Development of an HPLC-MS/MS Method for Chiral Separation and Quantitation of (R)- and (S)-Salbutamol and Their Sulfoconjugated Metabolites in Urine to Investigate Stereoselective Sulfonation
Abstract
:1. Introduction
2. Results
2.1. HPLC-MS/MS Method Development
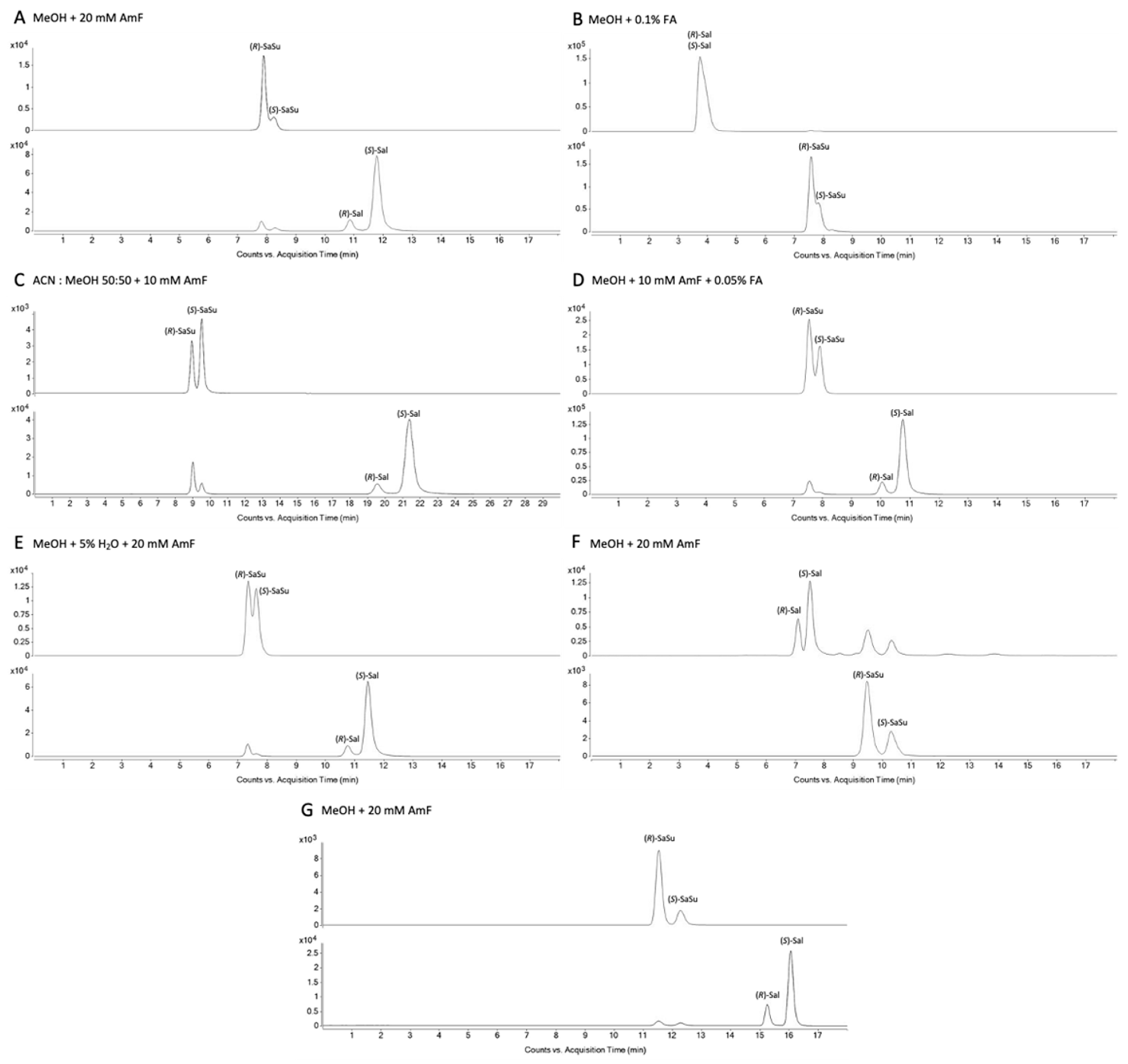
2.1.1. Chiral Chromatography
2.1.2. Electrospray Ionization
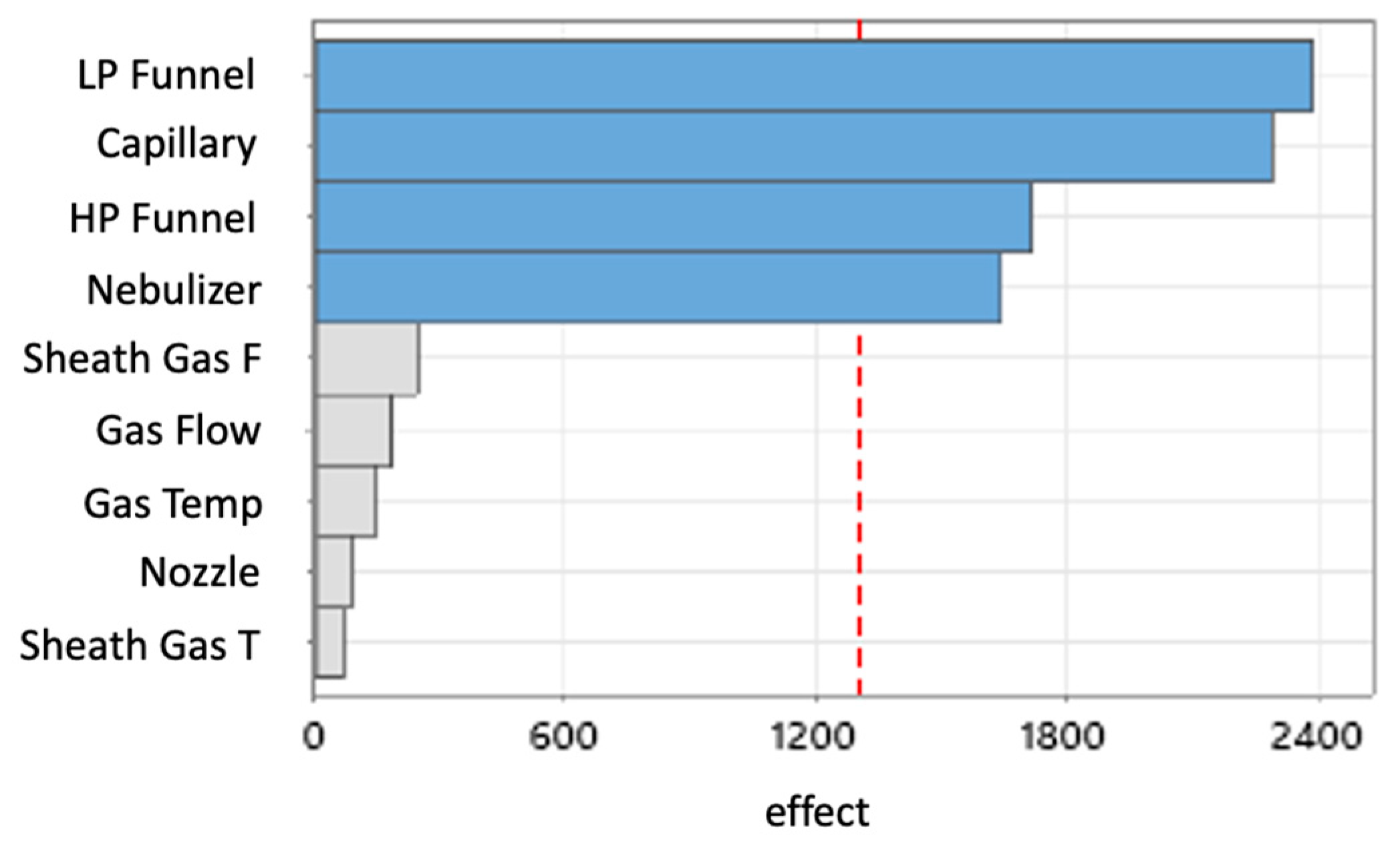
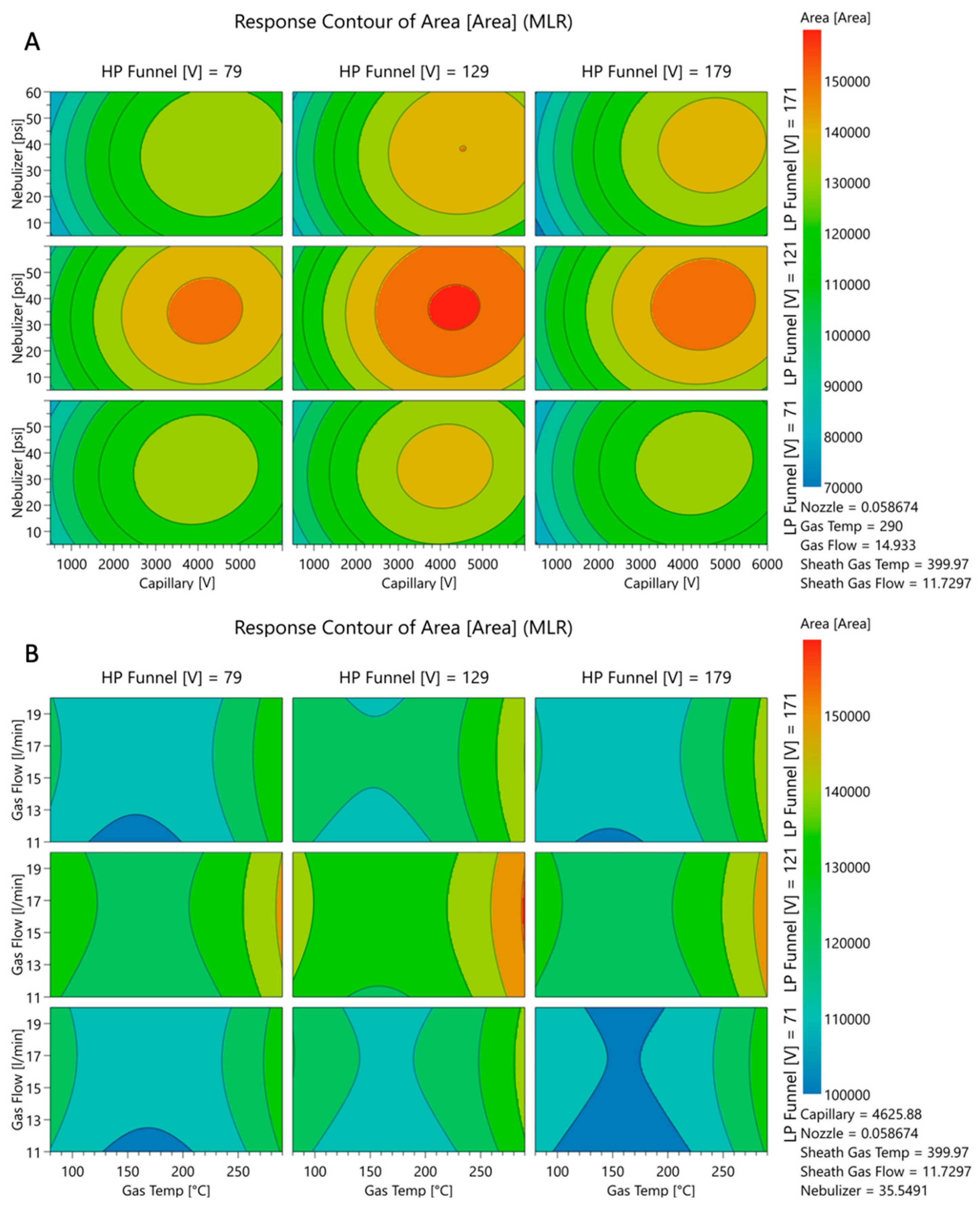
Multiple-Step Screening Design of Experiment (MSSD)
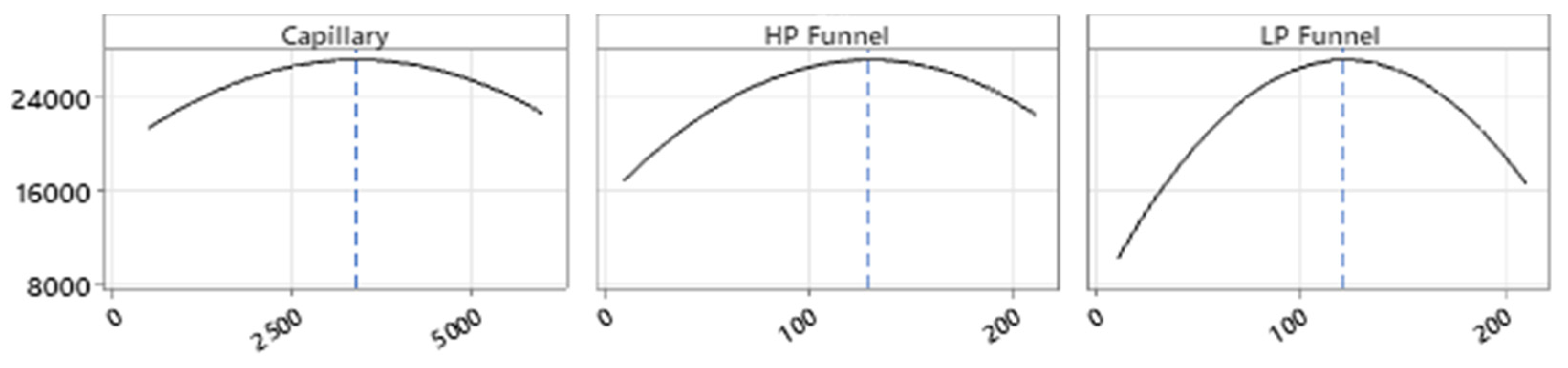
Full Factorial Design
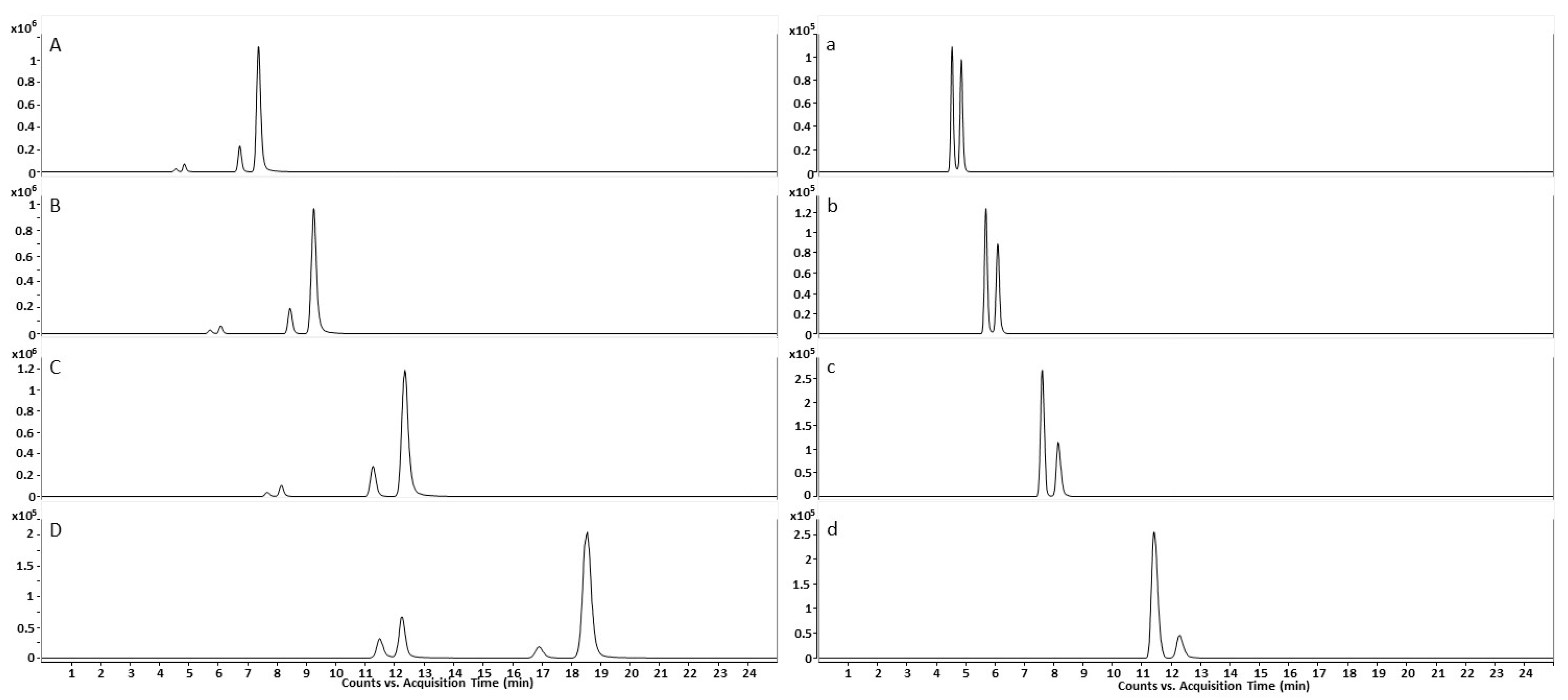
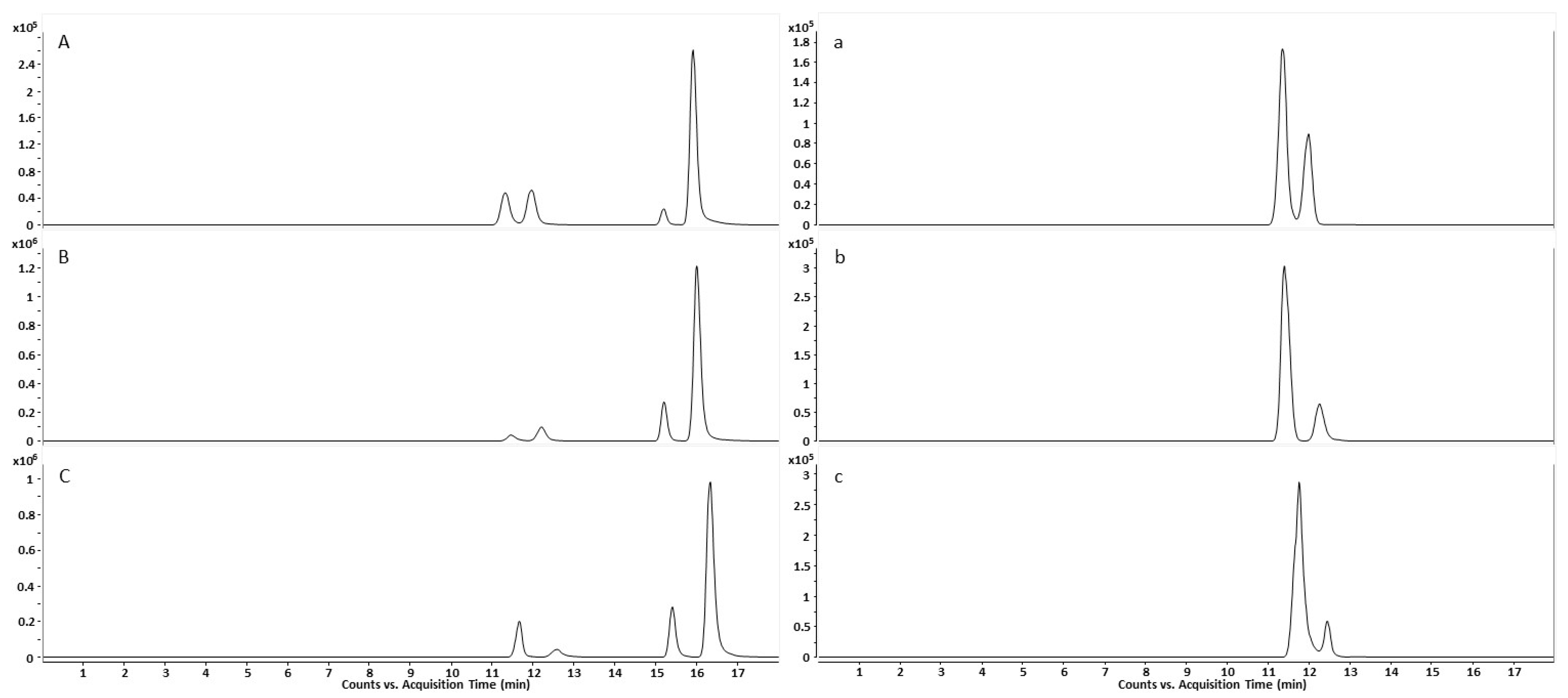
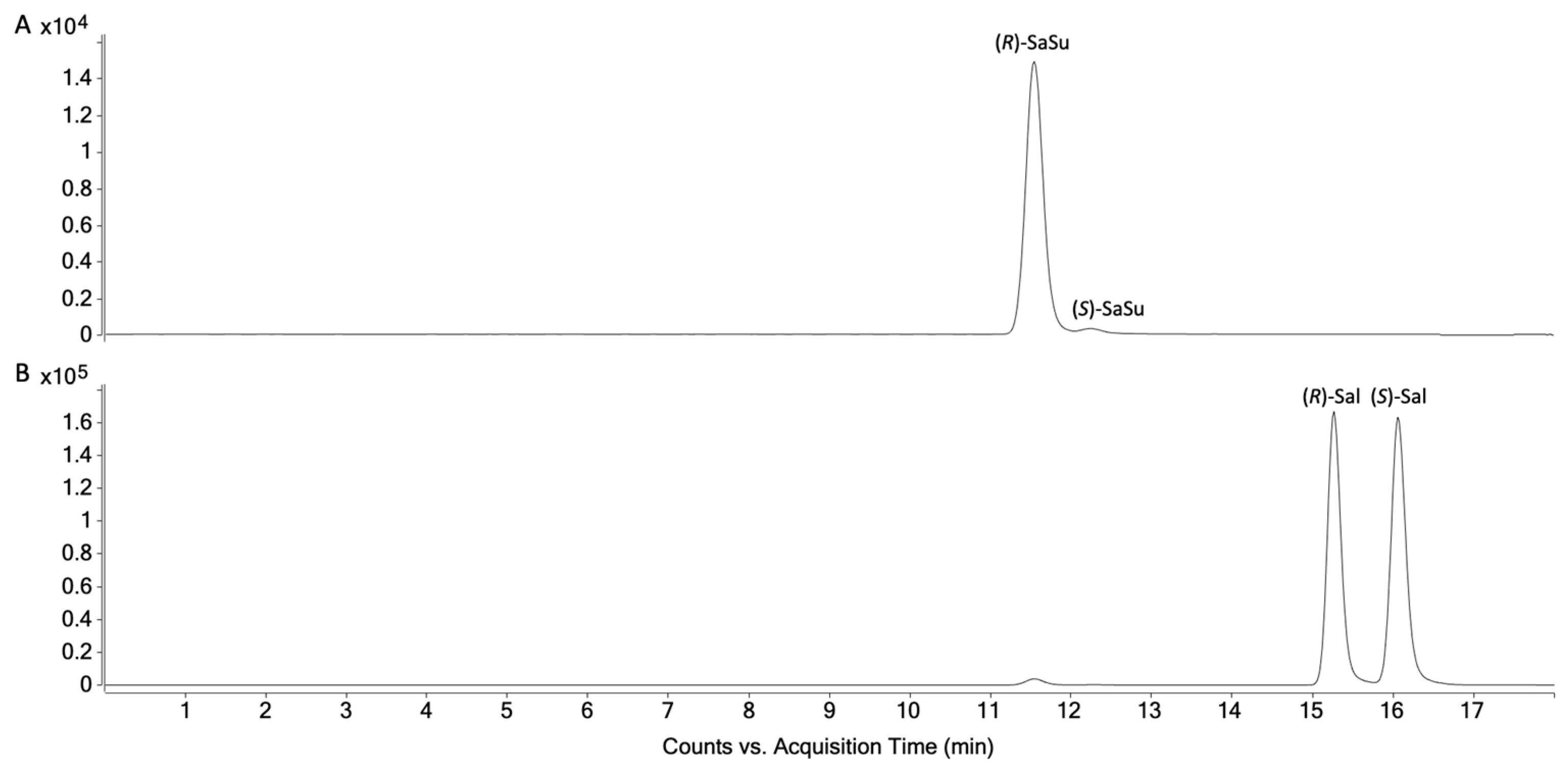
2.1.3. Peak Identification in Urine Samples through Qualifier-Quantifier Ratios
2.2. Chiral Analysis of Salbutamol-4′-O-sulfate Reference
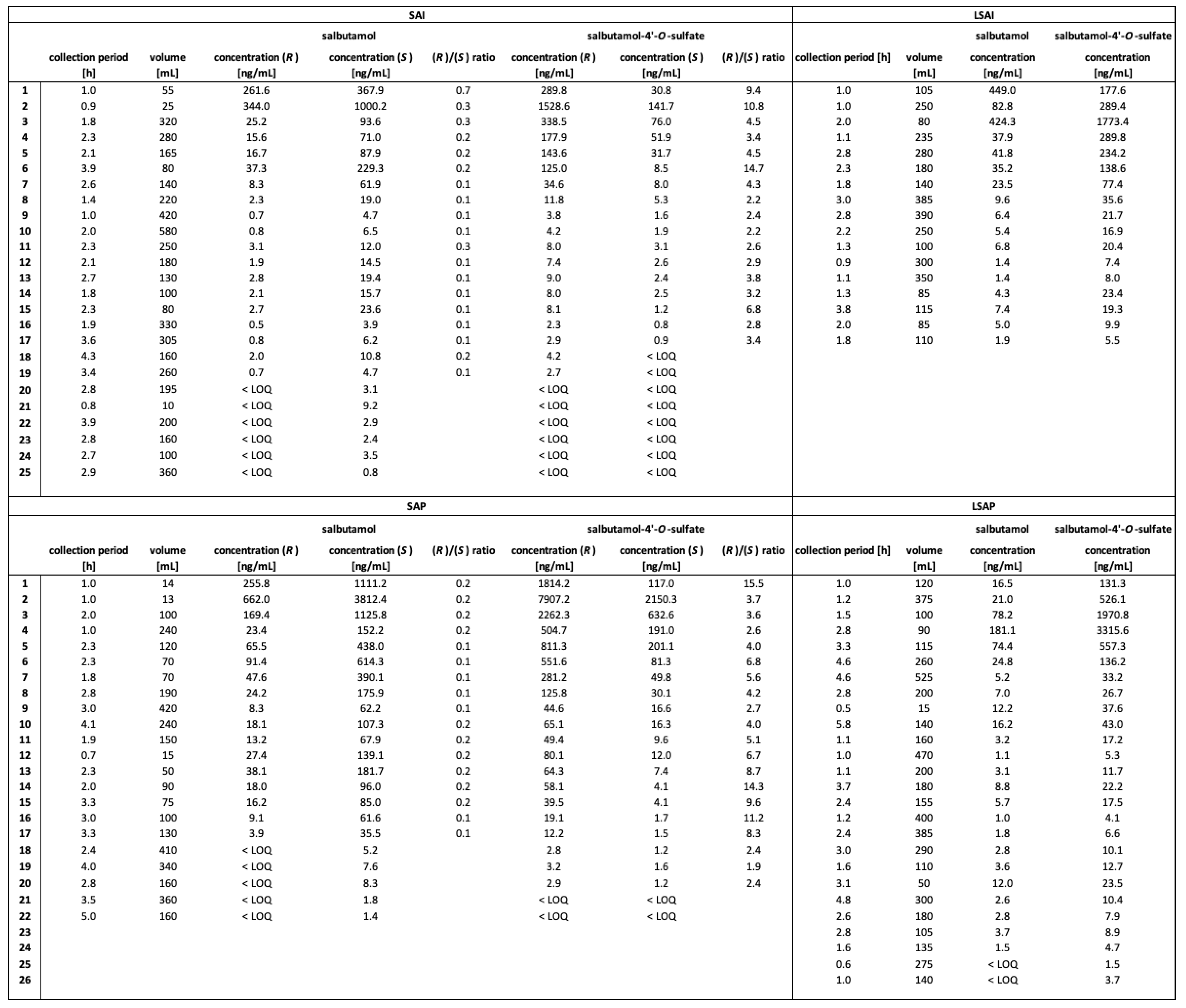
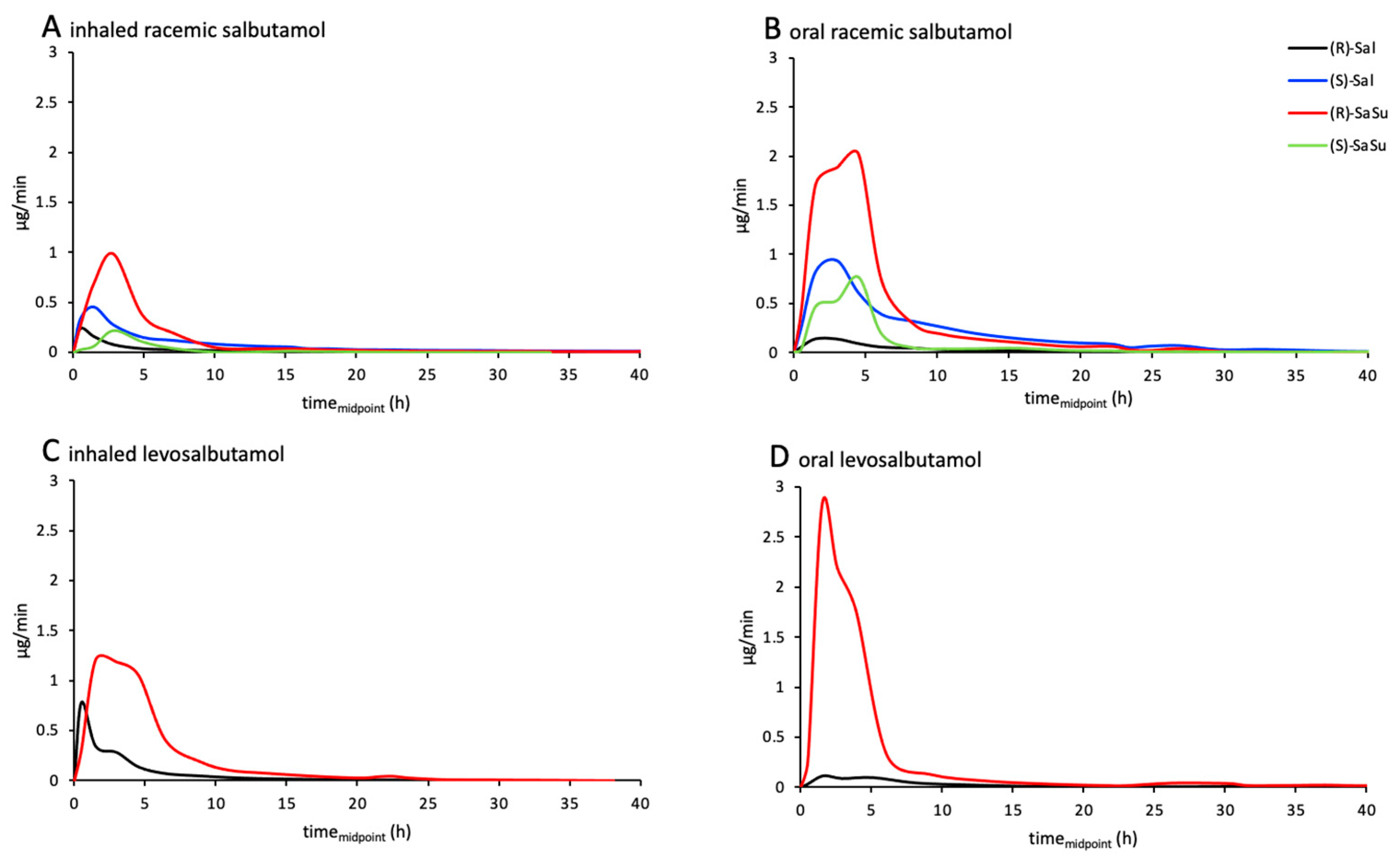
2.3. Excretion Rates of (R)-/(S)-Salbutamol and (R)-/(S)-Salbutamol-4′-O-sulfate
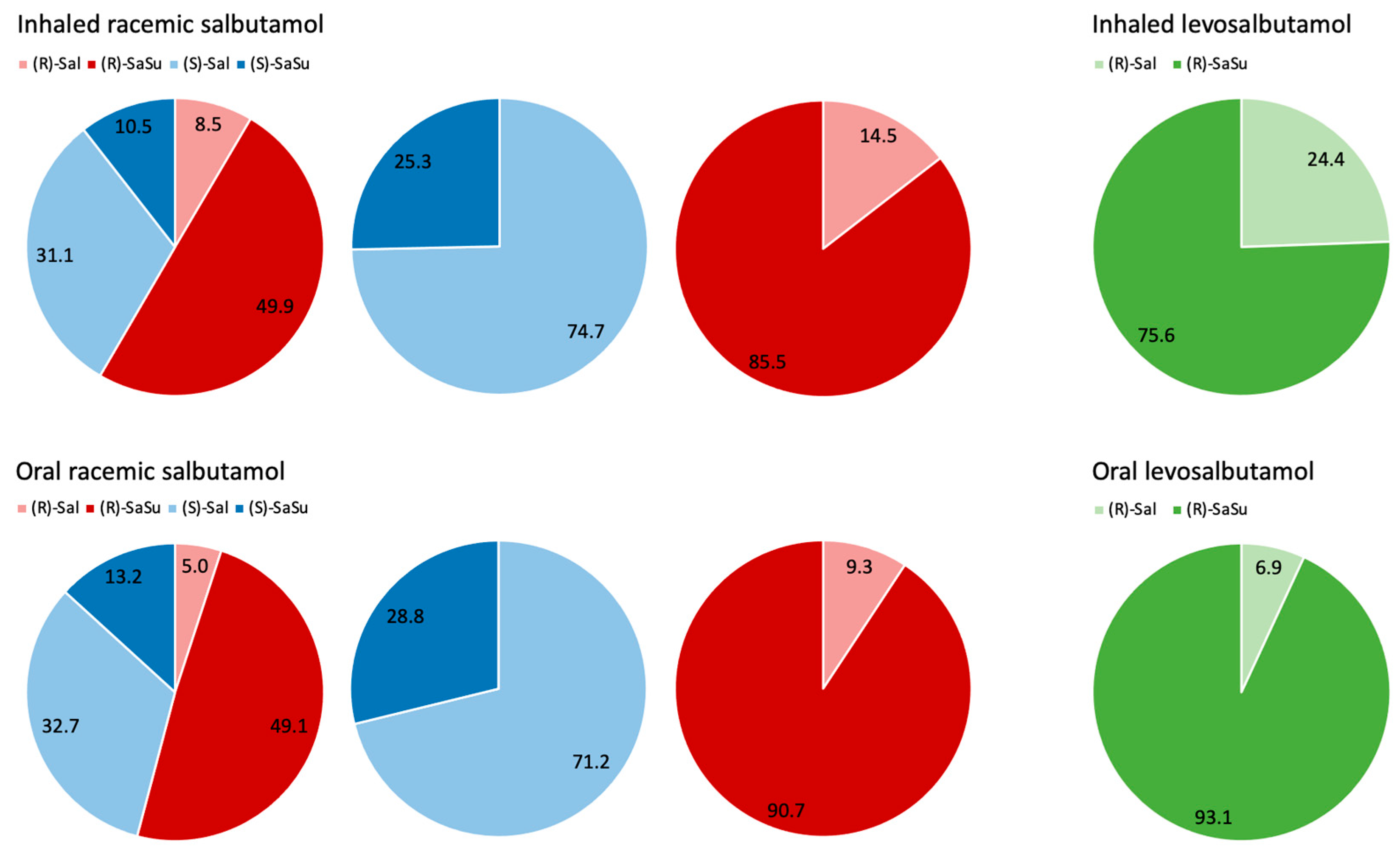
2.4. Enantiomerical Ratios in Urine
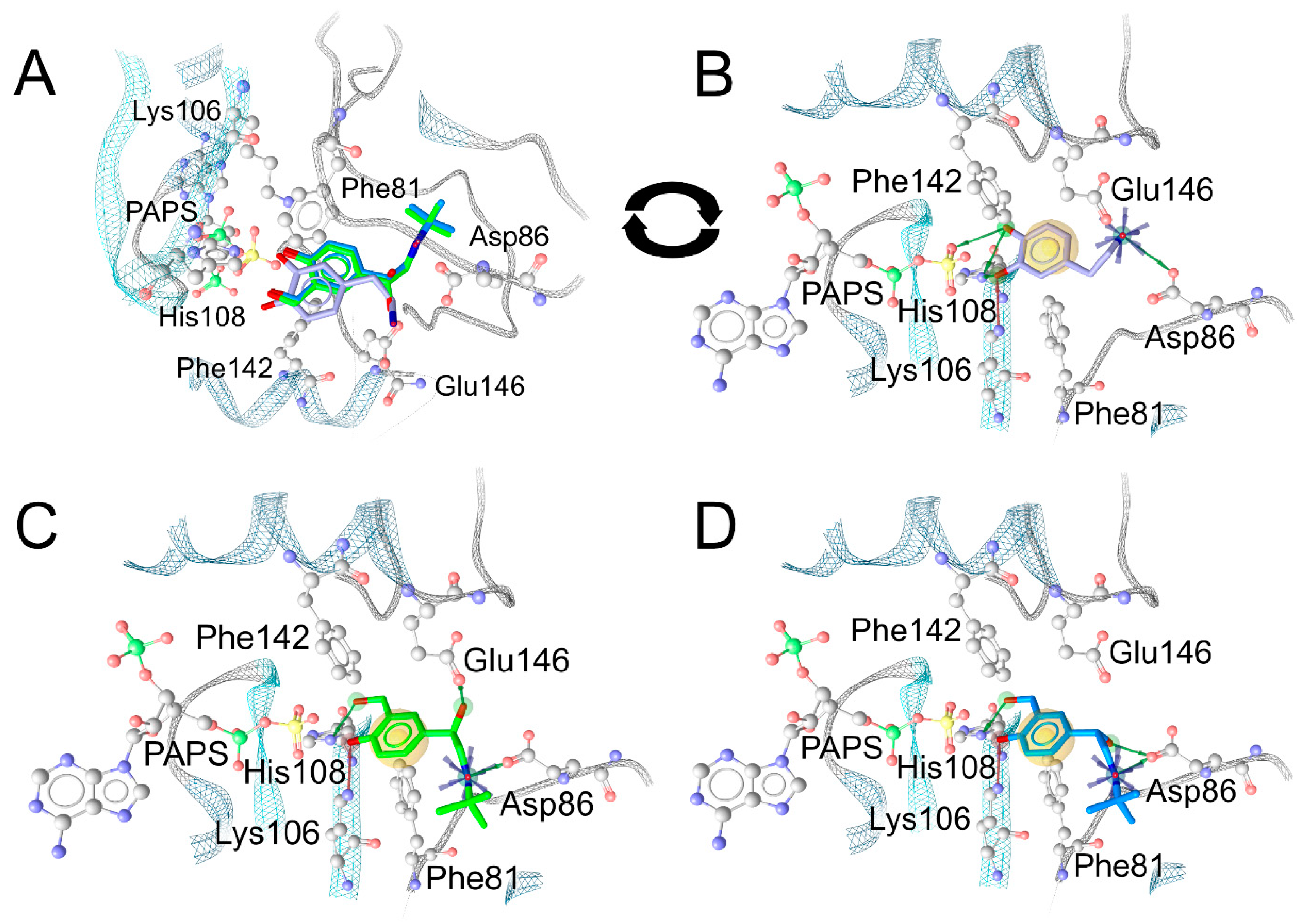
2.5. In-Silico Analysis of the Binding Mode of Salbutamol Enantiomers in SULT1A3
3. Discussion
3.1. Method Development
3.2. Stereoselectivity of Salbutamol Sulfonation
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Method Development Chiral Column Chromatography
4.2.1. Chiral Column and Mobile Phase Screening
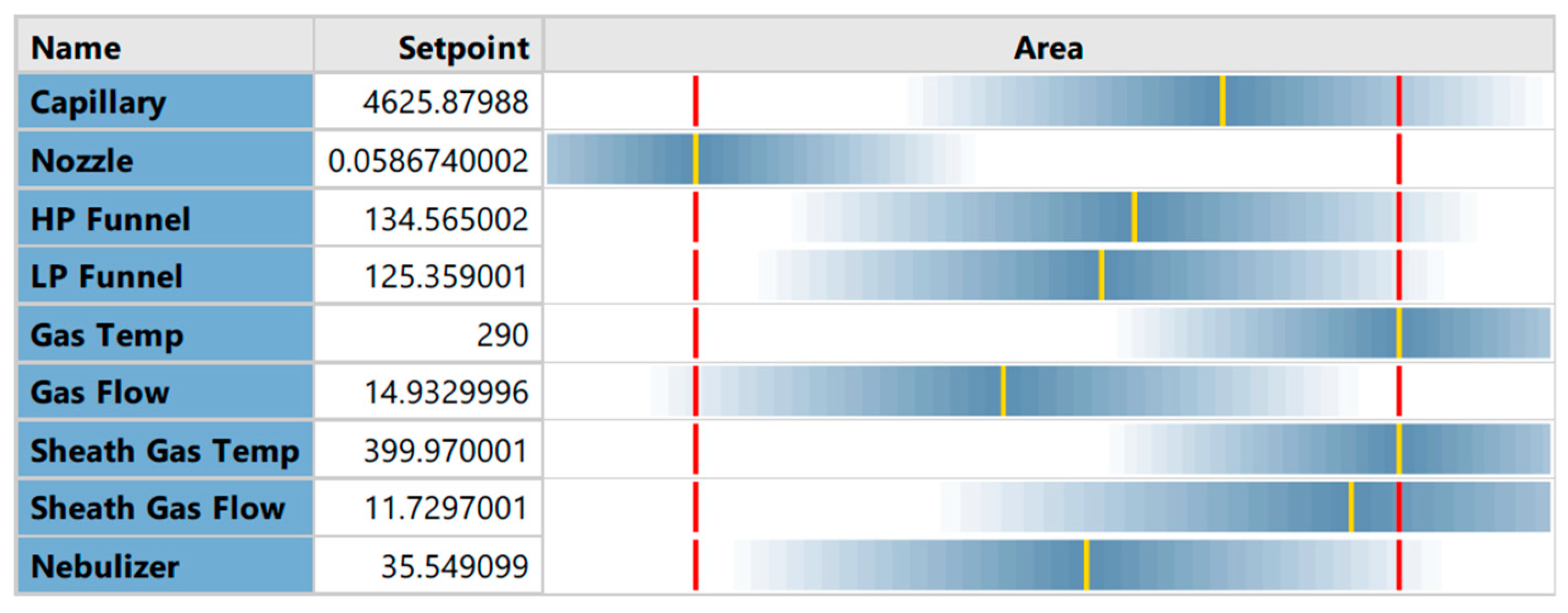
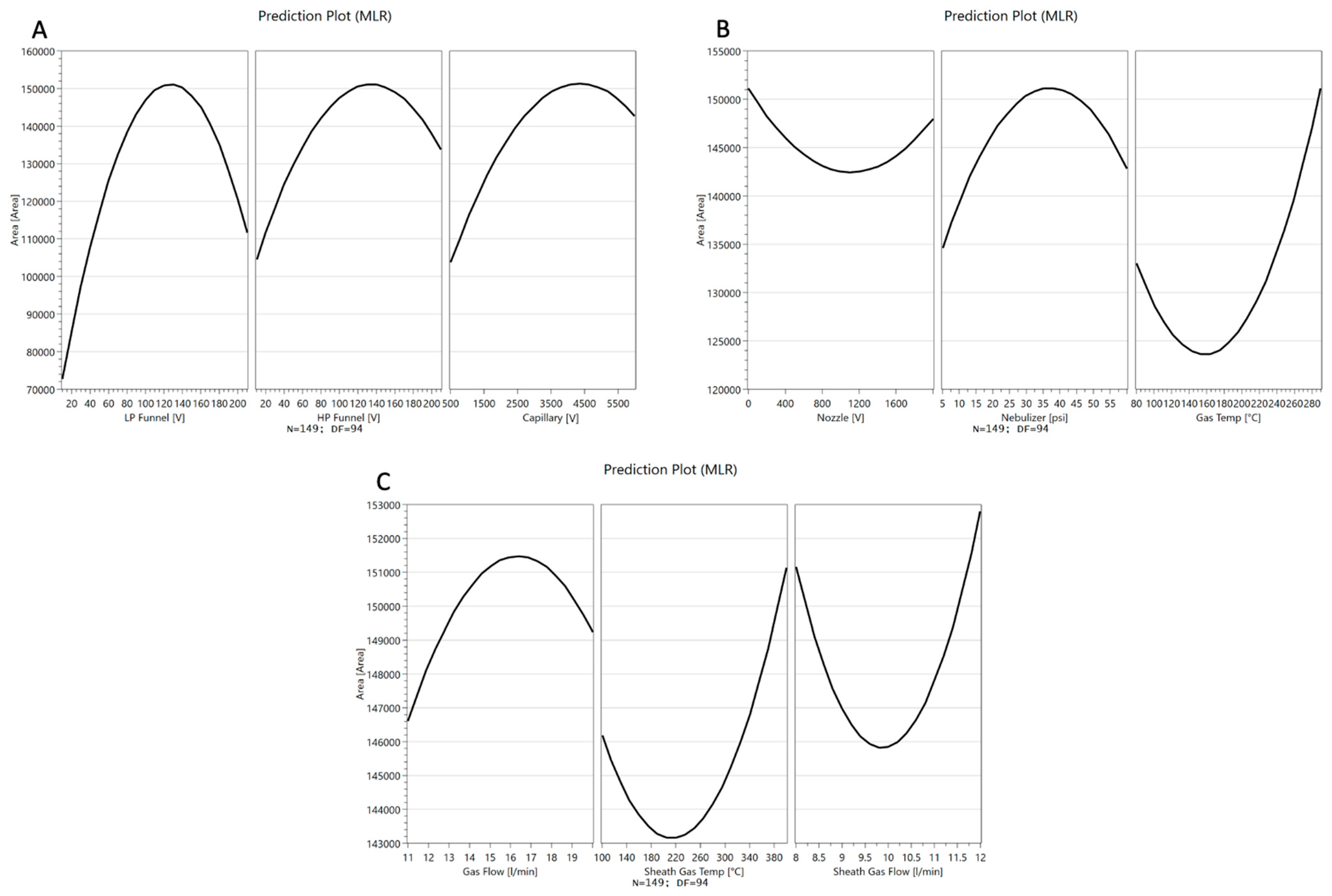
4.2.2. Optimization
4.3. Optimization of Electrospray Ionization Parameters Ion Source Optimization
4.4. Synthesis of Salbutamol-4′-O-sulfate Reference
4.5. Proof of Concept—Longitudinal Excretion Study
4.5.1. Study Design
4.5.2. Matrix-Assisted Calibration
4.5.3. Sample Preparation
4.5.4. Instruments and Chromatographic Conditions
4.6. Protein Preparation and Molecular Docking
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A

References
- Corda, L.; La Piana, G.; Bertella, E.; Taranto, M.; Pini, L.; Tantucci, C. Dose-response curve to salbutamol during acute and chronic treatment with formoterol in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 399–405. [Google Scholar] [CrossRef]
- Ahrens, R.C.; Smith, G.D. Albuterol: An Adrenergic Agent for Use in the Treatment of Asthma Pharmacology, Pharmacokinetics and Clinical Use. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1984, 4, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Thomson, N.C. (R)-salbutamol in the treatment of asthma and chronic obstructive airways disease. Expert Opin. Pharmacother. 2011, 12, 1133–1141. [Google Scholar] [CrossRef]
- Aliverti, A. Effect of salbutamol on lung function and chest wall volumes at rest and during exercise in COPD. Thorax 2005, 60, 916–924. [Google Scholar] [CrossRef]
- Griffin, J.; Lee, S.; Caiado, M.; Kesten, S.; Price, D. Comparison of tiotropium bromide and combined ipratropium/salbutamol for the treatment of COPD: A UK General Practice Research Database 12-month follow-up study. Prim. Care Respir. J. 2008, 17, 104–110. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency. The 2022 Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/2022-01/2022list_final_en_0.pdf (accessed on 11 February 2022).
- Mareck, U.; Guddat, S.; Schwenke, A.; Beuck, S.; Geyer, H.; Flenker, U.; Elers, J.; Backer, V.; Thevis, M.; Schänzer, W. Determination of salbutamol and salbutamol glucuronide in human urine by means of liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2011, 3, 820–827. [Google Scholar] [CrossRef]
- Ko, K.; Kurogi, K.; Davidson, G.; Liu, M.-Y.; Sakakibara, Y.; Suiko, M.; Liu, M.-C. Sulfation of ractopamine and salbutamol by the human cytosolic sulfotransferases. J. Biochem. 2012, 152, 275–283. [Google Scholar] [CrossRef]
- Boulton, D.W.; Fawcett, J.P. The pharmacokinetics of levosalbutamol: What are the clinical implications? Clin. Pharmacokinet. 2001, 40, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Harps, L.C.; Bureik, M.; Parr, M.K. Human Sulfotransferase Assays with PAPS Production in situ. Front. Mol. Biosci. 2022, 9, 827638. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.A.; Raidal, S.; Robson, K.; Narkowicz, C.K.; Nichols, D.S.; Haydn Walters, E. Bronchopulmonary pharmacokinetics of (R)-salbutamol and (S)-salbutamol enantiomers in pulmonary epithelial lining fluid and lung tissue of horses. Br. J. Clin. Pharmacol. 2017, 83, 1436–1445. [Google Scholar] [CrossRef]
- Walle, T.; Eaton, E.A.; Walle, U.K.; Pesola, G.R. Stereoselective metabolism ofRS-albuterol in humans. Clin. Rev. Allergy Immunol. 1996, 14, 101–113. [Google Scholar] [CrossRef]
- Boulton, D.W.; Fawcett, J.P. Pharmacokinetics and pharmacodynamics of single oral doses of albuterol and its enantiomers in humans. Clin. Pharmacol. Ther. 1997, 62, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Jendretzki, A.L.; Harps, L.C.; Sun, Y.; Bredendiek, F.; Bureik, M.; Girreser, U.; de la Torre, X.; Botrè, F.M.; Parr, M.K. Biosynthesis of Salbutamol-4′-O-sulfate as Reference for Identification of Intake Routes and Enantiopure Salbutamol Administration by Achiral UHPLC-MS/MS. Separations 2023, 10, 427. [Google Scholar] [CrossRef]
- Chan, S.H.; Lee, W.; Asmawi, M.Z.; Tan, S.C. Chiral liquid chromatography–mass spectrometry (LC–MS/MS) method development for the detection of salbutamol in urine samples. J. Chromatogr. B 2016, 1025, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zeng, J.; Liu, S.; Zhao, T.; Wu, J.; Lai, W.; He, M.; Xu, B.; Qu, S.; Xu, L.; et al. Study on the determination and chiral inversion of R-salbutamol in human plasma and urine by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2015, 1002, 218–227. [Google Scholar] [CrossRef]
- Jacobson, G.A.; Yee, K.C.; Wood-Baker, R.; Walters, E.H. SULT 1A3 single-nucleotide polymorphism and the single dose pharmacokinetics of inhaled salbutamol enantiomers: Are some athletes at risk of higher urine levels? Drug Test. Anal. 2015, 7, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bergés, R.; Segura, J.; Ventura, R.; Fitch, K.D.; Morton, A.R.; Farré, M.; Mas, M.; de La Torre, X. Discrimination of prohibited oral use of salbutamol from authorized inhaled asthma treatment. Clin. Chem. 2000, 46, 1365–1375. [Google Scholar] [CrossRef]
- Schmekel, B.; Rydberg, I.; Norlander, B.; Sjosward, K.N.; Ahlner, J.; Andersson, R.G. Stereoselective pharmacokinetics of S-salbutamol after administration of the racemate in healthy volunteers. Eur. Respir. J. 1999, 13, 1230. [Google Scholar] [CrossRef]
- Joyce, K.B.; Jones, A.E.; Scott, R.J.; Biddlecombe, R.A.; Pleasance, S. Determination of the enantiomers of salbutamol and its 4-O-sulphate metabolites in biological matrices by chiral liquid chromatography tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 1899–1910. [Google Scholar] [CrossRef]
- Ward, J.K.; Dow, J.; Dallow, N.; Eynott, P.; Milleri, S.; Ventresca, G.P. Enantiomeric disposition of inhaled, intravenous and oral racemic-salbutamol in man--no evidence of enantioselective lung metabolism. Br. J. Clin. Pharmacol. 2000, 49, 15–22. [Google Scholar] [CrossRef]
- Lu, J.H.; Li, H.T.; Liu, M.C.; Zhang, J.P.; Li, M.; An, X.M.; Chang, W.R. Crystal structure of human sulfotransferase SULT1A3 in complex with dopamine and 3′-phosphoadenosine 5′-phosphate. Biochem. Biophys. Res. Commun. 2005, 335, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Teubner, W. Charakterisierung von Sulfotransferasen im Gastrointestinaltrakt von Mensch und Ratte und Aktivierung von Promutagenen in V79- Zellen, die eine intestinale Form (1B1) des Menschen und der Ratte exprimieren. Ph.D. Thesis, Universität Potsdam, Potsdam, Germany, 2001. [Google Scholar]
- Yamamoto, T.; Yamatodani, A.; Nishimura, M.; Wada, H. Determination of dopamine-3- and 4-O-sulphate in human plasma and urine by anion-exchange high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. 1985, 342, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Itäaho, K.; Alakurtti, S.; Yli-Kauhaluoma, J.; Taskinen, J.; Coughtrie, M.W.; Kostiainen, R. Regioselective sulfonation of dopamine by SULT1A3 in vitro provides a molecular explanation for the preponderance of dopamine-3-O-sulfate in human blood circulation. Biochem. Pharmacol. 2007, 74, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, L.M.; McManus, M.E.; Gaedigk, A.; Kakuta, Y.; Negishi, M.; Pedersen, L.; Martin, J.L. Crystal structure of human catecholamine sulfotransferase. J. Mol. Biol. 1999, 293, 521–530. [Google Scholar] [CrossRef]
- Dajani, R.; Cleasby, A.; Neu, M.; Wonacott, A.J.; Jhoti, H.; Hood, A.M.; Modi, S.; Hersey, A.; Taskinen, J.; Cooke, R.M.; et al. X-ray crystal structure of human dopamine sulfotransferase, SULT1A3. Molecular modeling and quantitative structure-activity relationship analysis demonstrate a molecular basis for sulfotransferase substrate specificity. J. Biol. Chem. 1999, 274, 37862–37868. [Google Scholar] [CrossRef]
- Rana, D.; Alan, M.H.; Michael, W.H.C. A Single Amino Acid, Glu146, Governs the Substrate Specificity of a Human Dopamine Sulfotransferase, SULT1A3. Mol. Pharmacol. 1998, 54, 942. [Google Scholar] [CrossRef]
- Sun, Y.; Machalz, D.; Wolber, G.; Parr, M.K.; Bureik, M. Functional Expression of All Human Sulfotransferases in Fission Yeast, Assay Development, and Structural Models for Isoforms SULT4A1 and SULT6B1. Biomolecules 2020, 10, 1517. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Labute, P. Protonate3D: Assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins 2009, 75, 187–205. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Nachbar, R.B. Merck molecular force field. IV. conformational energies and geometries for MMFF94. J. Comput. Chem. 1996, 17, 587–615. [Google Scholar] [CrossRef]
- Wolber, G.; Langer, T. LigandScout: 3-D Pharmacophores Derived from Protein-Bound Ligands and Their Use as Virtual Screening Filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]


| MeOH | ACN | H2O | AmF | FA | |
|---|---|---|---|---|---|
| 1 | 50% | 50% | -- | 10 mM | -- |
| 2 | 50% | 50% | -- | 10 mM | 0.05% |
| 3 | 100% | -- | -- | 20 mM | -- |
| 4 | 100% | -- | -- | -- | 0.1% |
| 5 | 100% | -- | -- | 5 mM | 0.075% |
| 6 | 100% | -- | -- | 10 mM | 0.05% |
| 7 | 100% | -- | -- | 15 mM | 0.025% |
| 8 | 95% | -- | 5% | 20 mM | -- |
| 9 | 90% | -- | 10% | 20 mM | -- |
| 10 | 80% | -- | 20% | 20 mM | -- |
| 11 | 70% | -- | 30% | 20 mM | -- |
| Capillary voltage * | 500 V to 6000 V |
| Nozzle Voltage * | 0 V to 2000 V |
| High-pressure funnel voltage * | 10 V to 210 V |
| Low-pressure funnel voltage * | 10 V to 210 V |
| Drying gas temperature | 80 °C to 290 °C |
| Drying gas flow rate | 11 L/min to 20 L/min |
| Sheath gas temperature | 100 °C to 400 °C |
| Sheath gas flow rate | 8 L/min to 12 L/min |
| Nebulizer pressure | 5 psi to 60 psi |
| Compound | Precursor Ion [m/z] | Product Ion [m/z] | Collision Energy [eV] |
|---|---|---|---|
| (R)-/(S)-Salbutamol | [M + H]+ = 240.0 | 222.1 | 8 |
| 166.1 | 12 | ||
| 148.1 * | 16 | ||
| 121.1 | 25 | ||
| 91.0 | 48 | ||
| 77.1 | 56 | ||
| (R)-/(S)-Salbutamol-4′-O-sulfate | [M − H]– = 318.0 | 238.0 | 25 |
| 220.0 | 28 | ||
| 218.1 * | 32 | ||
| 190.0 | 44 | ||
| 97.0 | 25 | ||
| d9-Salbutamol | [M + H]+ = 249.0 | 231.1 | 8 |
| 166.1 | 12 | ||
| 148.1 | 16 | ||
| 121.1 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harps, L.C.; Jendretzki, A.L.; Wolf, C.A.; Girreser, U.; Wolber, G.; Parr, M.K. Development of an HPLC-MS/MS Method for Chiral Separation and Quantitation of (R)- and (S)-Salbutamol and Their Sulfoconjugated Metabolites in Urine to Investigate Stereoselective Sulfonation. Molecules 2023, 28, 7206. https://doi.org/10.3390/molecules28207206
Harps LC, Jendretzki AL, Wolf CA, Girreser U, Wolber G, Parr MK. Development of an HPLC-MS/MS Method for Chiral Separation and Quantitation of (R)- and (S)-Salbutamol and Their Sulfoconjugated Metabolites in Urine to Investigate Stereoselective Sulfonation. Molecules. 2023; 28(20):7206. https://doi.org/10.3390/molecules28207206
Chicago/Turabian StyleHarps, Lukas Corbinian, Annika Lisa Jendretzki, Clemens Alexander Wolf, Ulrich Girreser, Gerhard Wolber, and Maria Kristina Parr. 2023. "Development of an HPLC-MS/MS Method for Chiral Separation and Quantitation of (R)- and (S)-Salbutamol and Their Sulfoconjugated Metabolites in Urine to Investigate Stereoselective Sulfonation" Molecules 28, no. 20: 7206. https://doi.org/10.3390/molecules28207206
APA StyleHarps, L. C., Jendretzki, A. L., Wolf, C. A., Girreser, U., Wolber, G., & Parr, M. K. (2023). Development of an HPLC-MS/MS Method for Chiral Separation and Quantitation of (R)- and (S)-Salbutamol and Their Sulfoconjugated Metabolites in Urine to Investigate Stereoselective Sulfonation. Molecules, 28(20), 7206. https://doi.org/10.3390/molecules28207206







