Preparation of Low-Defect Manganese-Based Prussian Blue Cathode Materials with Cubic Structure for Sodium-Ion Batteries via Coprecipitation Method
Abstract
:1. Introduction
2. Results and Discussions
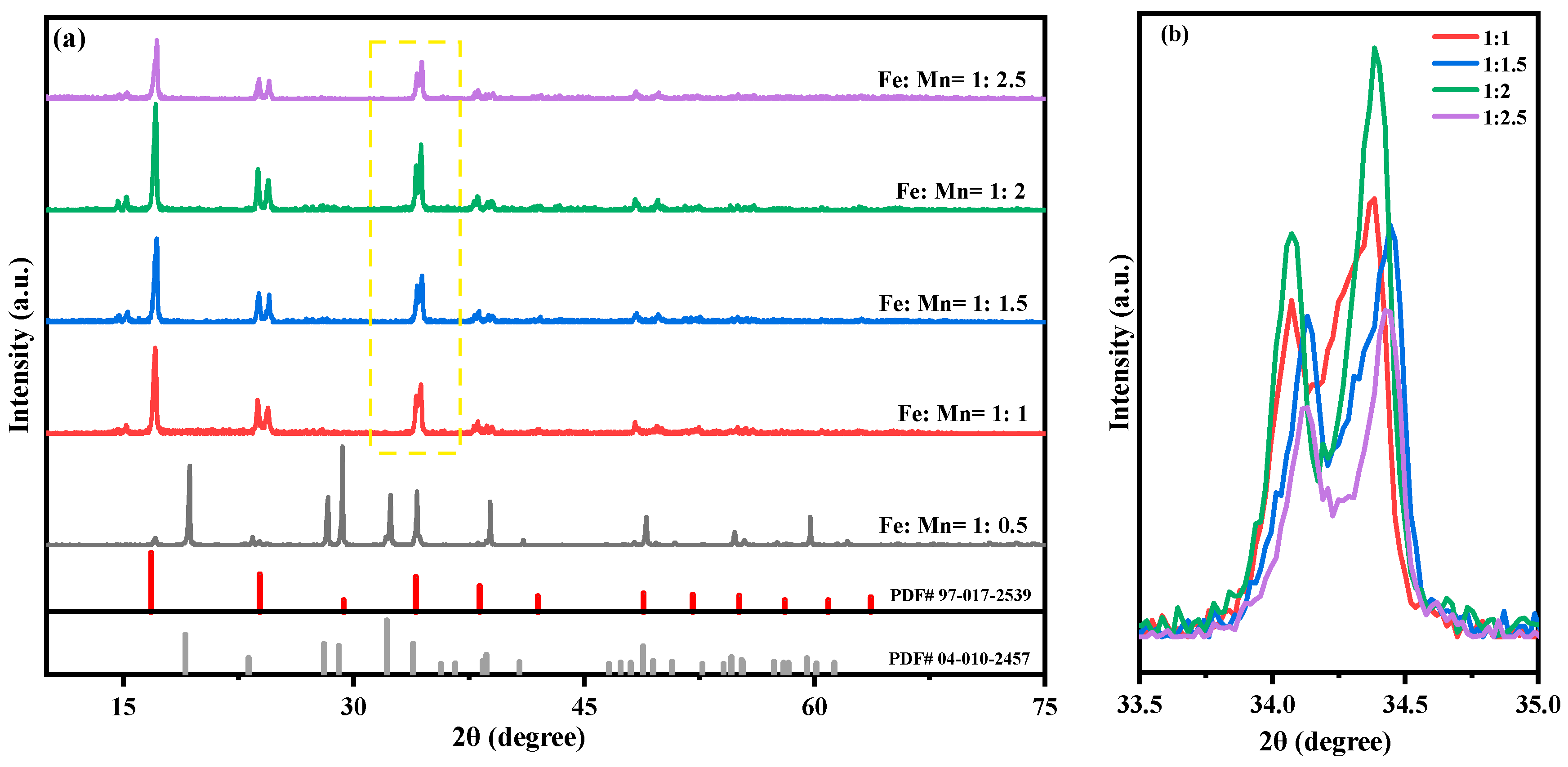
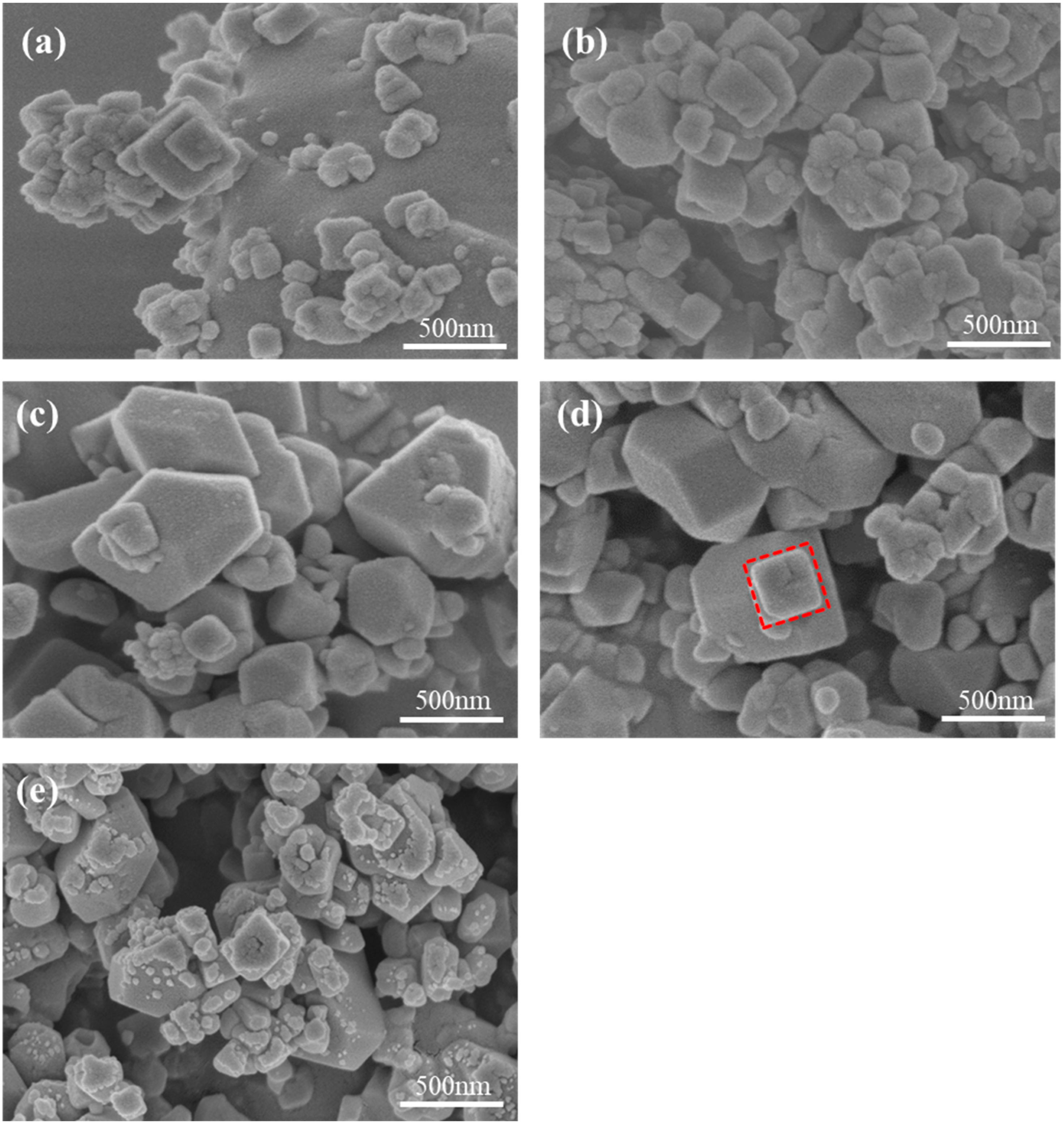
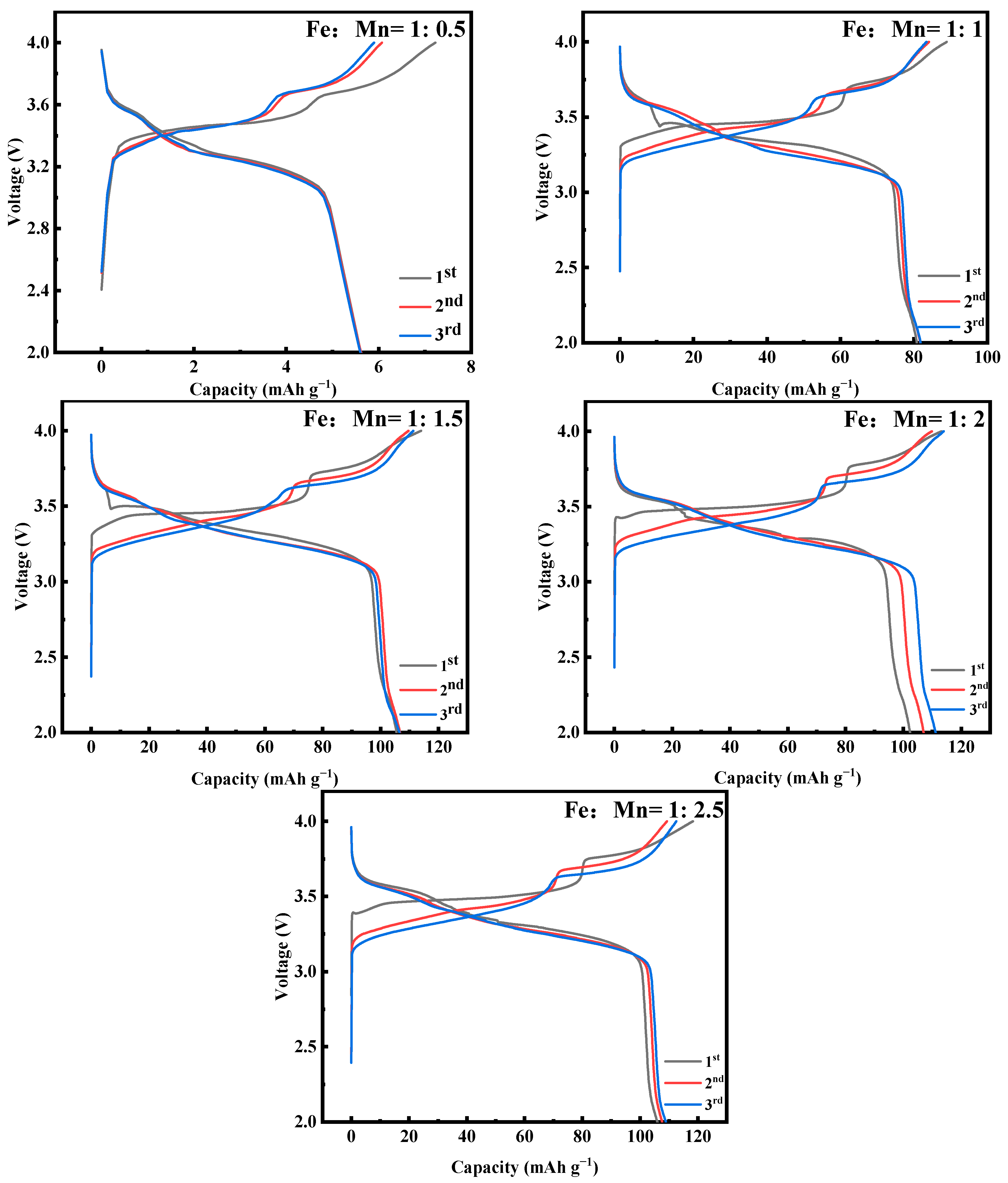
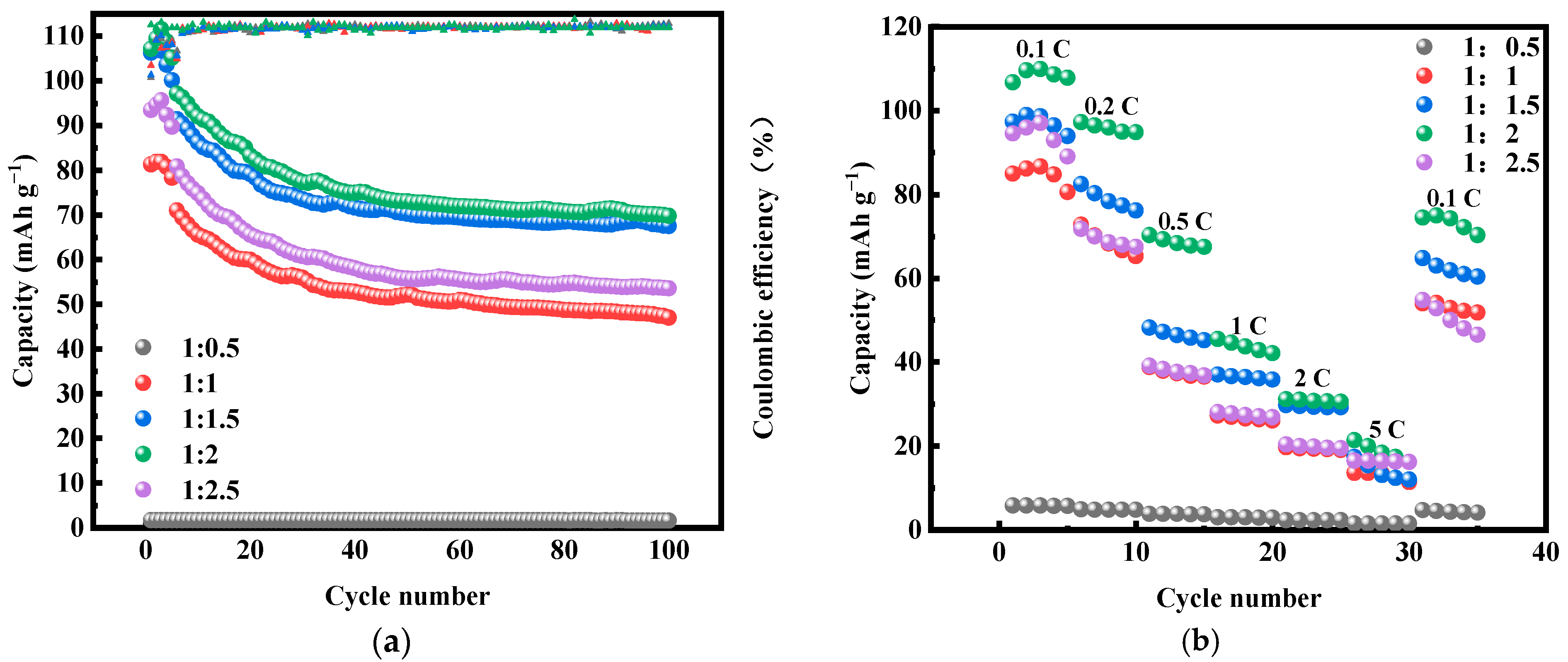
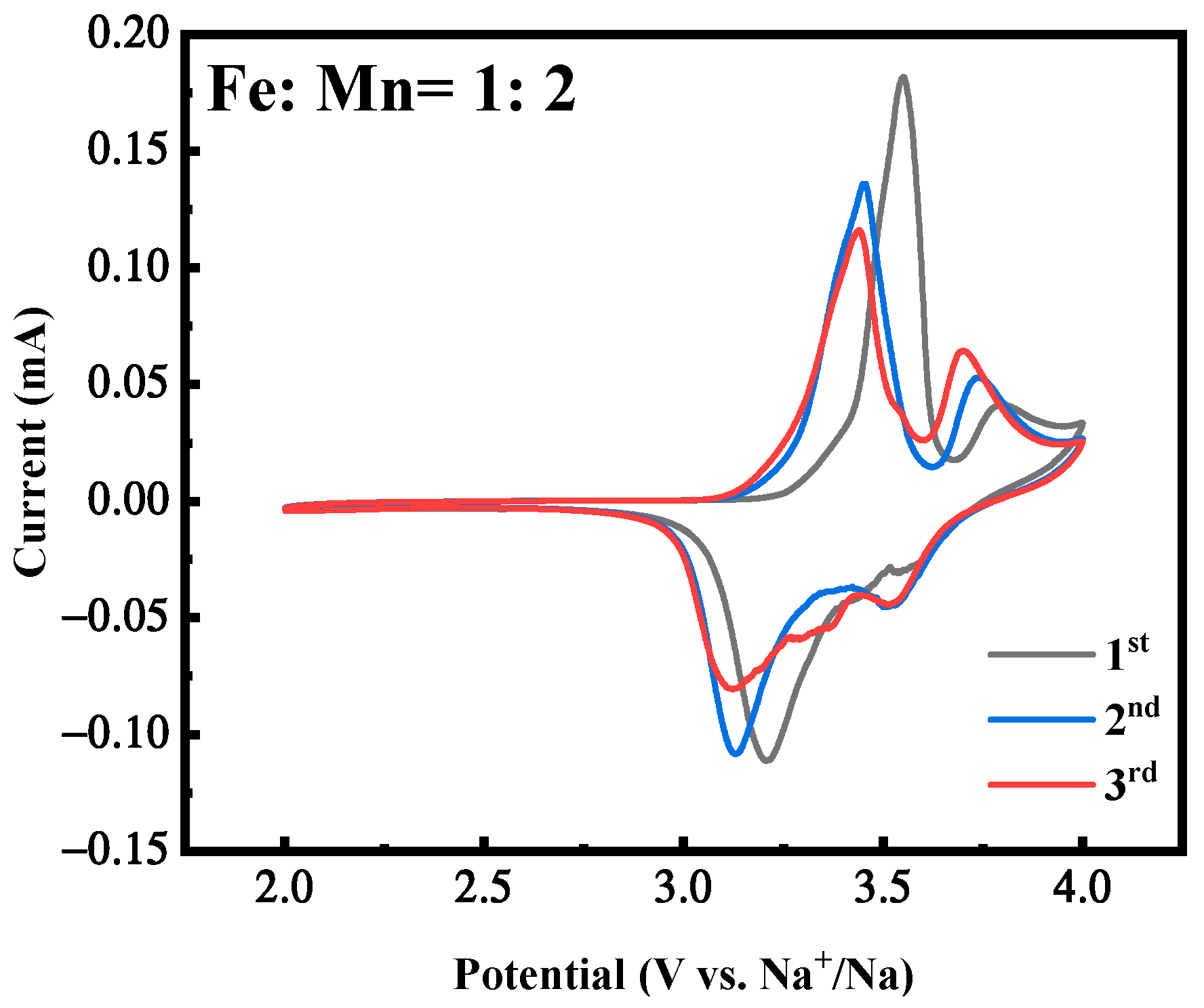
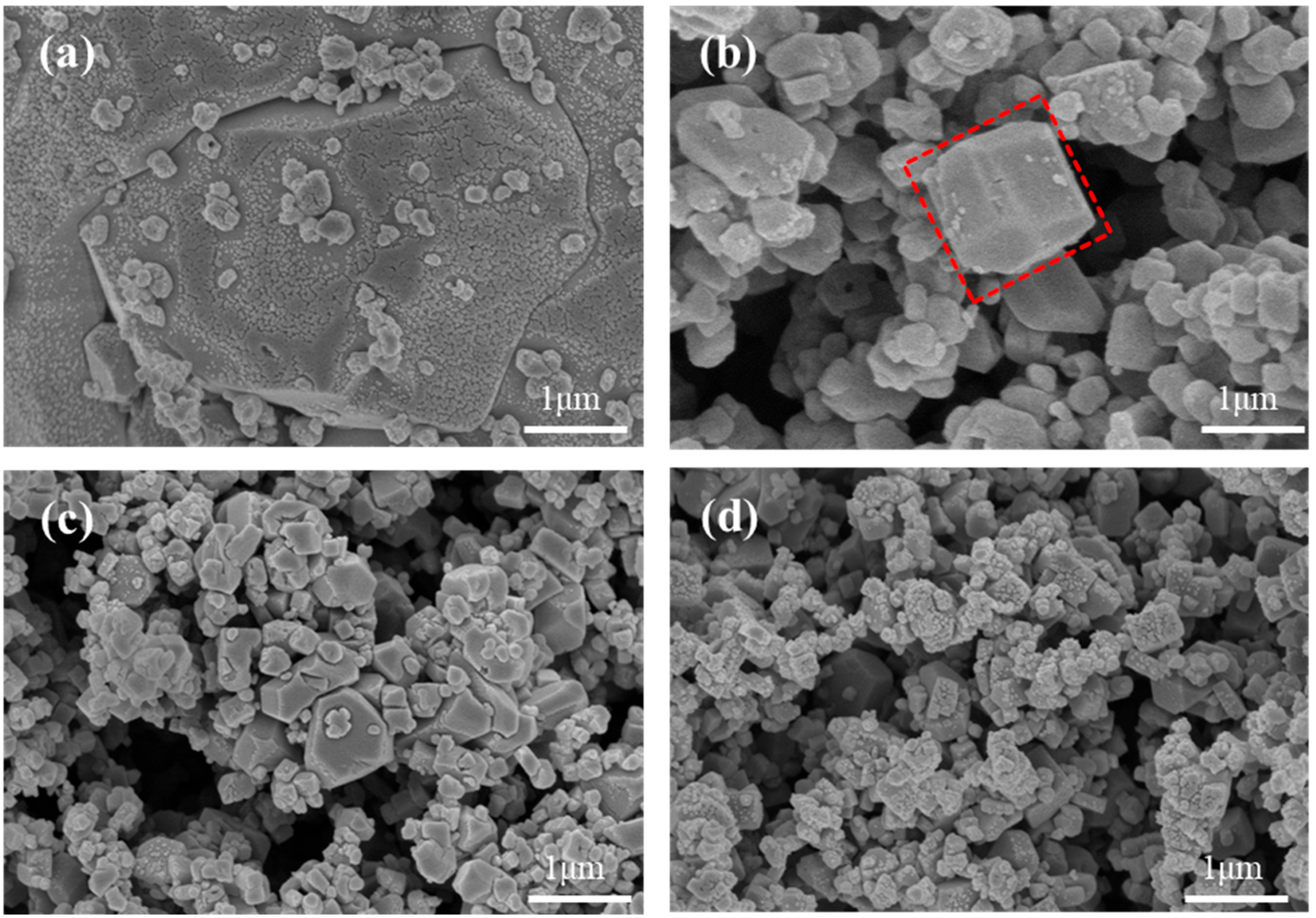
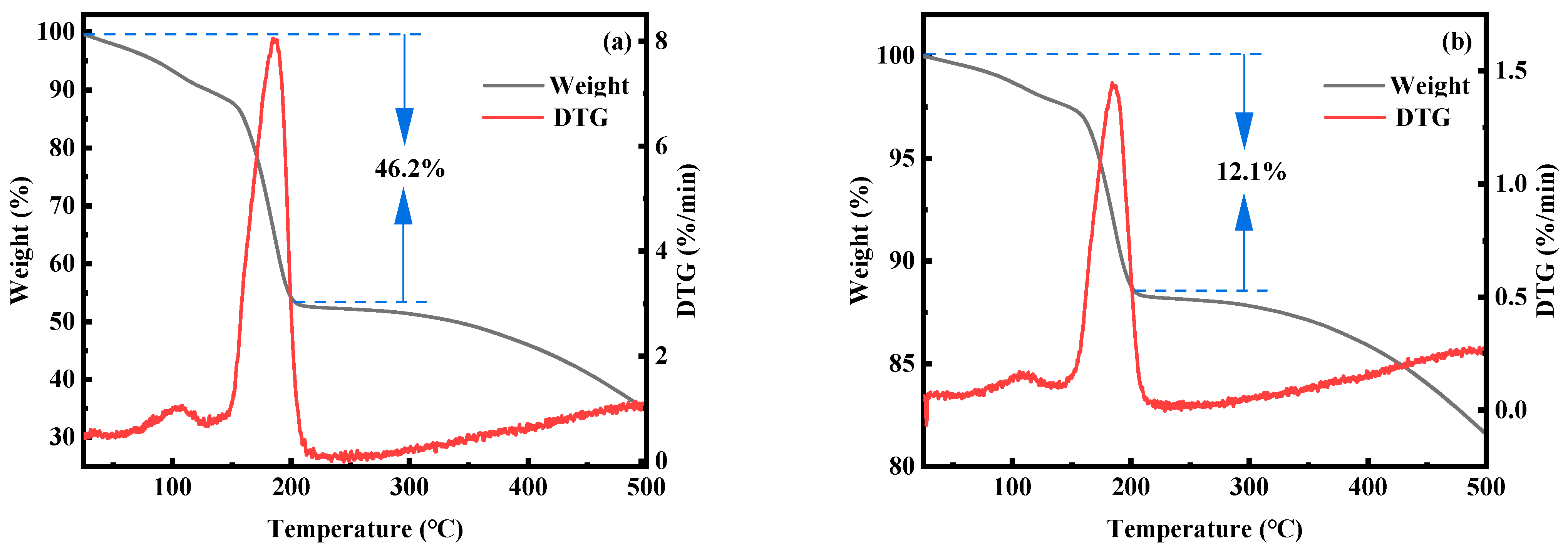
2.1. Effect of Fe-Mn Ratio on Properties of MnHCF
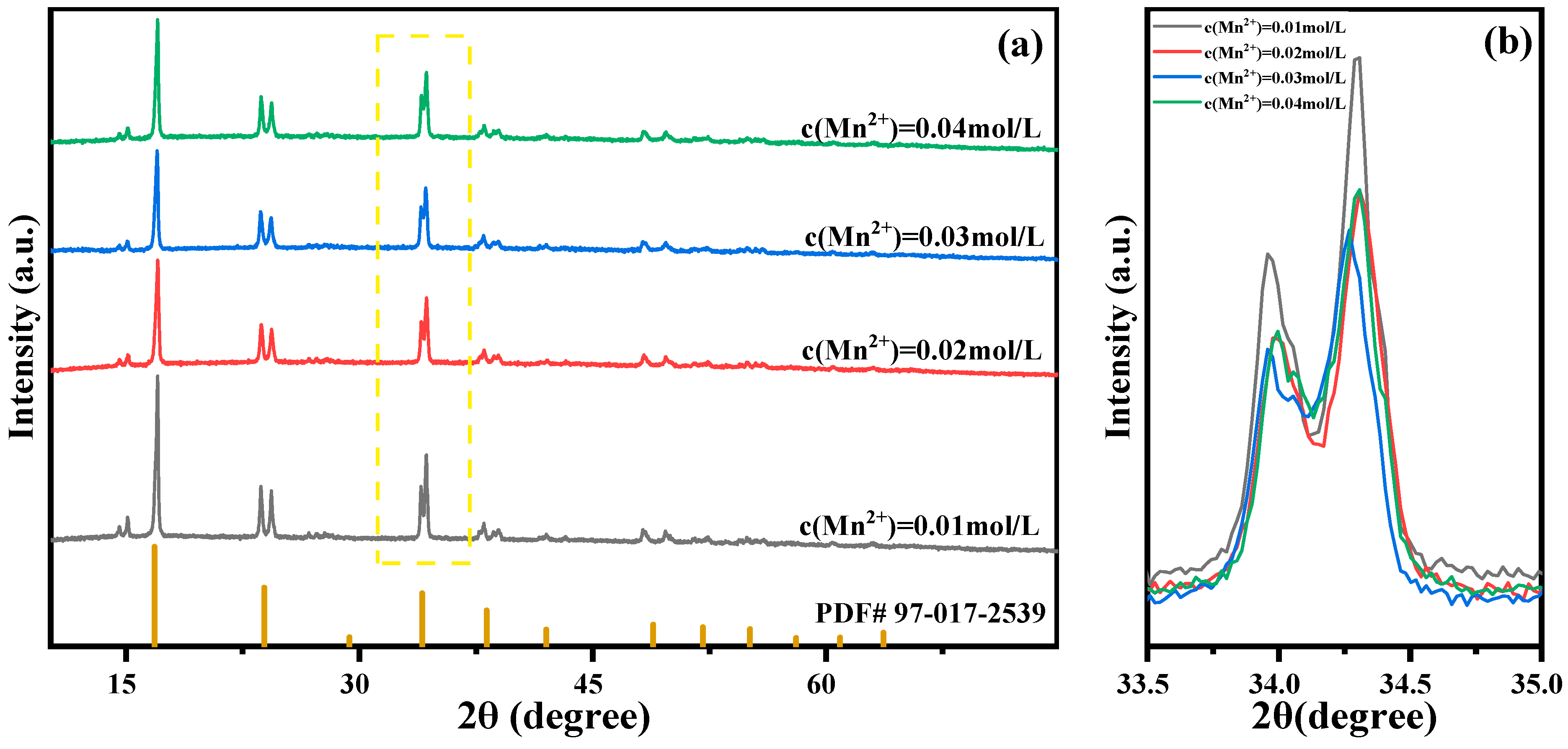
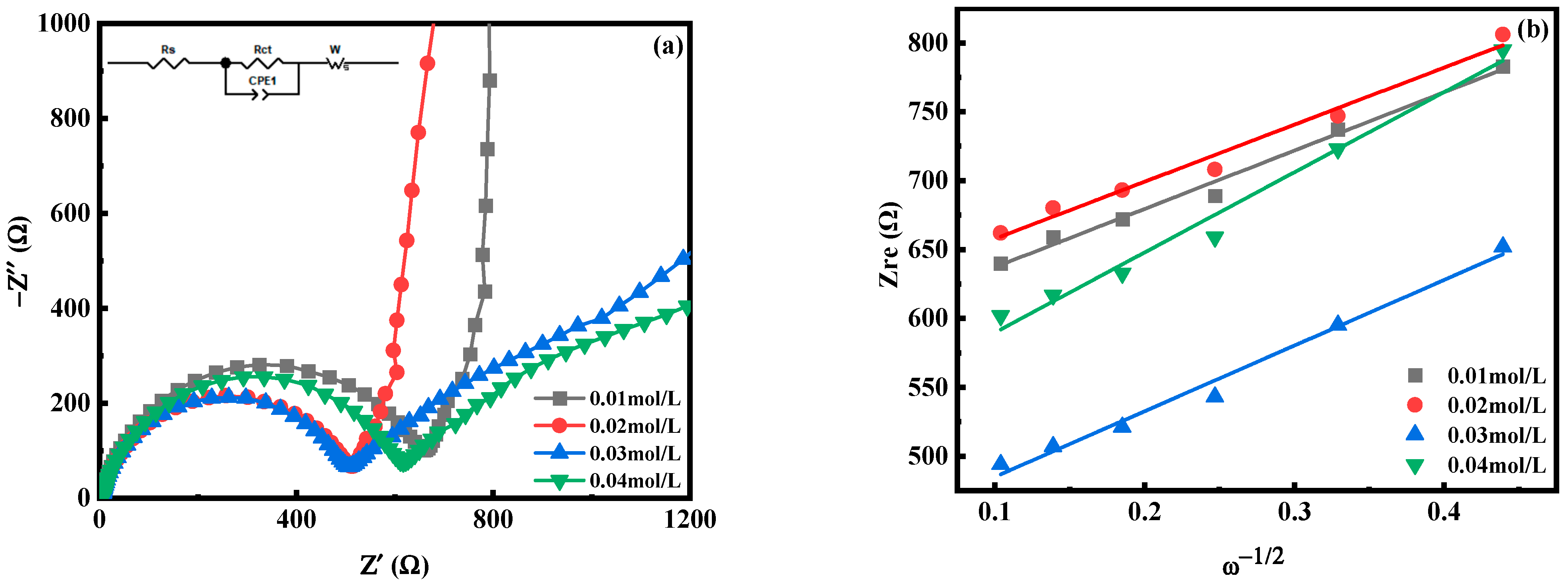
2.2. The Effect of Mn Ion Concentration on the Performance of MnHCF
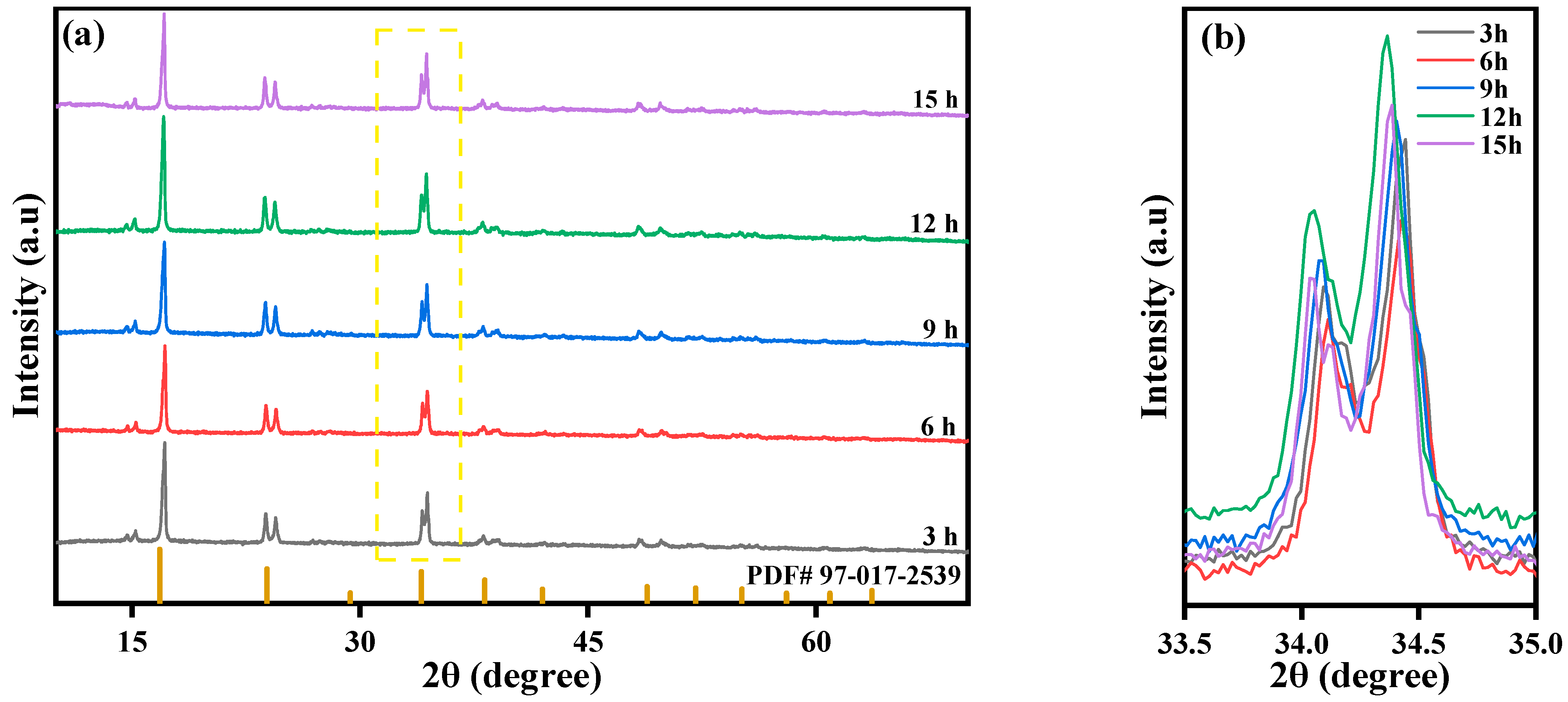
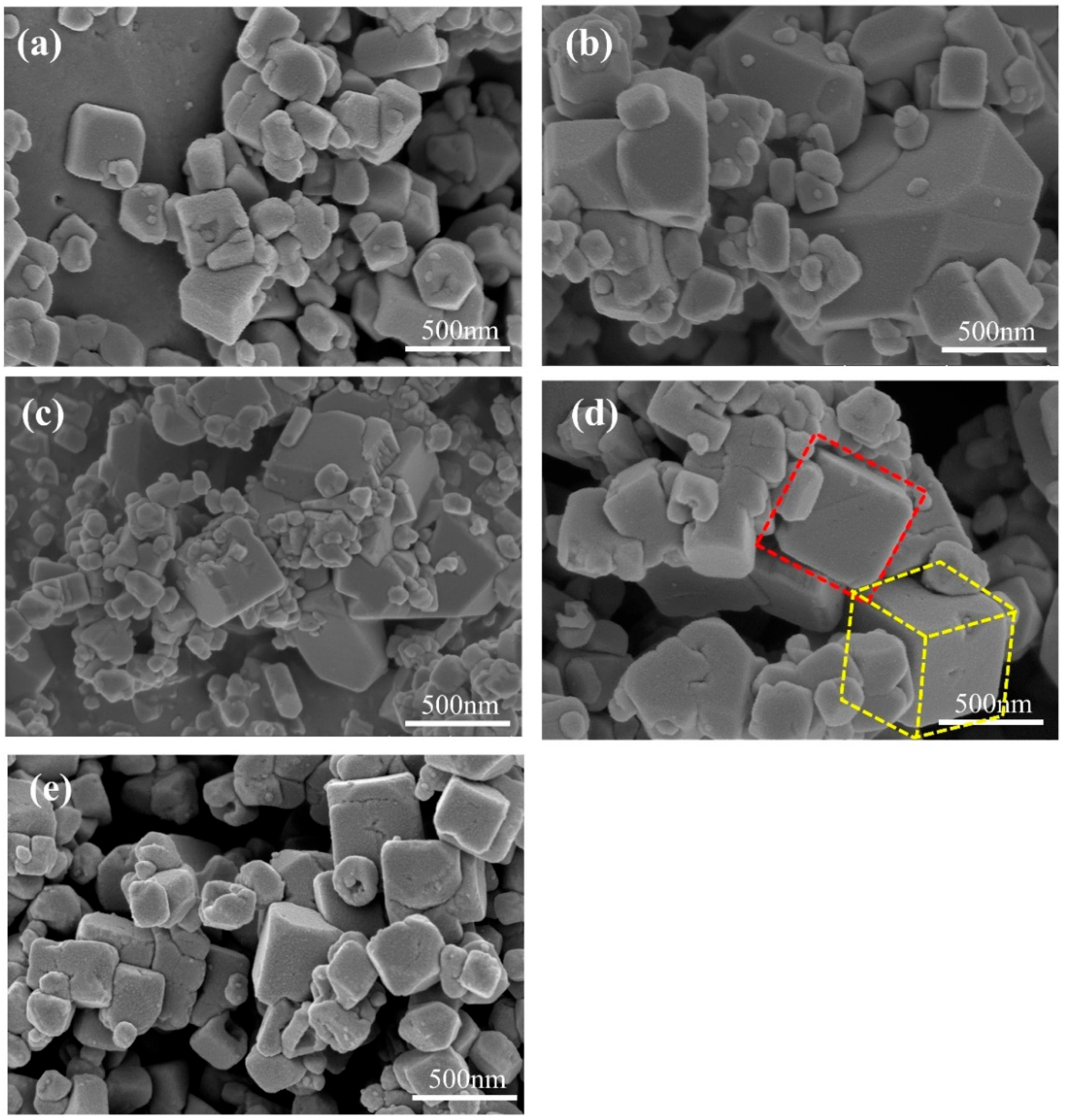
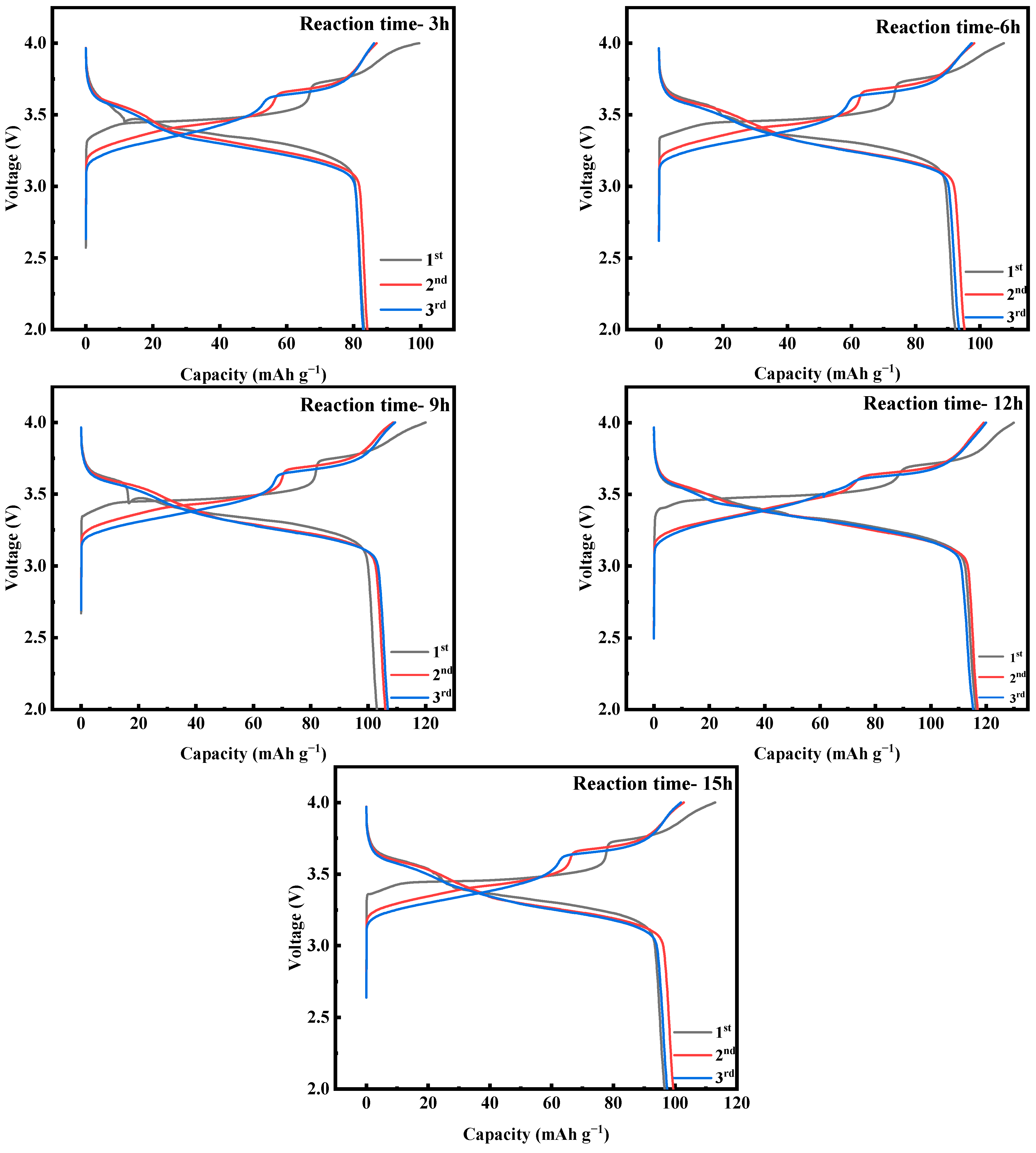
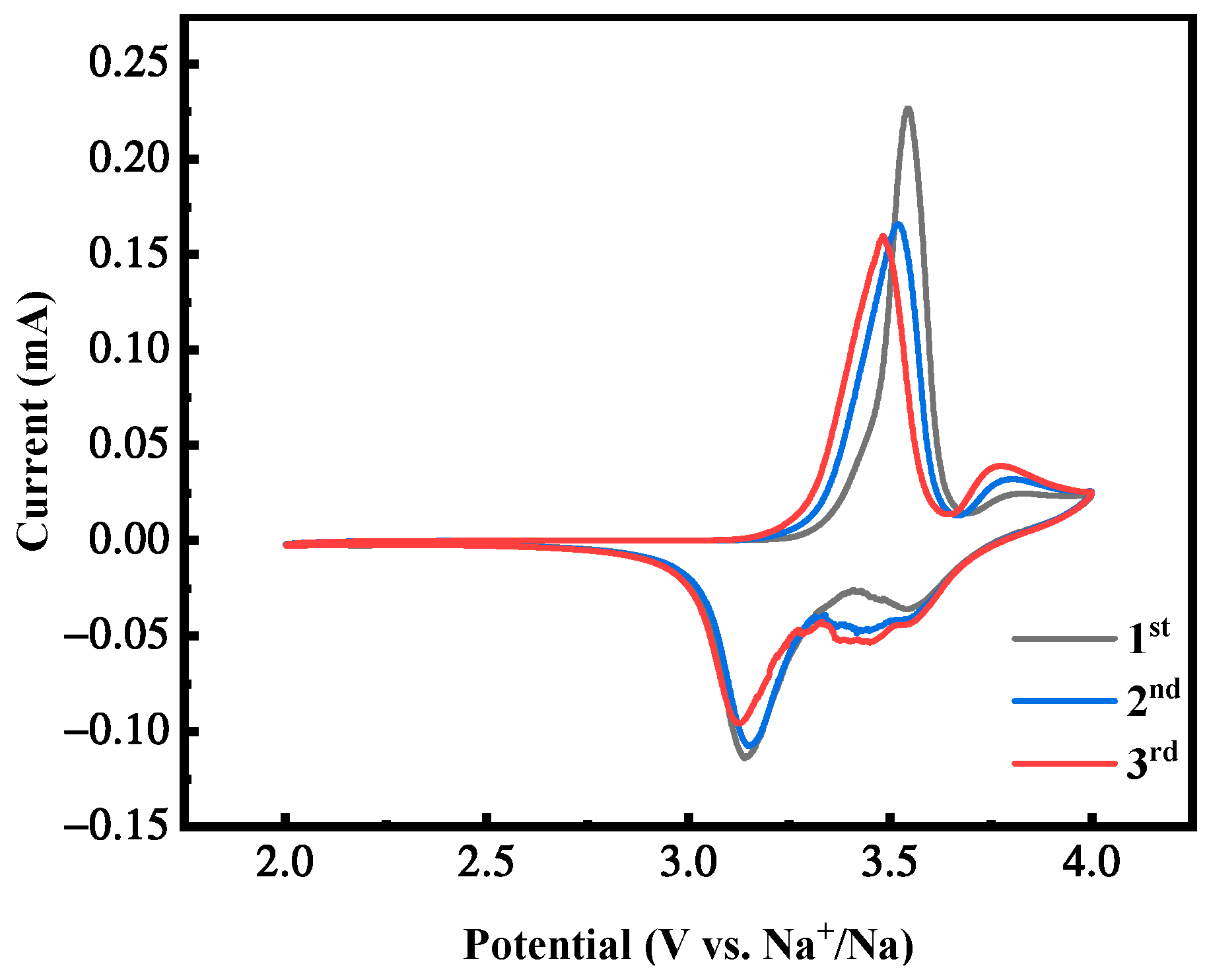
2.3. Effect of Reaction Time on the Performance of MnHCF
3. Experimental Details
3.1. Materials
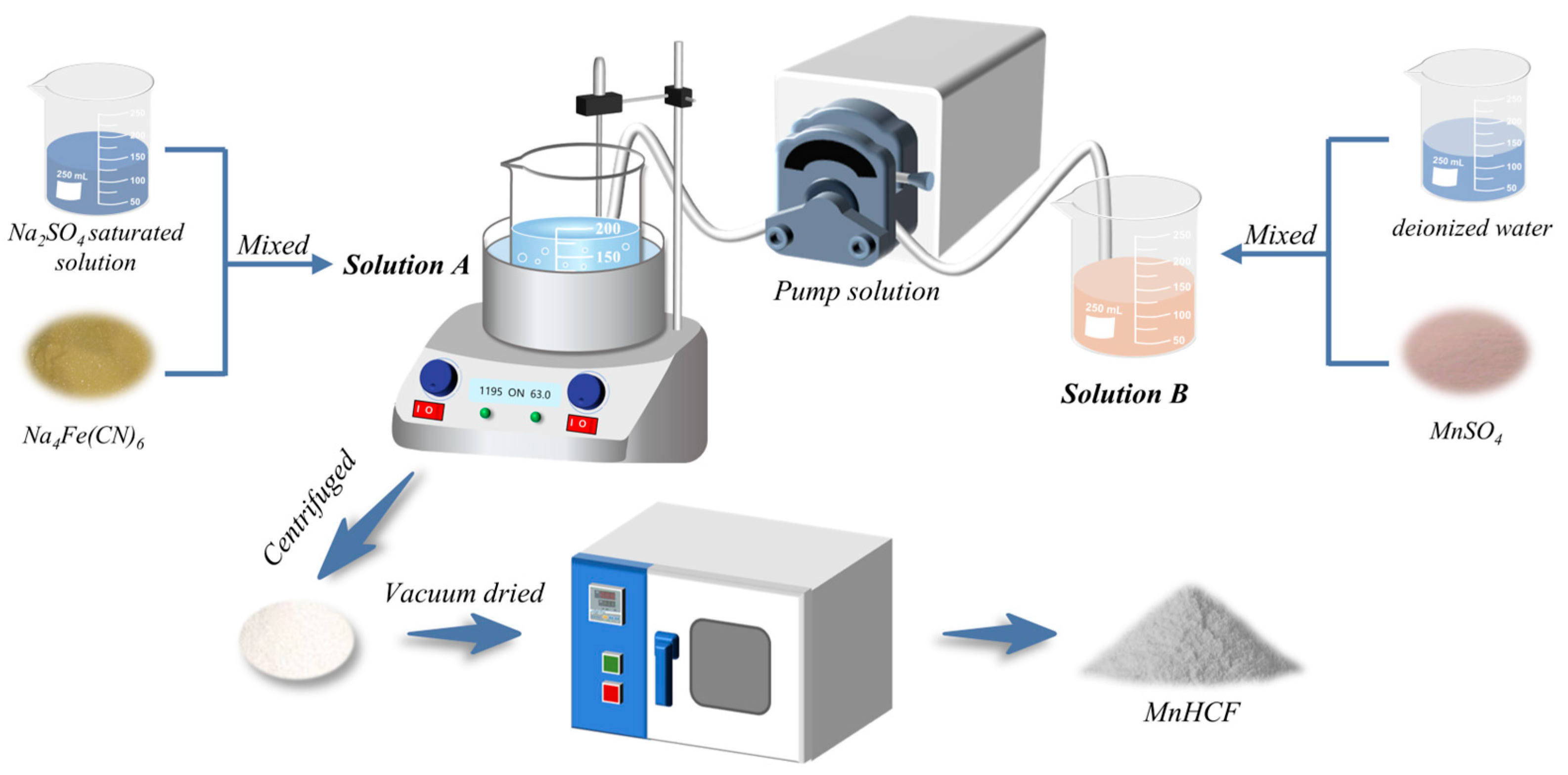
3.2. Material Synthesis
3.3. Material Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- An, X.; Zhu, W.; Tang, C.; Liu, L.; Chen, T.; Wang, X.; Zhao, J.; Zhang, G. Enhanced Electrochemical Water Oxidation Activity by Structural Engineered Prussian Blue Analogue/rGO Heterostructure. Molecules 2022, 27, 5472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-F.; Xiao, Y.; Dou, S.-X.; Kang, Y.-M.; Chou, S.-L. Spinel/post-spinel engineering on layered oxide cathodes for sodium-ion batteries. eScience 2021, 1, 13–27. [Google Scholar] [CrossRef]
- Xu, C.; Ma, Y.; Zhao, J.; Zhang, P.; Chen, Z.; Yang, C.; Liu, H.; Hu, Y.S. Surface Engineering Stabilizes Rhombohedral Sodium Manganese Hexacyanoferrates for High-Energy Na-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202217761. [Google Scholar] [CrossRef]
- Han, J.; Lin, Y.; Yang, Y.; Zuo, D.; Wang, C.; Liu, X. Dominant role of M element on the stability and properties of Prussian blue analogues NaxMFe(CN)6 (M = 3d transition metal) as cathode material for the sodium-ion batteries. J. Alloys Compd. 2021, 870, 159533. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Cheng, Y.; Li, L.; Lu, Z.; Liang, R.; Fan, Y.; Qiao, Y.; Chou, S. A Heterostructure Coupling of Bioinspired, Adhesive Polydopamine, and Porous Prussian Blue Nanocubics as Cathode for High-Performance Sodium-Ion Battery. Small 2020, 16, 1906946. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2017, 10, S3632–S3639. [Google Scholar] [CrossRef]
- Gao, C.; Lei, Y.; Wei, Y.; Wang, H.; Yuan, F.; Kang, F.; Zhai, D. Coexistence of two coordinated states contributing to high-voltage and long-life Prussian blue cathode for potassium ion battery. Chem. Eng. J. 2022, 431, 133926. [Google Scholar] [CrossRef]
- Yi, H.; Qin, R.; Ding, S.; Wang, Y.; Li, S.; Zhao, Q.; Pan, F. Structure and Properties of Prussian Blue Analogues in Energy Storage and Conversion Applications. Adv. Funct. Mater. 2020, 31, 2006970. [Google Scholar] [CrossRef]
- Bornamehr, B.; Presser, V.; Husmann, S. Mixed Cu–Fe Sulfides Derived from Polydopamine-Coated Prussian Blue Analogue as a Lithium-Ion Battery Electrode. ACS Omega 2022, 7, 38674–38685. [Google Scholar] [CrossRef]
- Chen, Y.; Woo, H.J.; Rizwan, M.; Yahya, R.b.; Cui, D.; Luo, D.; Chen, L.; Arof, A.K.M.; Wang, F. Nanoscale Morphology Control of Na-Rich Prussian Blue Cathode Materials for Sodium Ion Batteries with Good Thermal Stability. ACS Appl. Energy Mater. 2019, 2, 8570–8579. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Chen, Q.; Xu, C.; Cai, D.; Zhan, H. Resorcinol–Formaldehyde Resin-Coated Prussian Blue Core–Shell Spheres and Their Derived Unique Yolk–Shell FeS2@C Spheres for Lithium-Ion Batteries. Inorg. Chem. 2019, 58, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Liu, H.; Gao, J.; Xu, Y.; Chen, Z.; Li, X.; Liu, Y. Synthesis of Fe-doped Mn-based Prussian blue hierarchical architecture for high-performance sodium ion batteries. Electrochim. Acta 2023, 448, 142183. [Google Scholar] [CrossRef]
- Jiang, W.; Qi, W.; Pan, Q.; Jia, Q.; Yang, C.; Cao, B. Potassium ions stabilized hollow Mn-based prussian blue analogue nanocubes as cathode for high performance sodium ions battery. Int. J. Hydrog. Energy 2021, 46, 4252–4258. [Google Scholar] [CrossRef]
- Tang, Y.; Li, W.; Feng, P.; Zhou, M.; Wang, K.; Wang, Y.; Zaghib, K.; Jiang, K. High-Performance Manganese Hexacyanoferrate with Cubic Structure as Superior Cathode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1908754. [Google Scholar] [CrossRef]
- Yan, C.; Zhao, A.; Zhong, F.; Feng, X.; Chen, W.; Qian, J.; Ai, X.; Yang, H.; Cao, Y. A low-defect and Na-enriched Prussian blue lattice with ultralong cycle life for sodium-ion battery cathode. Electrochim. Acta 2020, 332, 135533. [Google Scholar] [CrossRef]
- Wang, P.-F.; Jin, T.; Zhang, J.; Wang, Q.-C.; Ji, X.; Cui, C.; Piao, N.; Liu, S.; Xu, J.; Yang, X.-Q.J.N.E. Elucidation of the Jahn-Teller effect in a pair of sodium isomer. Nano Energy 2020, 77, 105167. [Google Scholar] [CrossRef]
- Huang, T.; Du, G.; Qi, Y.; Li, J.; Zhong, W.; Yang, Q.; Zhang, X.; Xu, M. A Prussian blue analogue as a long-life cathode for liquid-state and solid-state sodium-ion batteries. Inorg. Chem. Front. 2020, 7, 3938–3944. [Google Scholar] [CrossRef]
- Feng, F.; Chen, S.; Zhao, S.; Zhang, W.; Miao, Y.; Che, H.; Liao, X.-Z.; Ma, Z.-F. Enhanced electrochemical performance of MnFe@NiFe Prussian blue analogue benefited from the inhibition of Mn ions dissolution for sodium-ion batteries. Chem. Eng. J. 2021, 411, 128518. [Google Scholar] [CrossRef]
- Shang, Y.; Li, X.; Song, J.; Huang, S.; Yang, Z.; Xu, Z.J.; Yang, H.Y. Unconventional Mn Vacancies in Mn–Fe Prussian Blue Analogs: Suppressing Jahn-Teller Distortion for Ultrastable Sodium Storage. Chem 2020, 6, 1804–1818. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef]
- Maisuradze, M.; Li, M.; Aquilanti, G.; Plaisier, J.; Giorgetti, M. Characterization of partially Ni substituted manganese hexacyanoferrate cathode material. Mater. Lett. 2023, 330, 133259. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, M.; Wang, Z.; Jiang, Y.; Yao, Y.; Li, S.; Li, Z.; Li, L.; Wu, F.; Chen, R. A Chemical Precipitation Method Preparing Hollow–Core–Shell Heterostructures Based on the Prussian Blue Analogs as Cathode for Sodium-Ion Batteries. Small 2018, 14, 1801246. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Yu, L.; Gao, P.; Liao, X.-Z.; Wen, J.; He, Y.-s.; Tan, G.; Ren, Y.; Ma, Z.-F. Highly crystalline sodium manganese ferrocyanide microcubes for advanced sodium ion battery cathodes. J. Mater. Chem. A 2019, 7, 22248–22256. [Google Scholar] [CrossRef]
- Peng, J.; Gao, Y.; Zhang, H.; Liu, Z.; Zhang, W.; Li, L.; Qiao, Y.; Yang, W.; Wang, J.; Dou, S.; et al. Ball Milling Solid-State Synthesis of Highly Crystalline Prussian Blue Analogue Na2−xMnFe(CN)6 Cathodes for All-Climate Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202205867. [Google Scholar] [CrossRef]
- Zuo, D.; Wang, C.; Wu, J.; Qiu, H.; Zhang, J.; Han, J.; Liu, X. Effect of co-precipitation pH on the electrochemical properties of Prussian blue electrode materials for sodium-ion batteries. Solid State Ion. 2019, 336, 120–128. [Google Scholar] [CrossRef]
- Shen, L.; Jiang, Y.; Liu, Y.; Ma, J.; Sun, T.; Zhu, N. High-stability monoclinic nickel hexacyanoferrate cathode materials for ultrafast aqueous sodium ion battery. Chem. Eng. J. 2020, 388, 124228. [Google Scholar] [CrossRef]
- Liu, X.; Gong, H.; Han, C.; Cao, Y.; Li, Y.; Sun, J. Barium ions act as defenders to prevent water from entering prussian blue lattice for sodium-ion battery. Energy Storage Mater. 2023, 57, 118–124. [Google Scholar] [CrossRef]
- Xi, Y.; Lu, Y. Interpretation on a Nonclassical Crystallization Route of Prussian White Nanocrystal Preparation. Cryst. Growth Des. 2021, 21, 1086–1092. [Google Scholar] [CrossRef]
- El-Hady, D.A.; Lyu, Y.; Zhan, S.; Yang, J.; Wang, Y.; Yang, F.; Zhao, Q.; Gu, M.; Shao, M. Vacancy and Composition Engineering of Manganese Hexacyanoferrate for Sodium-Ion Storage. ACS Appl. Energy Mater. 2022, 5, 8547–8553. [Google Scholar] [CrossRef]
- Hartmann, L.; Deshmukh, J.; Zhang, L.; Buechele, S.; Metzger, M. Reversing the Chemical and Structural Changes of Prussian White After Exposure to Humidity to Enable Aqueous Electrode Processing for Sodium-ion Batteries. J. Electrochem. Soc. 2023, 170, 3. [Google Scholar] [CrossRef]
- Shen, Z.; Guo, S.; Liu, C.; Sun, Y.; Chen, Z.; Tu, J.; Liu, S.; Cheng, J.; Xie, J.; Cao, G.; et al. Na-Rich Prussian White Cathodes for Long-Life Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 16121–16129. [Google Scholar] [CrossRef]
- Li, M.; Gaboardi, M.; Mullaliu, A.; Maisuradze, M.; Xue, X.; Aquilanti, G.; Rikkert Plaisier, J.; Passerini, S.; Giorgetti, M. Influence of Vacancies in Manganese Hexacyanoferrate Cathode for Organic Na-Ion Batteries: A Structural Perspective. ChemSusChem 2023, 16, e202300201. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, B.; Tang, Y.; Xu, B.B.; Liang, C.; Yan, M.; Jiang, Y. Manganese hexacyanoferrate reinforced by PEDOT coating towards high-rate and long-life sodium-ion battery cathode. J. Mater. Chem. A 2020, 8, 3222–3227. [Google Scholar] [CrossRef]
- Peng, J.; Huang, J.; Gao, Y.; Qiao, Y.; Dong, H.; Liu, Y.; Li, L.; Wang, J.; Dou, S.; Chou, S. Defect-Healing Induced Monoclinic Iron-Based Prussian Blue Analogs as High-Performance Cathode Materials for Sodium-Ion Batteries. Small 2023, 19, 2300435. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, X.; Ren, B.; Jiang, L.; Shu, H.; Yang, X.; Chen, Z.; Sun, X.; Liu, E.; Gao, P. High Rate and Long Lifespan Sodium-Organic Batteries Using Pseudocapacitive Porphyrin Complexes-Based Cathode. Nano-Micro Lett. 2021, 13, 71. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.-S.; Aravindan, V. Highly Reversible Na-Intercalation into Graphite Recovered from Spent Li–Ion Batteries for High-Energy Na-Ion Capacitor. ChemSusChem 2020, 13, 5654–5663. [Google Scholar] [CrossRef]
- Pan, Z.-T.; He, Z.-H.; Hou, J.-F.; Kong, L.-B. Designing CoHCF@FeHCF Core–Shell Structures to Enhance the Rate Performance and Cycling Stability of Sodium-Ion Batteries. Small 2023, e2302788. [Google Scholar] [CrossRef]
- Fu, H.; Xia, M.; Qi, R.; Liang, X.; Zhao, M.; Zhang, Z.; Lu, X.; Cao, G. Improved rate performance of Prussian blue cathode materials for sodium ion batteries induced by ion-conductive solid-electrolyte interphase layer. J. Power Sources 2018, 399, 42–48. [Google Scholar] [CrossRef]
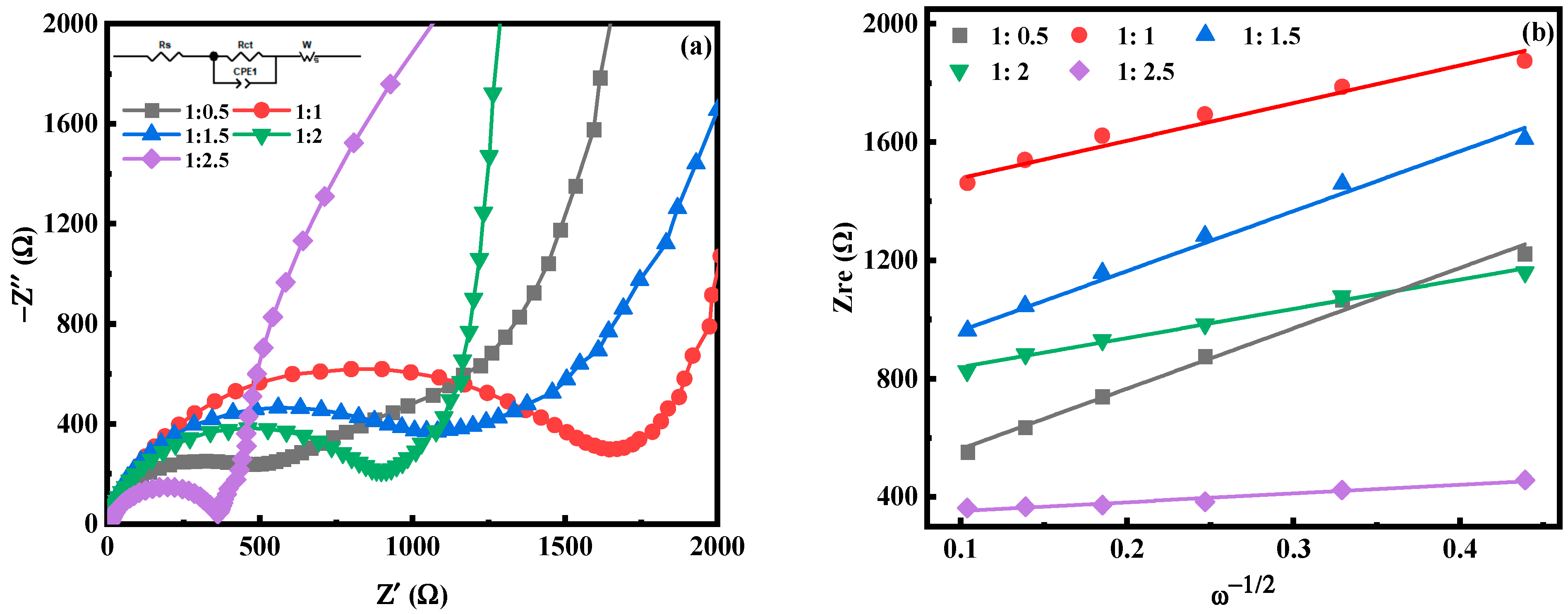







| Samples | Rs (Ω) | Rct (Ω) | DNa (cm2s−1) |
|---|---|---|---|
| Fe:Mn = 1:0.5 | 2.77 | 514 | 1.55 × 10−14 |
| Fe:Mn = 1:1 | 7.73 | 1530 | 4.20 × 10−14 |
| Fe:Mn = 1:1.5 | 3.53 | 1028 | 1.32 × 10−14 |
| Fe:Mn = 1:2 | 4.18 | 829 | 7.02 × 10−14 |
| Fe:Mn = 1:2.5 | 8.34 | 346 | 8.56 × 10−13 |
| Samples | Rs (Ω) | Rct (Ω) | DNa (cm2s−1) |
|---|---|---|---|
| c(Mn2+) = 0.01 mol/L | 4.20 | 654.6 | 3.81 × 10−13 |
| c(Mn2+) = 0.02 mol/L | 4.60 | 480.1 | 3.97 × 10−13 |
| c(Mn2+) = 0.03 mol/L | 4.25 | 483.3 | 3.01 × 10−13 |
| c(Mn2+) = 0.04 mol/L | 6.13 | 583.8 | 2.02 × 10−13 |
| Samples | Element Ratio | ||
|---|---|---|---|
| Na | Mn | Fe | |
| 3 h | 1.67 | 1 | 0.87 |
| 12 h | 1.95 | 1 | 0.95 |
| Samples | Rs (Ω) | Rct (Ω) | DNa (cm2s−1) |
|---|---|---|---|
| 3 h | 2.17 | 639.1 | 2.52 × 10−13 |
| 6 h | 2.71 | 606.9 | 3.63 × 10−13 |
| 9 h | 8.26 | 351.3 | 1.61 × 10−11 |
| 12 h | 10.65 | 297.5 | 2.25 × 10−11 |
| 15 h | 8.27 | 435.8 | 8.80 × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, H.; Wang, J.; Wang, Q.; Wang, H.; Hao, W.; Lu, F. Preparation of Low-Defect Manganese-Based Prussian Blue Cathode Materials with Cubic Structure for Sodium-Ion Batteries via Coprecipitation Method. Molecules 2023, 28, 7267. https://doi.org/10.3390/molecules28217267
Dong X, Wang H, Wang J, Wang Q, Wang H, Hao W, Lu F. Preparation of Low-Defect Manganese-Based Prussian Blue Cathode Materials with Cubic Structure for Sodium-Ion Batteries via Coprecipitation Method. Molecules. 2023; 28(21):7267. https://doi.org/10.3390/molecules28217267
Chicago/Turabian StyleDong, Xinyu, Haifeng Wang, Jiawei Wang, Qian Wang, Hao Wang, Wenhao Hao, and Fanghai Lu. 2023. "Preparation of Low-Defect Manganese-Based Prussian Blue Cathode Materials with Cubic Structure for Sodium-Ion Batteries via Coprecipitation Method" Molecules 28, no. 21: 7267. https://doi.org/10.3390/molecules28217267






