lowery, D.; Gonzalez-DeWhitt, P.; Greenberg, B.; Perry, G. Senile Plaque Neurites in Alzheimer Disease Accumulate Amyloid Precursor Protein. Proc. Natl. Acad. Sci. USA 1991, 88, 7552–7556. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Kempf, S.J.; Metaxas, A. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen. Res. 2016, 11, 1579. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Z.; Xia, Y.-Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal hyperphosphorylation of tau: Sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimer’s Dis. 2013, 33 (Suppl. S1), S123–S139. [Google Scholar] [CrossRef]
- Iqbal, K.; Gong, C.-X.; Liu, F. Hyperphosphorylation-induced tau oligomers. Front. Neurol. 2013, 4, 112. [Google Scholar] [CrossRef]
- Mudher, A.; Lovestone, S. Alzheimer’s disease—Do Taoists and Baptists finally shake hands? Trends Neurosci. 2002, 25, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef] [PubMed]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 1, CD005593. [Google Scholar] [CrossRef]
- Pohanka, M. Cholinesterases, a target of pharmacology and toxicology. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc 2011, 155, 219–223. [Google Scholar] [CrossRef]
- Robinson, D.M.; Keating, G.M. Memantine: A review of its use in Alzheimer’s disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef]
- McKeage, K. Memantine. CNS Drugs 2009, 23, 881–897. [Google Scholar] [CrossRef]
- Tariot, P.N.; Farlow, M.R.; Grossberg, G.T.; Graham, S.M.; McDonald, S.; Gergel, I.; for the Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil. JAMA 2004, 291, 317. [Google Scholar] [CrossRef]
- Lew, M. Overview of Parkinson’s disease. Pharmacotherapy 2007, 27 Pt 2, 155S160S. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and treatment of Parkinson disease. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Antony, P.M.A.; Diederich, N.J.; Krüger, R.; Balling, R. The hallmarks of Parkinson’s disease. FEBS J. 2013, 280, 5981–5993. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Greco, A.; Fabbrini, G.; Inghilleri, M.; Rizzo, M.I.; Gallo, A.; Conte, M.; Rosato, C.; Ciniglio Appiani, M.; de Vincentiis, M. Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 2016, 15, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Robinson, S.W.; Missale, C.; Caron, M.G. Dopamine receptors and brain function. Neuropharmacology 1996, 35, 1503–1519. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Dasgupta, P.S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 2000, 102, 113–124. [Google Scholar] [CrossRef]
- Reich, S.G.; Savitt, J.M. Parkinson’s disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s disease: A review. Front Biosci Schol Ed. 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Snowden, J.S. The neuropsychology of Huntington’s disease. Arch. Clin. Neuropsychol. 2017, 32, 876–887. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Langbehn, D.R.; Stout, J.C.; Aylward, E.; Ross, C.A.; Nance, M.; Guttman, M.; Johnson, S.; MacDonald, M.; Beglinger, L.J.; et al. Detection of Huntington’s disease decades before diagnosis: The predict-HD study. J. Neurol. Neurosurg. Psychiatry 2008, 79, 874–880. [Google Scholar] [CrossRef]
- Read, J.; Jones, R.; Owen, G.; Leavitt, B.R.; Coleman, A.; Roos, R.A.C.; Dumas, E.M.; Durr, A.; Justo, D.; Say, M.; et al. Quality of life in Huntington’s disease: A comparative study investigating the impact for those with pre-manifest and early manifest disease, and their partners. J. Huntington’s Dis. 2013, 2, 159–175. [Google Scholar] [CrossRef]
- Ghosh, R.; Tabrizi, S.J. Clinical features of Huntington’s disease. Adv. Exp. Med. Biol. 2018, 1049, 1–28. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2017, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Feigin, A. Huntington’s disease: New frontiers in therapeutics. Curr. Neurol. Neurosci. Rep. 2021, 21, 10. [Google Scholar] [CrossRef]
- Stoker, T.B.; Mason, S.L.; Greenland, J.C.; Holden, S.T.; Santini, H.; Barker, R.A. Huntington’s disease: Diagnosis and management. Pract. Neurol. 2022, 22, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Saleh, C.; Beyenburg, S. Is There an association between migraine and gastrointestinal disorders? J. Clin. Neurol. 2017, 13, 215. [Google Scholar] [CrossRef]
- Kountouras, J.M.D.P.; Tsolaki, M.; Gavalas, E.; Boziki, M.; Zavos, C.; Karatzoglou, P.; Chatzopoulos, D.; Venizelos, I. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology 2006, 66, 938–940. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Budzyński, J.; Kłopocka, M. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World J. Gastroenterol. 2014, 20, 5212. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Zhou, L.; Foster, J.A. Psychobiotics and the gut–brain axis: In the pursuit of happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2020, 19, 241–255. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The microbiota–gut–brain axis and Alzheimer’s disease: Neuroinflammation is to blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef]
- Iino, C.; Shimoyama, T. Impact of Helicobacter pylori infection on gut microbiota. World J. Gastroenterol. 2021, 27, 6224–6230. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Xu, J.; Wei, X.; Yang, J.; Liu, Y.; Li, H.; Zhao, C.; Wang, Y.; Zhang, L.; et al. Helicobacter pylori infection aggravates dysbiosis of gut microbiome in children with gastritis. Front. Cell. Infect. Microbiol. 2019, 9, 375. [Google Scholar] [CrossRef]
- Zheng, W.; Miao, J.; Luo, L.; Long, G.; Chen, B.; Shu, X.; Gu, W.; Peng, K.; Li, F.; Zhao, H.; et al. The effects of Helicobacter pylori infection on microbiota associated with gastric mucosa and immune factors in children. Front. Immunol. 2021, 12, 625586. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.-F. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef]
- Zou, B.; Li, J.; Ma, R.-X.; Cheng, X.-Y.; Ma, R.-Y.; Zhou, T.-Y.; Wu, Z.-Q.; Yao, Y.; Li, J. Gut microbiota is an impact factor based on the brain-gut axis to alzheimer’s disease: A systematic review. Aging Dis. 2023, 14, 964. [Google Scholar] [CrossRef]
- Doulberis, M.; Kotronis, G.; Thomann, R.; Polyzos, S.A.; Boziki, M.; Gialamprinou, D.; Deretzi, G.; Katsinelos, P.; Kountouras, J. Review: Impact of Helicobacter pylori on Alzheimer’s disease: What do we know so far? Helicobacter 2017, 23, e12454. [Google Scholar] [CrossRef]
- Tawfik, A.; Samra, Y.A.; Elsherbiny, N.M.; Al-Shabrawey, M. Implication of hyperhomocysteinemia in blood retinal barrier (BRB) dysfunction. Biomolecules 2020, 10, E1119. [Google Scholar] [CrossRef]
- Brustolin, S.; Giugliani, R.; Félix, T.M. Genetics of homocysteine metabolism and associated disorders. Braz. J. Med. Biol. Res. 2010, 43, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, C.; Di Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a risk factor and potential nutraceutical target for certain pathologies. Front. Nutr. 2019, 6, 49. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and dementia: An international consensus statement. J. Alzheimer’s Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Gavalas, E.; Boziki, M.; Zavos, C. Helicobacter pylori may be involved in cognitive impairment and dementia development through induction of atrophic gastritis, vitamin b-12–folate deficiency, and hyperhomocysteinemia sequence. Am. J. Clin. Nutr. 2007, 86, 805–806. [Google Scholar] [CrossRef]
- Kountouras, J.; Gavalas, E.; Zavos, C.; Stergiopoulos, C.; Chatzopoulos, D.; Kapetanakis, N.; Gisakis, D. Alzheimer’s disease and Helicobacter pylori infection: Defective immune regulation and apoptosis as proposed common links. Med. Hypotheses 2007, 68, 378–388. [Google Scholar] [CrossRef]
- Albaret, G.; Sifré, E.; Floch, P.; Laye, S.; Aubert, A.; Dubus, P.; Azzi-Martin, L.; Giese, A.; Salles, N.; Mégraud, F.; et al. Alzheimer’s disease and Helicobacter pylori infection: Inflammation from stomach to brain? J. Alzheimer’s Dis. 2020, 73, 801–809. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Bella, R.; Alagona, G.; Ferri, R.; Carnemolla, A.; Pennisi, G. Helicobacter pylori and Alzheimer’s disease: A possible link. Eur. J. Intern. Med. 2004, 15, 381–386. [Google Scholar] [CrossRef]
- Roubaud-Baudron, C.; Krolak-Salmon, P.; Quadrio, I.; Mégraud, F.; Salles, N. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: Preliminary results. Neurobiol. Aging 2012, 33, 1009.e11–1009.e19. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Zeng, J.; Yang, Y.; Xiong, Y.; Zhang, Z.-H.; Qiu, M.; Yan, X.; Sun, X.-Y.; Tuo, Q.-Z.; Liu, R.; et al. Helicobacter pylori filtrate induces Alzheimer-like tau hyperphosphorylation by activating glycogen synthase kinase-3β. J. Alzheimer’s Dis. 2014, 43, 153–165. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. Biomed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Toledo, A.R.L.; Monroy, G.R.; Salazar, F.E.; Lee, J.-Y.; Jain, S.; Yadav, H.; Borlongan, C.V. Gut–brain axis as a pathological and therapeutic target for neurodegenerative disorders. Int. J. Mol. Sci. 2022, 23, 1184. [Google Scholar] [CrossRef]
- Rhee, S.H. Lipopolysaccharide: Basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest. Res. 2014, 12, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Zielen, S.; Trischler, J.; Schubert, R. Lipopolysaccharide challenge: Immunological effects and safety in humans. Expert Rev. Clin. Immunol. 2015, 11, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Lubomski, M.; Tan, A.H.; Lim, S.-Y.; Holmes, A.J.; Davis, R.L.; Sue, C.M. Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. 2019, 267, 2507–2523. [Google Scholar] [CrossRef]
- Çamcı, G.; Oğuz, S. Association between Parkinson’s disease and Helicobacter pylori. J. Clin. Neurol. 2016, 12, 147. [Google Scholar] [CrossRef]
- Lahner, E.; Annibale, B.; Delle Fave, G. Systematic review: Heliocobacter pylori infection and impaired drug absorption. Aliment. Pharmacol. Ther. 2009, 29, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Leitzmann, C. Characteristics and health benefits of phytochemicals. Forsch. Komplementmed. 2016, 23, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2014, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Lachowicz, S.; Józef Gorzelany; Matłok, N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2017, 244, 705–719. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Phytochemical compounds and antioxidant activity in different cultivars of cranberry (Vaccinium macrocarpon L). J. Food Sci. 2017, 82, 2569–2575. [Google Scholar] [CrossRef]
- Kula, M.; Krauze-Baranowska, M. Rubus occidentalis: The black raspberry—Its potential in the prevention of cancer. Nutr. Cancer 2015, 68, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Giusti, M.M.; Stoner, G.D.; Schwartz, S.J. Characterization of a new anthocyanin in black raspberries (Rubus occidentalis) by liquid chromatography electrospray ionization tandem mass spectrometry. Food Chem. 2006, 94, 465–468. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Carotenoids: Health effects. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780123750839000453?via%3Dihub (accessed on 26 October 2022).
- Miller, E.C.; Giovannucci, E.; Erdman, J.W.; Bahnson, R.; Schwartz, S.J.; Clinton, S.K. Tomato products, lycopene, and prostate cancer risk. Urol. Clin. North Am. 2002, 29, 83–93. [Google Scholar] [CrossRef]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Structures and analysis of carotenoid molecules. Subcell. Biochem. 2016, 79, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N. 1.20—Carotenoids. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780080453828000095?via%3Dihub (accessed on 20 February 2023).
- Visioli, F.; Riso, P.; Grande, S.; Galli, C.; Porrini, M. Protective activity of tomato products on in vivo markers of lipid oxidation. Eur. J. Nutr. 2003, 42, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Catalano, A.; Simone, R.E.; Mele, M.C.; Cittadini, A. Effect of lycopene and tomato products on cholesterol metabolism. Ann. Nutr. Metab. 2012, 61, 126–134. [Google Scholar] [CrossRef]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef]
- Takagi, T.; Hayashi, R.; Nakai, Y.; Okada, S.; Miyashita, R.; Yamada, M.; Mihara, Y.; Mizushima, K.; Morita, M.; Uchiyama, K.; et al. Dietary intake of carotenoid-rich vegetables reduces visceral adiposity in obese Japanese men—A randomized, double-blind trial. Nutrients 2020, 12, 2342. [Google Scholar] [CrossRef]
- Wink, M. Alkaloids: Properties and Determination. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2010; Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780123849472000192 (accessed on 11 October 2023).
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Dmitrenok, A.S.; Dmitrenok, P.S.; Grebnev, B.B.; Stonik, V.A. Pibocin B, the first N-O-methylindole marine alkaloid, a metabolite from the far-eastern ascidian Eudistoma species. J. Nat. Prod. 2001, 64, 1559–1561. [Google Scholar] [CrossRef]
- Chay, C.; Cansino, R.; Pinzón, C.; Torres-Ochoa, R.; Martínez, R. Synthesis and anti-tuberculosis activity of the marine natural product caulerpin and its analogues. Mar. Drugs 2014, 12, 1757–1772. [Google Scholar] [CrossRef]
- Reyes, F.; Fernández, R.; Rodríguez, A.; Bueno, S.; de Eguilior, C.; Francesch, A.; Cuevas, C. Cytotoxic staurosporines from the marine ascidian Cystodytes solitus. J. Nat. Prod. 2008, 71, 1046–1048. [Google Scholar] [CrossRef]
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Lind, K.F.; Østerud, B.; Hansen, E.; Jørgensen, T.Ø.; Andersen, J.H. The immunomodulatory effects of barettin and involvement of the kinases CAMK1α and RIPK2. Immunopharmacol. Immunotoxicol. 2015, 37, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.-C.; Guo, Y.-W.; Shen, X. Two novel aromatic valerenane-type sesquiterpenes from the chinese green alga Caulerpa taxifolia. Bioorg. Med. Chem. Lett. 2006, 16, 2947–2950. [Google Scholar] [CrossRef] [PubMed]
- Güçlü-Ustündağ, O.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Yakindra Prasad Timilsena; Arissara Phosanam; Stockmann, R. Perspectives on saponins: Food functionality and applications. Int. J. Mol. Sci. 2023, 24, 13538. [Google Scholar] [CrossRef]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins: Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.-D.; Kengne, I.C.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of saponins from Melanthera elliptica and their synergistic effects with antibiotics against pathogenic phenotypes. Chem. Cent. J. 2018, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G. Effects of saponins on lipid metabolism: A review of potential health benefits in the treatment of obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L.; Yun, G.S.; Lu, Z.-Z.; Stoia, A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of CagA+ strains of Helicobacter pylori. Anticancer Res. 2003, 23, 3699–3702. [Google Scholar]
- Foryst-Ludwig, A.; Neumann, M.; Schneider-Brachert, W.; Naumann, M. Curcumin blocks NF-κB and the motogenic response in Helicobacter pylori infected epithelial cells. Biochem. Biophys. Res. Commun. 2004, 316, 1065–1072. [Google Scholar] [CrossRef]
- Lee, I.O.; Lee, K.H.; Pyo, J.H.; Kim, J.H.; Choi, Y.J.; Lee, Y.C. Anti-Inflammatory Effect of Capsaicin in Helicobacter pylori Infected Gastric Epithelial Cells. Helicobacter 2007, 12, 510–517. [Google Scholar] [CrossRef]
- Shih, Y.-T.; Wu, D.-C.; Liu, C.-M.; Yang, Y.-C.; Chen, I.-J.; Lo, Y.-C. San-Huang-Xie-Xin-Tang inhibits Helicobacter pylori induced inflammation in human gastric epithelial AGS cells. J. Ethnopharmacol. 2007, 112, 537–544. [Google Scholar] [CrossRef]
- Miguel, G.; Faleiro, L.; Cavaleiro, C.; Salgueiro, L.; Casanova, J. Susceptibility of Helicobacter pylori to essential oil of Dittrichia viscosa subsp. revoluta. Phytother. Res. 2008, 22, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-C.; Shun, C.-T.; Chien, C.-T.; Wang, T.-H. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS Cells and BALB/c mice. J. Nutr. 2008, 138, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Gaus, K.; Huang, Y.; Israel, D.A.; Pendland, S.L.; Adeniyi, B.A.; Mahady, G.B. Standardized ginger (Zingiber officinale) extract reduces bacterial load and suppresses acute and chronic inflammation in Mongolian gerbils infected with CagA+ Helicobacter pylori. Pharm. Biol. 2009, 47, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Pastene, E.; Speisky, H.; García, A.; Moreno, J.; Troncoso, M.; Figueroa, G. In vitro and in vivo effects of apple peel polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2010, 58, 7172–7179. [Google Scholar] [CrossRef]
- Huang, H.-L.; Ko, C.-H.; Yan, Y.-Y.; Wang, C.-K. Antiadhesion and Anti-Inflammation Effects of Noni (Morinda citrifolia) fruit extracts on AGS cells during Helicobacter pylori infection. J. Agric. Food Chem. 2014, 62, 2374–2383. [Google Scholar] [CrossRef]
- Pastene, E.; Parada, V.; Avello, M.; Ruiz, A.; García, A. Catechin-based procyanidins from Peumus boldus Mol. aqueous extract inhibit Helicobacter pylori urease and adherence to adenocarcinoma gastric cells. Phytother. Res. 2014, 28, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, H.; Li, X.; Xu, Z.; Chen, Y.-S.; Li, Y. Anti-Helicobacter pylori compounds from the ethanol extracts of Geranium wilfordii. J. Ethnopharmacol. 2013, 147, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, J.; Jafri, W.; Mehmood, M.H.; Abbas, Z.; Tariq, K. Immunomodulatory effects of Psyllium extract on Helicobacter pylori interaction with gastric epithelial cells. J. Evid.-Based Complement. Altern. Med. 2016, 21, NP18–NP24. [Google Scholar] [CrossRef]
- Kouitcheu Mabeku, L.B.; Eyoum Bille, B.; Tchouangueu, T.F.; Nguepi, E.; Leundji, H. Treatment of Helicobacter pylori infected mice with Bryophyllum pinnatum, a medicinal plant with antioxidant and antimicrobial properties, reduces bacterial load. Pharm. Biol. 2016, 55, 603–610. [Google Scholar] [CrossRef]
- Zhang, Q.; Yue, L. Inhibitory Activity of Mangiferin on Helicobacter pylori-induced inflammation in human gastric carcinoma ags cells. Afr. J. Tradit. Complement. Altern. Med. 2016, 14, 263–271. [Google Scholar] [CrossRef]
- Li, C.; Huang, P.; Wong, K.; Xu, Y.; Tan, L.; Chen, H.; Lu, Q.; Luo, C.; Tam, C.; Zhu, L.; et al. Coptisine-induced inhibition of Helicobacter pylori: Elucidation of specific mechanisms by probing urease active site and its maturation process. J. Enzyme Inhib. Med. Chem. 2018, 33, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-H.; Chiu, H.-F.; Huang, S.-Y.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Beneficial effect of burdock complex on asymptomatic Helicobacter pylori-infected subjects: A randomized, double-blind placebo-controlled clinical trial. Helicobacter 2018, 23, e12469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lim, J.W.; Kim, H. Astaxanthin prevents decreases in superoxide dismutase 2 level and superoxide dismutase ac-tivity in Helicobacter pylori-infected gastric epithelial cells. J. Cancer Prev. 2019, 24, 54–58. [Google Scholar] [CrossRef]
- Tian, J.; Si, X.; Wang, Y.; Gong, E.; Xie, X.; Zhang, Y.; Shu, C.; Li, B. Cyanidin-3-O-glucoside protects human gastric epithelial cells against Helicobacter pylori lipopolysaccharide-induced disorders by modulating TLR-mediated NF-κB pathway. J. Funct. Foods 2020, 68, 103899. [Google Scholar] [CrossRef]
- Goodman, C.; Lyon, K.N.; Scotto, A.; Smith, C.; Sebrell, T.A.; Gentry, A.B.; Bala, G.; Stoner, G.D.; Bimczok, D. A high-throughput metabolic microarray assay reveals antibacterial effects of black and red raspberries and blackberries against Helicobacter pylori infection. Antibiotics 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, L.; Hu, L.; Dong, W.; Zhang, M.; Liu, Y.; Li, P. Effect of Celastrus orbiculatus in inhibiting Helicobacter pylori induced inflammatory response by regulating epithelial mesenchymal transition and targeting mir-21/pdcd4 signaling pathway in gastric epithelial cells. BMC Complement. Altern. Med. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Eid, S.Y.; Alshammari, E.; Ashour, M.L.; Wink, M.; El-Readi, M.Z. Chrysanthemum indicum and Chrysanthemum morifolium: Chemical composition of their essential oils and their potential use as natural preservatives with antimicrobial and antioxidant activities. Foods 2020, 9, 1460. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Abdel-Aziz, M.M.; Elhady, S.S.; Bagalagel, A.A.; Malatani, R.T.; Elkady, W.M. Valorization of Pimenta racemosa essential oils and extracts: GC-MS and LC-MS phytochemical profiling and evaluation of Helicobacter pylori inhibitory activity. Molecules 2022, 27, 7965. [Google Scholar] [CrossRef]
- Kim, D.S.H.L.; Park, S.-Y.; Kim, J.-Y. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect pc12 rat pheochro-mocytoma and normal human umbilical vein endothelial cells from βa(1–42) insult. Neurosci. Lett. 2001, 303, 57–61. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Dang, Z.; Jia, Y. Berberine demonstrates anti-inflammatory properties in Helicobacter pylori-infected mice with chronic gastritis by attenuating the Th17 response triggered by the b cell-activating factor. J. Cell. Biochem. 2018, 119, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sun, Q.; Dong, H.; Qiao, J.; Lin, Y.; Yu, C.; Li, Y. Gastroprotective action of the extract of Corydalis yanhusuo in Helicobacter pylori infection and its bioactive component, dehydrocorydaline. J. Ethnopharmacol. 2023, 307, 116173. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, J.; Pan, K.; Go, V.L.W.; Chen, J.; You, W. Efficacy of cranberry juice on Helicobacter pylori infection: A dou-ble-blind, randomized placebo-controlled trial. Helicobacter 2005, 10, 139–145. [Google Scholar] [CrossRef]
- Shmuely, H.; Yahav, J.; Samra, Z.; Chodick, G.; Koren, R.; Niv, Y.; Ofek, I. Effect of cranberry juice on eradication of Helicobacter pylori in patients treated with antibiotics and a proton pump inhibitor. Mol. Nutr. Food Res. 2007, 51, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.-S.; Yang, K.-C.; Chen, J.-H.; Liu, Y.-H.; Hsu, Y.-H.; Lee, H.-C.; Huang, S.-Y. The efficacy of blueberry and grape seed extract combination on triple therapy for Helicobacter pylori eradication: A randomised controlled trial. Int. J. Food Sci. Nutr. 2016, 67, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ke, L.; Ni, Z.; Chen, Y.; Zhang, L.-H.; Zhu, S.-H.; Li, C.-J.; Shang, L.; Liang, J.; Shi, Y.-Q. Berberine containing quadruple therapy for initial Helicobacter pylori eradication. Medicine 2017, 96, e7697. [Google Scholar] [CrossRef]
- Surh, Y.-J. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem. Toxicol. 2002, 40, 1091–1097. [Google Scholar] [CrossRef]
- Burger, O.; Weiss, E.; Sharon, N.; Tabak, M.; Neeman, I.; Ofek, I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit. Rev. Food Sci. Nutr. 2002, 42 (Suppl. S3), 279–284. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Matsushima, M.; Suzuki, T.; Masui, A.; Kasai, K.; Kouchi, T.; Takagi, A.; Shirai, T.; Mine, T. Growth inhibitory action of cranberry on Helicobacter pylori. J. Gastroenterol. Hepatol. 2008, 23, S175–S180. [Google Scholar] [CrossRef]
- Malishev, R.; Shaham-Niv, S.; Nandi, S.; Kolusheva, S.; Gazit, E.; Jelinek, R. Bacoside-A, an Indian traditional-medicine sub-stance, inhibits β-amyloid cytotoxicity, fibrillation, and membrane interactions. ACS Chem. Neurosci. 2017, 8, 884–891. [Google Scholar] [CrossRef]
- Xu, T.-Z.; Shen, X.-Y.; Sun, L.-L.; Chen, Y.-L.; Zhang, B.-Q.; Huang, D.-K.; Li, W.-Z. Ginsenoside Rg1 protects against h2o2-induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int. J. Mol. Med. 2019, 43, 717–726. [Google Scholar] [CrossRef]
- Zolkiffly, S.Z.I.; Stanslas, J.; Abdul Hamid, H.; Mehat, M.Z. Ficus deltoidea: Potential inhibitor of pro-inflammatory mediators in lipopolysaccharide-induced activation of microglial cells. J. Ethnopharmacol. 2021, 279, 114309. [Google Scholar] [CrossRef]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and mitopark transgenic mouse models of Parkinson’s disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef]
- Zhang, M.; Qian, C.; Zheng, Z.-G.; Qian, F.; Wang, Y.; Thu, P.M.; Zhang, X.; Zhou, Y.; Tu, L.; Liu, Q.; et al. Jujuboside A promotes Aβ clearance and ameliorates cognitive deficiency in Alzheimer’s disease through activating Axl/HSP90/PPARγ pathway. Theranostics 2018, 8, 4262–4278. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, X.; Ren, F.; Yan, T.; Wu, B.; Bi, K.; Bi, W.; Jia, Y. Essential oil of Schisandra chinensis ameliorates cognitive decline in mice by alleviating inflammation. Food Funct. 2019, 10, 5827–5842. [Google Scholar] [CrossRef]
- Guan, L.; Mao, Z.; Yang, S.; Wu, G.; Chen, Y.; Yin, L.; Qi, Y.; Han, L.; Xu, L. Dioscin alleviates Alzheimer’s disease through regulating rage/nox4 mediated oxidative stress and inflammation. Biomed. Pharmacother. 2022, 152, 113248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Wang, S.; Song, S. Ginsenoside Rg3 prevents cognitive impairment by improving mitochondrial dysfunction in the rat model of Alzheimer’s disease. J. Agric. Food. Chem. 2019, 67, 10048–10058. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Weinreb, O.; Maor, G.; Youdim, M.B.H.; Mandel, S. Green tea polyphenol (−)-epigallocatechin-3-gallate prevents n-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001, 78, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar] [CrossRef]
- Koh, S.-H.; Lee, S.M.; Kim, H.Y.; Lee, K.-Y.; Lee, Y.J.; Kim, H.-T.; Kim, J.; Kim, M.-H.; Hwang, M.S.; Song, C.; et al. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci. Lett. 2006, 395, 103–107. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M.; Nadoushan, M.R.J.; Bagheri, M. Neuroprotective effect of genistein in 6-hydroxydopamine hemi-parkinsonian rat model. Phytother. Res. 2009, 23, 132–135. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington’s like symptoms in rats: Possible role of nitric oxide. Behav. Brain Res. 2010, 206, 38–46. [Google Scholar] [CrossRef]
- Pannangrong, W.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Tong-un, T. Purple rice berry is neuroprotective and en-hances cognition in a rat model of Alzheimer’s disease. J. Med. Food 2011, 14, 688–694. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J. Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale). Exp. Toxicol. Pathol. 2012, 64, 315–319. [Google Scholar] [CrossRef]
- Gopinath, K.; Sudhandiran, G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neu-rodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 2012, 227, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta 2013, 1832, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Arbabi, E.; Hamidi, G.; Talaei, S.A.; Salami, M. Estrogen agonist genistein differentially influences the cognitive and motor disorders in an ovariectomized animal model of parkinsonism. Iran. J. Basic Med. Sci. 2016, 19, 1285–1290. [Google Scholar] [CrossRef]
- El-Horany, H.E.; El-latif, R.N.A.; ElBatsh, M.M.; Emam, M.N. Ameliorative effect of quercetin on neurochemical and behav-ioral deficits in rotenone rat model of Parkinson’s disease: Modulating autophagy (quercetin on experimental Parkinson’s disease). J. Biochem. Mol. Toxicol. 2016, 30, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Chen, S.-L.; Chang, Y.-T.; Chyuan, J.-H.; Hsieh-Li, H.M. Administration of Momordica charantia enhances the neuroprotection and reduces the side effects of LiCl in the treatment of Alzheimer’s disease. Nutrients 2018, 10, 1888. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Kumar, G.; Gedda, M.R.; Tiwari, N.; Patnaik, R.; Singh, R.K.; Singh, S.P. Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Front. Pharmacol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, M.; Liang, Z. (−)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef]
- Xian, Y.-F.; Mao, Q.-Q.; Wu, J.C.; Su, Z.-R.; Chen, J.-N.; Lai, X.-P.; Ip, S.-P.; Lin, Z.-X. Isorhynchophylline treatment improves the amyloid-β-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. J. Alzheimer’s Dis. 2014, 39, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Jo, M.H.; Choe, K.; Khan, A.; Ahmad, S.; Saeed, K.; Kim, M.W.; Kim, M.O. Cycloastragenol, a triterpenoid saponin, regulates oxidative stress, neurotrophic dysfunctions, neuroinflammation and apoptotic cell death in neurodegenerative conditions. Cells 2021, 10, 2719. [Google Scholar] [CrossRef]
- Zhang, S.; Tomata, Y.; Sugiyama, K.; Sugawara, Y.; Tsuji, I. Citrus consumption and incident dementia in elderly Japanese: The Ohsaki cohort 2006 study. Br. J. Nutr. 2017, 117, 1174–1180. [Google Scholar] [CrossRef]
- Heo, J.-H.; Lee, S.-T.; Chu, K.; Oh, M.J.; Park, H.-J.; Shim, J.-Y.; Kim, M. An open-label trial of Korean red ginseng as an ad-juvant treatment for cognitive impairment in patients with Alzheimer’s disease. Eur. J. Neurol. 2008, 15, 865–868. [Google Scholar] [CrossRef]
- Lee, S.-T.; Chu, K.; Sim, J.-Y.; Heo, J.-H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alz-heimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2014, 29, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P.E. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2015, 55, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, D.S.H.L. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: a drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002, 65, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2004, 280, 5892–5901. [Google Scholar] [CrossRef]
- Ogunruku, O.O.; Oboh, G.; Passamonti, S.; Tramer, F.; Boligon, A.A. Capsicum annuum var. grossum (Bell Pepper) inhibits β-secretase activity and β-amyloid1–40 aggregation. J. Med. Food 2017, 20, 124–130. [Google Scholar] [CrossRef]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBio-Medicine 2016, 6, 42–49. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Zeng, Y. Overview of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2012, 11, 350–358. [Google Scholar] [CrossRef]
- Compagnoni, G.M.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The role of mitochondria in neurodegenerative diseases: The lesson from Alzheimer’s disease and Parkinson’s disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Sas, K.; Robotka, H.; Toldi, J.; Vécsei, L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007, 257, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Boziki, M.; Gavalas, E.; Zavos, C.; Grigoriadis, N.; Deretzi, G.; Tzilves, D.; Katsinelos, P.; Tsolaki, M.; Chatzopoulos, D.; et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J. Neurol. 2009, 256, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-P.; Chiu, G.-F.; Kuo, F.-C.; Lai, C.-L.; Yang, Y.-H.; Hu, H.-M.; Chang, P.-Y.; Chen, C.-Y.; Wu, D.-C.; Yu, F.-J. Eradication of Helicobacter pylori is associated with the progression of dementia: A population-based study. Gastroenterol. Res. Pract. 2013, 2013, e175729. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Visanji, N.P.; Liu, L.W.C.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef] [PubMed]


| Test Material | Activity | Findings | Source |
|---|---|---|---|
| Ginger (Gingerol) | Inhibit H. pylori growth | Inhibit growth of CagA+ H. pylori strains (MIC: 6.25–50 µg/mL) | [156] |
| Curcuma longa L. (Curcumin) | Anti-inflammatory properties |  IκBα degradation (up to 80 µM) IκBα degradation (up to 80 µM) IKKα and β activity (up to 80 µM) IKKα and β activity (up to 80 µM) NF-κB DNA-binding (up to 80 µM) NF-κB DNA-binding (up to 80 µM) | [157] |
| Chilli pepper (Capsaicin) | Anti-inflammatory properties |  H. pylori-induced IL-8 production in MKN45 and AGS cell (100 µM capsaicin, 43.2% and 70%, respectively, compared to control) H. pylori-induced IL-8 production in MKN45 and AGS cell (100 µM capsaicin, 43.2% and 70%, respectively, compared to control) IL-8 mRNA expression (100 µM capsaicin) IL-8 mRNA expression (100 µM capsaicin) Reduce H. pylori NF-κB activation (100 µM capsaicin) Reduce H. pylori NF-κB activation (100 µM capsaicin) | [158] |
| San-Huang-Xie-Xin-Tang (Coptis chinesis Franch, Scutellaria baicalensis Georgi, and Rheum officinale Baill) (Baicalin) | Anti-inflammatory properties |  H. pylori induced COX-2 enhancement (treatment vs. control group, p < 0.05) H. pylori induced COX-2 enhancement (treatment vs. control group, p < 0.05) IκBα degradation and nuclear translocation of NF-κB p50 subunit (treatment vs. control group, p < 0.05) IκBα degradation and nuclear translocation of NF-κB p50 subunit (treatment vs. control group, p < 0.05) iNOS and IL-8 mRNA expression (treatment vs. control group, p < 0.05) iNOS and IL-8 mRNA expression (treatment vs. control group, p < 0.05) decreased NO and IL-8 production (treatment vs. control group, p < 0.05) decreased NO and IL-8 production (treatment vs. control group, p < 0.05) | [159] |
| Dittrichia viscosa subsp. Revoluta (Essential oil (3-methoxy cuminyl isobutyrate, α-cadinol and α-eudesmol) | Inhibit H. pylori growth | Essential oil derived from Dittrichia viscosa especially fraction 5 and 7 show highest anti-H. pylori activity | [160] |
| Green tea (Catechin and pure sialic acid) | Antioxidant properties |  Reduce O2−, H2O2 count, NO production (treatment vs control group, p < 0.05) Reduce O2−, H2O2 count, NO production (treatment vs control group, p < 0.05) | [161] |
| Anti-inflammatory properties |  iNOS expression iNOS expression | ||
| Anti-apoptosis |  Inhibited apoptosis and reduced apoptosis related protein expression (treatment vs. control group, p < 0.05) Inhibited apoptosis and reduced apoptosis related protein expression (treatment vs. control group, p < 0.05) | ||
| Ginger (Gingerol) | Anti-inflammatory properties |  COX-2 (IC50: 8.5 µg/mL) COX-2 (IC50: 8.5 µg/mL) NF-κB transcription (IC50: 24.6 µg/mL) NF-κB transcription (IC50: 24.6 µg/mL) Inflammatory cytokine production (IL-1β, IL-6, IL-8, TNF-α (IC50: 3.89, 7.7, 8.5, and 8.37 µg/mL respectively)) Inflammatory cytokine production (IL-1β, IL-6, IL-8, TNF-α (IC50: 3.89, 7.7, 8.5, and 8.37 µg/mL respectively)) | [162] |
| Apple peel polyphenol | Anti-apoptosis |  H. pylori stimulated vacuolation in HeLa cell (IC50: 390 µg GAE/mL) H. pylori stimulated vacuolation in HeLa cell (IC50: 390 µg GAE/mL) | [163] |
| Anti-adhesion properties |  60% adhesion at concentration 5 mg GAE/mL 60% adhesion at concentration 5 mg GAE/mL | ||
| Noni fruit | Anti-adhesion properties |  Adhesion of H. pylori to AGS cell (treatment vs infected group, p < 0.05) Adhesion of H. pylori to AGS cell (treatment vs infected group, p < 0.05) Intracellular CagA level (treatment vs infected group, p < 0.05) Intracellular CagA level (treatment vs infected group, p < 0.05) | [164] |
| Anti-inflammatory properties |  Inflammatory markers (IL-8, iNOS, COX-2) and neutrophil chemotaxis (treatment vs. infected group, p < 0.05) Inflammatory markers (IL-8, iNOS, COX-2) and neutrophil chemotaxis (treatment vs. infected group, p < 0.05) | ||
| Peumus boldus Mol. (Catechin) | Inhibit urease activity |  Urease activity from H. pylori Urease activity from H. pylori | [165] |
| Anti-adhesion properties |  Adhesion ratio of H. pylori to AGS cell (treatment vs. infected group, p < 0.05) Adhesion ratio of H. pylori to AGS cell (treatment vs. infected group, p < 0.05) | ||
| Geranium wilfordii (Corilagin and 1,2,3,6-tetra-O-galloyl-b-D-glucose) | Inhibit H. pylori growth | Ethanol and ethyl acetate extract inhibited H. pylori growth (MIC: 40 and 30 μg/mL, respectively) | [166] |
| Plantago ovata | Anti-inflammatory properties |  Basal and H. pylori-stimulated IL-8 secretion up to 74.51% and 66.67%, respectively (p < 0.001) Basal and H. pylori-stimulated IL-8 secretion up to 74.51% and 66.67%, respectively (p < 0.001) CagA-positive H pylori–induced IL-8 mRNA expression up to 67.6% (p < 0.0001) CagA-positive H pylori–induced IL-8 mRNA expression up to 67.6% (p < 0.0001) Nf-κB activation (p = 0.0001) Nf-κB activation (p = 0.0001) | [167] |
| Bryophyllum pinnatum | Inhibit H. pylori growth | Bryophyllum pinnatum methanol extract showed anti-H. pylori activity (MIC: 32 μg/mL and MBC: 256 μg/mL) | [168] |
| Mangiferin indica (Mangiferin) | Inhibit H. pylori growth |  Growth of H. pylori (dose dependent, up to 100 μg/mL, p < 0.05) Growth of H. pylori (dose dependent, up to 100 μg/mL, p < 0.05) | [169] |
| Anti-adhesion properties |  H. pylori adhesion to AGS cell (p < 0.05, treatment vs. control group) H. pylori adhesion to AGS cell (p < 0.05, treatment vs. control group) | ||
| Anti-inflammatory properties |  Inflammatory cytokines (NF-κB p65 sub-unit, TNF-α, IL-1β, and IL-8) (p < 0.01) and enzyme expression (COX-2, iNOS) (p < 0.05) Inflammatory cytokines (NF-κB p65 sub-unit, TNF-α, IL-1β, and IL-8) (p < 0.01) and enzyme expression (COX-2, iNOS) (p < 0.05) | ||
| Coptis chinensis Franch (Berberine, palmatine, coptisine, jatrorrhizine, and epiberberine) | Inhibit H. pylori growth | Coptisine showed the highest anti-H. pylori activity with MIC and MBC 25 to 50 μg/mL and 37.5 to 125 μg/mL, respectively | [170] |
| Inhibit urease activity | Inhibit urease activity and maturation | ||
| Burdock complex (Arctium lappa, Angelica sinensis, Lithospermum erythrorhizon, and Sesamum indicum oil) | Anti-adhesion properties |  Adhesion of H. pylori to AGS cell (p < 0.05, compared with H. pylori-infected group) Adhesion of H. pylori to AGS cell (p < 0.05, compared with H. pylori-infected group) | [171] |
| Anti-inflammatory properties |  Inflammatory marker (IL-8, TNF-α) (p < 0.05, compared with H. pylori-infected group) Inflammatory marker (IL-8, TNF-α) (p < 0.05, compared with H. pylori-infected group) | ||
| Astaxanthin | Antioxidant properties | Prevent the SOD2 level decrease and increase SOD activity, and mitochondrial ROS production in AGS cell | [172] |
| Blueberry (Cyanindin-3-O-Glucoside) | Anti-inflammatory properties | C3G from blueberry suppressed abnormal DNA synthesis, inflammation, and TLR2 and TLR4 expression; induced apoptosis; and deactivated TLR-mediated NF-κB signaling in LPS-treated cell | [173] |
| Black raspberry (Anthocyanin) | Inhibit H. pylori growth | Inhibited growth of H. pylori without having side effects on AGS cell (MIC: 5 µg/mL) | [174] |
| Celastrus orbiculatus | Anti-inflammatory properties | Reduces inflammatory response by regulating epithelial–mesenchymal transition; suppressed methylation of PDCD4 promoter and inhibited microRNA-21, thus enhancing the PDCD4 expression | [175] |
| Chrysanthemum indicum and Chrysanthemum morifolium (Essential oil (major constituent camphor)) | Inhibit H. pylori growth | Both essential oil of C. indicum and C. morifolium showed potent anti-H. pylori activity with IC50 3.63 and 3.78 µg/mL, respectively | [176] |
| Pimenta racemosa (leaves and stem essential oil (eugenol) and methanolic extract) | Inhibit H. pylori growth | Pimenta racemosa stem essential oil showed the highest anti-H. pylori activity compared to others with MIC: 3.9 μg/mL and it inhibited H. pylori urease activity simulated with in silico molecular modelling | [177] |
 Indicating decrease in the issue.
Indicating decrease in the issue.| Test Material | Subject | Activity | Findings | Source |
|---|---|---|---|---|
| Green tea (Catechin and sialic acid) | Male BALB/c mice | Anti-inflammatory properties | Pre-treatment and post-treatment with catechin and/or sialic acid significantly reduced H. pylori infection, mucosal damage, and gastritits score (treatment vs. control group, p < 0.05) | [161] |
| Ginger (Gingerol) | Mongolian gerbils | Anti-inflammatory properties | Significantly reduces mucosal and submucosal inflammation, cryptitis, epithelial degeneration, and erosion due to H. pylori infection compared to control | [178] |
| Polyphenol rich apple peel extract | C57BL6/J mice | Anti-adhesion properties | Administration of apple peel polyphenol could reduce adhesion of H. pylori; reduced inflammation, lowering malonaldehyde levels and gastritis score in mice | [163] |
| Anti-inflammatory properties | ||||
| Bryophyllum pinnatum | Swiss mice | Inhibit H. pylori growth | Bryophyllum pinnatum significantly reduced bacterial colonization in gastric tissue and bacterial load in Swiss mice | [168] |
| Berberine | Male C57Bl/6 mice | Anti-inflammatory properties | Berberine treatment suppressed pro inflammatory cytokines and upregulated anti-inflammatory cytokines expression | [179] |
| Corydalis yanhusuo (Benzylisoquinoline alkaloids) | Male mice | Inhibit H. pylori growth | Two different extracts of Corydalis yanhusuo (ethanol and chloroform) inhibited the growth of H. pylori, with MIC ranging from 50 to 100 μg/mL and MBC ranging from 100 to 200 μg/mL; chloroform extract of Corydalis yanhusuo reduces survival ability of H. pylori in gastric mucosa and repairs gastric damage together with reduction of H. pylori IgG in infected mice | [180] |
| Cranberry (A-type proanthocyanidin) | H. pylori-positive adults | Anti-adhesion properties | Consumption of cranberry juice could significantly reduce H. pylori infection compared to placebo group | [181] |
| Cranberry (A-type proanthocyanidin) | H. pylori-positive adults | Anti-adhesion properties | Cranberry juice addition to standard triple therapy (Omeprazole, Amoxicillin, and Clarithromycin) could significantly improve H. pylori eradication rates in female subjects | [182] |
| Cranberry (A-type proanthocyanidin) and Lactobacillus johnsonii La1 | Asymptomatic H. pylori-positive children | Anti-adhesion properties | Combination of cranberry juice and L. johnsonii La1 reduced H. pylori infection compared to each test material alone and control group, but no synergistic inhibitory effect observed | [24] |
| Blueberry and grape seed extract (Proanthocyanidin) | H. pylori-positive patient | Antioxidant properties | Combination of blueberry and grape seed extract did not produce a significant change in eradication rate of H. pylori compared to placebo group | [183] |
| Berberine | H. pylori-positive patient | Antioxidant properties | No significant difference between berberine containing quadruple therapy eradication rate and adverse effect compared to bismuth containing quadruple therapy | [184] |
| Burdock complex (Arctium lappa, Angelica sinensis, Lithospermum erythrorhizon, and Sesamum indicum oil) | Asymptomatic H. pylori-positive subject | Anti-adhesion properties | Significantly reduced UBT value (compared to placebo, p < 0.05) | [171] |
| Anti-inflammatory properties | Significantly reduced inflammatory marker and (compared to placebo, p < 0.05) | |||
| Antioxidant properties | Improved antioxidant status and plasma phenolic level (compared to placebo, p < 0.05) and heal the ulcer in the stomach | |||
| Cranberry (A-type proanthocyanidin) | H. pylori positive adults | Anti-adhesion properties | Consumption of high-proanthocyanidin cranberry juice twice a day (44 mg/serving) for 8 weeks could significantly decrease H. pylori infection compared to placebo; consumption of encapsulated cranberry powder not significantly effective to reduce H. pylori infection | [20] |
| Test Material | Cell line | Activity | Findings | Source |
|---|---|---|---|---|
| Curcuma longa L. (Curcumin, demethoxycurcumin, and bisdemethoxycurcumin) | PC12 cells and human umbilical vein endothelial cells (HUVEC) | Anti-apoptosis activity | Three curcuminoids from Curcuma longa L. found to protect PC12 cells and HUVEC from Aβ insult | [162] |
| Curcuma longa L. (9 different isolated compounds) | PC12 cells | Anti-apoptosis activity | Five isolated compounds from Curcuma longa L. effectively protected PC12 cells from Aβ cytotoxicity | [163] |
| Capsicum annuum var. grossum (Polyphenol rich extract) | In vitro study | Reduce Aβ aggregation | Phenolic extract from bell pepper could counteract initial aggregation of Aβ and prevent further aggregation (fibril formation) | [164] |
| Bacopa monnieri (Bacoside-A) | SH-SY5Y cells | Anti-apoptosis activity | Reduced cell cytotoxicity and inhibited fibril formation both in buffer solution only and in the presence of membrane vesicles | [189] |
| Ginseng (Ginsenoside Rg1) | Primary hippocampal neurons | Anti-inflammatory properties | Ginsenoside Rg1 reduced ROS production, NOX2, and NLRP1 inflammasome due to H2O2 treatment. | [190] |
| Anti-apoptosis activity | Ginsenoside Rg1 also reduced apoptosis, activation of β-galactosidase, and neuronal damage after H2O2 treatment. | |||
| Ficus deltoidea Jack (Vitexin and isovitexin) | Mouse microglial (BV-2) cells | Anti-inflammatory properties | Treatment with Ficus deltoidea Jack extract significantly reduced ROS, NO, TNF-α, IL-1β, and IL-6 production from microglial cell after treatment with LPS | [191] |
| Quercetin | MN9D dopaminergic neuronal cells | Improve mitochondria function | Increased mitochondrial biogenesis and bioenergetics capacity of MN9D cell and reduced 6-hydroxydopmaine induced toxicity | [192] |
| Semen ziziphi spinosae (Jujuboside A) | BV-2 cells | Anti-apoptosis activity | JuA treatment upregulated expression of HSP90β, preserved PPARγ levels, promoted interaction between HSP90β and PPARγ, and promoted the clearance of Aβ42 | [193] |
| Schisandra chinensis (Essential oil) | BV-2 cells | Anti-inflammatory properties | Schisandra chinensis essential oil treatment decreased NO production and blocked MAPK activation in LPS-stimulated BV-2 microglial cell | [194] |
| Dioscoreae nipponicae (Dioscin) | SH-SY5Y cell | Anti-apoptosis activity | Dioscin improved cell viability | [195] |
| Antioxidant activity | Reduce ROS production due to H2O2 injury in SH-SY5Y cell line |
| Test Material | Compound | Subject | Findings | Source |
|---|---|---|---|---|
| Ginseng | Ginsenoside Rg3 | Male Wistar rats | Ginsenoside Rg3 significantly reduced neuronal apoptosis and apoptosis related protein after treatment of D-galactose; ginsenoside Rg3 also improved antioxidant status and mitochondrial function in D-galactose-induced AD rats | [196] |
| Green tea extract | (−) Epigallocatechin-3-gallate | Male C57/BL mice | Green tea extract treatment reduced N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity and prevented dopaminergic neuronal loss | [197] |
| Citrus | Tangeretin | Male Sprague-Dawley rats | Tangeretin can cross the blood–brain barrier and protect neuronal cells against 6-OHDA toxicity | [198] |
| Epigallocatechin gallate | Transgenic mice carrying human G93A mutated SOD1 gene | EGCG treatment prolonged lifespan and the symptoms onset and increased the survival rate of experimental mice | [199] | |
| Genistein | Male Sprague-Dawley rats | High dose genistein treatment showed neuroprotective effect against 6-OHDA toxicity | [200] | |
| Hesperidin and naringin | Wistar rats | Pre-treatment with hesperidin and naringin reduced behavioral alteration, oxidative stress, and mitochondrial enzyme dysfunction; this effect was further enhanced when combined with NOS inhibitor (L-NAME) | [201] | |
| Oryza sativa (Rice berry, purple) | Anthocyanin | Wistar rats | Prevented memory impairment and hippocampal neurodegeneration; decreased AChE activity and lipid peroxidation | [202] |
| Zingiber officinale (Red and White Ginger) | Wistar strain albino rats | Both extracts inhibited AChE individually and combined together, and both extracts significantly decreased the SNP and QA elevated brain MDA contents | [203] | |
| Naringin | Male Wistar rats | Improvement of glutathione/oxidized glutathione ratio and reduced free radical level due to 3-nitropropionic acid treatment through Nrf2 activation | [204] | |
| Quercetin | Female Wistar rats | Quercetin treatment improved mitochondrial function and antioxidant enzymes, as well as reducing astrogliosis and neurobehavioral deficits in experimental rats | [205] | |
| Genistein | Female Wistar rats | Improvement in Morris water maze result and neuroprotective effect on dopaminergic neuronal cells | [206] | |
| Quercetin | Albino rats | Significant reduction of behavioral impairment due to rotenone; reduced endoplasmic reticulum stress-induced apoptosis and oxidative stress | [207] | |
| Quercetin | MitoPark transgenic mice | Improved behavioral change, and reduced dopamine depletion and neuronal loss in MitoPark transgenic mice | [192] | |
| Momordica charantia | C57BL/6J and 3 × Tg-AD mice | Prevent memory deficits; reduced neuronal loss, gliosis, Aβ level, and tau hyperphosporylation; and increased synaptic-related protein and pS9-GSK3β expression | [208] | |
| Chlorogenic acid | Swiss albino male mice | Chlorogenic acid significantly improve motor coordination and antioxidant status. Chlorogenic acid also reduce neuroinflammation and inhibit release of proinflammatory cytokines | [209] | |
| (−) Epigallocatechin-3-gallate | C57BL/6J mice | Improvement in movement behavior and protection of tyrosine hydroxylase (+) cells against MPTP toxicity, increased CD3+/CD4+ and CD3+/CD8+ T lymphocyte ratio, and reduced pro-inflammatory cytokine production | [210] | |
| Uncaria rhynchophylla | Isorhynchophylline (IRN) | Male Sprague-Dawley rats | IRN treatment alleviated cognitive decline due to Aβ25-35, reduced neuronal apoptosis, and suppressed tau hyperphosphorylation; additionally, IRN also inhibited GSK-3β activity and activated PI3K phosphorylation, which play a role in neuroprotection | [211] |
| Semen ziziphi spinosae | Jujuboside A | APP/PS1 transgenic mice | JuA significantly reduced cognitive deficiency in APP/PS1 transgenic mice, and significantly reduced soluble Aβ42 levels and plaque numbers in the brain | [193] |
| Schisandra chinensis | Essential oil | Male KM mice | Schisandra chinensis essential oil can improve cognitive decline in mice, suppressed pro-inflammatory cytokines, and inhibited p38 activation in the mice model | [194] |
| Astragalus radix | Cycloastragenol | C57BL/6N mice | Cycloastragenol upregulated the expression of Nrf2, HO-1, p-TrKB, BDNF, and NeuN and downregulated the expression of p-JNK, p-P-38, and p-Erk; cycloastragenol reduced the activated microglia, inflammatory cytokines, apoptosis, and memory dysfunction | [212] |
| Dioscoreae nipponicae | Dioscin | C57BL/6 mice | Result from in vivo study showed dioscin improved spatial learning and memory; restored MDA, Aβ42, AChE, ACh, and SOD levels; and restored brain histopathological change; dioscin downregulated the expression of RAGE and NOX4 and upregulated Nrf2 and HO-1; dioscin also downregulated the levels of p-NF-κB(p-p65)/NF-κB(p65), AP-1, and inflammatory factors | [195] |
| Citrus | Men and women aged ≥65, living in Ohsaki City, Japan | Frequent consumption of citrus associated with lower risk of getting dementia | [213] | |
| Korean Red Ginseng (KRG) | High KRG dose (9 g/day), low KRG dose (4.5 g/day), control for 12 weeks intervention | 61 patients with AD | High dose KRG significantly improved Alzheimer’s Diseases Assessment Scale (ADAS) and Clinical Dementia Rating (CDR) compared to control; KRG group showed improvement on Mini Mental Status Examination (MMSE) but no significant difference with the control group | [214] |
| Panax Ginseng | Panax Ginseng powder (4.5 g/day) and control for 12 weeks | 97 patients with probable AD by NINDS-ADRDA criteria | Baseline MMSE and ADAS showed no difference between 2 groups; after intervention for 12 weeks, the group treated with panax ginseng showed MMSE and ADAS score improvement and after discontinuation of panax ginseng, MMSE and ADAS score declined to the level of the control group | [215] |
| Cherry juice | Anthocyanin (200 mL of cherry juice/day for 12 weeks) | Elder adult (age 70+) with mild to moderate dementia | Significantly improved verbal fluency, short-term and long-term memory, and reduction of systolic and diastolic blood pressure, but no alteration of inflammation markers | [22] |
| Curcumin | Healthy adults | Curcumin administration significantly improved sustained attention and working memory tasks compared to placebo; working memory and mood were significantly better after chronic treatment compared to placebo; curcumin treatment also significantly reduced total and LDL cholesterol | [216] | |
| Cocoa | Flavonol (1 dose daily for 8 weeks)
| Elder people with mild cognitive impairment | Time required to complete cognitive and verbal tests was significantly lower in the high and intermediate flavonol groups, compared to the low flavonol group | [21] |
| Orange Juice |
| Healthy older adults | High flavanone orange juice gives better improvement on global cognition score compared to the low flavanone group; no significant effect observed of flavanone consumption on mood changes | [217] |
| Resveratrol (500 mg/day of Resveratrol (with dose escalation by 500 mg increments every 13 weeks)) | People aged > 45 with:
| Resveratrol was safe and well tolerated and some alteration of AD biomarkers were observed but a further and bigger study is needed to find evidence | [218] | |
| Orange juice | Flavonoid-rich orange juice (272 mg/240 mL) or calorie-matched placebo | Males aged 30–65 years old | Flavonoid-rich orange juice improved cognitive function, psychomotor speed, and subjective alertness compared to placebo | [219] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tandoro, Y.; Chen, B.-K.; Ali, A.; Wang, C.-K. Review of Phytochemical Potency as a Natural Anti-Helicobacter pylori and Neuroprotective Agent. Molecules 2023, 28, 7150. https://doi.org/10.3390/molecules28207150
Tandoro Y, Chen B-K, Ali A, Wang C-K. Review of Phytochemical Potency as a Natural Anti-Helicobacter pylori and Neuroprotective Agent. Molecules. 2023; 28(20):7150. https://doi.org/10.3390/molecules28207150
Chicago/Turabian StyleTandoro, Yohanes, Bo-Kai Chen, Asif Ali, and Chin-Kun Wang. 2023. "Review of Phytochemical Potency as a Natural Anti-Helicobacter pylori and Neuroprotective Agent" Molecules 28, no. 20: 7150. https://doi.org/10.3390/molecules28207150
APA StyleTandoro, Y., Chen, B. -K., Ali, A., & Wang, C. -K. (2023). Review of Phytochemical Potency as a Natural Anti-Helicobacter pylori and Neuroprotective Agent. Molecules, 28(20), 7150. https://doi.org/10.3390/molecules28207150







