Abstract
Developing rapid and efficient analytical methods is of great importance for food safety Herein, we present a novel homogeneous electrochemical aptasensor for ultrasensitive quantitative determination of zearalenone (ZEN) based on a nanocomposite probe and silica nanochannel film. X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy and UV–Vis characterization techniques confirm that graphene oxide (GO) bears an aromatic conjugated structure, along with hydroxyl and carboxyl groups, facilitating the subsequent adsorption of cationic redox hexa-ammine-ruthenium (III) (Ru(NH3)63+) and anionic ZEN aptamer, to form a Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe in a homogeneous solution. Vertically-ordered mesoporous silica films (VMSF) bearing silanol groups can be simply grown on the solid indium tin oxide (ITO) electrode surface and enable the selective preconcentration of Ru(NH3)63+, eventually leading to signal amplification. Since the detachment of Ru(NH3)63+ from the GO surface by the recognized ZEN aptamer in the presence of ZEN, more free Ru(NH3)63+ is released in solution and produces enhanced redox signals at the VMSF modified ITO electrode, allowing quantitative detection of ZEN. On the basis of the above sensing strategy, the proposed homogeneity, due to the assistance of graphene, as well as of the signal amplification and anti-fouling effects of VMSF, accurate analysis of ZEN can be realized in maize and Chinese chestnut samples.
1. Introduction
Mycotoxins produced by fungal species are toxic and often contaminate nearly 25% of the world’s agricultural crops (e.g., wheat, corn and rice), leading to an annual loss of ~5 billion dollars [1]. Zearalenone (ZEN) is one of the most frequent mycotoxins and has severe effects on human health, including teratogenicity, abortion, liver toxicity, blood toxicity, and carcinogenicity [2]. ZEN, with high water insolubility and thermal stability (at 120 °C for 4 h, the toxicity cannot decrease), is difficult to be removed by conventional processing methods, which aggravate its contamination [3]. The International Agency for Research on Cancer has classified ZEN as a class III carcinogen [4]. In addition, the European Union sets the maximum residue levels of ZEN in human food at 75 μg/kg [5]. Due to this low permissible limit and harmfulness, rapid and efficient analytical methods for ZEN determination should be developed for food safety. To date, a great number of techniques have been utilized to detect ZEN, such as lateral flow immunoassays [6], liquid chromatography−tandem mass spectrometry [7], high performance liquid chromatography [8], enzyme-linked immunosorbent assay [9] and spectrometry [10]. These methods possess good accuracy but need complex processing steps, expensive instruments and technical staff. Electrochemical biosensors have received wide attention due to the benefits of convenience, easy miniaturization, good sensitivity, and economy, which have been employed to sensitively determine ZEN [11,12].
Electrochemical biosensors have received growing attraction due to their high specificity and sensitivity, rapidity, and ease of miniaturization and portability [13,14,15,16]. According to the category of biological recognition elements, electrochemical biosensors mainly fall into three kinds: electrochemical immunosensors, enzymatic biosensors and aptamer-based biosensors [17,18,19,20]. By contrast, aptamer-based biosensors are predominant and have been demonstrated to be useful for analytical applications due to their high specificity, chemical and thermal stability, inexpensiveness and successful applicability for diagnosis [21,22,23,24]. In comparison with most reported electrochemical aptasensors using the immobilized aptamer on the electrode surface, homogeneous electrochemical aptasensors are time-saving, free of immobilization steps and suitable for the formation of aptamer-target conjugations [25]. In this situation, all biochemical reactions occur in a homogeneous solution and an electrochemical signal of an indicator for quantification could be detected by the electrode. To improve the analytical sensitivity, nanostructured structures including graphene, metal nanoparticles and nano-porous materials with excellent perma-selectivity, have been applied to promote the electron transfer or the diffusion of indicator to the electrode surface [26,27,28]. Moreover, traditional homogeneous biosensors often require sandwich immunoassays to capture target molecules and probe-labeled antibodies, followed by magnetic separation using immunomagnetic beads before detection. This process involves multiple steps of incubation and washing. Therefore, integration of the amplification effect of nanomaterials with the high affinity of aptamers for the development of novel electrochemical aptasensors with sensitivity and simplicity is expected to bring about substantial advantages for ZEN determination, accompanied by ultra-trace analyses.
Porous materials possess a high specific surface area, adjustable structure and pore size, and excellent mass transfer ability, making them ideal matrices for integrating functional nanomaterials and developing high-performance sensing systems [29,30,31]. Recently, vertically-ordered mesoporous silica films (termed VMSF) with numerous tiny channels and tailored surface charges offer a confined space for gated or selective molecular transport, and have been widely employed for the development of novel electroanalytical sensors based on molecular size, charge, hydrogen bond and lipophilicity [32,33,34,35]. Thanks to the great number of silanol groups existing on the silica channel walls, VMSF shows a pronounced electrostatic perma-selectivity towards cationic species within ultrasmall channels at an appropriate pH, yielding significantly enhanced signals [36,37]. With this enhancement effect, excellent analytical performances in terms of increased sensitivity and decreased detection limit, as well as reduction in the amounts used of expensive signal molecules (e.g., tris(2,20-bipyridyl)ruthenium(II)) [38,39], can be achieved. Herein, we demonstrate a universal and simple homogeneous electrochemical aptasensor with inherent separation capability for sensitive quantitative analysis of ZEN, with the assistance of graphene oxide (GO) and VMSF. Two-dimensional GO nanosheets in a homogeneous solution primarily adsorb cationic Ru(NH3)63+ and anionic ZEN aptamer to form a Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe through electrostatic and π-π interactions, which can function as the specifically recognitive and signal element. VMSF with a negatively charged surface grown on the indium tin oxide (ITO) surface (termed the VMSF/ITO electrode) is capable of accelerating mass transport of the free hexa-ammine-ruthenium (III) (Ru(NH3)63+) electrochemical probe, with positive charge in solution, to the underlying ITO surface, generating amplified redox currents and greatly increasing sensitivity. Target ZEN could bind with ZEN aptamer and pull out Ru(NH3)63+ from the Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe, resulting in enhanced redox currents at the VMSF/ITO electrode and enabling the quantitative analysis of ZEN. Compared to traditional homogeneous electrochemical aptasensors, our proposed sensing strategy based on the large Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe can effectively reduce background signals through size exclusion and thereby eliminate the need for probe separation during detection. Furthermore, accurate quantification of ZEN in food samples (maize and Chinese chestnut) is realized by our simple homogeneous electrochemical strategy.
2. Results and Discussion
2.1. Principle of the Designed Electrochemical Aptasensor for ZEN Determination
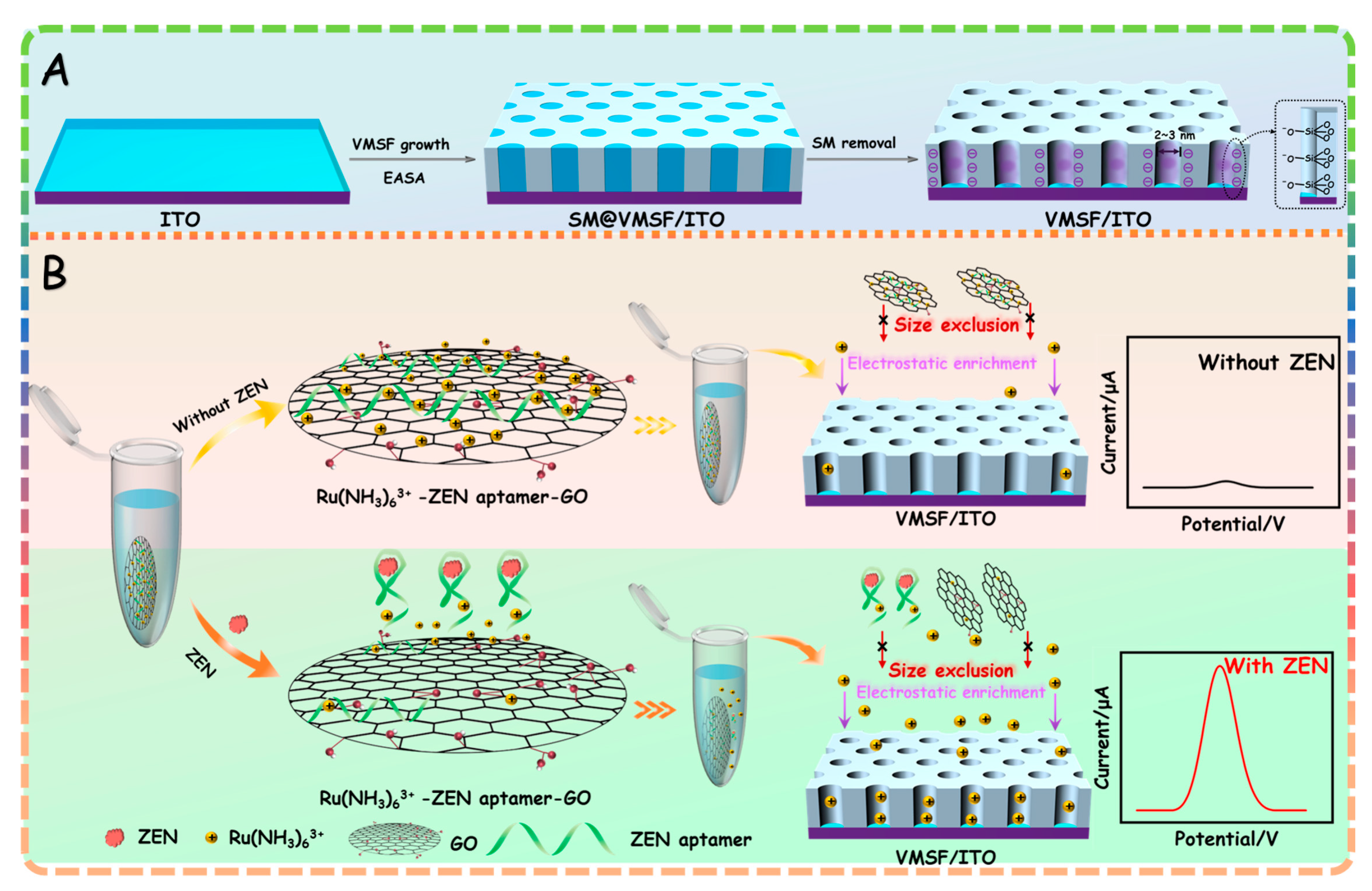
Growth of VMSF with an open channel on the ITO surface (termed VMSF/ITO) is performed by the previously reported electrochemically assisted self-assembly (EASA) approach and further exclusion of templated surfactant micelles (SM) from the silica channels (Scheme 1A). VMSF bearing silanol groups could provide numerous sites for the cationic probe in the buffer solution via the electrostatic effect, finally resulting in amplified signals and greatly improving the detection performance. Scheme 1B presents the principle of the proposed homogeneous electrochemical aptasensor by coupling the Ru(NH3)63+–ZEN aptamer–graphene oxide (GO)–nanocomposite probe and signal amplification of VMSF for Ru(NH3)63+. As shown, the Ru(NH3)63+ (signal probe) and ZEN aptamer (recognitive element) are first adsorbed onto the GO nanosheets through electrostatic and π–π interactions, in order to form the Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe. In the absence of ZEN, ZEN aptamer is adsorbed on the GO surface in the random coil state, leading to lesser amount of Ru(NH3)63+ in solution and generating the relatively low redox signals at the VMSF/ITO electrode. In the presence of ZEN, ZEN aptamer is able to specifically bind with ZEN, which could be pulled out from the GO surface due to the high affinity. In this case, Ru(NH3)63+ adsorbed on the GO surface is released to the homogeneous solution and displays an enhanced redox signal at the VMSF/ITO electrode. Such redox current variation of Ru(NH3)63+ generated with the help of GO could be accurately measured by VMSF/ITO electrode, allowing the quantitative determination of ZEN. This newly developed homogeneous electrochemical aptasensor is simple, without immobilization and labeling operations, offering a universal platform for the detection of various analytes by tailoring the suitable aptamer.
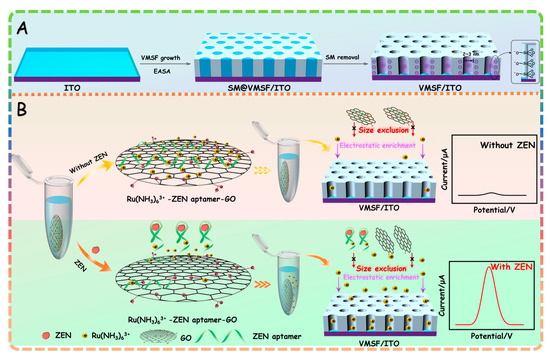
Scheme 1.
(A) Scheme of VMSF/ITO electrode prepared by using EASA method. (B) Schematic illustration for the homogeneous electrochemical aptasensor for the determination of ZEN.
2.2. Characterizations of GO and VMSF/ITO Electrode
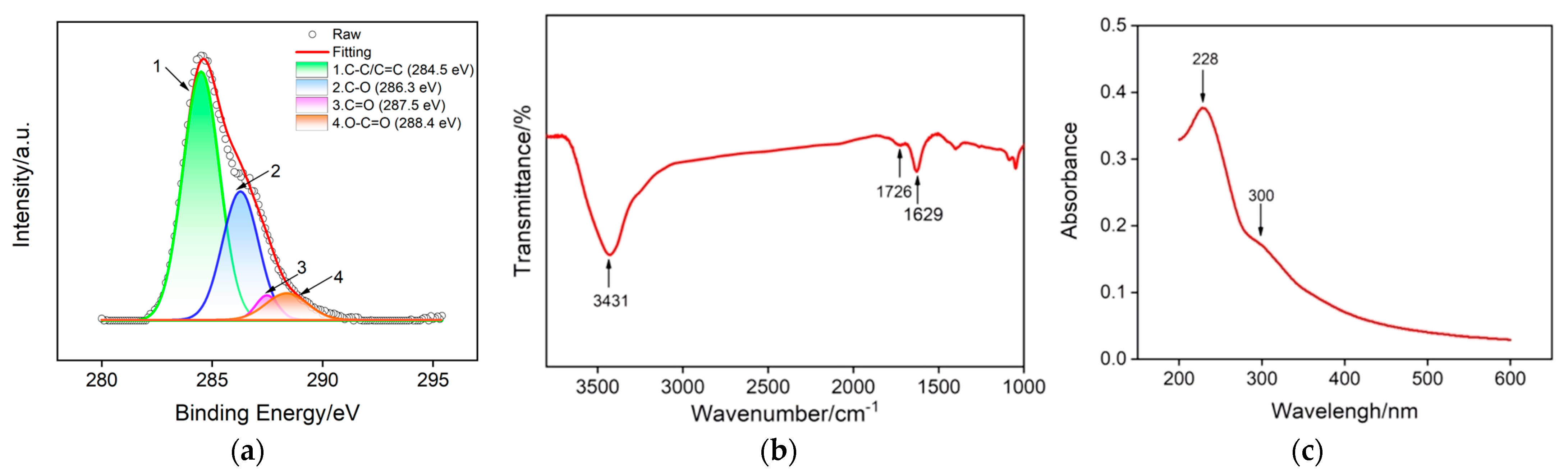
Graphene based nanomaterials with different dimensional structures (e.g., 0D quantum dots [40,41,42,43], 2D sheets [44], and 3D porous foam [45,46,47]) have been widely used in the construction of various novel chemo-/biosensors due to their unique structure and exceptional physico-chemical properties [48,49,50]. Among them, GO nanosheets have a high specific surface area and oxygen-containing groups on their basal plane and edges, which offer plentiful effective binding sites for the electrostatic adsorption of cationic species. Figure 1 shows the high-resolution carbon 1 s X-ray ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR) and UV–Vis spectra of GO. As presented in Figure 1a, four obvious characteristic peaks could be found in the carbon 1 s XPS profile of GO. The peak centered at 284.5 eV is attributed to the C-C/C=C of the aromatic rings, and the other three peaks located at 286.3 eV, 287.5 eV and 288.4 eV are assigned to the C-O, C=O and O-C=O, respectively, confirming the existence of oxygen-containing groups in GO nanosheets. Three absorption bands for hydroxyl group peak (3431 cm−1), carbonyl group peak (1629 cm−1) and carboxyl group (1726 cm−1) are found in the FTIR spectra of GO (Figure 1b), further proving the presence of oxygen-containing groups in GO. The UV–Vis spectrum of GO displayed in Figure 1c contains an adsorption peak at 228 nm corresponding to the π→π* transitions of the aromatic C-C bond and a shoulder peak at 300 nm corresponding to the n→π* transition of the C=O bond. All the above results are similar to previous reports and suggest the successful preparation of GO. Moreover, GO bearing an aromatic conjugated structure, hydroxyl and carboxyl groups facilitates the adsorption of cationic species with or without a π-conjugated structure.
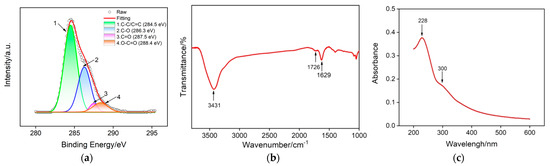
Figure 1.
Structure characterization of GO nanosheets: (a) high-resolution C 1 s XPS, (b) FTIR and (c) UV–Vis absorption spectra.
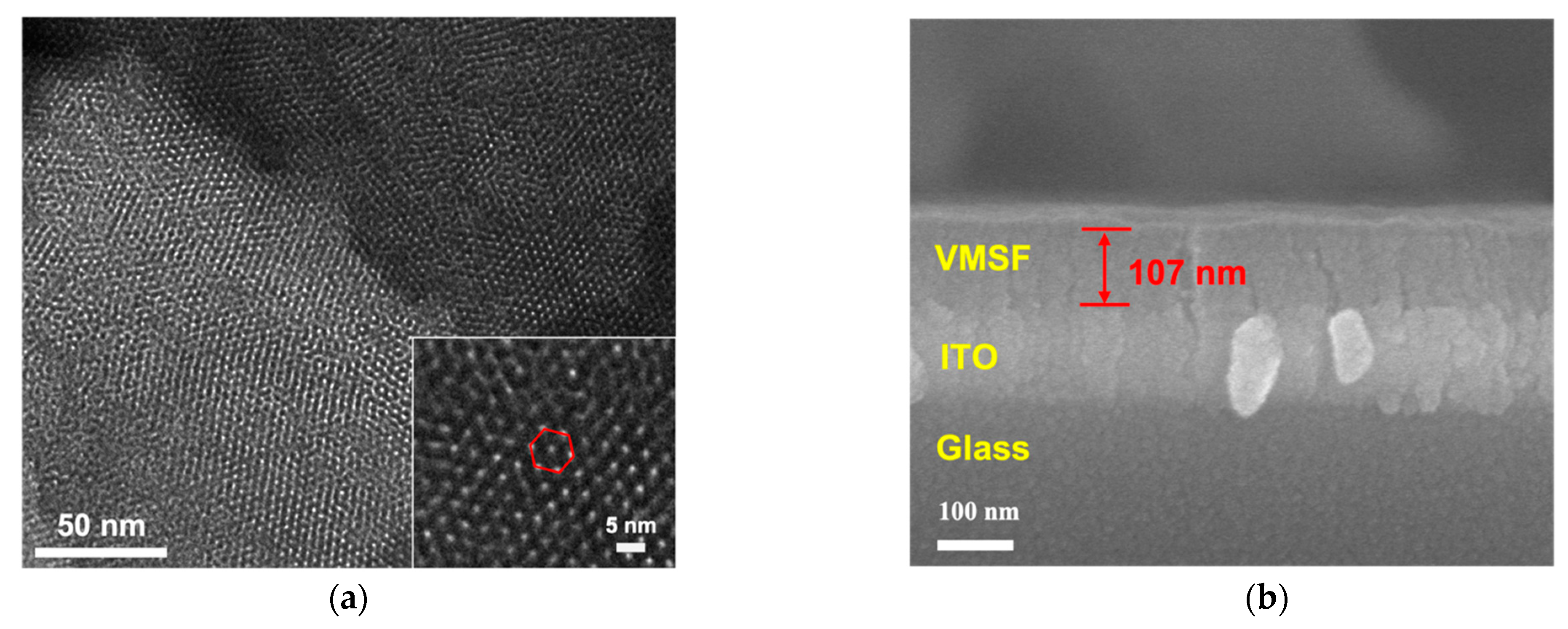
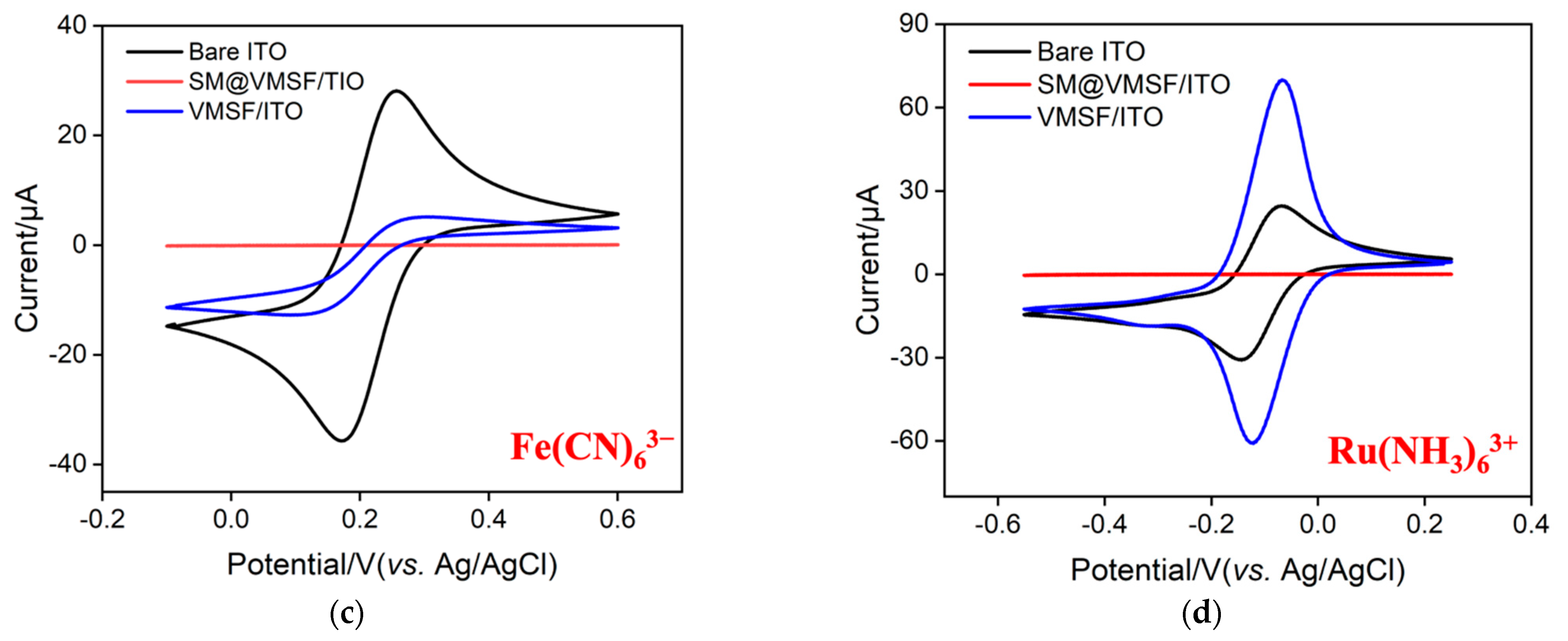
The morphology of VMSF grown on the ITO surface was characterized by electron microscope characterization. As seen in Figure 2a, plentiful and hexagonally aligned nanopores appear as bright spots and the as-prepared silica nanoporous film is intact without cracks over a large area. The diameter and thickness of VMSF are 2~3 nm and 107 nm, respectively (Figure 2a,b). The VMSF layer on the ITO coated glass surface is very homogeneous. The intactness and electrostatic perma-selectivity of VMSF were examined by electrochemical method and the results can be found in Figure 2c,d. Well-defined reversible redox peaks are shown at the bare ITO electrode, which are assigned to the electrochemical reaction of the Fe(CN)63−/Fe(CN)64− couple (Figure 2c) and Ru(NH3)63+/Ru(NH3)64+ couple (Figure 2d). As demonstrated, when the binary nanocomposite consisting of surfactant micelles (SM) and silica nanochannels was decorated on the ITO electrode surface, both anionic Fe(CN)63− and cationic Ru(NH3)63+ could not pass through the hydrophobic microenvironment formed by SM, resulting in the capacitive current at the SM@VMSF/ITO electrode and further suggesting the integrity of VMSF on the ITO electrode surface. After exclusion of SM from the silica nanochannels, enhanced redox currents for Ru(NH3)63+ and declined redox currents for Fe(CN)63− are shown at the VMSF/ITO electrode compared with that obtained at the bare ITO, which results from the electrostatic interaction between negatively charged silica walls and charged probes in the ultrasmall space. Note that VMSF is rich in silanol groups and can deprotonate to display negative charges. The above CV results imply that VMSF with open channels has excellent charge perma-selectivity and indeed shows the basis for the following homogeneous electrochemical aptasensor.
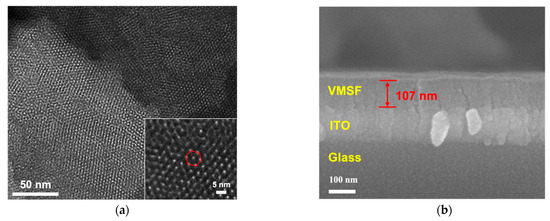
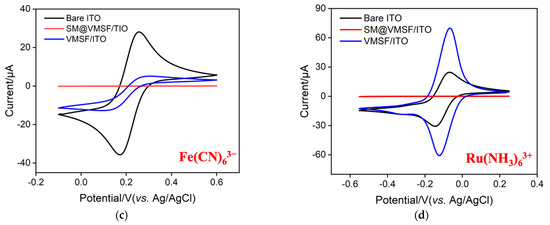
Figure 2.
(a) Top-view TEM micrographs of VMSF/ITO with various magnified views. The inset shows the hexagonally aligned nanopores. (b) SEM micrograph of VMSF/ITO displaying the cross-sectional topography. Cyclic voltammetry (CV) for the bare ITO, and the ITO decorated with SM@VMSF and VMSF recorded in the presence of 0.5 mM (c) Fe(CN)63− and (d) Ru(NH3)63+. Supporting electrolyte: 0.05 M KHP (pH = 7.4) solution.
2.3. Feasibility of the Homogeneous Electrochemical Aptasensor
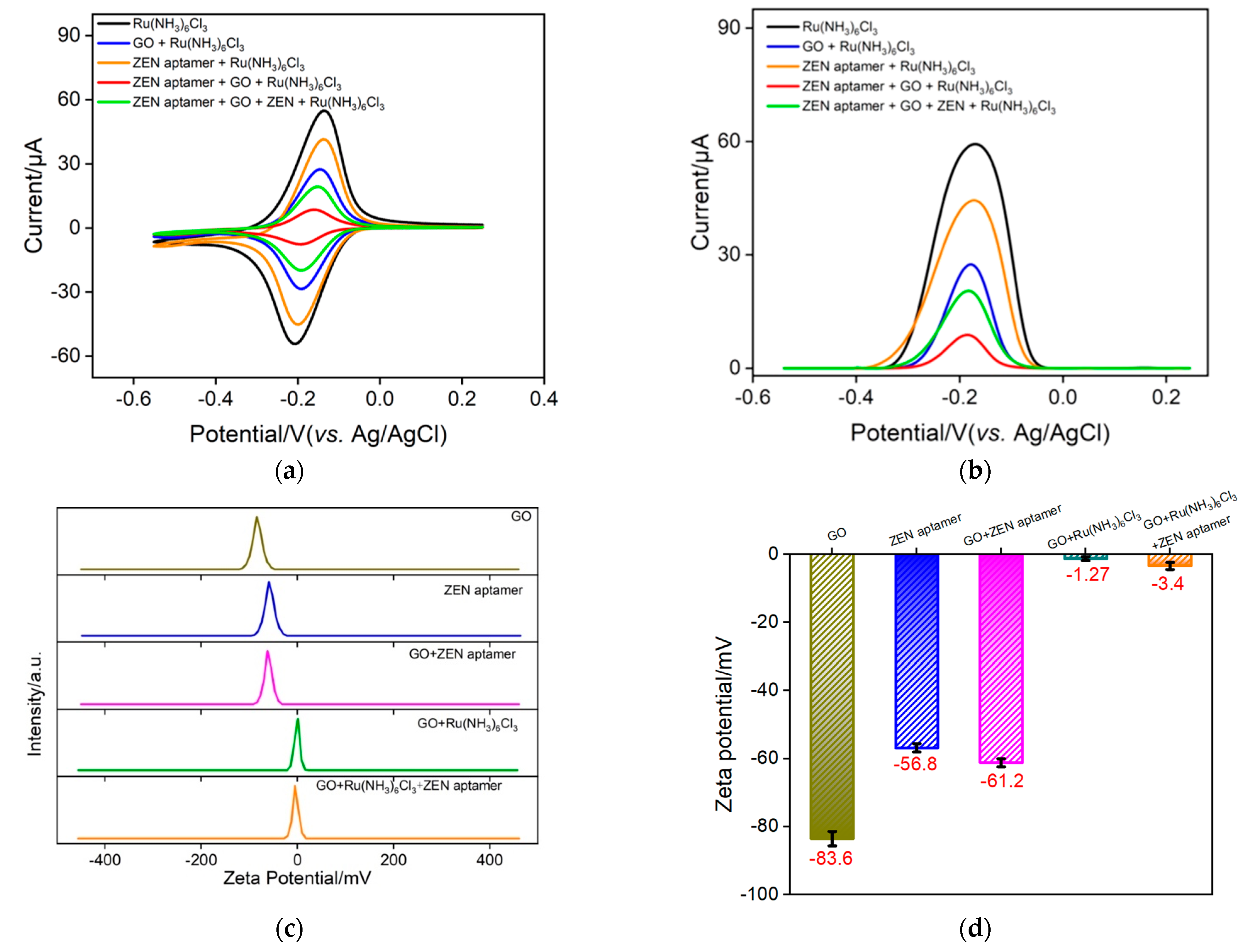
As illustrated in Scheme 1B, the Ru(NH3)63+–ZEN aptamer–GO nanocomposite electrochemical probe in a homogeneous solution could be employed to specifically recognize ZEN, leading to free Ru(NH3)63+ and further generating the electrochemical signal. Such redox current variation of Ru(NH3)63+ is able to be determined by VMSF/ITO owing to the electrostatic amplification effect. To verify this sensing strategy, an electrochemical method, including CV and the corresponding DPV, was first chosen. As revealed in Figure 3a, a pair of well-defined redox peaks due to the electrochemical reactions of Ru(NH3)63+ is observed at the VMSF/ITO electrode. When GO, ZEN aptamer, or their mixture is added to the solution, the redox signals of Ru(NH3)63+ dramatically decrease, which results from the electrostatic or π–π interactions between cationic Ru(NH3)63+ and negatively charged GO/ZEN aptamer, and thereby a smaller amount of free Ru(NH3)63+ in solution. When target ZEN is present in the solution, Ru(NH3)63+ could be released from the Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe and finally gives rise to the enhanced redox signals. The corresponding DPV curves are recorded in Figure 3b. In addition, zeta potential is used to confirm the successful formation of the Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe. As revealed in Figure 3c,d, zeta potentials of GO and ZEN aptamer in water are about −83.6 mV and −56.8 mV, exhibiting strong electronegativity. A mixture of GO and ZEN aptamer leads to −61.2 mV, which is comparable to that of ZEN aptamer and indicates the adsorption of ZEN aptamer through the π–π effect. In comparison with GO, ZEN aptamer and their mixture, the presence of cationic Ru(NH3)63+ could bring about an obvious enhancement in zeta potential, indicating the strong electrostatic adsorption of Ru(NH3)63+ on the GO nanosheet surface.
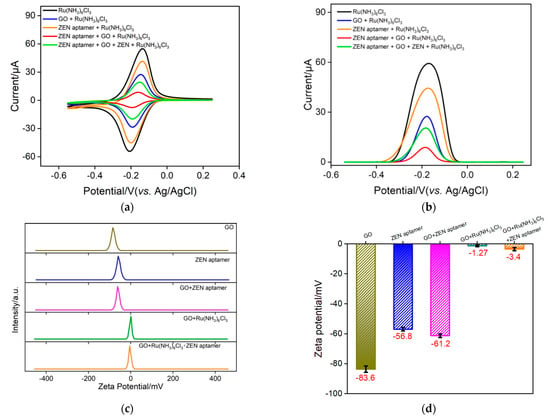
Figure 3.
CV (a) and DPV (b) responses of VMSF/ITO to free Ru(NH3)63+ in different detected solution. The concentrations of Ru(NH3)6Cl3, ZEN aptamer, GO and ZEN are 100 μM, 1 μΜ, 0.1 mg/mL and 1 ng/mL, respectively. (c,d) Zeta potential of GO, ZEN aptamer, GO + ZEN aptamer, GO + Ru(NH3)6Cl3 and GO + Ru(NH3)6Cl3 + ZEN aptamer in deionized water. Error bars denote the standard deviations of three measurements.
2.4. Detection of ZEN
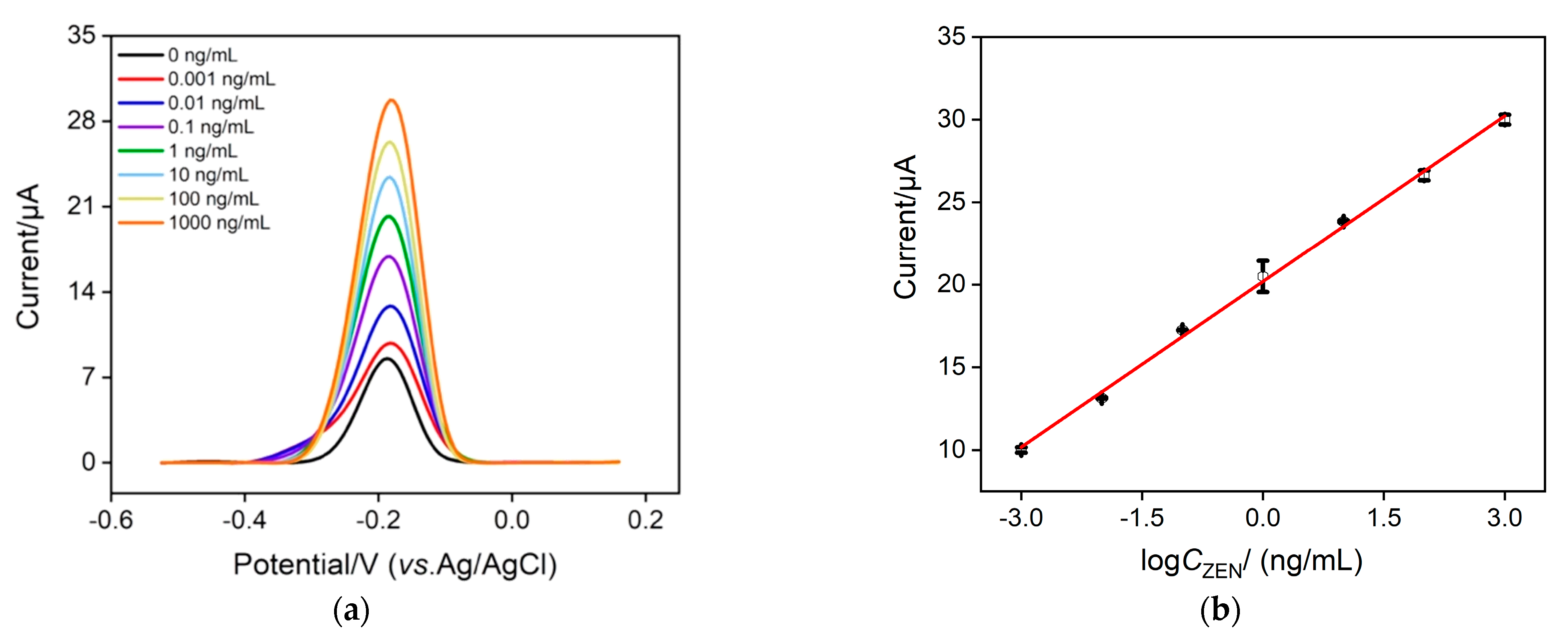
As Ru(NH3)63+ is chosen as the signal indicator, we investigate the optimal concentration of Ru(NH3)63+ to achieve the best analytical performance. Figure S1 records the anodic peak currents (left) and change of anodic peak current (ΔI = I − I0) (right) of the Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe with (I) and without (I0) 10 ng/mL ZEN at various concentrations of Ru(NH3)63+. As displayed, ΔI increases with the increasing concentration of Ru(NH3)63+ and reaches optimal value at a concentration of 100 μΜ, which is thereby selected as the best condition in this study. Under optimal experimental conditions, DPV curves at various concentrations of ZEN were recorded using our proposed homogeneous electrochemical aptasensor. As depicted in Figure 4a, when the ZEN concentration changes from 1 pg/mL to 1 μg/mL, the anodic current obtained at the VMSF/ITO electrode gradually enhances with increasing ZEN concentration. The magnitude of the anodic current is proportional to the logarithmic concentration of ZEN (Figure 4b), generating a well-fitting linear equation of I (μA) = 3.34 logCZEN + 20.2 (R2 = 0.998) and a low limit of detection (LOD) of 1.2 fg/mL (Signal/Noise = 3). By comparing the sensing performance of our proposed homogeneous strategy with other sensing approaches, a better linear dynamic range and a lower LOD are shown in Table 1. Moreover, most sensing methods reported previously involve complex labeling steps, which could be efficiently simplified by our proposed label-free homogeneous electrochemical sensor.
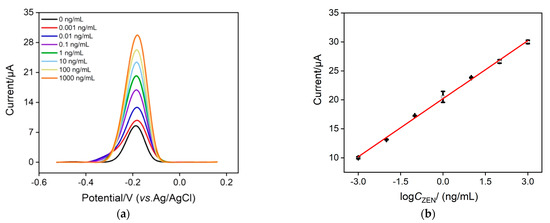
Figure 4.
(a) DPV curves obtained by our proposed homogenous electrochemical aptasensor in the presence of different concentrations of ZEN (from top to bottom: 0.001, 0.01, 0.1, 1, 10, 100, 1000 ng/mL). (b) The linear relationship between the anodic current and the logarithmic value of ZEN concentration. The composite probe solution contains 1 μΜ ZEN aptamer, 0.1 mg/mL GO and 100 μΜ Ru(NH3)6Cl3. Error bars denote the standard deviations of three measurements.

Table 1.
Comparison of linear range and LOD of the proposed homogeneous electrochemical aptasensor with previous sensing methods for the determination of ZEN.
Starch, ochratoxin (OTA), and aflatoxin (AFB1) were used to examine the selectivity of the proposed homogeneous electrochemical aptasensor combined VMSF/ITO and nanocomposite. Figure S2 shows the variation of anodic peak current response (I − I0) to the ZEN after the addition of interfering species. It could be found that the quantity of starch, OTA and AFB1 is remarkably smaller than that of ZEN or a mixture containing ZEN, suggesting the satisfactory selectivity of our proposed homogenous electrochemical sensing strategy. The reproducibility of the proposed homogeneous electrochemical aptasensor was studied by testing a known concentration of ZEN using five different VMSF/ITO electrodes. The relative standard deviation for five measured electrochemical signals is 3.7%, showing appreciable reproducibility of the proposed sensing strategy.
2.5. Analytical Application in Food Samples
To examine the feasibility of our designed homogeneous electrochemical aptasensor in practical application, maize and chestnut samples were employed. By mixing 5 g maize or chestnut powder with 20 mL acetonitrile solution of 8.5 mM NaCl under ultrasonication and centrifuging at 6000 rpm for 10 min, supernatant was achieved, followed by dilution using buffer solution (0.01 M PBS (pH = 7.4)). Thus, food samples for measurement were obtained. Various standard amounts of ZEN were artificially added into the above food samples and determined by the proposed homogeneous electrochemical aptasensor. As implied in Table 2, satisfactory recoveries in the range of 96.0–105% and low RSD value (less than 2.1%) were obtained, demonstrating the accuracy and successful applicability of the developed homogeneous electrochemical sensing strategy.

Table 2.
Analysis of ZEN in maize and chestnut samples.
3. Materials and Methods
3.1. Chemicals and Materials
Graphene oxide (GO) was prepared by a modified Hummers method [58]. Sodium dihydrogen phosphate dehydrate (NaH2PO4), sodium phosphate dibasic dodecahydrate (Na2HPO4), potassium ferricyanide (K3[Fe(CN)6]), potassium ferrocyanide (K4[Fe(CN)6]), tetraethyl orthosilicate (TEOS) and hexadecyl trimethyl ammonium bromide (CTAB) were purchased from Aladdin (Shanghai, China). Hexaammineruthenium (III) chloride (Ru(NH3)6Cl3) was bought from Sigma-Aldrich (Shanghai, China). Ethanol (EtOH) and sodium nitrate (NaNO3) were obtained from Hangzhou Gaojing Chemistry Co., Ltd. (Hangzhou, China). The ZEN aptamer (Apt) 5′-TCATCTATCTATGGTACATTACTATCTGTAATGTGATATG-3′ was synthesized by Sangon Biotech. Co., Ltd. (Shanghai, China). Aflatoxin (AFB1), ochratoxin (OTA) and zearalenone (ZEN) were provided by the College of Food Science, Zhejiang University (Hangzhou, China). Maize and chestnut were obtained from the local supermarket (Hangzhou, China). ITO coated glasses (<17 Ω/square, thickness: 100 ± 20 nm) were obtained from Zhuhai Kaivo Optoelectronic Technology, which were first cleaned using 1 M NaOH aqueous solution, and then sonicated in acetone, ethanol, and deionized water, respectively. Ultrapure water (18.2 MΩ cm) was used to prepare all aqueous solutions throughout this work.
3.2. Apparatus
A field-emission scanning electron microscope (SEM, S-4800, Hitachi, Tokyo, Japan) and a transmission electron microscope (TEM, JEM-2100, JEOL Ltd., Musashino, Japan) were used to investigate the morphology of VMSF. The acceleration voltages for SEM and TEM are 5 kV and 100 kV, respectively. Ultraviolet-Vis (UV–Vis) absorption spectra were performed on a UV–Vis spectrometer (UV-2450; Shimadzu, Kyoto, Japan). X-ray photoelectron spectroscopy (XPS) analysis (250 W, 14 kV, Mg Kα radiation) was carried out on a PHI5300 electron spectrometer (PE Ltd., Waltham, MA, USA). A Vertex 70 spectrometer (Bruker, Woodlands, TX, USA) was used to measure the Fourier transform infrared spectroscopy (FTIR) data through the KBr tablet method. The zeta potential characterization was taken on a SZ-100V2 nano particle analyzer (HORIBA, Tokyo, Japan) with a He-Ne laser (633 nm) at 90 angle collecting optics at 25 °C. Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were performed an Autolab PGSTAT302N electrochemical workstation (Metrohm, Herisau, Switzerland) with a three-electrode system consisting of bare or modified ITO as the working electrode, Ag/AgCl (saturated KCl solution) as the reference electrode and Pt wire electrode as the auxiliary electrode. The parameters for DPV tests are step (0.005 V), modulation amplitude (0.05 V), modulation time (0.05 s) and interval time (0.2 s).
3.3. Preparation of the VMSF/ITO
VMSF was grown on the cleaned ITO electrode (1 cm × 0.5 cm) by EASA method according to the previous reports [59]. Specially, 3.050 mL of TEOS and 1.585 g of CTAB were added to the mixture consisting of 20 mL of NaNO3 aqueous solution (0.1 M, pH = 2.6) and 20 mL of ethanol. Then the above solution required pre-hydrolyzation under stirring for 2.5 h to obtain the silica-based precursor. The bare ITO electrode underwent a cleaning procedure and was soaked in the above mixture under quiescent conditions along with the reference electrode (Ag/AgCl) and auxiliary electrode (Pt sheet). Experimental conditions for the growth of VMSF: a constant current density, −0.70 mA·cm−2, 10 s. Finally, the growth of VMSF was terminated immediately by rinsing with amounts of ultrapure water and the obtained modified electrode was further aged at 120 ◦C overnight. The modified electrode, keeping surfactant micelles (SMs) inside the nanochannels, was denoted as SM@VMSF/ITO. After exclusion of SMs by treatment of SM@VMSF/ITO electrode with 0.1 M HCl–ethanol solution under stirring for 5 min, VMSF with open channels on the ITO surface was achieved, named VMSF/ITO.
3.4. Preparation of the Ru(NH3)63+–ZEN Aptamer–GO Nanocomposite Probe and Detection of ZEN
10 μL of 10 mg/mL GO, 10 mM Ru(NH3)6Cl3 and 100 μM ZEN aptamer were mixed in a 1 mL 0.01 M PBS (pH = 7.4) solution and incubated at 37 °C for 2 h, to obtain the Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe. After mixing various concentrations of ZEN with the Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe and further incubating at 37 °C for 1 h, the VMSF/ITO electrode was used to measure the redox current variation of Ru(NH3)63+, allowing the quantitative detection of ZEN.
4. Conclusions
We demonstrated a simple homogeneous electrochemical aptasensor for ultrasensitive determination of ZEN in maize and Chinese chestnut samples in a combination of the assistance of GO and signal amplification of VMSF. The Ru(NH3)63+–ZEN aptamer–GO nanocomposite probe was formed in a homogeneous solution through electrostatic and π–π interactions, which could serve as the recognitive and signal element. When ZEN was present, ZEN aptamer was specifically dissociated from the nanocomposite probe and further triggered the release of Ru(NH3)63+, which could be determined by VMSF/ITO electrode and generate the increased redox currents. The VMSF could electrostatically preconcentrate the cationic Ru(NH3)63+ probe and captured the variation of Ru(NH3)63+ amounts in solution, enabling highly sensitive determination of ZEN. This sensing strategy could not only avoid the time-consuming complicated process of electrode modification and labeling, but also provide a highly sensitive and universal approach for a variety of analytes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28217241/s1, Figure S1: Optimization of experimental conditions; Figure S2: Anti-interference ability of homogeneous electrochemical aptasensor.
Author Contributions
Data curation, Z.H. and X.L.; validation, X.L.; methodology, Z.H.; writing—original draft preparation, Z.H. and X.L.; writing—review and editing, F.Y. and B.Z.; supervision, F.Y. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research and Development Program of Guangxi (AB23026079), the Zhejiang Provincial Natural Science Foundation of China (LY21B050003), and Guangxi Science and Technology Department Project (grant number GuikeAB21220062).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Viter, R.; Savchuk, M.; Iatsunskyi, I.; Pietralik, Z.; Starodub, N.; Shpyrka, N.; Ramanaviciene, A.; Ramanavicius, A. Analytical, thermodynamical and kinetic characteristics of photoluminescence immunosensor for the determination of Ochratoxin A. Biosens. Bioelectron. 2018, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Eremin, S.A.; Wen, K.; Yu, X.; Li, C.; Ke, Y.; Jiang, H.; Shen, J.; Wang, Z. Fluorescence polarization immunoassay based on a new monoclonal antibody for the detection of the zearalenone class of mycotoxins in maize. J. Agric. Food Chem. 2017, 65, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gao, L.; Yin, L.; Arslan, M.; El-Seedi, H.R.; Zou, X. Novel mesoporous silica surface loaded gold nanocomposites SERS aptasensor for sensitive detection of zearalenone. Food Chem. 2023, 403, 134384. [Google Scholar] [CrossRef]
- Hao, K.; Suryoprabowo, S.; Song, S.; Liu, L.; Kuang, H. Rapid detection of zearalenone and its metabolite in corn flour with the immunochromatographic test strip. Food Agric. Immunol. 2017, 29, 498–510. [Google Scholar] [CrossRef]
- Habler, K.; Gotthardt, M.; Schuler, J.; Rychlik, M. Multi-mycotoxin stable isotope dilution LC-MS/MS method for Fusarium toxins in beer. Food Chem. 2017, 218, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ren, Y.; Zhou, H.; Luan, L.; Cai, Z.; Wu, Y. A rapid method for simultaneous determination of zearalenone, alpha-zearalenol, beta-zearalenol, zearalanone, alpha-zearalanol and beta-zearalanol in traditional Chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2011, 879, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Dong, F.; Li, Y.; Guo, Y.; Liu, X.; Xu, J.; Wu, X.; Zheng, Y. Ultrasensitive immunoassay for detection of zearalenone in agro-products using enzyme and antibody co-embedded zeolitic imidazolate framework as labels. J. Hazard. Mater. 2021, 412, 125276. [Google Scholar] [CrossRef] [PubMed]
- Kalagatur, N.K.; Mudili, V.; Kamasani, J.R.; Siddaiah, C. Discrete and combined effects of Ylang-Ylang (Cananga odorata) essential oil and gamma irradiation on growth and mycotoxins production by Fusarium graminearum in maize. Food Control 2018, 94, 276–283. [Google Scholar] [CrossRef]
- Shkembi, X.; Svobodova, M.; Skouridou, V.; Bashammakh, A.S.; Alyoubi, A.O.; O’Sullivan, C.K. Aptasensors for mycotoxin detection: A review. Anal. Biochem. 2022, 644, 114156. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.Y.; Pike, A.; Tan, L.L. Recent advances in conventional methods and electrochemical aptasensors for mycotoxin detection. Foods 2021, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernandez, S.; Bertolin, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, L.; Huang, H.; Lv, N.; Liu, J.; Liu, Y. Nanochannel array on electrochemically polarized screen printed carbon electrode for rapid and sensitive electrochemical determination of clozapine in human whole blood. Molecules 2022, 27, 2739. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xu, S.; Xi, F. Disposal immunosensor for sensitive electrochemical detection of prostate-specific antigen based on amino-rich nanochannels array-modified patterned indium tin oxide electrode. Nanomaterials 2022, 12, 3810. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Kailasa, S.K.; Kumar, V.; Tsang, Y.F.; Lee, S.E.; Gobi, K.V.; Kim, K.H. Progress on nanostructured electrochemical sensors and their recognition elements for detection of mycotoxins: A review. Biosens. Bioelectron. 2018, 121, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, S.; Lin, X.; Liu, J.; Wang, K. Label-free electrochemical aptasensor based on the vertically-aligned mesoporous silica films for determination of aflatoxin B1. Biosensors 2023, 13, 661. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, J.; Xie, L.; Tang, H.; Wang, K.; Liu, J. One-step preparation of nitrogen-doped graphene quantum dots with anodic electrochemiluminescence for sensitive detection of hydrogen peroxide and glucose. Front. Chem. 2021, 9, 688358. [Google Scholar]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan–graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, S.; Liu, J.; Xing, J. Homogeneous electrochemical aptamer sensor based on two-dimensional nanocomposite probe and nanochannel modified electrode for sensitive detection of carcinoembryonic antigen. Molecules 2023, 28, 5186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless electrochemical detection of tumor biomarker based on stable confinement of electrochemical probe in bipolar silica nanochannel film. Nanomaterials 2023, 13, 1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Zhang, C.; Ji, H.; Pang, Q.; Li, X.; Luo, Z.; Wu, Q.; Zhang, L. Development of Aptamer-Based Molecular Tools for Rapid Intraoperative Diagnosis and In Vivo Imaging of Serous Ovarian Cancer. ACS Appl. Mater. Interfaces 2021, 13, 16118–16126. [Google Scholar] [CrossRef] [PubMed]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-based rapid diagnosis for point-of-care application. Microfluid. Nanofluid. 2023, 27, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, G.; Ni, J.; Wang, Q.; Lin, Z. From signal amplification to restrained background: Magnetic graphene oxide assisted homogeneous electrochemiluminescence aptasensor for highly sensitive detection of okadaic acid. Sens. Actuators B Chem. 2021, 327, 128872. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Design 2022, 215, 110506. [Google Scholar] [CrossRef]
- Medeiros, P.V.C.; Gueorguiev, G.K.; Stafström, S. Bonding, charge rearrangement and interface dipoles of benzene, graphene, and PAH molecules on Au(111) and Cu(111). Carbon 2015, 81, 620–628. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Rivelino, R.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Exploring 2D structures of indium oxide of different stoichiometry. CrystEngComm 2021, 23, 6661–6667. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Z.; Li, Y.; Xi, F.; Liu, J. Magnetic nanozyme based on loading nitrogen-doped carbon dots on mesoporous Fe3O4 nanoparticles for the colorimetric detection of glucose. Molecules 2023, 28, 4573. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, W.; Liu, X.; Xi, F.; Wu, J. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv. Funct. Mater. 2023, 2308183. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Su, R.; Lin, X.; Liu, J. Nanochannel array modified three-dimensional graphene electrode for sensitive electrochemical detection of 2,4,6-trichlorophenol and prochloraz. Front. Chem. 2022, 10, 954802. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef]
- Lv, N.; Qiu, X.; Han, Q.; Xi, F.; Wang, Y.; Chen, J. Anti-biofouling electrochemical sensor based on the binary nanocomposite of silica nanochannel array and graphene for doxorubicin detection in human serum and urine samples. Molecules 2022, 27, 8640. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zheng, Y.; An, L.; Liu, J. Ultrasensitive immunosensor for prostate-specific antigen based on enhanced electrochemiluminescence by vertically ordered mesoporous silica-nanochannel film. Front. Chem. 2022, 10, 851178. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J. Facile synthesis of iron and nitrogen co-doped carbon dot nanozyme as highly efficient peroxidase mimics for visualized detection of metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, C.; Ge, S.; Luo, T.; Chen, J.; Liu, J.; Xi, F.; Liu, J. Gram-scale synthesis of nitrogen doped graphene quantum dots for sensitive detection of mercury ions and L-cysteine. RSC Adv. 2019, 9, 32977–32983. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Z.; Zhao, R.; Lu, Y.; Shi, L.; Liu, J.; Dong, X.; Xi, F. Aqueous synthesis of amphiphilic graphene quantum dots and their application as surfactants for preparing of fluorescent polymer microspheres. Colloid Surface A 2019, 563, 77–83. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, Y.; Pang, Y.; Chen, J.; Zhang, Z.; Xi, F.; Chen, P. Graphene quantum dots as full-color and stimulus responsive fluorescence ink for information encryption. J. Colloid Interface Sci. 2020, 579, 307–314. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile pretreatment of three-dimensional graphene through electrochemical polarization for improved electrocatalytic performance and simultaneous electrochemical detection of catechol and hydroquinone. Nanomaterials 2022, 12, 65. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, H.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhao, J.; Ding, Y.; Tang, H.; Xi, F. Iron and nitrogen co-doped graphene quantum dots as highly active peroxidases for the sensitive detection of l-cysteine. New J. Chem. 2021, 45, 19056–19064. [Google Scholar] [CrossRef]
- Ge, S.; He, J.; Ma, C.; Liu, J.; Xi, F.; Dong, X. One-step synthesis of boron-doped graphene quantum dots for fluorescent sensors and biosensor. Talanta 2019, 199, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, J.; Deng, X.; Chen, J.; Xi, F.; Wang, X. Colorimetric and fluorescent dual-modality sensing platform based on fluorescent nanozyme. Front. Chem. 2021, 9, 774486. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhao, X.; Chen, L.J.; Yang, C.; Yin, X.B.; Yan, X.P. A dual-colored persistent luminescence nanosensor for simultaneous and autofluorescence-free determination of aflatoxin B1 and zearalenone. Talanta 2021, 232, 122395. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Wang, X.; Pasha, I.; Khan, I.M.; Zhao, S.; Shoaib, M.; Wu, S.; Wang, Z. A novel bioassay based on aptamer-functionalized magnetic nanoparticle for the detection of zearalenone using time resolved-fluorescence NaYF4: Ce/Tb nanoparticles as signal probe. Talanta 2018, 186, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lv, Y.; Qi, S.; Zhang, Y.; Wang, Z. Sensitive colorimetric aptasensor based on stimuli-responsive metal-organic framework nano-container and trivalent DNAzyme for zearalenone determination in food samples. Food Chem. 2022, 371, 131145. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Emrani, A.S.; Abnous, K. Novel colorimetric aptasensor for zearalenone detection based on nontarget-induced aptamer walker, gold nanoparticles, and exonuclease-assisted recycling amplification. ACS Appl. Mater. Interfaces 2018, 10, 12504–12509. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yu, C.; Wen, Y.; Chen, J.; Yu, Y.; Zhang, C.; Gao, R.; Mu, X.; He, J. Fabrication of pioneering 3D sakura-shaped metal-organic coordination polymers Cu@L-Glu phenomenal for signal amplification in highly sensitive detection of zearalenone. Biosens. Bioelectron. 2019, 129, 139–146. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yan, X. A “signal-on” voltammetric aptasensor fabricated by hcPt@AuNFs/PEI-rGO and Fe3O4NRs/rGO for the detection of zearalenone. Sens. Actuators B Chem. 2019, 290, 477–483. [Google Scholar] [CrossRef]
- He, B.; Yan, X. Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@Co-MOF networks for the detection of zearalenone. Sens. Actuators B Chem. 2020, 306, 127558. [Google Scholar] [CrossRef]
- Santhiago, M.; Maroneze, C.M.; Silva, C.C.C.; Camargo, M.N.L.; Kubota, L.T. Electrochemical oxidation of glassy carbon provides similar electrochemical response as graphene oxide prepared by Tour or Hummers routes. ChemElectroChem 2015, 2, 761–767. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).