Two New Lactam Derivatives from Micromelum falcatum (Lour.) Tan. with Brine Shrimp Larvae Toxicity
Abstract
:1. Introduction
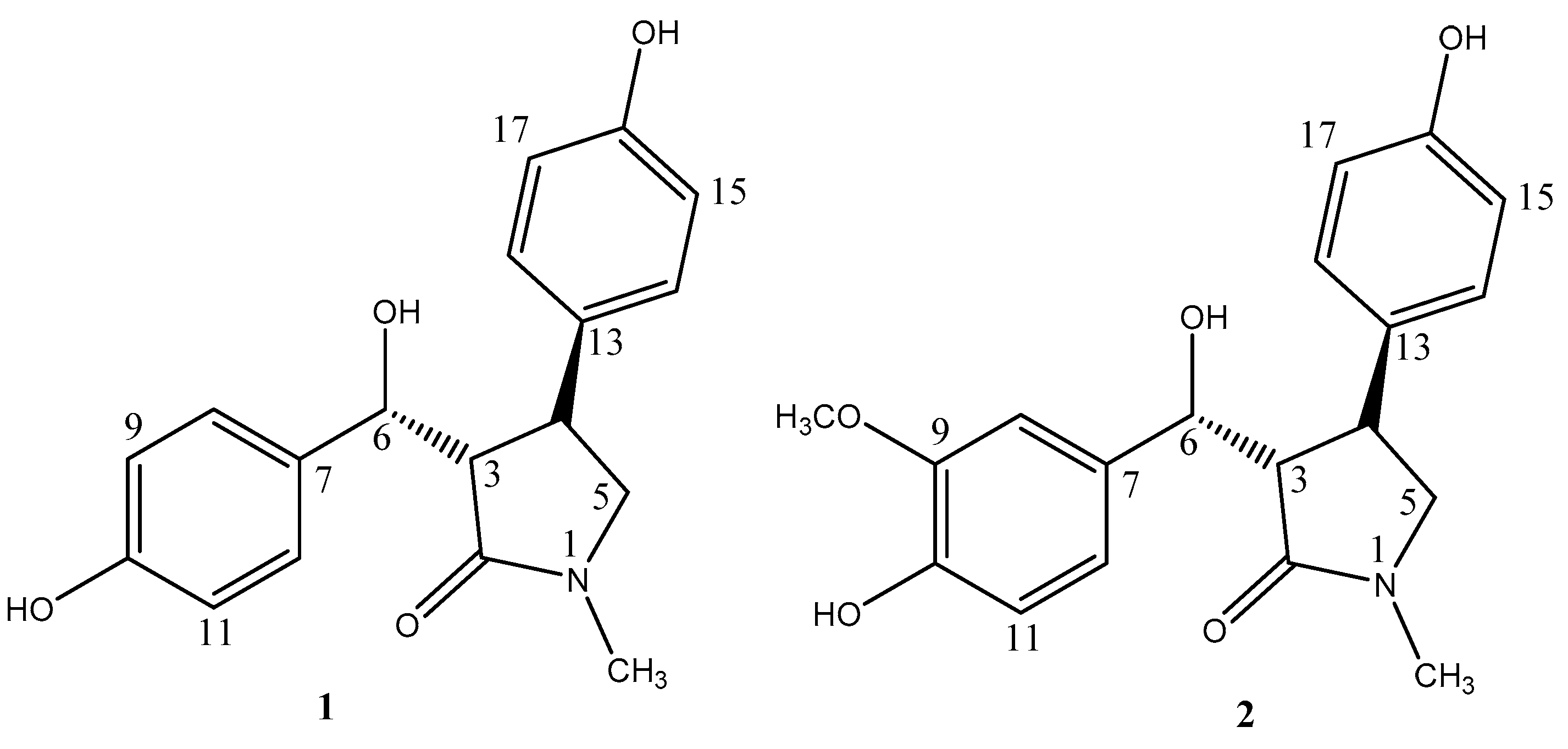
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Brine Shrimp Larvae Lethality Assays
4.5. Cytotoxicity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| EtOAc | Ethyl acetate |
| EtOH | Ethyl alcohol |
| AcOEt | Ethyl acetate |
| MeOH | Methanol |
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| HR-ESI-MS | High resolution electrospray ionization mass spectroscopy |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| HMBC | Heteronuclear multiple bond correlation |
| HSQC | Heteronuclear single quantum correlation |
| COSY | Homonuclear chemical shift Correlation Spectroscopy |
| NOESY | Nuclear Overhauser effect spectroscopy |
| ODS | Octadecyl silane |
| HPLC | High performance liquid chromatography |
| DMSO | Dimethyl sulfoxide |
| CCK-8 | Cell Counting Kit-8 |
References
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Qi, S.H.; Yin, H.; Gao, C.H.; Zhang, S. Alkaloids from the Stem Bark of Micromelum falcatum. Chem. Pharm. Bull. 2009, 57, 600. [Google Scholar] [CrossRef] [PubMed]
- Kamperdick, C.; Phuong, N.M.; Sung, T.V.; Schmidt, J.; Adam, G. Coumarins and dihydrocinnamic acid derivatives from Micromelum falcatum. Phytochemistry 1999, 52, 1671. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Ombito, J.O.; Chi, G.F.; Wansi, J.D. Ethnomedicinal uses, phytochemistry, and pharmacology of the genus Vepris (Rutaceae): A review. J. Ethnopharmacol. 2021, 267, 113622. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, Y.; He, W.J.; Zhao, S.X.; Yin, Z.Q.; Tan, N.H. Studies on Cyclic Peptides and Other Chemical Constituents in the Stem and Leaves of Xiaoyunmu. In Proceedings of the 10th National Natural Organic Chemistry Academic Conference of the Chinese Chemical Society—Part 1: Natural Product Isolation and Structural Identification, Guangzhou, China, 21–23 November 2014; p. 193. [Google Scholar]
- Suthiwong, J.; Sriphana, U.; Thongsri, Y.; Promsuwan, P.; Prariyachatigul, C.; Yenjai, C. Coumarinoids from the fruits of Micromelum falcatum. Fitoterapia 2014, 4, 134–141. [Google Scholar] [CrossRef]
- Luo, X.M.; Huang, Y.; Zhang, S.; Yin, H.; Li, C.R.; Li, Q.X. Five new phenethyl cinnamides from the mangrove associates Micro-melum falcatum. Biochem. Syst. Ecol. 2014, 56, 191–195. [Google Scholar] [CrossRef]
- Cao, N.K.; Chen, Y.M.; Zhu, S.S.; Zeng, K.W.; Zhao, M.B.; Li, J.; Tu, P.F.; Jiang, Y. Isolation and structure characterization of cytotoxic alkaloids from Micromelum integerrimum. Phytochemistry 2020, 178, 112463. [Google Scholar] [CrossRef]
- Kong, Y.C.; But, P.P.H.; Ng, K.H.; Li, Q.; Cheng, K.F.; Waterman, P.G. Micromelum: A key genus in the chemosystematics of the Clauseneae. Biochem. Syst. Ecol. 1988, 16, 485. [Google Scholar] [CrossRef]
- Ng, P.C.; Ho, D.D.; Ng, K.H.; Kong, Y.C.; Cheng, K.F.; Stone, G. Mixed estrogenic and anti-estrogenic activities of yuehchukene—A bis-indole alkaloid. Eur. J. Pharmacol. 1994, 264, 1–12. [Google Scholar]
- Oliveira, D.R.; Nepomuceno, D.D.; Castro, R.N.; Braz, R.F.; Carvalho, M.G. Special metabolites isolated from Urochloa humidicola (Poaceae). An. Acad. Bras. Cienc. 2017, 89, 789–797. [Google Scholar] [CrossRef]
- Orfali, R.; Perveen, S.; Khan, M.F.; Ahmed, A.F.; Wadaan, M.A.; Al-Taweel, A.M.; Alqahtani, A.S.; Nasr, F.A.; Tabassum, S.; Luciano, P.; et al. Antiproliferative Illudalane Sesquiterpenes from the Marine Sediment Ascomycete Aspergillus oryzae. Mar. Drugs 2021, 19, 333. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.X.; He, X.F.; Jiang, C.X.; Zhang, W.; Shi, Z.N.; Li, H.F.; Zhu, Y. Two novel bioactive sulfated guaiane sesquiterpenoid salt alkaloids from the aerial parts of Scorzonera divaricata. Fitoterapia 2018, 124, 113–119. [Google Scholar] [CrossRef]
- Elhady, S.S.; Abdelhameed, R.F.A.; El-Ayouty, M.M.; Ibrahim, A.K.; Habib, E.S.; Elgawish, M.S.; Hassanean, H.A.; Safo, M.K.; Nafie, M.S.; Ahmed, S.A. New Antiproliferative Triflavanone from Thymelaea hirsuta-Isolation, Structure Elucidation and Molecular Docking Studies. Molecules 2021, 26, 739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Woo, S.; Sung, S.H.; Yang, H. A new phenolic compound from Phedimus middendorffianus with antiproliferative activity. Nat. Prod. Res. 2020, 34, 1663–1668. [Google Scholar] [CrossRef]
- Ohta, T.; Inoue, H.; Kusano, G.; Oshima, Y. Lepiotins A and B, New Alkaloids from the Mushrooms, Macrolepiota neomastoidea and Chlorophyllum molybdites. Heterocycles 1998, 47, 883. [Google Scholar] [CrossRef] [PubMed]
- Caruano, J.; Muccioli, G.G.; Robiette, R. Biologically active γ-lactams: Synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. [Google Scholar] [CrossRef] [PubMed]
- Manam, R.R.; Teisan, S.; White, D.J.; Nicholson, B.; Grodberg, J.; Neuteboom, S.T.; Lam, K.S.; Mosca, D.A.; Lloyd, G.K.; Potts, B.C. Lajollamycin, a nitro-tetraene spiro-beta-lactone-gamma-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J. Nat. Prod. 2005, 68, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Lin, S.; Chen, M.; Liu, T.; Wang, A.; Li, J.; Guo, Q.; Shang, X. Monascustin, an Unusual γ-Lactam from Red Yeast Rice. J. Nat. Prod. 2017, 80, 201–204. [Google Scholar] [CrossRef]
- Fong, A.; Ross, M.; Boudreau, J.; Nokhbeh, R.; Tilbe, K.; Lee, H. Raja 42, a novel gamma lactam compound, is effective against Clostridioides difficile. PLoS ONE 2021, 9, e0257143. [Google Scholar] [CrossRef]
- Cheol, H.Y.; Advait, N.; Chiliu, C.; Drashti, G.; Kyung, W.J. γ-Lactam Synthesis via C−H Insertion: Elaboration of N-Benzyl Protecting Groups for High Regioselectivity toward the Total Synthesis of Rolipram. Org. Lett. 2003, 5, 2259–2262. [Google Scholar]
- Sflakidou, E.; Dalezis, P.; Trafalis Dimitrios, T.; Sarli, V. Synthesis and antiproliferative activities of steroidal lactam conjugates bearing a new nitrogen mustard. Eur. J. Med. Chem. 2023, 5, 249. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, S.; Huang, R.; Lu, Y.; Zheng, G. Efficient synthesis of the intermediate of abacavir and carbovir using a novel (+)-γ-lactamase as a catalyst. Bioorg. Med. Chem. Lett. 2015, 25, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ge, H.; Chen, Z.B.; Luo, X.M.; Su, F.J.; Liang, Y.B.; Li, Z.Y.; Wu, J.G.; Yang, Q.; Zeng, L.J.; et al. Micrometam C Protects against Oxidative Stress in Inflammation Models in Zebrafish and RAW264.7 Macrophages. Mar. Drugs 2015, 13, 5593–5605. [Google Scholar] [CrossRef] [PubMed]
- McChesney, J.D.; Rodenburg, D.L. Preparative chromatography and natural products discovery. Curr. Opin. Biotechnol. 2014, 25, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Periat, A.; Guillarme, D.; Veuthey, J.L.; Boccard, J.; Moco, S.; Barron, D.; Grand-Guillaume Perrenoud, A. Optimized selection of liquid chromatography conditions for wide range analysis of natural compounds. J. Chromatogr. A 2017, 1504, 91–104. [Google Scholar] [CrossRef]
- Namera, A.; Ota, S.; Tomioka, Y.; Saito, T.; Nagao, M. Facile determination of natural cannabinoids in cannabis products using a conventional fully porous particle column and isocratic high-performance liquid chromatography with diode-array detector. Forensic Toxicol. 2022, 40, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Wanyoike, G.N.; Chhabra, S.C.; Lang’at-Thoruwa, C.C.; Omar, S.A. Brine shrimp toxicity and antiplasmodial activity of five Kenyan medicinal plants. J. Ethnopharmacol. 2004, 90, 129–133. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Zhang, K.; Li, X.; Liu, J.; Zhang, W.J.; Yu, X.X. Cytotoxic Flavonoids from the Leaves and Twigs of Murraya tetramera. Molecules 2021, 26, 284. [Google Scholar] [CrossRef] [PubMed]


| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC (ppm) | δH (ppm) | δC (ppm) | δH (ppm) | |
| 2 | 176.9 | - | 177.0 | - |
| 3 | 59.0 | 2.93 (1H, dd, 6.0, 5.5 Hz) | 58.6 | 2.97 (1H, dd, 7.0, 5.5 Hz) |
| 4 | 39.7 | 3.25 (1H, m) | 40.2 | 3.21 (1H, m) |
| 5 | 57.4 | 3.15 (2H, d, 2.5 Hz) | 57.4 | 3.22 (2H, d, 2.0 Hz) |
| 6 | 75.3 | 4.95 (1H, d, 6.0 Hz) | 75.8 | 4.91 (1H, d, 7.0 Hz) |
| 7 | 132.6 | - | 133.2 | - |
| 8 | 129.1 | 7.13 (1H, d, 8.5 Hz) | 111.6 | 6.80 (1H, s) |
| 9 | 115.8 | 6.68 (1H, d, 8.5 Hz) | 148.8 | - |
| 10 | 158.3 | - | 147.5 | - |
| 11 | 115.8 | 6.68 (1H, d, 8.5 Hz) | 115.6 | 6.70 (1H, d, 8.0 Hz) |
| 12 | 129.1 | 7.13 (1H, d, 8.5 Hz) | 121.0 | 6.77 (1H, d, 8.0 Hz) |
| 13 | 135.7 | - | 135.3 | - |
| 14 | 129.1 | 6.88 (1H, d, 8.5 Hz) | 129.2 | 6.86 (1H, d, 8.5 Hz) |
| 15 | 116.4 | 6.64 (1H, d, 8.5 Hz) | 116.3 | 6.63 (1H, d, 8.5 Hz) |
| 16 | 157.1 | - | 157.1 | - |
| 17 | 116.4 | 6.64 (1H, d, 8.5 Hz) | 116.3 | 6.63 (1H, d, 8.5 Hz) |
| 18 | 129.1 | 6.88 (1H, d, 8.5 Hz) | 129.2 | 6.86 (1H, d, 8.5 Hz) |
| 1-Me | 29.7 | 2.80 (3H, s) | 29.8 | 2.84 (3H, s) |
| 9-OMe | - | 56.4 | 3.75 (3H, s) | |
| Compounds | LC50 (Brine Shrimp) μg mL−1 |
|---|---|
| 1 | 50.6 |
| 2 | 121.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Jin, X.; Chen, X.; Wang, X.; Zhang, W.; Luo, X. Two New Lactam Derivatives from Micromelum falcatum (Lour.) Tan. with Brine Shrimp Larvae Toxicity. Molecules 2023, 28, 7157. https://doi.org/10.3390/molecules28207157
Liu B, Jin X, Chen X, Wang X, Zhang W, Luo X. Two New Lactam Derivatives from Micromelum falcatum (Lour.) Tan. with Brine Shrimp Larvae Toxicity. Molecules. 2023; 28(20):7157. https://doi.org/10.3390/molecules28207157
Chicago/Turabian StyleLiu, Bin, Xiaobao Jin, Xiaohong Chen, Xin Wang, Wenbo Zhang, and Xiongming Luo. 2023. "Two New Lactam Derivatives from Micromelum falcatum (Lour.) Tan. with Brine Shrimp Larvae Toxicity" Molecules 28, no. 20: 7157. https://doi.org/10.3390/molecules28207157







