1. Introduction
B-cell lymphomas are the most frequent hematologic malignancies. The pharmaceutical therapy of B-cell lymphomas has changed significantly since the development of targeted drugs such as Bruton tyrosine kinase (BTK) inhibitors [
1]. BTK signaling is crucial for B-cell proliferation, differentiation, and survival, and it is also essential for leukemic cell survival [
2]. Thus, BTK inhibition is a developed therapeutic pathway for the treatment of B-cell malignancies. On 21 November 2019, acalabrutinib (Calquence, AstraZeneca, Cambridge, UK) was approved by the FDA for adults with small lymphocytic lymphoma (SLL) or chronic lymphocytic leukemia (CLL). The regiment of Calquence is that it is administered orally twice a day [
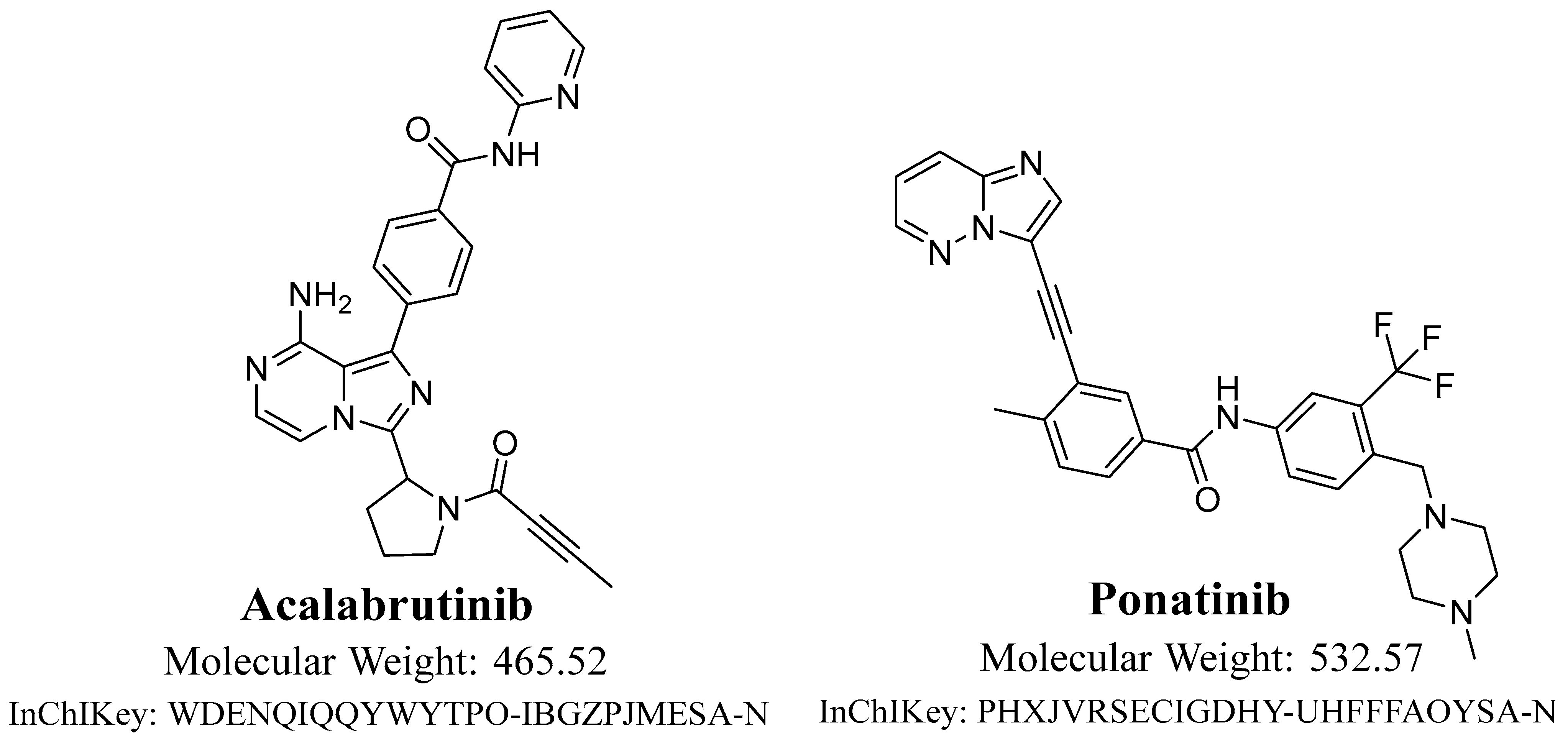
3]. Acalabrutinib (ACB;
Figure 1) is utilized in the treatment of numerous diseases, comprising mantle cell lymphoma and refractory/relapsed chronic lymphocytic leukemia (small lymphocytic lymphoma) [
4,
5,
6]. The development of ACB aimed to enhance the effectiveness and specificity of the first BTK inhibitors, like ibrutinib [
7]. ACB has a narrower spectrum of kinase inhibition compared to ibrutinib. It forms a covalent bond with the cystine 481 residue on the binding site of BTK [
8,
9].
Although ACB is considered a relatively safe drug in comparison with other anticancer agents, it is associated with numerous adverse effects. The most common and serious adverse effects caused by ACB include neutropenia (23%), serious infections (19%), major hemorrhage (3%), anemia (8%), cardiac arrhythmias (4%), thrombocytopenia (7%), and lymphopenia (7%) [
8]. Upon completing an extensive review of the available academic literature, it has been determined that there is a notable absence of published research specifically addressing the estimation of ACB in human liver microsomes (HLMs) in relation to the evaluation of ACB’s metabolic stability. Therefore, it is imperative to develop a methodology that utilizes ultraperformance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) with the dual objectives of achieving quick analysis and attaining a heightened level of sensitivity. The utilization of this methodology is crucial for accurately measuring the concentration of ACB in a wide range of matrices. The primary aim of the present study was to establish a rapid UPLC–MS/MS method that demonstrates high sensitivity (with a limit of detection as low as 1 ng/mL in a matrix of HLMs) and specificity for assessing the metabolic stability of ACB in a matrix of HLMs. Moreover, the results were validated using in vitro investigations, namely, metabolic incubations, along with in silico analysis employing StarDrop software (version 6.6) developed by Optibrium Ltd. (Cambridge, MA, USA). In contemporary times, there has been an increasing emphasis on the subject of green analytical chemistry (GAC), which aims to address the presence of harmful chemicals, decrease energy usage, and limit the production of waste by employing various analytical techniques [
10,
11]. The research integrates multiple approaches, specifically the Analytical Eco-Scale (AES), National Environmental Methods Index (NEMI), Red–Green–Blue (RGB), Green Analytical Procedures Index (GAPI), and Analytical Greenness Metric Approach (AGREE) [
10]. The AES, GAPI, NEMI, and RGB methodologies displayed a dependence on specific GAC values, as evidenced. The “AGREE” methodology was utilized to assess the degree of environmental sustainability through the evaluation of twelve Greenness Assessment Criteria (GAC) and the assignment of corresponding scores. The UPLC–MS/MS system showcased its environmental sustainability with the implementation of a decreased flow rate of 0.3 mL/min and an impressively short run time of 1 min. The linear relationship between the observed connection in this system was established by conducting experiments across a concentration range of 1 ng/mL to 3000 ng/mL.
In this present study, we investigated the in vitro metabolic stability of ACB utilizing a UPLC–MS/MS methodology. Additionally, we supplemented our findings with data obtained from the DEREK ((KB 2018 1.1) and P450 software (version 6.6) tools. The StarDrop software program, which incorporates the P450 metabolic module and DEREK software, was employed to assess the metabolic stability and identify potential hazardous alarms within the chemical structure of ACB. The assessment of in silico metabolic stability was conducted utilizing the StarDrop software package (version 6.6), with particular emphasis on the in silico P450 program. Metabolic lability refers to the quantification of the efficiency of the product generation phase within the catalytic cycle of CYP3A4. The presence of metabolic lability can be determined by assessing the composite site lability (CSL) value. A decreased CSL value indicates a higher probability of enhanced stability. The aforementioned value was employed as initial evidence to support the significance of conducting practical procedures, such as analytical method development and in vitro incubation with HLMs, in order to optimize resource allocation and time management [
12].
The Cl
int and in vitro t
1/2 of ACB were assessed in the present investigation using the UPLC–MS/MS approach [
13]. Three distinct models—dispersion, parallel tube, and venous equilibrium—were then used to estimate the in vivo metabolic rate using these parameters [
14,
15]. An in vitro method (the well-stirred model) was utilized to determine the in vitro t
1/2 and Cl
int of ACB [
14,
15]. Because of its intrinsic simplicity, this specific model is frequently used in studies pertaining to drug metabolism. According to earlier research [
16,
17,
18,
19], the ACB showed a moderate metabolism rate, which led to a favorable in vivo bioavailability and a moderate duration of action. The small extraction ratio of ACB was discovered to be in line with the frequently advised regular dosage of ACB (100 mg), which should be given twice daily to patients diagnosed with B-cell malignancies [
20]. The suggested theory for in silico metabolic stability, which matched the outcomes of the in vitro metabolic stability, was supported by screening for structural alarms inside the ACB chemical structure using DEREK software. The absorption, distribution, metabolism, and excretion (ADME) profile of ACB was ascertained by a computer study, which aimed to predict its drug-like characteristics.
3. Materials and Methods
3.1. Materials
Solvents of HPLC purity were used in the UPLC–MS/MS process. The investigation utilized analytical (AR)-grade reference powders, specifically acalabrutinib and ponatinib. Acalabrutinib (ACP-196; 99.88%), the target analyte, and ponatinib (AP24534; 99.43%), the internal standard (IS), were obtained from MedChem, a reliable supplier situated in Princeton, NJ, USA. Sigma-Aldrich, a reliable supplier with headquarters in St. Louis, Missouri, USA, provided the chemicals used in this investigation, which included acetonitrile (ACN), formic acid (HCOOH), ammonium formate (NH4COOH), and HLMs. Prior to being used, the HLMs (20 mg/mL) were kept in a refrigeration unit at a constant temperature of −78 °C. Using an internal Milli-Q water purification system from Millipore (Billerica, MA, USA), HPLC-grade water was produced.
3.2. UPLC–MS/MS Instrumental Features
The Acquity UPLC system, with a model code UPH and serial number of H10UPH, was employed to attain the chromatographic separation of ACB and IS peaks. The Acquity TQD MS instrument (with model code TQD and serial number QBB1203) was used to perform mass measurement of the target analyte peaks (ACB and IS) after extraction from the incubation HLMs matrix. The UPLC–MS/MS features were tuned to attain efficient separation and good sensitivity of the ACB and IS analytical peaks, as listed in
Table 5. The chromatographic properties of the HPLC system were attuned to attain the most favorable separation and sensitivity of the analytical peaks of ACB and IS. These modifications encompassed alterations in the mobile phase composition, pH conditions, and the stationary phase polarity, as outlined in
Table 5. The separation of ACB and IS was performed using a ZORBAX RRHD Eclipse Plus C18 column (95 angstroms, 2.1 × 50 mm, 1.8 micrometers) acquired from Agilent Technologies (Santa Clara, CA, USA) with the application of isocratic conditions. The mobile phase A was composed of a solution comprising 15% formic acid (HCOOH) with a concentration of 0.1% in water (H
2O) and a pH value of 3.2. The mobile phase B consisted of 85% ACN and was administered at a 0.3 mL/min flow rate. The examination of ACB indicated the occurrence of peak tailing and prolonged run time while analyzing a solution containing 10 mM of NH
4COOH with a pH above 3.2. When the proportion of ACN surpassed 85%, the ACB and IS analytical peaks were indistinguishable. Conversely, a decreased ratio caused a prolonged elution duration.
The mass spectrometry characteristics of the TQD MS were tuned to attain high sensitivity for the detection of ACB and IS analytical peaks. The ESI ionization source was employed in the positive ion mode to enable ion formation, as the chemical structure of ACB and IS contained basic nitrogen atoms capable of capturing protons and forming ions with positive charge. The droplet evaporation in the electrospray ionization (ESI) source was enabled by the use of nitrogen gas, which was formed by a nitrogen generator manufactured by Peak Scientific Company (Scotland, UK). The required vacuum inside the mass analyzer was established by utilizing a vacuum pump supplied by Sogevac, a company based in Murrysville, USA. The UPLC–MS/MS equipment was controlled utilizing the MassLynx package (Version 4.1, SCN 805; Milford, MA, USA) that was utilized as the managing program. The MassLynx software package has two significant software components, namely, IntelliStart
® and QuanLynx (Milford, MA, USA). The outputs that were created were analyzed and manipulated using QuanLynx software. The MS calibration of ACB (C
26H
23N
7O
2) and IS (C
29H
27F
3N
6O) was attained using IntelliStart
® software by employing the direct injection of the WKs of ACB and IS (10 µg/mL) into the mobile phase via combination feature. The mass analyzer detection function of the TQD was employed in multiple reaction monitoring (MRM) to estimate ACB and IS. This was carried out to enhance the selectivity and sensitivity of the UPLC–MS/MS technique that was developed. The analyte ions ACB and IS underwent fragmentation into their particular fragment ions in the collision cell (second quadrupole) of the TQD MS analyzer, utilizing collision gas (argon gas at 99.999% purity). The duration of the mass transition for ACB and IS (parent-to-fragment ions) was 0.025 s.
Table 6 shows the MRM features and mass transition properties of ACB and IS.
3.3. In Silico Study of ACB Metabolic Lability
The computational assessment of ACB’s metabolic stability was conducted using the P450 software (version 6.6) from Optibrium Ltd. (Cambridge, MA, USA) prior to performing the in vitro metabolic incubation experiment of ACB with HLMs. The significance of executing the in vitro metabolic incubations was confirmed by utilizing the results and data collected from the StarDrop™ software tool. The findings were further refined to determine the composite site lability (CSL), which serves as an indicator of ACB metabolic stability [
27]. The use of CSL was employed as a pivotal factor in evaluating the metabolic stability of ACB prior to conducting in vitro metabolic incubation, in order to ascertain the necessity of creating the proposed UPLC–MS/MS method for determining ACB metabolic stability. In order to identify ACB metabolic stability (CSL), the SMILES format of ACB (CC#CC(=O)N1CCC[C@H]1c2nc(c3n2ccnc3N)c4ccc(cc4)C(=O)Nc5ccccn5) was attached to the metabolic software (version 6.6). The metabolic lability at each atom was collected for calculating the CSL [
28,
29] using the following Equation (1):
where k
w is the rate constant for water formation.
The CSL is exhibited in the metabolic landscape and in the P450 column data set. It is an estimate of the ability of the product generation phase in the metabolic cycle of CYP3A4. Thus, a lower CSL value indicates a greater probability of better stability.
3.4. In Silico Expectation of the Toxicity of ACB using DEREK Software
The assessment of potential toxicity for ACB was conducted using the DEREK software. Additionally, the software was employed to identify structural warnings associated with ACB, with the aim of suggesting structural alterations that could mitigate the observed toxicity.
3.5. In Silico ADME Profile
The SwissADME software (version 1) developed by the Swiss Institute of Bioinformatics in Lausanne, Switzerland, was utilized to propose the Absorption, Distribution, Metabolism, and Excretion (ADME) features of ACB. The software under consideration can be accessed through the web platform located at (
http://www.swissadme.ch/ (accessed on 24 August 2023)).
3.6. ACB and IS Working Dilutions
At 250 mg/mL (ultrasonication required) and 50 mg/mL (ultrasonication required), respectively, ACB and IS showed their maximal solubility in DMSO. Thus, the stock solutions of IS and ACB, each at 1 mg/mL, were dissolved in DMSO. While the IS solution was created at 10 µg/mL, the ACB working solutions (WKs) were prepared at 1 µg/mL, 10 µg/mL, and 100 µg/mL. The stock solutions of ACB and IS, which were first made at 1 mg/mL, were diluted to create these solutions using the optimized mobile phase. Seven ACB calibration standards (CSs) and four quality controls (QCs) were produced using the WKs.
3.7. Establishing of ACB Calibration Standards
Before performing the validation steps for the established UPLC–MS/MS methodology, HLMs were made inactive by being exposed to a 2% concentration of DMSO. The deactivation step was conducted for 5 min at 50 °C. The objective of the deactivation phase was to reduce the potential influence of HLMs on the levels of ACB and IS by inhibiting their metabolic processes [
30,
31,
32]. So as to determine the ACB metabolic stability, a special matrix was developed for HLMs. The procedure consisted of the dilution of 30 µL of HLMs (deactivated) with a metabolic buffer. The metabolic buffer used was composed of 0.1 M of sodium phosphate at pH 7.4, along with 1 mM of NADPH (enzyme cofactor) and 3.3 mM of MgCl
2. The purpose of this dilution was to recreate the circumstances of the in vitro metabolic incubation of ACB and HLMs.
The ACB calibration standards (CSs) were made by diluting the ACB WKs in a sequential manner using the deactivated HLMs matrix. The aforementioned procedure led to the generation of seven distinct CSs at 1, 15, 100, 200, 500, 1500, and 3000 ng/mL. In addition, four QCs were generated at quantities of 1 ng/mL (representing the lower limit of quantification; LLOQ), 3 ng/mL (representing the lower QC; LQC), 900 ng/mL (representing the medium QC; MQC), and 2400 ng/mL (representing the higher QC; HQC). During the course of this experiment, the concentration of the HLMs matrix was consistently maintained at a level exceeding 90% in order to mitigate the potential effects of matrix dilution that may occur during the in vitro incubation period. QCs were utilized as representative samples with concentrations that were not known. The concentrations of these QCs were estimated by employing the regression equation which was generated from the concurrent injection of ACB CSs. A 100 µL volume of IS WK solution, at 10,000 ng/mL, was added into 1 mL of all ACB QCs and CSs as an IS.
3.8. Extraction of ACB and IS from the Incubation HLMs Matrix
ACB and IS were efficaciously separated from the incubation HLMs matrix via the employing of the protein precipitation methodology that involved the application of ACN as a precipitating (proteins) and quenching (metabolic reaction) agent for the HLMs matrix. Consequently, a 2 mL ACN was added to the ACB QCs and CSs. The samples underwent continuous shaking for 5 min to enable the extraction of ACB and IS from the precipitated proteins. Following this, centrifugation was conducted for 12 min using a temperature-regulated centrifuge operating at 4 °C at a rotational speed of 14,000 rpm. The purpose of this centrifugation stage was to facilitate the separation of proteins and achieve clarification of the supernatants. To guarantee the suitability and reliability of the samples for input into the UPLC–MS/MS apparatus, a filtration procedure was performed on all incubates using a syringe filter (0.22 µm pore size). The filtered samples were subsequently transferred into special vials with the aim of injecting them into the UPLC–MS/MS apparatus. Negative control and positive control samples were created utilizing the previously mentioned approach in order to validate that the HLMs matrix constituents do not cause any interference at the elution time of ACB and IS. The experimental sample was designated as the positive control composed of IS within the HLMs matrix. The construction of a calibration curve for ACB involved charting the nominal values of ACB on the x-axis and the peak area ratio of ACB to IS on the y-axis. The determination of the linearity range of the established ACB CSs was performed by assessing the different validation features and the linear regression equation (y = ax + b; r2) of the developed UPLC–MS/MS methodology.
3.9. Validation of the Current UPLC–MS/MS Method
The validation of the UPLC–MS/MS technique involved the assessment of its linearity, precision, accuracy, sensitivity, extraction recovery, specificity, stability, and matrix effect. This validation process adhered to the analytical method validation phases as specified in the guidelines provided by the FDA [
33,
34].
3.9.1. Specificity
The specificity of the present UPLC–MS/MS technology was evaluated by injecting six groups of blank HLM samples following the extraction technique. The purified extracts were loaded into the UPLC–MS/MS apparatus and subjected to analysis with the aim of ascertaining whether any interference was observed with the analytical peaks created by the matrix at the identical retention time as the ACB or IS peaks. Subsequently, the obtained data were juxtaposed with spiked HLM matrix samples that encompassed the desired analytes, namely, ACB and IS. The MRM mode was utilized to address the carryover impact of the targets, ACB and IS, in the MS/MS analyzer. The confirmation of this was achieved through the analysis of the negative control sample HLMs, that were lacking ACB and IS, and subsequent observation of the resultant consequences.
3.9.2. Sensitivity and Linearity
The assessment of linearity and sensitivity of the present UPLC–MS/MS approach involved the generation of 12 calibration curves, utilizing 7 calibration standards, for the analysis of ACB in the HLMs matrix. This assessment was conducted within a single day. Subsequently, the unidentified samples were back-calculated by employing the regression equation derived from the calibration curves. The computing of the LOD and LOQ involved the usage of the slope and the intercept SD of the constructed calibration curve in the HLMs matrix, as outlined in Equations (2) and (3), correspondingly [
24].
The estimation of UPLC–MS/MS method linearity was performed by calculating the coefficient of variation (R2) and employing the least-squared method (y = ax + b).
3.9.3. Precision and Accuracy
The assessment of accuracy and precision in the established UPLC–MS/MS technique involved the execution of multiple experiments spanning several consecutive days. In order to examine the precision and accuracy of the ACB QCs, a total of six sets were injected over the course of three consecutive days for inter-day evaluation. Additionally, 12 sets were performed in a single day to measure intra-day accuracy and precision. The assessment of the UPLC–MS/MS method’s accuracy and precision was performed by quantifying them in terms of percentage error (%E) and percentage relative SD (RSD), respectively. The estimated data were obtained through employing Equations (4) and (5), correspondingly.
3.9.4. Matrix Effect and Extraction Recovery
The evaluation of the influence of the HLMs matrix on the ionization of ACB and IS was conducted by segregating the samples into two separate cohorts. Group 1′s HLMs matrix was enhanced with the addition of the ACB LQC at 3 ng/mL and IS at 1000 ng/mL. Alternatively, group 2 was created by utilizing the mobile phase in place of the metabolic HLMs matrix. The determination of the normalized matrix effect (ME) of the internal standard (IS) was conducted utilizing Equation (6), whereas the ME for ACB and IS was obtained utilizing Equation (7).
The assessment of the efficiency of the ACB extraction method from the HLMs matrix and the influence of HLMs on the extent of ACB ionization were carried out by administering four quality control samples through injection. The efficacy of protein precipitation as the chosen extraction technique for ACB and IS was validated through the execution of six sets of four quality control samples in the HLMs (B), followed by a comparison with four quality control samples obtained in the mobile phase (A). The quantification of the extraction recoveries of ACB and IS was achieved by computing the ratio of B to A, multiplied by 100.
3.9.5. Stability
The running of the current study aimed to assess the stability of ACB in the HLMs matrix by evaluating its concentration at two different quality control levels (LQC and HQC). This analysis was conducted to validate the robustness and dependability of the UPLC–MS/MS methodology under various storage conditions. The estimation was conducted through five repetitions under various laboratory conditions, including storage for both short and long durations, storage in the autosampler, and subjecting the samples to three cycles of freezing and thawing. To assess the freeze–thaw stability, the LQC and HQC samples underwent a series of three freeze–thaw cycles. Every cycle involved subjecting the samples to freezing conditions at −80 °C in a refrigerator, followed by thawing at room temperature prior to further processing. In the context of short-term stability experiments, the LQC and HQC samples underwent processing and analysis subsequent to an 8 h period of storage at ambient temperature on the laboratory benchtop. The assessment of long-term stability involved the placement of the spiked HLMs matrix in a refrigerator set at −80 °C for three months before conducting the analysis. The stability of the prepared samples in the autosampler was determined by subjecting the prepared sample to a storage period of 24 h at 10 °C, followed by loading into the UPLC–MS/MS apparatus.
3.10. In Vitro Estimation of the Metabolic Stability of ACB
The determination of Cl
int and the in vitro t
1/2 of ACB was conducted by assessing the remaining ratio of ACB after in vitro metabolism. The accomplishment was realized through the use of an active HLMs incubation matrix comprising NADPH (enzyme cofactor) and MgCl
2. The experimental protocol for in vitro metabolic incubation consisted of four consecutive steps. During the initial phase of the experiment, a 1 µL volume of ACB was subjected to preincubation with metabolic HLMs matrix. The aforementioned procedure was conducted at a temperature of 37 °C for a duration of 10 min, employing a water bath equipped with a temperature control system. During the initiation stage, a concentration of 1 mM of NADPH was introduced to each individual sample. Subsequently, the samples were transferred to a thermostatic shaking water bath, where they were kept at a constant temperature of 37 °C. During the third step of the experiment, a solution of IS with a volume of 100 µL and a concentration of 1000 ng/mL was introduced into the system prior to the addition of acetonitrile (ACN), which served as the solvent for terminating the reaction. The use of this procedure aimed to achieve a consistent concentration of the IS and minimize the potential impact of metabolic reactions on the IS concentration. In the fourth step, known as termination, a volume of 2 mL of ACN was introduced into all samples at predetermined time intervals. The intervals observed in this study were as follows: 0, 2.5, 7.5, 15, 20, 30, 40, 50, 60, and 70 min. The purpose of this procedure was to inhibit the metabolic reaction and cause the excess proteins to form a solid substance. This step is the first in the extraction process of ACB and IS, as stated in
Section 3.6. A negative control was performed by incubating ACB with HLMs and no NADPH, following the protocol previously stated. This was carried out to assess the impact of incubation conditions or matrix components on the concentration of ACB during the in vitro incubation tests.
The equation of the regression line obtained from the concurrent injection of ACB control samples was employed to calculate the residual concentration of ACB. The inclusion of specific time intermissions (ranging from 0 to 70 min) on the
x-axis constituted a crucial component in the formulation of the ACB stability curve. The
y-axis of the graph represented the relative concentration of ACB surviving compared to its initial concentration at time zero, which was set as 100%. Subsequently, the segment of the preceding metabolic curve spanning from 0 to 40 min was selected to generate a natural logarithmic curve. A graphical representation was generated to depict the relationship between the natural logarithm (ln) of ACB concentrations and the metabolic incubation time points ranging from 0 to 40 min. By examining the slope of the aforementioned curve, it was possible to approximate the rate constant associated with the metabolic stability of ACB. The in vitro half-life (t
1/2) was determined using the formula ln2/slope, where the slope obtained from the data was employed for the calculation of the in vitro t
1/2. The liver tissue mass (26 g) per kg of body weight and the HLMs (45 mg) per gram of liver tissue were incorporated into the calculation of the ACB Cl
int (mL/min/kg) [
35], as indicated in Equation (8) [
25].
4. Conclusions
The current investigation focused on the development and verification of a UPLC–MS/MS technique for measuring ACB concentration in the metabolic HLMs matrix. The aforementioned technique was later utilized to estimate the metabolic stability of ACB. The utilization of UPLC–MS/MS technology exhibited advantageous characteristics such as high sensitivity, specificity, eco-friendliness, and effective retrieval of ACB and IS from the HLMs matrix. Protein precipitation was utilized as the preferable extraction procedure. The use of a low flow rate of 0.3 mL/min, along with a decreased amount of ACN and a significantly shorter elution time of 1 min, has rendered the current UPLC–MS/MS methodology environmentally sustainable. The results derived from the metabolic P450 software were verified by comparing them to the results obtained from in vitro HLMs incubation assays. The findings from the investigation on metabolic stability reveal that ACB possesses a half-life (t1/2) of 20.45 min and a moderate clearance (Clint) of 39.65 mL/min/kg. These results suggest that ACB can be categorized as a drug exhibiting a moderate extraction ratio. Consequently, it is assumed that the administration of ACB to patients may not result in the accumulation of doses within the body. Based on the results obtained from computational P450 metabolic and DEREK software analyses, it is recommended that introducing minor structural alterations to the alpha, beta-unsaturated amide moiety or replacing the group during the drug design process could potentially enhance the metabolic stability and safety profile of unique derivatives, in comparison to ACB. Potential future study could be conducted using the current methodology, which involves the use of in vitro metabolic incubations and in silico software tools. These strategies play a crucial role in the progress of innovative pharmaceutical development, specifically in terms of augmenting metabolic stability. The findings derived from the analysis of ACB through in silico software and in vitro incubation studies have proved the effectiveness of utilizing various in silico metabolic software techniques in conserving supplies and reducing labor.