Abstract
Selective dehydrogenative silylation is one of the most valuable tools for synthesizing organosilicon compounds. In this study, a regio- and stereoselective ruthenium-catalyzed dehydrogenative intermolecular silylation was firstly developed to access (E)-alkenyl silyl-ether derivatives and silyl-ether heterocycles with good functional group tolerance. Furthermore, two pathways for RuH2(CO)(PPh3)3/NBE-catalyzed dehydrogenative intermolecular silylation of alcohols and alkenes as well as intermolecular silylation of naphthol derivatives were investigated with H2SiEt2 as the hydrosilane reagent.
1. Introduction
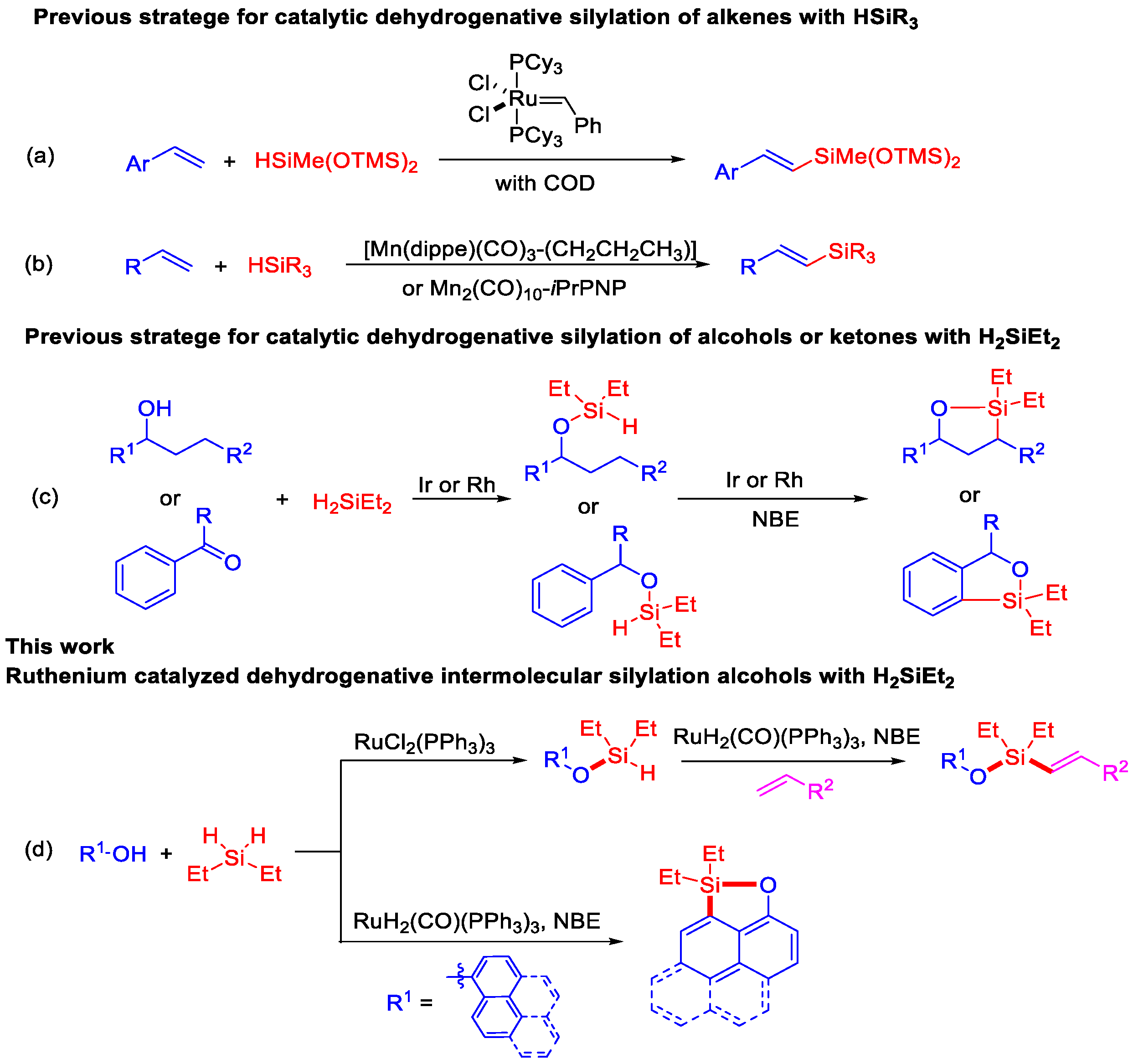
Alkenylsilanes are one of the most versatile building blocks in numerous polymers, semi-conductors, and medicinal compounds due to their low toxicity and favorable metabolic profiles [1,2]. They can also serve as synthetic intermediates for useful moieties, including allylic alcohols, allylic amines, and alkenyl halides via oxidation with H2O2, aminations, and halogenations [3,4,5,6,7]. Compared to the alkenylsilanes, synthetic methodologies with the silyl–Heck reaction [8,9,10], alkyne hydrosilylation [11,12], and dehydrogenative silylation of alkenes, direct dehydrogenative silylation of alkenes has attracted attention as a competitive alternative process because alkenes are less expensive and more readily available [13,14]. The competitive alkene hydrosilylation to generate alkylsilanes is still a challenging step during the dehydrogenative silylation process to produce alkenylsilanes [15,16,17]. Recently, several precious metals, such as rhodium [18,19,20], iridium [21], ruthenium [22], and earth-abundant metals, including iron [23], copper [24], and manganese [25,26], were developed for the selective dehydrogenative silylation of alkenes. For example, Zhang reported efficient [RhCl(COD)]2/PPh3-catalyzed dehydrogenative silylation derivatives with trisubstituted hydrosilanes as the hydrosilane reagents [18]. Jeon demonstrated regio- and stereoselective dehydrogenative silylation and hydrosilylation of vinylarenes with alkoxysilanes by using a ruthenium alkylidene catalyst (Scheme 1a) [22]. Very recently, [Mn(dippe)(CO)3-(CH2CH2CH3)] developed by Kirchner [25] and the Mn2(CO)10-iPrPNP catalytic system by Jin Xie [26] were successfully applied in the dehydrogenative silylation of alkenes, with trisubstituted hydrosilanes as the hydrosilane reagents (Scheme 1b).
Scheme 1.
Strategy for transition metal-catalyzed dehydrogenative silylation.
In spite of the significant progress in the direct dehydrogenative silylation of alkenes, HSiEt3 and HSiMe(OSiMe3)2 as the hydrosilane reagents are always used, whereas H2SiEt2 has still not been developed in dehydrogenative silylation of alkenes mainly because H2SiEt2 is more difficult to control and easily generates other silane-byproducts during the catalytic reaction. In addition, H2SiEt2 constitutes two Si–H bonds, which could provide more possibilities and molecule diversity to produce organosilicon compounds. Recently, Hartwig demonstrated efficient synthetic methods to afford five-membered silyl ether heterocycles via Rh or Ir-catalyzed dehydrogenative silylation of alcohols or ketones with H2SiEt2 (Scheme 1c) [27,28].
In our previous study, we demonstrated that the selective C–H silylation of N-heterocycles, amides, and anilides utilizing the RuHCl(CO)(PPh3)3/KOAc catalytic system was feasible to achieve silylated compounds with HSiEt3 [29,30,31]. Herein, we report Ru-catalyzed regio- and stereoselective dehydrogenative intermolecular silylations to synthesize (E)-alkenyl silyl-ether derivatives and silyl-ether heterocycles with H2SiEt2 as the hydrosilane reagent (Scheme 1d).
2. Results
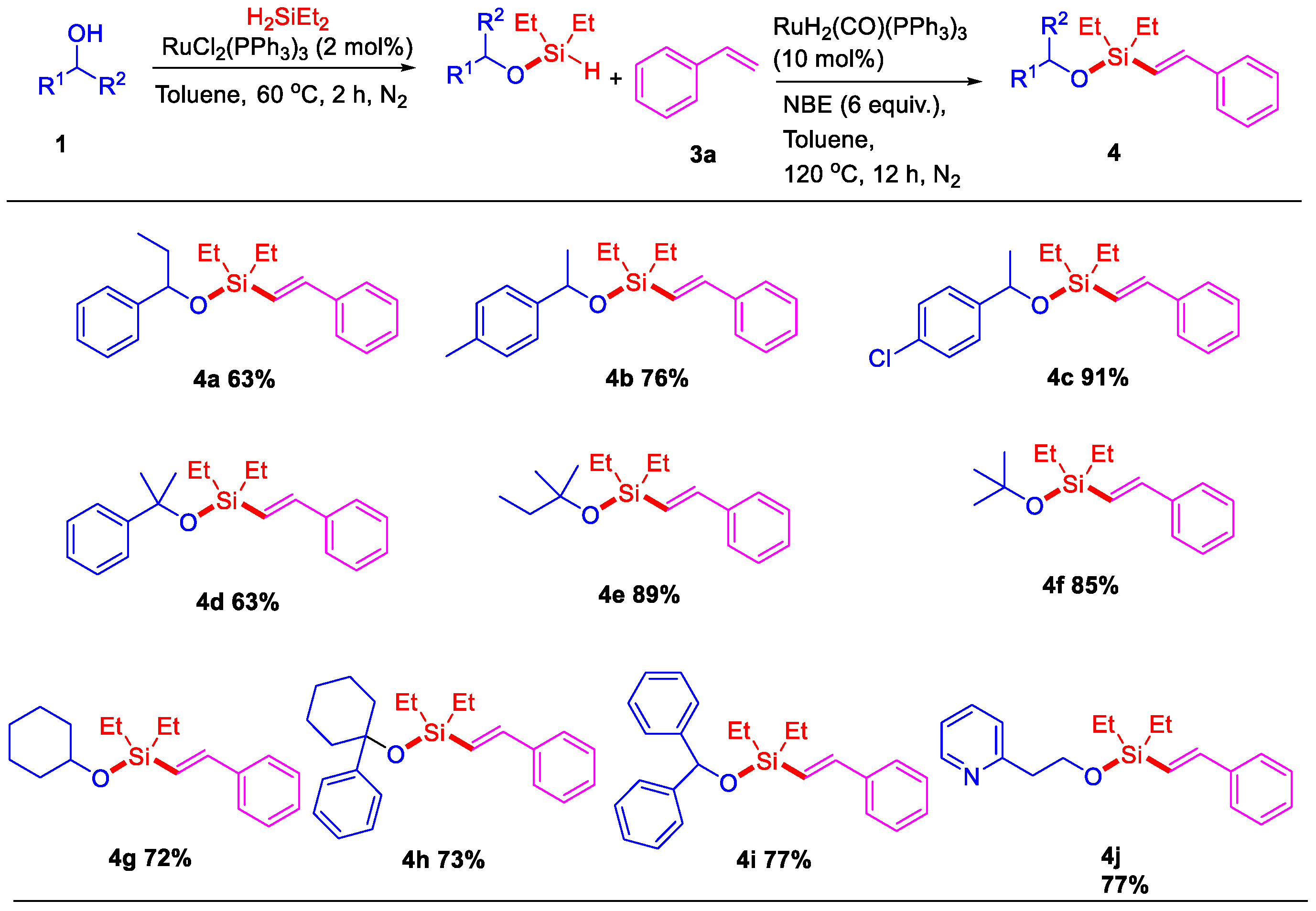
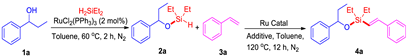
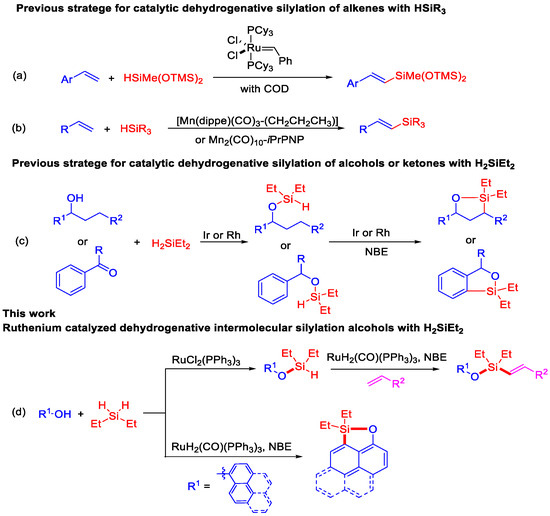
To attempt the alkenyl silyl-ether, a two-step sequential ruthenium-catalyzed dehydrogenative intermolecular silylation of 1-phenylpropan-1-ol (1a) with styrene (3a) has been evaluated. Based on our previous experience and literature on dehydrogenative silylation of O–H/Si–H bonds [32], full conversion of intermediate silyl-ether (2a) was obtained from the reaction of 1-phenylpropan-1-ol (1a) with H2SiEt2 in the presence of 2 mol% of RuCl2(PPh3)3 in toluene at 60 °C under N2 (more details see Table S1). After 2 h, the reaction conditions of the in situ-generated silyl-ether (2a) with styrene (3a) were optimized and shown in Table 1. Firstly, 88% yield of (E)-alkenyl silyl-ether 4a was observed by using 10 mol% of RuHCl(CO)(PPh3)3 as catalyst, 6 equiv. of norbornene (NBE) as hydrogen acceptor at 120 °C for 12 h (Table 1, entry 1). Then, other ruthenium complexes, such as [RuCl2(p-cymene)]2, [RuCl2(COD)]n, RuH2(CO)(PPh3)3, RuCl2(PPh3)3, and Ru3(CO)12 were tested for this dehydrogenative intermolecular Si–H/C–H silylation, and RuH2(CO)(PPh3)3 was found to the best catalyst and led to almost full conversion to (E)-alkenyl silyl-ether 4a (Table 1, entry 4). Other hydrogen trappers such as cyclohexene, t-butyl acrylate, and ɑ-methylstyrene did not give better results, and the yields of (E)-alkenyl silyl-ether 4a were below 44% (Table 1, entries 8–10), which is mainly because the NBE gave significant ring tension and high reactivity. Decreasing the NBE amount or the temperature did not improve the yield of (E)-alkenyl silyl-ether (Table 1, entries 7 and 11). Then, some other solvents, including MeOH, THF, CH3CN, and 1,4-dioxane, were also tested and did not show favorable effects for the reaction (Table 1, entries 12 to 15).

Table 1.
Optimization of reaction conditions for Ru-catalyzed dehydrogenative intermolecular silylation of 1-phenylpropan-1-ol (1a) with styrene (3a) [a].
We subsequently investigated the effect of various alcohols on the efficiency of this ruthenium-catalyzed dehydrogenative intermolecular silylation with the optimized conditions in entry 4 of Table 1 (Scheme 2). Firstly, secondary alcohols, such as 1-phenylethanol with para-Me (3b) and -Cl (3c) substituents, were applied to synthesize the corresponding (E)-alkenyl silyl-ethers 4b and 4c in 76% and 91% yields with the reaction of styrene. Tertiary alcohols with phenyl or alkyl group were able to give the desired (E)-alkenyl silyl-ethers 4d–4f in good yields. Additionally, other functional alcohols, including cyclohexanol, 1-phenylcyclohexanol, diphenylmethanol, and 2-(pyridin-2-yl)ethanol, showed good reactivities in the production of (E)-alkenyl silyl-ethers (3g–3j).
Scheme 2.
Sequential Ru-catalyzed dehydrogenative intermolecular silylation of alcohols with styrene a,b. a Reaction conditions: Step 1: 1-phenylpropan-1-ol 1 (0.5 mmol), RuCl2(PPh3)3 (2 mol%), H2SiEt2 (0.55 mmol), toluene (2 mL), 60 °C for 2 h, under N2. Step 2: styrene 3a (0.6 mmol), RuH2(CO)(PPh3)3 (10 mol%), NBE (6 equiv.), at 120 °C under N2, 12 h. b Isolated yield.
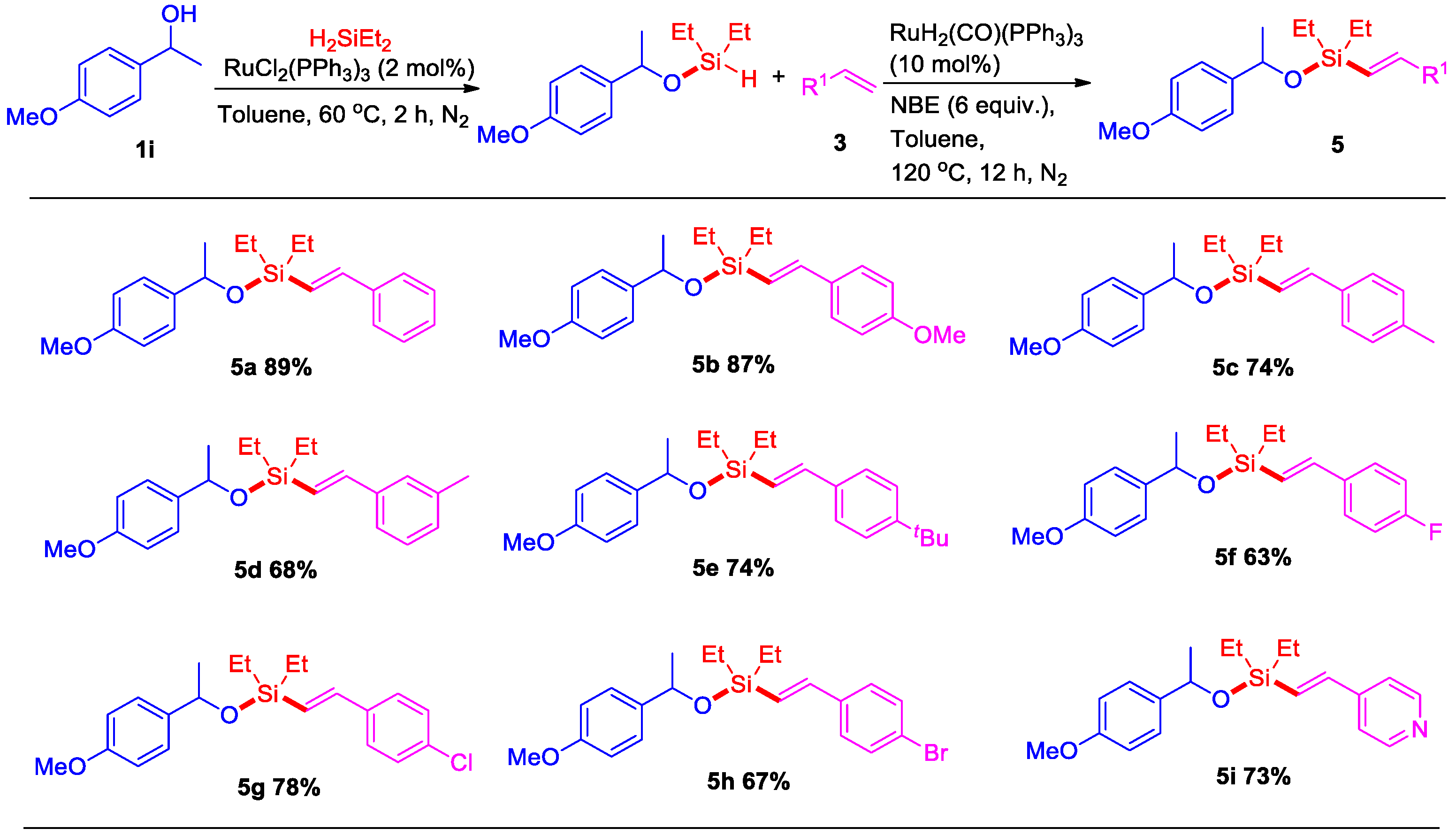
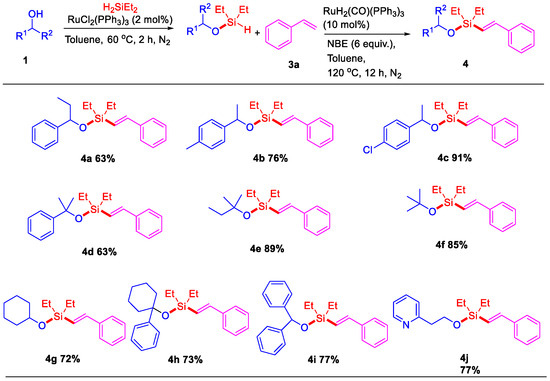
We next studied the tolerance and selectivity of the reaction of alkene derivatives (Scheme 3). (E)-Alkenyl silyl-ethers with a 4-methoxyphenyl group could be easily prepared through this sequential ruthenium-catalyzed dehydrogenative intermolecular silylation. The corresponding alkenyl silyl-ethers bearing substituents, including -OMe, -Me, -tBu, -F, -Cl, and -Br at the para or meta position on the styrene ring, could be produced in 63–87% yields under similar conditions (5b–5h). Analogously, the pyridine ring instead of the phenyl ring also proceeded well via this sequential ruthenium-catalyzed dehydrogenative intermolecular silylation.
Scheme 3.
Sequential Ru-catalyzed dehydrogenative intermolecular silylation of 1-(4-methoxyphenyl)ethanol with alkenes a,b. a Reaction conditions: Step 1: 1-(4-methoxyphenyl)ethanol 1i (0.5 mmol), RuCl2(PPh3)3 (2 mol%), H2SiEt2 (0.55 mmol), toluene (2 mL), 60 °C for 2 h, under N2. Step 2: alkene 3 (0.6 mmol), RuH2(CO)(PPh3)3 (10 mol%), NBE (6 equiv.), at 120 °C under N2, 12 h. b Isolated yield.
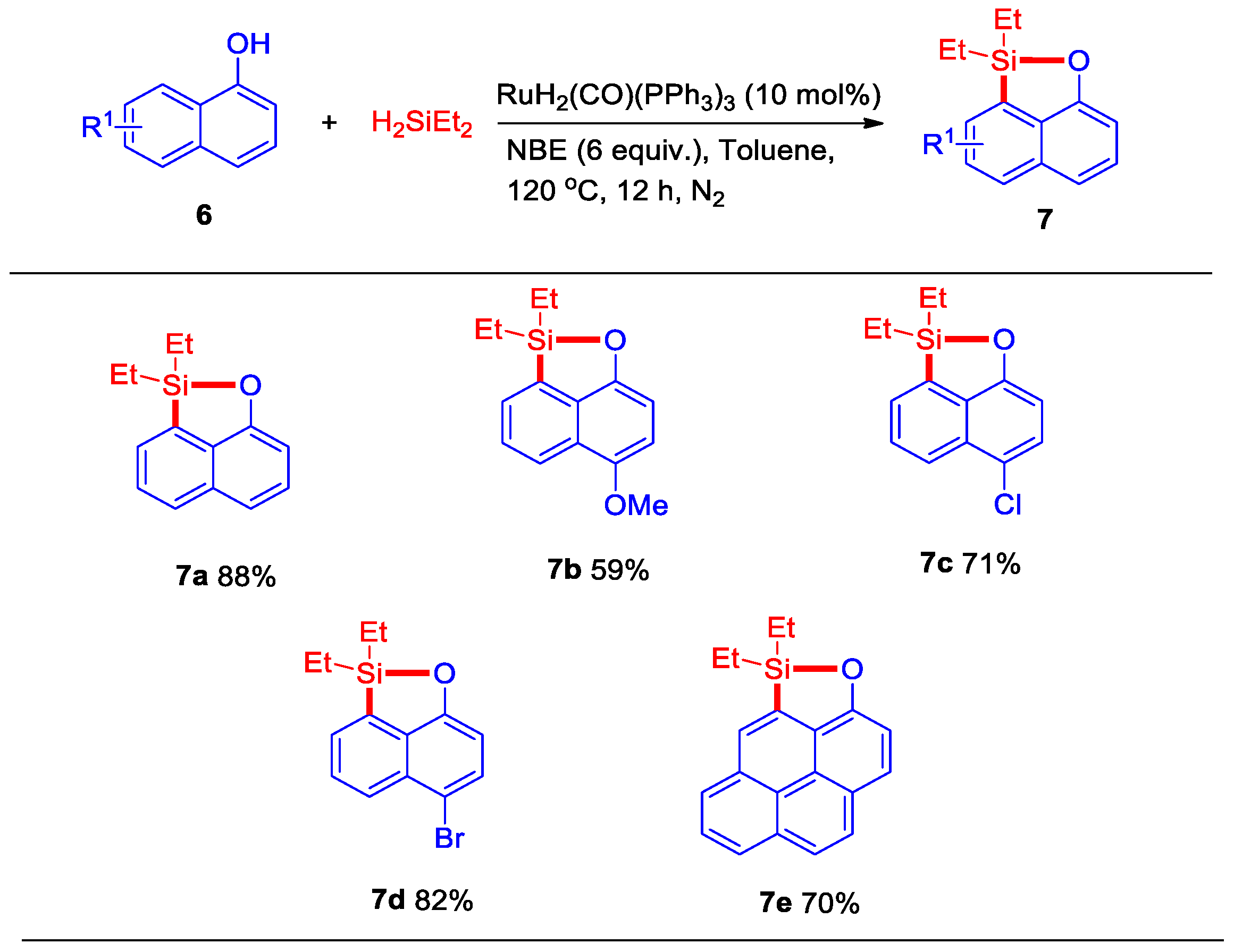
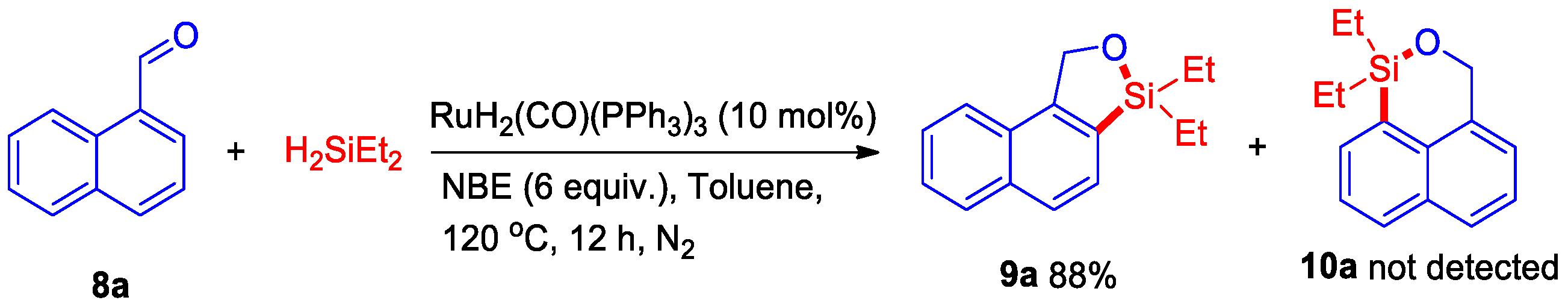
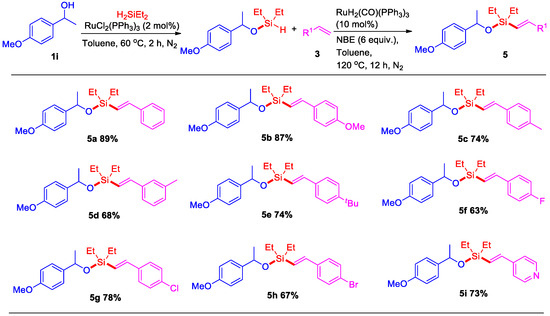
Then, the above-described catalytic system was evaluated for dehydrogenative C–H silylation of naphthol with styrene, however, an important silyl-ether heterocycle 7a product was observed, which indicated that styrene did not play a role in this dehydrogenative intermolecular silylation. After our evaluation, an 88% yield of silyl-ether heterocycle 7a was isolated in the presence of 10 mol% of RuH2(CO)(PPh3)3 and 6 equiv. of NBE at 120 °C for 12 h under N2 (Scheme 4). Then, some naphthols bearing -OMe, -Cl, and -Br substituents were also successfully transferred to the corresponding silyl-ether heterocycles 7b–7d in 59–82% yields. Interestingly, this dehydrogenative intermolecular C–H silylation catalytic system could also be applied to synthesize pyren silyl-ether 7e at a good yield. Moreover, when this reaction reacted with 1-naphthaldehyde, the C–H bond at position eight of the naphthalene ring was not activated, whereas position two of the C–H bond was activated and generated silyl-ether heterocycle 9a at an 88% yield (Scheme 5). This result indicated that the five-membered silyl-ether heterocycle is easier to produce than the six-membered silyl-ether heterocycle.
Scheme 4.
Ru-catalyzed dehydrogenative intermolecular silylation of naphthalen-1-ol derivatives a,b. a Reaction conditions: naphthalen-1-ols 6 (0.5 mmol), RuH2(CO)(PPh3)3 (10 mol%), NBE (6 equiv.), H2SiEt2 (0.55 mmol), toluene (2 mL), at 120 °C under N2, 12 h. b Isolated yield.
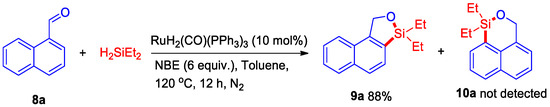
Scheme 5.
Ru-catalyzed dehydrogenative intermolecular silylation of 1-naphthaldehyde.
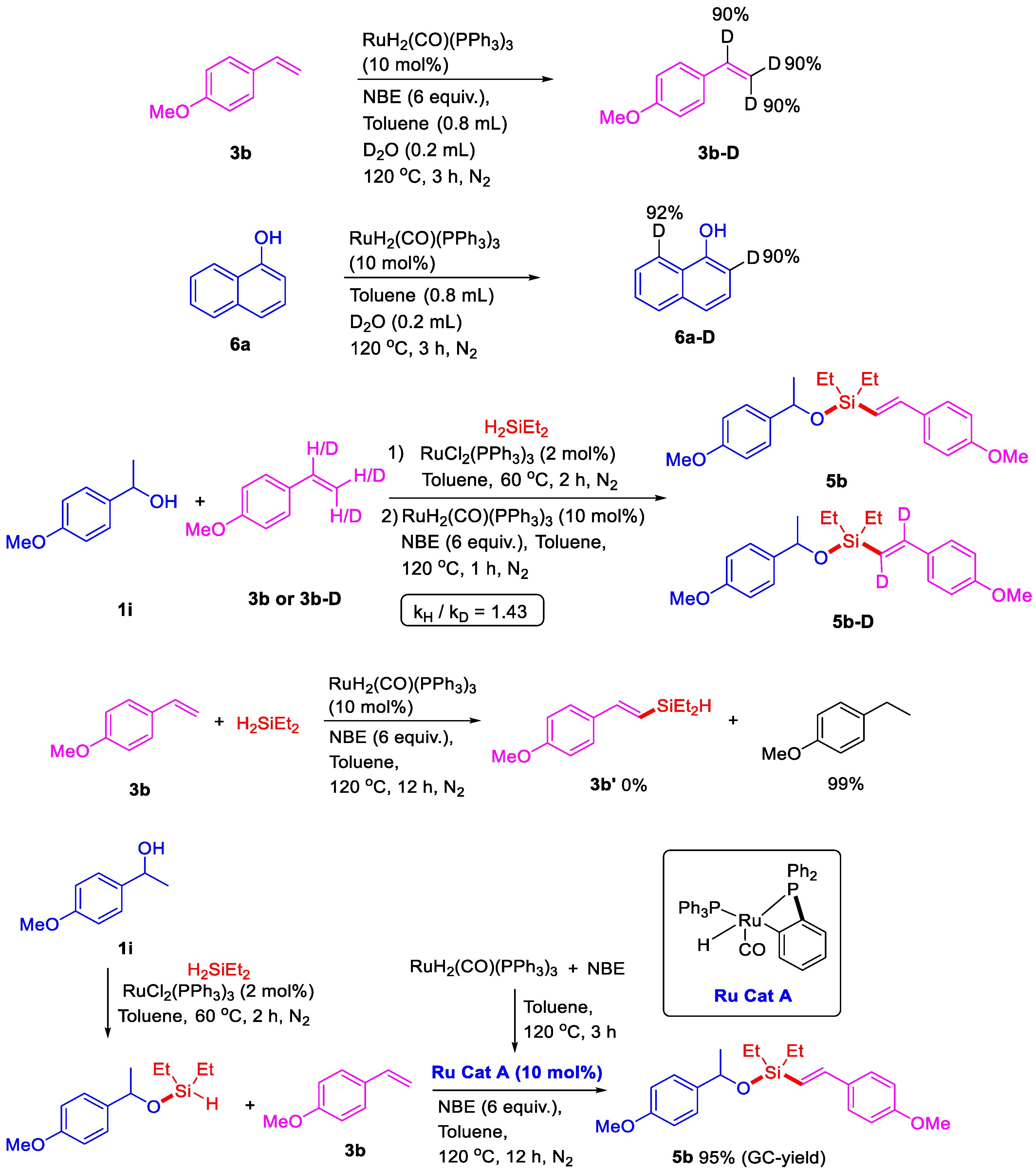
To gain further insight into the reaction mechanism, several controlled experiments were performed (Scheme 6). Two H/D exchange reactions were conducted in the presence of D2O (0.2 mL) under the above conditions for 3 h. A 90% H/D exchange took place at the C–H bonds of 4-methoxystyrene 3b. In the H/D exchange reaction of naphthol 6a, 90% of the H/D exchange took place at position two, and a 92% H/D exchange was observed at position eight. These results indicated that C–H bonds at both position two and position eight in naphthol could be reactive. However, the ruthenium-catalyzed intermolecular silylation was performed at position eight in naphthol, not at position two, mainly because the four-membered ruthenacycle activated at position two is more difficult to generate and unstable during the catalytic cycle. In addition, the KIE value of the C–H activation in this dehydrogenative silylation of 3b and 3b-D with 1i and Et2SiH2 was 1.43, revealing that the C–H cleavage is not the rate-determining step. No dehydrogenative silylated compound 3b was detected and full conversion of reductive product 4-ethylanisole was obtained from the reaction of 4-methoxystyrene 3b with H2SiEt2 in the presence of RuH2(CO)(PPh3)3 and NBE, which indicated that the silyl ether would reduce the reactivity of the Si–H bond and play an important role for dehydrogenative silylation. Furthermore, Murai described a complex Ru(H)(o-C6H4PPh2)(PPh3)2(CO), which could be easily synthesized from the reaction with RuH2(CO)(PPh3)3 with trimethylsilane [33]. With this important result, the reaction of RuH2(CO)(PPh3)3 with NBE was performed at 120 °C for 3 h, and a mixture with the major Ru complex A Ru(H)(o-C6H4PPh2)(PPh3)(CO) was obtained and detected by HR-MS (See in Supplementary Materials). Then, Ru complex A could be successfully applied to dehydrogenative intermolecular silylation and give 95% GC-yield of alkenyl silyl-ether 5b. These results indicated that the Ru(H)(o-C6H4PPh2)(PPh3)(CO) may be the active ruthenium catalyst for the dehydrogenative intermolecular silylation.
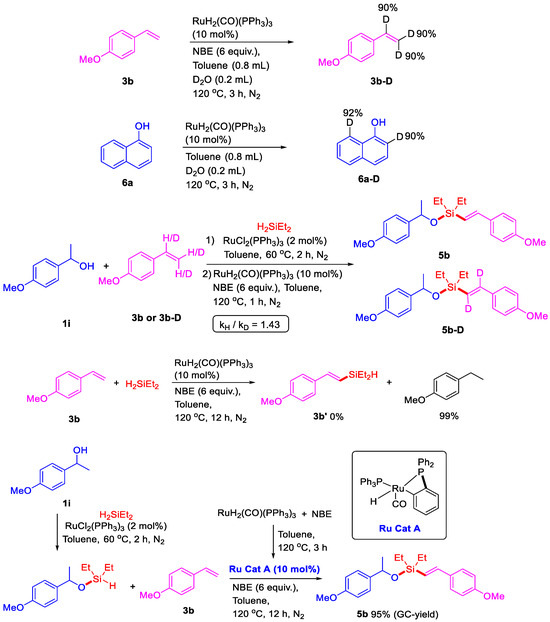
Scheme 6.
Controlled experiments.
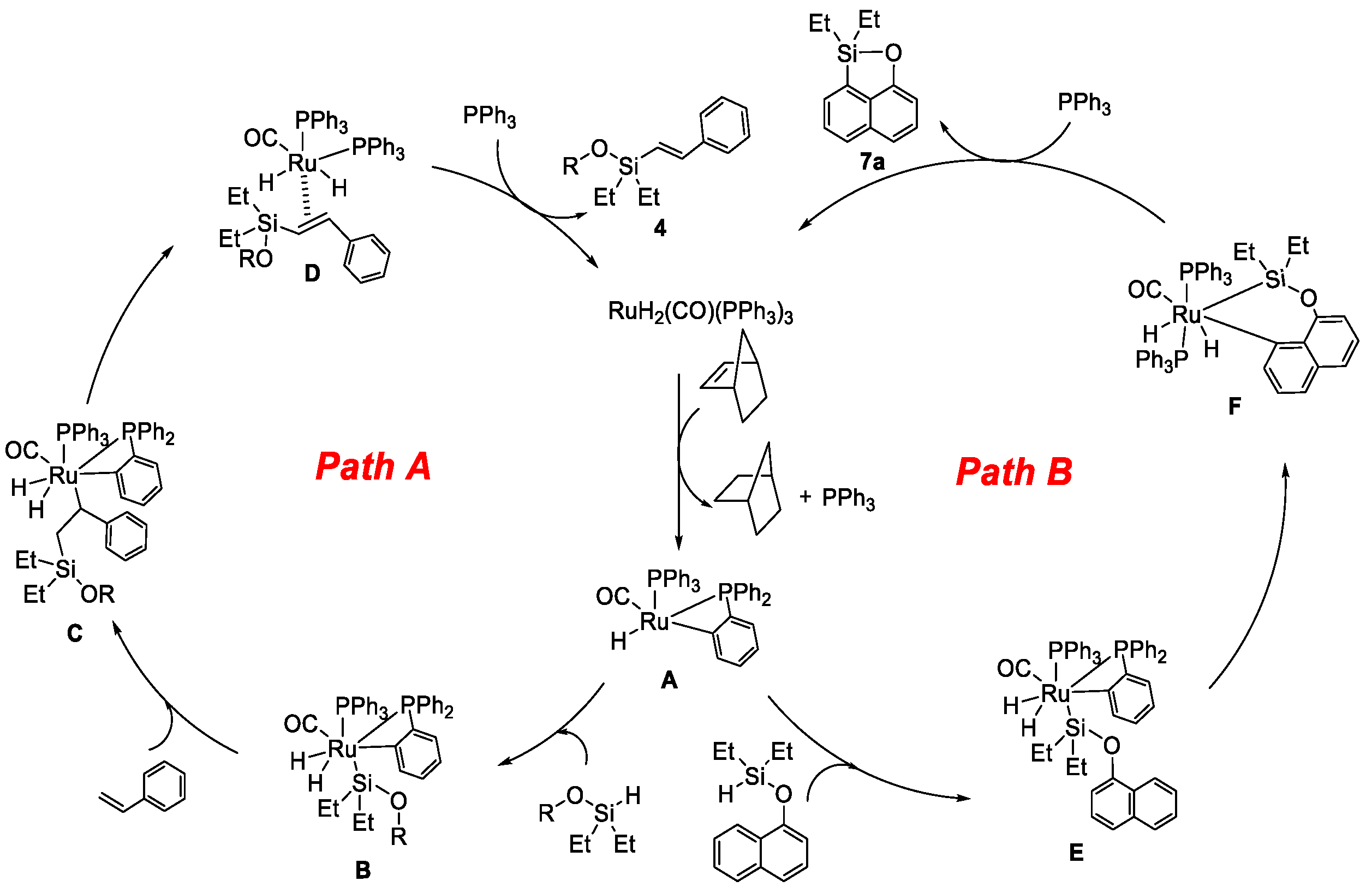
On the basis of the above results and previous studies [29,33,34,35] in the literature, we proposed mechanisms for the RuH2(CO)(PPh3)3/NBE-catalyzed silylation of alkene with alcohol and the silylation of naphthol through the intermolecular pathway, as illustrated in Scheme 7. In the Ru-catalyzed intermolecular silylation cycle of alkene with alcohol, the active Ru(H)(o-C6H4PPh2)(PPh3)(CO) species A was generated in situ after the release of one PPh3 and cyclometallation from the reaction of RuH2(CO)(PPh3)3 with NBE [29,33,34]. Then, the oxidative addition of the above-generated ROSi(Et2)H compound led to “Ru–Si” intermediate B [36]. Next, following the insertion of the Ru–Si bond to styrene [18] and β-elimination of Ru, intermediate C led to Ru intermediate D. Finally, (E)-alkenyl silyl-ether product 4 was produced via the decoordination of Ru intermediate D, and RuH2(CO)(PPh3)3 was regenerated with the previous release of PPh3 for the next catalytic cycle. On the other hand, in the Ru-catalyzed dehydrogenative intermolecular silylation cycle of naphthol, the active Ru(H)(o-C6H4PPh2)(PPh3)(CO) species A reacted with the in situ generated “O–Si–H” compound to afford “Ru–Si” intermediate E. Then, a six-membered “Ru–Si–O” intermediate F was generated via C–H bond deprotonation and cycloruthenation. The silyl-ether heterocycle 7a was finally obtained by reductive elimination and released RuH2(CO)(PPh3)3 for the next catalytic cycle.
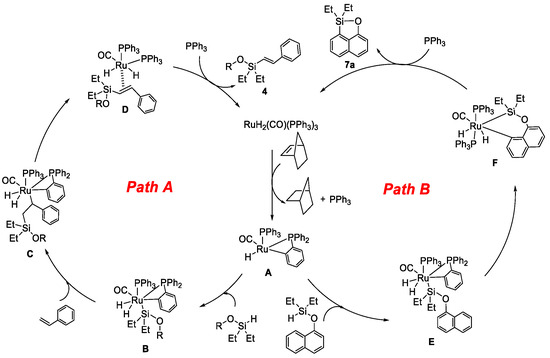
Scheme 7.
Proposed mechanism.
3. Materials and Methods
3.1. General Information
All reagents were obtained from commercial sources and used as received. Ethanol (anhydrous) was used as received. Technical grade petroleum ether (40–60 °C bp.) and ethyl acetate were used for chromatography column.
1H NMR spectra were recorded in CDCl3 at ambient temperature on Bruker AVANCE I 400 or 500 spectrometers at 400.1 or 500.1 MHz, using the solvent as internal standard (7.26 ppm). 13C NMR spectra were obtained at 100 MHz or 125 MHz and referenced to the internal solvent signals (central peak is 77.2 ppm). Chemical shift (δ) and coupling constants (J) are given in ppm and in Hz, respectively. The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; and br. for broad.
GC analyses were performed with GC-7890A (Agilent, Santa Clara, CA, USA) equipped with a 30 m capillary column (HP-5ms, fused silica capillary column, 30 M × 0.25 mm × 0.25 mm film thickness) with N2/air as vector gas. GCMS was measured by GCMS-7890A-5975C (Agilent) with GC-7890A equipped with a 30 m capillary column (HP-5ms, fused silica capillary column, 30 M × 0.25 mm × 0.25 mm film thickness) with helium as vector gas. HRMS were measured by MAT 95XP (Termol, Vinkovci, Croatia) (LCMS-IT-TOF).
The following GC conditions were used: Method A: initial temperature 100 °C for 1.7 min, then rate 10 °C/min until 250 °C and 250 °C for 13 min. Method B: initial temperature 120 °C for 2 min, then rate 10 °C/min until 280 °C and 280 °C for 15 min.
3.2. General Procedure for Ruthenium-Catalyzed Dehydrogenative Intermolecular O–H/Si–H/C–H Silylation of Alcohols with Alkenes
Alcohol (0.5 mmol), RuCl2(PPh3)3 (2 mol%), H2SiEt2 (0.55 mmol), and toluene (2 mL) were introduced in a tube under N2, equipped with magnetic stirring bar and stirred at 60 °C. After 2 h, alkene (0.6 mmol), RuH2(CO)(PPh3)3 (10 mol%), and norbornene (3 mmol) were introduced in a tube under N2 and stirred at 120 °C for 12 h; then the conversion of the reaction was analyzed by gas chromatography. The solvent was then evaporated under vacuum, and the desired product was purified by using a silica gel chromatography column and a mixture of petrol ether/ethyl acetate as eluent.
3.3. General Procedure for Ruthenium-Catalyzed Dehydrogenative Intermolecular O–H/Si–H/C–H silylation of naphthalen-1-ol Derivatives
Naphthalen-1-ol derivative (0.5 mmol), RuH2(CO)(PPh3)3 (10 mol%), norbornene (3 mmol), H2SiEt2 (0.55 mmol), and toluene (2 mL) were introduced in a tube under N2, equipped with magnetic stirring bar and stirred at 120 °C for 12 h; then the conversion of the reaction was analyzed by gas chromatography. The solvent was then evaporated under vacuum, and the desired product was purified by using a silica gel chromatography column and a mixture of petrol ether/ethyl acetate as eluent.
3.4. Characterization Data of Substrates
(E)-diethyl(1-phenylpropoxy)(styryl)silane (4a) Light yellow oil, yield = 63%, 102 mg, 1H NMR (500 MHz, CDCl3): δ = 7.46 − 7.32 (m, 10H), 7.01–6.96 (m, 1H), 6.40–6.35 (m, 1H), 4.72–4.69 (m, 1H), 1.89–1.77 (m, 2H), 1.08–1.04 (m, 3H), 1.00–0.95 (m, 6H), 0.83–0.70 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 146.1, 145.5, 138.3, 128.6, 128.4, 128.2, 127.1, 126.7, 126.3, 124.7, 76.7, 33.6, 10.3, 6.9, 6.8, 5.7, 5.6. GC: tR = 7.96 min (Method A). HRMS (EI): m/z calcd for C21H28NaOSi [M+Na]+ 347.1802, found 347.1801.
(E)-diethyl(styryl)(1-(p-tolyl)ethoxy)silane (4b) Light yellow oil, yield = 76%, 123 mg, 1H NMR (500 MHz, CDCl3): δ = 7.53 − 7.25 (m, 9H), 7.08 (d, 1H, J = 19.5 Hz), 6.46 (d, 1H, J = 19.5 Hz), 5.06–5.01 (m, 1H), 2.46 (s, 3H), 1.59 (d, 3H, J = 6.5 Hz), 1.16–1.07 (m, 6H), 0.90–0.79 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 146.1, 143.8, 138.2, 136.5, 129.0, 128.6, 128.4, 126.7, 125.5, 124.6, 71.0, 27.2, 21.3, 7.0, 6.9, 5.7, 5.5. GC: tR = 8.07 min (Method A). HRMS (EI): m/z calcd for C21H28NaOSi [M + Na]+ 347.1802, found 347.1799.
(E)-(1-(4-chlorophenyl)ethoxy)diethyl(styryl)silane (4c) Light yellow oil, yield = 91%, 157 mg, 1H NMR (500 MHz, CDCl3): δ = 7.457.29 (m, 9H), 7.00 (d, 1H, J = 19.0 Hz), 6.36 (d, 1H, J = 19.5 Hz), 4.97–4.91 (m, 1H), 1.48 (d, 3H, J = 6.5 Hz), 1.07–0.98 (m, 6H), 0.82–0.70 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 146.4, 145.3, 138.1, 132.6, 128.7, 128.5, 128.4, 127.0, 126.7, 124.2, 70.5, 27.2, 6.9, 6.8, 5.6, 5.5. GC: tR = 8.05 min (Method A). HRMS (EI): m/z calcd for C20H25ClNaOSi [M + Na]+ 367.1255, found 367.1260.
(E)-diethyl((2-phenylpropan-2-yl)oxy)(styryl)silane (4d) Light yellow oil, yield = 63%, 103 mg, 1H NMR (500 MHz, CDCl3): δ = 7.55–7.53 (m, 2H), 7.44–7.25 (m, 8H), 6.97 (d, 1H, J = 19.5 Hz), 6.38 (d, 1H, J = 19.5 Hz), 1.66 (s, 3H), 1.06 (t, 6H, J = 7.5 Hz), 0.88–0.77 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 150.2, 145.1, 138.4, 128.7, 128.3, 128.1, 126.8, 126.7, 126.5, 124.9, 75.4, 32.7, 7.2, 7.1. GC: tR = 8.12 min (Method A). HRMS (EI): m/z calcd for C21H28NaOSi [M + Na]+ 347.1802, found 347.1798.
(E)-diethyl(tert-pentyloxy)(styryl)silane (4e) Light yellow oil, yield = 89%, 123 mg, 1H NMR (500 MHz, CDCl3): δ = 7.51 (d, 2H, J = 7.5 Hz), 7.41–7.30 (m, 3H), 7.02 (d, 1H, J = 19.0 Hz), 6.52 (d, 1H, J = 19.5 Hz), 1.57–1.53 (m, 2H), 1.28 (s, 6H), 1.06 (t, 6H, J = 7.5 Hz), 0.96 (t, 3H, J = 7.5 Hz), 0.80–0.76 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 144.8, 138.6, 128.7, 128.2, 127.4, 126.6, 74.4, 37.6, 29.6, 9.0, 7.3, 7.2. GC: tR = 4.23 min (Method B). HRMS (EI): m/z calcd for C17H28NaOSi [M + Na]+ 299.1802, found 299.1800.
(E)-tert-butoxydiethyl(styryl)silane (4f) Colorless oil, yield = 85%, 111 mg, 1H NMR (500 MHz, CDCl3): δ = 7.51 (d, 2H, J = 7.5 Hz), 7.39 (t, 2H, J = 7.0 Hz), 7.32–7.29 (m, 1H), 7.02 (d, 1H, J = 19.0 Hz), 6.52 (d, 1H, J = 19.5 Hz), 1.33 (s, 9H), 1.06 (t, 6H, J = 7.5 Hz), 0.80–0.76 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 144.9, 138.6, 128.7, 128.2, 127.4, 126.7, 72.3, 32.3, 7.2, 7.1. GC: tR = 4.11 min (Method B). HRMS (EI): m/z calcd for C19H26OSiK [M+K]+ 301.1384, found 301.1382.
(E)-(cyclohexyloxy)diethyl(styryl)silane (4g) Colorless oil, yield = 72%, 104 mg, 1H NMR (500 MHz, CDCl3): δ = 7.46 (d, 2H, J = 7.0 Hz), 7.34 (t, 2H, J = 7.5 Hz), 7.28–7.24 (m, 1H), 7.01 (d, 1H, J = 19.5 Hz), 6.42 (d, 1H, J = 19.5 Hz), 3.67–3.62 (m, 1H), 1.84–1.81 (m, 2H), 1.74–1.71 (m, 2H), 1.35–1.19 (m, 6H), 1.01 (t, 6H, J = 8.0 Hz), 0.77–0.72 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 145.9, 138.4, 128.7, 128.35, 126.7, 125.2, 71.4, 36.2, 25.7, 24.6, 7.0, 5.7. GC: tR = 5.05 min (Method B). HRMS (EI): m/z calcd for C18H28OSiK [M+K]+ 327.1541, found 327.1541.
(E)-diethyl((1-phenylcyclohexyl)oxy)(styryl)silane (4h) Light yellow oil, yield = 73%, 133 mg, 1H NMR (500 MHz, CDCl3): δ = 7.56 − 7.54 (m, 2H), 7.39–7.28 (m, 8H), 6.81–6.76 (m, 1H), 6.06–6.01 (m, 1H), 2.13–2.11 (m, 2H), 1.93–1.83 (m, 4H), 1.72–1.70 (m, 1H), 1.62–1.60 (m, 2H), 1.34–1.32 (m, 1H), 1.00–0.96 (m, 6H), 0.64–0.58 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 148.4, 144.3, 138.6, 128.6, 128.1, 127.1, 126.8, 126.6, 126.2, 75.5, 39.2, 26.0, 22.7, 7.1, 6.6. GC: tR = 10.41 min (Method A). HRMS (EI): m/z calcd for C24H32NaOSi [M+Na]+ 387.2115, found 387.2115.
(E)-(benzhydryloxy)diethyl(styryl)silane (4i) Light yellow oil, yield = 77%, 143 mg, 1H NMR (500 MHz, CDCl3): δ = 7.49 − 7.32 (m, 15H), 7.04–7.00 (m, 1H), 6.42–6.38 (m, 1H), 5.95 (s, 1H), 1.08–1.01 (m, 6H), 0.83–0.81 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 146.5, 145.1, 138.2, 128.7, 128.42, 128.39, 128.36, 128.3, 127.9, 127.2, 126.7, 126.6, 126.5, 124.2, 76.8, 6.9, 5.7. GC: tR = 11.57 min (Method A). HRMS (EI): m/z calcd for C25H28NaOSi [M+Na]+ 395.1802, found 395.1801.
(E)-2-(2-((diethyl(styryl)silyl)oxy)ethyl)pyridine (4j) Colorless oil, yield = 77%, 120 mg, 1H NMR (500 MHz, CDCl3): δ = 8.54 − 8.53 (m, 1H), 7.64–7.61 (m, 1H), 7.43 (d, 2H, J = 7.0 Hz), 7.35 (d, 2H, J = 7.0 Hz), 7.29–7.26 (m, 2H), 7.16–7.13 (m, 1H), 6.93 (d, 1H, J = 19.5 Hz), 6.29 (d, 1H, J = 19.5 Hz), 4.07 (t, 2H, J = 6.5 Hz), 308 (t, 2H, J = 6.5 Hz), 0.97 (t, 6H, J = 8.0 Hz), 0.73–0.68 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 159.4, 149.0, 146.3, 138.2, 136.6, 128.7, 128.4, 126.7, 124.4, 124.0, 121.6, 62.8, 41.6, 6.8, 5.1. GC: tR = 5.08 min (Method B). HRMS (EI): m/z calcd for C19H26NOSi [M+H]+ 312.1778, found 312.1778.
(E)-diethyl(1-(4-methoxyphenyl)ethoxy)(styryl)silane (5a) Light yellow oil, yield = 89%, 151 mg, 1H NMR (300 MHz, CDCl3): δ = 7.45 − 7.30 (m, 7H) 7.01–6.88 (m, 3H), 6.36 (d, 1H, J = 19.2 Hz), 5.02-4.86 (m, 1H), 3.83 (s, 3H), 1.48 (d, 3H, J = 6.0 Hz), 1.06–0.96 (m, 6H), 0.81–0.70 (m, 4H). 13C{1H} NMR (75 MHz, CDCl3): δ = 158.7, 146.1, 139.0, 138.3, 128.7, 128.4, 126.8, 126.7, 124.7, 113.7, 70.8, 55.4, 27.2, 6.9, 6.8, 5.7, 5.6. GC: tR = 9.67 min (Method A). HRMS (EI): m/z calcd for C21H28NaO2Si [M+Na]+ 363.1751, found 363.1748.
(E)-diethyl(1-(4-methoxyphenyl)ethoxy)(4-methoxystyryl)silane (5b) Light yellow oil, yield = 87%, 161 mg, 1H NMR (500 MHz, CDCl3): δ = 7.39 − 7.31 (m, 4H), 6.94–6.88 (m, 5H), 6.19 (d, 1H, J = 19.5 Hz), 4.95–4.91 (m, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 1.49 (d, 3H, J = 6.5 Hz), 1.05-0.96 (m, 6H), 0.79–0.67 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 159.9, 158.6, 145.6, 139.1, 131.3, 128.0, 126.8, 121.7, 114.0, 113.6, 70.6, 55.5, 55.4, 27.2, 7.0, 6.9, 5.7, 5.6. GC: tR = 10.04 min (Method A). HRMS (EI): m/z calcd for C22H31O3Si [M+H]+ 371.2037, found 371.2036.
(E)-diethyl(1-(4-methoxyphenyl)ethoxy)(4-methylstyryl)silane (5c) Light yellow oil, yield = 74%, 131 mg, 1H NMR (500 MHz, CDCl3): δ = 7.36−7.28 (m, 4H), 7.18 (d, 2H, J = 7.5 Hz), 6.98–6.89 (m, 3H), 6.31 (d, 1H, J = 19.5 Hz), 4.96–4.92 (m, 1H), 3.84 (s, 3H), 2.39 (s, 3H), 1.49 (d, 3H, J = 6.5 Hz), 1.05–0.97 (m, 6H), 0.80–0.68 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 158.6, 146.0, 139.0, 138.3, 135.6, 129.4, 126.8, 126.6, 123.2, 113.6, 70.7, 55.4, 27.2, 21.4, 7.0, 6.9, 5.7, 5.6. GC: tR = 9.61 min (Method A). HRMS (EI): m/z calcd for C22H30NaO2Si [M+Na]+ 377.1907, found 377.1904.
(E)-diethyl(1-(4-methoxyphenyl)ethoxy)(3-methylstyryl)silane (5d) Light yellow oil, yield = 68%, 120 mg, 1H NMR (500 MHz, CDCl3): δ = 7.36−7.34 (m, 2H), 7.30–7.28 (m, 2H), 7.16–7.15 (m, 1H), 7.00–6.92 (m, 3H), 6.38 (d, 1H, J = 19.5 Hz), 4.98–4.95 (m, 1H), 3.86 (s, 3H), 2.43 (s, 3H), 1.52 (d, 3H, J = 6.5 Hz), 1.09–1.00 (m, 6H), 0.83–0.70 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 158.6, 146.2, 139.0, 138.2, 129.2, 128.5, 127.4, 126.8, 126.6, 124.3, 123.9, 113.6, 70.7, 55.3, 27.2, 21.5, 7.0, 6.9, 5.6, 5.5. GC: tR = 9.59 min (Method A). HRMS (EI): m/z calcd for C22H30NaO2Si [M+Na]+ 377.1907, found 377.1903.
(E)-(4-(tert-butyl)styryl)diethyl(1-(4-methoxyphenyl)ethoxy)silane (5e) Light yellow oil, yield = 74%, 147 mg, 1H NMR (500 MHz, CDCl3): δ = 7.39 − 7.29 (m, 6H), 6.96–6.88 (m, 3H), 6.30 (d, 1H, J = 19.5 Hz), 4.93–4.89 (m, 1H), 3.83 (s, 3H), 1.47 (d, 3H, J = 6.0 Hz), 1.35 (s, 9H), 1.03–0.94 (m, 6H), 0.78–0.66 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 158.6, 151.6, 145.9, 139.0, 135.6, 126.8, 126.4, 125.6, 123.6, 113.6, 70.7, 55.4, 34.8, 31.5, 27.2, 7.0, 6.9, 5.7, 5.6. GC: tR = 10.29 min (Method A). HRMS (EI): m/z calcd for C25H36NaO2Si [M+Na]+ 419.2377, found 419.2373.
(E)-diethyl(4-fluorostyryl)(1-(4-methoxyphenyl)ethoxy)silane (5f) Light yellow oil, yield = 63%, 113 mg, 1H NMR (500 MHz, CDCl3): δ = 7.39−7.37 (m, 2H), 7.31–7.28 (m, 2H), 7.06–7.02 (m, 2H), 6.92–6.87 (m, 3H), 6.23 (d, 1H, J = 19.0 Hz), 4.93-4.89 (m, 1H), 3.82 (s, 3H), 1.47 (d, 3H, J = 6.0 Hz), 1.02–0.95 (m, 6H), 0.77–0.67 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 161.9 (JCF = 246.3 Hz), 158.7, 144.8, 138.9, 134.5 (JCF = 3.4 Hz), 128.3 (JCF = 8.0 Hz), 126.8, 124.4 (JCF = 2.3 Hz), 115.6 (JCF = 21.1 Hz), 113.6, 70.8, 55.4, 27.2, 7.0, 6.9, 5.6, 5.5. 19F NMR (470 MHz, CDCl3): δ = 113.6 Hz. GC: tR = 8.76 min (Method A). HRMS (EI): m/z calcd for C21H27FNaO2Si [M+Na]+ 381.1657, found 381.1651.
(E)-(4-chlorostyryl)diethyl(1-(4-methoxyphenyl)ethoxy)silane (5g) Light yellow oil, yield = 78%, 146 mg, 1H NMR (500 MHz, CDCl3): δ = 7.35−7.28 (m, 6H), 6.90–6.86 (m, 3H), 6.30 (d, 1H, J = 19.5 Hz), 4.93–4.89 (m, 1H), 3.83 (s, 3H), 1.47 (d, 3H, J = 6.5 Hz), 1.03–0.95 (m, 6H), 0.78–0.66 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 158.7, 144.7, 138.9, 136.7, 134.0, 128.8, 127.9, 126.8, 125.6, 113.6, 70.8, 55.4, 27.2, 6.9, 6.8, 5.6, 5.5. GC: tR = 9.63 min (Method A). HRMS (EI): m/z calcd for C21H27ClNaO2Si [M+Na]+ 397.1361, found 397.1360.
(E)-(4-bromostyryl)diethyl(1-(4-methoxyphenyl)ethoxy)silane (5h) Light yellow oil, yield = 67%, 140 mg, 1H NMR (500 MHz, CDCl3): δ = 7.48 (d, 2H, J = 8.5 Hz) 7.32–7.27 (m, 4H), 6.90–6.86 (m, 3H), 6.33 (d, 1H, J = 19.5 Hz), 4.94–4.90 (m, 1H), 3.83 (s, 3H), 1.49 (d, 3H, J = 6.0 Hz), 1.07–0.97 (m, 6H), 0.81–0.67 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 158.7, 144.7, 138.8, 137.2, 131.7, 128.2, 126.8, 125.8, 122.2, 113.6, 70.8, 55.4, 27.2, 6.9, 6.8, 5.6, 5.5. GC: tR = 9.95 min (Method A). HRMS (EI): m/z calcd for C21H27BrNaO2Si [M+Na]+ 441.0856, found 441.0853.
(E)-4-(2-(diethyl(1-(4-methoxyphenyl)ethoxy)silyl)vinyl)pyridine (5i) Light yellow oil, yield = 73%, 124 mg, 1H NMR (500 MHz, CDCl3): δ = 8.57 (d, 2H, J = 5.5 Hz), 7.29–7.23 (m, 4H), 6.88–6.82 (m, 3H), 6.55 (d, 1H, J = 19.5 Hz), 4.91-4.87 (m, 1H), 3.81 (s, 3H), 1.47 (d, 3H, J = 6.0 Hz), 1.01–0.95 (m, 6H), 0.78-0.68 (m, 4H). 13C{1H} NMR (125 MHz, CDCl3): δ = 158.8, 150.2, 145.2, 143.3, 138.7, 131.2, 126.8, 121.1, 113.7, 71.0, 55.4, 27.2, 6.9, 6.8, 5.5, 5.4. GC: tR = 9.73 min (Method A). HRMS (EI): m/z calcd for C20H28NO2Si [M+H]+ 342.1884, found 342.1882.
2,2-diethyl-2H-naphtho [1,8-cd][1,2]oxasilole (7a) Light yellow oil, yield = 88%, 115 mg, 1H NMR (500 MHz, CDCl3): δ = 7.77 (d, 1H, J = 8.0 Hz), 7.60 (d, 1H, J = 6.5 Hz), 7.48-7.46 (m, 1H), 7.32–7.26 (m, 2H), 6.83–6.81 (m, 1H), 0.97–0.91 (m, 10H). 13C{1H} NMR (125 MHz, CDCl3): δ = 159.1, 134.3, 132.4, 132.1, 129.3, 128.1, 128.0, 127.5, 118.0, 106.9, 6.4, 6.2. GC: tR = 4.37 min (Method B). HRMS (EI): m/z calcd for C14H17OSi [M+H]+ 229.1043, found 229.1042.
2,2-diethyl-6-methoxy-2H-naphtho [1,8-cd][1,2]oxasilole (7b) Light yellow oil, yield = 59%, 76 mg, 1H NMR (500 MHz, CDCl3): δ = 8.23 − 8.21 (m, 1H), 7.77–7.76 (m, 1H), 7.63–7.60 (m, 1H), 6.84 (d, 1H, J = 8.0 Hz), 6.72 (d, 1H, J = 8.5 Hz), 3.99 (s, 3H), 1.09–1.03 (m, 10H). 13C{1H} NMR (125 MHz, CDCl3): δ = 152.7, 148.8, 134.9, 131.8, 129.9, 126.9, 124.4, 123.2, 105.4, 104.9, 55.9, 6.5, 6.2. GC: tR = 8.25 min (Method A). HRMS (EI): m/z calcd for C15H19O2Si [M+H]+ 259.1149, found 259.1149.
6-chloro-2,2-diethyl-2H-naphtho [1,8-cd][1,2]oxasilole (7c) Light yellow oil, yield = 71%, 93 mg, 1H NMR (500 MHz, CDCl3): δ = 8.18−8.17 (m, 1H), 7.78–7.68 (m, 2H), 7.45 (d, 1H, J = 8.0 Hz), 6.84 (d, 1H, J = 8.0 Hz), 1.08–1.00 (m, 10H). 13C{1H} NMR (125 MHz, CDCl3): δ = 158.2, 135.1, 132.6, 130.1, 129.8, 128.4, 127.6, 125.3, 121.2, 107.2, 6.3, 6.2. GC: tR = 7.29 min (Method A). HRMS (EI): m/z calcd for C14H16ClOSi [M+H]+ 263.0653, found 263.0648.
6-bromo-2,2-diethyl-2H-naphtho [1,8-cd][1,2]oxasilole (7d) Light yellow oil, yield = 82%, 126 mg, 1H NMR (500 MHz, CDCl3): δ = 8.14−8.12 (m, 1H), 7.77–7.65 (m, 3H), 6.83 (d, 2H, J = 8.0 Hz), 1.09–1.01 (m, 10H). 13C{1H} NMR (125 MHz, CDCl3): δ = 158.9, 135.3, 132.7, 131.2, 131.1, 130.2, 128.7, 127.7, 110.7, 108.0, 6.3, 6.2. GC: tR = 7.79 min (Method A). HRMS (EI): m/z calcd for C14H16BrOSi [M+H]+ 307.1498, found 307.1502.
4,4-diethyl-4H-pyreno [10,1-cd][1,2]oxasilole (7e) Light yellow oil, yield = 70%, 106 mg, 1H NMR (500 MHz, CDCl3): δ = 8.33 (s, 1H), 8.21-8.12 (m, 3H), 8.04-8.02 (m, 2H), 7.92 (d, 1H, J = 9.0 Hz), 7.63 (d, 1H, J = 8.5 Hz), 1.21-1.13 (m, 10H). 13C{1H} NMR (125 MHz, CDCl3): δ = 157.3, 132.5, 131.8, 131.1, 131.0, 127.5, 127.1, 126.5, 126.2, 125.6, 124.8, 124.6, 124.4, 123.5, 111.4, 6.5, 6.3. GC: tR = 14.09 min (Method A). HRMS (EI): m/z calcd for C20H19OSi [M+H]+ 303.1199, found 303.1201.
3,3-diethyl-1,3-dihydronaphtho [2,1-c][1,2]oxasilole (9a) Light yellow oil, yield = 88%, 106 mg, 1H NMR (500 MHz, CDCl3): δ = 7.95−7.93 (m, 1H), 7.82 (d, 1H, J = 8.0 Hz), 7.74–7.72 (m, 1H), 7.64 (d, 1H, J = 8.0 Hz), 7.58–7.56 (m, 2H), 1.02–0.90 (m, 10H). 13C{1H} NMR (125 MHz, CDCl3): δ = 148.1, 134.2, 130.6, 128.8, 128.1, 127.5, 127.4, 126.7, 126.5, 123.2, 7.8, 7.3, 6.7. GC: tR = 5.68 min (Method A). HRMS (EI): m/z calcd for C15H19OSi [M+H]+ 243.1199, found 243.1203.
4. Conclusions
In summary, we have described a highly regio- and stereoselective sequential ruthenium-catalyzed dehydrogenative intermolecular silylation to access (E)-alkenyl silyl-ether derivatives with alcohols and alkenes and H2SiEt2 as the hydrosilane reagent. Furthermore, a regioselective intermolecular C-H silylation of naphthol derivatives with H2SiEt2 to synthesize silyl-ether heterocycles was also developed using the RuH2(CO)(PPh3)3/NBE catalytic system. Various (E)-alkenyl silyl-ether derivatives and silyl-ether heterocycles bearing tolerated functional groups were successfully synthesized in moderate to excellent yields. Additionally, two pathways of Ru-catalyzed dehydrogenative intermolecular silylations were proposed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28207186/s1. Table S1. Optimization of reaction conditions for Ru catalyzed dehydrogenative silylation of 1-phenylpropan-1-ol (1a) with EtSiH2[a].
Author Contributions
Methodology, Q.L.; Formal analysis, J.L. and S.L.; Investigation, S.X.; Writing—original draft, Z.H.; Writing—review & editing, F.X., J.W. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded the Foundation of the Department of Education of Guangdong Province (No: 2021ZDZX2045), the Foundation of Wuyi University (No: 2021WGALH06), the Foundation of Graduate Education Innovation Plan of Guangdong Province (No: YJS-JGXM-21-03, 2021JGXM111), and the Science and Technology Planning Project of Guangdong Province (2021B1212040016).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Min, G.K.; Hernandez, D.; Skrydstrup, T. Efficient Routes to Carbon-Silicon Bond Formation for the Synthesis of SiliconContaining Peptides and Azasilaheterocycles. Acc. Chem. Res. 2013, 46, 457–470. [Google Scholar] [CrossRef]
- Hofmann, R.J.; Vlatkovic, M.; Wiesbrock, F. Fifty Years of Hydrosilylation in Polymer Science: A Review of Current Trends of Low-Cost Transition-Metal and Metal-Free Catalysts, NonThermally Triggered Hydrosilylation Reactions, and Industrial Applications. Polymers 2017, 9, 534–571. [Google Scholar] [PubMed]
- Cheng, C.; Hartwig, J.F. Catalytic Silylation of Unactivated C-H Bonds. Chem. Rev. 2015, 115, 8946–8975. [Google Scholar] [PubMed]
- Li, B.; Dixneuf, P.H. Metal-Catalyzed Silylation of sp3C-H Bonds. Chem. Soc. Rev. 2021, 50, 5062–5085. [Google Scholar] [PubMed]
- Ge, Y.; Huang, X.; Ke, J.; He, C. Transition-Metal-Catalyzed Enantioselective C-H Silylation. Chem. Catal. 2022, 2, 2898–2928. [Google Scholar]
- Ren, L.-Q.; Li, N.; Ke, J.; He, C. Recent Advances in Photo- and Electro-Enabled Radical Silylation. Org. Chem. Front. 2022, 9, 6400–6415. [Google Scholar]
- Hou, J.; Han, X.; Zhang, Y.; Huang, J.; Wang, J.; Yuan, K. Triflic Acid/Silane Promoted Deoxygenative Transformation of Ketones via Carbocations. Org. Lett. 2023, 25, 5709–5713. [Google Scholar] [CrossRef]
- McAtee, J.R.; Martin, S.E.S.; Ahneman, D.T.; Johnson, K.A.; Watson, D.A. Preparation of Allyl and Vinyl Silanes by the Palladium-Catalyzed Silylation of Terminal Olefins: A Silyl-Heck Reaction. Angew. Chem. Int. Ed. 2012, 51, 3663–3667. [Google Scholar] [CrossRef]
- Martin, S.E.S.; Watson, D.A. Preparation of Vinyl Silyl Ethers and Disiloxanes via the Silyl-Heck Reaction of Silyl Ditriflates. J. Am. Chem. Soc. 2013, 135, 13330–13333. [Google Scholar] [CrossRef]
- Matsumoto, K.; Huang, J.; Naganawa, Y.; Guo, H.; Beppu, T.; Sato, K.; Shimada, S.; Nakajima, Y. Direct Silyl-Heck Reaction of Chlorosilanes. Org. Lett. 2018, 20, 2481–2484. [Google Scholar] [CrossRef]
- Rémond, E.; Martin, C.; Martinez, J.; Cavelier, F. Silicon-Containing Amino Acids: Synthetic Aspects, Conformational Studies, and Applications to Bioactive Peptides. Chem. Rev. 2016, 116, 11654–11684. [Google Scholar]
- Pérez-Torrente, J.J.; Nguyen, D.H.; Jiménez, M.V.; Modrego, F.J.; Puerta-Oteo, R.; Gómez-Bautista, D.; Iglesias, M.; Oro, L.A. Hydrosilylation of Terminal Alkynes Catalyzed by a ONOPincer Iridium(III) Hydride Compound: Mechanistic Insights into the Hydrosilylation and Dehydrogenative Silylation Catalysis. Organometallics 2016, 35, 2410–2422. [Google Scholar]
- Marciniec, B. Catalysis by Transition Metal Complexes of Alkene Silylation-Recent Progress and Mechanistic Implications. Coordin. Chem. Rev. 2005, 249, 2374–2390. [Google Scholar]
- Zhang, L.; Hang, Z.; Liu, Z.-Q. A Free-Radical-Promoted Stereospecific Decarboxylative Silylation of α,β-Unsaturated Acids with Silanes. Angew. Chem. Int. Ed. 2016, 55, 236–239. [Google Scholar]
- Lu, C.; Lin, Y.; Wang, M.; Zhou, J.; Wang, S.; Jiang, H.; Kang, K.; Huang, L. Nickel-Catalyzed Ring-Opening of Benzofurans for the Divergent Synthesis of ortho-Functionalized Phenol Derivatives. ACS Catal. 2023, 13, 2432. [Google Scholar] [CrossRef]
- Liu, W.; Lu, W.; Yang, L.; Wu, X.; Zhang, Z. Rhodium-Catalyzed anti-Markovnikov Hydrosilylation of Alkenes. Tetrahedron 2022, 109, 132632. [Google Scholar]
- Greenhalgh, M.D.; Frank, D.J.; Thomas, S.P. Iron-Catalysed Chemo-, Regio-, and Stereoselective Hydrosilylation of Alkenes and Alkynes using a Bench-Stable Iron(II) Pre-Catalyst. Adv. Synth. Catal. 2014, 356, 584–590. [Google Scholar]
- Lu, W.; Li, C.; Wu, X.; Xie, X.; Zhang, Z. [Rh(COD)Cl]2/PPh3-Catalyzed Dehydrogenative Silylation of Styrene Derivatives with NBE as a Hydrogen Acceptor. Organometallics 2020, 39, 3780–3788. [Google Scholar] [CrossRef]
- Zhu, F.; Spannenberg, A.; Wu, X. Rhodium-Catalyzed Carbonylative Synthesis of Silyl-substituted Indenones. Chem. Commun. 2017, 53, 13149–13152. [Google Scholar]
- Chen, B.; Wu, X. Rhodium-Catalyzed Carbonylative Synthesis of Benzosilinones. Org. Lett. 2019, 21, 2899–2902. [Google Scholar] [CrossRef]
- Cheng, C.; Simmons, E.M.; Hartwig, J.F. Iridium-Catalyzed, Diastereoselective Dehydrogenative Silylation of Terminal Alkenes with (TMSO)2MeSiH. Angew. Chem. Int. Ed. 2013, 52, 8984–8989. [Google Scholar]
- Bokka, A.; Jeon, J. Regio- and Stereoselective Dehydrogenative Silylation and Hydrosilylation of Vinylarenes Catalyzed by Ruthenium Alkylidenes. Org. Lett. 2016, 18, 5324–5327. [Google Scholar] [PubMed]
- Naumov, R.N.; Itazaki, M.; Kamitani, M.; Nakazawa, H. Selective Dehydrogenative Silylation-Hydrogenation Reaction of Divinyldisiloxane with Hydrosilane Catalyzed by an Iron Complex. J. Am. Chem. Soc. 2012, 134, 804–807. [Google Scholar] [CrossRef]
- Gu, J.; Cai, C. Stereoselective Synthesis of Vinylsilanes via Copper-Catalyzed Silylation of Alkenes with Silanes. Chem. Commun. 2016, 52, 10779–10782. [Google Scholar]
- Weber, S.; Glavic, M.; Stöger, B.; Pittenauer, E.; Podewitz, M.; Veiros, L.F.; Kirchner, K. Manganese-Catalyzed Dehydrogenative Silylation of Alkenes Following Two Parallel Inner-Sphere Pathways. J. Am. Chem. Soc. 2021, 143, 17825–17832. [Google Scholar]
- Dong, J.; Yuan, X.-A.; Yan, Z.; Mu, L.; Junyang, M.J.; Zhu, C.; Xie, J. Manganese-Catalysed Divergent Silylation of Alkenes. Nat. Chem. 2021, 13, 182–190. [Google Scholar] [PubMed]
- Li, B.; Driess, M.; Hartwig, J.F. Iridium-Catalyzed Regioselective Silylation of Secondary Alkyl C-H Bonds for the Synthesis of 1,3-Diols. J. Am. Chem. Soc. 2014, 136, 6586–6589. [Google Scholar]
- Lee, T.; Wilson, T.W.; Berg, R.; Ryberg, P.; Hartwig, J.F. Rhodium-Catalyzed Enantioselective Silylation of Arene C-H Bonds: Desymmetrization of Diarylmethanols. J. Am. Chem. Soc. 2015, 137, 6742–6745. [Google Scholar] [PubMed]
- Lin, Q.; Lin, Z.; Pan, M.; Zheng, Q.; Li, H.; Chen, X.; Darcel, C.; Dixneuf, P.H.; Li, B. Alkene as Hydrogen Trapper to Control the Regio-Selective Ruthenium(II) Catalyzed Ortho C-H Silylation of Amides and Anilides. Org. Chem. Front. 2021, 8, 514–521. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Lin, Q.; Huang, Y.; Li, B. Ruthenium(II) Acetate Catalyzed Synthesis of Silylated Oxazoles via C-H Silylation and Dehalogenation. Org. Lett. 2019, 21, 1134–1138. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Q.; Liao, C.; Chen, J.; Zhang, K.; Liu, Q.; Li, B. Ruthenium(II)/Acetate Catalyzed Intermolecular Dehydrogenative Ortho C-H Silylation of 2-Aryl N-Containing Heterocycles. Org. Biomol. Chem. 2019, 17, 4115–4120. [Google Scholar] [PubMed]
- Chatterjee, B.; Gunanathan, C. Ruthenium Catalyzed Selective Hydrosilylation of Aldehydes. Chem. Commun. 2014, 50, 888–890. [Google Scholar]
- Kakiuchi, F.; Kochi, T.; Mizushima, E.; Murai, S. Room-Temperature Regioselective C-H/Olefifin Coupling of Aromatic Ketones Using an Activated Ruthenium Catalyst with a Carbonyl Ligand and Structural Elucidation of Key Intermediates. J. Am. Chem. Soc. 2010, 132, 17741–17750. [Google Scholar] [CrossRef] [PubMed]
- Christ, M.L.; Sabo-Etienne, S.; Chaudret, B. Highly Selective Dehydrogenative Silylation of Ethylene Using the Bis(dihydrogen) Complex RuH2(H2)2(PCy3)2 as Catalyst Precursor. Organometallics 1995, 14, 1082–1084. [Google Scholar] [CrossRef]
- Parija, A.; Sunoj, R.B. Mechanism of Catalytic Functionalization of Primary C-H Bonds Using a Silylation Strategy. Org. Lett. 2013, 15, 4066–4069. [Google Scholar] [PubMed]
- Delpech, F.; Mansas, J.; Leuser, H.; Sabo-Etienne, S.; Chaudret, B. Ruthenium-Catalyzed Silylation of Ethylene by Disilanes. Organometallics 2000, 19, 5750–5757. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).